Vegetal Waste
-
Upload
douglas-pereira -
Category
Documents
-
view
67 -
download
0
Transcript of Vegetal Waste

Review paper
Transformation of vegetable waste into value added products:
(A) the upgrading concept; (B) practical implementations
G€uunther Laufenberg a,*, Benno Kunz a, Marianne Nystroem b
a Department of Food Technology, University Bonn, Roemerstr. 164, D-53117 Bonn, Germanyb Department of Chemical Technology, Lappeenranta University of Technology, Postbox 20, FIN-53851 Lappeenranta, Finland
Abstract
Waste can contain many reusable substances of high value. Depending on there being an adequate technology this residual
matter can be converted into commercial products either as raw material for secondary processes, as operating supplies or as in-
gredients of new products. Numerous valuable substances in food production are suitable for separation and recycling at the end of
their life cycle, even though present separation and recycling processes are not absolutely cost efficient.
In Part A a need statement is visualised––based on a holistic concept of food production––for the vegetable industry, recording
occurrence, quantities and utilisation of the residual products. A literature survey, covering more than 160 articles from all over the
world, plus our own investigations summarises the latest knowledge in the above-mentioned field and outline prospects for future
economic treatment of vegetable ‘co-products’.
The main goal of a clean production process is demonstrated by three practical implementations in Part B:
1. Upgrading of vegetable residues for the production of novel types of products: multifunctional food ingredients in fruit juice and
bakery goods.
2. Bioconversion via solid-state fermentation: vegetable residues as an exclusive substrate for the generation of fruity food flavours.
3. Conversion of vegetable residues into operating supplies: bioadsorbents for waste water treatment.
The investigations are promising with regard to future application in the mentioned industrial branch. The outlined concept can
be naturally transferred to several areas of industrial food production. The intentions of this research area are located at the de-
velopment of techniques, which fulfil the conditions of environmental protection with costs to a minimum. The prospect of several
new niche markets is worthwhile indeed.
� 2002 Elsevier Science Ltd. All rights reserved.
Keywords: Green productivity; Vegetable waste treatment; Clean production; Valuable substances; Bioadsorbents; Upgrading; Recycling;
Bioflavours; Multifunctional food ingredient; Review
Part A
1. Introduction
A thing is right when it tends to preserve the inte-grity, stability and beauty of the biotic community.
It is wrong when it tends to do otherwise.
(Aldo Leopold)
Today’s society, in which there is a great demand for
appropriate nutritional standards, is characterized by
rising costs and often decreasing availability of rawmaterials together with much concern about environ-
mental pollution. Consequently there is a considerable
emphasis on the recovery, recycling and upgrading of
wastes. This is particularly valid for the food and food
processing industry in which wastes, effluents, residues,
and by-products can be recovered and can often be
upgraded to higher value and useful products.
The food industry produces large volumes of wastes,both solids and liquids, resulting from the production,
preparation, and consumption of food. These wastes
pose increasing disposal and potentially severe pollution
Bioresource Technology 87 (2003) 167–198
*Corresponding author. Tel.: +49-228-734-274; fax: +49-228-734-
429.
E-mail address: [email protected] (G. Laufenberg).
0960-8524/03/$ - see front matter � 2002 Elsevier Science Ltd. All rights reserved.
PII: S0960-8524 (02 )00167-0

problems and represent a loss of valuable biomass andnutrients. In the past they often have been dumped or
used without treatment for animal feed or as fertilizers.
In the last few years, however, owing to the increasing
necessity to take into consideration aspects aimed at
preventing pollution of the environment as well as for
economic motives, and the need to conserve energy and
new materials, new methods and policies for waste
handling and treatment have been introduced in therecovery, bioconversion, and utilization of valuable con-
stituents from food processing wastes. Besides their
pollution and hazardous aspects, in many cases, food
processing wastes might have a potential for recycling
raw materials or for conversion into useful products of
higher value as a by-product, or even as raw material for
other industries, or for the use as food or feed/fodder
after biological treatment. Particularly, the bioconver-sion of food processing residues is receiving increased
attention regarding the fact that these residual matters
represent a possible and utilizable resource for conver-
sion to useful products (Martin, 1998, p. 316ff).
1.1. Clean production strategy
Clean production can be considered so far as a strategic
element in manufacturing technology for present and
future products in several industrial branches. Demand is
focused on the development of cost effective technology,
the optimisation of processes including separation steps,alternative processes for the reduction of wastes, optimi-
sation of the use of resources and improvement in pro-
duction efficiency (Paul and Ohlrogge, 1998).
Hence current industrial waste management tech-
niques can be classified into three options: source re-
duction via in-plant modification, waste recovery/recycle
or waste treatment by detoxifying, neutralising or des-
troying the undesirable compounds.The first two options plant modification and waste
recovery/recycle represent the most promising waste
management strategies. Indeed waste recovery is a par-
ticularly attractive option. Significant environmental
and economic benefits can accrue from separating in-
dustrial wastes with the objective of recycling/reusing
these valuable components and/or the bulk of water.
Promising concepts include pervaporation in hybridprocesses (Hausmanns et al., 1999) or the upgrading of
vegetable residues to create a secondary use for the
‘‘waste products’’ (Laufenberg et al., 1999).
It has become apparent that the current practices of
pollution control and waste management cannot com-
pletely meet the increasingly stringent requirements for
the reduction of environmental contamination. There-
fore the manufacturing industry has to include theoptimisation of product-integrated environmental pro-
tection into strategic planning, research and develop-
ment. Beside these strategies green productivity can play
an important role. This paper will report on the occur-rence, quantities and current utilisation routes for solid
vegetable waste, the transformation into value added
products and the practical implementation represented
by three possible applications.
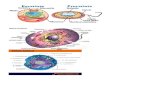
1.2. The ‘‘Holistic concept of food production’’
Present R&D in food technology is unthinkablewithout taking environmental aspects into account. A
responsible management of scarce resources is needed
especially in view of tighter living spaces. Based on these
considerations the holistic concept of food production,
shown in Fig. 1 has been developed. What does it mean?
This approach tries to connect differing goals, such as
highest product quality and safety, highest production
efficiency and the integration of environmental aspectsinto product development and food production. Within
the concept every factor and aspect should be taken into
account in a coherent manner.
The recycling of residues is important to every man-
ufacturing branch and includes high developing poten-
tial. A systematic reduction of product losses and
emissions is profitable under both economical and eco-
logical aspects.Concepts like the differentiation and separate treat-
ment of waste water streams and a task oriented
by-product management support this trend, in this
connection special attention is drawn to the recovery of
valuable substances or product losses and internal pro-
cess water recycling.
A Greenpeace briefing published on the web (Kru-
szewska and Thorpe, 1995) defines clean production in asimilar way.
‘‘The goal of clean production is to fulfil our need
for products in a sustainable way i.e., using re-
newable, non-hazardous materials and energy
Fig. 1. The holistic concept of food production.
168 G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198

efficiently while conserving biodiversity. Clean pro-duction systems are circular and use fewer materials
and less water and energy. Resources flow through
the production–consumption cycle at slower rates.
In the first place, a clean production approach
questions the very need for the product or looks
at how else that need could be satisfied or reduced.
Clean production implements the precautionary
principle––it is a new holistic and integrated ap-proach to environmental issues centred around
the product. This approach recognises that most
of our environmental problems––for example
global warming, toxic pollution, loss of biodiver-
sity––are caused by the way and rate at which we
produce and consume resources. It also acknowl-
edges the need for public participation in political
and economic decision-making.’’
Fig. 2 exhibits suggestions for a sustainable economy,beside the preventative approach waste reduction and
recycling is the other most important goal in future.
1.2.1. Development of clean production processes
The outlined system approach results in an opera-
tional program, which is not defined by technological
areas, but by short, medium and long term goals.
Short term goals
• Waste reduction and recycling of valuable sub-stances, by-products and residues.
• Enlargement and adjustment of existing technology
to the application area in particular (e.g., hybrid pro-
cesses).
Outcome: a reduction of emission and risk.
Medium term goals
• Development and application of new and efficient
production processes.
• Adding value to by-products.
Outcome: higher environmental responsibility for the
companies is accompanied by competitive advantages.
Long term goals
• Step by step implementation of environmentally be-
nign manufacturing.
• Development of ‘innovative’ products.
Outcome: Innovative food products like functional/
designer food will open new market segments and addi-
tionally meet clean/green productivity objectives.
1.2.2. Challenge for the vegetable/beverage industry
Considering the vegetable industry the mentioned
goals could be fulfilled by the usual approaches such
as minimisation, disposal, feeding, fertilisation/compo-sting, closed loop production, or conversion.
At present there are few possibilities for the utilisa-
tion or recycling for most of these wastes, the residues
are thus disposed or fed to animals. Transport costs and
sales problems due to the low quality of the residual
matter have led to alternative utilisation concepts, like
the use as a building material, or conversion concepts
like composting and biogas production. Incineration hasbeen largely investigated but not strongly pursued due
to the low calorific value 1 and high water content. An
electric power station in Nimwegen/NL has recently
started to incinerate 40 t of dried coffee grounds from an
instant coffee production plant. Besides the low ‘‘com-
bustion’’ value, a crucial point for all vegetable residues,
the formation of off-odours, bothering the nearby resi-
dents, appears to be another serious problem (Tages-schau, 1999).
Focused on the feeding concept there are further
problems mentioned in the literature. Not every animal
can take every food/residue. Laufenberg et al. (1996)
described that protein concentrate made of potato fruit
water could only be fed to cattle due to the high po-
tassium content, Clemente et al. (1997) found that olive
cake is not recommended for feeding because of itslow digestibility. Sugarcane bagasse has a high lignin
content of 22%, which forms a protective association
with cellulose, thereby causing low digestibility for ani-
mal foodstuff (Purchase, 1995).
According to a survey of the United States Depart-
ment of Agriculture (USDA) estimating and addressing
America’s Food losses (Scott Kantor et al., 1997), about
50 million US$ annually could be saved alone in solidwaste disposal costs for landfills if 5% of processing,
retail, food service and consumer food losses in 1995
were recovered (total amount of loss was 43:54� 109 kg
that year!).
Fig. 2. Circular structure of a sustainable economy (source: adapted
from Stahel Walter R. The Product-life Institute, Geneva).
1 Energetic utilisation is only recommended if the calorific value is
beyond 11,000 kJ kg�1 waste (Kuper-Theodoritis, 1996), which is even
not the fact for fat-containing residues. It has to be doubted if 18,840
kJ kg�1 olive press cakes (Vlyssides et al., 1999) are profitable. Abu-
Qudais (1996) classified the combustion of olive cake as technically
inefficient and economically unacceptable.
G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198 169

1.2.3. End of pipe solution?
The waste to be treated is already produced, a pre-
cautionary approach is possible to a certain extent, but
beyond that the vegetable industry, and especially bev-
erage industry, will always produce residues. The up-
grading concept tries to add value to the by-products
and residues. This medium term goal results in the cre-
ation of innovative products like
• dietary fibres as matrices for flavours, dyes or anti-
oxidants,
• pectin and gelling agents with defined properties
using synergetic effects,
• designer dietary fibres for application in bread or bev-
erages,
• bioflavours produced by bioconversion of waste
material or smart technology like• effective and low cost bioadsorbents, which can be
easily desorbed or biodegraded after use,
• hybrid processes combining adsorption and mem-
brane processes for an advanced wastewater treat-
ment and internal process water recycle.
Thus the introduced concept is a further step to-
wards environmentally benign manufacturing. Theconcept does not present any immediate patent solu-
tions or recipes, because industrial food production
is an interactive process, which needs to fulfil all
three conditions, quality, efficiency and environmental
protection as aforementioned. The upgrading concept
is a continuing development and research strategy,
keeping in mind this interrelated character of produc-
tion.Instead of just blaming the industry to develop a so
far unknown standby preventative solution for the
waste, the outlined concept tries to combine economical
aspects too. The result is a step by step waste reduction
with simultaneously rising productivity, not obtained by
restrictions but by opportunities, advantages are sum-
marised in Table 1.
Consequently the concept follows a three steps app-roach:
1. Evaluation as state of the art, visualising vegetable
waste in its occurrence, quantities and current utilisa-
tion routes.
2. Introducing the upgrading concept.
3. Technical implementation by three selected examples.
2. Need statement: the vegetable waste situation
2.1. Vegetable waste quantities in several countries
The scale of the problem is illustrated by looking at
the total amounts of waste materials produced by dif-
ferent states. Table 2 is a list of waste quantities men-
tioned in the literature.
2.2. Strategies and utilisation routes: state of the art
Special attention was given to publications, which
focus on ideas beyond fodder/feed and composting/fer-
tilisation.
Not covered in this literature survey are
• liquid vegetable waste streams,• any solid or liquid waste stream related to animal
production, slaughtering, meat and meat product
processing.
Vegetable residues mostly contain considerable
amounts of potentially interesting compounds. Due to
legislation and environmental reasons the industry is
more and more forced to find an alternative use for theresidual matter. The recovery of high value compounds
is an elegant way to reuse waste streams, while being
economically interesting on the other hand. Several fruit
and vegetable residues are listed in Table 3.
In the last decade the interest in the alternative use of
waste streams beyond disposal or fertilisation has in-
creased drastically. Further to rising disposal costs the
economic interest has appeared as well. A new nichemarket for residual matter recently appeared in choco-
late production. After a four years discussion the EC has
dropped the purity law for chocolate. The European
Parliament decided on March 8th 2000, that chocolate
manufacturing industry is allowed to add up to 5% other
fat types besides cocoa fat to their chocolate products
(ZDF.MSNBC, 2000). One of the legalised cocoa butter
substitutes is mango kernel, which contains 12% fat(Nanjundaswamy, 1997).
The utilisation of a waste stream as raw material for
new products needs to be economically attractive as
aforementioned. The selection of high value products
reaches from natural bioflavours over food colours to
biocontrolling agents for food preservation.
A real bulk application for vegetable waste––with
minimised further treatment steps––could be the use as abioadsorbent for the pre-treatment of aromatic waste
waters. Activated carbon being used so far is relatively
expensive. In order to obtain cheaper adsorbents,
Table 1
Advantages for industry and environment
� Closed loop of valuable constituents
� Preservation of resources
� Discovery of niche markets
� Environmental protection combined with
� Reduced waste disposal costs
170 G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198

lignocellulosic materials have been studied. Low cost
and simplicity of the modification methods are also
desirable for applications (Peternele et al., 1999).
Another branch for a further use of vegetable resi-
dues is their availability as a source of potential phyto-chemicals. Olive pomace is used as a nematodes
controlling agent for tomatoes (Rodriguez-Kabana
et al., 1995), citrus waste streams are used in horticulture
(Widmer and Montanari, 1995) and mandarin peel
flavonoids are interesting due to their fungistatic activity
(Chkhikvishvili and Gogiya, 1995) which may be ap-
plied to naturally protect vegetables and fruits from
moulding. The limonoid compounds in citrus peel andseeds have recently been found to have important
pharmacological properties as well as potential in the
use as an insect antifeedant for agricultural crops
(Manthey and Grohmann, 1996).
Despite the studies cited and their potentially prom-
ising results, no systematic investigation on poten-
tial utilisation routes and innovative concepts has
been completed yet. Furthermore mechanisms for im-proved yields of existing recycling strategies are not
known.
The improved utilisation of vegetable waste, outlined
here, should lead to a more efficient use of resources
and less negative environmental impact (Sriroth et al.,
2000).
3. The upgrading concept
Important factor for the upgrading process is the
development of a procedure using technical standard
equipment. Goal of the upgrading is a product with
desired, reproducible properties designed under eco-
nomical and ecological conditions.Most of the vegetable residues consist mainly of
water and cellulose and have a poor microbiological
quality because of numerous spoilage bacteria on the
surface, particularly if stored in the production unit
prior to use; thus they quickly decompose in an un-
controlled way. A pre-treatment step in the form of ino-
culation with lactic acid bacteria may produce a more
stable substrate, which should be dried to further en-hance shelf and storage life. An alternative to the fer-
mentation is the acidification by acids like citric, acetic
or ascorbic acid. For sensorial reasons and because of
Table 2
Waste quantities in different countries (selection)
Country/state Quantity and waste type
Germany, 1997 (Henn, 1998) 380,000 t/a organic waste only from potato, vegetable and fruit processing
1,954,000 t/a spent malt and hops (breweries)
1,800,000 t/a grape pomace (viniculture)
3,000,000 t/a crude fibre residues (sugar production)
100,000 t of wet apple pomace (ffi25,000 t dry apple pomace) remain if 400,000 t
of apples are processed into apple juice (Henn and Kunz, 1996)
Belgium, 1992 (Lucas et al., 1997) 105,000 t/a biowaste (vegetable, garden and fruit waste)
280,000 t/a estimations due to legislation of separate household collection
Thailand, 1993 (Prasertsan and Prasertsan, 1996) palm oil
production
386,930 t/a empty fruit bunches
165,830 t/a palm press fibre
110,550 t/a palm kernel shells
1,000,000 t/a cassava pulp (1994, Sriroth et al., 2000)
Spain, 1997 (Clemente et al., 1997) >250,000 t/a olive pomace
EEC, 1996 (Dronnet et al., 1998a) 14,000,000 t/a sugar beet pulp (dry matter!)
Portugal, 1994 (Carvalheiro et al., 1994) 14,000 t/a tomato pomace
Jordan, 1999 (Haddadin et al., 1999) 36,000 t/a olive pomace
Malaysia, 1996 (Hussein et al., 1996) palm oil production 2,520,000 t/a palm mesocarp fibre
1,440,000 t/a oil palm shells
4,140,000 t/a empty fruit bunches
Australia, 1995 (Tran and Mitchell, 1995) 400,000 t/a pineapple peel
USA 300,000 t/a grape pomace in California only (1994) (Nakata, 1994)
9,525 t/a cranberry pomace (1998) (Zheng and Shetty, 1998)
200,000 t/a almond shells (1997) (Toles et al., 2000)
3,300,000 t/a orange peel in Florida (1994) (Manthey and Grohmann, 1996)
G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198 171

