LIPIDS. -Lipids are an amphiphilic class of hydrocarbon-containing organic compounds. -they have...
-
Upload
cecilia-kelley -
Category
Documents
-
view
212 -
download
0
Transcript of LIPIDS. -Lipids are an amphiphilic class of hydrocarbon-containing organic compounds. -they have...

LIPIDS

-Lipids are an amphiphilic class of hydrocarbon-containing organic compounds.
-they have complicated solvation properties
-Lipid molecules have these properties because they consist largely of long hydrocarbon tails which are lipophilic in nature as well as polar headgroups (e.g. phosphate-based functionality)
-In living organisms, lipids are used for energy storage, serve as the structural components of cell membranes, and constitute important signalling molecules.
- Although the term lipid is often used as a synonym for fat, the latter is in fact a subgroup of lipids called triglycerides
Lipids

LipidsSaponified lipids
- I. Energy storing lipids: triacylgricerols or Glycerides-II.Structural lipids : waxes
phospholipids sphingolipids
non-saponifiable lipids - (poly)terpenes and derivatives
-fatty acid derivatives: prostaglandins

Fats Lipids

Saponified lipids
- Energy storing lipids: triacylgricerols: fats
Lipids
Glycerides are lipids
From a glycerol (propan-1, 2, 3-triol) core structure with one or more different fatty acyl groups (3)
these fatty acid-derived chains attached to the glycerol backbone by ester linkages
Glycerides with three acyl groups (triglycerides or neutral fats) are the main storage form of fat in animals and plants.

Saponified lipids
- Energy storing lipids: triacylgricerols: fats(glycerol + fatty acids)
Lipids
Fatty acids-Chemically, fatty acids can be described as long-chain monocarboxylic acids and have a general structure of CH3(CH2)nCOOH.
-The length of the chain usually ranges from 12 to 24, always with an even number of carbons.
-When the carbon chain contains no double bonds,it is a saturated chain.
-If it contains one or more double bonds, it is unsaturated

-The presence of double bonds generally reduces the melting point of fatty acids
-Fats contain usually saturated fatty acids
- Oils contain (poly)unsaturated fatty acids (e.g. Vegetable oils are rich in various polyunsaturated fatty acids )
- unsaturated fatty acids can occur either in cis or trans geometric isomers.
- In naturally occurring fatty acids, the double bonds are always in the cis-configuration
LipidsFat

Lipids
Fatty acids in animals: double bonds in the first half of the fatty acidsIn plants: double bonds in the second half of the fatty acids
Essential fatty acids: e.g. oleic acid (ω-9)linoleic acid (ω-6)linolenic acid (ω-3)
Only plants can produce them- double bonds in the 2nd half of the fatty acid

LipidsFat
the fatty acid oxidation is the main energy source of animalsthe oxidation number of the carbon in the fatty acids is -2The oxidation number of the carbon in the glucose „chain” is 0 When we oxidaze the carbon to CO2 (oxidation number +4)We can get more energy from fats
the fat is the most concentrated form of energy in living organisms
lipid molecules are so hydrophobe, they exclude everything – the adipose tissue contain only fat, pure substanceThe fat is stored separated from the place where E is neededThe transport of fatty acids is compicated.
the free fatty acids are highly toxic compounds

Lipids
Chemical properties
1. saponification
CH2-O-C-R1
CH-O-C-R2
CH2-O-C-R3
║
║
║
O
O
O
+ NaOHheat
CH2-OH NaO-C-R1
CH-OH + NaO-C-R2
CH2-OH NaO-C-R3
║
║
║
O
O
O
Fat

Inorganic esters
In aquaeus solutions they form micells
Amphipate molecules : likes polar and non polar as well
Detergent effect: they separate the non-polar substances from each other – cleaning effect
e.g. washing powder
Lipids

Other saponified fatty group: Inorganic esters
e.g. inorganic phospate ester : glucose 6 phosphate
Sulfate ester : ester of alcohol and sulfuric acid
R-CH2-O-S║
O
O-
O║
R-CH2-O-P║
O
O-
O-
Long carbon chain = non-polar part
Long carbon chain = non-polar part
Polar head
Polar head
Lipids

Lipids
II. Structural lipids :
1.waxes: long chain alcohols with long chain fatty acids (protection in the plant world)
2. phospholipids
Phospholipids are a class of lipids, and a major component of all biological membranes, along with glycolipids, cholesterol and proteins

2. phospholipids
Lipids

2. phospholipidsLipids
They are built upon one of a backbone:glycerol backbone, derivatives known as glycerophospholipids (or phosphoglycerides) The following components are attached to the carbons on the backbone:- a saturated fatty acid- an unsaturated fatty acid - a negatively-charged phosphate group,Which is usually attached to a nitrogen containing alcohol like ethanolamine or an organic compound such as choline.
e.g. X: -CH2-CH2-NH3+
Ethanolamine
Forming phosohatidyl ethanolamine

Lipids2. phospholipids
Phosphoglycerides are found in biological membranes, such as the cell's plasma membrane and the intracellular membranes of organelles

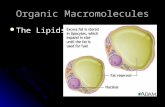
-Formation of lipid bilayers is an energetically-favoured process when the glycerophospholipids are in an aqueous environment. In an aqueous system, the polar heads of lipids orientate towards the polar, aqueous environment, while the hydrophobic tails minimise their contact with water. The lipophilic tails of lipids (U) tend to cluster together, forming a lipid bilayer (1) or a micelle (2) (for U; 1; 2 see image before).
Lipids2. phospholipids
the non polar part:the phospholipid unsaturated carbohydrate tails are in cis coformation They can interact with each other- the membrane is more stable and they can keep the mobility of the molecules in the membrane- fluid, not rigid membrane
biological membranes

