glomerulonephritis
-
Upload
cheska-tumulak -
Category
Documents
-
view
602 -
download
2
Transcript of glomerulonephritis

1
MED II-B Group 6 Notes
Level II Block V Module 2 – URINARY SYSTEM
Case 1: “Puffy Raffy”
1. Recall the anatomic and histologic features of the kidneys.
Each human adult kidney weighs about 150 gm. As the ureter enters the kidney at the hilum, it dilates into a funnel-shaped cavity, the
pelvis, from which derive two or three main branches, the major calyces; each of these subdivides again into three or four minor calyces.
There are about 12 minor calyces in the human kidney. On the cut surface, the kidney is made up of a cortex and a medulla, the former 1.2
to 1.5 cm in thickness. The medulla consists of renal pyramids, the apices of which are called papillae, each
related to a calyx. Cortical tissue extends into spaces between adjacent pyramids as the renal columns
of Bertin. From the standpoint of its diseases, the kidney can be divided into four components:
blood vessels, glomeruli, tubules, and interstitium.
Blood Vessels
The kidney is richly supplied by blood vessels, and although both kidneys make up only 0.5% of the total body weight, they receive about 25% of the cardiac output. The cortex is by far the most richly vascularized part of the kidney, receiving 90% of the total renal blood supply.
The main renal artery divides into anterior and posterior sections at the hilum. From these, interlobar arteries emerge, course between lobes, and give rise to the
arcuate arteries, which arch between cortex and medulla, in turn giving rise to the interlobular arteries.
From the interlobular arteries, afferent arterioles enter the glomerular tuft, where they progressively subdivide into 20 to 40 capillary loops arranged in several units or lobules architecturally centered by a supporting mesangial stalk.
Capillary loops merge to exit from the glomerulus as efferent arterioles. In general, efferent arterioles from superficial nephrons form a rich vascular network
that encircles cortical tubules (peritubular vascular network), and deeper juxtamedullary glomeruli give rise to the vasa recta, which descend as straight vessels to supply the outer and inner medulla.
These descending arterial vasa recta then make several loops in the inner medulla and ascend as the venous vasa recta.
The anatomy of renal vessels has several important implications. First, because the arteries are largely end-arteries, occlusion of any branch usually
results in infarction of the specific area it supplies. Glomerular disease that interferes with blood flow through the glomerular capillaries
has profound effects on the tubules, within both the cortex and the medulla, because all tubular capillary beds are derived from the efferent arterioles.
The peculiarities of the blood supply to the renal medulla render them especially vulnerable to ischemia; the medulla does not have its own arterial blood supply but is dependent on the blood emanating from the glomerular efferent arterioles.
The blood in the capillary loops in the medulla has a remarkably low level of oxygenation.
Thus, minor interference with the blood supply of the medulla may result in medullary necrosis from ischemia.
Glomeruli
The glomerulus consists of an anastomosing network of capillaries lined by fenestrated endothelium invested by two layers of epithelium.
The visceral epithelium is incorporated into and becomes an intrinsic part of the capillary wall, separated from endothelial cells by a basement membrane.
The parietal epithelium, situated on Bowman's capsule, lines the urinary space, the cavity in which plasma filtrate first collects.
The glomerular capillary wall is the filtering membrane and consists of the following structures: A thin layer of fenestrated endothelial cells, each fenestrum being about 70 to
100 nm in diameter. A glomerular basement membrane (GBM) with a thick electron-dense central
layer, the lamina densa, and thinner electron-lucent peripheral layers, the lamina rara interna and lamina rara externa. The GBM consists of collagen (mostly type IV), laminin, polyanionic proteoglycans (mostly heparan sulfate), fibronectin, entactin, and several other glycoproteins. Type IV collagen forms a network suprastructure to which other glycoproteins attach. The building block (monomer) of this network is a triple-helical molecule made up of three α-chains, composed of one or more of six types of α-chains (α1 to α6 or COL4A1 to COL4A6), the most common consisting of α1, α2, α1. Each molecule consists of a 7S domain at the amino terminus, a triple-helical domain in the middle, and a globular noncollagenous domain (NC1) at the carboxyl terminus. The NC1 domain is important for helix formation and for assembly of collagen monomers into the basement membrane suprastructure. Glycoproteins (laminin, entactin) and acidic proteoglycans (heparan sulfate, perlecan) attach to the collagenous suprastructure3-5. These biochemical determinants are critical to understanding glomerular diseases. For example, as we shall see, the antigens in the NC1
domain are the targets of antibodies in anti-GBM nephritis; genetic defects in the α-chains underlie some forms of hereditary nephritis; and the acidic porous nature of the GBM determines its permeability characteristics. The visceral epithelial cells (podocytes), are structurally complex cells that
possess interdigitating processes embedded in and adherent to the lamina rara externa of the basement membrane. Adjacent foot processes (pedicels) are separated by 20- to 30-nm-wide filtration slits, which are bridged by a thin diaphragm.
The entire glomerular tuft is supported by mesangial cells lying between the capillaries. Basement membrane-like mesangial matrix forms a meshwork through which the mesangial cells are centered (Fig. 20-1). These cells, of mesenchymal origin, are contractile, phagocytic, and capable of proliferation, of laying down both matrix and collagen, and of secreting a number of biologically active mediators. Biologically, they are most akin to vascular smooth muscle cells and pericytes. They are, as we shall see, important players in many forms of human glomerulonephritis.
The major characteristics of normal glomerular filtration are an extraordinarily high permeability to water and small solutes, because of the highly fenestrated nature of the endothelium, and impermeability to proteins, such as molecules of the size of albumin (+3.6-nm radius; 70 kilodaltons [kDa] molecular weight) or larger.
The latter property, called glomerular barrier function, discriminates among various protein molecules, depending on their size (the larger, the less permeable) and charge (the more cationic, the more permeable).
This size- and charge-dependent barrier function is accounted for by the complex structure of the capillary wall, the collagenous porous and charged structure of the GBM, and the many anionic moieties present within the wall, including the acidic proteoglycans of the GBM and the sialoglycoproteins of epithelial and endothelial cell coats.
The charge-dependent restriction is important in the virtually complete exclusion of albumin from the filtrate, because albumin is an anionic molecule of a pI 4.5. The visceral epithelial cell, also known as a podocyte, is important for the maintenance of glomerular barrier function; its slit diaphragm presents a size-selective distal diffusion barrier to the filtration of proteins, and it is the cell type that is largely responsible for synthesis of GBM components.
Proteins located in the slit diaphragm control glomular permeability. While the details are imcomplete, three important proteins have been identified (Fig.
20-5). Nephrin is a transmembrane protein with a large extracellular portion made up of immunoglobulin (Ig)-like domains. Nephrin molecules extend towards each other from neighboring foot processes and dimerize across the slit diaphragm.
Within the cytoplasm of the foot processes, nephrin forms molecular connections with podocin, CD2-associated protein, and ultimately the actin cytoskeleton. The importance of these proteins in maintaining glomerular permeability is demonstrated by the observation that mutations in the genes encoding them give rise to nephrotic syndrome (discussed later).
This has resulted in renewed appreciation of the importance of the slit diaphragm in glomerular barrier function and its contribution to protein leakage in disease states.
Tubules
The structure of renal tubular epithelial cells varies considerably at different levels of the nephron and, to a certain extent, correlates with function.
For example, the highly developed structure of the proximal tubular cells, with their abundant long microvilli, numerous mitochondria, apical canaliculi, and extensive intercellular interdigitations, is correlated with their major functions: reabsorption of two-thirds of filtered sodium and water as well as glucose, potassium, phosphate, amino acids, and proteins.
The proximal tubule is particularly vulnerable to ischemic damage. Furthermore, toxins are frequently reabsorbed by the proximal tubule, rendering it
also susceptible to chemical injury. The juxtaglomerular apparatus snuggles closely against the glomerulus where the
afferent arteriole enters it. The juxtaglomerular apparatus consists of:
1. The juxtaglomerular cells, modified granulated smooth muscle cells in the media of the afferent arteriole that contain renin.
2. The macula densa, a specialized region of the distal tubule as it returns to the vascular pole of its parent glomerulus, where the tubular cells are more crowded and the cells are somewhat shorter and possess distinct patterns of interdigitation between adjacent membranes.
3. The lacis cells or nongranular cells, which reside in the area bounded by the afferent arteriole, the macula densa, and the glomerulus.
They resemble mesangial cells and appear to be continuous with them. The juxtaglomerular apparatus is a small endocrine organ, the juxtaglomerular cells
being the principal sources of renin production in the kidney.
Interstitium
In the normal cortex, the interstitial space is compact, being occupied by the fenestrated peritubular capillaries and a small number of fibroblast-like cells. Any obvious expansion of the cortical interstitium is usually abnormal; this expansion can be due to edema or infiltration by acute inflammatory cells, as in acute interstitial diseases, or it may be caused by accumulation of chronic inflammatory cells and fibrous tissue, as in chronic interstitial diseases.
The amounts of proteoglycans in the interstitial tissue of the medulla increase with age and in the presence of ischemia.
(Robbins and Cotran Pathologic Basis of Therapeutics 7th Edition)2. Identify Acute Glomerulonephritis as to:
2.1 Pathophysiology
Although we know little of etiologic agents and triggering events, it is clear that immune mechanisms underlie most forms of primary and many of the secondary glomerular disorders.
Glomerulonephritis can be readily induced experimentally by antigen-antibody reactions.
Furthermore, glomerular deposits of immunoglobulins, often with various components of complement, are found in the majority of patients with glomerulonephritis.
Cell-mediated immune reactions also clearly play a role, usually in concert with antibody-mediated events.
We therefore begin this discussion with a review of antibody-instigated injury.

2
Two forms of antibody-associated injury have been established: (1) injury by antibodies reacting in situ within the glomerulus, either with insoluble fixed (intrinsic) glomerular antigens or with molecules planted within the glomerulus, and (2) injury resulting from deposition of circulating antigen- antibody complexes in the glomerulus. In addition, there is experimental evidence that cytotoxic antibodies directed against glomerular cell components may cause glomerular injury.
These pathways are not mutually exclusive, and in humans, all may contribute to injury.
In this form of injury, antibodies react directly with intrinsic tissue antigen, or antigens "planted" in the glomerulus from the circulation.
There are two well-established experimental models for anti-tissue antibody-mediated glomerular injury, for which there are counterparts in human disease: antiglomerular basement membrane (anti-GBM) antibody- induced nephritis and Heymann nephritis.
(Robbins and Cotran Pathologic Basis of Therapeutics 7th Edition)
Glomerular lesions in acute glomerulonephritis are the result of glomerular deposition or in situ formation of immune complexes.
On gross appearance, the kidneys may be enlarged up to 50%. Histopathologic changes include swelling of the glomerular tufts and
infiltration with polymorphonucleocyte. Immunofluorescence reveals deposition of immunoglobulins and complement. With the exception of poststreptococcal glomerulonephritis, the exact triggers
for the formation of the immune complexes are unclear. In streptococcal infection, involvement of derivatives of streptococcal proteins
has been reported. A streptococcal neuramidase may alter host immunoglobulin G (IgG). IgG combines with host antibodies. IgG/anti-IgG immune complexes are formed and then collect in the glomeruli. In addition, elevations of antibody titers to other antigens, such as
antistreptolysin O or antihyaluronidase, DNAase-B, and streptokinase, provide evidence of a recent streptococcal infection.
(http://www.emedicine.com/emerg/topic219.htm)
2.2 Incidence
Frequency
United States
Glomerulonephritis represents 10-15% of glomerular diseases. Variable incidence has been reported due in part to the subclinical nature of
the disease in more than one half the affected population. Despite sporadic outbreaks, incidence of poststreptococcal
glomerulonephritis has fallen over the last few decades. Factors responsible for this decline may include better health care delivery
and improved socioeconomic conditions.
International
With some exceptions, a reduction in the incidence of poststreptococcal glomerulonephritis has occurred in most western countries.
It remains much more common in regions such as Africa, the Caribbean, India, Pakistan, Malaysia, Papua New Guinea, and South America.
Immunoglobulin A (IgA) nephropathy glomerulonephritis (ie, Berger disease) is the most common cause of glomerulonephritis worldwide.
(http://www.emedicine.com/emerg/topic219.htm)
Incidence of AGN is decreasing in western countries, and it is typically sporadic.
Epidemic cases are still seen, though less commonly. Typically affects children between the ages of 2 and 14 years old, but 10% of
the patients are older than 40 y.o. More common in males and the familial or cohabitant incidence is high as
40%.
(Harrison’s Principles of Medicine 16th Edition)2.3 Clinical Manifestation
Classic presentation is acute nephritic picture with hematuria, pyuria,, RBC casts, edema , HPN, and oliguric renal failure which may be severe enough to appear as RPGN
Systemic SX include headache, malaise, anorexia, and flank pain are reported in as many as 50% of cases
5% of children and 20% of adults have proteinuria in the nephritic change In the 1st week of Sx, 90% of patients will have a depressed CH50 and
decreased levels of C3 with normal levels of C4 A subclinical disease is reported in some series to be four or five times as
common as clinical nephritis, and these latter cases are characterized by asymptomatic microscopic hematuria with low level serum complement levels.
(Harrison’s Principles of Medicine 16th Edition)
A thorough history should focus on the identification of an underlying systemic disease (if any) or recent infection.
Most often, the patient is a boy, aged 2-14 years, who suddenly develops puffiness of the eyelids and facial edema in the setting of a poststreptococcal
infection. The urine is dark and scanty, and the blood pressure may be elevated.
Onset of symptoms is usually abrupt. Nonspecific symptoms include weakness, fever, abdominal pain, and
malaise. In the setting of a postinfectious acute nephritis, a latent period of up to 3
weeks occurs before onset of symptoms. However, the latent period may vary; typically 1-2 weeks for postpharyngitis cases and 2-4 weeks for cases of postdermal infection (ie, pyoderma).
Onset of nephritis within 1-4 days of streptococcal infection suggests preexisting renal disease.
Symptoms of acute glomerulonephritis include the following:o Hematuria is a universal finding, even if it is microscopic. Gross
hematuria is reported in 30% of pediatric patients.o Oliguriao Edema (peripheral or periorbital) is reported in approximately 85% of
pediatric patients; edema may be mild (involving only the face) to severe, bordering on a nephrotic appearance.
o Headache may occur secondary to hypertension; confusion secondary to malignant hypertension may be seen in as many as 5% of patients.
o Shortness of breath or dyspnea on exertion secondary to heart failure or pulmonary edema; usually uncommon, particularly in children.
o Possible flank pain secondary to stretching of the renal capsule. Patients may also present with symptoms specific to an underlying systemic
disease that can precipitate an acute glomerulonephritis. These disease entities are briefly described in Causes. Classic presentations include the following:
o Triad of sinusitis, pulmonary infiltrates, and nephritis suggesting Wegener granulomatosis
o Nausea/vomiting, abdominal pain, and purpura observed with Henoch-Schönlein purpura
o Arthralgias associated with systemic lupus erythematosus (SLE)o Hemoptysis occurring with Goodpasture syndrome or idiopathic
progressive glomerulonephritiso Skin rashes observed with a hypersensitivity vasculitis or systemic
lupus erythematosus; also possibly due to the purpura that can occur in hypersensitivity vasculitis, cryoglobulinemia, and Henoch-Schönlein purpura.
Physical Examination
This description does not include all the physical findings that can be associated with the nonnephritic features of an infectious process (eg, fever), renal etiology, or systemic etiology, as such a description is beyond the scope of this article.
Patients often have a normal physical examination and blood pressure; most frequently, however, patients present with a combination of edema, hypertension, and oliguria.o Edema frequently involves the face, specifically the periorbital area.o Hypertension is seen in as many as 80% of affected patients.o Hematuria, either macroscopic (gross) or microscopic, may be noted.o Skin rashes (ie, malar rash frequently seen with lupus nephritis) may
be observed.o Abnormal neurologic examination or altered level of consciousness
occurring because of malignant hypertension or hypertensive encephalopathy.
o Arthritis may be noted.o Other signs:
Pharyngitis Impetigo Respiratory infection Pulmonary hemorrhage Heart murmur may indicate endocarditis Scarlet fever Weight gain Abdominal pain Anorexia Back pain Skin pallor Palpable purpura in patients with Henoch-Schönlein purpura Oral ulcers
(http://www.emedicine.com/emerg/topic219.htm)
2.4 Differentiate nephrotic syndrome from nephritic syndrome.
The nephrotic syndrome is characterized by heavy proteinuria (more than 3.5 gm/day), hypoalbuminemia, severe edema, hyperlipidemia, and lipiduria (lipid in the urine) while nephritic syndrome (acute) is a glomerular syndrome dominated by the acute onset of usually grossly visible hematuria (red blood cells in urine), mild to moderate proteinuria, and hypertension; it is the classic presentation of acute poststreptococcal glomerulonephritis.
(Robbins and Cotran Pathologic Basis of Therapeutics 7th Edition)
NEPHROTIC SYNDROME
Nephrotic syndrome is urinary excretion of > 3 g of protein/day due to glomerular disease.
It is more common in children and has both primary and secondary causes.

