Solution Thermodynamic: Vapor/Liquid Equilibrium (VLE) PTT 201/4 THERMODYNAMICS SEM 1 (2013/2014)
-
Upload
antonia-small -
Category
Documents
-
view
225 -
download
2
Transcript of Solution Thermodynamic: Vapor/Liquid Equilibrium (VLE) PTT 201/4 THERMODYNAMICS SEM 1 (2013/2014)

Solution Thermodynamic:
Vapor/Liquid Equilibrium (VLE)
PTT 201/4 THERMODYNAMICSSEM 1 (2013/2014)

Nature of Equilibrium– Definition– Measures of composition
VLE : Qualitative behaviorSimple Models for VLE - Raoult’s Law - Dewpoint & Bubblepoint Calculations with Raoult’s Law - Henry’s LawVLE by modified Raoult’s lawVLE from K-value correlations
Chapter Outline (Smith)

THE NATURE OF EQUILIBRIUM
Equilibrium : A static condition in which no changes occur in the macroscopic properties of a system with time.
The T, P, composition reaches final value which will remain fixed: equilibrium

m
m
m
mx iii
V
xC ii
iiiMxM
Measures of composition

VLE: State of coexistence of L & V phases A condition where a liquid phase and vapor phase
are in equilibrium with each other At this condition: rate of evaporation (liquid → vapor) = rate of condensation (vapor → liquid)
VLE: QUALITATIVE BEHAVIOR
Binary mixture: Mixture that contains two constituents e.g: mixture of liquid and vapor at an equilibrium level takes place when liquid and vapor are allowed to contact to each other in a closed location

• Under surface- sat. V states (P-T-y1)
• Upper surface- sat. L states (P-T-x1)
• Liquid at F, reduces pressure at constant T & composition along FG, the first bubble appear at L – bubble point: a point when a liquid forms the first bubble of vapor and begins to evaporate
• As pressure reduces, more & more L vaporizes until completed at W; point where last drop of L (dew) disappear – dew point: a point when a vapor forms the first droplet of liquid and begins to condense
Fig. 10.1 – Shows the P-T-composition surfaces of equilibrium states of
saturated V & saturated L of a binary system



SIMPLE MODELS FOR VLE

Raoult’s Law
• V phase is an ideal gas– Applicable for low to moderate
pressure• L phase is an ideal solution
– Valid only if the species are chemically similar (size, same chemical nature e.g. isomers such as ortho-, meta- & para-xylene)
Assumptions;

NiPxPy satiii ,...,2,1
Where;
pressure Total :
species pure of pressureVapor :
fraction mole phase:
fraction mole phase:
P
iP
Vy
Lx
sati
i
i
1

BUBL P: Calculate {yi} and P, given {xi} and T
DEW P: Calculate {xi} and P, given {yi} and T
BUBL T: Calculate {yi} and T, given {xi} and P
DEW T: Calculate {xi} and T, given {yi} and P
Dewpoint & Bubblepoint Calculations with Raoult’s Law
FIND GIVEN

For binary systems to solve for bubblepoint calculation (T is given);
1i iy
i
satiiPxP 1212 xPPPP satsatsat
PPx
ysat
11
1
2
3

i
satii Py
P1
Raoult’s law equation can be solved for xi to solve for dewpoint calculation (T is given) 1i i
x
satsat PyPyP
2211//
1
satPPy
x1
1
1
4
5

Example 1
Binary system acetonitrile(1)/nitromethane(2) conforms closely to Raoult’s law. Vapor pressure for the pure species are given by the following Antoine equations:
00.209
64.972,22043.14ln
00.224
47.945,22724.14ln
02
01
CtkPaP
CtkPaP
sat
sat
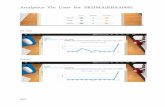
a)Prepare a graph showing P vs. x1 and P vs. y1 at temperature 750C
b)Prepare a graph showing t vs. x1 and t vs. y1 for a pressure of 70 kPa
i
ii

a) BUBL P calculations are required. Since this is a binary system, Eq. 2 may be used.
)(1212 AxPPPP satsatsat
At 750C, the saturated pressure is given by Antoine equation;
98.4121.83 21 satsat PP
Substitute both values in (A) to find P;
kPaP
P
72.66
6.098.4121.8398.41

