2008 Library Prize submission: “Comparison of the ... · Here we report on tests of the efficacy...
Transcript of 2008 Library Prize submission: “Comparison of the ... · Here we report on tests of the efficacy...
2008 Library Prize submission:
“Comparison of the Inhibitory Effects of Tea Tree Oil, Fresh Garlic and Commercial Garlic on Bacterial Growth”
Biology 212, Winter 2008
Susannah L. Bodman
Comparison of the Inhibitory Effects of Tea Tree Oil, Fresh Garlic and Commercial Garlic on Bacterial Growth
Abstract
Tea tree oil and garlic preparations have shown early promise as agents for effectively controlling the growth of a wide variety of bacteria, including some drug-resistant strains. Exploring the extent and limits of their efficacy will become increasingly important as the numbers of drug-resistant microbes rise and the public becomes increasingly interested in “natural” methods of controlling bacteria. To increase understanding of the extent of their efficacy, the ability of tea tree oil, fresh garlic and commercial garlic on to inhibit bacterial growth was further tested here on three species of gram-positive and gram-negative bacteria — Bacillus subtilis, Escherichia coli and Serratia marcescens . Of these, tea tree oil shows the greatest promise for inhibiting growth across a broad spectrum of bacteria, followed by fresh garlic. Commercial garlic, however, appears to be highly variable in its inhibitory effects and is therefore unreliable as an antibacterial agent.
Introduction
The efficacy of essential oils and garlic at inhibiting bacterial growth has been well-tested across a variety of gram-positive and gram-negative bacterial species. In recent years, compounds in the oil of Melaleuca alternifolia (aka tea tree), as well as fresh and commercial garlic preparations, have been tested by numerous researchers.
Here we report on tests of the efficacy of tea tree oil, fresh garlic and commercial garlic against gram-positive Bacillus subtilis a and gram-negative Escherichia coli and Serratia marcescens .
Tea tree oil has been shown in other studies to have broad-spectrum inhibitory activity against numerous bacteria and other organisms.
In gram-negative bacterium E. coli , tea tree oil was found to disrupt the permeability of cell membrane structures, stimulate leakage of cellular potassium ions and inhibit respiration with lethal effects, which may explain tea tree oil’s antimicrobial activity. In this and other bacteria, H+, K+, Na+ and Ca2+ ions play key roles in allowing cells and their organelles to regulate the exit and entry of different compounds (Cox, Mann, Markham, Bell, Gustafson, Warmington, et al., 2000).
A cell membrane’s role in acting as a permeable barrier is integral to key cellular functions, including regulating the cell’s energy states, energy transduction processes, transportation of solutes, metabolism and turgor pressure. It’s the toxic effects that essential oils and their components have on cell membrane structure and function that have been used to explain their antimicrobial action (Cox, Mann, Markham, Bell, Gustafson, Warmington, et al., 2000).
Commercial preparations of tea tree oil, such as the product Burnaid, which is used to topically treat burn wounds, also have been shown to have inhibitory activity against E. coli and other bacteria, such as the gram-positive Staphylococcus aureus. However, it fails to inhibit the bacteria Enterococcus faecalis (gram-positive) and Pseudomonas aeruginosa (gram-negative) (Faoagali, George & Leditschke, 1997).
Overall, gram-negative bacteria has been found to be more resistant to the effects of essential oils, including tea tree, than are gram-positive strains. Tea tree oil has been included in a group of substances exhibiting the highest antimicrobial properties and broadest ranges of inhibition, producing zones of inhibition between 2 mm and 16 mm for the gram-positive Bacillus cereus, Micrococcus luteus, S. aureus, Streptococcus faecalis and the gram-negative Enterobacter cloacae, Alcaligenes faecalis, E. coli, P. aeruginosa (Chao, Young & Oberg, 2000).
Meanwhile, research into the efficacy of garlic (Allium sativum) or garlic-derived compounds (e.g. allicin and ajoene) as antibacterial agents has focused on the effects of fresh garlic preparations (including juices), aqueous and alcoholic extracts, lyophilized powders, steam-distilled oil and commercial garlic preparations ( Sivam, 2001).