3
Diagnosis is by measurement of a spot urine protein/creatinine ratio or a 24-h urinary protein; underlying causes are diagnosed based on history, physical examination, and renal biopsy.
Treatment and prognosis vary by cause. Nephrotic syndrome is urinary excretion of > 3 g of protein/day due to
glomerular disease. It is more common in children and has both primary and secondary causes. Diagnosis is by measurement of a spot urine protein/creatinine ratio or a 24-h
urinary protein; underlying causes are diagnosed based on history, physical examination, and renal biopsy.
Treatment and prognosis vary by cause.
NEPHRITIC SYNDROME
Nephritic syndrome is defined by hematuria and RBC casts on microscopic examination of urinary sediment.
Often one or more elements of mild to moderate proteinuria, edema, hypertension, elevated serum creatinine, and oliguria are also present.
It has both primary and secondary causes. Diagnosis is based on history, physical examination, and sometimes renal
biopsy. Treatment and prognosis vary by cause. Nephritic syndrome is a manifestation of glomerular inflammation
(glomerulonephritis [GN]) and occurs at any age. Causes differ by age and mechanisms differ by cause. Acute and chronic
forms exist. Postinfectious GN is the prototype of acute GN, but the condition may be
caused by other glomerulopathies and by systemic diseases such as connective tissue disorders and paraproteinemias.
Glomerular Diseases: Causes of Glomerulonephritis Chronic GN has features similar to acute GN but develops slowly and may
display mild to moderate proteinuria. Examples include IgA nephropathy and hereditary nephritis.(www.merk.com)
3. Identify the morphology and pathogenesis of the different causes of acute glomerulonephritis.3.1 Primary Causes
3.1.1 Acute Diffuse Proliferative Glomerulonephritis3.1.1.1 Post-streptococcal3.1.1.2 Viral 3.1.1.3 Fungal and Parasitic
(3.1.1-3.1.1.3 Fused)
ACUTE GLOMERULONEPHRITIS
Mechanisms of Chronic Tubulointerstitial Injury in Glomerulonephritis
o Various components of the protein-rich filtrate and cytokines derived from leukocytes cause tubular cell activation and secretion of cytokines, growth factors, and other mediators.
o These, together with products of macrophages, incite interstitial inflammation and fibrosis.
o ET-1, endothelin-1, PAI-1, plasminogen activator inhibitor-1; TIMP-1, tissue inhibitor of metalloproteinases
o This group of glomerular diseases is characterized anatomically by inflammatory alterations in the glomeruli and clinically by the syndrome of acute nephritis.
o The nephritic patient usually presents with hematuria, red cell casts in the urine, azotemia, oliguria, and mild to moderate hypertension.
o The patient also commonly has proteinuria and edema, but these are not as severe as those encountered in the nephrotic syndrome, discussed later.
o The acute nephritic syndrome may occur in such multisystem diseases as SLE and microscopic polyarteritis.
o Typically, however, it is characteristic of acute proliferative glomerulonephritis and is an important component of crescentic glomerulonephritis, which is described later.
Acute Proliferative (Poststreptococcal, Postinfectious) Glomerulonephritis
o As the name implies, this cluster of diseases is characterized histologically by diffuse proliferation of glomerular cells, associated with influx of leukocytes.
o These lesions are typically caused by immune complexes. o The inciting antigen may be exogenous or endogenous. o The prototypic exogenous antigen-induced disease pattern is
postinfectious glomerulonephritis, whereas that produced by an endogenous antigen is the nephritis of systemic lupus erythematosus.
o The most common infections are streptococcal, but the disorder has also been associated with other infections.
Poststreptococcal Glomerulonephritis
o This glomerular disease is decreasing in frequency in the United States but continues to be a fairly common disorder worldwide.47 It usually appears 1 to 4 weeks after a streptococcal infection of the pharynx or skin (impetigo).
o Skin infections are commonly associated with overcrowding and poor hygiene.
o Poststreptococcal glomerulonephritis occurs most frequently in children 6 to 10 years of age, but adults of any age can be affected.
Etiology and Pathogenesis
o Only certain strains of group A β-hemolytic streptococci are nephritogenic, more than 90% of cases being traced to types 12, 4, and 1, which can be identified by typing of M protein of the cell wall.
o Poststreptococcal glomerulonephritis is an immunologically mediated disease.
o The latent period between infection and onset of nephritis is compatible with the time required for the production of antibodies and the formation of immune complexes.
o Elevated titers of antibodies against one or more streptococcal antigens are present in a great majority of patients.
o Serum complement levels are low, compatible with activation of the complement system and consumption of complement components.
o The presence of granular immune deposits in the glomeruli demonstrates an immune complex-mediated mechanism, and so does the finding of electron-dense deposits.
o The streptococcal antigenic component responsible for the immune reaction has eluded identification for years.
o A cytoplasmic antigen called endostreptosin and several cationic antigens, including a proteinase (nephritis strain-associated protein, NSAP) related to streptokinase and unique to nephritogenic strains of streptococci, can be present in affected glomeruli.
o It is not known if these represent planted antigens, part of circulating immune complexes, or both. GBM proteins altered by streptococcal enzymes have also been implicated as antigens at one time or another.
Morphology
o The classic diagnostic picture is one of enlarged, hypercellular glomeruli.
o The hypercellularity is caused by (1) infiltration by leukocytes, both neutrophils and monocytes; (2) proliferation of endothelial and mesangial cells; and (3) in severe cases by crescent formation.
o The proliferation and leukocyte infiltration are diffuse, that is, involving all lobules of all glomeruli.
o There is also swelling of endothelial cells, and the combination of proliferation, swelling, and leukocyte infiltration obliterates the capillary lumens.
o There may be interstitial edema and inflammation, and the tubules often contain red cell casts.
o By immunofluorescence microscopy, there are granular deposits of IgG, IgM, and C3 in the mesangium and along the basement membrane.
o Although almost universally present, they are often focal and sparse. o The characteristic electron microscopic findings are discrete,
amorphous, electron-dense deposits on the epithelial side of the membrane, often having the appearance of "humps", presumably representing the antigen- antibody complexes at the epithelial cell surface.
o Subendothelial and intramembranous deposits are also commonly seen, and mesangial deposits may be present.
o There is often swelling of endothelial and mesangial cells.
Clinical Course
o In the classic case, a young child abruptly develops malaise, fever, nausea, oliguria, and hematuria (smoky or cocoa-colored urine) 1 to 2 weeks after recovery from a sore throat.
o The patients exhibit red cell casts in the urine, mild proteinuria (usually less than 1 gm/day), periorbital edema, and mild to moderate hypertension.
o In adults, the onset is more likely to be atypical, with the sudden appearance of hypertension or edema, frequently with elevation of BUN.
o During epidemics caused by nephritogenic streptococcal infections, glomerulonephritis may be asymptomatic, discovered only on screening for microscopic hematuria. Important laboratory findings include elevations of antistreptococcal antibody (ASO) titers and a decline in the serum concentration of C3 and other components of the complement cascade and the presence of cryoglobulins in the serum.
o More than 95% of affected children eventually recover totally with conservative therapy aimed at maintaining sodium and water balance.
o A small minority of children (perhaps less than 1%) do not improve, become severely oliguric, and develop a rapidly progressive form of glomerulonephritis (described later). Some of the remaining patients may undergo slow progression to chronic glomerulonephritis with or without recurrence of an active nephritic picture.
o Prolonged and persistent heavy proteinuria and abnormal GFR mark patients with an unfavorable prognosis.
o In adults, the disease is less benign. o Although the overall prognosis in epidemics is good, in only about 60%
of sporadic cases do the patients recover promptly. In the remainder, the glomerular lesions fail to resolve quickly, as manifested by persistent proteinuria, hematuria, and hypertension.

4
o In some of these patients, the lesions eventually clear totally, but others develop chronic glomerulonephritis.
o Some patients will develop a syndrome of rapidly progressive glomerulonephritis.
Nonstreptococcal Acute Glomerulonephritis (Postinfectious Glomerulonephritis)
o A similar form of glomerulonephritis occurs sporadically in association with other bacterial infections (e.g., staphylococcal endocarditis, pneumococcal pneumonia, and meningococcemia), viral disease (e.g., hepatitis B, hepatitis C, mumps, human immunodeficiency virus [HIV] infection, varicella, and infectious mononucleosis), and parasitic infections (malaria, toxoplasmosis).
o In this setting, granular immunofluorescent deposits and subepithelial humps characteristic of immune complex nephritis are present.
(Robbins and Cotran Pathologic Basis of Therapeutics 7th Edition)
Morphology:
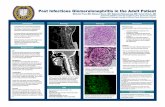
o Typical findings on immunofluorescence microscopy of renal biopsy specimens from patients with anti-glomerular basement membrane antibody disease, immune complex–mediated glomerulonephritis, and pauci-immune glomerulonephritis.
o Specimens in the upper and middle panels were stained for immunoglobulin and show the classic linear “ribbon-like” pattern of anti-GBM disease (A) and granular pattern of immune complex–mediated glomerulonephritis (B).
o Immunoglobulin is sparse or absent in patients with pauci-immune glomerulonephritis (not shown); however, abundant fibrin is detected in crescents (C). (Micrographs courtesy of Dr. Helmut Rennke.)
CLINICAL PRESENTATIONS
ACUTE NEPHRITIC SYNDROME AND RAPIDLY PROGRESSIVE GLOMERULONEPHRITIS CLINICAL FEATURES AND CLINICOPATHOLOGIC CORRELATES
o The acute nephritic syndrome is the clinical correlate of acute glomerular inflammation.
o In its most dramatic form, the acute nephritic syndrome is characterized by sudden onset (i.e., over days to weeks) of acute renal failure and oliguria (400 mL/day of urine).
o Renal blood flow and GFR fall as a result of obstruction of the glomerular capillary lumen by infiltrating inflammatory cells and proliferating resident glomerular cells.
o Renal blood flow and GFR are further compromised by intrarenal vasoconstriction and mesangial cell contraction that result from local imbalances of vasoconstrictor (e.g., leukotrienes, platelet-activating factor, thromboxanes, endothelins) and vasodilator substances (e.g., nitric oxide, prostacyclin) within the renal microcirculation.
o Extracellular fluid volume expansion, edema, and hypertension develop because of impaired GFR and enhanced tubular reabsorption of salt and water. As a result of injury to the glomerular capillary wall, urinalysis typically reveals red blood cell casts, dysmorphic red blood cells, leukocytes, and subnephrotic proteinuria of <3.0 g per 24 h (“nephritic urinary sediment”). Hematuria is often macroscopic.
o The classic pathologic correlate of the nephritic syndrome is proliferative glomerulonephritis.
o The proliferation of glomerular cells is due initially to infiltration of the glomerular tuft by neutrophils and monocytes and subsequently to true proliferation of resident glomerular endothelial and mesangial cells (endocapillary proliferation).
o In its most severe form, the nephritic syndrome is associated with acute inflammation of most glomeruli, i.e., acute diffuse proliferative glomerulonephritis.
o When less vigorous, <50% of glomeruli may be involved, i.e., focal proliferative glomerulonephritis.
o In milder forms of nephritic injury, cellular proliferation may be confined to the mesangium, i.e., mesangioproliferative glomerulonephritis.
o RPGN is the clinical correlate of more subacute glomerular inflammation.
o Patients develop renal failure over weeks to months in association with a nephritic urinary sediment, subnephrotic proteinuria and variable oliguria, hypervolemia, edema, and hypertension.
o The classic pathologic correlate of RPGN is crescent formation involving most glomeruli (crescentic glomerulonephritis).
o In practice, the clinical term rapidly progressive glomerulonephritis and the pathologic term crescentic glomerulonephritis are often used interchangeably.
o In addition to classic crescentic glomerulonephritis, in which crescents dominate the glomerular pathology, crescents can also develop concomitantly with proliferative glomerulonephritis or as a complication of membranous glomerulopathy and other more indolent forms of glomerular inflammation.
o The acute nephritic syndrome and RPGN are part of a spectrum of presentations of immunologically mediated proliferative glomerulonephritis.
o Studies of experimental models suggest that nephritic syndrome and diffuse proliferative glomerulonephritis represent an acute immune response to a sudden large antigen load, whereas RPGN and crescentic glomerulonephritis represent a more subacute immune response to a smaller antigen load in presensitized individuals.
o At the other end of the spectrum, chronic low-grade immune injury presents with slowly progressive renal insufficiency or asymptomatic hematuria in association with focal proliferative or mesangioproliferative glomerulonephritis.
o These more indolent forms of immune-mediated glomerulonephritis are discussed later in this chapter.
ETIOLOGY AND DIFFERENTIAL DIAGNOSIS
o Acute nephritic syndrome and RPGN can result from renal-limited primary glomerulopathy or from secondary glomerulopathy complicating systemic disease.
o In general, rapid diagnosis and prompt treatment are critical to avoid the development of irreversible renal failure.
o Renal biopsy remains the “gold standard” for diagnosis. Immunofluorescence microscopy is particularly helpful and identifies three major patterns of deposition of immunoglobulin that define three broad diagnostic categories: 1. Scattered granular deposits of immunoglobulin, a hallmark of
immunecomplex glomerulonephritis.2. More discrete linear deposition of immunoglobulin along the
GBM, characteristic of anti-GBM disease.3. Paucity or absence of immunoglobulin—pauci-immune
glomerulonephritiso Most patients (>70%) with full-blown acute nephritic syndrome have
immune-complex glomerulonephritis. o Pauci-immune glomerulonephritis is less common in this setting
(<30%), and anti-GBM disease is rare (<1%). o Among patients with RPGN, immune-complex glomerulonephritis and
pauci-immune glomerulonephritis are equally prevalent (~45% each), whereas anti- GBM disease again accounts for a minority of cases (<10%).
o Three serologic markers often predict the immunofluorescence microscopy findings in nephritic syndrome and RPGN and may obviate the need for renal biopsy in classic cases.
o They are the serum C3 level and titers of anti-GBM antibody and ANCA.
o As discussed in previous sections, the kidney is host to immune attack in immune-complex glomerulonephritis, most cases being initiated either by in situ formation of immune complexes or less commonly by glomerular trapping of circulating immune complexes.
o These patients typically have hypocomplementemia (low C3 in 90%) and negative anti-GBM and ANCA serology, the major exception being IgA nephropathy/Henoch Scho¨nlein purpura where complement levels are typically normal.
o The glomerulus is the direct target of immune attack in anti-GBM disease, glomerular inflammation being initiated by an autoantibody directed at a 28-kDa autoantigen on the alpha3 chain of type IV collagen.
o Approximately 90 to 95% of patients with anti- GBM disease have circulating anti-GBM autoantibodies detectable by immunoassay; serum complement levels are typically normal, and ANCA are usually not detected.
o The pathogenesis of pauci-immune glomerulonephritis is still being defined; however, most patients have circulating ANCA. Serum complement levels are typically normal, and anti-GBM titers are usually negative in ANCA-associated renal disease.
o It should be noted, however, that there may be some serologic overlap, with as many as 20% of patients with immune complex or anti-GBM glomerulonephritis also having at least low levels of circulating ANCA.
NEPHRITIC SYNDROME AND RPGN DUE TO IMMUNE-COMPLEX GLOMERULONEPHRITIS
o Nephritic syndrome induced by immune- complex glomerulonephritis may be (1) be idiopathic, (2) represent a response to a known antigenic stimulus (e.g., glomerulonephritis triggered by bacterial endocarditis or streptococcal infection, or hepatitis B or C infection in cryoglobulinemic glomerulonephritis), or (3) form part of a multisystem immune-complex disorder (e.g., lupus nephritis, Henoch-Scho¨nlein purpura)
INFECTION-ASSOCIATED GLOMERULONEPHRITIS INCLUDING GLOMERULONEPHRITIS ASSOCIATED WITH STREPTOCOCCAL INFECTION AND INFECTIVE ENDOCARDITIS
o A variety of infections can precipitate immune-complex glomerulonephritis.
o The most common clinicopathologic lesion in this setting is acute diffuse proliferative glomerulonephritis presenting as the acute nephritic syndrome; however, depending on the speed of onset and site and extent of immune complex formation, infection-associated immune complex formation can trigger mesangioproliferative, focal proliferative, membranoproliferative, or membranous glomerulopathy.

5
o Poststreptococcal glomerulonephritis is the prototypical postinfectious glomerulonephritis and a leading cause of acute nephritic syndrome.
o Most cases are sporadic, though the disease can occur as an epidemic.
o Glomerulonephritis develops, on average, 10 days after pharyngitis or 2 weeks after a skin infection (impetigo) with a nephritogenic strain of group A beta-hemolytic streptococcus.
o The known nephritic strains include M types 1, 2, 4, 12, 18, 25, 49, 55, 57, and 60.
o Immunity to these strains is type-specific and long-lasting, and repeated infection and nephritis are rare.
o Epidemic poststreptococcal glomerulonephritis is most commonly encountered in children of 2 to 6 years of age with pharyngitis during the winter months.
o This entity appears to be decreasing in frequency, possibly due to more widespread and prompt use of antibiotics.
o Poststreptococcal glomerulonephritis in association with cutaneous infections usually occurs in a setting of poor personal hygiene or streptococcal superinfection of another skin disease.
o The classic clinical presentation of poststreptococcal glomerulonephritis is full-blown nephritic syndrome with oliguric acute renal failure; however, most patients have milder disease. Indeed, subclinical cases outnumber overt cases by four- to tenfold during epidemics.
o Patients with overt disease present with gross hematuria (red or “smoky” urine), headache, and generalized symptoms such as anorexia, nausea, vomiting, and malaise. Swelling of the renal capsule can cause flank or back pain.
o Physical examination reveals hypervolemia, edema, and hypertension. o The urinary sediment is nephritic, with dysmorphic red blood cells, red
cell casts, leukocytes, occasionally leukocyte casts, and subnephrotic proteinuria.
o Fewer than 5% of patients develop nephrotic-range proteinuria. The latter may only manifest as acute nephritis resolves and renal blood flow and GFR recover.
o Coexistent rheumatic fever is extremely rare.o The serum creatinine is often mildly elevated at presentation. o Serum C3 levels and CH50 are depressed within 2 weeks in ~90% of
cases. o C4 levels are characteristically normal, indicating activation of the
alternate pathway of complement. o Complement levels usually return to normal within 6 to 8 weeks.
Persistently depressed levels after this period should suggest another cause, such as the presence of a C3 nephritic factor (see “Membranoproliferative Glomerulonephritis,” p. 1687).
o The majority of patients (>75%) have transient hypergammaglobulinemia and mixed cryoglobulinemia.
o The antecedent streptococcal infection may still be evident or may have resolved either spontaneously or in response to antibiotic therapy.
o Most patients (>90%) have circulating antibodies against streptococcal exoenzymes such as antistreptolysin O (ASO), anti-deoxyribonuclease B (anti-DNAse B), antistreptokinase (ASKase), anti-nicotinyl adenine dinucleotidase (anti-NADase), and antihyaluronidase (AHase).
o Acute poststreptococcal glomerulonephritis is usually diagnosed on clinical and serologic grounds, without resort to renal biopsy, especially in children with a typical antecedent history. The characteristic lesion on light microscopy is diffuse proliferative glomerulonephritis.
o Crescents are uncommon, and extraglomerular involvement is usually mild. Immunofluorescence microscopy reveals diffuse granular deposition of IgG and C3, giving rise to a “starry sky”.
o The characteristic finding on electron microscopy is the presence of large electron-dense immune deposits in the subendothelial, subepithelial, and mesangial areas.
o In addition to poststreptococcal glomerulonephritis, the nephritic syndrome and RPGN can complicate acute immune-complex glomerulonephritis due to other viral, bacterial, fungal, and parasitic infections.
o Diffuse proliferative immune-complex glomerulonephritis is a well-described complication of acute and subacute infective endocarditis and is usually associated with hypocomplementemia.
o The glomerular lesion typically resolves following eradication of the cardiac infection.
(Harrison’s Principles of Internal Medicine 16th Edition)
3.1.2 Rapidly Progressive (Cresentric Glomerulonephritis)
RAPIDLY PROGRESSIVE (CRESCENTIC) GLOMERULONEPHRITIS
o Rapidly progressive glomerulonephritis (RPGN) is a syndrome associated with severe glomerular injury and does not denote a specific etiologic form of glomerulonephritis.
o It is characterized clinically by rapid and progressive loss of renal function associated with severe oliguria and (if untreated) death from renal failure within weeks to months.
o Regardless of the cause, the classic histologic picture is characterized by the presence of crescents in most of the glomeruli (crescentic glomerulonephritis).
o As discussed earlier, these are produced in part by proliferation of the parietal epithelial cells lining Bowman capsule and in part by infiltration of monocytes and macrophages.
Classification and Pathogenesis
o RPGN may be caused by a number of different diseases, some restricted to the kidney and others systemic.
o Although no single mechanism can explain all cases, there is little doubt that in most cases, the glomerular injury is immunologically mediated.
o Thus, a practical classification divides RPGN into three groups on the basis of immunologic findings (Table 20-7). In each group, the disease may be associated with a known disorder, or it may be idiopathic.
o The first type of RPGN is best remembered as anti-GBM antibody-induced disease and hence is characterized by linear deposits of IgG and, in many cases, C3 in the GBM, as previously described.48 In some of these patients, the anti-GBM antibodies cross-react with pulmonary alveolar basement membranes to produce the clinical picture of pulmonary hemorrhage associated with renal failure (Goodpasture syndrome).
o Plasmapheresis to remove the pathogenic circulating antibodies is usually part of the treatment, which also includes therapy to suppress the underlying immune response.
o The Goodpasture antigen, as was noted earlier, is a peptide within the noncollagenous portion of the α3-chain of collagen type IV.
o What triggers the formation of these antibodies is unclear in most patients. Exposure to viruses or hydrocarbon solvents (found in paints and dyes) has been implicated in some patients, as have various drugs and cancers.
o There is a high prevalence of certain HLA subtypes and haplotypes (e.g., HLA-DRB1) in affected patients, a finding consistent with the genetic predisposition to autoimmunity.
o The second type of RPGN is the result of immune complex-mediated disease.
o It can be a complication of any of the immune complex nephritides, including postinfectious glomerulonephritis, SLE, IgA nephropathy, and Henoch-Schönlein purpura.
o In all of these cases, immunofluorescence studies reveal the granular pattern of staining characteristic of immune complex deposition. These patients cannot usually be helped by plasmapheresis, and they require treatment for the underlying disease.
o The third type of RPGN, also called pauci-immune type, is defined by the lack of anti-GBM antibodies or immune complexes by immunofluorescence and electron microscopy.
o Most patients with this type of RPGN have antineutrophil cytoplasmic antibodies (ANCA), of cytoplasmic (C) or perinuclear (P) patterns, in the serum, which, as we have seen (Chapter 11), play a role in some vasculitides.
o Hence, in some cases, this type of RPGN is a component of a systemic vasculitis such as Wegener granulomatosis or microscopic polyarteritis.
o In many cases, however, pauci-immune crescentic glomerulonephritis is isolated and hence idiopathic.
o More than 90% of such idiopathic cases have c-ANCA or p-ANCA in the sera.
o The presence of circulating ANCAs in both idiopathic RPGN and cases of RPGN that occur as a component of systemic vasculitis, and the similar pathologic features in either setting, have led to the idea that these disorders are pathogenetically related.
o According to this concept, all cases of RPGN of the pauci-immune type are manifestations of small vessel vasculitis or polyangiitis, which is limited to glomerular and perhaps peritubular capillaries in cases of idiopathic crescentic glomerulonephritis.
o The clinical distinction between systemic vasculitis with pauci-immune renal involvement and idiopathic crescentic glomerulonephritis accordingly has become deemphasized, as these entities are viewed as part of a spectrum of vasculitic disease.
o ANCAs have proved to be invaluable as a highly sensitive diagnostic marker for pauci-immune RPGN, but proof of their role as a direct cause of RPGN has been elusive.
o Recent strong evidence of their pathogenic potential has been obtained by studies in which antibodies against myeloperoxidase (the target antigen of most p-ANCAs) are transferred into mice.
o To summarize, all three types of RPGN may be associated with a well-defined renal or extrarenal disease, but in many cases (approximately 50%), the disorder is idiopathic. Of the patients with this syndrome, about one fifth have anti-GBM antibody-induced disease without lung involvement; another one fourth have immune complex-mediated disease RPGN; and the remainder are of the pauci-immune type.
o The common denominator in all types of RPGN is severe glomerular injury.
Morphology
o The kidneys are enlarged and pale, often with petechial hemorrhages on the cortical surfaces.
o Depending on the underlying cause, the glomeruli may show focal necrosis, diffuse or focal endothelial proliferation, and mesangial proliferation.
o The histologic picture, however, is dominated by the formation of distinctive crescents.