Table 3
Current utilisation concepts for vegetable pomace (selection)
Residual matter/co-product Pre-treatment Results Application/secondary use Reference
Almond shells M: grinding, C: phosphoric acid
pre-treatment, P: heat activation
Superior to commercial carbons in metal
uptake, 85–92% with organic solutions
Op: wastewater treatment, metal and or-
ganics adsorption
Toles et al. (2000)
Apple pomace B: SSF Candida, Sacch., Torula
spp., P: drying
Crude protein three times higher, fat 1.5–2
times higher, vitamin C two times higher,
minerals and fibres content higher
O: ethanol, Ff Joshi and Sandhu (1996)
Apple pomace P: drying, M: powdering Enhanced fibre content in food, Sensorial
tests: moderately liked
Fi: pie filling, oatmeal crackers Carson et al. (1994)
Apple pomace B: degradation of linoleic acid by
intrinsic enzyme system of po-
mace
Better results by adding SO2 and Vitamin C O: flavours, volatile aldehydes for chemical
industry
Almosnino and Belin (1991)
Apple pomace – O: Fine chemicals, polyphenols Lu and Foo (1997)
Apple pomace, Pepper
peels
C: CO2 flavour extraction, frac-
tionated precipitation
Fi: flavour extract Bundschuh et al. (1988), Bund-
schuh et al. (1986)
Apple pomace, cassava
bagasse
M: grinding, B: SSF with 4
Rhizopus strains
Highest flavour production with amaranth
plus mineral precursor and apple/cassava/
soybean
Fi: food flavours Christen et al. (2000)
Apple pomace, spent malt
grain
M, B: SSF with Thamnidium
elegans
Highest product yields with ratio of 3 to 1
(AP to SMG), precursor peanut oil further
improved yield
Fi: food supplement c-linolenic acid,
O: pharmaceutical application
Stredansky et al. (2000)
Apricot seeds B: enzymatic degradation Protein substitute only after degradation of
cyanogenic glycosides
Fi: substitute for marzipan, oil for bitter
almond oil, protein enhancer
Tuncel et al. (1998)
Banana pith waste (banana
stem marrow)
M, P: drying, grinding Parameters: agitation time, adsorbent dos-
age, pH-value, initial conc.
O: dye removal in wastewater treatment Namasivayam and Kanchana
(1992)
Blackcurrant and apple
pomace
C, M, P Fibres (60%) are useful for binding Cd
(>30%) and Pb (>40%). Blackcurrant po-
mace binding capacity was higher than for
apple. Ca binding was low, good for food
application
Fi: dietary fibre in food, nutritional value
and healthy food, binding metals, O: binding
capacities for toxic metals potential for
adsorption?
Borycka and Zuchowski (1998)
Carrot pomace M: grinding, B: SSF lactic acid
hygenisation with L. farciminis
In bread: improved nutritional value, fresh-
ness, water binding capacity, sourdough
functions, positive on porosity
Dietary fibre enhancement in bread, sour-
dough substitute in rye and white bread
Filipini and Hogg (1997)
Carrot pomace Fi: cake, dressings, pickles, Fi: bread Ohsawa et al. (1995), Ohsawa
et al. (1994)
Carrot pomace M, P Development of the upgrading process, good
results for stabilisation of several properties
Fi: multifunctional ingredient in beverages Henn and Kunz (1996), Henn
(1998)
Carrot pomace, citrus
and pineapple peels and
pomace
B: SSF A. niger mass multipli-
cation
Biocontrolling agent against Fusarium oxy-
sporum on muskmelon, especially citrus
pomace (20%)
O: biocontrol agent in cultivation of melons Mukherjee and Sen (1998)
Carrot residue, orange
waste, mango peel and
stone
– Egg size and production enhanced with
carrot and orange, neutral with mango stone
Ff: Layer’s hen diet Zia-ur-Rehman et al. (1994)
Cauliflower leaves, cabbage
leaves
B: 1. cellulolytic degradation by
A. niger, 2. Torulopsis utilis, P: 3.
drying
Protein content rose from 14.5% to 22.6% Ff: cattle and poultry Majid et al. (1995)
Citrus by products and
wastes
M, P Influencing the texture and viscosity of the
beverage
Fi: clouding agent in beverages Sreenath et al. (1995)
172
G.Laufen
berg
etal./Bioreso
urce
Tech
nology87(2003)167–198

Citrus peel – Pectin extraction, phytochemicals Fi: stabiliser, thickening agent, gelling agent,
soluble fibre, O: several applications
Widmer and Montanari (1995)
Citrus residues, apple po-
mace, sugar beet pulp
B: enzymatic, cellulolytic and
pectinolytic hydrolysis, micro-
bial conversion
O: Pectin, liquid biofuel, substrate for bio-
conversions
Grohmann and Bothast (1994)
Cocoa pod husk, bean
shells and germ
M, P Pectin and protein extraction, germ oil with
high oleic and linoleic acid content
Fi: stabiliser, thickening agent, gelling agent,
soluble fibre, Ff: protein rich pod husk
Nambudiri and Shivashankar
(1985)
Corncob shreds, wheat
straw, wood chips
M: chopping, B: SSF with
Phaenerochaete chrysosporium
and C. versicolor
Corncob shreds and wheat straw showed 70–
75% adsorption rate for textile dyes. In SSF
both substrates have been degraded and dyes
metabolised. Wood chips did not work for
adsorption nor SSF
Op: bioadsorbents for wastewater treatment,
O: soil conditioner after SSF
Nigam et al. (2000)
Corncobs M, P: drying, pyrolysis, C: ZnCl2as activator
O: general use in wastewater treatment Tsai et al. (1998)
Corncobs and onion skin M, P Cu ion adsorption is lignin and cellulose
dependent, simple modifications necessary,
packed bed investigations
Op: alternative bioadsorbents for wastewater
treatment
Odozi and Emelike (1985),
Hawthorne Costa et al. (1995)
Cranberry processing waste B O: SSF, fungal inoculate production Zheng and Shetty (1998)
Fruit pomace – Investigated new dietary fibre compositions
for the application in food
Fi: health quality improvement by adding
fibre product
Borycka (1996)
Galgal peel (citrus pseudo-
limon)
M: powdering, C: pectin extrac-
tion
Fi: as stabilisers, thickening agent, jellies, etc. Attri and Maini (1996)
Grape pomace M: grinding, P: drying Fi: dietary fibre supplement, insoluble is
major fraction
Valiente et al. (1995)
Grape pomace – Nutritional value improved, physiological
properties influenced
Fi Martin-Carron et al. (1997)
Grape pomace Combination of the antioxiden-
tial potential of polyphenols in
grape pomace with the dietary
fibre matrix
Future idea to use the carrier function of
dietary fibres as a matrix for other techno-
logically useful substances (antioxidants,
flavours, dyes, emulsifiers) plus improvement
in nutritional value (fibre, vitamins)
Both fibre enhancement and antioxidants in
food, carrier idea
Saura-Calixto (1998)
Grape pomace, Carrot po-
mace
M: grinding, P: drying, B: SSF,
UASB reactor
O: substrate in bioreactor UASB, Fi: bread
improver, sourdough substitute, dietary fibre
and b-Carotene enhancer
Lucas et al. (1997)
Hawthorn pulp (Mexican
fruit)
– Pectin extraction Fi: stabiliser, thickening agent, gelling agent,
soluble fibre
Higareda et al. (1995)
Jack fruit, pineapple (skin,
stem, leaf) and mango
waste (skin, kernel)
C, M, P Determination of nutritional value, several
analyses
Fi: possible application as food ingredients Haque et al. (1997)
Lemon peel and pulp, olive,
apple and grape pomace
C, P Interactions of dietary fibres with Fe and Ca O: possible adsorbents, Fi: importance for
human nutrition
Torre et al. (1995)
Mandarin fruit waste C: flavonoid extraction High fungistatic activity towards Phoma
tracheiphila, causing citrus malsecco
O: natural fungicide in citrus fruit cultivation Chkhikvishvili and Gogiya
(1995)
Mango kernel C, M Flour substitute with moderate sensory ac-
ceptability, higher calories and protein
Fi: for biscuits Arogba (1999)
Mango peel – Pectin extraction Fi: stabiliser, thickening agent, gelling agent,
soluble fibre
Srirangarajan and Shrikhade
(1976)
G.Laufen
berg
etal./Bioreso
urce
Tech
nology87(2003)167–198
173

Table 3 (continued)
Residual matter/co-product Pre-treatment Results Application/secondary use Reference
Mango peel and stone – 15% pectin in the peel, 20-fold flavour
concentrate can be recovered from peel,
stone kernel is rich source of carbohydrates,
protein and fat (12%).
Fi and O: pectin, Kernel fat: use in soap
manufacturing or as cocoa butter substitute,
potential for the preparation of sweetmakers
Nanjundaswamy (1997)
Oat, corn rice, soybean
hulls; pea pods, wheat and
corn bran
M: peeling, purifying, milling,
P: drying
Fi: Z-trim, a fat replacer and texturizing
agent, lowering the calorie content of food
and fibre enhancer
Inglett (1998)
Oil palm shells M, P: drying, pyrolysis, C:
ZnCl2, CO2, as activators
ZnCl2 0–15% impregnation produces decent
microporous carbons
O: activated carbon for adsorption in
chemical industry
Hussein et al. (1996)
Olive cake P: drying, C: extraction with
hexane
Fat oxidation during drying process, hexane
extraction was on no influence on oxidation
Gomes and Caponio (1997)
Olive cake, sugarcane bag-
asse
B: SSF Lipase can degrade the fat in olive cake O: lipase use in chemical, food and phar-
maceutical industry
Cordova et al. (1998)
Olive oil industry waste – Liquid and solid fractions Vitolo et al. (1998)
Olive pomace In mixtures with several chemi-
cals
Phytotoxic if used as an exclusive substance,
in combination with chemicals potential
nematodes controller
O: nematodes controlling agent for tomato
cultivation, potential phytochemical
Rodriguez-Kabana et al. (1995)
Olive pomace Main component is fibre > 70%.
C: extraction, P: drying, chemi-
cal analysis
Analysis used for possible sec. use: nitrogen
value low, amino acid composition well
balanced (except lysine), soluble sugars and
organic acids
Clemente et al. (1997)
Olive pomace P: drying, M: grinding, B: delig-
nification, saccharification
Degradation of lignin, crude protein en-
hancement from 5.9% to 40.3%
Ff: fodder enhancement Haddadin et al. (1999)
Onion skin M, C Stirred tank and packed bed investigations,
parameters are agitation, pH-value, initial
conc., high adsorption rates with a selectivity
towards the heavy metals
Op: alternative bioadsorbents for wastewater
treatment
Kumar and Dara (1981), Bankar
and Dara (1982)
Onions, cull M, C: extraction Transferable to garlic or fruit waste Fi: onion oil flavour Brose (1993)
Orange and mango skin,
apple pomace, wheat bran
M: grinding, powdering, P: dry-
ing
New technology in dietary fibre production Fi: General application for dietary fibre
enhancement
Larrauri et al. (1999)
Orange peel M: washing followed by leach
liquid treatment, B: pectinolytic
enzyme treatment to recover
soluble solids
Wastewater treatment concept for the pectin
production industry. Material balance de-
veloped
O: pectin wastewater treatment El-Nawawi and Heikal (1996)
Orange peel M, P: cutting, drying, grinding Dye removal with cellulosic material, pa-
rameters: initial conc. of dye important,
particle sizes of adsorbent, pH-value
Op: dye removal in wastewater treatment Namasivayam et al. (1996)
Palm kernel husk M, C Pb is preferably adsorbed to Zn Op: alternative bioadsorbents for wastewater
treatment
Omgbu and Iweanya (1990)
Palm oil mill waste – Quantities and potential usage O: empty fruit bunches for mushroom cul-
tivation, decomposed rest as fertiliser, Op:
palm press fibre for pulp and paper, Op:
palm kernel shell as activated carbon
Prasertsan and Prasertsan (1996)
Peanut and walnut shells – Rich in tannin, possible use in wastewater
treatment
O: heavy metal ions removal in wastewaters Randall et al. (1974)
Peanut skin M, C Stirred tank and packed bed investigations,
two types untreated and treated peanut skin
Op: alternative bioadsorbents for wastewater
treatment
Randall et al. (1975)
174
G.Laufen
berg
etal./Bioreso
urce
Tech
nology87(2003)167–198

Pear and kiwi pomace C, M Major analysis of several compounds Fi: sugar source, fibre enhancement insolu-
ble/soluble, pectin
Martin-Cabrejas et al. (1995)
Pineapple cannery waste P: heat treatment, M: centrifu-
gation, B: ethanol fermentation
Continuous fermentation substrate, high
syrup reduces fermenter size, enhances
ethanol production
Op: liquid fuel production Nigam (1999)
Pineapple peel B: SSF Aspergillus foetidus ACM
3996
Substrate is superior to rice or wheat bran,
16.1 g citric acid per 100 g dry waste: 62.4%
yield
Fi: citric acid, O: pharmaceuticals Tran and Mitchell (1995)
Potato peel M, P Baking experiments showed that potato peel
is superior to wheat bran in minerals content,
water holding capacity and lack of phytate
Fi: bread fibre improvement Toma et al. (1979)
Potato starch waste, carrot
pomace
M, P, B Potato fruit water: protein content, peel and
pulp: fibre content, especially soluble frac-
tion, carrot pomace: colour stabilisation,
nutritional value, preservation, viscosity
Fi: multifunctional ingredient in general,
focus on bread and beverages
Laufenberg et al. (1996)
Spent malt B: Ceratocystes fimbriata Good utilisation potential for the formation
of bioflavours even without precursors, sub-
strate screening with several waste types was
done
Fi: general application, O: pharmaceutical
industry
Fischbach et al. (2000)
Sugar beet pulp, cereal
bran
M and P: free ferulic acid from
pectin, B: SSF with two micro-
organisms
1. A. niger to transfer ferulic into vanillic
acid, 2. Pycnoporus cinnabarius into vanillin
Fi: vanillin as a food flavour, O: flavour
compound for chemical use
Asther et al. (1997)
Sugar beet pulp P, C, B Special attention to exploit the hemicellulotic
fraction, gum arabic substitute and fat
replacer has been developed
Fi: dietary fibre, esp. soluble. Two novel
food ingredients were developed, FF: en-
zymes for use in feeds
Broughton et al. (1995a,b)
Sugar beet pulp C: washing with ethanol,
P: drying, M: milling
Coarse (600 lm) and medium (355 lm)
particle sizes showed good cookie properties.
Corn grids gave better colour. In sensory
evaluation cookies with up to 6% sugar beet
were even favoured against plain cookies
Fi: dietary supplement in cookies K€ooksel and €OOzboy (1999)
Sugar beet pulp C, M, P: saponification or pre-
treatment with formaldehyde or
epichlorohydrin
Due to the pre-treatment increasing ion-
exchange capacities and reduced hydration.
Epichlorohydrin treatment seems to be most
efficient, even if sorption/desorption cycles
are suggested
Op: heavy metal removal in wastewater
treatment
Dronnet et al. (1997, 1998a,b)
Sugarcane bagasse, bark
and onion skin
M, C Stirred tank and packed bed investigations,
seven heavy metals tested
Op: alternative bioadsorbents for wastewater
treatment
Kumar and Dara (1982)
Sugarcane bagasse C: lignin extraction Parameters: pH-value, ionic strength, temp. O: heavy metal bioadsorbent for wastewater
treatment
Peternele et al. (1999)
Sugarcane bagasse, pecan
shells
P, P, C: grinding, pyrolysis,
phosphoric acid activation
Comparable efficiency to commercial acti-
vated carbons in decolourisation of raw
sugar
Op: GACs Ahmenda et al. (2000a,b),
Pendyal et al. (1999)
Sunflower heads – Pectin extraction Fi: stabiliser, thickening agent, gelling agent,
soluble fibre
Wang et al. (1997)
Tomato pomace M: grinding, B: fungal cultures,
pure and mixed
Increasing the protein and lignin content,
hence digestibility by micro-organism cul-
tures
Ff: feed stuff or fodder enhancement Carvalheiro et al. (1994)
Tomato skins and seeds P: drying, C: extraction Proteins, minerals, and dyes (lycopenes) Ff, Fi: biocolorants Al-Wandawi et al. (1985)
Urban organic waste B: mesophilic fermentation Volatile fatty acids as possible flavours Fi or O: chemical industry Sans et al. (1995)
G.Laufen
berg
etal./Bioreso
urce
Tech
nology87(2003)167–198
175