Allicin (Fig. 1), which is an active derivative of freshly crushed garlic, has been shown to have antibacterial activity in its pure form on a range of gram-negative and gram-positive bacteria, including multi-drug resistant strains of E. coli. Allicin’s activity appears to come from its chemical reaction with the thiol groups of various enzymes, including RNA polymerase, which plays a key role in transcribing DNA into RNA in cells (Ankri & Mirelman, 1999; Campbell & Reece, 2005; Sivam, 2001).
Amounts of allicin and other compounds in different strains of garlic have been found to be variable, ranging from 2.8 mg/g to 7.7 mg/g. Allicin is produced in garlic cloves apparently as a defense mechanism against fungi and other microbial soil pathogens, which invade cloves and destroy membranes enclosing compartments containing the compound alliin and the enzyme allinase. When allin and the enzyme interact, this results in the rapid production of allicin, which in turn inactivates the invading pathogen. Allicin molecules, however, have short half-lives due to further reactions with surrounding proteins. If not for this half-life, as well as limited and localized production in the area of microbial attack, allicin could have a “quasi-suicidal” effect on the garlic plant itself, as this compound is also toxic in massive quantities to various plant tissues and enzymes (Ankri & Mirelman, 1999).
Reaction of allicin with various thiol-containing enzymes in microorganisms may contribute to the mechanism of its antimicrobial activity. Allicin’s inhibitory activity also may be tied to acetyl-CoA-forming systems, DNA and protein synthesis (e.g. in the gram-negative Salmonella typhimurium), and RNA synthesis. In fact, the activity on the latter in RNA is immediate, suggesting that RNA polymerase could be the primary target of allicin action, especially in E. coli. Allicin’s condensation product, ajoene, has also been shown to inhibit proliferation of the protist Trypanosoma cruzi. Overall, various garlic preparations have shown wide spectrum antibacterial activity against gram-negative and gram-positive bacteria, including species of Escherichia, Salmonella, Staphylococcus, Streptococcus, Klebsiella, Proteus, Bacillus and Clostridium. Also showing sensitivities to garlic are Mycobacterium tuberculosis (weakly gram-positive), Helicobacter pylori (gram-negative), and various species or strains of drug-resistant microbes. However, garlic appears to not be effective against the gram-positive Clostridium botulinum (Ankri & Mirelman, 1999).
Garlic, which may contain 0.3 percent to 0.5 percent allicin, also has been shown to be effective enough against food-borne pathogens S. aureus, E. coli, Salmonella enterica typhi (gram-negative) and Listeria monocytogenes (gram-positive) that use of garlic has been recommended for increasing the shelf-life and retarding growth of pathogens in processed foods. Minimum inhibitory concentrations of the four pathogens listed above were found to be 5.0, 3.95, 7.0 and 8.8 percent, respectively (Kumar & Berwal, 1998; Sivam, 2001).
Ajoene, meanwhile, is a sulfur-containing compound obtained from allicin that prevents platelet aggregation and exhibits broad-spectrum antimicrobial activity, including inhibiting growth of gram-positive B. cereus, Bacillus subtilis, Mycobacterium smegmatis, S. aureus and Lactobacillus plantarum and gram-negative E. coli, Klebsiella pneumoniae and Xanthomonas maltophilia. Among these bacteria, B. subtilis was found to require one of the lowest concentrations of ajoene to inhibit growth (minimum inhibitory concentration), while other gram-positive bacteria were generally more
sensitive at lower concentrations (except for M. luteus) than gram-negative bacteria, such as E. coli (Naganawa, Iwata, Ishikawa, Fukuda, Fujino, & Suzuki, 1996).
Ajoene shares a chemical structure with that of allicin, including two allyl groups, sulfur and a sulfinyl group. In fact, the disulfide bond (S-S) may be important to the antimicrobial activity of ajoene and allicin (Naganawa, Iwata, Ishikawa, Fukuda, Fujino, & Suzuki, 1996).