6
o Crescents are formed by proliferation of parietal cells and by migration of monocytes and macrophages into the urinary space.
o Neutrophils and lymphocytes may be present. o The crescents eventually obliterate Bowman space and compress the
glomerular tuft. o Fibrin strands are prominent between the cellular layers in the
crescents; indeed, as discussed earlier, the escape of fibrin into Bowman space is an important contributor to crescent formation.
o Electron microscopy may, as expected, disclose subepithelial deposits in some cases, but in many cases, it shows distinct ruptures in the GBM, the severe injury that allows leukocytes, proteins, and inflammatory mediators into the urinary space, where they trigger the crescent formation.
o In time, most crescents undergo sclerosis, but restoration of normal glomerular architecture marks a successful clinical outcome in some patients, particularly those with an infection-associated immune complex etiology.
o By immunofluorescence microscopy, postinfec-tious cases exhibit granular immune deposits; Goodpasture syndrome cases show linear fluorescence for immunoglobulin and complement, and pauci-immune cases have little or no deposition of immune reactants.
Clinical Course
o The renal manifestations of all forms include hematuria with red cell casts in the urine, moderate proteinuria occasionally reaching the nephrotic range, and variable hypertension and edema.
o In Goodpasture syndrome, the course may be dominated by recurrent hemoptysis or even life-threatening pulmonary hemorrhage.
o Serum analyses for anti-GBM antibodies, antinuclear antibodies, and ANCA are helpful in the diagnosis of specific subtypes.
o Although milder forms of glomerular injury may subside, the renal involvement is usually progressive over a matter of weeks and culminates in severe oliguria.
o Recovery of renal function may follow early intensive plasmapheresis (plasma exchange) combined with steroids and cytotoxic agents in Goodpasture syndrome.
o This therapy appears to reverse both pulmonary hemorrhage and renal failure.
o Other forms of RPGN also respond well to steroids and cytotoxic agents.
o Despite therapy, patients may eventually require chronic dialysis or transplantation.
(Robbins and Cotran Pathologic Basis of Disease 7th Edition)
3.1.3 Membranous Glomerulopathy
MEMBRANOUS GLOMERULOPATHY (MEMBRANOUS NEPHROPATHY)
o Membranous glomerulopathy is the most common cause of the nephrotic syndrome in adults.
o It is characterized by diffuse thickening of the glomerular capillary wall and the accumulation of electron-dense, immunoglobulin-containing deposits along the subepithelial side of the basement membrane.
o Membranous glomerulopathy occurring in association with other systemic diseases and a variety of identifiable etiologic agents is referred to as secondary membranous glomerulopathy.
o The most notable such associations are as follows: Drugs (penicillamine, captopril, gold, nonsteroidal anti-
inflammatory drugs [NSAIDs]): 1% to 7% of patients with rheumatoid arthritis treated with penicillamine or gold (drugs now used infrequently for this purpose) develop membranous glomerulopathy. NSAIDs, as we shall see, also cause minimal change disease.
Underlying malignant tumors, particularly carcinoma of the lung and colon and melanoma. According to some investigators, these are present in up to 5% to 10% of adults with membranous glomerulopathy.
SLE: About 15% of glomerulonephritis in SLE is of the membranous type.
Infections (chronic hepatitis B, hepatitis C, syphilis, schistosomiasis, malaria)
Other autoimmune disorders, such as thyroiditis o In about 85% of patients, no associated condition can be uncovered,
and the disease is considered idiopathic.
Etiology and Pathogenesis
o Membranous glomerulopathy is a form of chronic immune complex-mediated disease. In secondary membranous glomerulopathy, particular antigens can sometimes be identified in the immune complexes.
o For example, membranous glomerulopathy in SLE is associated with deposition of autoantigen-antibody complexes.
o Exogenous (hepatitis B, Treponema antigens) or endogenous (thyroglobulin) antigens have been identified within deposits in some patients.
o The lesions bear a striking resemblance to those of experimental Heymann nephritis, which, as you might recall, is induced by antibodies to a megalin antigenic complex.
o A similar but still unidentified antigen is presumed to be present in most cases of idiopathic membranous glomerulopathy in humans.
o Susceptibility to Heymann nephritis in rats and membranous glomerulopathy in humans is linked to the MHC locus, which influences the ability to produce antibodies to the nephritogenic antigen.
o Thus, idiopathic membranous glomerulopathy, like Heymann nephritis, is considered an autoimmune disease linked to susceptibility genes and caused by antibodies to a renal autoantigen.
o How does the glomerular capillary wall become leaky in membranous glomerulopathy?
o There is a paucity of neutrophils, monocytes, or platelets in glomeruli and the virtually uniform presence of complement, and experimental work suggests a direct action of C5b-C9, the pathway leading to the formation of the membrane attack complex.
o C5b-C9 causes activation of glomerular epithelial and mesangial cells, inducing them to liberate proteases and oxidants, which cause capillary wall injury and increased protein leakage.
Morphology
o By light microscopy, the glomeruli either appear normal in the early stages of the dis-ease or exhibit uniform, diffuse thickening of the glomerular capillary wall.
o By electron microscopy, the thickening is seen to be caused by irregular dense deposits between the basement membrane and the overlying epithelial cells, the latter having effaced foot processes.
o Basement membrane material is laid down between these deposits, appearing as irregular spikes protruding from the GBM.
o These spikes are best seen by silver stains, which color the basement membrane black.
o In time, these spikes thicken to produce domelike protrusions and eventually close over the immune deposits, burying them within a markedly thickened, irregular membrane. Immunofluorescence microscopy demonstrates that the granular deposits contain both immunoglobulins and various amounts of complement.
o As the disease advances, the membrane thickening progressively encroaches on the capillary lumens, and sclerosis of the mesangium may occur; in the course of time, glomeruli may become totally sclerosed.
o The epithelial cells of the proximal tubules contain protein reabsorption droplets, and there may be considerable mononuclear cell interstitial inflammation.
Clinical Course
o In a previously healthy individual, this disorder usually begins with the insidious onset of the nephrotic syndrome or, in 15% of patients, with non-nephrotic proteinuria.
o Hematuria and mild hypertension are present in 15% to 35% of cases. o It is necessary in any patient to first rule out the secondary causes
described earlier, since treatment of the underlying condition (malignant neoplasm, infection, or SLE) or discontinuance of the offending drug can reverse progression.
o The course of the disease is variable but generally indolent. o In contrast to minimal change disease, described later, the proteinuria
is nonselective and does not usually respond well to corticosteroid therapy.
o Progression is associated with increasing sclerosis of glomeruli, rising BUN reflecting renal insufficiency, and development of hypertension.
o Although proteinuria persists in more than 60% of patients, only about 10% die or progress to renal failure within 10 years, and no more than 40% eventually develop renal insufficiency. Concurrent sclerosis of glomeruli in the renal biopsy at the time of diagnosis is a predictor of worse prognosis.
o Spontaneous remissions and a relatively benign outcome occur more commonly in women and in those with proteinuria in the non-nephrotic range.
o Because of the variable course of the disease, it has been difficult to evaluate the overall effectiveness of corticosteroids or other immunosuppressive therapy in controlling the proteinuria or progression.
(Robbins and Cotran Pathologic Basis of Disease 7th Edition)
o MGN, or membranous nephropathy as it is sometimes called, accounts for approximately 30% of cases of nephrotic syndrome in adults, with a peak incidence between the ages of 30–50 years and a male to female ratio of 2:1.
o It is rare in childhood and by far the most common cause of nephrotic syndrome in the elderly.
o In 25–30% of cases, MGN is secondary to malignancy (solid tumors of the breast, lung, colon), infection (hepatitis B, malaria, schistosomiasis), or rheumatologic disorders like lupus or rarely rheumatoid arthritis (Table 277-6).
o Uniform thickening of the basement membrane along the peripheral capillary loops is seen by light microscopy on renal biopsy, this thickening needs to be distinguished from that seen in diabetes and amyloidosis.

7
o Immunofluorescence demonstrates diffuse granular deposits of IgG and C3, and electron microscopy typically reveals electron-dense subepithelial deposits.
o While different stages (I–V) of progressive membranous lesions have been described, some published analyses indicate the degree of tubular atrophy or interstitial fibrosis is more predictive of progression than is the stage of glomerular disease.
o The presence of subendothelial deposits or the presence of tubuloreticular inclusions strongly points to a diagnosis of membranous lupus nephritis, which may precede the extrarenal manifestations of lupus.
o Work in Heyman nephritis, an animal model of MGN, suggests that glomerular lesions result from in situ formation of immune complexes with megalin receptor–associated protein as the putative antigen.
o This antigen is not found in human podocytes, but human antibodies have been described against neutral endopeptidase expressed by podocytes, hepatitis antigens B/C, Helicobacterpylori antigens, tumor antigens, and thyroglobulin.
o Eighty percent of patients with MGN present with nephrotic syndrome and nonselective proteinuria.
o Microscopic hematuria is seen in up to 50% of patients. Spontaneous remissions occur in 20–33% of patients and often occur late in the course after years of nephrotic syndrome.
o One-third of patients continue to have relapsing nephrotic syndrome but maintain normal renal function, and approximately another third of patients develop renal failure or die from the complications of nephrotic syndrome. Male gender, older age, hypertension, and the persistence of proteinuria are associated with worse prognosis.
o Although thrombotic complications are a feature of all nephrotic syndromes, MGN has the highest reported incidences of renal vein thrombosis, pulmonary embolism, and deep vein thrombosis. Prophylactic anticoagulation is controversial but has been recommended for patients with severe or prolonged proteinuria in the absence of risk factors for bleeding.
o In addition to the treatment of edema, dyslipidemia, and hypertension, inhibition of the renin-angiotensin system is recommended.
o Therapy with immunosuppressive drugs is also recommended for patients with primary MGN and persistent proteinuria (>3.0 g/24 h).
o The choice of immunosuppressive drugs for therapy is controversial, but current recommendations based on small clinical studies are to treat with steroids and cyclophosphamide, chlorambucil, or cyclosporine.
o Experience with mycophenolate mofetil or anti-CD20 antibody is even more limited.
(Harrison’s Principles of Internal Medicine 17th Edition)
3.1.4 Minimal Change
MINIMAL CHANGE DISEASE (LIPOID NEPHROSIS)
o This relatively benign disorder is the most frequent cause of nephrotic syndrome in children, but it is less common in adults.
o It is characterized by diffuse effacement of foot processes of epithelial cells in glomeruli that appear virtually normal by light microscopy.
o The peak incidence is between 2 and 6 years of age. o The disease sometimes follows a respiratory infection or routine
prophylactic immunization. o Its most characteristic feature is its usually dramatic response to
corticosteroid therapy.
Etiology and Pathogenesis
o Although the absence of immune deposits in the glomerulus excludes classic immune complex mechanisms, several features of the disease point to an immunologic basis, including (1) the clinical association with respiratory infections and prophylactic immunization; (2) the response to corticosteroids and/or other immunosuppressive therapy; (3) the association with other atopic disorders (e.g., eczema, rhinitis); (4) the increased prevalence of certain HLA haplotypes in patients with minimal change disease associated with atopy (suggesting a genetic predisposition); (5) the increased incidence of minimal change disease in patients with Hodgkin disease, in whom defects in T cell-mediated immunity are well recognized; and (6) reports of proteinuria-inducing factors in the plasma or lymphocyte supernatants of patients with minimal change disease and focal glomerulosclerosis.
o The current leading hypothesis is that minimal change disease involves some immune dysfunction, eventually resulting in the elaboration of a cytokine that damages visceral epithelial cells and causes proteinuria.
o The ultrastructural changes point to a primary visceral epithelial cell injury, and studies in animal models suggest the loss of glomerular polyanions.
o Thus, defects in the charge barrier may contribute to the proteinuria. o The actual route by which protein traverses the epithelial cell portion of
the capillary wall remains an enigma. o Possibilities include transcellular passage through the epithelial cells,
passage through residual spaces between remaining but damaged foot processes, or leakage through foci in which the epithelial cells have become detached from the basement membrane.
o Additional insight into mechanisms by which epithelial cell injury results in proteinuria in minimal change disease, focal and segmental
glomerulosclerosis, and related entities should come from the recent discovery of mutations in several glomerular proteins, including nephrin, discussed in the section on focal glomerulosclerosis below.
o A mutation in the nephrin gene causes a hereditary form of congenital nephrotic syndrome (Finnish type) with minimal change glomerular morphology.
o Such mutations and the proteinuria they engender demonstrate that at least some cases of nephrotic syndrome with minimal change disease morphology can occur in the absence of abnormal responses of the immune system.
Morphology
o The glomeruli are normal by light microscopy. o By electron microscopy, the basement membrane appears normal,
and no electron-dense material is deposited. o The principal lesion is in the visceral epithelial cells, which show a
uniform and diffuse effacement of foot processes, these being replaced by a rim of cytoplasm often showing vacuolization, swelling, and hyperplasia of villi.
o This change, often incorrectly termed "fusion" of foot processes, actually represents simplification of the epithelial cell architecture with flattening, retraction, and swelling of foot processes.
o Foot process effacement is also present in other proteinuric states (e.g., membranous glomerulopathy, diabetes); it is only when effacement is associated with normal glomeruli by light microscopy that the diagnosis of minimal change disease can be made.
o The visceral epithelial changes are completely reversible after corticosteroid therapy, concomitant with remission of the proteinuria.
o The cells of the proximal tubules are often laden with lipid and protein, reflecting tubular reabsorption of lipoproteins passing through diseased glomeruli (thus, the historical term lipoid nephrosis).
o Immunofluorescence studies show no immunoglobulin or complement deposits.
Clinical Course
Despite massive proteinuria, renal function remains good, and there is commonly no hypertension or hematuria.
The proteinuria usually is highly selective, most of the protein consisting of albumin.
Most children (more than 90%) with minimal change disease respond rapidly to corticosteroid therapy.
However, the nephrotic phase may recur, and some patients may become steroid dependent or resistant.
Nevertheless, the long-term prognosis for patients is excellent, and even steroid-dependent disease resolves when children reach puberty.
Although adults are slower to respond, the long-term prognosis is also excellent.
As has been noted, minimal change disease in adults can be associated with Hodgkin disease and, less frequently, other lymphomas and leukemias.
(Robbins and Cotran Pathologic Basis of Disease 7th Edition)
Minimal Change Disease
o MCD, sometimes known as nil lesion, causes 70–90% of nephrotic syndrome in childhood but only 10–15% of nephrotic syndrome in adults.
o MCD usually presents as a primary renal disease but can be associated with several other conditions, including Hodgkin's disease, allergies, or use of nonsteroidal anti-inflammatory agents; significant interstitial nephritis often accompanies cases associated with nonsteroidal use.
o MCD on renal biopsy shows no obvious glomerular lesion by light microscopy and is negative for deposits by immunofluorescent microscopy, or occasionally shows small amounts of IgM in the mesangium.
o Electron microscopy, however, consistently demonstrates an effacement of the foot process supporting the epithelial podocytes with weakening of slit-pore membranes.
o The pathophysiology of this lesion is uncertain. o Most agree there is a circulating cytokine, perhaps related to a T cell
response that alters capillary charge and podocyte integrity. o The evidence for cytokine-related immune injury is circumstantial and
is suggested by the presence of preceding allergies, altered cell-mediated immunity during viral infections, and the high frequency of remissions with steroids.
o MCD presents clinically with the abrupt onset of edema and nephrotic syndrome accompanied by acellular urinary sediment.
o Less common clinical features include hypertension (30% in children, 50% in adults), microscopic hematuria (20% in children, 33% in adults), atopy or allergic symptoms (40% in children, 30% in adults), and decreased renal function (<5% in children, 30% in adults).
o The appearance of acute renal failure in adults is usually caused by intrarenal edema (nephrosarca) that is responsive to intravenous albumin and diuretics.
o This presentation must be distinguished from acute renal failure secondary to hypovolemia.