the influence on colour stability an application of thelatter ascorbic acid would be most useful for food app-
lications.
Hence almost any recycling process will start with the
steps pre-treatment (ensiling), drying, size deduction and
fractionation.
The overall recycling strategy, described in Fig. 3, is
designed in a modular manner, thus subdivided into
substance characterisation, definition of objectives,product and process design and application and opti-
misation. The result is a final product which is opti-
mised, in regard to the requested product properties, in
the exhibited way a multifunctional food ingredient.
The first phase is mainly the substance characteri-
sation, based on these data the optimal recycling and
application areas and possibilities are worked out. Par-
ticle classification, chemical analysis and physicochemi-cal properties are the important steps.
Following the definition of objectives will enclose the
desired properties of the future food ingredient as well
as the food to be applied to. At this point a decision has
to be made about the use in theory. Based on these ‘‘key
properties’’ advantages will arise for technological ben-
efit, health or taste of a product.
Product and process design covers product and dis-persion properties as well as their changes depending on
the process parameters. Obvious examples are desirable
or undesirable interactions between the food ingredients
in general or during processing and interactions with
surrounding and processing factors.
The range of possible interactions is enormous, thus a
concentration on the valuable ingredients as well as on
the desired technological, sensorial and physiologicalproperties is useful. A continuous control and improve-
ment of the upgrading process and product can be
gained by prototype development, definitions of partial
qualities as well as incorporation of feed back circles.
At the application phase food product and newly de-
signed food ingredient will be combined. At this inter-
action point the estimated use and practical application
in a real food system meet each other. Quality relatedproperties of the new product have to be assessed and
compared with similar products being already on the
market. Hence a successful launch may be forecasted.
The sensorial quality is the most important criteria
for a multifunctional food ingredient applied in a new
product. Since sensorial, technological and nutritional
quality of the new product is compared to a so-called
‘gold standard’, the optimisation is nearly completed.Final investigations into product properties will answer
questions, which are useful to point out the consumers’
benefit or even the unique selling position. The latter is
often science based and hence measurable.
Instead of producing a multifunctional food ingredi-
ent the goal could be alternatively the bioconversion
into a food flavour or the development of an operationalTable
3(continued)
Residualmatter/co-product
Pre-treatm
ent
Results
Application/secondary
use
Reference
Vanilla
shells
CO
2flavourextraction
Patent
Fi:Naturalvanilla
flavour
Schutz
etal.(1982)
Vegetable
raw
materials
–O:bioplasticswithspecialproperties
Feil(1995)
Vegetable
residues
(not
specified)
M,P,B
Porouscarbohydrate
ingredientswithcarrier
functionto
encapsulate
flavours
Fi:dietary
fibre
enhancement,flavouren-
capsulationandapplicationin
variousfood
Zeller(1999)
Vegetable
waste,
24types
–Analysisofthenutritivepotential,cauli-
flower
leaves
bestnutritivescore
Ff:forruminants
Gupta
etal.(1993)
Wheatbran
M:debranning,polishing
Fi:multifunctionalfoodingredientwith
specified
physicochem
icalandnutritional
properties
DexterandWood(1996)
Wheatstraw,corn
stalks
B:biodegradationofhem
icellu-
losicfractionbyL.edodes
SSFissuperiorto
SMFforbiodegradation,
mechanicalcharacteristics
ofthepaperswere
improved
Op:paper
andpulp
fibre
resource(lignin)
Giovannozzi-Sermanniet
al.
(1995)
Wheatstraw,insoluble
straw
xanthane
M:grinding,C:alkalitreatm
ent
>90%
removaleffi
ciency
inheavymetal
solutions,75–95%
intannerywastew
ater
Op:wastew
atertreatm
entadsorbent
Kumaret
al.(2000)
Woolfibre
M,P:washing,drying,C:
petroleum
ether
degreasing
Parameters:fast
rate
uptake,
notemp.
dependency
Op:heavymetalcationsremovalin
waste-
watertreatm
ent
Balk€ oose
andBaltacioglu
(1992)
C:chem
ical,M:mechanical,P:physical,B:biotechnical;Ff:fodder/feed,Op:operationalsupply,Fi:foodingredient,O:other
and(–):nodata
available.
176 G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198

supply like a bioadsorbent. Hence different objectives
will affect the product and process conception and the
application phase.
In the following the theoretical description of theupgrading concept will be verified at three implemen-
tation examples.
Part B
4. Target state: Selected practical implementations
4.1. Novel types of products: multifunctional food ingre-
dients
Let your food be your first medicine
(Hippocrate, 377 B.C.)
A promising possibility for the utilisation of organicresidues in the frame of green productivity is the de-
velopment of innovative products. In the mentioned
context multifunctional food ingredients have to be
understood as natural ingredients taking over food ad-
ditive functions during processing and/or add a further
benefit to the final product.
Several research groups have been working on thedevelopment of multifunctional ingredients from vege-
table residues and its application in different food
products. The crude fibre content combined with at least
one other property enables them to fulfil several func-
tions in food as exhibited in Table 4. A couple of quality
Fig. 3. Strategy for the development of multifunctional food ingredients made of vegetable residues: the upgrading concept (modified after Henn
(1998)).
Table 4
Food properties and quality influenced by multifunctional food in-
gredients (Laufenberg et al., 1996)
Operating areas of multifunctional food ingredients due to food
properties and quality
(1) Nutritional and healthy quality, e.g. vitamin content, dietary
fibre content
(2) Food product structure, e.g. porosity, network structure
(3) Sensorial properties, e.g. texture/structure, mouth feel, freshness
(4) Physical properties, e.g. density, viscosity
(5) Processing properties, e.g. water binding ability, emulsifying
properties
G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198 177

determining food properties can be governed by theapplication of these food ingredients. The raw material
mostly used is carrot pomace (Filipini and Hogg, 1997;
Henn and Kunz, 1996; Henn, 1998; Lucas et al., 1997;
Ohsawa et al., 1994;Ohsawa et al., 1995; Laufenberg et al.,
1996), followed by citrus waste (Sreenath et al., 1995;
Widmer and Montanari, 1995), grape or apple pomace
(Borycka, 1996; Carson et al., 1994; Lucas et al., 1997;
Saura-Calixto, 1998; Masoodi and Chauhan, 1998),sugar beet pomace (Broughton et al., 1995a,b; K€ookseland €OOzboy, 1999), orange, mango and apple peel
(Larrauri et al., 1999), mango kernel flour (Arogba,
1999) (as a wheat flour substitute), potato peel (Toma
et al., 1979), sugarcane bagasse (Clarke, 1995) or mix-
tures of oat, rice, corn hulls and pea pods (Inglett, 1998).
They are applied in pie fillings (Carson et al., 1994),
crackers (Carson et al., 1994; Joshi and Sandhu, 1996),bread (Filipini and Hogg, 1997; Lucas et al., 1997;
Ohsawa et al., 1994; Clarke, 1995), cookies (Clarke,
1995; K€ooksel and €OOzboy, 1999), beverages (Henn and
Kunz, 1996; Henn, 1998; Laufenberg et al., 1996; Sree-
nath et al., 1995), jam (Grigelmo-Miguel and Martin-
Belloso, 1999), and cakes, dressings and pickles (Ohsawaet al., 1995). New approaches try to use the dietary fibre
as a matrix for the encapsulation of antioxidants (Saura-
Calixto, 1998) or flavours (Zeller, 1999), using both the
physiological effects and the technological advantages in
the form of a controlled release.
Almost every flavour company is nowadays inter-
ested in microscopically encapsulated aromas, which do
not escape directly but under precisely defined circum-stances, for example under mechanical stress such as
chewing the chewing gum or at a certain temperature
while baking cake mixtures (Stock, 1999; Schr€ooder,1999).
Beside the application as a texturing or gelling agent
the fat replacement function in diet food is an impor-
tant advantage of fibres. Recently new food additives
have been developed on the basis of vegetable residues(Broughton et al., 1995a,b; Inglett, 1998).
The high crude fibre content of the vegetable po-
mace, see Table 5, suggests its utilisation as a crude
fibre ‘‘bread improver’’. One reason for the low di-
etary fibre uptake is the non-acceptance of whole meal
Table 5
Content and composition of dietary fibre of some residues (Laufenberg et al., 1996; Martin-Cabrejas et al., 1995; Torre et al., 1995; Seibel and
Hanneforth, 1994)
Residues Fibre Pectin Lignin Cellulose
Total Insoluble Soluble
Apple pomace 62.5 48.3 14.2 15.69 18.2 –
Barley pomace 65.3 62.1 3.2 – – –
Carrot pomace 29.6 18.9 10.7 22–25 – –
Cocoa pod husks/bean
shells (Nambudiri and
Shivashankar, 1985)
36.3 – – 6 – 13.7
Corncobs – – 43a – 17 (Hawthorne Costa
et al., 1995)
32
Kiwi pomace 25.8 18.7 7.1 7.25 3.2 –
Lemon peel 50.9 28.2 22.7 25.23 5.5 –
Lemon pulp 45.8 26.0 19.8 12.02 2.9 –
Olive cake 69.4 65.7 3.7/15.5 (Haddadin
et al., 1999)a4.10 37.2/35.4 (Haddadin
et al., 1999)
18.4 (Haddadin et al.,
1999)
Pea pots 90.1 84.7 5.4 – – –
Peach pomace (Pag�aan
and Ibarz, 1999)
54.5 35.4 19.1 – – –
Pear pomace 43.9 36.3 7.6 7.05 5.2 –
Potato peel (Toma
et al., 1979)
73 6.2a 16 13.8 16
Potato pulp 15.8 9.4 6.4 �15 (Tuncel et al.,
1998)
– –
Soybean shells 64.6 56.9 7.7 – – –
Sugar beet pulp 75.3 50.1 25.2/22.1 (K€ooksel and€OOzboy, 1999)
30 (Purchase, 1995)/26
(Broughton et al.,
1995b)
1.85 (K€ooksel and€OOzboy, 1999)/4.56
(Broughton et al.,
1995b)
23/27.2 (K€ooksel and€OOzboy, 1999)
White wine pomace 58.6 56.3 2.3 3.9 (Torre et al., 1995)/
5.5 (Valiente et al.,
1995)
41.2 (Torre et al.,
1995)/53.6 (Valiente
et al., 1995)
–
Results expressed as percentage of original dry matter, (–): no data available.aAs hemicellulose.
178 G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198

products in large parts of the population. An enrich-ment of different products with crude fibre compounds
can thus raise the dietary fibre uptake, if the food
products are not modified too much. The macro-
molecular structure of the fibre must not be changed
during the transformation of the residue into a food
compound, and the fibre material has to be of food
grade.
In bread and bakery goods, as well as in pastry, ce-reals and dairy products, the investigated carrot pomace
works as a stabiliser. Beside crude fibre it is rich in pro-
vitamins, colour and natural acids. It takes over several
functional properties mentioned in Table 4, additionally
substitutes sourdough in bread, is acidifying agent,
preservative or antioxidant in several food products
(Filipini and Hogg, 1997; Lucas et al., 1997; Ohsawa
et al., 1994, 1995; Toma et al., 1979; Masoodi andChauhan, 1998).
In beverages, carrot pomace or citrus waste will sta-
bilise the natural colour, improve the vitamin and fibre
content, enhance the viscosity (mouthfeel) (Laufenberg
et al., 1996; Henn and Kunz, 1996; Henn, 1998), and
enrich or adjust the cloudy appearance (Sreenath et al.,
1995). The organoleptic and chemical properties offer a
widespread use in healthy and functional drinks andselected fruit juices.
Several series of experiments were done to determine
the influence of different pre-treatment/preservation
methods on the physicochemical properties of carrot
pomace when applied in food. Therefore common
acidifying agents, i.e., citric, acetic or ascorbic acid, were
applied to stabilise and preserve the fresh pomace, as
well the carrot pomace was fermented by Lactobacillus
farciminis.
Processing of the vegetable residue can be done by
fermentation with lactic acid bacteria leading to a
suitable transformation of low molecular materials
like sugars and to a microbial stabilisation, because
enterobacteriaceae and moulds present on the po-
mace are inhibited by the lactic acid formed. Thiseffect is already used for several vegetables in-
cluding carrots with the task of preservation, e.g.
pickles. The pomace is fermented by Solid-State-
Fermentation, which does not need as much free
liquid phase as submerged fermentation and thus
makes downstream processing of the crude fibre
product easier and cheaper. After lactic acid fer-
mentation of carrot and grape pomace the productis rich in crude fibre, shows an acidic pH and can be
used as a bread improver and for crude fibre enrich-
ment of bakery goods.
Afterwards the pomace was treated with common
drying operations, i.e., spray, freeze or oven drying. It
could be determined that the colour stability was mostly
improved by the addition of ascorbic acid (avoids the
formation of free radicals), which improves the nutri-
tional value too. Fermented samples were superior to
non-fermented samples as clouding agents and showed agood shelf life too.
The ideal dietary fibre should meet specific require-
ments, residues own natural properties, this relation
could be tailored during the different processing steps, as
exhibited in Fig. 4.
Currently there is a great variety of raw materials
from which dietary fibres are obtained such as wheat
or rice, etc. Useful alternatives/substitutes could begained from vegetable residues like orange peels, mango
peels, grains, soybean or oat hulls, cereal bran, etc.
(Larrauri et al., 1999), fruit pomace (Borycka, 1996),
grape pomace (Martin-Carron et al., 1997; Valiente
et al., 1995), pear and kiwi pomace (Martin-Cabrejas
et al., 1995), wheat bran (Dexter and Wood, 1996), or
sugar beet pulp (Broughton et al., 1995a,b; K€ooksel and€OOzboy, 1999).
Several treatments could be performed to improve the
functionality of the insoluble fibre, which is the main
component of the residual matter, mentioned in Table 5,
as
Fig. 4. Natural properties of vegetable waste (average) and food properties and quality being influenced by multifunctional food ingredients.
G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198 179

• partial delignification of lignocellulose by alkalineH2O2 treatment,
• extrusion,
• encapsulation with soluble fibre to produce a product
with better textural properties,
• enzymatic modification to improve sensorial proper-
ties.
Dietary fibre from cereals are more frequently usedthan those from fruits, although fruit fibres have better
quality due to higher total and soluble fibre contents,
lower phytic acid contents, colonic fermentability and
water and oil holding capacities. The latter is of par-
ticular interest for the carrier function of dietary fibres
which has been already used for antioxidants (Saura-
Calixto, 1998), encapsulation of flavours (Zeller, 1999)
or dyes (Filipini and Hogg, 1997; Henn, 1998). There isa future need to develop processes for the preparation of
fruit fibres that minimise the losses of associated natural
bioactive compounds which may exert higher health
promoting effects than the dietary fibre itself. The higher
concentration of health promoting flavonoids compared
to traditional wine growing is often mentioned in con-
nection with ecological viniculture. Besides environ-
mental benefits the quality of the wines is improved,resulting in higher prices for these wines and special
recommendations in wine guide journals (Kriener,
1999).
Saura-Calixto (1998) produced a dietary fibre rich in
associated polyphenolic compounds combining in a
single material the physiological effects of both dietary
fibre and antioxidants. Fibre matrices could act as
support for biocolourants made of anthocyanins fromolive cake (Clemente et al., 1997), lycopenes from to-
mato skins (Al-Wandawi et al., 1985) or b-carotenefrom carrot pomace (Henn and Kunz, 1996). Phenolic
compounds are powerful antioxidants and may possess
potential pharmacological properties, already widely
used with green tea catechins (Nwuha et al., 1999) or
ferulic acid extracted from sugar beet pulp (Couteau and
Mathaly, 1998) which could make them desirable in-gredients in the developing market of ‘functional foods’
for health. Bioflavonoids like hesperidin, naringin or
rutin are able to normalise capillary permeability and
vascular brittleness, therefore they are frequently called
vitamin P factors. Hesperidin is applied in vein medi-
cation, acts antiviral in flue therapy and owns artificial
sweetener properties; hydrated naringin is �300 times
Table 6
Content of several relevant compounds in vegetable residues (Al-Wandawi et al., 1985; Clemente et al., 1997; Henn, 1998; Larrauri et al., 1999; Lu
and Foo, 1997; Saura-Calixto, 1998)
Residue Plant phenols (flavonoids, phenol carboxylic acids)
Colourless Coloured
Apple pomace 0.724%a (Lu and Foo, 1997); 350.6 mg/kgb, FA 8.0 mg/kgb
(Lucarini et al., 1999)
Carrot pomace b-carotenea 3 mg/kg
Chokeberry
pomace
Anthocyanins 9.1 g/kg (M�aari�aassyov�aa et al., 1999b)
Cocoa bean shells Tannins 3.1%a Leucoanthocyanidin (Nambudiri and Shivashankar, 1985)
Elderberry pomace Anthocyanins 16.6 g/kg (M�aari�aassyov�aa et al., 1999b)
Grape pomace 2%a; 11.7%a (Zeller, 1999) Anthocyanins
Grape skins 25–35%a (Anon., 1999) Anthocyanins
Grape fruit peel Naringin 0.07–1.7%b Carotenoids
Green tea 10.1–21.6%a ;c
Honeysuckle
pomace
Anthocyanins 8.0 g/kg (M�aari�aassyov�aa et al., 1999b)
Mango peel 5.5%a Carotenoids
Olive press cake 0.3%b Anthocyanins
Orange peel Hesperidin 1.3–2.4%b/1.7–2%a (Manthey and Grohmann,
1996); Nobiletind 32% (Manthey and Grohmann, 1996)
Carotenoids
Red beet pomace Betanine 414.3 mg/kgb (M�aari�aassyov�aa et al., 1999a)
Sugar beet pulp FA 0.36%a (Couteau and Mathaly, 1998)/8 g kga
(Thibault et al., 1998)
Tomato skins 210.8 mg/kgb FA 3.7 mg/kgb (Lucarini et al., 1999) Lycopenesb 120 mg/kg (Al-Wandawi et al., 1985), 80 mg/kgb
(Lucarini et al., 1999)
FA¼ ferulic acid.aRelated to dry mass.bRelated to fresh good.cDepending on the tea species and seasonal changes. Tea flavonoids are catechin, gallocatechin, epicatechin, epicatechin gallate, epigallocatechine,
epigallocatechin gallate, the latter of which is always the largest fraction (Chu and Juneja, 1997).d In orange peel oil solids (hexane extracted).
180 G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198

