fracture healing 2
-
Upload
faris-aziz-pridianto -
Category
Documents
-
view
58 -
download
9
description
Transcript of fracture healing 2

72
1 Biology of fracture healing in unstable fractures 73
1.1 Healing under uncontrolled motion 731.1.1 Inflammatory phase 731.1.2 Repair phase 751.1.3 Remodeling phase 761.2 Healing under restricted motion 77
2 Biology of fracture healing in stable fractures 78
2.1 Contact healing 792.2 Gap healing 80
3 Stimulation of fracture healing 81
4 Radiographic evaluation of fracture healing 88
5 Implant removal 91
6 Bibliography 92
3 Fracture healing

73
Author Dominique J Griffon
3 Fracture healing
Fracture healing shares many similarities with soft-tissue healing but its ability to be completed without the formation of a scar is unique. There-fore, any tissue other than bone which persists within a fracture gap represents incomplete healing [1]. If adequate vascularity is present, the pattern of fracture repair is dictated by the bio mechanical environment. Indeed, bone can only be produced after restoration of mechanical stability. This may be achieved by a natural process of healing or by osteosynthesis, with partial or complete stabilization of the frac-ture fragments. These healing mechanisms have unique histological features, and each can occur in isolation or in concert with the other to achieve bone union.
1 Biology of fracture healing in unstable fractures
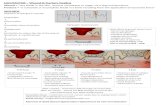
Healing of unstable fractures is characterized by the formation of an intermediate callus prior to bone formation. This type of healing is referred to as indirect or secondary healing. It is arbitrarily divided into three overlapping phases: infl amma-tion, repair, and remodeling (Fig 3-1). This passage through different tissue stages of increasing stiff-ness and strength eventually leads to a biome-chanical environment permitting bone formation and union. The amount of callus produced depends on the stability of the fracture, and increases with greater instability.
1.1 Healing under uncontrolled motion
Spontaneous healing of complete fractures typically occurs in the presence of highly unstable fragment ends. Bone repair must develop in spite of high
interfragmentary strain, defi ned as the deformation occurring at the fracture site relative to the size of the gap. However, bone formation can only occur in a stable biomechanical environment with an interfragmentary strain lower than 2% [2, 3]. Various processes occur to overcome this un-favorable situation. They include initial contraction of muscles surrounding the fracture, resorption of fragment ends, orderly repair with tissues suitable for the mechanical envi ronment, and formation of a prominent external callus.
1.1.1 Infl ammatory phaseThe infl ammatory phase begins immediately after the initial disruption of bone and surrounding soft tissues, and persists until the formation of cartilage or bone is initiated. This phase therefore lasts 3–4 days and potentially longer, depending on the amount of force that caused the fracture. Clinically, the end of the infl ammatory stage coincides with a decrease in pain and swelling.
Fractures inevitably cause a disruption of the medullary vessels and subsequent extravasation of blood. Although contraction and thrombosis of disrupted vessels minimize blood loss, the trau-matic interruption of the blood fl ow leads to ische-mic necrosis of bone, characterized histologically by the presence of empty lacunae. A fi brin-rich clot forms at the fracture site, initiating spontaneous fracture healing. The lack of mechanical support provided by this blood coagulum is well recog-nized. However, its biological contribution to frac-ture healing remains somewhat controversial. In 1969, Ham observed that much of the frac -ture repair process took place around, rather than within, the interfragmentary hematoma and questioned its signifi cance [4]. In fact, large clots persisting unchanged at the fracture site for an extended period of time have been described as
Interfragmentary strain, defi ned as the deformation occurring at the fracture site relative to the size of the gap, infl uences thetype of tissue which forms in the fracture gap.