8
o In children, the abnormal urine principally contains albumin with minimal amounts of higher molecular weight proteins, and is sometimes called selective proteinuria.
o Although up to 30% of children have a spontaneous remission, all children today are treated with steroids; only children who are nonresponders are biopsied in this setting.
o Primary responders are patients who have a complete remission (<0.2 mg/24 h of proteinuria) after a single course of prednisone; steroid-dependent patients relapse as their steroid dose is tapered. Frequent relapsers have two or more relapses in the 6 months following taper, and steroid-resistant patients fail to respond to steroid therapy.
o Ninety to 95% of children will develop a complete remission after 8 weeks of steroid therapy, and 80–85% of adults will achieve complete remission, but only after a longer course of 20–24 weeks.
o Patients with steroid resistance can develop FSGS on repeat biopsy. o Some hypothesize that if the first renal biopsy does not have a sample
of deeper glomeruli, then the correct early diagnosis of FSGS may be missed.
o Relapses occur in 70–75% of children after the first remission, and early relapse predicts multiple subsequent relapses.
o The frequency of relapses decreases after puberty, although there is an increased risk of relapse following the rapid tapering of steroids in all groups.
o Relapses are less common in adults but are more resistant to subsequent therapy.
o Prednisone is first-line therapy, and other immunosuppressive drugs, such as cyclophosphamide, chlorambucil, and mycophenolate mofetil, are saved for frequent relapsers, steroid-dependent, or steroid-resistant patients.
o Cyclosporine can induce remission, but relapse is also common when cyclosporine is withdrawn. The long-term prognosis in adults is less favorable when acute renal failure or steroid resistance occurs.
(Harrison’s Principles of Internal Medicine 17th Edition)
3.1.5 Focal Segmental Glomerulosclerosis
FOCAL SEGMENTAL GLOMERULOSCLEROSIS
o As the name implies, this lesion is characterized by sclerosis of some, but not all, glomeruli (thus, it is focal); and in the affected glomeruli, only a portion of the capillary tuft is involved (thus, it is segmental).
o Focal segmental glomerulosclerosis is frequently accompanied clinically by the nephrotic syndrome or heavy proteinuria.
Classification and Types
o Focal segmental glomerulosclerosis (FSGS) occurs in the following settings: In association with other known conditions, such as HIV
infection (HIV nephropathy), heroin addiction (heroin nephropathy), sickle cell disease, and massive obesity
As a secondary event, reflecting glomerular scarring, in cases of focal glomerulonephritis (e.g., IgA nephropathy)
As a component of the adaptive response to loss of renal tissue (renal ablation, described earlier) in advanced stages of other renal disorders, such as reflux nephropathy, hypertensive nephropathy, or with unilateral renal agenesis
In certain inherited forms of nephrotic syndrome where the disease, in some pedigrees, has been linked to mutations in genes encoding nephrin, podocin, or α-actinin 4
As a primary disease (idiopathic focal segmental glomerulosclerosis)
o Idiopathic focal segmental glomerulosclerosis accounts for up to 10% and 35% of cases of nephrotic syndrome in children and adults in many series, respectively. FSGS (both primary and secondary forms) has increased in incidence and is now the most common cause of nephrotic syndrome in adults in the United States.
o It is a particularly common cause of nephrotic syndrome in Hispanic and African American patients.
o The clinical signs differ from those of minimal change disease in the following respects: (1) there is a higher incidence of hematuria, reduced GFR, and hypertension; (2) proteinuria is more often nonselective; (3) there is poor response to corticosteroid therapy; (4) there is progression to chronic glomerulosclerosis, with at least 50% developing end-stage renal disease within 10 years; and (5) immunofluorescence microscopy may show nonspecific deposition ("trapping") of IgM and C3 in the sclerotic segment.
Morphology
o By light microscopy, the segmental lesions may involve only a minority of the glomeruli and may be missed if the biopsy specimen contains an insufficient number of glomeruli.
o The lesions initially tend to involve the juxtamedullary glomeruli, although they subsequently become more generalized.
o In the sclerotic segments, there is collapse of basement membranes, increase in matrix, and segmental insudation of plasma proteins along the capillary wall (hyalinosis), which may extend to aggregates within glomerular capillaries that occlude the lumina.
o Lipid droplets and foam cells are often present.
o Glomeruli that do not exhibit segmental lesions either appear normal on light microscopy or may show increased mesangial matrix and mesangial proliferation.
o On electron microscopy, both sclerotic and nonsclerotic areas show the diffuse effacement of foot processes characteristic of minimal change disease, but in addition, there may be focal detachment of the epithelial cells with denudation of the underlying GBM.
o By immunofluorescence microscopy, IgM and C3 may be present in the sclerotic areas and/or in the mesangium. In addition to the focal sclerosis, there may be pronounced hyalinosis and thickening of afferent arterioles.
o With the progression of the disease, increased numbers of glomeruli become involved, sclerosis spreads within each glomerulus, and there is an increase in mesangial matrix.
o In time, this leads to total sclerosis of glomeruli, with pronounced tubular atrophy and interstitial fibrosis.
o A morphologic variant of focal segmental glomerulosclerosis, called collapsing glomerulopathy, is characterized by collapse and sclerosis of the entire glomerular tuft in addition to the usual focal segmental glomerulosclerosis lesions.
o A characteristic feature is proliferation and hypertrophy of glomerular visceral epithelial cells.
o This lesion may be seen in situations in which it is idiopathic, but it is the most characteristic lesion of HIV-associated nephropathy.
o In both cases, there is associated prominent tubular injury with formation of microcysts. It has a particularly poor prognosis.
Pathogenesis
o Whether idiopathic focal segmental glomerulosclerosis represents a distinct disease or is simply a phase in the evolution of a subset of patients with minimal change disease remains unresolved.
o The characteristic degeneration and focal disruption of visceral epithelial cells are thought to represent an accentuation of the diffuse epithelial cell change typical of minimal change disease.
o It is this epithelial damage that is the hallmark of focal segmental glomerulosclerosis.
o The hyalinosis and sclerosis represent entrapment of plasma proteins in extremely hyperpermeable foci with increased ECM deposition.
o The recurrence of proteinuria, sometimes within 24 hours after transplantation, suggests that a circulating factor, perhaps a cytokine, may be the cause of the epithelial damage.
o An approximately 50-kDa nonimmunoglobulin factor causing proteinuria has been isolated from sera of such patients.
o The recent discovery of a genetic basis for some cases of FSGS has improved the understanding of the pathogenesis of proteinuria in the nephrotic syndrome and has provided new methods for diagnosis and prognosis of affected patients.
o The first relevant gene to be identified, NPHS1, maps to chromosome 19q13 and encodes the protein nephrin.
o Nephrin is a key component of the slit diaphragm, the zipper-like structure between podocyte foot processes that might control glomerular permeability.
o Several types of mutations of the NPHS1 gene have been identified, and they give rise to congenital nephrotic syndrome of the Finnish type.
o Prenatal diagnosis of CNF is now possible based on analysis of the NPHS1 gene.
o A distinctive pattern of autosomal recessive FSGS results from mutations in the NPHS2 gene, which maps to chromosome 1q25-31 and encodes the protein product podocin.
o Podocin has also been localized to the slit diaphragm. o Mutations in NPHS2 result in a syndrome of steroid-resistant nephrotic
syndrome of childhood onset. o Affected children usually show pathologic features of FSGS but
sometimes of minimal change disease. o Podocin mutations may account for up to 30% of cases of steroid-
resistant nephrotic syndrome in children. o A third set of mutations in the gene encoding the podocyte actin-
binding protein α-actinin 4 underlies some cases of autosomal dominant FSGS, which can be insidious in onset but has a high rate of progression to renal insufficiency.
o What these proteins have in common is their localization to the slit diaphragm and to adjacent podocyte cytoskeletal structures such as actin.
o Their specific functions and interactions are incompletely understood, but it is clear that the integrity of each is necessary to maintain the normal glomerular filtration barrier.
o Additional components of the podocyte/slit diaphragm apparatus, such as CD2-associated protein (CD2AP), have been identified that may also contribute to proteinuria, as has been suggested in studies of knockout mice (but not yet demonstrated in humans).
o While identification of these genetic defects has clarified the pathogenesis of some cases of the so-called idiopathic nephrotic syndrome, many other factors contribute to permeability defects.
o These include cell-cell and cell-matrix interactions, particularly those mediated by α3β1 integrins and dystroglycans. Defects in these interactions may also cause a loss of podocyte adhesion to the glomerular basement membrane.

9
o Renal ablation focal segmental glomerulosclerosis occurs as a complication of glomerular and nonglomerular diseases causing reduction in functioning renal tissue, particularly reflux nephropathy and unilateral agenesis.
o These may lead to progressive glomerulosclerosis and renal failure. o The pathogenesis of focal segmental glomerulosclerosis in this setting
has been described earlier in this chapter.
Clinical Course
o There is little tendency for spontaneous remission in idiopathic focal segmental glomerulosclerosis, and responses to corticosteroid therapy are variable.
o In general, children have a better prognosis than adults do. o Progression of renal failure occurs at variable rates. o About 20% of patients follow an unusually rapid course, with
intractable massive proteinuria ending in renal failure within 2 years. o Recurrences are seen in 25% to 50% of patients receiving allografts.
(Robbins and Cotran Pathologic Basis of Disease 7th Edition)
o FSGS refers to a pattern of renal injury characterized by segmental glomerular scars that involve some but not all glomeruli; the clinical findings of FSGS largely manifest as proteinuria.
o When the secondary causes of FSGS are eliminated (Table 277-5), the remaining patients are considered to have FSGS.
o The incidence of this disease is increasing, and it now represents up to one-third of cases of nephrotic syndrome in adults and one-half of cases of nephrotic syndrome in African Americans, in whom it is seen more commonly.
o The pathogenesis of FSGS is probably multifactorial. o Possible mechanisms include a T cell–mediated circulating
permeability factor, TGF-–mediated cellular proliferation and matrix synthesis, and podocyte abnormalities associated with genetic mutations.
o The pathologic changes of FSGS are most prominent in glomeruli located at the corticomedullary junction (Fig. e9-2), so if the renal biopsy specimen is from superficial tissue, the lesions can be missed, which sometimes leads to a misdiagnosis of MCD.
o In addition to focal and segmental scarring, other variants have been described, including cellular lesions with endocapillary hypercellularity and heavy proteinuria; collapsing glomerulopathy (Fig. e9-3) with segmental or global glomerular collapse and a rapid decline in renal function; or the glomerular tip lesion, which seems to have a better prognosis.
o FSGS can present with any level of proteinuria, hematuria, hypertension, or renal insufficiency. Nephrotic range proteinuria, African-American race, and renal insufficiency are associated with a poor outcome, with 50% of patients reaching renal failure in 6–8 years.
o FSGS rarely remits spontaneously, but treatment-induced remission of proteinuria significantly improves prognosis.
o Treatment of patients with primary FSGS should include inhibitors of the renin-angiotensin system. Based on retrospective studies, patients with nephrotic range proteinuria can be treated with steroids but respond far less often than patients with MCD.
o Proteinuria remits in only 20–45% of patients receiving a course of steroids over 6–9 months.
o Limited evidence suggests that the use of cyclosporine in steroid-responsive patients helps ensure remissions, while other cytotoxic agents confer little added benefit over steroid therapy. Primary FSGS recurs in 25–40% of patients given allografts at end-stage disease, leading to graft loss in half of those cases.
o The treatment of secondary FSGS typically involves treating the underlying cause and controlling proteinuria. There is no role for steroids or other immunosuppressive agents in secondary FSGS.
(Harrison’s Principles of Internal Medicine 17th Edition)
3.2 Systemic Causes
3.2.1 Wegener Granulomatosis
o Wegener’s granulomatosis is a distinct clinicopathologic entity characterized by granulomatous vasculitis of the upper and lower respiratory tracts together with glomerulonephritis.
o In addition, variable degrees of disseminated vasculitis involving both small arteries and veins may occur.
o Renal injury occurs in 80% of patients with Wegener’s granulomatosis and varies from indolent smoldering inflammation to rapidly progressive renal failure.
o Cytoplasmic ANCA are detected at presentation in 80% of patients with renal disease and in 10% more on follow-up.
o Renal biopsy typically reveals focal, segmental, necrotizing pauci-immune glomerulonephritis with crescent formation.
o In contrast to the lung, granulomas are rarely seen in the kidney.
(Harrison’s Principles of Internal Medicine 16th Edition)
3.2.2 Hypersensitivity
o Most noninfectious vasculitides appear to be initiated by one of several immunologic mechanisms.
o Such processes often induce relatively distinctive clinicopathologic entities, in which the vasculitis is widespread.
o Of these so-called systemic necrotizing vasculitides, several types affect the aorta and medium-sized vessels; most affect vessels smaller than arteries, such as arterioles, venules, and capillaries (designated small vessel vasculitis).
Immune Complexes
o The evidence for involvement of immune complexes in vasculitides can be summarized as follows:
o The vascular lesions resemble those found in experimental immune complex-mediated conditions, such as the local Arthus phenomenon and serum sickness.
o Immune reactants and complement can be detected in the serum or vessels of patients with vasculitis.
o For example, DNA-anti-DNA complexes are present in the vascular lesions of systemic lupus erythematosus-associated vasculitis; IgG, IgM, and complement in cryoglobulinemic vasculitis; and a number of other antigens in isolated cases.
o Hypersensitivity to drugs causes approximately 10% of vasculitic skin lesions.
o Some, such as penicillin conjugate serum proteins, whereas others such as streptokinase are foreign proteins; both can lead to vascular deposits of immune complexes.
o The most impressive evidence comes from vasculitis associated with viral infections, particularly hepatitis.
o There is a high incidence of hepatitis B antigen (HBsAg) and HBsAg-anti-HBsAg immune complexes in the serum and, with complement, in the vascular lesions of some patients with vasculitis, particularly those with large vessel polyarteritis nodosa and less commonly in those with membranous or membranoproliferative glomerulonephritis or leukocytoclastic vasculitis.
o Importantly, immunosuppressive treatment results in a remission of the vasculitis but perpetuates the hepatitis B virus infection.
o Chronic hepatitis C virus (HCV) infection leads to glomerulonephritis, in which HCV/RNA and cryoprecipitates containing anti-HCV antibodies are detected in glomeruli.
Antineutrophil Cytoplasmic Antibodies
o Serum from many patients with vasculitis in small vessels reacts with cytoplasmic antigens in neutrophils, indicating the presence of antineutrophil cytoplasmic autoantibodies (ANCA).
o ANCA comprise a heterogeneous group of autoantibodies against enzymes mainly found within the azurophil or primary granules in neutrophils but also found in the lysosomes of monocytes and in endothelial cells.
o ANCA can be detected in serum by immunofluorescent microscopy of ethanol-fixed neutrophils and by immunochemical assays.
o Two main patterns of immunofluorescent staining distinguish different ANCA types.
o One ANCA type shows cytoplasmic localization of the staining (c-ANCA), and the most common target antigen is proteinase 3 (PR-3), a neutrophil granule constituent.
o The second type shows perinuclear staining (p-ANCA) and is usually specific for myeloperoxidase (MPO).
o Either ANCA specificity may occur in a patient with ANCA-associated small vessel vasculitis, but c-ANCA (PR-3 specificity) are typically found in Wegener granulomatosis and p-ANCA (MPO specificity) are found in most cases of microscopic polyangiitis and Churg-Strauss syndrome. Approximately 10% of patients with these disorders, however, do not demonstrate ANCA by typical assays.
o ANCA serve as useful quantitative diagnostic markers for these disorders, and their discovery has led to segregation of a group of these disorders as the ANCA-associated vasculitides.
o The close association between ANCA titers and disease activity, particularly c-ANCA in Wegener granulomatosis, suggests that they may be important in the pathogenesis of this disease, but the precise mechanisms by which ANCA induce injury are unknown.
o One scenario currently being pursued postulates the following events: o An autoimmune process of yet uncertain cause and mechanism
initiates the formation of ANCA.o Proinflammatory cytokines produced during an infection, by
malignancy, or possibly triggered by drugs induce surface expression of the ANCA target antigens PR-3 and MPO on susceptible cells, thereby making them accessible to the respective antibodies.
o Binding of circulating ANCA to these antigens leads to neutrophil degranulation and endothelial cell injury with subsequent vascular damage.
Other Mechanisms
o Antibodies to endothelial cells, perhaps induced by defects in immune regulation, may predispose to certain vasculitides, such as those associated with systemic lupus erythematosus and Kawasaki disease.
o Additionally, in Goodpasture syndrome the glomerulitis and pneumonitis are caused by anti-glomerular basement membrane antibodies.
o Finally, there is experimental evidence that certain viruses (e.g., herpes, coxsackievirus) may cause vasculitis by immune mechanisms involving T-cell action and gamma-interferon.

10
(Robbins and Cotran Pathologic Basis of Disease 7th Edition)
3.2.3 Cryoglobulinemia
o Renal involvement is most common with the mixed cryoglobulinemias (types II and III), which are more common in females and usually begin in the sixth decade.
o Most patients present with a variable combination of leukocytoclastic vasculitis, skin ulcerations, arthralgias, fatigue, and Raynaud’s phenomenon.
o Renal disease is a complication in 50% of patients and usually develops after 12 to 24 months.
o The typical clinical renal manifestations are nephrotic-range proteinuria, microscopic hematuria, and hypertension.
o Acute nephritic syndrome occurs in 20 to 30%, and oliguric acute renal failure in about 5% of patients with renal disease.
o Circulating levels of C3, C4, and CH50 are depressed in about 80% of patients with renal involvement, and a transient ANA (speckled pattern) is sometimes detected. Hepatitis C virus (HCV) RNA has been isolated from the serum of patients with essential mixed cryoglobulinemia (EMC).
(Harrison’s Principles of Internal Medicine 16th Edition)
3.2.4 Systemic Lupus Erythematous
o Renal involvement is clinically evident in 40 to 85% of patients with SLE; it varies from isolated abnormalities of the urinary sediment to full-blown nephritic or nephrotic syndrome or chronic renal failure.
o Most glomerular injury is triggered by the formation of immune complexes within the glomerular capillary wall; however, thrombotic microangiopathy may be the dominant reason for renal dysfunction in a small subset of patients with the antiphospholipid antibody syndrome.
o Renal biopsy has proven very useful for identifying the different patterns of immune-complex glomerulonephritis in SLE, which are diverse, portend different prognoses, and do not necessarily correlate with the clinical findings.
o Indeed, clinically silent lupus nephritis is well described as having a urinalysis virtually normal but renal biopsy demonstrating varying degrees of injury.
o Patients with active lupus nephritis have a range of serologic abnormalities. Hypocomplementemia is present in 75 to 90% of patients and is most striking with diffuse proliferative glomerulonephritis.
o Antinuclear antibodies (ANA) are usually detected (95 to 99%), although not specific for SLE. ANA titers tend to fall with treatment, and ANA may not be detected during remissions.
o Anti-double-stranded DNA (dsDNA) antibodies are highly specific for SLE, and changes in their titers correlate with the activity of lupus nephritis.
o Patients with the lupus-related antiphospholipid antibody syndrome can develop a variable degree of renal impairment due to thrombotic microangiopathy.
o The latter typically affects the interlobular arteries, arterioles, and glomerular capillaries and is characterized by intravascular microthrombi and swelling of endothelial cells.
o Decreased levels of tissue plasminogen activator and increased levels of α2-antiplasmin, both of which would tend to promote thrombosis, have been described in this syndrome.
(Harrison’s Principles of Internal Medicine 16th Edition)
3.2.5 Polyarteritis nodosa
o Polyarteritis nodosa is a systemic vasculitis manifested by transmural necrotizing inflammation of small or medium-sized muscular arteries, typically involving renal and visceral vessels and sparing the pulmonary circulation.
o Neither glomerulonephritis nor vasculitis of arterioles, capillaries, or venules is present.
o The involvement is peculiarly focal, random, and episodic. It often produces irregular aneurysmal dilation, nodularity, and vascular obstruction and sometimes infarctions.
o To differentiate this disorder from other similar vasculitides, which are now thought to be distinct entities, the term classic is sometimes added to the designation.
MORPHOLOGY
o In classic cases, polyarteritis nodosa involves arteries of medium to small size in any organ, with the possible exception of the lung.
o The distribution of lesions, in descending order of frequency is kidneys, heart, liver, and gastrointestinal tract, followed by pancreas, testes, skeletal muscle, nervous system, and skin.
o Individual lesions are sharply segmental, may involve only a portion of the vessel circumference, and have a predilection for branching points and bifurcations.
o Segmental erosion with weakening of the arterial wall owing to the inflammatory process may cause aneurysmal dilation or localized
rupture that is perceived clinically as a palpable nodule and can be demonstrated by arteriography.
o Impairment of perfusion causing ulcerations, infarcts, ischemic atrophy, or hemorrhages in the area supplied by these vessels may provide the first clue to the existence of the underlying disorder. Sometimes, however, the lesions are exclusively microscopic and produce no gross changes.
o Histologically the vasculitis during the acute phase is characterized by transmural inflammation of the arterial wall with a heavy infiltrate of neutrophils, eosinophils, and mononuclear cells, frequently accompanied by fibrinoid necrosis of the inner half of the vessel wall.
o Typically the inflammatory reaction permeates the adventitia. o The lumen may become thrombosed. o In some lesions, only a portion of the circumference is affected,
leaving segments of normal arterial wall juxtaposed to areas of vascular inflammation.
o At a later stage, the acute inflammatory infiltrate begins to disappear and is replaced by fibrous thickening of the vessel wall accompanied by a mononuclear infiltrate.
o The fibroblastic proliferation may extend into the adventitia, contributing to the firm nodularity that sometimes marks the lesions.
o At a still later stage, all that remains is marked fibrotic thickening of the affected vessel, devoid of significant inflammatory infiltration. Particularly characteristic of polyarteritis nodosa is that all stages of activity may coexist in different vessels or even within the same vessel. Thus, whatever the inflammatory insult, it is apparently recurrent and strangely haphazard.
Clinical Course
o Classic polyarteritis nodosa is a disease of young adults, although it may occur in children and older individuals.
o The course may be acute, subacute, or chronic and is frequently remittent, with long symptom-free intervals.
o Because the vascular involvement is widely scattered, the clinical signs and symptoms of this disorder may be varied and puzzling.
o The most common manifestations are malaise, fever of unknown cause, and weight loss; hypertension, usually developing rapidly; abdominal pain and melena (bloody stool) owing to vascular lesions in the alimentary tract; diffuse muscular aches and pains; and peripheral neuritis, which is predominantly motor.
o Renal involvement is one of the prominent manifestations of polyarteritis nodosa and a major cause of death.
o Because small vessel involvement is absent, however, there is no glomerulonephritis.
o About 30% of patients with polyarteritis nodosa have hepatitis B antigen in their serum. Unlike microscopic polyarteritis (microscopic polyangiitis, see later), classic polyarteritis nodosa has little association with ANCA.
o The diagnosis can usually be definitely established by the identification of necrotizing arteritis on tissue biopsy specimens, particularly medium-sized arteries of clinically involved tissue, such as kidney and nodular skin lesions.
o Angiography shows vascular aneurysms or occlusions of main visceral arteries in 50% of cases. Untreated, the disease is fatal in most cases, either during an acute fulminant attack or after a protracted course, but therapy with corticosteroids and cyclophosphamide results in remissions or cures in 90%.
o Effective treatment of the hypertension is a prerequisite for a favorable prognosis.
(Robbins and Cotran Pathologic Basis of Disease 7th Edition)
3.2.6 Henoch-Sch lein Purpuraӧ
o This syndrome consists of purpuric skin lesions characteristically involving the extensor surfaces of arms and legs as well as buttocks; abdominal manifestations including pain, vomiting, and intestinal bleeding; nonmigratory arthralgia; and renal abnormalities.
o The renal manifestations occur in one-third of patients and include gross or microscopic hematuria, proteinuria, and nephrotic syndrome.
o A small number of patients, mostly adults, develop a rapidly progressive form of glomerulonephritis with many crescents.
o Not all components of the syndrome need to be present, and individual patients may have purpura, abdominal pain, or urinary abnormalities as the dominant feature.
o The disease is most common in children 3 to 8 years old, but it also occurs in adults, in whom the renal manifestations are usually more severe.
o There is a strong background of atopy in about one-third of patients, and onset often follows an upper respiratory infection. IgA is deposited in the glomerular mesangium in a distribution similar to that of IgA nephropathy.
o This has led to the concept that IgA nephropathy and Henoch-Schönlein purpura are spectra of the same disease.68
Morphology
o On histologic examination, the renal lesions vary from mild focal mesangial proliferation to diffuse mesangial proliferation to crescentic glomerulonephritis.