sweeter than saccharose, neohesperidin almost 2000times.
The array of different compounds existing in a di-
versity of free and bound forms is considerable, see
Table 6. Grape skin extract in powder form is com-
mercially available as a natural food colorant. Besides
the blue–red colour the food will be enriched with
‘‘healthy’’ polyphenols (Anon., 1999). The fermentation
of dietary fibre increases digestibility, shelf life andpreserves the bioactivity of the components. It is often
recommended as a hygienisation step prior to drying
and milling (see above).
The use of fibres from new origins that are cur-
rently not fully exploited and the possibility of mod-
ifying the fibres, by chemical, enzymatic and/or
physical treatments, combining them with other com-
ponents and enhancing their nutritional and sensorycharacteristics, will probably widen the fields of ap-
plication for dietary fibres. Thus this market shows a
reasonable utilisation potential for vegetable residues,
adding value to by-products, most of which are re-
garded as waste to be discharged so far (Theobaudin
et al., 1997).
Several residues have already been characterised by
their pectin content, as shown in Table 5. Pectins arelinear polymers of a-DD-galacturonic acid in which the DD-
galacturonic acid units are linked by 1 ! 4 glycosidic
linkages. Pectic substances have an important influence
on food texture and are used in products like jams, jel-
lies, dairy products, beverages, pastries and confection-
eries. More and more they are used in pharmaceutics
and cosmetics as well. Pectin is located in the cell walls
of vegetables and fruits, commercially and environ-mentally interesting is the use of residual matter as
a potential pectin source. The gelation mechanism of
pectins is mainly governed by their degree of esterifica-
tion (DE). Commonly, two types of pectin gels are dis-
tinguished. The first type made from high methoxyl
pectins (DE beyond 50%) form gels in an acidic envi-
ronment and in the presence of sucrose. The second type
of pectin gel is composed of low methoxyl pectins (DEbelow 50%). These pectins form gels in presence of al-
kaline earth elements, especially calcium. In both cases
gelation and gel properties depend on many factors,
including pH, temperature, DE, sugar, calcium, and
pectin content (Tuncel et al., 1998).
The presence of up to 30% pectin in dried residual
matters like sugar beet pulp, carrot pomace, potato pulp
or lemon peel and its availability in large quantities havemade extraction worthwhile. Several other sources of
pectin are reported beside the mentioned ones in Table
5, e.g., citrus peel (Widmer and Montanari, 1995), cit-
rus, apple and sugar beet pulp (Grohmann and Bothast,
1994), cocoa husk (Nambudiri and Shivashankar, 1985),
galgal (citrus fruit) peel (Attri and Maini, 1996), haw-
thorn (Mexican fruit) peel (Higareda et al., 1995),
mango peel (Srirangarajan and Shrikhade, 1976) orsunflower heads (Wang et al., 1997).
However, no pectins have ever been extracted
from vegetable residues with gel forming proper-
ties comparable to those of pectins extracted from
apple pomace. Turqouis et al. (1999) recently developed
an alkaline extraction process for pectin from sugar
beet pulp and potato pulp. A high gelling ability
was proved using 2% extract from sugar beet and po-tato pulp with 172 mg CaSO4 H2O per g extracted
product.
Kahlert (1999) tried to overcome the low gelling
properties by the combination of gelling agents using
their synergetic effects. A lot of structuring substances
like cellulose, pectin, carrageen, agar-agar, alginate can
be taken from vegetable waste and composed to new
multifunctional food ingredients and act as stabilisers,thickeners, fat replacement, etc. Pectins of different or-
igin could be mixed due to the requested application and
properties, examples are mentioned in Higareda et al.
(1995), Nambudiri and Shivashankar (1985), Srirang-
arajan and Shrikhade (1976), Wang et al. (1997) and
Widmer and Montanari (1995).
4.2. Bioconversion via solid-state fermentation: the gen-
eration of flavours
The tongue cannot be betrayed permanently.
(K€ooster, E. International FoodTec Congress,
Cologne/D 1994, giving a lecture about natural
and synthetic food additives.)
Biological conversion processes of fruit processing
wastes into various value added products through solid-
state fermentation (SSF) has been of major interest to
many laboratories around the world. SSF deals with theutilisation of water-insoluble materials for microbial
growth and metabolism, and it is usually carried out in
solid or semi-solid systems in the near absence of free
water or reduced water content compared with sub-
merged fermentation (SMF). Many of the potential
products from fruit and vegetable residues have been
developed using the SSF technique, and such products
include ethanol, methane, lactic acid, citric acid, mush-rooms, enzymes and food ingredients (Zheng and
Shetty, 1998). As shown in Table 9 most of the research
in SSF of residual matter has been done in the last seven
years. Still there is a lack of proper modelling and
process parameters.
The world market of aroma chemicals, fragrances
and flavours has world-wide a growth rate of 4–5% per
year. In 1995 it was worth 9:6� 109 US$ and in 2000it is expected to be 12� 109 US$ (Hartman, 1996).
Because of a higher consumer acceptance there is an
increasing economic interest in natural flavours.
G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198 181

The European Community guidelines 88/388/EWGand 9/71/EWG subdivide flavours/aromas into six
categories, the first of which is describing regula-
tions for the food labelling ‘‘natural flavour’’. Nat-
ural flavours are chemical substances with aroma
properties which are produced from feedstock of
plant or animal origin by means of physical, enzy-
matic or microbiological processing (Huber and
Fo�aa, 1999).
The microbial synthesis of these natural flavours is
generally carried out by SMF. Due to the high costs of
this currently used technology on an industrial scale
there is a need of developing low cost processes even forcheaper molecules like benzaldehyde (�US$ 198 per kg
(Sigma, 2000)). This could be achieved by exploration of
the metabolic pathways and by alternative technology
such as SSF (Feron et al., 1996).
Suitable for SSF is every vegetable waste in principle.
In case of bioflavour production the SSF of residual
matter is a fairly new technology of waste utilisation,
based on a very old preservation method, which bio-converts secondary raw materials to natural flavours, as
shown in Table 11.
The microbial synthesis on solid substrates offers
some advantages compared with conventional SMF
such as
• The conditions of the SSF-process are well adapted
to the requirements of moulds, which represent about60% of the micro-organisms used in flavour produc-
tion.
• The higher space-time yield leads to smaller reactor
volumes compared with SMF. The SSF reactor with
the same yield as an SMF reactor can lead to a three
times smaller amount of investment costs (Vollbrecht,
1997).
Most important flavour is currently vanillin, the an-
nual world-wide use exceeds 12,000 t/a, 20 t of which are
produced from natural extract (equivalent to 1800 t of
vanilla beans) (Asther et al., 1997). Vanillin is followed
by benzaldehyde, used in an amount of more than 1000
t/a. Vanillin is currently synthesised from petrochemical
substances like guajacol, or lignin, both of which are
produced as co-products of pulp manufacture in huge
amounts. The microbiological production is based on
the precursors eugenole or ferulic acid, the latter oftenfound in vegetable residues and pomaces in reasonable
amounts. So far there are only few effective bioconver-
sion processes available, hence an industrial application
is limited.
Tables 7 and 8 are an overview of profit margins
possible provided that an effective production path is
found. Table 9 lists recent progress in SSF of vegetable
residues, obviously most research in flavour fermenta-tion via SSF was done in the last seven years.
In order to introduce an economically competitive
biological process, three major drawbacks must be
overcome:
II(I) The high costs of the substrate (e.g., molasses).
I(II) The low product concentration (about 2% for ace-
tone–butanole–isopropanole (ABI) fermentationbecause of solvent toxicity).
Table 7
Selected flavours, production rates and selling prices
Flavour Feed conc.a Selling price in US$/kg Year
Vanillin 230 mg/l (Asther et al., 1997) Natural extract 4000 (Asther et al., 1997) 1996
560 mg/l (Thibault et al., 1998) 1998
c-Deca lactone 6 g/l Biotechnical 6000 1992
Isoamyl acetate 5.22 mg/kg DM 1999
6.25 mg/l (Christen et al., 1997) 1997
b-Phenethyl alcohol 2 g/l Biotechnical 2500 1994
2-Heptanone 17 g/l Synthetic 39 1991
Ethyl butyrate – Biotechnical 180 1996
(–) No data available.aRelated to biotechnical production.
Table 8
Selected flavours and selling prices in US$ per kg
Flavour/aroma Description Synthetic Natural
c-Deca lactone Peach, fruity 75.00 1400.00
d-Deca lactone Coconut, creamy 130.00 5500.00
1-Octen-3-ol Mushroom 184.00 –
2-Heptanone Cheesy, spicy 39.00 –
Benzaldehyde Almond 31.00 198.00
Ethyl butyrate Fruity, pineapple 31.00 55.00
Isoamyl acetate Banana, fruity,
sweet
31.00 31.00
Isoamyl butyrate Banana, fruity,
pineapple
31.00 345.00
Phenethyl alcohol Roses 31.00 2050.00
Raspberry ketone Raspberries 58.00 3000.00
Vanillin Vanilla 31.00 685.00
Vanillic acid Vanilla 410.00 –
Source: Flavors & Fragrances 2000, Aldrich Milwaukee WI USA,
(Sigma, 2000).
182 G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198

(III) The high product recovery costs (distillation hasbeen used in the past).
The provision of substrates makes up about 63% of
the total costs of ABI production, therefore a variety of
alternative compounds have been checked out for their
ability to replace the now expensive molasses (Duerre,
1998). The high raw material costs could be drastically
reduced by using vegetable co-products as substrates,see Table 9. Christen et al. (2000) mixed various pro-
portions of vegetable residues and added selected
precursors to additionally influence metabolism and
product concentration. They found that the volatile
carbon production by fungi can be significantly influ-
enced, leading to manifold enhanced production rates.
Remaining problem besides the production of a cer-
tain flavour is its isolation and purification (downstream processing), which is again strongly dependent
on the produced concentration of the ‘‘biochemical’’.
Therefore research groups have been looking for com-bined solutions of highly concentrated production and
on-line separation (Kunz, 1999).
A pilot plant for acetone and butanol fermentation in
Starrein/Austria is described in (Duerre, 1998), the op-
erational start was planned for spring 1998. As sub-
strates have been used agricultural ‘starchy’ materials
like low grade potatoes, potato cutting waste, potato
pulp and fruit water from starch production, maize andrye. The product separation is done by gas stripping
with heating of the effluent to �70 �C and condensation
of the solvent/water vapours.
Membrane based systems such as reverse osmosis,
perstraction, pervaporation and membrane evaporation,
as well as liquid/liquid extraction, adsorption and gas
stripping have been compared by Duerre (1998) towards
their employment in downstream processing, but thereis no precise statement as to the most suitable one.
Membrane systems show a high selectivity for solvents,
Table 9
Flavours and biofine chemicals produced by SSF of vegetable residues (selection)
Year Residual matter Description/conversion principle Product
1991 Apple pomace (Almosnino and Belin, 1991) Enzyme system to degrade the precursors
linoleic and linolenic acid
Volatile aldehydes, alcohols
2000 Apple pomace, spent malt grains (Stredan-
sky et al., 2000)
T. elegans CCF 1456 degraded the substrate
in a ratio of 3 to 1 (AP to SMG), precursor
peanut oil even increased the yield
c-Linolenic acid was produced in a yield of
5.17 g per kg dry substrate; with peanut oil
precursor 8.75 g per kg DM
1998 Carrot, citrus, pineapple pomace (Mukher-
jee and Sen, 1998)
Aspergillus spp. Mass multiplication Biocontrolling agent in cultivation of mel-
ons
2000 Cassava bagasse, apple pomace (Christen
et al., 2000)
Four strains of Rhizopus, two residues and
two precursors, mixed substrate combina-
tions
Volatile carbons as flavours; acetaldehyde,
ethanol, propanol, esters
1997 Cassava bagasse, wheat bran and sugarcane
bagasse (Christen et al., 1997; Bramorski
et al., 1998)
C. fimbriata, ability to generate fruity aro-
mas in dependence on the substrate used
Banana flavour and fruity complex flavours,
up to 10-fold higher production compared
to ripe bananas
1994 Citrus, apple, sugar beet pomace (Groh-
mann and Bothast, 1994)
Microbial conversion by enzymatic hydro-
lysis
Pectin, substrate, liquid biofuel
1998 Cranberry pomace (fish offal) (Zheng and
Shetty, 1998)
Trichoderma viride, Rhizopus CaCO3 was
added as neutraliser, water for aw adjust-
ment
Polymeric dye decolourising isolate for
wastewater treatment, extracellular enzymes
2001 Linseed cake, castor oil cake, olive press
cake, sunflower cake (Laufenberg et al.,
2001)
Moniliella suaveolens, Trichoderma harzia-
num, Pityrosporum ovale and Ceratocytis
moniliformis form decalactones (problems
with phenolic components)
Acceptable yields on olive press cake and
castor oil cake. d- and c-decalactone (up to
1 g per kg DM) are produced
1998 Olive cake, sugarcane bagasse (Cordova
et al., 1998)
Lipase degrading fat in olive cake Enzyme product applied in bakery goods,
confectionery, pharmaceuticals
1999 Olive pomace (Haddadin et al., 1999) Four micro-organisms, delignification, sac-
charification with Trichoderma spp., bio-
mass formation with Candida utilis and
Saccharomyces cerevisiae
Crude protein enriched from 5.9% to 40.3%.
Source for animal fodder
1995 Pineapple waste (Tran and Mitchell, 1995) A. foetidus produces citric acid 16.1 g/100 g
DM and 3% methanol
Pharmaceuticals, food industry, preserving
agent
1997 Potato waste (Lucas et al., 1997) Amylases Bakery goods, breweries, textile industry
1997 Sugar beet pulp, cereal bran (Asther et al.,
1997)
Commensalism of two micro-organisms de-
grading the substrate
Flavour vanillin
1994 Tomato pomace (Carvalheiro et al., 1994) Co-cultures of Trichoderma reesei and
Sporotrichum sp. are degrading cellulose and
hemicellulose fraction
67% less cellulose, 73% less hemicellulose,
enhanced lignin and protein content
DM ¼ dry matter.
G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198 183

but might suffer from clogging and fouling, liquid/liquidextractions might form emulsions, reducing their effec-
tiveness, gas stripping does not lead to complete removal
of solvents and adsorption materials are quite expensive.
The raw material costs for the latter adsorption mate-
rials could be drastically reduced by the use of bioad-
sorbents made of vegetable waste, which is explained in
detail below.
The restrictions might be overcome by the com-bination of SSF with hollow fibre contained liquid
membranes for the production and separation of vola-
tile food flavours like isoamyl acetate or 1-octen-3-ol
(mushroom), recently described in Laufenberg and
Cussler (1999).
The bioconversion of vegetable residues is economi-
cally attractive only if high value products are produced.
The market prices for biotechnically produced bulkchemicals are fairly low, e.g., 0.53 per kg butanol, 0.44
per kg acetone or 0.40 per kg ethanol (prices in US$ in
1980 (Duerre, 1998)). The selling prices for bioflavours
are many times higher, as shown in Table 8. Highlights
are of course fruity/flowery flavours like peach, rose or
vanilla, but also for banana, produced by Ceratocystis
fimbriata as a complex bioflavour with improved qual-
ity, the fermented flavour will reach higher market pri-ces.
The variety of vegetable residues as substrates could
be even broadened by using co-cultures of micro-
organisms, called commensalism. C. acetobutylicum for
example is unable to degrade cellulose, but in co-culture
with a mesophilic cellulolytic Clostridium spp. even cel-
lulose-enriched rice hulls, orange peel or sugar beet pulp
could be metabolised (Duerre, 1998). The researchgroup of Asther et al. (1997), Bonnin et al. (1999) and
Lesage-Meessen et al. (1999) employed a co-culture of
Aspergillus niger and Pycnoporus cinnabarius to trans-
form ferulic acid from sugar beet pulp via vanillic acid
into vanillin.
Ferulic acid is found associated with the cell wall of
very few dicots, including sugar beets (0.36% related to
dry weight) (Broughton et al., 1995a,b; Couteau andMathaly, 1998) and many monocots like wheat or maize
(1–2% related to dry weight) (Asther et al., 1997; Dexter
and Wood, 1996). It is ester-linked to pectic sidechains
in beets and ether-linked to lignin in cereals. Besides
sugar beet pulp carrot pomace contains of reason-
able amounts of pectin, thus of ferulic acid. For
olive press cake and corncobs a lignin content of �35%
rsp. 17% indicates high precursor rates too, see Table 5;remarkable amounts of ferulic acid are mentioned
for palm press fibre by Prasertsan and Prasertsan
(1996).
Almosnino and Belin (1991) described the use of the
intrinsic enzyme system of apple pomace for the bio-
transformation of fatty acids into potential flavours. By
the use of these lipolytic enzymes the precursors linoleic
and linolenic acid were converted into alcohols andvolatile aldehydes. A possible substrate to use instead of
the precursors would be olive cake with its amounts of
74.0% oleic acid, 11.7% linoleic acid and 0.8% linolenic
acid (Clemente et al., 1997). Thus a mixture of these
residues would result in a useful substrate for a bio-
conversion of flavours. The addition of SO2 and ascor-
bic acid combined with micronization of the pomace
enhanced the flavour yield significantly up to 90%. Theaddition of ascorbic acid may be replaced by a lactic
acid bacterial fermentation of the pomace, which will
enhance shelf life of the pomace and possibly flavour
yield.
A few other studies have shown the importance of the
media in the specific development of a defined aroma
(Christen et al., 1994, 1997; Meza et al., 1998). They
found that adding a nitrogen source enhances the for-mation of total volatiles up to 10 times, which is 10-fold
higher than that of ripe bananas too, as listed in Table
10.
Cassava bagasse with leucine supplement seems to be
the optimal substrate for banana flavour production.
Spent malt appears to be an even better substrate for
total volatile carbon formation. Fermentation with this
substrate has reached almost double production yieldswithout any additional supplement. Biomass develop-
ment and isoamyl acetate formation profiles are shown
Table 10
Isoamyl acetate formation depending on the substrate used (based on
(Christen et al., 1997))
Residual matter Isoamyl acetate formation
in lmol l�1
Wheat bran/no supplement Not detectable
Sugarcane bagasse/no supplement Not detectable
Cassava bagasse/no supplement 0.45
Spent malt/no supplement (Fischbach
et al., 2000)
0.8
Wheat bran plus leucine 2.1
Sugarcane bagasse plus leucine 9.5
Cassava bagasse plus leucine 48
Fig. 5. SSF of spent malt with C. fimbriata (Fischbach et al., 2000;
Laufenberg et al., 1999).
184 G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198

