Chapter 19 Lipids Chemistry 203. Lipids - Family of bimolecules. - They are not defined by a...
-
Upload
caroline-hodges -
Category
Documents
-
view
221 -
download
4
Transcript of Chapter 19 Lipids Chemistry 203. Lipids - Family of bimolecules. - They are not defined by a...
Lipids
- Family of bimolecules.
- They are not defined by a particular functional group, thus they have a variety of structures and functions.
- They are soluble in organic solvents but not in water (nonpolar).
- They contain many nonpolar C—C and C—H bonds and few polar bonds resulting in their water insolubility.
1. Store energy: fat cells
2. Chemical messengers: find in nerve fibers and hormones.
3. Parts of membranes: insoluble in water
Lipids
Lipids
1. Simple lipids: (Waxes, Fats & Oils)
2. Complex lipids (Glycerophospholipids)
3. Steroid (Cholesterol & steroid hormones)
4. Eicosanoids
Store energy, insulation
Cell membrane
Chemical messengerCell membrane
Pain, fever, inflammation
Lipids can be categorized as:
1. Hydrolyzable lipids can be converted into small molecules by aqueous hydrolysis.
Lipids
Lipids can be categorized as:
2. Nonhydrolyzable lipids cannot be cleaved into smaller molecules by aqueous hydrolysis.
Lipids
Hydrolysis: reaction with water.
(breaking a bond and adding the elements of water)
RCOR 'O
RC-OHO
H- O R '
= =An alcoholA carboxylic acidAn ester
+ H2O +Heat
H+ or enzyme
Hydrolysis
Most hydrolyzable lipids contain an ester.
Fatty acids
Fatty acids are:
• Long-chain unbranched carbon attached to a carboxyl group (-COOH).
• Typically 12-20 carbon atoms.
• They have an even number of C atoms.
• Insoluble in water.
COOH
COOH
COOH
COOH
Stearic acid (18:0)(mp 70°C)
Oleic acid (18;1)(mp 16°C)
Linoleic acid (18:2)(mp-5°C)
Linolenic acid (18:3)(mp -11°C)
COOH
COOH
COOH
COOH
Stearic acid (18:0)(mp 70°C)
Oleic acid (18;1)(mp 16°C)
Linoleic acid (18:2)(mp-5°C)
Linolenic acid (18:3)(mp -11°C)
Cis
Hydrolyzable lipids are derived from fatty acids.
Fatty acids
nonpolar portion = hydrophobic
polar portion = hydrophillic
CH3(CH2)14COOH (palmitic acid)
Hydrophobic portion is much bigger than hydrophilic portion. Insoluble in water
Saturated and unsaturated Fatty acids
Saturated fatty acids have no double bonds in their long hydrocarbon chains.
COOH
COOH
COOH
COOH
COOH
Packed together Maximum London dispersion forces
They are solids at room temperature.
Stearic acid: CH3(CH2)16COOH
Unsaturated fatty acids have 1 or more double bonds (generally cis) in their long hydrocarbon chains.
Saturated and unsaturated Fatty acids
COOH
COOH
COOH
COOH
COOH
They can not pack together London dispersion forces
They are liquids at room temperature.
Oleic acid: CH3(CH2)7CH=CH(CH2)7COOH
• The human body is capable of synthesizing most fatty acids from
carbohydrates or other fatty acids.
• Humans do not synthesize sufficient amounts of fatty acids that
have more than one double bond.
• More than one double bond fatty acids are called essential fatty
acids and they must be provided by the diet.
Fatty acids
Linoleic acid linolenic acid
Linoleic acid is called an omega-6 acid, because of the position of the first C=C in the nonpolar chain.
Essential Fatty acids
Omega-n acids n: the position of the first double bond
Linolenic acid is called an omega-3 acid, because of the position of the first C=C in the nonpolar chain.
Essential Fatty acids
Long-chain alcohol Fatty acidEster bond
Waxes
Wax is an ester of saturated fatty acid and long chain alcohol.
Acid
For example, shown below is the formation of spermaceti wax, isolated from the heads of sperm whales.
Waxes
Acid
Because of their long nonpolar C chains, waxes are very hydrophobic.
Waxes
Beeswax(myricyl palmitate)
CH3(CH2)14 C
O
O(CH2)29CH3
hydrophobic region
hydrophobic region
They form protective coatings:
- In plants, they help prevent loss of water and damage from pests.
- In humans and animals, provide waterproof coating on skin and fur.
Beeswax CarnaubaCoating
Jojoba
Lanolin from wool lotions
Triacylglycerols are:
• Fats and oils (are stored in the body).
• Triesters of glycerol.
• Produced by Fischer esterification.