11
o Whatever the histologic lesions, the prominent feature by fluorescence microscopy is the deposition of IgA, sometimes with IgG and C3, in the mesangial region.
o The skin lesions consist of subepidermal hemorrhages and a necrotizing vasculitis involving the small vessels of the dermis.
o IgA is also present in such vessels. o Vasculitis also occurs in other organs, such as the gastrointestinal
tract, but is rare in the kidney.o The course of the disease is variable, but recurrences of hematuria
may persist for many years after onset. o Most children have an excellent prognosis. o Patients with the more diffuse lesions, crescents, or the nephrotic
syndrome have a somewhat poorer prognosis.
(Robbins and Cotran Pathologic Basis of Disease 7th Edition)
3.2.7 Goodpasture Syndrome
o Goodpasture syndrome, microscopic polyarteritis, and Wegener granulomatosis (Chapter 11) are commonly associated with glomerular lesions, as described in the discussion of these diseases.
o Suffice it to say here that the glomerular lesions in these three conditions can be histologically similar and are principally characterized by foci of glomerular necrosis and crescent formation.
o In the early or mild forms of involvement, there is focal and segmental, sometimes necrotizing, glomerulonephritis, and most of these patients will have hematuria with mild decline in GFR.
o In the more severe cases associated with RPGN, there is more extensive necrosis, fibrin deposition, and extensive formation of epithelial (cellular) crescents, which can become organized to form fibrocellular and fibrous crescents if the glomerular injury evolves into segmental or global scarring (sclerosis).
(Robbins and Cotran Pathologic Basis of Disease 7th Edition)
3.3 Renal Diseases
3.3.1 Membranoproliferative Glomerulonephritis
o Membranoproliferative glomerulonephritis (MPGN) is characterized histologically by alterations in the basement membrane, proliferation of glomerular cells, and leukocyte infiltration.
o Because the proliferation is predominantly in the mesangium, a frequently used synonym is mesangiocapillary glomerulonephritis.
o MPGN accounts for 10% to 20% of cases of nephrotic syndrome in children and young adults.
o Some patients present only with hematuria or proteinuria in the non-nephrotic range, and others have a combined nephrotic-nephritic picture.
o Like many other glomerulonephritides, MPGN either can be associated with other systemic disorders and known etiologic agents (secondary MPGN) or may be idiopathic (primary MPGN).
o Primary MPGN is divided into two major types on the basis of distinct ultrastructural, immunofluorescent, and pathologic findings: type I and type II MPGN (dense-deposit disease).
Morphology
o By light microscopy, both types are similar. o The glomeruli are large and hypercellular. o The hypercellularity is produced both by proliferation of cells in the
mesangium and so-called endocapillary cell proliferation involving capillary endothelium and infiltrating leukocytes. Parietal epithelial crescents are present in many cases.
o The glomeruli have a "lobular" appearance accentuated by the proliferating mesangial cells and increased mesangial matrix
o The GBM is clearly thickened, often focally; this is most evident in the peripheral capillary loops.
o The glomerular capillary wall often shows a "double-contour" or "tram-track" appearance, especially evident in silver or PAS stains.
o This is caused by "duplication" of the basement membrane, usually as the result of new basement membrane synthesis.
o Within the besement membrane there is inclusion or interposition of cellular elements, which can be of mesangial, endothelial, or leukocytic origin.
o Such interposition gives rise to the appearance of "split" basement membranes.
o Types I and II MPGN differ in their ultrastructural and immunofluorescent features (Fig. 20-24).
o Type I MPGN (the great majority of cases) is characterized by the presence of subendothelial electron-dense deposits. Mesangial and occasional subepithelial deposits may also be present (Fig. 20-24A). By immunofluorescence, C3 is deposited in a granular pattern, and IgG and early complement components (C1q and C4) are often also present, suggesting an immune complex pathogenesis.
o In dense-deposit disease (type II MPGN) a relatively rare entity, the lamina densa of the GBM is transformed into an irregular, ribbon-like, extremely electron-dense structure because of the deposition of dense material of unknown composition in the GBM proper, giving rise to the term dense-deposit disease.
o C3 is present in irregular granular or linear foci in the basement membranes on either side but not within the dense deposits.
o C3 is also present in the mesangium in characteristic circular aggregates (mesangial rings).
o IgG is usually absent, as are the early-acting complement components (C1q and C4).
Pathogenesis
o In most cases of type I MPGN there is evidence of immune complexes in the glomerulus and activation of both classical and alternative complement pathways.
o The antigens involved in idiopathic MPGN are unknown. o In many cases, they are believed to be proteins derived from infectious
agents such as hepatitis C and B viruses, which presumably behave either as "planted" antigens after first binding to or becoming trapped within glomerular structures or are contained in preformed immune complexes deposited from the circulation.
o Most patients with dense-deposit disease (type II MPGN) have abnormalities that suggest activation of the alternative complement pathway.
o These patients have a consistently decreased serum C3 but normal C1 and C4, the immune complex-activated early components of complement.
o They also have diminished serum levels of factor B and properdin, components of the alternative complement pathway.
o In the glomeruli, C3 and properdin are deposited, but IgG is not. Recall that in the alternative complement pathway, C3 is directly cleaved to C3b (Fig. 20-25; see also Chapter 2, Fig. 2-14).
o The reaction depends on the initial interaction of C3 with such substances as bacterial polysaccharides, endotoxin, and aggregates of IgA in the presence of factors B and D.
o This leads to the generation of C3bBb, the alternative pathway C3 convertase.
o This C3 convertase is labile, being degraded by factors I and H, but it can be stabilized by properdin.
o More than 70% of patients with dense-deposit disease have a circulating antibody termed C3 nephritic factor (C3NeF), which is an autoantibody that binds to the alternative pathway C3 convertase.
o Binding of the antibody stabilizes the convertase, protecting it from enzymatic degradation and thus favoring persistent C3 degradation and hypocomplementemia.
o There is also decreased C3 synthesis by the liver, further contributing to the profound hypocomplementemia.
o Precisely how C3NeF is related to glomerular injury and the nature of the dense deposits is unknown.
o C3NeF activity also occurs in some patients with a genetically determined disease, partial lipodystrophy, some of whom develop dense-deposit disease (type II MPGN).
Clinical Course
o The principal mode of presentation is the nephrotic syndrome occurring in older children or young adults (idiopathic MPGN type I and cases of type II), but usually with a nephritic component manifested by hematuria or, more insidiously, as mild proteinuria.
o Few remissions occur spontaneously in either type, and the disease follows a slowly progressive but unremitting course.
o Some patients develop numerous crescents and a clinical picture of RPGN.
o About 50% develop chronic renal failure within 10 years. o Treatments with steroids, immunosuppressive agents, and antiplatelet
drugs have not been proved to be materially effective. There is a high incidence of recurrence in transplant recipients, particularly in dense-deposit disease; dense deposits may recur in 90% of such patients, although renal failure in the allograft is much less common.
Secondary MPGN
o Secondary MPGN (invariably type I) is more common in adults and arises in the following settings: Chronic immune complex disorders, such as SLE; hepatitis B
infection; hepatitis C infection, usually with cryoglobulinemia; endocarditis; infected ventriculoatrial shunts; chronic visceral abscesses; HIV infection; and schistosomiasis
α1-Antitrypsin deficiency Malignant diseases (chronic lymphocytic leukemia and
lymphoma) Hereditary deficiencies of complement regulatory proteins
o The mechanisms underlying the process of immune complex deposition in the last three categories above remain unknown.
(Robbins and Cotran Pathologic Basis of Disease 7th Edition)
3.3.2 Berger Disease (IgG-immunoglobulin A [IgA] nephropathy)
IGA NEPHROPATHY (BERGER DISEASE)
o This form of glomerulonephritis is characterized by the presence of prominent IgA deposits in the mesangial regions, detected by immunofluorescence microscopy.
o The disease can be suspected by light microscopic examination, but diagnosis is made only by immunocytochemical techniques.

12
o IgA nephropathy is a frequent cause of recurrent gross or microscopic hematuria and is probably the most common type of glomerulonephritis worldwide.
o Mild proteinuria is usually present, and the nephrotic syndrome may occasionally develop.
o Rarely, patients may present with rapidly progressive crescentic glomerulonephritis.
o Whereas IgA nephropathy is typically an isolated renal disease, similar IgA deposits are present in a systemic disorder of children, Henoch-Schönlein purpura, to be discussed later, which has many overlapping features with IgA nephropathy.
o In addition, secondary IgA nephropathy occurs in patients with liver and intestinal diseases, as discussed in the section on pathogenesis.
Pathogenesis
o IgA, the main immunoglobulin in mucosal secretions, is at low levels in normal serum, where it is present mostly in monomeric form, the polymeric forms being catabolized in the liver.
o In patients with IgA nephropathy, serum polymeric IgA is increased, and circulating IgA-containing immune complexes are present in some patients.
o However, it is clear that increased production of IgA cannot itself cause this disease.
o Although there are two subclasses of IgA molecules in humans (IgA1 and IgA2), only IgA1 forms the nephritogenic deposits of IgA nephropathy.
o A genetic influence is suggested by the occurrence of this condition in families and in HLA-identical brothers and the increased frequency of certain HLA and complement phenotypes in some populations.
o The prominent mesangial deposition of IgA suggests entrapment of IgA immune complexes in the mesangium, and the presence of C3 combined with the absence of C1q and C4 in glomeruli points to activation of the alternative complement pathway.
o Taken together, these clues suggest a genetic or acquired abnormality of immune regulation leading to increased mucosal IgA synthesis in response to respiratory or gastrointestinal exposure to environmental agents (e.g., viruses, bacteria, food proteins).
o IgA1 and IgA1-containing immune complexes are then trapped in the mesangium, where they activate the alternative complement pathway and initiate glomerular injury.
o In support of this scenario, IgA nephropathy occurs with increased frequency in patients with gluten enteropathy (celiac disease), in whom intestinal mucosal defects are well defined, and in liver disease, in which there is defective hepatobiliary clearance of IgA complexes (secondary IgA nephropathy).
o The nature of the initiating antigens is unknown, and several infectious agents and food products have been implicated.
o The deposited IgA appears to be polyclonal, and it may be that a variety of antigens are involved in the course of the disease. Alternatively, there is evidence that qualitative alterations in the IgA1 molecule itself, specifically a defect in normal galactosylation, make it more likely to bind to mesangial antigens or form mesangial deposits owing to other as yet unidentified mechanisms.
Morphology
o On histologic examination, the lesions vary considerably. o The glomeruli may be normal or may show mesangial widening and
proliferation (mesangioproliferative glomerulonephritis), segmental proliferation confined to some glomeruli (focal proliferative glomerulonephritis), or rarely, overt crescentic glomerulonephritis.
o The presence of leukocytes within glomerular capillaries is a variable feature.
o The mesangial widening may be the result of cell proliferation, accumulation of matrix, or both.
o Healing of the focal proliferative lesion may lead to focal segmental sclerosis.
o The characteristic immunofluorescent picture is of mesangial deposition of IgA often with C3 and properdin and lesser amounts of IgG or IgM.
o Early complement components are usually absent. o Electron microscopy confirms the presence of electron-dense deposits
in the mesangium.
Clinical Course
o The disease affects people of any age, but older children and young adults are most commonly affected.
o Many patients present with gross hematuria after an infection of the respiratory or, less commonly, gastrointestinal or urinary tract; 30% to 40% have only microscopic hematuria, with or without proteinuria; and 5% to 10% develop a typical acute nephritic syndrome.
o The hematuria typically lasts for several days and then subsides, only to return every few months.
o The subsequent course is highly variable.o Many patients maintain normal renal function for decades. o Slow progression to chronic renal failure occurs in 15% to 40% of
cases over a period of 20 years. o Onset in old age, heavy proteinuria, hypertension, and the extent of
glomerulosclerosis on biopsy are clues to an increased risk of progression.
o Recurrence of IgA deposits in transplanted kidneys is frequent. o In approximately 15% of those with recurrent IgA deposits, there is
resulting clinical disease, which most frequently runs the same indolent, slowly progressive course as that of the primary IgA nephropathy.
(Robbins and Cotran Pathologic Basis of Disease 7th Edition)
4. Identify the different diagnostic modalities for patients with glomerulonephritis.
4.1 Laboratory Work-up
Complete blood cell counto A decrease in hematocrit may demonstrate a dilutional anemia.o In the setting of an infectious etiology, pleocytosis may be evident.
Electrolytes, including BUN and creatinine (to estimate the glomerular filtration rate [GFR]): The BUN and creatinine levels will exhibit a degree of renal compromise.
Urinalysiso Urine is dark.o Specific gravity is greater than 1020 osm.o Proteinuria is observed.o RBCs and red cell casts are present.o Although not indicated in the ED setting, a 24-hour urine protein
excretion and creatinine clearance may be helpful to document the degree of renal dysfunction and proteinuria.
Streptozyme test: This test includes many streptococcal antigens that are sensitive for screening but are not quantitative.
Antistreptolysin O (ASO)o This quantitative titer is increased in 60-80% of patients.o Increase begins in 1-3 weeks, peaks in 3-5 weeks, and returns to
normal in 6 months.o Antistreptolysin O titer is unrelated to severity, duration, or prognosis
of renal disease. Erythrocyte sedimentation ratio (ESR) usually is increased. Urine or plasma creatinine level greater than 40; decreased renin level is
noted. Cultures of throat and skin lesions to rule out Streptococcus species may be
obtained. Blood cultures
o Indicated in patients with fever, immunosuppression, intravenous drug use history, indwelling shunts, or catheters.
o Blood culture may indicate hypertriglyceridemia, decreased glomerular filtration rate, or anemia.
(http://www.emedicine.com/emerg/topic219.htm)
4.2 Imaging Studies
Radiography
Chest radiography is needed in patients with a cough, with or without hemoptysis (ie, Wegener granulomatosis, Goodpasture syndrome, pulmonary congestion).
Abdominal radiographic imaging (ie, computed tomography) is needed if visceral abscesses are suspected; also look for chest abscesses.o Echocardiography in patients with a new cardiac murmur or a positive
blood culture to rule out endocarditis or a pericardial effusion.o Bedside renal ultrasonography may be appropriate to evaluate kidney
size as well as to determine the extent of fibrosis. A kidney size of less than 9 cm is suggestive of extensive scarring and a low
likelihood of reversibility.
(http://www.emedicine.com/emerg/topic219.htm)
4.3 Serology and Complement Levels
4.3.1 Antinuclear Antibody
o Immunofluorescence microscopy is particularly helpful and identifies three major patterns of deposition of immunoglobulin that define three broad diagnostic categories: (1) scattered granular deposits of immunoglobulin, a hallmark of immunecomplex glomerulonephritis; (2) more discrete linear deposition of immunoglobulin along the GBM, characteristic of anti-GBM disease; and (3) paucity or absence of immunoglobulin—pauci-immune glomerulonephritis.
o Most patients (>70%) with full-blown acute nephritic syndrome have immune-complex glomerulonephritis.
o Pauci-immune glomerulonephritis is less common in this setting (<30%), and anti-GBM disease is rare (<1%).
o Among patients with RPGN, immune-complex glomerulonephritis and pauci-immune
o glomerulonephritis are equally prevalent (~45% each), whereas anti-GBM disease again accounts for a minority of cases (<10%).
o Three serologic markers often predict the immunofluorescence microscopy findings in nephritic syndrome and RPGN and may obviate the need for renal biopsy in classic cases.
o They are the serum C3 level and titers of anti-GBM antibody and ANCA
o As discussed in previous sections, the kidney is host to immune attack in immune-complex glomerulonephritis, most cases being initiated