Lipids2. phospholipids
biological membranes
The fluidity of the mambrane depends on the conformation of the non polar tails ( cis) and the quality of the fatty acids:More saturated membrane, more rigid, the melting point is higherMore unsaturated membrane is more fluide , the melting point is lowerIn cold and warm periodes we have to compensate the fluidity of our membranes: in winter we need more unsaturated fatty acidsIn warm periode we need more saturated fatty acids

Lipids3. Sphingolipids
- are a class of lipids derived from the aliphatic amino alcohol sphingosine.- Sphingolipids are often found in neural tissue, and play an important role in both signal transmission and cell recognition.
The sphingosine backbone is O-linked to a (usually) charged head group such as ethanolamine or choline.The backbone is also amide-linked to an acyl group, such as a fatty acid.

Lipids3. Sphingolipids
Ceramides are the simplest type of sphingolipid.They consist simply of a fatty acid chain attached through an amide linkage to sphingosine.
R= H

Lipids3. Sphingolipids
Function
-Sphingolipids are commonly believed to protect the cell surface against harmful environmental factors by forming a mechanically stable and chemically resistant outer leaflet of the plasma membrane lipid bilayer
-Certain complex glycosphingolipids were found to be involved in specific functions, such as cell recognition and signaling.
Recently, relatively simple sphingolipid metabolites, such as ceramide and sphingosine-1-phosphate, have been shown to be important mediators in the signaling cascades involved in apoptosis, proliferation, and stress responses

Lipids4. Terpenes
Terpenes are a large and varied class of hydrocarbons, produced primarily by plantsThey are the major components of resin, and of turpentine produced from resin. The name "terpene" is derived from the word "turpentine".
When terpenes are modified chemically, such as by oxidation or rearrangement of the carbon skeleton, the resulting compounds are generally referred to as terpenoids. Terpenes and terpenoids are the primary constituents of the essential oils of many types of plants and flowers.Essential oils are used widely as natural flavor additives for food, as fragrances in perfumery, in aromatherapy, and in traditional and alternative medicines.Synthetic variations and derivatives of natural terpenes and terpenoids also greatly expand the variety of aromas used in perfumery and flavors used in food additives.

plant world: di, tri and sesqui terpens: fragrances, flavours, aromas polyterpens milks, resins
animal world: steroids (mostly free forms, sometimes esters or salts) structural and signalling functions
Lipids4. Terpenes

4. Terpenes Lipids
-Terpenes are derived biosynthetically from units of isoprene, which has the molecular formula C5H8.
-The basic molecular formulas of terpenes are multiples of that, (C5H8)n where n is the number of linked isoprene units.
-This is called the isoprene rule or the C5 rule.
- The isoprene units may be linked together "head to tail" to form linear chains or they may be arranged to form rings.
Isoprene unit
As chains of isoprene units are built up, the resulting terpenes are classified sequentially by size as hemiterpenes, monoterpenes, sesquiterpenes, diterpenes, sesterterpenes, triterpenes, and tetraterpenes.

Lipids4. Terpenes
Hemiterpenes consist of a single isoprene unit. Isoprene itself is considered the only hemiterpeneMonoterpenes consist of two isoprene units and have the molecular formula C10H16. Sesquiterpenes consist of three isoprene units and have the molecular formula C15H24
The sesqui- prefix means one and a half. Diterpenes are composed for four isoprene units and have the molecular formula C20H32. Examples: taxadiene (precursor of taxol). Diterpenes also form the basis for biologically important compounds such as retinol, retinal, and phytol. Sesterterpenes, terpenes having 25 carbons and five isoprene units, are rare relative to the other sizes. The sester- prefix means half to three, i.e. two and a half. Triterpenes consist of six isoprene units and have the molecular formula C30H48.
Tetraterpenes contain eight isoprene units and have the molecular formula C40H56. Biologically important tetraterpenes include the acyclic lycopene, the monocyclic gamma-carotene, and the bicyclic alpha- and beta-carotenes
Polyterpenes consist of long chains of many isoprene units.

4. Terpenes Lipids
Polyterpenes consist of long chains of many isoprene units.Sterane sceletons
e.g. Cholesterol
Sterane sceleton + -OH
Cholesterol is a sterol (a combination steroid and alcohol), a lipid found in the cell membranes of all body tissues, and is transported in the blood plasma of all animalsIts level in blood can be raised beyond natural levels by eating food in excess of daily requirements, especially with cholesterol-containing foods.
Cholesterol plays a central role in many biochemical processes, but is best known for the association of cardiovascular disease with various lipoprotein cholesterol transport patterns and high levels of cholesterol in the blood. Cholesterol is insoluble in blood, but is transported in the circulatory system bound to one of the varieties of lipoprotein, spherical particles which have an exterior composed mainly of water-soluble proteins.

4. Terpenes Lipids
Steroid hormons: different substituent on the sterane sceleton
e.g. aldosteron testosteron progesteron

testosteroneprogesterone














![Stimuli-responsive mesoporous silica nanoparticles for ... · polymers, lipids, self-assembling amphiphilic molecules, metals and other inorganic materials [14]. The raw material](https://static.fdocuments.in/doc/165x107/5fa6ffd31f655536fd2de42c/stimuli-responsive-mesoporous-silica-nanoparticles-for-polymers-lipids-self-assembling.jpg)