In commercial garlic preparations, water and phosphoric acid (a weak acid) are sometimes used as a buffer (to maintain pH) in preserved (canned or bottled) garlic. This buffering activity results from the tendency of phosphoric acid (H3PO4) to dissociate in the presence of water (H2O) into hydronium ions (H3O+) and various anionic phosphoric compounds (ending in phosphate, PO43-, as the final product) (Spencer, Bodner & Rickard, 2006).
Based on its impacts on cell membranes and ion leakage, tea tree oil was expected to be the most effective agent for inhibiting bacterial growth in the current study involving B. subtilis , E. coli and S. marcescens (e.g. showing the largest zones of inhibition across all species and/or consistently across the greatest number of the samples). Based on its impacts on RNA polymerase but the short-lived nature of allicin, fresh garlic was expected to be the second-most effective agent. Commercial garlic, especially as prepared in water and phosphoric acid, was expected to be the least effective antibacterial agent.
Methods
B. subtilis , E. coli and S. marcescens were cultured (from stocks maintained at Portland Community College) in standard tryptic soy nutrient broth (which was inoculated with 1 mL of a broth already inoculated several days prior) in test tubes by PCC biology laboratory staff and transferred to petri dishes (aka plates) containing standard tryptic soy agar, using aseptic techniques throughout. Agars were inoculated by using sterile swabs to spread bacteria in a standard lawn formations.
A commercial preparation of tea tree oil of unknown concentration (but potentially as high as 100 percent) was obtained from Desert Essence. A single-plant clove of fresh elephant garlic purchased from Barbur Foods was chopped finely by PCC biology lab staff using a sterile scalpel and cutting paper. Commercial garlic was obtained from Spice World Inc. in the form of its Minced Garlic product, which contains garlic, water and phosphoric acid in unknown concentrations.
Disks (6 mm in width) of Ahlstrom standard filter paper were sterilized in an autoclave and then aseptically transferred and soaked for several hours in distilled water, tea tree oil and solutions of fresh garlic and commercial garlic in containers with lids to minimize airborne contamination.
For tea tree oil tests, one tea tree oil-soaked disk and one water-soaked disk (used as a control) were placed on inoculated agars. For garlic tests, one fresh garlic-soaked disk, one commercial garlic-soaked disk and one water-soaked disk were placed on inoculated agars. In all cases, aseptic techniques were used in transferring the disks to the agars.
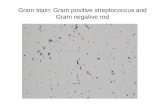
Each prepared disk was incubated at about 36° C for seven days. Fig. 2 shows images of the resulting bacterial growth in some of the petri dishes.
The inhibitory effects of the agents tested were quantified by measuring zones of inhibition in millimeters.
Results
Four groups tested the inhibitory effects of tea tree oil, fresh garlic, commercial garlic and sterile controls against samples of B. subtilis, E. coli and S. marcescens. The zone of inhibition (ZOI) measurements were recorded in Table 1; 0 mm ZOIs indicate no inhibition was present.
Two problems occurred during the experiment. Group 1’s B. subtilis sample did not have sufficient growth to assess inhibitory effects, while group 4 experienced spillage of tea tree oil into its control test area, producing a 10 mm ZOI. Meanwhile, no one group tested all agents on all types of bacteria, and some groups did not record specific data for commercial garlic and control tests.
After excluding these problematic results, the following was observed:Tea tree oil inhibited growth of bacteria in five of the six samples in which it was tested; group
3’s sample of S. marcescens was the only one not displaying inhibition. ZOIs ranged from 5 mm to covering two-thirds of a plate for B. subtilis, from 10 mm to 17 mm for E. coli and was 6 mm for group 4’s S. marcescens sample. The size of the inhibition zone for group 3’s B. subtilis sample was the largest of all the samples — the ZOI whose diameter ranged up to two-thirds of the plate.
Fresh garlic, meanwhile, created inhibition in two of eight samples tested, producing ZOIs of 3 mm in one B. subtilis sample, 5 mm for one E. coli and 2 mm for one S. marcescens. All samples with inhibitions zones came from group 4.