13
either by in situ formation of immune complexes or less commonly by glomerular trapping of circulating immune complexes.
o These patients typically have hypocomplementemia (low C3 in 90%) and negative anti-GBM and ANCA serology, the major exception being IgA nephropathy/Henoch Schonlein purpura where complement levels are typically normal.
o The glomerulus is the direct target of immune attack in anti-GBM disease, glomerular inflammation being initiated by an autoantibody directed at a 28-kDa autoantigen on the α3 chain of type IV collagen.
o Approximately90 to 95% of patients with anti-GBM disease have circulating anti-GBM autoantibodies detectable by immunoassay; serum complement levels are typically normal, and ANCA are
o usually not detected. o The pathogenesis of pauci-immune glomerulonephritis is still being
defined; however, most patients have circulating ANCA. o Serum complement levels are typically normal, and anti-GBM titers are
usually negative in ANCA-associated renal disease. o It should be noted, however, that there may be some serologic
overlap, with as many as 20% of patients with immune complex or anti-GBM glomerulonephritis also having at least low levels of circulating ANCA.
(Harrison’s Principles of Internal Medicine 16th Edition)
4.4 Renal Biopsy
A renal biopsy in the setting of glomerulonephritis can quickly identify the type of glomerular injury and often suggests a course of treatment.
The biopsy is processed for light microscopy using stains for hematoxylin and eosin (H&E) to assess cellularity and architecture, periodic acid-Schiff (PAS) to stain carbohydrate moieties in the membranes of the glomerular tuft and tubules, Jones-methenamine silver to enhance basement membrane structure,
Congo red for amyloid deposits, and Masson's trichrome to identify collagen deposition and assess the degree of glomerulosclerosis and interstitial fibrosis.
Biopsies are also processed for direct immunofluorescence using conjugated antibodies against IgG, IgM, and IgA to detect the presence of "lumpy-bumpy" immune deposits or "linear" IgG or IgA antibodies bound to GBM, antibodies against trapped complement proteins (C3 and C4), or specific antibodies against a relevant antigen. High-resolution electron microscopy can clarify the principal location of immune deposits and the status of the basement membrane.
Each region of a renal biopsy is assessed separately. By light microscopy, glomeruli (at least 10 and ideally 20) are reviewed
individually for discrete lesions; <50% involvement is considered focal, and >50% is diffuse. Injury in each glomerular tuft can be segmental, involving a portion of the tuft, or global, involving most of the glomerulus.
Glomeruli can have proliferative characteristics, showing increased cellularity. When cells in the capillary tuft proliferate, it is called endocapillary, and when cellular proliferation extends into Bowman's space, it is called extracapillary.
Synechiae are formed when epithelial podocytes attach to Bowman's capsule in the setting of glomerular injury; crescents, which in some cases may be the extension of synechiae, develop when fibrocellular/fibrin collections fill all or part of Bowman's space; and sclerotic glomeruli show acellular, amorphous accumulations of proteinaceous material throughout the tuft with loss of functional capillaries and normal mesangium.
Since age-related glomerulosclerosis is common in adults, one can estimate the background percentage of sclerosis by dividing the patient's age in half and subtracting 10.
Immunofluorescent and electron microscopy can detect the presence and location of subepithelial,subendothelial, or mesangial immune deposits, or reduplication or splitting of the basement membrane.
In the other regions of the biopsy, the vasculature surrounding glomeruli and tubules can show angiopathy, vasculitis, the presence of fibrils, or thrombi.
The tubules can be assessed for adjacency to one another; separation can be the result of edema, tubular dropout, or collagen deposition resulting from interstitial fibrosis. Interstitial fibrosis is an ominous sign of irreversibility and progression to renal failure.
(Harrison’s Principles of Internal Medicine 17th Edition)
Renal biopsy remains the gold standard” for diagnosis.
(Harrison’s Principles of Internal Medicine 16th Edition)
CLINICAL INDICATIONS FOR A RENAL BIOPSY
Proteinuria with impairment of renal function Symptomless isolated proteinuria if more than 2g/day Nephrotic syndrome in adults
In children it is most likely to be due to steroid sensitiveminimal change g/n so trial of steroids is less risky than biopsy.
Persistent haematuria with proteinuria especially if there is renal impairment. Acute renal failure if likely to be renal on origin, as opposed to pre-renal
(hypovolaemic shock) or post-renal (obstructive) in origin. Chronic renal failure with normal sized kidneys Renal transplant dysfunction Systemic disorders with features that include haematuria, proteinuria and
renal failure.
SOME CONTRA-INDICATIONS FOR A RENAL BIOPSY
Asymmetric kidneys Tiny shrunken kidneys Obstructive renal disease Coagulation defects Acute infections
(Dr. Bigornia’s Lecture)
Additional Information
Electron microscopy makes some important contributionso To the understanding of the glomerulus in health.o To the understanding of mechanisms of disease in diagnosis.
In diagnostic pathology transmission electron microscopy is usually used, rather than scanning electron microscopy.
Although immune complexes can be identified by immunofluoresence, it is sometimes helpful to know exactly where these deposits are in relation to the basement membranes.o Sub-epithelial deposits in post-streptococcal g/n.o Deposits within thickened basement membranes in membranous g/n.
IMMUNOLOGY
Antibodies are prepared, specific for abnormal things that might be present in the diseased glomerulus.
A fluorescent molecule is attached to the back end of the antibody molecule so that the molecule will be visible down the light microscope under ultra-violet light.
Thin sections are cut from the frozen fresh material. These are then “stained” using specific antibodies prepared in the laboratory. The “stained” sections are viewed under ultra-violet light. Positive “staining” appears as green fluorescence on a black background
Usually three variables are scored.
1. Identity of abnormal materials in glomerulus.
o Usually patient’s own immune reactants eg. IgG, IgM, IgA, C3.
2. Where is it?o Eg. In capillary loops. In mesangium.
3. What is it like? o Eg. lumpy-bumpy granular, linear.
Example 1: Which immune reactants?
IgG +/- IgMComplement C3Where?In walls of glomerular capillaries.Pattern?Lumpy-bumpy granularInterpretation?Immune complex deposition
Example 2:Which immune reactants?IgG +/- IgMComplement C3Where?In walls of glomerular capillaries.Pattern?LinearInterpretation?Anti-basement membrane disease (Goodpasture’s).
Example 3:Which immune reactants?IgAComplement C3Where?In mesangial stalks.Pattern?Granular
Interpretation ?Berger’s IgA disease
CLINICAL INDICATIONS FOR A RENAL BIOPSY
Proteinuria with impairment of renal function Symptomless isolated proteinuria if more than 2g/day Nephrotic syndrome in adults
In children it is most likely to be due to steroid sensitiveminimal change g/n so trial of steroids is less risky than biopsy.
Persistent haematuria with proteinuria especially if there is renal impairment. Acute renal failure if likely to be renal on origin, as opposed to pre-renal
(hypovolaemic shock) or post-renal (obstructive) in origin. Chronic renal failure with normal sized kidneys Renal transplant dysfunction Systemic disorders with features that include haematuria, proteinuria and
renal failure.

14
SOME CONTRA-INDICATIONS FOR A RENAL BIOPSY
Asymmetric kidneys Tiny shrunken kidneys Obstructive renal disease Coagulation defects Acute infections
(Dr. Bigornia’s Lecture)
5. Identify the different treatment modalities for acute glomerulonephritis.
5.1 Antibiotics Therapy
Streptococcal Pharyngitis (Including Scarlet Fever)
This is the most common disease produced by S. pyogenes (group A b-hemolytic streptococcus).
Penicillin-resistant isolates have yet to be observed for S. pyogenes. The preferred oral therapy is with penicillin V, 500 mg every 6 hours for 10
days. Equally good results are produced by the administration of 600,000 units of
penicillin G procaine intramuscularly once daily for 10 days or by a single injection of 1.2 million units of penicillin G benzathine.
Parenteral therapy is preferred if there are questions of patient compliance. Penicillin therapy of streptococcal pharyngitis reduces the risk of subsequent
acute rheumatic fever; however, current evidence suggests that the incidence of glomerulonephritis that follows streptococcal infections is not reduced to a significant degree by treatment with penicillin.
The administration of penicillin to individuals exposed to S. pyogenes affords protection from infection.
The oral ingestion of 200,000 units of penicillin G or penicillin V twice a day or a single injection of 1.2 million units of penicillin G benzathine is effective.
Indications for this type of prophylaxis include outbreaks of streptococcal disease in closed populations, such as boarding schools or military bases. Patients with extensive deep burns are at high risk of severe wound infections with S. pyogenes; "low dose" prophylaxis for several days appears to be effective in reducing the incidence of this complication.
(Goodman and Gilman’s The Pharmacological Basis of Therapeutics 11th Edition)
Therapy must cover all likely pathogens in the context of the clinical setting. Penicillin is the DOC in treating acute glomerulonephritis of a
poststreptococcal group A beta-hemolytic etiology.
Drug Name Penicillin V (Veetids)
Description
Derivative of 6-aminopenicillanic acid with a beta-lactam ring structure essential for bactericidal activity. Inhibits enzymes and cell wall receptors, resulting in cell wall synthesis inhibition. Other autolytics enzymes are also activated, degrading the bacterial cell wall. Bacterial resistance via beta-lactamase can be prevented with addition of clavulanic acid, sulbactam, or tazobactam. Other forms of bacterial resistance include alteration of bacterial PBPs and decreased permeability of cell wall to penicillin.
Adult Dose 500 mg PO q6h
Pediatric Dose<12 years: 40 mg/kg/d PO divided q4-6h; not to exceed adult dose>12 years: Administer as in adults
Contraindications
Documented hypersensitivity; renal function impairment; bleeding disorder; congestive heart failure; cystic fibrosis; GI disease or antibiotic-associated colitis; mononucleosis
Interactions
Probenecid may increase effectiveness by decreasing clearance; tetracyclines are bacteriostatic, causing decrease in effectiveness of penicillins when administered concurrently
PregnancyC - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus
Precautions Caution in renal impairment
(http://www.emedicine.com/emerg/topic219.htm)
5.2 Non-selective β-blocker with Cardioselective α-1 Blocker
β-ADRENERGIC RECEPTOR AGONISTS
Introduction
β-adrenergic receptor agonists have been utilized in many clinical settings but now play a major role only in the treatment of bronchoconstriction in patients with asthma (reversible airway obstruction) or chronic obstructive pulmonary disease (COPD).
Minor uses include management of preterm labor, treatment of complete heart block in shock, and short-term treatment of cardiac decompensation after surgery or in patients with congestive heart failure or myocardial infarction.
Epinephrine first was used as a bronchodilator at the beginning of the past century, and ephedrine was introduced into western medicine in 1924, although it had been used in China for thousands of years.
The next major advance was the development in the 1940s of isoproterenol, a b receptor-selective agonist; this provided a drug for asthma that lacked a receptor activity.
The recent development of b2-selective agonists has resulted in drugs with even more valuable characteristics, including adequate oral bioavailability, lack of a adrenergic activity, and diminished likelihood of some adverse cardiovascular effects.
β-receptor agonists may be used to stimulate the rate and force of cardiac contraction.
The chronotropic effect is useful in the emergency treatment of arrhythmias such as torsades de pointes, bradycardia, or heart block (see Chapter 34), whereas the inotropic effect is useful when it is desirable to augment myocardial contractility.
The therapeutic uses of b receptor agonists are discussed later in the chapter.
Isoproterenol
Isoproterenol (isopropylarterenol, isopropyl norepinephrine, isoprenaline, isopropyl noradrenaline, d,l-b-[3,4-dihydroxyphenyl]-a-isopropylaminoethanol) (Table 10-1) is a potent, nonselective b receptor agonist with very low affinity for a receptors.
Consequently, isoproterenol has powerful effects on all b receptors and almost no action at a receptors.
Pharmacological Actions
The major cardiovascular effects of isoproterenol (compared with epinephrine and norepinephrine) are illustrated in Figure 10-3.
Intravenous infusion of isoproterenol lowers peripheral vascular resistance, primarily in skeletal muscle but also in renal and mesenteric vascular beds.
Diastolic pressure falls. Systolic blood pressure may remain unchanged or rise, although mean
arterial pressure typically falls. Cardiac output is increased because of the positive inotropic and chronotropic
effects of the drug in the face of diminished peripheral vascular resistance. The cardiac effects of isoproterenol may lead to palpitations, sinus
tachycardia, and more serious arrhythmias; large doses of isoproterenol may cause myocardial necrosis in animals.
Isoproterenol relaxes almost all varieties of smooth muscle when the tone is high, but this action is most pronounced on bronchial and GI smooth muscle.
It prevents or relieves bronchoconstriction. Its effect in asthma may be due in part to an additional action to inhibit
antigen-induced release of histamine and other mediators of inflammation; this action is shared by b2 receptor-selective stimulants.
Absorption, Fate, and Excretion
Isoproterenol is readily absorbed when given parenterally or as an aerosol. It is metabolized primarily in the liver and other tissues by COMT. Isoproterenol is a relatively poor substrate for MAO and is not taken up by
sympathetic neurons to the same extent as are epinephrine and norepinephrine.
The duration of action of isoproterenol therefore may be longer than that of epinephrine, but it still is brief.
Toxicity and Adverse Effects
Palpitations, tachycardia, headache, and flushing are common. Cardiac ischemia and arrhythmias may occur, particularly in patients with
underlying coronary artery disease.
Therapeutic Uses
Isoproterenol (ISUPREL, others) may be used in emergencies to stimulate heart rate in patients with bradycardia or heart block, particularly in anticipation of inserting an artificial cardiac pacemaker or in patients with the ventricular arrhythmia torsades de pointes.
In disorders such as asthma and shock, isoproterenol largely has been replaced by other sympathomimetic drugs (see below and Chapter 27).
Dobutamine
Dobutamine resembles dopamine structurally but possesses a bulky aromatic substituent on the amino group (Table 10-1).
The pharmacological effects of dobutamine are due to direct interactions with α and β receptors; its actions do not appear to result from release of norepinephrine from sympathetic nerve endings, nor are they exerted via dopaminergic receptors.
Although dobutamine originally was thought to be a relatively selective β1 receptor agonist, it now is clear that its pharmacological effects are complex.
Dobutamine possesses a center of asymmetry; both enantiomeric forms are present in the racemic mixture used clinically.
The (-) isomer of dobutamine is a potent agonist at a1 receptors and is capable of causing marked pressor responses.

15
In contrast, (+)-dobutamine is a potent a1 receptor antagonist, which can block the effects of (-)-dobutamine. The effects of these two isomers are mediated via b receptors.
The (+) isomer is a more potent b receptor agonist than the (-) isomer (approximately tenfold). Both isomers appear to be full agonists.
Cardiovascular Effects
Cardiovascular effects of racemic dobutamine represent a composite of the distinct pharmacological properties of the (-) and (+) stereoisomers.
Dobutamine has relatively more prominent inotropic than chronotropic effects on the heart compared to isoproterenol.
Although not completely understood, this useful selectivity may arise because peripheral resistance is relatively unchanged.
Alternatively, cardiac a1 receptors may contribute to the inotropic effect. At equivalent inotropic doses, dobutamine enhances automaticity of the sinus
node to a lesser extent than does isoproterenol; however, enhancement of atrioventricular and intraventricular conduction is similar for both drugs.
In animals, administration of dobutamine at a rate of 2.5 to 15 mg/kg per minute increases cardiac contractility and cardiac output.
Total peripheral resistance is not greatly affected. The relatively constant peripheral resistance presumably reflects
counterbalancing of a1 receptor-mediated vasoconstriction and b2 receptor-mediated vasodilation (Ruffolo, 1987).
Heart rate increases only modestly when the rate of administration of dobutamine is maintained at less than 20 mg/kg per minute.
After administration of b receptor antagonists, infusion of dobutamine fails to increase cardiac output, but total peripheral resistance increases, confirming that dobutamine has modest direct effects on a adrenergic receptors in the vasculature.
Adverse Effects
In some patients, blood pressure and heart rate increase significantly during dobutamine administration; this may require reduction of the rate of infusion.
Patients with a history of hypertension may exhibit such an exaggerated pressor response more frequently.
Since dobutamine facilitates atrioventricular conduction, patients with atrial fibrillation are at risk of marked increases in ventricular response rates; digoxin or other measures may be required to prevent this from occurring. Some patients may develop ventricular ectopic activity.
As with any inotropic agent, dobutamine potentially may increase the size of a myocardial infarct by increasing myocardial oxygen demand.
This risk must be balanced against the patient's overall clinical status. The efficacy of dobutamine over a period of more than a few days is
uncertain; there is evidence for the development of tolerance.
Therapeutic Uses
Dobutamine (DOBUTREX, others) is indicated for the short-term treatment of cardiac decompensation that may occur after cardiac surgery or in patients with congestive heart failure or acute myocardial infarction. Dobutamine increases cardiac output and stroke volume in such patients, usually without a marked increase in heart rate.
Alterations in blood pressure or peripheral resistance usually are minor, although some patients may have marked increases in blood pressure or heart rate.
Clinical evidence of longer-term efficacy remains uncertain. An infusion of dobutamine in combination with echocardiography is useful in the noninvasive assessment of patients with coronary artery disease (Madu et al., 1994).
Stressing of the heart with dobutamine may reveal cardiac abnormalities in carefully selected patients.
Dobutamine has a half-life of about 2 minutes; the major metabolites are conjugates of dobutamine and 3-O-methyldobutamine.
The onset of effect is rapid. Consequently, a loading dose is not required, and steady-state
concentrations generally are achieved within 10 minutes of initiation of the infusion.
The rate of infusion required to increase cardiac output typically is between 2.5 and 10 mg/kg per minute, although higher infusion rates occasionally are required.
The rate and duration of the infusion are determined by the clinical and hemodynamic responses of the patient.
(Goodman and Gilman’s The Pharmacological Basis of Therapeutics 11th Edition)
Labetalol is used for hypertensive encephalopathy and malignant hypertension.
Drug Name Labetalol (Normodyne)
Description
Has nonselective beta-antagonist and cardioselective alpha1-antagonist effects. Beta-blocking effects predominate, particularly when used IV. Low lipid solubility means bioavailability is reduced by first pass metabolism and enhanced by coadministration of food. Drug is not removed by hemodialysis.
Adult Dose
20 mg (0.25 mg/kg for 80-kg patient) IV microdrip labetalol hydrochloride injection slowly over 2 min; desired BP may be achieved with continued injections of 40-80 mg at 10-min intervals or until 300 mg has been administered prn; to reduce possibility of postural hypotension, patients should remain supine for 3 h after administration
Pediatric DoseNot establishedSuggested dose: 0.4-1 mg/kg/h IV; not to exceed 3 mg/kg/h
Contraindications
Documented hypersensitivity; cardiogenic shock; atrioventricular block; uncompensated congestive heart failure; pulmonary edema; bradycardia; reactive airway disease; severe bradycardia
Interactions
Decreases effect of diuretics and increases toxicity of methotrexate, lithium, and salicylates; may diminish reflex tachycardia, resulting from nitroglycerin use, without interfering with hypotensive effects; cimetidine may increase labetalol blood levels; glutethimide may decrease labetalol effects by inducing microsomal enzymes
Pregnancy B - Fetal risk not confirmed in studies in humans but has been shown in some studies in animals
Precautions
Caution in impaired hepatic function; discontinue therapy if signs of liver dysfunction; in elderly patients, lower response rate and higher incidence of toxicity may be observed
(http://www.emedicine.com/emerg/topic219.htm)
5.3 Loop Diuretics
INHIBITORS OF NA+-K+-2CL- SYMPORT (LOOP DIURETICS, HIGH-CEILING DIURETICS)
Drugs in this group of diuretics inhibit the activity of the Na+-K+-2Cl- symporter in the thick ascending limb of the loop of Henle; hence these diuretics also are referred to as loop diuretics.
Although the proximal tubule reabsorbs approximately 65% of the filtered Na+, diuretics acting only in the proximal tubule have limited efficacy because the thick ascending limb has a great reabsorptive capacity and reabsorbs most of the rejectate from the proximal tubule.
Diuretics acting predominantly at sites past the thick ascending limb also have limited efficacy because only a small percentage of the filtered Na+ load reaches these more distal sites.
In contrast, inhibitors of Na+-K+-2Cl-symport in the thick ascending limb are highly efficacious, and for this reason, they sometimes are called high-ceiling diuretics.
The efficacy of inhibitors of Na+-K+-2Cl- symport in the thick ascending limb of the loop of Henle is due to a combination of two factors: (1) Approximately 25% of the filtered Na+ load normally is reabsorbed by the thick ascending limb, and (2) nephron segments past the thick ascending limb do not possess the reabsorptive capacity to rescue the flood of rejectate exiting the thick ascending limb.
Chemistry
Inhibitors of Na+-K+-2Cl- symport are a chemically diverse group. Only furosemide (LASIX), bumetanide (BUMEX), ethacrynic acid (EDECRIN),
and torsemide (DEMADEX) are available currently in the United States. Furosemide and bumetanide contain a sulfonamide moiety.
Ethacrynic acid is a phenoxyacetic acid derivative and torsemide is a sulfonylurea.
Mechanism and Site of Action
Inhibitors of Na+-K+-2Cl- symport act primarily in the thick ascending limb. Micropuncture of the DCT demonstrates that loop diuretics increase the
delivery of solutes out of the loop of Henle. Also, in situ microperfusion of the loop of Henle and in vitro microperfusion of
the CTAL indicate inhibition of transport by low concentrations of furosemide in the perfusate.
Some inhibitors of Na+-K+-2Cl- symport may have additional effects in the proximal tubule; however, the significance of these effects is unclear.
It was thought initially that Cl- was transported by a primary active electrogenic transporter in the luminal membrane independent of Na+.
Discovery of furosemide-sensitive Na+-K+-2Cl- symport in other tissues prompted a more careful investigation of the Na+ dependence of Cl- transport in the isolated perfused rabbit CTAL.
Scrupulous removal of Na+ from the luminal perfusate demonstrated the dependence of Cl- transport on Na+.
It is now well accepted that flux of Na+, K+, and Cl- from the lumen into the epithelial cells in the thick ascending limb is mediated by a Na+-K+-2Cl- symporter.
This symporter captures the free energy in the Na+ electrochemical gradient established by the basolateral Na+ pump and provides for "uphill" transport of K+ and Cl- into the cell.
K+ channels in the luminal membrane (called ROMK) provide a conductive pathway for the apical recycling of this cation, and basolateral Cl- channels (called CLC-Kb) provide a basolateral exit mechanism for Cl-.
The luminal membranes of epithelial cells in the thick ascending limb have a large conductive pathway (channels) for K+; therefore, the apical membrane