• Formed when the hydroxyl groups of glycerol react with the carboxyl groups of fatty acids.
Triacylglycerols (Triglycerides)
glycerol three fatty acids triacylglycerol
OHCH2
OH
OHCH2
CHO
(CH2)14CH3CHO
O
(CH2)14CH3CHO
O
(CH2)14CH3CHO
+ 3H2O
O
O
C (CH2)14CH3
CH O
O
C (CH2)14CH3
CH2 O
O
C (CH2)14CH3
CH2
Esterification
Acid
CH(CH2)7CH3(CH2)5CH
O
C
CH(CH2)7CH3(CH2)5CH
O
C
CH(CH2)7CH3(CH2)5CH
O
C
O
O
OCH2
CH2
CH
GLYCEROL
Fatty acid
Fatty acid
Fatty acid
Triacylglycerols (Triglycerides)
Produced by esterification of glycerol (a trihydroxyl alcohol).
CH2
CH
CH2
OH
OH
OH
Glycerol
Simple triacylglycerols have three identical fatty acid side chains.
Triacylglycerols (Triglycerides)
Mixed triacylglycerols have two or three different fatty acids.
Triacylglycerols (Triglycerides)
Monounsaturated triacylglycerols have 1 C=C bond.
Polyunsaturated triacylglycerols have many C=C bonds.
Increasing the number of double bonds in the fatty acid chain decreases the melting point of the triacylglycerol.
Saturated triacylglycerols contain only saturated fatty acids.
Triacylglycerols (Triglycerides)
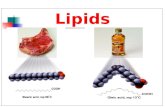
Fat: is a triacylglycerol that is solid at room temperature.
Made by more saturated fatty acids (Saturated triacylglycerols).
Meat, milk, butter and cheese (animal sources).
Oil: is a triacylglycerol that is liquid at room temperature.
Made by more unsaturated fatty acids (Unsaturated triacylglycerols).
Corn, cotton seed, safflower and sunflower (plant sources).
Both are colorless, odorless, and tasteless.
- Fats are used to build cell membranes, insulate the body, and store energy for later use.
- It is recommended that no more than 20-35% of a person’s caloric intake should come from lipids.
- A high intake of saturated triacylglycerols is linked to heart disease.
- Saturated fats stimulate cholesterol synthesis in the liver, which can lead to cholesterol plaques building up inside arteries.
- The result is high blood pressure, heart attack, and even stroke.
Fat & Health
- Unlike other vegetable oils, oils from palm and coconut trees are very high in saturated fats.
- Unsaturated triacylglycerols (omega-3 fatty acids from fish) lower the risk of heart disease by decreasing the level of cholesterol in the blood.
- However, if the double bond of the unsaturated triacylglycerol is trans, the beneficial effect is lost.
- Trans fats, which are primarily synthesized instead of naturally occurring, act like saturated fats and increase the cholesterol levels in the blood.
Fat & Health
- Hydrogen adds to the double bonds of unsaturated fats (using transition
metal catalyst such as Ni).
- Melting point is increased.
- Liquid oils are converted to semi-solid fats.
1- Hydrogenation
_C=C_ + H2 → _C_C_Ni
H H
H H
H H
Ni
+ 3H2
glyceryl Trioleate (triolein)
glyceryl tristearate (tristearin)
O
(CH2)14CH3C
O
(CH2)14CH3C
O
(CH2)14CH3C
O
O
OCH2
CH2
CH
CH(CH2)7CH3(CH2)5CH
O
C
CH(CH2)7CH3(CH2)5CH
O
C
CH(CH2)7CH3(CH2)5CH
O
C
O
O
OCH2
CH2
CH
1- Hydrogenation
__
2- Hydrolysis
Triacylglycerols are hydrolysis (split by water) in the presence of strong acid or lipase (digestive enzyme).
O
O
C (CH2)14CH3
CH O
O
C (CH2)14CH3
CH2 O
O
C (CH2)14CH3
CH2
+ 3H2O
Na+ -O
O
C (CH2)14CH3
OH
CH OH
CH2 OH
CH2
3H+H+ or Lipase
H
H
H
O
- Humans store energy as triacylglycerols in adipose cells below the surface of the skin, in the breast area, and surrounding internal organs.
- The number of adipose cells is constant; weight gained or lost causes them to swell or shrink, but not decrease or increase in number.
- To metabolize triacylglycerols for energy, the esters are hydrolyzed by enzymes called lipases.
- Complete metabolism of a triacylglycerol yields CO2, H2O, and a great deal of energy.
Metabolism of tricaylglycerols
- Is the process of forming “soaps” (salts of fatty acids).
- Is the reaction of a fat with a strong base (NaOH).
- Splits triacylglycerols into glycerol and the salts of fatty acids.