in Figs. 5 and 6. First experiments have shown that
spent malt has a good utilisation potential as a substrate
for the production of fruit flavours. A substrate
screening including spent malt, rape seed oil cake, soy-
bean coarse meal and different kinds of sugar beet pel-
lets and chips was done, where spent malt reached the
highest product yields, see Table 11. Therefore, the
following experiments were carried out using spent maltas an exclusive substrate.
Additional optimisation of the media could be
reached by the combination of spent malt with potato
pulp consisting of remaining 6.1% (w/w) raw protein
(Laufenberg et al., 1996). Further investigations havenot been published yet.
4.3. Vegetable residues as operating supplies: bioadsor-
bents for wastewater treatment
Realising the maximum potential using the mini-
mum amount of material.
To view vegetable waste recovery processes as po-
tential goldmines is typically overly optimistic, as the
costs of extraction and purification of the components
generally reduce the profit margins available to levelsthat are barely economic, as already described. For this
reason further effort is focused on the creation of ‘bio-
adsorbents’ with improved functionality, using their
natural content of adsorptive components or enhancing
their adsorption rate by combination of favoured raw
materials.
Adsorption is almost always a process involving a
fluid and a solid. This solid can adsorb mere traces ofsolute, making this method especially useful for dilute
solutions, including those streams requiring treatment
for pollution control. Molecules adsorb on virtually all
surfaces, the amount they adsorb is roughly propor-
tional to the amount of surface. As a result, commercial
adsorbents are extremely porous with surface areas
typically of several hundred square metres per gram. In
general the energy intensive and sophisticated materialrequiring treatment tends to be more expensive than
other separations.
Adsorbents are conveniently divided into three clas-
ses: inorganic materials, synthetic polymers and car-
bons. Inorganic materials vary widely. Activated
alumina with polar surface is used as a desiccant, as well
as silica gel. Clays are used as inexpensive adsorbents for
some petroleum based applications, mostly they are usedonce then discarded. Fuller’s earth is used to purify oils.
The most important class of inorganics is probably the
zeolites, a subclass of molecular sieves, their specific pores
are located within small crystals. Adsorbents based on
synthetic polymers, like ion exchange or acrylic ester
polymers are commonly used in wastewater treatment.
Most interesting in this connection are carbons. The
carbons have non-polar surfaces that are used to adsorbnon-polar molecules, especially hydrocarbons. They are
manufactured from both organic and inorganic sources,
and can be used to recover solvents, to filter gases or to
purify water. Overall carbons make a broad and im-
portant class of adsorbents (Cussler, 1997).
Conventional methods for treating wastewater con-
taining dyes, aromatic compounds or heavy metals are
coagulation, flocculation, reverse osmosis, nanofiltra-tion and pervaporation (Paul and Ohlrogge, 1998), and
activated carbon adsorption, the latter of which is
combined with membrane processes like nanofiltration
Table 11
Potential product substrate combinations (Fischbach et al., 2000;
Laufenberg et al., 1999)
Substrate Flavour Micro-organism
Apple pomace, spent
malt, spent hops,
carrot pomace
Ethyl butyrate
(pineapple), ethyl
pentanoate (apple),
isoamyl acetate
(banana)
C. fimbriata
Sugar beet pulp
(Asther et al., 1997;
Bonnin et al., 1999;
Lesage-Meessen
et al., 1999)
Vanillin Pycnoporus
cinnabarius, A. niger
Ricinus oil cake
(Feron et al., 2000;
Ferreira et al., 2000)
c-decalactone(peach)
Yarrovia lipolytica
1-Octen-3-ol
(mushroom)
P. pulmonarius
Castor oil cake
(Laufenberg et al.,
2001)
c-decalactone,6-pentyl-a-pyrone(nutty)
M. suaveolens,
T. harzianum
Sunflower seed cake
(Laufenberg et al.,
2001)
c-decalactone T. harzianum
Olive press cake
(Laufenberg et al.,
2001)
c-decalactone,d-decalactone(coconut)
P. ovale, Ceratocys-
tis moniliformis
Soybean coarse meal Pyrazine
(roast flavour)
Bacillus subtilis
Fig. 6. Generation of isoamyl acetate by SSF of spent malt (Fischbach
et al., 2000; Laufenberg et al., 1999).
G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198 185

(Eilers and Melin, 1999; Nicolet and Rott, 1999) orultrafiltration (Lenggenhager and Lyndon, 1997) as
well.
The mentioned processes do not show significant ef-
ficiency or economic advantage. Low cost treatment
methods have therefore been investigated for a long
time. A number of low cost adsorbents have been tried
for wastewater treatment like wool fibres (Balk€oose and
Baltacioglu, 1992), microbial biosorbents (Xie et al.,1996), pillared clays (Baksh et al., 1992), coir pith
untreated (Namasivayam and Kadirvelu, 1996) or acti-
vated (Namasivayam and Kadirvelu, 1997), banana pith
(Namasivayam and Kanchana, 1992), orange peel
(Namasivayam et al., 1996), peanut and walnut shells
(Randall et al., 1974), modified onion skin (Bankar and
Dara, 1982; Kumar and Dara, 1981) corncobs (Haw-
thorne Costa et al., 1995; Tsai et al., 1998), the combi-nation of onion skin with corncobs (Odozi and Emelike,
1985), peanut skin (Randall et al., 1975), palm kernel
husk (Omgbu and Iweanya, 1990), pecan (Ahmenda
et al., 2000a,b) and almond shells (Toles et al., 2000),
sugarcane bagasse (Kumar and Dara, 1982) or func-
tionalised lignin extracted from sugarcane bagasse
(Peternele et al., 1999). Suitable is even black currant
and apple dietary fibre because of its binding capacityfor cadmium and lead (Borycka and Zuchowski, 1998).
Furthermore Namasivayam et al. (1996) mentions peat,
biogas waste slurry, Shukla and Sakhardande (1990)
used cotton and jute fibres, bamboo pulp and saw dust.
The pre-treatment methods for these materials differ,
reaching from chemical extraction of lignin (Peternele
et al., 1999) to adding chemicals and further pyrolysis
(Hussein et al., 1996; Toles et al., 2000; Ahmenda et al.,2000a,b) as included in Table 14, polymerisation
(Bankar and Dara, 1982; Kumar and Dara, 1981;
Randall et al., 1975) or just cutting, drying and grinding
(Namasivayam and Kanchana, 1992; Namasivayam
et al., 1996), see Table 15.
Adsorbents attach atoms, molecules, ions and radi-
cals from their surrounding gaseous or liquid phase onto
their surface. Due to the loose binding forces the layer
thickness on the surface is monomolecular. Adsorptionhappens on the interface, therefore an important crite-
rion for the effectiveness of an adsorbent is its surface
area. Several methods are available to reach as large as
possible surface area like fine grinding, chemical or
biochemical modification, or creating a specific struc-
ture. Hence there is an relation between the natural
properties of vegetable material and the requirements
for high quality adsorbents which could be matchedduring adaptation processing, as visualised in Fig. 7.
Besides a large surface area the optimum adsorbent
has to possess adequate surface chemistry and pore size
distribution to adsorb targeted species. Macropores lead
the component to the micropores where the actual ad-
sorption takes place. Hence the macropores are impor-
tant for diffusion velocity and adsorption kinetics. The
ratio of micro and macropores and total surface areacan be influenced by ZnCl2 addition to the residual
matter. Hussein et al. (1996) described that an addition
of ZnCl2 solution (10 w/w%) to oil palm shells resulted
in enhanced surface area from 950 to 1200 m2 g�1 and
25% micropores, which are important for adsorption
capacity.
Granulated active carbons (GAC) with moderate
surface area (300 m2 g�1), as the case for sugarcanebagasse plus binder corn syrup, exhibit a good pore size
distribution and relatively low surface charge which
explains their good sugar decolourising capacities (Ah-
menda et al., 2000b). Compared to the latter the surface
area of unpyrolysed sugar beet pulp is fairly low, see
Table 12. Physical and chemical activation changes the
Fig. 7. Natural properties of vegetable waste (average) and expected product profile for carbons in wastewater treatment.
Table 12
Surface areas of selected carbon adsorbents
Residual matter Surface area in m2 g�1
Pecan shells (Ahmenda et al., 2000a,b) 1200
Oil palm shell (Hussein et al., 1996) 1200
Sugarcane bagasse and corn syrup
binder (Ahmenda et al., 2000b)
300
Sugar beet pulp (Dronnet et al., 1997) 3
186 G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198

adsorption behaviour of the raw material strongly,Table 14 exhibits a selection of used residues.
Toles et al. (2000) investigated the adsorptive prop-
erties of air-activated almond shells towards several
organics and copper. The almond shell carbon could
remove more than 400% of Cu2þ from the solution
compared to commercial carbon Norite RO3515. The
organic adsorption of almond shell carbon was lower
compared to Filtrasorbe 400, ranging between 84% and92% of the Calgone carbon total adsorption. Con-
vincing as well is the cost estimation: commercial car-
bons are produced for US$ 3.30 per kg, almond shell
carbons for US$ 2.45 per kg. Johns et al. (1998) com-
pared seven commercial GAC with GAC’s made of re-
sidual matter mentioned in Table 14. Both CO2 and
steam activated nutshell carbons consistently removed
more total organics than the commercial GAC’s.The soybean hull based GAC’s showed three or four
times higher copper adsorption compared with all other
commercial or co-product based GACs.
Effective adsorption is as well feasible without phys-
ical or chemical activation. Table 15 is a selection of
vegetable residues used as bioadsorbents for wastewater
treatment so far. The raw material has only been cut,
dried, and ground before the experiments; importantinfluencing parameters arise.
A first series of experiments was done with three
different residues, checking their ability to adsorb
aromatic wastewater components by Laufenberg and
Filipini (2002). Vanillin, representing a substance of
oecotoxic relevance together with benzaldehyde, phenol
and humic acid, has been tested in aqueous solution.
The adsorbing conditions were determined whilechanging the influencing process parameters. As bioad-
sorbents the residues carrot pomace, corncob, and sugar
beet pulp, dried and ground to the particle sizes 125–180
and 710–1500 lm were used.
In a second test series carrot pomace and sugar beet
pulp were inoculated with L. farciminis and fermented
for 20 h. This pre-treatment step was supposed to en-
hance the shelf life of the fresh pomace and to partlydegrade the components. The tests should reveal if there
is any correlation between the targeted metabolism of
lactic acid fermentation and the adsorbent capacity.
Current research in this area is versatile, employing
several residues and experimental conditions, and fo-
cused on different operational modes. In the following
structure and differentiation is given by subdividing the
whole material via the varying process parameters.
4.3.1. Residues, combinations, and synergies
The natural composition of the residual matter deci-sively influences the adsorption capacity. Kumar et al.
(2000) found better removal efficiency for insoluble
straw xanthate (82–99%) than for alkali treated straw
(77–87%) while treating heavy metal solutions. Laufen-berg and Filipini (2002) determined for the adsorption
rate in order of carrot pomace > corncob > sugar beet
pulp. Carrot pomace adsorbed vanillin best at pH 4,
corncob at pH 7 and sugar beet pulp at pH 10.
Kahlert (1999) found that the combination of gelling
agents, using synergetic effects, improves the techno-
logical properties towards a widespread application in
food. These facts may enhance the adsorption capacityand effectiveness too. Odozi and Emelike (1985) com-
bined corncob (lignin and furfural source) and red onion
skin (source of phenolic compounds) and could enhance
the adsorption rate by 20%. The ideal adsorbents should
be rich in lignin and rich in phenols, therefore very
effective residue combinations would be lignin-rich
grape pomace (�45%), olive cake (37%), corncob (17%)
or apple pomace (18%) combined with polyphenolrich green tea waste, mango, orange or grapefruit
peel (Manthey and Grohmann, 1996), see Tables 5 and
6.
A selectivity for special substances may be reached by
combining residues with different binding mechanisms.
Some residues preferably adsorb components by com-
plex formation, others by physical adsorption depending
on the lignin content, as described in Torre et al. (1995).Peternele et al. (1999) describe synergetic effects while
treating wastewater mixtures. The effect was explained
by competition towards the binding sites. The following
selectivity scale was found in adsorption tests for the
divalent metals by Dronnet et al. (1997): Cu2þ PPb2þ � Cd2þ � Zn2þ > Ni2þ > Ca2þ.
In dependency to the application a variety of options
arise. Different residue types may be combined, possiblypre-treated biochemically, or adsorption promoters like
fat and/or fatty acids may be added. Clemente et al.
(1997) suggested olive pomace as a suitable substrate for
adsorption. The natural fat content may even enhance
the efficiency by promoting the monolayer formation.
4.3.2. Particle size
Particle sizes range between 53 and 1500 lm as
exhibited in Table 15. So far only little dependency be-
tween particle size and adsorption rate could be identi-
fied. Ho and McKay (1999) could determine a reducedsorption capacity for colour removal with rising particle
size of sugarcane bagasse pith due to reduced surface
area; as well did Liversidge et al. (1997) with colour
adsorption on linseed cake.
Furthermore the suspension behaviour of particles in
solution is a limiting factor to the process. Contact area
is reduced if the particles sediment too fast. With most
of the investigations the effect was compensated by ag-itation. Laufenberg and Filipini (2002) have used the
polymer Galactomannan (carob flour, molecular weight
310,000 gmol�1), a food thickening agent and stabiliser,
G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198 187

to keep the particles in suspension. Besides the suspen-
sion behaviour the enhanced surface area is an impor-
tant parameter for the adsorption. Table 13 determines
the reduction in surface area per kg carrot pomace in
dependency of the particle sizes. Between the corn
classes 16 and 1700 lm the surface area is reduced by the
factor 100.
Further experiments should determine the effect ofparticle size on adsorption rate. Another possibility to
enhance the contact area could be the fixation of the
bioadsorbents in a packed bed as suggested in Fig. 8.
4.3.3. Adsorbent dosage
At least 2 g residual matter per liter solution was used
in all experiments. An increase of the metal binding with
rising sugar beet pulp concentration was naturally ob-
served by Dronnet et al. (1997). While changing the
adsorbent dosage from 3.64 to 14.55 g l�1 the totally
removed amount of metal was higher, but the slope was
regressive. Nevertheless the concentration of sugar beet
pulp needed for total saturation of all available carboxylfunctions by Cu2þ was not reached. The authors deter-
mined that the complete binding of Cu2þ by carboxy-
lates would occur for an adsorbent dosage of �50 g l�1.
Laufenberg and Filipini (2002) simultaneously varied
adsorption time and adsorbents dosage. Their optimum
adsorbent dosage was determined as 20 g l�1 with a
contact time of 5 min.
4.3.4. Removed component
Most experiments were determined treating dye so-
lutions of different origin or heavy metal solutions,
preferring combinations of copper, cadmium, lead, zinc,
chrome, and nickel. So far only Laufenberg and Filipini
(2002) have removed organics from aqueous solution.
4.3.5. Initial concentration
The initial substance concentration to be treated is
naturally dependent on its solubility in aqueous solu-
tion, it ranges from 5 to 740 mg l�1. For vanillin it couldbe determined that all applied residues adsorbed 10 or
20 mg l�1 vanillin with a similar adsorption rate (Lau-
fenberg and Filipini, 2002). The total vanillin uptake
increased with an increased initial concentration, al-
though the percentage removal decreased. The following
example will clarify:
Table 13
Surface area reduction in dependency of particle size, determined for carrot pomace
Particle size (lm) Mean d (lm) Density (gcm�3) Volume (m3) Surface (m2) Surface (m2 kg�1) Surface (m2 g�1)
0–32 16 1.43 1.71573E)14 3.21699E)09 131.118 0.131
32–63 47.5 1.41 4.48921E)13 2.83529E)08 44.792 0.044
63–90 76.5 1.39 1.87531E)12 7.35415E)08 28.212 0.028
90–125 107.5 1.4 5.20372E)12 1.4522E)07 19.933 0.019
125–180 152.5 1.39 1.48559E)11 2.92247E)07 14.152 0.014
180–250 215 1.39 4.16298E)11 5.8088E)07 10.038 0.010
250–355 302.5 1.39 1.15948E)10 1.1499E)06 7.134 0.007
355–500 427.5 1.37 3.27263E)10 2.29658E)06 5.122 0.005
500–710 605 1.37 9.27587E)10 4.59961E)06 3.619 0.003
710–1000 855 1.35 2.6181E)09 9.18633E)06 2.599 0.002
1000–1400 1200 1.35 7.23823E)09 1.80956E)05 1.851 0.001
1400–2000 1700 1.34 2.05795E)08 3.63168E)05 1.316 0.001
Fig. 8. Design considerations for future packed bed adsorption: (a) vertical adsorber with high volume packed bed (b) ring adsorber and (c)
horizontal thick layer adsorber.
188 G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198