16
voltage is determined by the equilibrium potential for K+ (EK) and is hyperpolarized.
In contrast, the basolateral membrane has a large conductive pathway (channels) for Cl-, so the basolateral membrane voltage is less negative than EK; i.e., conductance for Cl- depolarizes the basolateral membrane.
Hyperpolarization of the luminal membrane and depolarization of the basolateral membrane result in a transepithelial potential difference of approximately 10 mV, with the lumen positive with respect to the interstitial space.
This lumen-positive potential difference repels cations (Na+, Ca2+, and Mg2+) and thereby provides an important driving force for the paracellular flux of these cations into the interstitial space.
As the name implies, inhibitors of Na+-K+-2Cl- symport bind to the Na+-K+-2Cl- symporter in the thick ascending limb and block its function, bringing salt transport in this segment of the nephron to a virtual standstill.
The molecular mechanism by which this class of drugs blocks the Na+-K+-2Cl- symporter is unknown, but evidence suggests that these drugs attach to the Cl--binding site.
Inhibitors of Na+-K+-2Cl- symport also inhibit Ca2+ and Mg2+ reabsorption in the thick ascending limb by abolishing the transepithelial potential difference that is the dominant driving force for reabsorption of these cations.
Na+-K+-2Cl- symporters are an important family of transport molecules found in many secretory and absorbing epithelia.
Effects on Urinary Excretion
Owing to blockade of the Na+-K+-2Cl- symporter, loop diuretics increase in the urinary excretion of Na+ and Cl- profoundly (i.e., up to 25% of the filtered load of Na+).
Abolition of the transepithelial potential difference also results in marked increases in the excretion of Ca2+ and Mg2+.
Some (e.g., furosemide) but not all (e.g., bumetanide) sulfonamide-based loop diuretics have weak carbonic anhydrase-inhibiting activity.
Drugs with carbonic anhydrase-inhibiting activity increase the urinary excretion of HCO3 - and phosphate. The mechanism by which inhibition of carbonic anhydrase increases phosphate excretion is not known.
All inhibitors of Na+-K+-2Cl- symport increase the urinary excretion of K+ and titratable acid.
This effect is due in part to increased delivery of Na+ to the distal tubule. The mechanism by which increased distal delivery of Na+ enhances excretion
of K+ and H+ is discussed in the section on inhibitors of Na+ channels. Other mechanisms contributing to enhanced K+ and H+ excretion include
flow-dependent enhancement of ion secretion by the collecting duct, nonosmotic vasopressin release, and activation of the renin-angiotensin-aldosterone axis.
Acutely, loop diuretics increase the excretion of uric acid, whereas chronic administration of these drugs results in reduced excretion of uric acid.
The chronic effects of loop diuretics on uric acid excretion may be due to enhanced transport in the proximal tubule secondary to volume depletion, leading to increased uric acid reabsorption, or to competition between the diuretic and uric acid for the organic acid secretory mechanism in the proximal tubule, leading to reduced uric acid secretion.
By blocking active NaCl reabsorption in the thick ascending limb, inhibitors of Na+-K+-2Cl- symport interfere with a critical step in the mechanism that produces a hypertonic medullary interstitium.
Therefore, loop diuretics block the kidney's ability to concentrate urine during hydropenia.
Also, since the thick ascending limb is part of the diluting segment, inhibitors of Na+-K+-2Cl- symport markedly impair the kidney's ability to excrete a dilute urine during water diuresis.
Effects on Renal Hemodynamics
If volume depletion is prevented by replacing fluid losses, inhibitors of Na+-K+-2Cl- symport generally increase total RBF and redistribute RBF to the midcortex.
However, the effects on RBF are variable. The mechanism of the increase in RBF is not known but may involve
prostaglandins. Nonsteroidal antiinflammatory drugs (NSAIDs) attenuate the diuretic
response to loop diuretics in part by preventing prostaglandin-mediated increases in RBF.
Loop diuretics block TGF by inhibiting salt transport into the macula densa so that the macula densa no longer can detect NaCl concentrations in the tubular fluid.
Therefore, unlike carbonic anhydrase inhibitors, loop diuretics do not decrease GFR by activating TGF.
Loop diuretics are powerful stimulators of renin release. This effect is due to interference with NaCl transport by the macula densa
and, if volume depletion occurs, to reflex activation of the sympathetic nervous system and to stimulation of the intrarenal baroreceptor mechanism.
Prostaglandins, particularly prostacyclin, may play an important role in mediating the renin-release response to loop diuretics.
Other Actions
Loop diuretics may cause direct vascular effects. Loop diuretics, particularly furosemide, acutely increase systemic venous
capacitance and thereby decrease left ventricular filling pressure. This effect, which may be mediated by prostaglandins and requires intact
kidneys, benefits patients with pulmonary edema even before diuresis ensues.
Furosemide and ethacrynic acid can inhibit Na+,K+-ATPase, glycolysis, mitochondrial respiration, the microsomal Ca2+ pump, adenylyl cyclase,
phosphodiesterase, and prostaglandin dehydrogenase; however, these effects do not have therapeutic implications.
In vitro, high doses of inhibitors of Na+-K+-2Cl- symport can inhibit electrolyte transport in many tissues.
Only in the inner ear, where alterations in the electrolyte composition of endolymph may contribute to drug-induced ototoxicity, is this effect important clinically.
Absorption and Elimination
Because furosemide, bumetanide, ethacrynic acid, and torsemide are bound extensively to plasma proteins, delivery of these drugs to the tubules by filtration is limited.
However, they are secreted efficiently by the organic acid transport system in the proximal tubule and thereby gain access to their binding sites on the Na+-K+-2Cl- symport in the luminal membrane of the thick ascending limb.
Probenecid shifts the plasma concentration-response curve to furosemide to the right by competitively inhibiting furosemide secretion by the organic acid transport system.
Approximately 65% of furosemide is excreted unchanged in the urine, and the remainder is conjugated to glucuronic acid in the kidney.
Accordingly, in patients with renal, but not liver, disease, the elimination half-life of furosemide is prolonged.
In contrast, bumetanide and torsemide have significant hepatic metabolism, so the elimination half-lives of these loop diuretics are prolonged by liver, but not renal, disease.
Although the average oral availability of furosemide is approximately 60%, oral availability of furosemide varies from 10% to 100%.
In contrast, oral availabilities of bumetanide and torsemide are reliably high. Heart failure patients have fewer hospitalizations and better quality of life with torsemide than with furosemide perhaps because of the more reliable absorption of torsemide (Shankar and Brater, 2003).
As a class, loop diuretics have short elimination half-lives, and prolonged-release preparations are not available. Consequently, often the dosing interval is too short to maintain adequate levels of loop diuretics in the tubular lumen. Note that torsemide has a longer t1/2 than other agents available in the United States.
As the concentration of loop diuretic in the tubular lumen declines, nephrons begin to avidly reabsorb Na+, which often nullifies the overall effect of the loop diuretic on total-body Na+.
This phenomenon of "postdiuretic Na+ retention" can be overcome by restricting dietary Na+ intake or by more frequent administration of the loop diuretic (Ellison, 1999).
Toxicity, Adverse Effects, Contraindications, Drug Interactions
Adverse effects unrelated to the diuretic efficacy are rare, and most adverse effects are due to abnormalities of fluid and electrolyte balance.
Overzealous use of loop diuretics can cause serious depletion of total-body Na+.
This may be manifest as hyponatremia and/or extracellular fluid volume depletion associated with hypotension, reduced GFR, circulatory collapse, thromboembolic episodes, and in patients with liver disease, hepatic encephalopathy.
Increased delivery of Na+ to the distal tubule, particularly when combined with activation of the renin-angiotensin system, leads to increased urinary excretion of K+ and H+, causing a hypochloremic alkalosis.
If dietary K+ intake is not sufficient, hypokalemia may develop, and this may induce cardiac arrhythmias, particularly in patients taking cardiac glycosides.
Increased Mg2+ and Ca2+ excretion may result in hypomagnesemia (a risk factor for cardiac arrhythmias) and hypocalcemia (rarely leading to tetany).
Recent evidence suggests that loop diuretics should be avoided in postmenopausal osteopenic women, in whom increased Ca2+ excretion may have deleterious effects on bone metabolism.
Loop diuretics can cause ototoxicity that manifests as tinnitus, hearing impairment, deafness, vertigo, and a sense of fullness in the ears.
Hearing impairment and deafness are usually, but not always, reversible. Ototoxicity occurs most frequently with rapid intravenous administration and
least frequently with oral administration. Ethacrynic acid appears to induce ototoxicity more often than do other loop
diuretics and should be used only in patients who cannot tolerate the other loop diuretics.
Loop diuretics also can cause hyperuricemia (occasionally leading to gout) and hyperglycemia (infrequently precipitating diabetes mellitus) and can increase plasma levels of low-density lipoprotein (LDL) cholesterol and triglycerides while decreasing plasma levels of high-density lipoprotein (HDL) cholesterol.
Other adverse effects include skin rashes, photosensitivity, paresthesias, bone marrow depression, and gastrointestinal disturbances.
Contraindications to the use of loop diuretics include severe Na+ and volume depletion, hypersensitivity to sulfonamides (for sulfonamide-based loop diuretics), and anuria unresponsive to a trial dose of loop diuretic.
Drug interactions may occur when loop diuretics are coadministered with (1) aminoglycosides (synergism of ototoxicity caused by both drugs), (2) anticoagulants (increased anticoagulant activity), (3) digitalis glycosides (increased digitalis-induced arrhythmias), (4) lithium (increased plasma levels of lithium), (5) propranolol (increased plasma levels of propranolol), (6) sulfonylureas (hyperglycemia), (7) cisplatin (increased risk of diuretic-induced ototoxicity), (8) NSAIDs (blunted diuretic response and salicylate toxicity when given with high doses of salicylates), (9) probenecid (blunted diuretic response), (10) thiazide diuretics (synergism of diuretic activity of both drugs leading to profound diuresis), and (11) amphotericin B (increased potential for nephrotoxicity and toxicity and intensification of electrolyte imbalance).

17
Therapeutic Uses
A major use of loop diuretics is in the treatment of acute pulmonary edema. A rapid increase in venous capacitance in conjunction with a brisk natriuresis
reduces left ventricular filling pressures and thereby rapidly relieves pulmonary edema. Loop diuretics also are used widely for the treatment of chronic congestive heart failure when diminution of extracellular fluid volume is desirable to minimize venous and pulmonary congestion.
In this regard, a meta-analysis of randomized clinical trials demonstrates that diuretics cause a significant reduction in mortality and the risk of worsening heart failure, as well as an improvement in exercise capacity.
Diuretics are used widely for the treatment of hypertension and controlled clinical trials demonstrating reduced morbidity and mortality have been conducted with Na+-Cl- symport (thiazides and thiazidelike diuretics) but not Na+-K+-2Cl- symport inhibitors.
Nonetheless, Na+-K+-2Cl- symport inhibitors appear to lower blood pressure as effectively as Na+-Cl- symport inhibitors while causing smaller perturbations in the lipid profile.
However, the short elimination half-lives of loop diuretics render them less useful for hypertension than thiazide-type diuretics.
The edema of nephrotic syndrome often is refractory to other classes of diuretics, and loop diuretics often are the only drugs capable of reducing the massive edema associated with this renal disease.
Loop diuretics also are employed in the treatment of edema and ascites of liver cirrhosis; however, care must be taken not to induce encephalopathy or hepatorenal syndrome. In patients with a drug overdose, loop diuretics can be used to induce a forced diuresis to facilitate more rapid renal elimination of the offending drug.
Loop diuretics, combined with isotonic saline administration to prevent volume depletion, are used to treat hypercalcemia.
Loop diuretics interfere with the kidney's capacity to produce a concentrated urine.
Consequently, loop diuretics combined with hypertonic saline are useful for the treatment of life-threatening hyponatremia.
Loop diuretics also are used to treat edema associated with chronic renal insufficiency.
However, animal studies have demonstrated that loop diuretics increase PGC by activating the renin-angiotensin system, an effect that could accelerate renal injury.
Most patients with ARF receive a trial dose of a loop diuretic in an attempt to convert oliguric ARF to nonoliguric ARF.
However, there is no evidence that loop diuretics prevent ATN or improve outcome in patients with ARF.
(Goodman and Gilman’s The Pharmacological Basis of Therapeutics 11th Edition)
Loop diuretics are used for hypertensive encephalopathy with CNS signs and circulatory congestion or pulmonary edema.
Furosemide is DOC for this indication.
Drug Name Furosemide (Lasix)
Description
Inhibits resorption of sodium and water in ascending limb of loop of Henle by interfering with Na+/K+/Cl- channel. An antihypercalcemic effect is mediated by an increased excretion of calcium.Plasma volume, blood pressure, and cardiac output are reduced. Calcium excretion is increased.Absorption of oral furosemide is reduced with renal disease or nephrotic syndrome as a result of edematous bowel. Parenteral administration may be indicated in patients with compromised kidneys; metabolized by hepatic biotransformation and renal excretion; onset of action is 20-60 min PO and 5 min IV. Children with nephrotic syndrome may require higher dosing beyond the scope of ED care.
Adult Dose20-80 mg PO/IV once initially, followed by once qd, or once qod after titrating for optimum efficacy, or by dividing daily dose bid/tid
Pediatric Dose Initially: 2 mg/kg PO/IV once; titrate with additional 1-2 mg/kg q6h.
ContraindicationsDocumented hypersensitivity; hepatic coma; anuria; renal function impairment; diabetes mellitus; gout; MI; pancreatitis; state of severe electrolyte depletion.
Interactions
Metformin decreases furosemide concentrations; furosemide interferes with hypoglycemic effect of antidiabetic agents and antagonizes muscle-relaxing effect of tubocurarine; auditory toxicity appears to be increased with coadministration of aminoglycosides and furosemide (hearing loss of varying degrees may occur); anticoagulant activity of warfarin may be enhanced when taken concurrently; increased plasma lithium levels and toxicity are possible when taken concurrently.
(http://www.emedicine.com/emerg/topic219.htm)
5.4 Corticosteroids
General Mechanisms for Corticosteroid Effects
Corticosteroids interact with specific receptor proteins in target tissues to regulate the expression of corticosteroid-responsive genes, thereby changing the levels and array of proteins synthesized by the various target tissues.
As a consequence of the time required to modulate gene expression and protein synthesis, most effects of corticosteroids are not immediate but become apparent after several hours.
This fact is of clinical significance, because a delay generally is seen before beneficial effects of corticosteroid therapy become manifest.
Although corticosteroids predominantly act to increase expression of target genes, there are well-documented examples in which glucocorticoids decrease transcription of target genes.
The receptors for corticosteroids are members of the nuclear receptor family of transcription factors that transduce the effects of a diverse array of small, hydrophobic ligands, including the steroid hormones, thyroid hormone, vitamin D, and retinoids.
These receptors share two highly conserved domains: a region of approximately 70 amino acids forming two zinc-binding domains, called zinc fingers, that are essential for the interaction of the receptor with specific DNA sequences, and a region at the carboxyl terminus that interacts with ligand (the ligand-binding domain).
Although complete loss of glucocorticoid receptor (GR) function apparently is lethal, mutations leading to partial loss of GR function have been identified in rare patients with generalized glucocorticoid resistance.
These patients harbor mutations in the GR that impair glucocorticoid binding and decrease transcriptional activation.
As a consequence of these mutations, cortisol levels that normally mediate feedback inhibition fail to suppress the HPA axis completely.
In this setting of partial loss of GR function, the HPA axis resets to a higher level to provide compensatory increases in ACTH and cortisol secretion.
Because the GR defect is partial, adequate compensation for the end-organ insensitivity can result from the elevated cortisol level, but the excess
ACTH secretion also stimulates the production of mineralocorticoids and adrenal androgens.
Because the mineralocorticoid receptor (MR) and the androgen receptor are intact, these subjects present with manifestations of mineralocorticoid excess (hypertension and hypokalemic alkalosis) and/or of increased androgen levels (acne, hirsutism, male pattern baldness, menstrual irregularities, anovulation, and infertility). In children, the excess adrenal androgens can cause precocious sexual development.
Glucocorticoids
Patients with nephrotic syndrome secondary to minimal change disease generally respond well to steroid therapy, and glucocorticoids clearly are the first-line treatment in both adults and children. Initial daily doses of prednisone are 1 to 2 mg/kg for 6 weeks, followed by a gradual tapering of the dose over 6 to 8 weeks, although some nephrologists advocate alternate-day therapy.
Objective evidence of response, such as diminished proteinuria, is seen within 2 to 3 weeks in 85% of patients, and more than 95% of patients will have remission within 3 months.
Cessation of steroid therapy frequently is complicated by disease relapse, as manifested by recurrent proteinuria.
Patients who relapse repeatedly are termed steroid-resistant and often are treated with other immunosuppressive drugs such as azathioprine or cyclophosphamide.
Patients with renal disease secondary to systemic lupus erythematosus also are generally given a therapeutic trial of glucocorticoids.
Studies with other forms of renal disease, such as membranous and membranoproliferative glomerulonephritis and focal sclerosis, have provided conflicting data on the role of glucocorticoids.
In clinical practice, patients with these disorders often are given a therapeutic trial of glucocorticoids with careful monitoring of laboratory indices of response. In the case of membranous glomerulonephritis, many nephrologists recommend a trial of alternate-day glucocorticoids for 8 to 10 weeks (e.g., prednisone, 120 mg every other day), followed by a 1- to 2-month period of tapering.

