Root-Knot and Reniform Nematode Infection of Cotton Hairy Roots Martin J. E. Wubben and Franklin E....
-
Upload
maritza-carpenter -
Category
Documents
-
view
212 -
download
0
Transcript of Root-Knot and Reniform Nematode Infection of Cotton Hairy Roots Martin J. E. Wubben and Franklin E....
- Slide 1
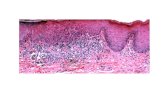
Root-Knot and Reniform Nematode Infection of Cotton Hairy Roots Martin J. E. Wubben and Franklin E. Callahan USDA-ARS Crop Science Research Laboratory Genetics and Precision Agriculture Research Unit Mississippi State, MS Slide 2 Plant-parasitic nematodes of cotton Root-Knot Nematode Meloidogyne incognita Reniform Nematode Rotylenchulus reniformis Sedentary root parasites Require living cells to siphon nutrients from the host root Inflict significant annual yield losses Incorporation of genetic resistance into elite cultivars is ongoing Molecular aspects of their interaction with cotton remain largely unstudied Slide 3 Advantages of an in vitro system Provide a controlled environment - limit abiotic stresses - eliminate secondary infections - requires a growth chamber and not a greenhouse (saves space) Feeding reniform nematodes can more easily be collected for functional genomic studies Candidate resistance gene screening Increase throughput for testing alternative biotech- driven control methods (example: RNAi) Slide 4 Cotton hairy roots Established for soybean, tomato, tobacco, coffee and others in studying nematode parasitism Arabidopsis lacks resistance to root-knot and reniform nematode Cheaper and easier to generate transgenic hairy roots versus whole plant transformation Hairy root initiation on cotton cotyledons Slide 5 Optimize hairy root growth conditions for propagation and nematode inoculation Optimize protocol for inoculum collection and surface-sterilization of infective nematode life- stages Verify nematode life-cycle completion on the susceptible hairy root culture DP90 Primary objectives Slide 6 Root-knot nematode inoculations NaOCl extraction Sugar floatation Hatch chamber Surface sterilization Suspend in 1.5 % low-melting-point agarose Apply nematode / LMP suspension directly to the root tips Slide 7 7 days after inoculation with infective RKN J2 10 days after inoculation with infective RKN J2 RKN infection of cotton hairy roots Galls Slide 8 RKN infection of cotton hairy roots J2 J3 17 days after inoculation roots stained with acid fuchsin Slide 9 RKN infection of cotton hairy roots 17 days after inoculation roots stained with acid fuchsin Slide 10 RKN fourth-stage juvenileRKN third-stage juvenile Slide 11 Gelatinous matrix exuded by RKN female (45 dai) RKN egg production on cotton hairy root culture (45 dai) Slide 12 Reniform nematode inoculations Surface sterilization Suspend in 1.5 % low-melting-point agarose Apply nematode / LMP suspension directly to the root tips Baerman funnel - Autoclaved sand - Growth chamber - Egg inoculum Slide 13 11 days after inoculation roots stained with acid fuchsin Reniform nematode infection of cotton hairy roots Slide 14 Reniform nematode on cotton hairy roots 24 days after inoculation roots stained with acid fuchsin Slide 15 live feeding nematodes 32 days after inoculation Slide 16 mature female complete egg mass 32 days after inoculation Slide 17 Research in progress Hairy root lines from root-knot and reniform nematode resistant plants are being propagated Determine best method for measuring nematode reproduction (galls, eggs, females, .) Histopathology of susceptible and resistant interactions Candidate gene over-expression in susceptible root culture Slide 18 Acknowledgements USDA-ARS, MS State Dr. Russel Hayes Dr. Johnie Jenkins Mr. Kimber Gorley USDA-ARS, Lubbock Dr. Forest Robinson USDA-ARS, Stoneville Dr. Erik Sacks USDA-ARS, New Orleans Dr. Barbara Triplett