Chapter 21 Lipids Chemistry B11. Lipids - Family of bimolecules. - They are not defined by a...
Transcript of Chapter 21 Lipids Chemistry B11. Lipids - Family of bimolecules. - They are not defined by a...

Chapter 21
Lipids
Chemistry B11

Lipids
- Family of bimolecules.
- They are not defined by a particular functional group, thus they have a variety of structures and functions.
- They are soluble in organic solvents but not in water (nonpolar).
- They contain many nonpolar C—C and C—H bonds and few polar bonds resulting in their water insolubility.

Lipids
1. Store energy: fat cells
2. Chemical messengers: find in nerve fibers and hormones.
3. Parts of membranes: insoluble in water
Lipids

Lipids
1. Simple lipids: (Waxes, Fats & Oils)
2. Complex lipids (Glycerophospholipids)
3. Steroid (Cholesterol & steroid hormones)
4. Eicosanoids
Store energy, insulation
Cell membrane
Chemical messengerCell membrane
Pain, fever, inflammation

Fatty acids
Fatty acids are:
• Long-chain unbranched carbon attached to a carboxyl group.
• Typically 12-18 carbon atoms.
• Insoluble in water.
• Saturated or unsaturated. COOH
COOH
COOH
COOH
Stearic acid (18:0)(mp 70°C)
Oleic acid (18;1)(mp 16°C)
Linoleic acid (18:2)(mp-5°C)
Linolenic acid (18:3)(mp -11°C)
Cis
COOH
COOH
COOH
COOH
Stearic acid (18:0)(mp 70°C)
Oleic acid (18;1)(mp 16°C)
Linoleic acid (18:2)(mp-5°C)
Linolenic acid (18:3)(mp -11°C)

Saturated and unsaturated Fatty acids
COOH
COOH
COOH
COOH
COOH
COOH
COOH
COOH
COOH
COOH
Saturated fatty acids are solids at room temperature.
Packed together Maximum London dispersion forces
Unsaturated fatty acids are liquids at room temperature.
Can not pack together London dispersion forces

• The human body is capable of synthesizing most fatty acids from
carbohydrates or other fatty acids.
• Humans do not synthesize sufficient amounts of fatty acids that
have more than one double bond.
• More than one double bond fatty acids are called essential fatty
acids and they must be provided by the diet.
Fatty acids

Long-chain alcohol Fatty acidEster bond
Waxes
- are found in many plants and animals (or humans).
- In plants, they help prevent loss of water and damage from pests.
- In humans and animals, provide waterproof coating on skin and fur.
Wax is an ester of saturated fatty acid and long chain alcohol.

For example, shown below is the formation of spermaceti wax, isolated from the heads of sperm whales.
Waxes
Acid

Beeswax CarnaubaCoating
Jojoba
Lanolin from wool lotions

Triacylglycerols are:
• Fats and oils (are stored in the body).
• Triesters of glycerol.
• Produced by Fischer esterification.
• Formed when the hydroxyl groups of glycerol react with the carboxyl groups of fatty acids.
Triacylglycerols (Triglycerides)

glycerol three fatty acids triacylglycerol
OHCH2
OH
OHCH2
CHO
(CH2)14CH3CHO
O
(CH2)14CH3CHO
O
(CH2)14CH3CHO
+ 3H2O
O
O
C (CH2)14CH3
CH O
O
C (CH2)14CH3
CH2 O
O
C (CH2)14CH3
CH2
Esterification
Acid

CH(CH2)7CH3(CH2)5CH
O
C
CH(CH2)7CH3(CH2)5CH
O
C
CH(CH2)7CH3(CH2)5CH
O
C
O
O
OCH2
CH2
CH
GLYCEROL
Fatty acid
Fatty acid
Fatty acid
Triacylglycerols (Triglycerides)
Produced by esterification of glycerol (a trihydroxyl alcohol).
CH2
CH
CH2
OH
OH
OH
Glycerol

Triacylglycerols (Triglycerides)
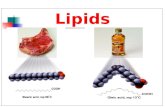
Fat: is a triacylglycerol that is solid at room temperature.
Made by more saturated fatty acids (Saturated triacylglycerols).
Meat, milk, butter and cheese (animal sources).
Oil: is a triacylglycerol that is liquid at room temperature.
Made by more unsaturated fatty acids (Unsaturated triacylglycerols).
Corn, cotton seed, safflower and sunflower (plant sources).
Both are colorless, odorless, and tasteless.