The corresponding value of y1 is found from Eq. 1, sat
iii PxPy
x1 y1 P/kPa
0.0 0.0000 41.98
0.2 0.3313 50.23
0.4 0.5692 58.47
x1 y1 P/kPa
0.6 0.7483 66.72
0.8 0.8880 74.96
1.0 1.0000 83.21
7483.0
72.66
21.836.0111
P
Pxy
sat


At point c, the vapor composition is y1=0.6, but the composition of liquid at c’ and the pressure must read from graph or calculated. Thus DEW P calculations are required. By using Eq. 3;
satsat PyPyP
2211
1
For y1=0.6 and t=750C
kPaP 74.5998.414.021.836.0
1

And by Eq. 1,
4308.0
21.83
74.596.0
1
11
satP
Pyx
This is the liquid-phase composition at point c’
b) When P is fixed, the T varies along T1sat and
T2sat, with x1 & y1. T1sat & T2sat are calculated
from Antoine equation;
ii
isati C
PA
Bt
ln

For P=70kPa, T1sat=69.840C, T2sat=89.580C. Select T between these two temperatures and calculate P1sat &
P2sat for the two temperatures.
Evaluate x1 by Eq. (A). For example;
satsat
sat
PP
PPx
21
21
5156.0
84.4676.91
84.46701
x
Get y1 from Eq. 1
6759.0
70
76.915156.0111
P
Pxy
sat
e.g; select T= 78˚C
Substituting T= 78˚C into (i) and (ii)
P1sat = 91.76 kPa
P2sat = 46.84 kPa

Summary;
x1 y1 T/˚C
0.0000 0.0000 89.58 (t2sat)
0.1424 0.2401 86
0.3184 0.4742 82
0.5156 0.6759 78
0.7378 0.8484 74
1.0000 1.0000 69.84 (t1sat)


1. For pressure low It is so low that it can be assume as ideal gas
2. For species present as a very dilute solution in liquid phase
Assumptions;
Henry’s Law

NiHxPy iii ,...,2,1
Where;
pressure Total :
constant sHenry' :
fraction mole phase:
fraction mole phase:
P
H
Vy
Lx
i
i
i
Henry’s Law
6


Example 2
Assuming that carbonated water contains only CO2(1) and H2O(2), determine the compositions of the V & L phases in a sealed can of ‘soda’ & the P exerted on the can at 100C. Henry’s constant for CO2 in water at 100C is about 990 bar and x1=0.01.

Henry’s law for species 1 & Raoult’s law for species 2 are written;
111 HxPy satPxPy 222
With H1=990 bar & P2sat = 0.01227 bar (from steam tables at 100C)
barP
P
912.9
01227.099.099001.0
satPxHxP 2211

Then by Raoult’s law, Eq. 1 written for species 2;
0012.0
912.9
01227.099.0222
P
Pxy
sat
Whence y1=1-y2=0.9988, and the vapor phase is nearly pure CO2, as expected.

The 2nd assumption of Raoult’s Law is abandoned, taking into account the deviation from solution
ideality in L phase.
Thus, activity coefficient is introduced in Raoult’s Law
NiPxPy satiiii ,...,2,1
VLE BY MODIFIED RAOULT’S LAW
7

Activity coefficients are function of T & liquid phase composition, x
1i iy
i
satiii PxP
i
satiii Py
P
1
For bubble point
For dew point
Since;
1i ix
8
9

AZEOTROPE
A mixture that has a constant composition of liquid and vapor phase
When x1=y1, the dew point and bubble point curves are tangent to the same horizontal line
A boiling L of this composition produce a vapor exactly the same composition; L does not change in composition as it evaporates

VLE FROM K-VALUE CORRELATTIONS
The partition between liquid and vapor phases of a chemical species is equilibrium ratio, Ki.
i
ii x
yK
This quantity is called K-value.
10

satiii PxPy K-value for Raoult’s Law
P
PK
sati
i
K-value for modified Raoult’s Lawsatiiii PxPy
P
PK
satii
i
11
12

Hence,
For binary systems to solve for bubble point calculation;
1i iy
1 ii ixK
For binary systems to solve for dew point calculation;
1i ix
Hence, 1ii
i
K
y
13
14

K-value from DePriester chart-Low T range

K-value from DePriester chart-High T range

When given a mixture of composition at certain T or P;
Bubble point
- System is almost vaporized
- The given mole fraction is yi
- Need to satisfy equation 13
- Composition of dew is xi=yi/Ki
Dew point
- System is almost condensed
- The given mole fraction is xi
- Need to satisfy equation 14
- Composition of bubble is yi=Kixi

The End