This equilibrium effect is described in several publi-cations (Kumar and Dara, 1981; Bankar and Dara,
1982; Dronnet et al., 1997; Laufenberg and Filipini,
2002).
4.3.6. Agitation type and contact time
Different methods are applied ranging from no agi-
tation (soft mixing at the beginning), over gently mixing
to continuously stirring for 24 h. Very often the particlesare not very stable and tend to leak. Randall et al. (1975)
described the problem while employing peanut skin for
the adsorption of cupric ions; due to the leaking it lost
most of the tannin, too. They suggest a pre-treatment
mixing 1 kg peanut skin with 10 l 0.2 N H2SO4 and 500 g
35% formaldehyde, stirring it for 2 h at 50 �C. Due to
the polymerisation the separated liquid is clear. The
treated peanut skins were more stable, showed no sign
of disintegration even when immersed in water for long
periods. Untreated peanut skins were soft and tended todisintegrate, producing very fine particles which were
difficult to filter.
A contact time of five minutes is sufficient to adsorb
75–85% of the chemical (Laufenberg and Filipini, 2002);
Bankar and Dara (1982) found in equilibrium experi-
ments the maximum sorption for Ca2þ and Mg2þ within
5 min as well.
4.3.7. pH-value
A clear pH dependency was identified, changing withthe chemical adsorbed. Best adsorption results were
achieved for vanillin at an acidic pH, the sequence is
pH 4 > pH 7 > pH 10 (Laufenberg and Filipini, 2002).
Solution Adsorbent
dosage (g)
Bound
chemical
Percentage
removal
10 g
Cu2þ l�11 5.0 g
Cu2þ50
20 g
Cu2þ l�11 7.5 g
Cu2þ37.5
Table 14
Physically and chemically activated carbons made from vegetable residues as a feedstock (selection)
Material/
adsorbents
Particle size Adsorbent
dosage (g l�1)
Removed
component
Initial conc. Agitation type
and time
pH-value Temp.
Almond shells
(Toles et al.,
2000)
10� 20 mesh
size
1 benzene, tolu-
ene, 1,4-di-
oxane, CuCl2,
acetone metha-
nol, acetonitrile
80 mg l�1 Continuously
stirred 24 h
Room temp.
Oil palm shell
(Hussein et al.,
1996)
Characterisation and preparation. ZnCl2 addition of 10 w/w% resulted in higher surface area of 1200 m2 g�1 and 25%
micropores, which are important for adsorption capacity
Sugarcane bag-
asse, pecan
shells, rice hulls
and strawa
(Ahmenda
et al., 2000a,b;
Pendyal et al.,
1999)
12–40 mesh 10 Sugar decolo-
urisation
– Batch test with-
out agitation
Rice straw,
soybean hull,
sugarcane bag-
asse, peanut,
pecan and wal-
nut shells and
seven commer-
cial GACs
(Johns et al.,
1998) (molasses
as binder)
<5 mm, pelle-
tised, activated
with CO2 or
steam, physical
and chemical
activation
100 Benzene,
toluene,
1,4-dioxane,
acetonitrile,
acetone,
methanol
80 mg l�1 500 rpm, 24 h 6–7 23 �C
10 Cu2þ, Cd2þ,
Zn2þ, Ni2þ,
ionic strength
0.03
0.25 mmol of
each metal
250 rpm, 2 h
aAs binders were used sugarcane molasses, sugar beet molasses, corn syrup, coal tar pitch. These binders are necessary to enhance the physical and
chemical properties of the resulting carbon, whilst the adsorption behaviour is still dictated by the residual matter used.
G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198 189

Namasivayam and Kadirvelu (1996) determined an al-kaline pH as a promoter for the adsorption of heavy
metals and alkalis on coir pith, the effect is further de-
scribed for heavy metal adsorption on wool fibres
(Balk€oose and Baltacioglu, 1992), on lignin from sugar-
cane bagasse (Peternele et al., 1999), and the carboni-
sation of oil palm shells (Hussein et al., 1996). For acid
or dye removal the pH should be acidic as described for
orange peel (Namasivayam et al., 1996), or bananapith (Namasivayam and Kanchana, 1992). Dronnet
et al. (1997) realised that positively charged metals are
attracted to negative charges on the adsorbing pomace,
the effect becomes stronger in alkaline solutions.
Torre et al. (1995) investigated the adsorption be-
haviour of grape and olive pomace and lemon peel to-
wards Fe3þ, Fe2þ and Ca2þ. They found with increasing
pH an increase of bound mineral, in addition they de-termined that the quantity of polyvalent cations bound
by dietary fibre materials increases with a higher level of
mineral addition, which is in compliance with other re-
sults. Binding isotherms of Zn2þ increased as the initial
pH of the suspension varied from 3.5 to 7.2. As the pH is
lowered, the overall surface charge on sugar beet pulp
becomes less negative which reduces the attraction of
positively charged metal ions (Dronnet et al., 1997,1998a,b).
4.3.8. Targeted metabolism
Giovannozzi-Sermanni et al. (1995) found with a
targeted metabolism of Lentinus edodes on wheat straw
and corn stalks changing material properties. The mi-
cro-organism, mainly degrading the carbohydrates,
changed the lignin ratio and availability of the raw
material, which resulted in a higher lignin extractability
for alkaline cooking. These observations match with
results obtained by Peternele et al. (1999). Lignin is apotential adsorbents, hence a targeted metabolism of the
vegetable waste prior to the application as a bioadsor-
bents will enhance its adsorption capacity; see in this
connection Table 5 and the differing lignin content of
vegetable residues.
Similar effects are described by Carvalheiro et al.
(1994). Their aim was to enhance protein and lignin
content of tomato pomace to improve its digestibility asa fodder. After SSF for 10 days with a co-culture of T.
reesei and Sporotrichum sp. the cellulose and hemicel-
lulose content was decreased by 67% and 73% re-
spectively, on the other hand the lignin and protein
content rose manifold. A targeted metabolism towards
an enhancement of certain components, i.e., lignin or
cellulose, and thus changed particle properties is feasi-
ble.A targeted metabolism may even govern the ratio
lignin:cellulose (cellulose is used as a chromatography
adsorbent as well) towards the compound to be sepa-
rated. Cellulosic material predominately adsorbs alka-line components as determined by Nawar and Doma
(1989) for the basic dye sandocryl orange. The high af-
finity of the orange dye to cellulosic material like rice
hulls or orange peel is the result of ionic interactions
between the cationic centres on the dyestuff and acidic
sites, mostly carboxylate groups, on the fibres.
The adsorption rate is decisively influenced by the
method of acidification, as aforementioned. Hydro-chloric acid enhances the capacity due to a shift of pH-
value, but the metabolism of selected micro-organisms
additionally influences the particle character (e.g., dif-
ferent configuration of carbohydrates and lignin frac-
tion and/or degradation in total). This targeted
metabolism is superior. The fermented samples did not
need any pH adjustment, were stable in shape and initial
pH and tended to adsorb more substance than the HCl-adjusted samples (Laufenberg and Filipini, 2002).
4.3.9. Surface area
Ahmenda et al. (2000a,b) described a low surface area
for their soft materials sugarcane bagasse, rice hulls andstraw independently of chemical or physical activation.
They found that the total surface area does not correlate
with adsorption efficiency and suggested pore size dis-
tribution as well as surface charges play an important
role too. It seems that the low surface area of untreated
residues does not have the major effect on the adsorp-
tion process (Tables 12 and 13), other parameters may
be stronger as expected, see Fig. 7. Removal rates inTable 15 up to 90% are convincing.
4.3.10. Binding mechanisms
Kumar et al. (2000) observed that basic groups of
straw and straw xanthate interact with Cr3þ via an ionexchange route. For each Cr3þ exchanged, one equiva-
lent of Naþ is released into the solution, hence the so-
lution became alkaline.
Laufenberg and Filipini (2002) noted a clear tendency
of the pH-adjusted carrot pomace and sugar beet pulp
to return to its initial pH after a certain time due to the
ion exchange mechanism. Kumar and Dara (1981) tes-
ted the binding capacity of polymerised onion skinstowards several heavy metals in aqueous solutions.
During their equilibrium experiments they determined
that in all studied cases the final pH of the metal-
adsorbent-solution was always less than the initial pH.
This effect indicates that, as the metal ions are bound on
the substrate, hydrogen ions are released into the solu-
tion. The authors concluded that the onion skin sub-
strate probably acts as an acid-form ion exchanger. Thetheory was confirmed for other materials like sugarcane
bagasse (Kumar and Dara, 1982; Dronnet et al., 1997),
onion skin (Bankar and Dara, 1982), onion skin and
190 G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198

Table 15
Vegetable residues as bioadsorbents: influencing process parameters
Material/adsorbents Particle size
(lm)
Adsor-
bent
dosage
(g l�1)
Removed component Initial concentration Agitation type/time pH-value Temp. (�C) Ionic strength
Apple/black currant
pomace (Borycka and
Zuchowski, 1998)
6 180 and
710–1500
10 and
20
Cd, Pb, Ca, low 30–40% – – – – –
Banana pith (Namasiva-
yam and Kanchana, 1992)a53–1000 0.5–6 Textile dyes, up to 80% 20–100 mg l�1 140 rpm/20–200 min. 2"–11# 30� 2 –
Carrot pomace and sugar
beet pulpb (Laufenberg and
Filipini, 2002)
125–180 and
710–1500
10 and
20
Vanillin, humic acid 10 and 20 mg l�1 No agitation/5, 10,
20 min, and 1, 8, 24
h
4", 7, 10# 23� 2 –
Coconut coir pith
(Kadirvelu et al., 2001)
53–1000 2.5, 5, 6,
9
Hg2þ, Pb2þ, Cd2þ, Cu2þ,
Ni2þ 73–100%
126, 709, 996 mg l Batch mode 2#–10" – –
Corncobs (Hawthorne
Costa et al., 1995)
– 30 Cu2þ 6� 103; 0:5� 104 mole l�1 Continuous/15 min. 6 35 0.1, 0.5 and 0.9
mol l�1
Corncobs, wood chips,
wheat straw (Nigam et al.,
2000)
3, 0.3, and 0.2
mm3
100 Several red, blue and
black dyes; 70–75% re-
moval
200–500 mg l�1 Soaking for 48 h – 20, 30 or 48 –
Corncobs mixed with onion
skin (Odozi and Emelike,
1985)c ;d ;e
<200 10 Ni, Mg, Zn, Pb, Cd, Ca,
Hg, Mn-cations
142–740 mg l�1 Continuous/24 h "6 and
higher,
lower #
Room temp. –
Linseed cake and peat
(Liversidge et al., 1997)
180–1000 2 Basic blue 41–95% re-
moval, acid blue 148, re-
active red 184
50–2000 mg l�1 120 rpm/90 min. 30"þ50#strange!
–
Onion skin, sugarcane
bagasse, bark (Kumar and
Dara, 1981)f ;c
– 10 Hg, Cd, Ni, Zn, Cr, Pb 45–100 mg l�1 – 2–11 – –
Onion skins (Bankar and
Dara, 1982)c ;gPowdered 40 Ca2þ, Mg2þ 0.4–20� 103 mg l�1 Ca2þ,
0.2–1200 mg l�1 Mg2þGently agitated/10
min.
4–10", less#
30, 50, 100 –
Orange peel (Namasivayam
et al., 1996)
75–500 2–10 Dyes, 73–92% 10–60 mg l�1 140 rpm/20–90 min 3"–12# 29� 2 –
Palm kernel husk (Omgbu
and Iweanya, 1990)c>500 2 Pb2þ 60–80%, Zn2þ 13–
24%
5–25 mg l�1 – – – –
Peanut skins (Randall
et al., 1975)c<1000 10 Cu2þ 10 mg l�1 Gently agitated/30
min
6 or higher Room temp. –
Rice hulls, peat and acti-
vated carbon (Nawar and
Doma, 1989)c
160, 300, and
1200
10 Sandocryl orange 98%
and lanasyn black 78%
50 mg l�1 15"–120 min Black 3–6,
orange 2–
11
Room temp. –
Sugar beet pulpd ;f (Dron-
net et al., 1997, 1998a,b)
200–500 1.82,
7.27,
14.55
Ca2þ, Pb2þ Cd2þ, Zn2þ,
Ni2þ, Ca2þ, 72% removal,
10 min
0.01 M solution Constantly stirred/2
h
3.5–7" 25� 1 –
Sugarcane bagasse
(Peternele et al., 1999)d– 5 Cd2þ, Pb2þ 1–6 mmol dm�3 Shaking/8 h "5 or 6" 30, 40, "50 "0.5 and 1.0
mol dm�3
G.Laufen
berg
etal./Bioreso
urce
Tech
nology87(2003)167–198
191

corncobs (Odozi and Emelike, 1985), peanut skins
(Randall et al., 1975), palm kernel husks (Omgbu and
Iweanya, 1990) or pure corncobs (Hawthorne Costa
et al., 1995). Since the latter residue is mainly lignin and
cellulose based several residual matters, e.g., olive cake
or white wine pomace, could be taken into account for
future adsorption of dyes, heavy metals and chemicals,
see Table 5.Certain mechanisms for the uptake of components
from solutions are discussed according to the type of
substrate used (Dronnet et al., 1997). An overview of
binding mechanisms is given in Table 16. Some simple
chemical or physical modifications may improve the
adsorbent behaviour of these materials several times.
Modification reactions include crosslinking and/or
functionalisation to enhance the adsorbent stability and/or capacity (Peternele et al., 1999).
4.3.11. Packed bed design
Adsorption is much more effective in a packed bedthan in a stirred tank, which is an equivalent to count-
ercurrent flow in extraction. A packed bed will permit
faster mass transfer and higher conversion. Assumed is
that a large volume of solution is to be fed through a
small bed of adsorbing solid. The bed is completely
uniformly packed and the flow moving evenly, without
dispersion and independent of the bed’s radius. Hence in
the packed bed the concentration in the solid is inequilibrium with the high feed concentration. In stirred
tank loaded solid reaches equilibrium with depleted so-
lution which is less than with the feed solution. There-
fore yields are much less effective (Cussler, 1997).
The results of several investigations match with the
described relations. Namasivayam et al. (1996), Na-
masivayam and Kanchana (1992) and Peternele et al.
(1999) realised that an increase in the initial substanceconcentration increased the amount of substance ad-
sorbed. It was clearly shown that the removal of dye or
metal is dependent on its initial concentration in solu-Table
15(continued)
Material/adsorbents
Particle
size
(lm)
Adsor-
bent
dosage
(gl�
1)
Rem
oved
component
Initialconcentration
Agitationtype/time
pH-value
Tem
p.(�C)
Ionic
strength
Sugarcanebagassepith
(HoandMcK
ay,1999)
150"–1000#
0.251.5
Basicred22andacidred
114
50–300mgl�
1–
–20–80"
–
Wheatstraw
andinsoluble
straw
xanthate
(Kumar
etal.,2000)
1000
20
Cr3
þ,Cr6
þCr,Pb,Cu,
Ni,Fe,
Mn,Zn
37.3
mgl�
1Cr64mgl�
1others
�0.5
mgl�
1
Continuous/15min
3.6
25�1
–
Woolfibres(Balk€ oose
and
Baltacioglu,1992)
17onaverage
15
Ni,Cu,Zn,Cd,Hg,Pb
70%
50–200mgl�
1Constantly/upto
60
min
–25or50,no
effect
–
(")increasedadsorption,(#)decreasedadsorption,(–)nodata
available.
aWithincreasingagitationtimetherewasadecrease
indiffusionlayer
thickness.
bTargeted
metabolism
hasledto
differentpH
values
andmaterialproperties.
cDid
additionalcolumntests.
dSelectivityscale,synergisticeffects.
e20%
higher
adsorptionrate
onaveragedueto
columntest
andcombinationofresidues.
fSelectivityfortheionsare
dependingonthetypeofpomace
tooandabsolute
adsorptionrate
increasedwithhigher
initialconcentration.
gPreferred
adsorptionofCa2þ.
Table 16
Physicochemical mechanisms for the uptake of components from so-
lutions
Residue or main component Mechanism
Sugar beet pulp; responsible are the
galacturonosyl units of the pectic chains
(Dronnet et al., 1997, 1998a)
Ion exchange
Soybean hulls, peanut shells, sugar cane
bagasse, rice straw (Johns et al., 1998)
Ion exchange, chelation,
and coordination, but
without modification also
surface precipitation
Corncobs (Hawthorne Costa et al.,
1995): carboxylate groups
Ion exchange
Straw and straw xanthane (Kumar
et al., 2000)
Ion exchange
192 G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198

tion due to the change in equilibrium limit. This is the
aforesaid disadvantage of the stirred tank. It could be
transferred into an advantage if the adsorption would
take place in a packed bed. Possible design consider-
ations are given in Fig. 8. Similarities are described by
Balk€oose and Baltacioglu (1992), they suggest further
experiments with controlled flow rates and tightly
packed wool beds. Kumar and Dara (1982) achievedhigher adsorption results in a column for all tested
heavy metals, also did Bankar and Dara (1982) and
Randall et al. (1975).
4.3.12. Post-treatment
Nigam et al. (2000) removed several textile dyes fromeffluents using the residues wheat straw, wood chips and
corncob shreds. The adsorption rate was rather good,
up to 75% of the dyes could be removed. The authors
additionally investigated the suitability of the dye-
adsorbed residues for SSF by the white-rot fungi
Phanerochaete chrysosporium and Coriolus versicolor,
both possessing dye degradation capabilities. It could be
determined that both dye adsorbed residues wheat strawand corncob shreds were heavily colonised and hence
are suitable substrates for fungal colonisation. Visual
substrate decolourisation occurred; which has to be
precisely determined in future experiments. The fer-
mented residue is useful as a soil conditioner. Other
groups have used Pleurotus pulmonarius for the degra-
dation of antrazine (Masaphy et al., 1996).
Depending on the recycling method for the spentadsorbents an equal ratio between adsorption and de-
sorption becomes an important goal. The high adsorp-
tion rate is the only point of interest if the laden
adsorbents is afterwards metabolised by micro-organ-
isms. A regeneration/desorption has to be taken into
account, if the substances should be recycled, several
methods are described (Hamer and P€uuschner, 1997;
Urano and Tachikawa, 1992; Urano et al., 1992).
4.3.13. Future considerations
Depending on the origin the residues adsorb more or
less water. Oil press cakes, like olive or sunflower cake,
exhibit little water binding capacity, beverage residues
like carrot or grape pomace swell considerably in water.
Hydration properties can be reduced by establishing
cross-links between the cell wall polysaccharides using
the pre-treatment chemicals formaldehyde (Kumar
and Dara, 1982; Randall et al., 1975; Dronnet et al.,
1998a,b), epichlorohydrin, divinylsulfone, glutaric di-
aldehyde, or citric acid. The saponification doubledthe cation-exchange capacity, and epichlorohydrin was
most effective improving the binding properties of the
investigated beet pulp per unit of hydrated volume, i.e.,
decreased its hydration properties and increased metal
binding capacities (Dronnet et al., 1998a,b).
Laufenberg and Filipini (2002) found that their bio-
adsorbents carrot pomace and sugar beet pulp exhibit
some properties which are difficult to handle, these arein particular: no stable size, the particles are brittle, the
colour leaking distorts the results, and the phase sepa-
ration is difficult. Corrective actions in future experi-
ments should include
• mechanical pre-treatment to enlarge surface area and/
or stabilize the suspension,
• chemical pre-treatment to prevent colour leaking,• packed bed technology to enhance effectiveness to-
wards the equilibrium limit, enlarge contact area
and to simplify phase separation, desorption, and re-
cycling,
• SSF to change particle composition and naturally in-
fluence pH-value,
• extrusion to add promoters, concentrate existing pro-
moters, remove disturbing components, combine twomaterials with differing properties, coat or line com-
ponents, and influence the suspension behaviour,
• adding fat containing residues like olive cake to re-
duce the brittleness.
5. Conclusions and outlook
This clean production concept shows a good utilisa-
tion potential for solid vegetable waste. It could achieve
a reduction of investment and raw material costs and
Table 17
Adsorption parameters and economic evaluation of three adsorbents (Nawar and Doma, 1989)
Compound used Adsorbents material,
particle size 0.3–0.4 mm
Adsorbent dosage (g l�1) Comparative cost
per kg adsorbent
Comparative cost
to remove kg dye
Comparative cost to
remove kg dye in (%)
Sandocryl orange Peat 1.8 0.04 1.46 0.53
Rice hulls 16.3 0.01 3.3 1.52
Activated carbon 13.6 1 277 –
Lanasyn black Peat 72 0.04 58 1.4
Rice hulls 77.7 0.01 15.8 0.38
Activated carbon 204 1 4163
G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198 193