18
Ethylprednisolone is used for nonstreptococcal etiologies of acute glomerulonephritis, particularly in lupus nephritis and in idiopathic progressive glomerulonephritis.
(Goodman and Gilman’s The Pharmacological Basis of Therapeutics 11th Edition)
Drug Name Methylprednisolone (Medrol)
Description
Has anti-inflammatory effect and is immunosuppressive. Metabolized by hepatic transformation and renal excretion.
Adult Dose Pulse therapy of 30 mg/kg IV over minimum of 30 min
Pediatric Dose Administer as in adults
Contraindications Documented hypersensitivity; viral, fungal, or tubercular skin infections
Interactions
Coadministration with digoxin may increase digitalis toxicity secondary to hypokalemia; estrogens may increase levels of methylprednisolone; phenobarbital, phenytoin, and rifampin may decrease levels of methylprednisolone (adjust dose); monitor patients for hypokalemia when taking medication concurrently with diuretics
PregnancyC - Fetal risk revealed in studies in animals but not established or not studied in humans; may use if benefits outweigh risk to fetus
Precautions
Adverse effects include allergy, cataracts, Cushing syndrome, severe acne, GI irritation, and pancreatitisHyperglycemia, edema, osteonecrosis, peptic ulcer disease, hypokalemia, osteoporosis, euphoria, psychosis, growth suppression, myopathy, and infections are possible complications of glucocorticoid use
(http://www.emedicine.com/emerg/topic219.htm)
5.5 Antineoplastics and Immunosuppressants
Antineoplastic Agents (Cyclophosphamide)
Pharmacological and Cytotoxic Actions
The general cytotoxic action of this drug is similar to that of other alkylating agents.
The drug is not a vesicant, and produces no local irritation.
Absorption, Fate, and Excretion
Cyclophosphamide is well absorbed orally. The drug is activated by CYP2B to 4-hydroxycyclophosphamide, which is in a
steady state with the acyclic tautomer aldophosphamide. A closely related oxazaphosphorine, ifosfamide, is hydroxylated by CYP3A4. This difference may account for the somewhat slower activation of ifosfamide
in vivo, and the interpatient variability in toxicity of both molecules. The rate of metabolic activation of cyclophosphamide exhibits significant
interpatient variability and increases with successive doses in high-dose regimens, but appears to be saturable above infusion rates of 4 g/90 minutes and concentrations of parent compound above 150 uM.
4-Hydroxycyclophosphamide may be oxidized further by aldehyde oxidase, either in liver or in tumor tissue, and perhaps by other enzymes, yielding the inactive metabolites carboxyphosphamide and 4-ketocyclophosphamide, and ifosfamide is inactivated in an analogous reaction.
The active cyclophosphamide metabolites such as 4-hydroxycyclophosphamide and its tautomer, aldophosphamide, are carried in the circulation to tumor cells where aldophosphamide cleaves spontaneously, generating stoichiometric amounts of phosphoramide mustard and acrolein.
Phosphoramide mustard is responsible for antitumor effects, while acrolein causes hemorrhagic cystitis often seen during therapy with cyclophosphamide.
As mentioned above, cystitis can be reduced in intensity or prevented by the parenteral coadministration of mesna. Mesna does not negate the systemic antitumor activity of the drug.
For routine clinical use, ample fluid intake is recommended and vigorous intravenous hydration is required during high-dose treatment.
Brisk hematuria in a patient receiving daily oral therapy should lead to immediate drug discontinuation.
Refractory bladder hemorrhage may require cystectomy for control of bleeding.
The syndrome of inappropriate secretion of antidiuretic hormone has been observed in patients receiving cyclophosphamide, usually at doses higher than 50 mg/kg.
It is important to be aware of the possibility of water intoxication, since these patients usually are vigorously hydrated to prevent bladder toxicity.
Pretreatment with CYP inducers such as phenobarbital enhances the rate of activation of the azoxyphosphorenes but does not alter total exposure to active metabolites over time and does not affect toxicity or therapeutic activity in humans.
Cyclophosphamide can be used in full doses in patients with renal dysfunction, as it is eliminated by hepatic metabolism.
Urinary and fecal recovery of unchanged cyclophosphamide is minimal after intravenous administration. Maximal concentrations in plasma are achieved 1 hour after oral administration, and the half-life of parent drug in plasma is about 7 hours.
Therapeutic Uses
Cyclophosphamide (CYTOXAN, NEOSAR, others) is administered orally or intravenously.
Recommended doses vary widely, and published protocols for the dosage of cyclophosphamide and other chemotherapeutic agents and for the method and sequence of administration should be consulted.
As a single agent, a daily oral dose of 100 mg/m2 for 14 days has been recommended as adjuvant therapy for breast cancer, and for patients with lymphomas and chronic lymphocytic leukemia.
A higher dosage of 500 mg/m2 intravenously every 2 to 4 weeks in combination with other drugs often is employed in the treatment of breast cancer and lymphomas.
The neutrophil nadir of 500 to 1000 cells per mm3 generally serves as a guide to dosage adjustments in prolonged therapy. In regimens associated with bone marrow or peripheral stem cell rescue, cyclophosphamide may be given in total doses of 5 to 7 g/m2 over a 3- to 5-day period.
Gastrointestinal ulceration, cystitis (counteracted by mesna and diuresis), and less commonly pulmonary, renal, hepatic, and cardiac toxicities (a hemorrhagic myocardial necrosis) may occur after high-dose therapy with total doses above 200 mg/kg.
The clinical spectrum of activity for cyclophosphamide is very broad. It is an essential component of many effective drug combinations for non-
Hodgkin's lymphomas, ovarian cancers, and solid tumors in children. Complete remissions and presumed cures have been reported when
cyclophosphamide was given as a single agent for Burkitt's lymphoma. It frequently is used in combination with methotrexate (or doxorubicin) and
fluorouracil as adjuvant therapy after surgery for carcinoma of the breast. Because of its potent immunosuppressive properties, cyclophosphamide has
been used to prevent organ rejection after transplantation. It has activity in nonneoplastic disorders associated with altered immune
reactivity, including Wegener's granulomatosis, rheumatoid arthritis, and the nephrotic syndrome.
Caution is advised when the drug is considered for use in these conditions, not only because of its acute toxic effects but also because of its potential for inducing sterility, teratogenic effects, and leukemia.
Immunosuppressants
Calcineurin Inhibitors
Perhaps the most effective immunosuppressive drugs in routine use are the calcineurin inhibitors, cyclosporine and tacrolimus, which target intracellular signaling pathways induced as a consequence of T-cell-receptor activation.
Although they are structurally unrelated and bind to distinct, albeit related molecular targets, they inhibit normal T-cell signal transduction essentially by the same mechanism.
Cyclosporine and tacrolimus do not act per se as immunosuppressive agents. Instead, these drugs bind to an immunophilin (cyclophilin for cyclosporine or
FKBP-12 for tacrolimus), resulting in subsequent interaction with calcineurin to block its phosphatase activity.
Calcineurin-catalyzed dephosphorylation is required for movement of a component of the nuclear factor of activated T lymphocytes (NFAT) into the nucleus.
NFAT, in turn, is required to induce a number of cytokine genes, including that for interleukin-2 (IL-2), a prototypic T-cell growth and differentiation factor.
Cyclosporine
Chemistry
Cyclosporine (cyclosporin A), a cyclic polypeptide consisting of 11 amino acids, is produced by the fungus species Beauveria nivea.
Of note, all amide nitrogens are either hydrogen bonded or methylated, the single D-amino acid is at position 8, the methyl amide between residues 9 and 10 is in the cis configuration, and all other methyl amide moieties are in the trans form.
Because cyclosporine is lipophilic and highly hydrophobic, it is formulated for clinical administration using castor oil or other strategies to ensure solubilization.
Mechanism of Action
Cyclosporine suppresses some humoral immunity, but is more effective against T-cell-dependent immune mechanisms such as those underlying transplant rejection and some forms of autoimmunity.
It preferentially inhibits antigen-triggered signal transduction in T lymphocytes, blunting expression of many lymphokines including IL-2, and the expression of antiapoptotic proteins.
Cyclosporine forms a complex with cyclophilin, a cytoplasmic receptor protein present in target cells.
This complex binds to calcineurin, inhibiting Ca2+-stimulated dephosphorylation of the cytosolic component of NFAT.
When cytoplasmic NFAT is dephosphorylated, it translocates to the nucleus and complexes with nuclear components required for complete T-cell activation, including transactivation of IL-2 and other lymphokine genes.
Calcineurin phosphatase activity is inhibited after physical interaction with the cyclosporine/cyclophilin complex.

19
This prevents NFAT dephosphorylation such that NFAT does not enter the nucleus, gene transcription is not activated, and the T lymphocyte fails to respond to specific antigenic stimulation.
Cyclosporine also increases expression of transforming growth factor-b (TGF-b), a potent inhibitor of IL-2-stimulated T-cell proliferation and generation of cytotoxic T lymphocytes (CTL).
Disposition and Pharmacokinetics
Cyclosporine can be administered intravenously or orally. The intravenous preparation (SANDIMMUNE Injection) is provided as a
solution in an ethanol-polyoxyethylated castor oil vehicle that must be further diluted in 0.9% sodium chloride solution or 5% dextrose solution before injection.
The oral dosage forms include soft gelatin capsules and oral solutions. Cyclosporine supplied in the original soft gelatin capsule (SANDIMMUNE) is absorbed slowly with 20% to 50% bioavailability.
A modified microemulsion formulation (NEORAL) is available. It has more uniform and slightly increased bioavailability compared to
SANDIMMUNE and is provided as 25-mg and 100-mg soft gelatin capsules and a 100-mg/ml oral solution.
Since SANDIMMUNE and NEORAL are not bioequivalent, they cannot be used interchangeably without supervision by a physician and monitoring of drug concentrations in plasma.
Comparison of blood concentrations in published literature and in clinical practice must be performed with a detailed knowledge of the assay system employed.
Generic preparations of both NEORAL and SANDIMMUNE are available that are bioequivalent by FDA criteria.
The generic preparations for NEORAL have been shown to be bioequivalent in normal volunteers, and, in some studies, also in transplant recipients.
After oral administration of cyclosporine (as NEORAL), the time to peak blood concentrations is 1.5 to 2 hours.
Administration with food delays and decreases absorption. High- and low-fat meals consumed within 30 minutes of administration
decrease the AUC by approximately 13% and the maximum concentration by 33%. This makes it imperative to individualize dosage regimens for outpatients.
Cyclosporine is distributed extensively outside the vascular compartment. After intravenous dosing, the steady-state volume of distribution is reportedly
as high as 3 to 5 L/kg in solid-organ transplant recipients. Only 0.1% of cyclosporine is excreted unchanged in urine. Cyclosporine is extensively metabolized in the liver by CYP3A and to a lesser
degree by the gastrointestinal tract and kidneys (Fahr, 1993). At least 25 metabolites have been identified in human bile, feces, blood, and
urine. Although the cyclic peptide structure of cyclosporine is relatively resistant to
metabolism, the side chains are extensively metabolized. All of the metabolites have reduced biological activity and toxicity compared
to the parent drug. Cyclosporine and its metabolites are excreted principally through the bile into
the feces, with only about 6% being excreted in the urine. Cyclosporine also is excreted in human milk. In the presence of hepatic dysfunction, dosage adjustments are required. No
adjustments generally are necessary for dialysis or renal failure patients.
Therapeutic Uses
Clinical indications for cyclosporine are kidney, liver, heart, and other organ transplantation; rheumatoid arthritis; and psoriasis.
Cyclosporine generally is recognized as the agent that ushered in the modern era of organ transplantation, increasing the rates of early engraftment, extending kidney graft survival, and making cardiac and liver transplantation possible.
Cyclosporine usually is combined with other agents, especially glucocorticoids and either azathioprine or mycophenolate mofetil, and most recently, sirolimus.
The dose of cyclosporine varies, depending on the organ transplanted and the other drugs used in the specific treatment protocol(s).
The initial dose generally is not given before the transplant because of the concern about nephrotoxicity.
Especially for renal transplant patients, therapeutic algorithms have been developed to delay cyclosporine introduction until a threshold renal function has been attained.
The amount of the initial dose and reduction to maintenance dosing is sufficiently variable that no specific recommendation is provided here.
Dosage is guided by signs of rejection (too low a dose), renal or other toxicity (too high a dose), and close monitoring of blood levels. Great care must be taken to differentiate renal toxicity from rejection in kidney transplant patients.
Ultrasound-guided allograft biopsy is the best way to assess the reason for renal dysfunction.
Because adverse reactions have been ascribed more frequently to the intravenous formulation, this route of administration is discontinued as soon as the patient is able to take the drug orally.
Toxicity
The principal adverse reactions to cyclosporine therapy are renal dysfunction, tremor, hirsutism, hypertension, hyperlipidemia, and gum hyperplasia.
Hyperuricemia may lead to worsening of gout, increased P-glycoprotein activity, and hypercholesterolemia.
Nephrotoxicity occurs in the majority of patients treated and is the major indication for cessation or modification of therapy.
Hypertension occurs in approximately 50% of renal transplant and almost all cardiac transplant patients.
Combined use of calcineurin inhibitors and glucocorticoids is particularly diabetogenic, although this apparently is more problematic in patients treated with tacrolimus (see below).
Especially at risk are obese patients, African-American or Hispanic recipients, or those with family history of type II diabetes or obesity.
Cyclosporine, as opposed to tacrolimus, is more likely to produce elevations in LDL cholesterol.
(Goodman and Gilman’s The Pharmacological Basis of Therapeutics 11th Edition)
Cyclophosphamide is used for etiology-dependent treatment of acute glomerulonephritis due to Wegener granulomatosis.
Drug Name Cyclophosphamide (Cytoxan, Neosar, Procytox)
Description
Acts as alkylating agent that cross-links strands of DNA and RNA. Other actions include inhibition of protein synthesis, immunosuppression, and cholinesterase inhibition. Not within the scope of ED care.
Adult Dose
For long-term therapy, the following doses are used:400-1800 mg/m2 (30-40 mg/kg) IV in divided doses over 2-5 d; may repeat at 2- to 4-wk intervals; alternatively, 10-15 mg/kg IV q7-10d or 3-5 mg/kg twice weekly
Pediatric Dose Long-term therapy: Administer as in adults
Contraindications Documented hypersensitivity; severely depressed bone marrow function
Interactions
Allopurinol may increase risk of bleeding or infection and enhance myelosuppressive effects; may potentiate doxorubicin-induced cardiotoxicity; may reduce digoxin serum levels and antimicrobial effects of quinolonesChloramphenicol may increase half-life while decreasing metabolite concentrations; may increase effect of anticoagulants; coadministration with high doses of phenobarbital may increase rate of metabolism and leukopenic activity; thiazide diuretics may prolong cyclophosphamide-induced leukopenia and neuromuscular blockade by inhibiting cholinesterase activity
Pregnancy D - Fetal risk shown in humans; use only if benefits outweigh risk to fetus
Precautions
Regularly examine hematologic profile (particularly neutrophils and platelets) to monitor for hematopoietic suppression; regularly examine urine for RBCs, which may precede hemorrhagic cystitis
(http://www.emedicine.com/emerg/topic219.htm)
6. Identify the complications of glomerulonephritis.
Progression of Glomerular Disease
Persistent glomerulonephritis that worsens renal function is always accompanied by interstitial nephritis, renal fibrosis, and tubular atrophy.
What is not so obvious, however, is that renal failure in glomerulonephritis best correlates histologically with the appearance of tubulointerstitial nephritis rather than with the type of inciting glomerular injury.
Loss of renal function due to interstitial damage can be explained hypothetically by several mechanisms.
The simplest explanation is that urine flow is impeded by tubular obstruction as a result of interstitial inflammation and fibrosis.
Thus, obstruction of the tubules with debris or by extrinsic compression results in aglomerular nephrons.
A second mechanism suggests that interstitial changes, including interstitial edema or fibrosis, alter tubular and vascular architecture and thereby compromise the normal tubular transport of solutes and water from tubular lumen to vascular space.
This failure increases the solute and water content of the tubule fluid, resulting in isothenuria and polyuria.
Adaptive mechanisms related to tubuloglomerular feedback also fail, resulting in a reduction of renin output from the juxtaglomerular apparatus of glomeruli trapped by interstitial inflammation.
Consequently, the local vasoconstrictive influence of angiotensin II on the glomerular arterioles decreases, and filtration drops owing to a generalized decrease in arteriolar tone.
A third mechanism involves changes in vascular resistance due to damage of peritubular capillaries.
The cross-sectional volume of these capillaries is decreased in areas of interstitial inflammation, edema, or fibrosis.
These structural alterations in vascular resistance affect renal function through two mechanisms.
First, tubular cells are very metabolically active, and, as a result, decreased perfusion could lead to ischemic injury.

20
Second, impairment of glomerular arteriolar outflow leads to increased intraglomerular hypertension in less involved glomeruli; this selective intraglomerular hypertension aggravates and extends mesangial sclerosis and glomerulosclerosis to less-involved glomeruli.
Regardless of the exact mechanism, early acute tubulointerstitial nephritis suggests potentially recoverable renal function, while the development of chronic interstitial fibrosis prognosticates a permanent loss.
Persistent damage to glomerular capillaries spreads to the tubulointerstitium in association with proteinuria.
There is an untested hypothesis that efferent arterioles leading from inflamed glomeruli carry forward inflammatory mediators, which induces downstream interstitial nephritis, resulting in fibrosis.
Glomerular filtrate from injured glomerular capillaries adherent to Bowman's capsule may also be misdirected to the periglomerular interstitium.
Most nephrologists believe, however, that proteinuric glomerular filtrate forming tubular fluid is the primary route to downstream tubulointerstitial injury, although none of these hypotheses are mutually exclusive.
The simplest explanation for the effect of proteinuria on the development of interstitial nephritis is that increasingly severe proteinuria, carrying activated cytokines and lipoproteins producing reactive oxygen species, triggers a downstream inflammatory cascade in and around epithelial cells lining the tubular nephron.
These effects induce T lymphocyte and macrophage infiltrates in the interstitial spaces along with fibrosis and tubular atrophy.
Tubules disappear following direct damage to their basement membranes, leading to decondensation and epithelial-mesenchymal transitions forming more interstitial fibroblasts at the site of injury.
Transforming growth factor (TGF-), fibroblast growth factor 2, and platelet-derived growth factor (PDGF) are particularly active in this transition.
With persistent nephritis, fibroblasts multiply and lay down tenascin and a fibronectin scaffold for the polymerization of new interstitial collagens I/III.
These events form scar tissue through a process called fibrogenesis. In experimental studies, bone morphogenetic protein 7 and hematopoietic growth
factor can reverse early fibrogenesis and preserve tubular architecture. When fibroblasts outdistance their survival factors, they apoptose, and the
permanent renal scar becomes acellular, leading to irreversible renal failure.
(Harrison's Principles of Internal Medicine 17th Edition)
Complications
Progression to sclerosis is rare in the typical patient; however, in 0.5-2% of patients with acute glomerulonephritis, the course progresses toward renal failure, resulting in kidney death in a short period.
Abnormal urinalysis (ie, microhematuria) may persist for years. Marked decline in glomerular filtration rate is rare. Other complications, resulting in relevant end-organ damage in the central nervous
and cardiopulmonary systems, can develop in patients who present with severe hypertension, encephalopathy, and pulmonary edema.
Those complications include the following: Hypertensive retinopathy Hypertensive encephalopathy Rapidly progressive glomerulonephritis Chronic renal failure
(http://www.emedicine.com/emerg/topic219.htm)
7. Identify the prognosis of acute glomerulonephritis.
Prognosis (Acute Glomerulonephritis)
Acute poststreptococcal glomerulonephritis resolves completely in most cases, especially in children.
About 0.1% of children and 25% of adults develop chronic kidney failure. The prognosis for people with rapidly progressive glomerulonephritis depends on the
severity of glomerular scarring and whether the underlying disease, such as infection, can be cured.
In about 75% of the people who are treated early (within weeks to a few months), kidney function is preserved and dialysis is not needed.
However, because the early symptoms can be subtle and vague, many people who have rapidly progressive glomerulonephritis are not aware of the underlying disease and do not seek medical care until kidney failure develops.
If treatment occurs late, the person is more likely to develop chronic kidney failure. The prognosis also depends on the cause, the person's age, and any other diseases
the person might have. When the cause is unknown or the person is older, the prognosis is worse.
In some children and adults who do not recover completely from acute glomerulonephritis, other types of kidney disorders develop, such as asymptomatic proteinuria and hematuria syndrome or nephrotic syndrome.
Other people with acute glomerulonephritis, especially older adults, often develop chronic glomerulonephritis.
(http://www.merck.com/mmhe/sec11/ch144/ch144b.html)
Prognosis (Acute Post-Streptococcal Glomerulonephritis) In poststreptococcal nephritis, the long-term prognosis generally is good. More than
98% of individuals are asymptomatic after 5 years, with chronic renal failure reported 1-3% of the time.
The prognosis for nonstreptococcal postinfectious glomerulonephritis depends on the underlying agent, which must be identified and addressed.
Generally, the prognosis is worse in patients with heavy proteinuria, severe hypertension, and significant elevations of creatinine level.
Other causes of acute glomerulonephritis have outcomes varying from complete recovery to complete renal failure. Prognosis depends on the underlying disease and the overall health of the patient.
Occurrence of cardiopulmonary or neurologic complications worsens the prognosis.
(http://www.emedicine.com/emerg/topic219.htm)
Prognosis (Acute Post-Streptococcal Glomerulonephritis)
Epidemic PSAGN appears to end in virtually complete resolution and healing in all patients, and the prognosis is favorable for most children with sporadic PSAGN. Edema usually resolves within 5-10 days, and BP usually returns to normal
after 2-3 weeks, even though persistence of elevated pressures for as many as 6 weeks is compatible with complete resolution.
Gross hematuria usually disappears within 1-3 weeks but may be exacerbated by physical activity.
C3 concentration returns to normal in more than 95% of patients by the end of 8-10 weeks.
Urinary abnormalities resolve at various times after onset. Proteinuria may disappear within the first 2-3 months or may decrease slowly
over 6 months. Intermittent or postural proteinuria has been noted for 1-2 years after onset.
Microscopic hematuria usually disappears after 6 months, but its presence for as long as 1 year should not cause undue concern, and even more prolonged hematuria (1-3 y) has been observed.
Consider the possibility of chronic renal disease when both hematuria and proteinuria persist longer than 12 months.
The ultimate prognosis in individuals with PSAGN depends largely on the severity of the initial insult. In a few hospitalized patients, the initial injury is so severe that either
persistent renal failure or progressive renal failure ensues. Clinical manifestations of the disease rarely recur after the first 3 months, and
second episodes of AGN are rare.
(http://www.emedicine.com/ped/topic27.htm)