Commercial garlic produced inhibition in three of six plates tested and encouraged growth in
Finally, except for the contamination in group 4’s control, all the control samples for which data were recorded showed no inhibition, as expected.one of the six. ZOIs ranged from 7 mm for E. coli to 0.5 mm and 3 mm for S. marcescens. Group 1’s E. coli sample encouraged growth around its garlic-soaked disk.
Discussion
The results for tea tree oil were consistent with previous experiments in the literature in that the oil produced broad-spectrum inhibition across the three types of bacteria tested. In producing the largest ZOI in the B. subtilis test, it may also be consistent with other findings that gram-positive bacteria are more sensitive to tea tree oil’s inhibitory effects than gram-negative varieties, such as E. coli and S. marcescens. The size range of the zones of inhibition of all three bacterial types was also
generally consistent with that reported by other researchers (2 mm to 16 mm ZOIs) (Chao, Young & Oberg, 2000).
The mechanism by which tea tree oil achieved inhibitory effects, however, was not tested in this study, so it is cannot be determined whether the oil’s lethality here is due to disrupting cell membrane structures and causing potassium ion leakage in E. coli and S. marcescens, as reported in Cox, Mann, Markham, Bell, Gustafson, Warmington, et al. (2000).
Fresh garlic showed inhibitory action in each type of bacteria tested, which is consistent with findings in the literature in the broad-spectrum effects of garlic components allicin and ajoene (Ankri & Mirelman, 1999; Sivam, 2001; Naganawa, Iwata, Ishikawa, Fukuda, Fujino, & Suzuki, 1996). However, only group 4’s tests obtained ZOIs, while other groups experienced no inhibition with fresh garlic. Perhaps group 4’s methods of applying the antibacterial agent differed slightly from other groups, such as applying chunks of garlic instead of the paper disks or perhaps applying paper disks that had a greater saturation of fresh garlic solution.
Also for fresh garlic, the mechanism of inhibition was not tested, so it is unclear whether the observed inhibition was due to interference with RNA polymerase by allicin or ajoene (Ankri & Mirelman, 1999) or due to other mechanisms.
Commercial garlic resulted in ZOIs only in E. coli and S. marcescens, ranging from 0.5 mm to 7 mm in size. In one of the E. coli samples (group 1’s), it appeared to encourage bacterial growth immediately around the garlic-soaked disk. The photos in Fig. 2 for fresh and commercial garlic tests also show two other groups (although it is unclear which from the images) experienced similar growth around their commercial garlic tests for B. subtilis and E. coli, albeit this growth was not noted in the data. It is possible the growth is due to contamination of the commercial garlic sample by another microbe other than the bacteria being tested; however, further tests (beyond visual inspection) were not conducted to determine the identity of the organism growing around the disks.
Even so, careful aseptic procedures were used to prevent contamination of all samples, so contamination by another microbe is less likely, unless this contamination occurred during processing. However, such contamination during processing would seem to contradict the findings in the literature of garlic’s usefulness as a preservative that discourages bacterial growth and inhibits pathogens (Kumar & Berwal, 1998; Sivam, 2001).
Instead, it seems more likely that the growth suggests that commercial garlic’s inhibitory effects may be highly variable and therefore unreliable as an antibacterial agent. This variability may be due to how it was processed. Whereas the fresh garlic in this test came from a single plant, commercially processed garlic may contain minced cloves from several different plants with variable levels of allicin and other compounds (e.g. the 0.3 percent to 0.5 percent variation in allicin content found by Kumar & Berwal, 1998). Additionally, preservation of garlic in a solution of water and phosphoric acid, which dissociate into hydronium cations and various phosphoric anions, may react with the charged portions of allicin’s sulfinyl group (Fig. 1) or other portions of this molecule, breaking it apart or otherwise altering its molecular geometry so that it loses its ability to act on bacterial cells and inhibit their growth or reproduction. Further testing of the chemical reactions taking place between garlic compounds in a water and phosphoric acid solution and their impacts on mechanisms of inhibition of bacteria are needed to reach a solid conclusion.