can contribute to a waste minimised food production.Especially the development of bioadsorbents is a prom-
ising area to add value to vegetable residues. They will
appear as a cheap and environmentally safe alternative
to commercial ion-exchange resins.
Nawar and Doma (1989) have made economical
considerations for three bioadsorbents at equilibrium
under similar experimental conditions in 1989. They
used peat, rice hulls and activated carbon for stirredtank and fixed bed experiments to remove dyes from
wastewaters. The costs of removal of 1 kg of each of the
studied dyes via different adsorbents showed that for
sandocryl orange the costs of peat are 0.53% and of rice
hulls 1.52% of the costs of activated carbon. For lanosyl
black removal the values are 1.4% and 0.38%, respec-
tively, see Table 17.
The cheapness of rice hulls in particular and vegeta-ble residues in general means that regeneration is a
further goal in clean production but under economic
considerations not absolutely necessary.
Acknowledgements
Special thanks to Conny Schnitter, MEng, who spentfour weeks in Lappeenranta/Finland doing the adsorp-
tion experiments and setting up the analysis.
References
Abu-Qudais, M., 1996. Fluidized-bed combustion for energy produc-
tion from olive cake. Energy 21 (3), 173–178.
Ahmenda, M., Marshall, W.E., Rao, R.M., 2000a. Production of
granular activated carbons from select agricultural by-products
and evaluation of their physical, chemical and adsorption proper-
ties. Bioresource Technology 71, 113–123.
Ahmenda, M., Marshall, W.E., Rao, R.M., 2000b. Surface properties
of granular activated carbons from agricultural by-products and
their effects on raw sugar decolorization. Bioresource Technology
71, 103–112.
Almosnino, A.M., Belin, J.M., 1991. Apple pomace: an enzyme system
for producing aroma compounds from polyunsaturated fatty acids.
Biotechnology Letters 13 (12), 893–898.
Al-Wandawi, H. et al., 1985. Tomato processing wastes as essential
raw material source. Journal of Agriculture and Food Chemistry
33, 804–807.
Anon., 1999. Gesunde Rott€oone. Lebensmitteltechnik 7–8, 38.
Arogba, S.S., 1999. The performance of processed mango (Mangifera
indica) kernel flour in a model food system. Bioresource Techno-
logy 70, 277–281.
Asther, M. et al., 1997. Fungal biotransformation of European
agricultural by-products to natural vanillin: a two-step process.
Food Ingredients Porte de Versailles, Paris, France, 12–14
November 1996, pp. 123–125.
Attri, B.L., Maini, S.B., 1996. Pectin from galgal (Citrus pseudolimon
tan.) peel. Bioresource Technology 55 (1), 89–91.
Baksh, M.S., Kikkinides, E.S., Yang, R.T., 1992. Characterization by
physisorption of a new class of microporous adsorbens: Pillared
clays. Industrial Engineering and Chemical Research 31 (9), 2181–
2189.
Balk€oose, D., Baltacioglu, H., 1992. Adsorption of heavy metal cations
from aqueous solutions by wool fibers. Journal of Chemical
Technology and Biotechnology 54, 393–397.
Bankar, D.B., Dara, S.S., 1982. Binding of calcium and magnesium by
modified onion skins. Journal of Applied Polymer Science 27,
1727–1733.
Bonnin, E. et al., 1999. Enhanced bioconversion of vanillic acid into
vanillin by the use of ‘natural’ callobiose. Journal of the Science of
Food and Agriculture 79, 484–486.
Borycka, B., 1996. Fruit pomace in new dietary fiber compositions.
Przemysl-Fermentacyjny-i-Owocowo-Warzywny (Poland) 40 (12),
37–39.
Borycka, B., Zuchowski, J., 1998. Metal sorption capacity of fibre
preparations from fruit pomace. Polish-Journal-of-Food-and-Nu-
trition-Sciences 7 (48), 67–76.
Bramorski, A., Soccol, C.R., Christen, P., Revah, S., 1998. Fruity
aroma production by Ceratocystis fimbriata in solid cultures
from agro-industrial wastes. Revista de Microbiologia 28 (3),
208–212.
Brose, D.J., 1993. Novel process technology for utilisation of fruit and
vegetable waste. SBIR Phase I project USDA ICSRS, Washington,
DC.
Broughton, N.W., Dalton, C.C., Jones, G.C., Williams, E.L., 1995a.
Adding value to sugar beet pulp. Hoehere Erloese aus Zuckerrue-
benschnitzeln. Zuckerindustrie 120 (5), 411–416.
Broughton, N.W., Dalton, C.C., Jones, G.C., Williams, E.L., 1995b.
Adding value to sugar beet pulp. International Sugar Journal 97
(1154), 57–60, 93–95.
Bundschuh, E., Baumann, G., Gierschner, K., 1988. Untersuchungen
zur CO2-Hochdruckextraktion von Aromastoffen aus Reststoffen
der Apfelverarbeitung. Deutsche Lebensmittelrundschau 84, 205–
210.
Bundschuh, E. et al., 1986. Gewinnung von nat€uurlichen Aromen aus
Reststoffen der Lebensmittelproduktion mit Hilfe der CO2-Ho-
chdruckextraktion. Lebensmittelwissenschaft und Technologie 19,
493–496.
Carson, K.J., Collins, J.L., Penfield, M.P., 1994. Unrefined, dried
apple pomace as a potential food ingredient. Journal of Food
Science 59 (6), 1213–1215.
Carvalheiro, F., Roseiro, J.C., Collaco, M.T.A., 1994. Biological
conversion of tomato pomace by pure and mixed fungal cultures.
Process biochemistry 29 (7), 601–605.
Chkhikvishvili, I.D., Gogiya, N.N., 1995. Flavonoids of mandarin
fruit wastes and their fungistatic effect on the fungus Phoma
tracheiphila. Applied Biochemistry and Microbiology 31 (3), 292–
296.
Christen, P., Bramorski, A., Revah, S., Soccol, C.R., 2000. Charac-
terization of volatile compounds produced by Rhizopus strains
grown on agro-industrial solid wastes. Bioresource Technology 71,
211–215.
Christen, P., Meza, J.C., Revah, S., 1997. Fruity aroma production in
solid state fermentation by Ceratocystis fimbriata: influence of the
substrate type and the presence of precursors. Mycological
Research 101 (8), 911–919.
Christen, P., Villegas, E., Revah, S., 1994. Growth and Aroma
production by Ceratocystis fimbriata in various fermentation
media. Biotechnology letters 16 (11), 1183–1188.
Chu, D.C., Juneja, L.R., 1997. General chemical composition of green
tea and its infusion. In: Yamamoto, T. et al. (Eds.), Chemistry and
applications of Green tea. CRC Press, New York (Chapter 2).
Clarke, S., 1995. Eat bagasse. Sugar Journal 58, 12.
Clemente, A., Sanchez-Vioque, R., Vioque, J., Bautista, J., Millan, F.,
1997. Chemical composition of extracted dried olive pomaces
containing two and three phases. Food-Biotechnology 11 (3), 273–
291.
194 G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198

Cordova, J. et al., 1998. Lipase production by solid state fermentation
of olive cake and sugar cane bagasse. Journal of Molecular
Catalysis B: Enzymatic 5 (1–4), 75–78.
Couteau, D., Mathaly, P., 1998. Fixed-bed purification of ferulic acid
from sugar beet pulp using activated carbon: optimization studies.
Bioresource Technology 60, 17–25.
Cussler, E.L., 1997. Diffusion, second ed. Cambridge University Press,
New York.
Dexter, J.E., Wood, P.J., 1996. Recent applications of debranning of
wheat before milling. Trends in Food Science and Technology 7
(2), 35–41.
Dronnet, V.M., Axelos, M.A.V., Renard, C.M., Thibault, J.F., 1997.
Binding of divalent metal cations by sugar-beet pulp. Carbohydrate
Polymers 34, 73–82.
Dronnet, V.M., Axelos, M.A.V., Renard, C.M., Thibault, J.F., 1998a.
Improvement of the binding capacity of metal cations by sugar-
beet pulp. 1. Impact of cross-linking treatments on composition,
hydration and binding properties. Carbohydrate polymers 35, 29–
37.
Dronnet, V.M., Axelos, M.A.V., Renard, C.M., Thibault,
J.F., 1998b. Improvement of the binding capacity of metal cations
by sugar-beet pulp. 2. Binding of divalent metal cations
by modified sugar-beet pulp. Carbohydrate polymers 35, 239–
247.
Duerre, P., 1998. New insights and novel developments in clostridial
acetone/butanol/isopropanol fermentation. Applied Microbiology
and Biotechnology 49, 639–648.
Eilers, L., Melin, T., 1999. Nanofiltration kombiniert mit Adsorption
an Pulverkohle f€uur die Abwasserreinigung. In: 7th Aachener
Membrankolloquium 9–13.03.1999 (D), pp. 343–346.
El-Nawawi, S.A., Heikal, Y.A., 1996. Production of pectin pomace
and recovery of leach liquids from orange peel. Journal of Food
Engineering 28 (3/4), 341–347.
Feil, H., 1995. Biodegradable plastics from vegetable raw materials.
Agro-Food-Industry-Hi-Tech. 6 (4), 25–32.
Feron, G., Bonnarme, P., Durand, A., 1996. Prospects for the
microbial production of food flavours. Trends in Food Science
and Technology 7, 285–293.
Feron, G., Wang, X.-D., Viel, C., Perrin, C., Mauvais, G., Cachon, R.,
Divi�ees, C., 2000. Influence of a reduction agent on the bioconver-
sion of ricinoleic acid to y-decalactone by the yeast Sporidiobolus
ruinenii: cellular and enzymatic approaches. Biotechnology 2000:
Book of Abstracts 3 Poster VI, 146, p. 604.
Ferreira, P., Diez, N., Soilveri, J., Copa-Patino, J.L., 2000. Screening
of different Streptomycetes for the ability to produce ferulic acid
from sugar beet pulp. Biotechnology 2000: Book of Abstracts 3
Poster V. 84, p. 276.
Filipini, M., Hogg, T., 1997. Upgrading of vegetable wastes and
applications in the food industry. In: 11 Forum for Ap-
plied Biotechnology. Gent (Belgium). 25–26 September 1997.
Mededelingen-Faculteit-Landbouwkundige-en-Toegepaste-Biolog-
ische-Wetenschappen-Universiteit-Gent (Belgium). 62(4a) pp.
1329–1331.
Fischbach, R., Laufenberg, G., Kunz, B., 2000. Generation of
natural flavours by solid-state fermentation of food industry
by-products. In: Proceedings of Biotechnology 2000, 03–
08.09.00, Berlin, Frankfurt/Main: Dechema e.V., vol. 4. pp. 266–
268.
Giovannozzi-Sermanni, G. et al., 1995. Paper biopulping of agricul-
tural wastes by Lentinus edodes (Chapter 22). In: Sadler, J.N.,
Penner, M. (Eds.), Degradation of insoluble Carbohydrates. ACS
Press, Washington, DC, pp. 339–351.
Gomes, T., Caponio, F., 1997. Evaluation of the state of oxidation of
crude olive-pomace oils. Influence of olive-pomace drying and oil
extraction with solvent. Journal of Agriculture and Food Chem-
istry 45 (4), 1381–1384.
Grigelmo-Miguel, N., Martin-Belloso, O., 1999. Influence of fruit
dietary fibre addition on physical and sensorial properties of
strawberry jams. Journal of Food Engineering 41, 13–21.
Grohmann, K., Bothast, R.J., 1994. Pectin-rich residues generated by
processing of citrus fruits, apples, and sugar beets: enzymatic
hydrolysis and biological conversion to value-added products.
ACS-symp-ser, vol. 566. American Chemical Society, Washington,
DC, pp. 372–390.
Gupta, R., Chauhau, T.R., Lall, D., 1993. Nutritional potential of
vegetable waste products for ruminants. Bioresource Technology
44 (3), 263–265.
Haddadin, M.S., Abdulrahim, S.M., Al-Kawaldeh, G.Y., Robinson,
R.K., 1999. Solid state fermentation of waste pomace from olive
processing. Journal of Chemical Technology and Biotechnology
74, 613–618.
Hamer, A., P€uuschner, H., 1997. Untersuchungen zur L€oosungsmit-
telr€uuckgewinnung aus der w€aassrigen Phase durch Adsorption und
Desorption mit Mikrowellenenergie. Chemie Ingenieur Technik 69,
480–483.
Haque, K.S., Tareque, A.M., Zaman, M.A., 1997. Chemical analysis
of nutritive values of fruit wastes. Bangladesh Veterinary Journal
31 (1–2), 54–56.
Hartman, H., 1996. Scent and taste. Perfumer and Flavorist 21 (2), 21–
24.
Hausmanns, S., Laufenberg, G., Lipnitzki, F., Field, R., 1999.
Prospects and performance of hydrophobic pervaporation in the
concept of clean production. In: 2nd Asia-Pacific Cleaner Pro-
duction Roundtable and Trade Expo, Brisbane April 21–24,
1999.
Hawthorne Costa, E.T. et al., 1995. Removal of cupric ions from
aqueous solutions by contact with corncobs. Separation Science
and Technology 30 (12), 2593–2602.
Henn, T., 1998. Untersuchungen zur Entwicklung und Bewertung
funktioneller Lebensmittelzutaten aus Reststoffen am Beispiel von
M€oohrentrestern und ihrer Anwendung in Getr€aanken (Thesis Bonn/
D 1998) Cuvillier Verlag G€oottingen.
Henn, T., Kunz, B., 1996. Zum Wegwerfen zu schade. ZFL 47 (1/2),
21–23.
Higareda, R.A. et al., 1995. Commercial pectin concentrate from
hawthorn pulp. Revista Chapingo, Serie Horticultura 1 (4), 155–
157.
Ho, Y.S., McKay, G., 1999. A kinetic study of dye sorption by
biosorbent waste product pith. Resources, Conservation and
Recycling 25, 171–193.
Huber, W., Fo�aa, D., 1999. Eine reine Geschmacksfrage. Lebensmit-
teltechnik 9, 52–54.
Hussein, M.Z., Tarmizi, R.S.H., Zainal, Z., Ibrahim, R., 1996.
Preparation and characterization of active carbons from oil palm
shells. Carbon 34 (11), 1447–1454.
Inglett, G.E., 1998. New cereal products with health benefits. Food
Ingredients Europe, Conference Proceedings, 17–19.
Johns, M.M., Marshall, W.E., Toles, C.A., 1998. Agricultural by-
products as granular activated carbons for adsorbing dissolved
metals and organics. Journal of Chemical Technology and
Biotechnology 71, 131–140.
Joshi, V.K., Sandhu, D.K., 1996. Preparation and evaluation of an
animal feed byproduct produced by solid-state fermentation of
apple pomace. Bioresource Technology 56 (2/3), 251–255.
Kadirvelu, K., Thamaraiselvi, K., Namasivayam, C., 2001. Removal
of heavy metals from industrial wastewaters by adsorption onto
activated carbon prepared from an agricultural solid waste.
Bioresource Technology 76, 63–65.
Kahlert, S., 1999. Stabile IQF-Produkte. Lebensmitteltechnik 4, 68–69.
K€ooksel, H., €OOzboy, €OO., 1999. Effects of sugar beet fiber on cookie
quality Einfluß von Zuckerr€uu benfaserstoffen auf die Qualit€aat von
Cookie-Keksen. Zuckerindustrie 124 (7), 542–544.
G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198 195