74
potentially hindering bone repair [5, 6]. Others suggest that the hematoma acts as a scaffold for cells, and a spacer guiding the size and shape of the callus [7, 8]. However, there is growing evidence to support the concept that the hematoma sets the stage for the repair phase by releasing growth factors, thereby stimulating angiogenesis and bone formation. Transplantation of fracture hematoma has been found to induce endochondral bone formation in ectopic sites, which would be consistent with the presence of osteoinductive growth factors within the hematoma [9, 10]. Platelets are likely to be the fi rst source of mito-genic factors at a traumatized site [11]. In addition to coagulation factors, they release platelet-derived growth factor (PDGF) and transforming growth factor-β1 (TGF-β1), both of which stimulate bone production [12].
Fig 3-1 Secondary bone healing in unstable fractures: The passage through different tissue stages of increasing stiffness and strength leads to a biomechanical environment permit-ting bone formation. Inflammatory phase: The defect is initially filled with hematoma and there is intense in-flammation (1). Repair phase: This is quickly replaced by gra n-ulation tissue (2). Remodeling phase: Over the weeks a fibrocar-tilaginous callus is formed (3).Mineralization leads to formation of a hard callus, becoming fusiform and slowly disap-pearing as Haversian remodeling progresses (4).
The angiogenic properties of fracture hematoma appear to be mediated via vascular endothelial growth factor (VEGF) [10]. Local acidity and cyto-kines, contained in the exudate accumulating in the injured area, complement this effect. Indeed, infl ammatory mediators such as prostaglandins E1 and E2 may stimulate angiogenesis, and may also be responsible for signaling early bone resorption by osteoclasts and proliferation of osteoprogenitor cells [13]. Finally, mast cells containing vasoactive substances are abundant during this stage and con-tribute to the formation of new vessels [14–16]. Within hours, a transient extraosseous blood supply emerges from surrounding soft tis sues, revascularizing the hypoxic fracture site [17] .
Mononuclear phagocytes delivered by these new vessels assist in the removal of necrotic bone and aid in construction of the callus.

3 Fracture healing
75
Resorption of fragment ends is particularly obvious in spontaneous fracture healing—when the fracture gap widens, thereby lower-ing interfragmentary strain and minimizing the deformation of local tissues [7] . Macro-phages are also believed to orchestrate the orderly sequence of cutaneous wound healing and would play a similar role in fracture repair. They contain several growth factors, such as fi broblast growth factor (FGF), and initiate fi broplasia both in soft tissue as well as in bone repair [12, 14, 16, 18]. The proliferative vascular response and the degree of bone resorption may be affected by soft-tissue com-promise, either traumatic or iatrogenic. On the other hand, angiogenesis has been enhanced by a muscle fl ap and has improved the healing of experimental tibial osteotomies in dogs [19]. This illustrates the importance of optimizing the role of the soft tissues sur roun ding the fracture. As medullary blood fl ow resumes, this extra-osseous blood supply subsides. The hematoma is resorbed by the end of the fi rst week unless infection, excessive motion, or extensive necrosis of the sur-rounding soft tissues persist at the fracture site [1].
1.1.2 Repair phaseWithin a few days, capillary ingrowth, together with mononuclear cells and fi broblasts, begins the transformation of a hematoma into granulation tissue (Fig 3-1). This initial stage of the repair phase coincides with a slight gain in mechanical strength, since granulation tissue can withstand a tensile force up to 0.1 Nm/mm2 [7, 20]. The ability of granulation tissue to elongate to twice its origi-nal length explains its formation at this stage since interfragmentary deformation remains high. As granulation tissue matures into connective tissue, collagen fi bers become more abundant. They have an ultimate tensile strength of 1–60 Nm/mm2 and resist elongation up to a maximum of 17%.
Types I, II, and III collagen are initially deposited, but as the maturation process continues, type I col-lagen predominates [21, 22]. This interfragmentary fi brous tissue is organized in a diagonal pattern, optimizing its ability to elongate [7]. Low oxygen tension, poor vascularity, growth factors, and interfragmentary strain infl uence the elaboration of a cartilaginous callus [22–24].
Mesenchymal cells within the cambium layer of the periosteum, the endosteum, the bone marrow, and adjacent soft tissues start proliferating during the infl ammatory phase and differentiate into chondrocytes during the repair phase. Chemo -taxis, proliferation, coordination and differentia-tion of these stem cells into chondrocytes or osteo-blasts are orchestrated by numerous growth fac -tors, among which TGF-β and bone morphogenic proteins (BMPs) play a major role. Although the exact timing of this induction phase remains unclear, it may be initiated during the infl amma-tory phase and is crucial to the orderly formation and maturation of tissues within the fracture gap [14]. The periosteum surrounding the fracture site thickens prior to undergoing chondrogenic trans-formation, thereby producing an external callus entirely vascularized by extraosseous vessels [22, 25]. An internal or medullary callus develops from the endosteal cell layer and is confi ned to the medullary canal and receives its blood supply de-rived from medullary arterioles [25]. The presence of a fi brocartilage layer within the medullary canal temporarily interrupts the medullary blood fl ow across the fracture gap. The external callus and the internal callus both constitute the “bridging callus” [25, 26]. This early “soft callus” formed during the fi rst three weeks after injury resists compression, but its ultimate tensile strength (4–19 Nm/mm2) and elongation at rupture (10–12.8%) are similar to those of fi brous tissue [27].
As the hematoma transforms into granulation tissue, which matures into connective tissue, interfragmentary strain decreases.
The repair phase of secondary bone healing includes: hematoma granulation tissue connective tissue cartilage cartilage miner -al ization woven bone formation.