Additionally, it is possible that the presence of water, phosphoric acid and their dissociated products may be beneficial in and of themselves to certain bacterial types. Perhaps additional reactions occur that lead to the formation of phosphates or phosphate groups that the bacteria can use in their respiratory processes, encouraging growth. Again, further chemical tests are needed to assess this possibility.
Overall, the test results seem to support tea tree oil as being the most effective agent (among those studied) for inhibiting bacterial growth. This is based on the broad-spectrum inhibition observed for the oil across all three bacterial types, inhibition in more than one sample of each bacterial type and the large sizes of ZOIs compared to those of other agents, especially in B. subtilis. Meanwhile, fresh garlic appeared to be the second-most effective agent in that it produced ZOIs in one sample of each type, but that these ZOIs were generally smaller in size than those produced by tea tree oil.
Finally, the variability of the results in commercial garlic and its encouragement of bacterial growth make it appear to be the least effective agent in this test.
However, it should be noted that these findings are based on a small sample size involving only three types of bacteria. Additionally, the agents tested were not applied in a range of known and controlled concentrations, so it’s difficult to know whether different results would have been achieved if a variety of concentrations of each agent had been tested.
Therefore, these findings are limited in scope and should not be considered statistically significant. Further testing is needed — including larger sample sizes, using a greater variety of bacterial species and a variety of controlled concentrations for antibacterial agents, analysis of the mechanisms of inhibition, and the effects of water and phosphoric acid solutions on allicin and ajoene chemistry — to fully and confidently assess the efficacy of these antibacterial agents.
Conclusion
Tea tree oil shows promise as a broad-spectrum antibacterial agent that should continue to be investigated for topical antibacterial or disinfectant uses, whereas fresh garlic also shows promise as an antibacterial agent for internal use as a dietary aid or food preparation agent that may discourage bacterial growth in foods for human consumption. Further investigation of fresh garlic is also encouraged. These investigations may become increasingly relevant as the numbers of drug-resistant bacteria increase and the need to find alternative means of controlling them rises.
Commercial garlic, meanwhile, shows considerably less promise and effectiveness as an antibacterial agent; it’s use should remain limited to a seasoning additive. Furthermore, the effects of water and phosphoric acid preparations should be investigated to see whether they create conditions that encourage growth of bacteria after canned garlic products are opened and whether this poses any pathogenic risks for humans.
Acknowledgments
I thank Portland Community College biology instructor Kelly Ballew and biology lab technician Cathie Cookus for their assistance during the research for this paper.
References
Ankri, S., & Mirelman, D., (1999). Antimicrobial properties of allicin from garlic. Microbes and
Infection, 2, 125-129.
Campbell, N.A., & Reece, J.B. (2005). Biology. San Francisco: Pearson Education Inc./Benjamin
Cummings.
Chao, S.C., Young, D.G., & Oberg, C.J. (2000). Screening for inhibitory activity of essential oils on
selected bacteria, fungi and viruses. Journal of Essential Oil Research, 12, 639-649.
Cox, S.D., Mann, C.M., Markham, J.L., Bell, H.C., Gustafson, J.E., Warmington, J.R., et al. (2000). The
mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). Journal of
Applied Microbiology, 88, 170-175.
Faoagali, J., George, N., & Leditschke, J.F. (1997). Does tea tree oil have a place in the topical
treatment of burns? Burns, 23, 349-351.
Kumar, M., & Berwal, J.S. (1998). Sensitivity of food pathogens to garlic (Allium sativum). Journal of
Applied Microbiology, 84, 213-215.
Naganawa, R., Iwata, N., Ishikawa, K., Fukuda, H., Fujino, T., & Suzuki, A. (1996). Inhibition of
microbial growth by ajoene, a sulfur-containing compound derived from garlic. Applied and
Environmental Microbiology, 62, 4238-4242.
Sivam, G.P. (2001). Protection against Helicobater pylori and other bacterial infections by garlic.
Journal of Nutrition, 131, 1106S-1108S.
Spencer, J.N, Bodner, G.M., & Rickard, L.H. (2006). Chemistry: Structure and Dynamics. Hoboken, N.J.:
John Wiley & Sons Inc.