- With KOH or the oils that are polyunsaturated gives softer soaps (liquid soaps).
- Soaps are typically made from lard (from hogs), tallow (from cows or sheep), coconut oil, or palm oil.
- All soaps work in the same way, but have different properties depending on the lipid source, length of C chain, and degree of unsaturation.
3- Saponification (Basic Hydrolysis)
O
O
C (CH2)14CH3
CH O
O
C (CH2)14CH3
CH2 O
O
C (CH2)14CH3
CH2
+ 3NaOH
Na+ -O
O
C (CH2)14CH3
OH
CH OH
CH2 OH
CH2
+
“soap”
Heat
Salt of fatty acid
3- Saponification (Basic Hydrolysis)
H
H
H
Na+-O3
Sodium soaps1,2,3-Propanetriol(Glycerol; glycerin)
A triglyceride( a triester of glycerol)
+saponification
+
CH2OCR
CH2OCR
CHOH
CH2OH
CH2OH
RCOCH 3NaOH 3RCO-Na
+O
O
O
O
Soaps
Hydrophobic part: nonpolar
Hydrophilic part: polar (remains in contact with environment)
When soap is mixed with dirt (grease, oil, and …), soap
micelles “dissolve” these nonpolar, water-insoluble molecules.
Soaps
They are the main component of most cell membranes.
Structurally, they resemble a triacylglycerol, except the third fatty acid has been replaced with a phosphodiester bonded to an alcohol.
phospholipids
1. Phosphoacylglycerols:
Amino alcohol
Fatty acid
Fatty acid
1. Phosphoacylglycerols:
phospholipids
There are two types of phosphoacylglycerols:
CholineEthanolamine
phospholipids
2. Sphingomyelins: They differ in two ways:
1. They do not contain a glycerol backbone, they have a sphingosine backbone instead.
sphingosine
2. They do not contain an ester; their single fatty acid is bonded to the backbone by an amide bond.
phospholipids
2. Sphingomyelins:
The myelin sheath, the coating that surrounds nerve cells, is rich in sphingomyelins.
Interact with both polar and nonpolar substances.
1. Most abundant lipids in cell membranes (semipermeable).
2. Combine with less polar triglycerides and cholesterol to make them soluble.
phosphoacylglycerols
O R Polar
Nonpolar
Cell Membrane
Semipermeable: selected nutrients can enter and waste products can leave.
Fluid mosaic model
NonpolarHydrophobic
PolarHydrophilic
Phospholipidbilayer
Carbohydrate
- Peripheral proteins are embedded within the membrane and extend outward on one side only.
- Integral proteins extend through the entire bilayer.
- Sometimes carbohydrates are attached to the exterior of the cell forming glycolipids and glycoproteins.
Cell Membrane
Transport Across a Cell Membrane
Simple Diffusion: Small molecules like O2 and CO2 can diffuse through the cell membrane, traveling from higher to lower concentration.
Facilitated Transport: Larger polar molecules (glucose) and ions (Cl- and HCO3
-) travel through integral protein channels.
Active Transport: Other ions, Na+, K+, and Ca2+, move against the concentration gradient; this required energy input.
Steroids have:
• A steroid nucleus which is 4 carbon rings.
• Attached groups that make the different types of compounds.
• No fatty acids.
Steroids
(steroid nucleus)
Cholesterol:
• Is the most abundant steroid in the body.
• Insoluble in water (need a water soluble carrier).
• Has methyl CH3- groups, alkyl chain, and -OH attached to the steroid nucleus.
CH3
CH3CH3
CH3
HO
CH3
Cholesterol
Cholesterol:
• Is obtained from meats, milk, and eggs.
• Is synthesized in the liver from fats, carbohydrates and proteins.
• Is needed for cell membranes, brain and nerve tissue, steroid hormones, and Vitamin D.
• Clogs arteries when high levels form plaque (because it is insoluble in blood).
• No cholesterol in vegetable and plants.
At artery clogged by cholesterol plaque
Cholesterol
Gallstones form in gallbladder
Triacylglycerols
Lipoproteins
Transporting lipids through the bloodstream to tissues where they are stored, Used for energy, or to make hormones.
Spherical particles
Polar surface and nonpolar inner
Water-soluble form of lipids
(soluble in blood)
Lipoproteins
VLDL: very-low-density lipoprotein
Liver Fat storage cells
Heart and muscles
LDL
VLDL
HDLEnergyIntestine
andelimination
Triglycerides and Cholesterol
LDL: low-density lipoprotein (bad Cholesterol) Cholesterol
Chylomicrons Triglycerides and Cholesterol
HDL: high-density lipoprotein (good Cholesterol) Cholesterol
Recommended levels are: HDL > 40 mg/dL, LDL < 100 mg/dL, total serum cholesterol < 200 mg/dL.