76
Production of a prominent external callus is a common fi nding in well-vascularized unstable fractures. The resulting enlargement in the cross-sectional diameter of the injured area greatly in-creases its resistance to bending, since its strength effi ciency increases by the third power of the dis-tance to the neutral axis of the bone and its rigidity increases by the fourth power [7, 28]. Increasing proteoglycan concentrations within the fi brocarti-lage also contribute to the stiffening of the inter-fragmentary gap [23]. Although the mechanical properties of this calcifi ed fi brocartilaginous tissue have not been reported, these structures contribu te greatly to the restoration of strength and stiffness within the fracture gap, thus allowing formation of compact bone. Mineralization of the soft callus proceeds from the fragment ends toward the center of the fracture site and forms a “hard callus” [7]. Rather than being a uniform process, chondrocytes initiate and control the formation of mineralized clusters [23]. Although the exact mechanism of this calcifi cation remains unclear, it is thought that mitochondria in the fracture gap behave as they do in growth plates [29]. They appear to accumulate calcium-containing granules that are released in the hypoxic environment created by anaerobic me-tabolism. Intramitochondrial deposits of calcium phosphate are released in the extracellular matrix and become the seeds for growth of apatite micro-crystallites. The other steps of bony substitution at the fracture site closely resemble endochondral os-sifi cation. Vascular invasion of fi brocartilage is coupled with the degradation of nonmineralized matrix compartments by macrophages. Following this resorbing front, blood vessels and osteo-progenitor cells form new trabeculae. Provided there is suffi cient vascularity and mechanical sup-port from mineralized callus, fi brous tissue within the fracture gap can undergo intramembranous (direct) bone formation [7, 23].
The ultimate tensile strength of compact bone is around 130 Nm/mm2, but its modulus of elasticity (resistance to deformation) is high (10,000 Nm/mm2) and its ability to elongate is limited to 2% [7].
At the end of the repair phase, bone union is achieved, but the structure of the fracture site dif-fers from that of the original bone. The time re-quired to achieve union varies greatly according to fracture confi guration and location, status of the adjacent soft tissues, as well as patient character-istics (species, age, health status, concurrent in-juries/diseases). At the end of the repair phase, the injured bone has regained enough strength and rigidity to allow low impact exercise [30] .
1.1.3 Remodeling phase This fi nal phase of fracture repair is characterized by a morphological adaptation of bone to regain optimal function and strength. This slow process may last for 6–9 years in humans, representing 70% of the total healing time of a fracture (Fig 3-1) [22, 31]. The balanced action of osteoclastic resorp-tion and osteoblastic deposition is governed by Wolff’s law and modulated by piezoelectricity, a phenomenon in which electrical polarity is created by pressure exerted in a crystalline environment [22, 32, 33]. Axial loading of long bones creates an electropositive convex surface, on which osteo-clastic ac tivity predominates. On the opposite, concave surface, electronegativity is associated with increased osteoblastic activity. The external callus becomes more fusiform and gradually disappears. Re mo d eling of the internal callus allows reestablishment of a continuous medullary cavity in the diaphysis of the bone. In spontaneous healing of a fracture, progression from soft to hard callus depends upon an adequate blood supply and a gradual increase in stability at the fracture site. The tolerance for interfragmen-
The external callus enlarges the cross-sectional diameter of the fracture, greatly increasing the resistance to bending and decreasing the interfragmentary strain at the fracture.
In the remodeling phase, the woven bone is replaced by cortical bone and the medullary cavity is restored.