Kriener, M., 1999. Naturnaher Pflanzenbau-€OOkoweine sind nicht nur
gesund, sie schmecken auch besser. K€oolner Stadtanzeiger 237 (9/
10), 28.
Mukherjee, K., Sen, B., 1998. Biological control of Fusarium wilt of
muskmelon by formulations of Aspergillus niger. Israel Journal of
Plant Sciences 46 (1), 67–72.
Kruszewska, I., Thorpe, B., 1995. What is clean production? HTML
version of a Greenpeace Briefing, http://www.rec.org/Poland/wpa/
cpb.htm.
Kumar, A., Rao, N.N., Kaul, S.N., 2000. Alkali-treated straw and
insoluble straw xanthate as low cost adsorbents for heavy metal
removal-preparation, characterization and application. Biore-
source Technology 71, 133–142.
Kumar, P., Dara, S.S., 1981. Binding heavy metal ions with polymer-
ized onion skin. Journal of Polymer Science: Polymer Chemistry
edition 19, 397–402.
Kumar, P., Dara, S.S., 1982. Utilisation of agricultural wastes for
decontaminating industrial/domestic wastewaters from toxic met-
als. Agricultural Wastes 4, 213–223.
Kunz, B., 1999. €AApfel f€uur Bananenduft. Der Spiegel 48, 273.
Kuper-Theodoritis, S., 1996. Emissionen der Lebensmittelindust-
rie, ihre Vermeidung, Verwertung und Entsorgung. In: Heiss,
R. (Ed.), Lebensmitteltechnologie. Springer, New York (Chapter
47).
Larrauri, J.A. et al., 1999. New approaches in the preparation of high
dietary fibre powders from food by-products. Trends in Food
Science and Technology 10 (1), 3–8.
Laufenberg, G., Rosato, P., Kunz, B., 2001. Conversion of vegetable
waste into value added products: oil press cake as an exclusive
substrate for microbial d-decalactone production. In: Lecture at
Lipids, Fats, and Oils: Reality and public perception. 24th world
congress and exhibition of the ISF, 16–20.09.01. BerlinD, AOCS
press, pp.10ff (ISBN: 1-893997-16-x).
Laufenberg, G., Filipini, M., 2002. Adding value to vegetable waste:
particle characterization and adsorption properties of food indus-
try residues. Environ. Prog. (submitted).
Laufenberg, G., Gr€uuß, O., Kunz, B., 1996. Neue Konzepte der
Reststoffverwertung in der Lebensmittelindustrie––Chancen f€uur dieKartoffelst€aarkeindustrie. New concepts for the utilisation of
residual products from food industry––Prospects for the potato
starch industry. Starch-St€aarke 48, 315–321.
Laufenberg, G., Hausmanns, S., Kunz, B., Nystroem, M., 1999. Green
productivity concept for the utilisation of residual products from
food industry––trends and performance. In: Lecture at the 2nd
Asia-Pacific Cleaner Production Roundtable and Trade Expo,
Brisbane 21–24 April, 1999.
Laufenberg, G.H., Cussler, E.L., 1999. Recovery of bioflavours by
hollow fibre membrane contactors. In: Euromembrane 99, 19–22
September, 1999. Leuven, Belgium, pp. 317–318.
Lenggenhager, T., Lyndon, R., 1997. Profit-generating benefits of
ultrafiltration and adsorber technology. Fruit Processing 7, 250–
256.
Lesage-Meessen, L. et al., 1999. Fungal transformation of ferulic acid
from sugar beet pulp to natural vanillin. Journal of the Science of
Food and Agriculture 79, 487–490.
Liversidge, R.M., Lloyd, G.J., Wase, D.A.J., Forster, C.F., 1997.
Removal of basic blue 41 dye from aqueous solution by linseed
cake. Process Biochemistry 32 (6), 473–477.
Lu, Y., Foo, L.Y., 1997. Identification and quantification of ma-
jor polyphenols in apple pomace. Food Chemistry 59 (2), 187–
194.
Lucarini, M. et al., 1999. Endogenous markets for organic versus
conventional plant products. In: H€aagg, M. et al. (Eds.) Agri-Food
Quality II, Quality management of Fruits and Vegetables. Confer-
ence proceedings of a congress held on 22–25.04.1998 in Turku,
Finland. Royal society of Chemistry, Cambridge, UK, pp. 306–
310.
Lucas, J. et al., 1997. Fermentative utilization of fruit and vegetable
pomace (biowaste) for the production of novel types of products–
results of an air project. In: Proceedings of the eleventh forum
for applied biotechnology, Gent, Belgium, 25–26 September,
1997, Part II. Mededelingen-Faculteit-Landbouwkundige-en-Toe-
gepaste-Biologische-Wetenschappen, Universiteit-Gent. 62(4b),
1865–1867.
Majid, A., Haroon, S., Joarder, G.K., 1995. High protein feed from
vegetable waste. Bangladesh Journal of Scientific and Industrial
Research 30 (2–3), 1–11.
Manthey, J.A., Grohmann, K., 1996. Concentrations of Hesperi-
din and other orange peel flavonoids in citrus processing
byproducts. Journal of Agriculture and Food Chemistry 44, 811–
814.
M�aari�aassyov�aa, M. et al., 1999a. Red beet as source of pigments. In:
H€aagg, M. et al. (Eds.) Agri-Food Quality II, Quality management
of Fruits and Vegetables. Conference Proceedings of a Congress
held on 22–25.04.1998 in Turku, Finland. Royal society of
Chemistry, Cambridge, UK, pp. 306–310.
M�aari�aassyov�aa, M., �SSilh�aar, S., Kov�aac, M., 1999b. New sources for
anthocyanins. In: H€aagg, M. et al. (Eds.) Agri-Food Quality II,
Quality management of Fruits and Vegetables. Conference Pro-
ceedings of a Congress held on 22–25.04.1998 in Turku, Finland.
Royal society of Chemistry, Cambridge, UK, pp. 311–313.
Martin, A.M. (Ed.), 1998. Bioconversion of waste materials to
industrial products, second ed. Blackie Academic & Professional,
London.
Martin-Cabrejas, M.A., Esteban, R.M., Lopez-Andreu, F.J., Wal-
dron, K., Selvendran, R.R., 1995. Dietary fiber content of pear and
kiwi pomaces. Journal of Agriculture and Food chemistry 43 (3),
662–666.
Martin-Carron, N., Garcia-Alonso, A., Goni, I., Saura-Calixto, F.,
1997. Nutritional and physiological properties of grape pomace as
a potential food ingredient. American Journal of Enology and
Viticulture 48 (3), 328–332.
Masaphy, S., Levanon, D., Henis, Y., 1996. Degradation of antrazine
by the Lignocelluloytic Fungus Pleurotus pulmonarius during solid-
state fermentation. Bioresource Technology 56, 207–214.
Masoodi, F.A., Chauhan, G.S., 1998. Use of apple pomace as a source
of dietary fiber in wheat bread. Journal of Food Processing and
Preservation 22, 255–263.
Meza, J.C., Christen, P., Revah, S., 1998. Effect of added amino acids
on the production of a fruity aroma by Ceratocystis fimbriata.
Sciences des Aliments 18, 627–636.
Nakata, B., 1994. Recycling by-products on California vineyards.
Biocycle 4, 61.
Namasivayam, C., Kanchana, N., 1992. Waste Banana Pith as
Adsorbent for color removal from wastewaters. Chemosphere 25
(11), 1691–1705.
Namasivayam, C., Kadirvelu, K., 1996. Uptake of mercury (II) from
wastewater by activated carbon from an unwanted agricultural
solid by-product: coirpith. Carbon 1073(SGML) C, 1–6.
Namasivayam, C., Kadirvelu, K., 1997. Activated carbons prepared
from coir pith by physical and chemical activation methods.
Bioresource Technology 62, 123–127.
Namasivayam, C., Muniasamy, N., Gayatri, K., Rani, M., Rangana-
than, K., 1996. Removal of dyes from aqueous solutions by
cellulosic waste orange peel. Bioresource Technology 57, 37–43.
Nambudiri, E.S., Shivashankar, S., 1985. Cocoa waste and its
utilization. Indian Cocoa, Arecanut and Spices Journal 8 (3), 78–
80.
Nanjundaswamy, A.M., 1997. Processing. In: R.E. Litz (Ed.), The
Mango, Botany, Production and Uses, Cab International, Wal-
lingford, pp. 535–539.
Nawar, S.S., Doma, H.L., 1989. Removal of dyes from effluents using
low-cost agricultural by-products. The Science of the Total
Environment 79, 271–279.
196 G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198

Nicolet, L., Rott, U., 1999. Einsatz der Membranfiltration zur
Trennung von Pulveraktivkohle aus Wasser und Abwasser. In:
7th Aachener Membrankolloquium, 9–13.03.1999, (D), pp. 287–
290.
Nigam, J.N., 1999. Continuous ethanol production from pineapple
cannery waste. Journal of Biotechnology 72, 197–202.
Nigam, P., Armour, G., Banat, I.M., Singh, D., Marchant, R., 2000.
Physical removal of textile dyes from effluents and solid-state
fermentation of dye-adsorbed agricultural residues. Bioresource
Technology 72, 219–226.
Nwuha, V. et al., 1999. Solubility studies of green tea extracts in pure
solvents and edible oils. Journal of Food Engineering 40, 161–165.
Odozi, T., Emelike, R., 1985. The sorption of heavy metals with
corncob hydroxylate-red onion skin resins. Journal of Applied
Polymer Science 30, 2715–2719.
Ohsawa, K., Chinen, C., Takanami, S., Kuribayashi, T., Kurokouchi,
K., 1994. Studies on effective utilization of carrot pomace, 1:
Effective utilization to bread. Research Report of the Nagano State
Laboratory of Food Technology Japan (22), 24–28.
Ohsawa, K., Chinen, C., Takanami, S., Kuribayashi, T., Kurokouchi,
K., 1995. Studies on effective utilization of carrot pomace, 2:
Effective utilization to cake, dressing and pickles. Research Report
of Food Technology Research Institute of Nagano Prefecture,
Japan 4 (23), 15–18.
Omgbu, J.A., Iweanya, V.I., 1990. Dynamic sorption of Pb2þ and Zn2þ
ions with palm Elaesis guineensis kernel husk. Journal of Chemical
Education 67 (9), 800–801.
Pag�aan, J., Ibarz, A., 1999. Extraction and rheological properties of
pectin from fresh peach pomace. Journal of Food Engineering 39,
193–201.
Paul, D., Ohlrogge, K., 1998. Membrane separation processes for
clean production. Environmental progress 17 (3), 137–141.
Pendyal, B. et al., 1999. Removal of sugar colorants by granular
activated carbons made from binders and agricultural by-products.
Bioresource Technology 69, 45–51.
Peternele, W.S., Winkler-Hechenleitner, A.A., Pineda, E.A., 1999.
Adsorption of Cd(II) and Pb(II) onto functionalized formic
lignin from sugar cane bagasse. Bioresource Technology 68, 95–
100.
Prasertsan, S., Prasertsan, P., 1996. Biomass residues from palm oil
mills in Thailand: an overview on quantity and potential usage.
Biomass and Bioenergy 11 (5), 387–395.
Purchase, B., 1995. Products from sugarcane. International Sugar
Journal 97 (1154), 70–71.
Randall, J.M., Hautala, E., Waiss, A.C., 1974. Removal and
recycling of heavy metal ions from mining and industrial
waste streams with agricultural by-products. In: SO Proceed-
ings of Minerals Waste Utilization Symposium 4th. pp. 329–
334.
Randall, J.M., Reuter, F.W., Waiss, A.C., 1975. Removal of cupric ion
from solution by contact with peanut skins. Journal of Applied
Polymer Science 19, 1563–1571.
Rodriguez-Kabana, R., Estaun, V., Pinochet, J., Marfa, O., 1995.
Mixtures of olive pomace with different nitrogen sources for the
control of Meloidogyne spp. on tomato. Journal of Nematology 27
(45), 575–584.
Sans, C., Mata-Alvarez, J., Cecchi, F., Pavan, P., Bassetti, A., 1995.
Volatile fatty acids production by mesophilic fermentation of
mechanically-sorted urban organic wastes in a plug-flow Reactor.
Bioresource Technology 51 (1), 89–96.
Saura-Calixto, F., 1998. Antioxidant dietary fiber product: a new
concept and a potential food ingredient. Journal of Agricultural
and Food Chemistry 46 (10), 4303–4306.
Schr€ooder, T., 1999. Verkapselt und funktionell. Lebensmitteltechnik 6,
48–50.
Schutz, E. et al., 1982. Verfahren zur Extraktion der Aromastoffe aus
der Vanillekapsel, EP 0075134 A2.
Scott Kantor, L., Lipton, K., Manchester, A., Oliveira, V., 1997.
Estimating and addressing america’s food losses. Economic
Research Service, United States Department of Agriculture,
prepublished on the web. URL: http://151.121.66.126:80/whats-
new/feature/ARCHIVES/JULAUG97/INDEX.HTM.
Seibel, W., Hanneforth, U., 1994. Ballaststoffkonzentrate. Lebensmit-
teltechnik 4, 14–16.
Shukla, S.R., Sakhardande, V.D., 1990. Cupric ion removal by dyed
cellulosic materials. Journal of Applied Polymer Science 41, 2655–
2663.
Sigma, A., 2000. Flavors & Fragrances, Catalogue and Price list 2000.
Milwaukee, WI, USA.
Sreenath, H.K., Crandall, P.G., Baker, R.A., 1995. Utilization of
citrus by-products and wastes as beverage clouding agents. Journal
Of Fermentation And Bioengineering 80 (2), 190–194.
Srirangarajan, A.N., Shrikhade, A.J., 1976. Mango peel waste as a
source of pectin. Current science 45 (17), 620–621.
Sriroth, K. et al., 2000. Processing of cassava waste for improved
biomass utilization. Bioresource Technology 71, 63–69.
Stock, U., 1999. Am K€oorper der Suppe Wo kommt eigentlich der
Geschmack her? Die. ZEIT 52, 36, vom 22.12.99.
Stredansky, M., Conti, E., Stredanska, S., Zanetti, F., 2000. c-Linolenic acid production with Thamnidium elegans by solid-state
fermentation on apple pomace. Bioresource Technology 73, 41–
45.
Tagesschau vom 2.11.1999, Strom aus Kaffeesatz. HTML version der
Tagesschau/ARD, http://www.tagesschau.de/archiv/1999/11/02/
akruell/meldungen /kaffee?
Theobaudin, J.Y. et al., 1997. Dietary fibres: nutritional and techno-
logical interest. Trends in Food Science and Technology 8, 41–
48.
Thibault, J.F. et al., 1998. Fungal bioconversion of agricultural by-
products to vanillin. Lebensmittel, Wissenschaft und Technologie
31, 530–536.
Toles, C.A. et al., 2000. Acid-activated carbons from almond shells:
physical, chemical and adsorptive properties and estimated cost of
production. Bioresource Technology 71, 87–92.
Toma, R.B. et al., 1979. Physical and chemical properties of potato
peel as a source of dietary fiber in bread. Journal of Food Science
44, 1403–1407, 1417.
Torre, M., Rodriguez, A.R., Saura-Calixto, F., 1995. Interactions of
Fe(II), Ca(II) and Fe(III) with high dietary fibre materials: a
physicochemical approach. Food Chemistry 54 (1), 23–31.
Tran, C.T., Mitchell, D.A., 1995. Pineapple waste––a novel substrate
for citric acid production by solid-state fermentation. Biotechno-
logy Letters 17 (10), 1107–1110.
Tsai, W.T., Chang, C.Y., Lee, S.L., 1998. A low cost adsorbent from
agricultural waste corn cob by zinc chloride activation. Bioresource
Technology 64, 211–217.
Tuncel, G., Nout, M.J.R., Brimer, L., 1998. Degradation of cyano-
genic gycosides of bitter apricot seeds (Prunusi iarmeniacai) by
endogenous and added enzymes as affected by heat treatments and
particle size. Food Chemistry 63 (1), 65–69.
Turqouis, T., Rinaudo, M., Taravel, F.R., Heyraud, A., 1999.
Extraction of highly gelling pectic substances from sugar beet pulp
and potato pulp: influence of extrinsic parameters on their gelling
properties. Food Hydrocolloids 13, 255–262.
Urano, K., Tachikawa, H., Kitajima, M., 1992. Process development
for removal and recovery of phosphorus from wastewater by a new
adsorbent. 4. Recovery of phosphate and aluminium from desorb-
ing solution. Industrial Engineering and Chemical Research 31,
1513–1515.
Urano, K., Tachikawa, H., 1992. Process development for removal
and recovery of phosphorus from wastewater by a new ad-
sorbent. 3. Desorption of phosphate and regeneration of ad-
sorbent. Industrial Engineering and Chemical Research 31,
1510–1513.
G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198 197

Valiente, C., Arrigoni, E., Esteban, R.M., Amado, R., 1995. Grape
pomace as a potential food fiber. Journal of Food Science 60 (4),
818–820.
Vitolo, S., Petarca, L., Bresci, B., 1998. Treatment of olive oil industry
wastes. Bioresource Technology 67 (2), 129–137.
Vlyssides, A.G., Loizidou, M., Zorpas, A.A., 1999. Characteristics of
solid residues from olive oil processing as bulking material for co-
composting with industrial wastewaters. Journal of Environment,
Science and Health 34 (3), 737–748.
Vollbrecht, D., 1997. Feststoff-Fermentation-Ein historischer €UUberb-
lick. Chemie Ingenieur Technik 69 (10), 1403–1408.
Wang, J. et al., 1997. Continuous countercurrent extraction of pectin
from sunflower heads. Transactions of the ASAE 40 (6), 1649–
1654.
Widmer, W., Montanari, A.M., 1995. Citrus waste streams as a source
of phytochemicals. In: 107th Annual Meeting of the Florida State
Horticultural Society, Orlando/Florida, USA, vol. 107. pp. 284–
288.
Xie, J.Z., Chang, H.-L., Kilbane II, J.J., 1996. Removal and
recovery of metal ions from wastewater using biosorbents and
chemically modified biosorbents. Bioresource Technology 57, 127–
136.
ZDF.MSNBC vom 10.03.00, Reinheitsgebot f€uur Schokolade f€aallt.
Internetseite des ZDF, http://www.zdf.msnbc.de/news/50614.asp.
Zeller, B.L., 1999. Development of porous carbohydrate food ingre-
dients for the use in flavour encapsulation. Trends in Food Science
and Technology 9 (11–12), 389–394.
Zheng, Z., Shetty, K., 1998. Cranberry processing waste for solid state
fungal inoculant production. Process Biochemistry 33 (3), 323–329.
Zia-ur-Rehman, Ali, S., Khan, A.D., Shah, F.H., 1994. Utilisation of
fruit and vegetable wastes in layers diet. Journal of Science in Food
Agriculture 65 (4), 381–383.
198 G. Laufenberg et al. / Bioresource Technology 87 (2003) 167–198