- Hydrogen adds to the double bonds of unsaturated fats (using transition
metal catalyst such as Ni).
- Melting point is increased.
- Liquid oils are converted to semisolid fats.
Hydrogenation
_C=C_ + H2 → _C_C_Ni
H H
H H
H H

Ni
+ 3H2
glyceryl Trioleate (triolein)
glyceryl tristearate (tristearin)
O
(CH2)14CH3C
O
(CH2)14CH3C
O
(CH2)14CH3C
O
O
OCH2
CH2
CH
CH(CH2)7CH3(CH2)5CH
O
C
CH(CH2)7CH3(CH2)5CH
O
C
CH(CH2)7CH3(CH2)5CH
O
C
O
O
OCH2
CH2
CH
1- Hydrogenation

2- Hydrolysis
Triacylglycerols are hydrolysis (split by water) in the presence of strong acid or lipase (digestive enzyme).
O
O
C (CH2)14CH3
CH O
O
C (CH2)14CH3
CH2 O
O
C (CH2)14CH3
CH2
+ 3H2O
Na+ -O
O
C (CH2)14CH3+H+ or Lipase
OH
CH OH
CH2 OH
CH2
3HO

• Is the process of forming “soaps” (salts of fatty acids).
• Is the reaction of a fat with a strong base (NaOH).
• Splits triacylglycerols into glycerol and the salts of fatty acids.
• With KOH or the oils that are polyunsaturated gives softer soaps (liquid soaps).
• Name of soap gives the source of the oil.
3- Saponification
Like coconut or avocado soap

O
O
C (CH2)14CH3
CH O
O
C (CH2)14CH3
CH2 O
O
C (CH2)14CH3
CH2
+ 3NaOH
Na+ -O
O
C (CH2)14CH33
OH
CH OH
CH2 OH
CH2
+
“soap”
Heat
Salt of fatty acid
3- Saponification (Basic Hydrolysis)

GLYCEROL
Fatty acid
Fatty acid
phosphateAminoalcohol
Glycerophospholipids
Polar part (polar head) and nonpolar part (nonpolar tail)
P
O
OO
O_
N
CH3
HO – CH2 _ CH2
CH3
CH3
+
Interact with both polar and nonpolar substances.
1. Most abundant lipids in cell membranes (semipermeable).
2. Combine with less polar triglycerides and cholesterol to make them soluble.
Choline

Steroids have:
• A steroid nucleus which is 4 carbon rings.
• Attached groups that make the different types of compounds.
• No fatty acids. steroid nucleus
Steroids

Cholesterol:
• Is the most abundant steroid in the body.
• Insoluble in water (need a water soluble carrier).
• Has methyl CH3- groups, alkyl chain, and -OH attached to the steroid nucleus.
CH3
CH3CH3
CH3
HO
CH3
Cholesterol

Cholesterol:
• Is obtained from meats, milk, and eggs.
• Is synthesized in the liver from fats, carbohydrates and proteins.
• Is needed for cell membranes, brain and nerve tissue, steroid hormones, and Vitamin D.
• Clogs arteries when high levels form plaque.
• No cholesterol in vegetable and plants.
At artery clogged by cholesterol plaque
Cholesterol
Gallstones form in gallbladder

Steroid hormones are:
Chemical messengers in body
Sex hormones
Testosterone & androsterone in males Estrogen & progesterone in females
OHCH3
CH3
HO
OHCH3
CH3
O
Testosterone (androgen)Male sex hormone
EstrogenFemale sex hormone
Steroids

Triacylglycerols
Lipoproteins
Transporting lipids through the bloodstream to tissues where they are stored, Used for energy, or to make hormones.
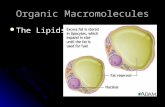
Spherical particles
Polar surface and nonpolar inner
Water-soluble form of lipids
(soluble in blood)

Lipoproteins
VLDL: very-low-density lipoprotein
Liver Fat storage cells
Heart and muscles
LDL
VLDL
HDLEnergyIntestine
andelimination
Triglycerides and Cholesterol
LDL: low-density lipoprotein (bad Cholesterol) Cholesterol
Chylomicrons Triglycerides and Cholesterol
HDL: high-density lipoprotein (good Cholesterol) Cholesterol
Recommended levels are: HDL > 40 mg/dL, LDL < 100 mg/dL, total serum cholesterol < 200 mg/dL.

Cell Membrane
Semipermeable: nutrients can enter and waste products can leave.
Fluid mosaic model
NonpolarPolar
Phospholipidbilayer
Carbohydrate