1. Sex hormones
2. Adrenal Cortical Steroids
Steroid Hormones
A hormone is a molecule that is synthesized in one part of an organism, which then elicits a response at a different site.
Two types of steroids hormones:
Estrogens & progestins in females
Androgens in males
Sex Hormones
The estrogens estradiol and estrone control development of secondary sex characteristics, regulate the menstrual cycle, and are made in the ovaries.
Estrogens (Female Sex Hormones):
Sex Hormones
Progestins (Female Sex Hormones):
The progestin progesterone is called the “pregnancy hormone”; it is responsible for the preparation of the uterus for implantation of a fertilized egg.
Sex Hormones
Androgens (Male Sex Hormones):
Testosterone and Androsterone are androgens made in the testes.They control the development of secondary sex characteristics in males.
- Synthetic androgen analogues, called anabolic steroids, promote muscle growth.
- They have the same effect as testosterone, but are more stable, so they are not metabolized as quickly.
- They have come to be used by athletes and body builders, but are not permitted in competitive sports.
- Prolonged use of anabolic steroids can cause physical and psychological problems.
Sex Hormones
Adrenal Cortical Steroids
Aldosterone regulates blood pressure and volume by controlling the concentration of Na+ and K+ in body fluids.
Cortisone and cortisol serve as anti-inflammatory agents, which also regulate carbohydrate metabolism.
aldosteronecortisone
cortisol
Prolonged use of these steroids can have undesired side effects, including bone loss and high blood pressure.
Adrenal Cortical Steroids
Prednisone, a synthetic alternative, has similar anti-inflammatory properties but can be taken orally.
Cortisone are used to suppress organ rejection after transplant surgery and to treat many allergic and autoimmune disorders.
They are organic compounds required in small quantities for normal metabolism.
Vitamins are either water soluble or fat soluble.
The four fat-soluble vitamins (A, D, E, and K) are lipids and nonpolar.
Excess vitamins are stored in adipose cells to be used when needed.
Vitamins
They must be obtained from the diet (our cells cannot synthesize them).
They are found in fruits, vegetables, fish, liver, and dairy products.
Vitamins
It is found in liver, fish, and dairy products, and is made from β-carotene (the orange pigment in carrots).
It is needed for vision and for healthy mucous membranes.
Vitamin A deficiency causes night blindness and dry eyes and skin.
Vitamin A
Vitamins
Vitamin D
Vitamin D can be synthesized from cholesterol.
It can be obtained in the diet from many foods, especially milk, and helps regulate Ca and P metabolism.
A deficiency of vitamin D causes rickets (bone malformation).
Vitamins
Vitamin E
Vitamin E is an antioxidant, protecting unsaturated side chains in fatty acids from unwanted oxidation.
Deficiency of vitamin E causes numerous neurological problems, although it is rare.
Vitamin K
Vitamin K regulates the synthesis of clotting proteins (prothrombin), and deficiency of this leads to excessive or fatal bleeding.
Vitamins
Prostaglandins and Leukotrienes are two types of eicosanoids (20 C atoms derived from the fatty acids).
Eicosanoids
- All eicosanoids are very potent compounds, which are not stored in cells, but rather synthesized in response to external stimulus.
- Unlike hormones they are local mediators, performing their function in the environment in which they are synthesized.
Prostaglandins are carboxylic acids that contain a five-membered ring and have a wide range of biological activities.
Prostaglandins
Prostaglandins
Prostaglandins are responsible for inflammation.
- Aspirin and ibuprofen relieve pain and inflammation by blocking the synthesis of these molecules.
- Prostaglandins also decrease gastric secretions, inhibit blood platelet aggregation, stimulate uterine contractions, and relax smooth muscles.
- There are two different cylcooxygenase enzymes responsible for prostaglandin synthesis called COX-1 and COX-2.
Prostaglandins
COX-1 is involved in the usual production of prostaglandins.
COX-2 is responsible for additional prostaglandins in inflammatory diseases like arthritis.
- Nonsteroidal anti-inflammatory drugs (NSAIDs) like aspirin and ibuprofen inactivate both COX-1 and -2, but increase risk for stomach ulcer formation.
- Drugs sold as Vioxx, Bextra, and Celebrex block only the COX-2 enzyme without affecting gastric secretions.
Leukotrienes are molecules that contribute to the asthmatic response by constricting smooth muscle of the lung.
Asthma is characterized by chronic inflammation, so inhaled steroids to reduce this inflammation are commonly used.
New asthma drugs act by blocking the synthesis of leukotriene C4, which treat the disease instead of just the inflammation symptoms.
Leukotrienes