3 Fracture healing
77
tary motion varies: ribs are more likely to heal in the presence of persistent instability than long bones [34]. Compromised vascularization and ex-cessive instability will merely permit the formation of fi brous tissue and the development of an atro-phic nonunion. If the fracture gap is well vascular-ized, but there is uncontrolled interfragmentary motion, the fracture will progress to a cartilagi-nous callus, but this may be unable to stabilize the fragments. Thus a hypertrophic nonunion or pseudo arthrosis may develop. On the other hand a stable fracture with an adequate blood supply will allow the formation of mineralized callus. Nev er-theless, initial displacement of bone fragments due to trauma and muscle contraction frequently results in malunion.
1.2 Healing under restricted motion
The pattern of healing under restricted motion is intermediate to biological immobilization by a callus formed in spontaneous healing and callus-free repair obtained after absolute stabilization. Any intermediate between these two extremes can be found, depending on the fracture confi guration and implant rigidity. Fracture healing after exter-nal coaptation resembles spontaneous bone repair except that malalignment of fragments is mini-mized by closed reduction (Fig 3-2).
Gliding implants such as intramedullary pins and nails typically allow some motion. Indirect healing proceeds with some resorption of the fragment ends. Intramedullary implants also temporarily disturb medullary blood fl ow. Reaming of the med-ullary canal and contact between implant and end-osteum interfere with the vascularization of the inner cortex [35]. It induces a reversed, centripetal blood fl ow and generates intense remodeling of the site [7, 35]. Unreamed intramedullary nails have
consequently been developed. In experimental tibial fractures, unreamed nails caused a 30% attenuation in blood supply compared with 70% in the reamed procedure [35, 36]. Unreamed nails were also shown to improve healing of simple diaphyseal osteotomies compared with reamed nails [37, 38]. However they still limited the endo-steal osteogenesis associated with healing of ex-perimental comminuted fractures to about 10% of that produced after stabilization with a plate or external fi xator [38].
External fi xators interfere minimally with the blood supply to the healing bone, especially if the fracture has been reduced in a closed fashion or through a limited exposure. In these cases, the
a b
Fig 3-2a–ba Fracture healing under high inter-
fragmentary strains. Callus formation and fragment displacements twelve weeks after external coap tation of a radial fracture in a young dog.
b This type of healing resembles spon-taneous bone repair except that mis-alignment of bone fragments has been minimized by closed reduction.

78
amount of callus produced is highly variable, depending on the fracture confi guration and the rigidity of the frame applied.
Callus formation after plate fi xation may occur when the implant is not placed on the tension side of the bone, fracture reduction is not perfect or when the plate lacks rigidity [7, 39]. This is espe-cially relevant in biological fi xation of comminuted fractures. In these cases, perfect apposition of frag-ments is unlikely and the surgeon chooses biologi-cal factors over anatomical reduction and mechani-cal stability [40, 41]. The general alignment of the joints is restored but manipulation of fragments and adjacent soft tissues is minimized. Instead, a bridging plate is applied across the fracture gap, spanning the entire length of the bone. This less invasive mode of surgical fi xation is not as stable as traditional application of a compression plate and therefore results in increased callus production.
In a recent study, the bone density and osteogenesis in comminuted fractures were increased 12 weeks after application of a bridging plate, an intramedul-lary nail, or an external fi xator compared with the application of lag screws and compression plate [38]. Similar results have been reported in clin ical studies in both humans and animals: Biological fi xation of comminuted fractures is associated with increased callus production, accelerated ra dio gra-phic union, earlier gain in biomechanical strength, and therefore, earlier return to function [41, 42].
2 Biology of fracture healing in stable fractures
The lack of callus formation between two bone fragments apposed under a rigid plate was fi rst not-ed by Danis in 1949 [43]. He termed this mode of repair “primary healing”, referring to direct fi lling of the fracture site with bone, without formation of mechanically relevant periosteal or endosteal cal-lus. Schenk and Willenegger later confi rmed that healing under these conditions occurred by direct osteonal proliferation [16, 44]. Very stable inter-digitation of bone fragments is clinically achieved by application of rigid, nongliding implants, such as compression plates or lag screws [3, 27]. Precise reduction and rigid fi xation appear to eliminate the biological signals that are known to attract osteoprogenitor cells from surrounding soft tissues and which contribute to callus formation in sec-ondary healing [45, 46].
Even under conditions of interfragmentary com-pression, the microenvironment differs within the fracture site and infl uences the process by which bone is produced (Fig 3-3). Indeed, full congruency between the fragment ends is never achieved, even after meticulous reduction. Instead contact and compression are obtained in circumscribed zones (contact points), separated by areas where frag-ment ends are separated by small gaps [23].
The type of bone healing observed is infl uenced by the type of fracture fi xation.

3 Fracture healing
79
The application of a plate also tends to create dif-ferent biomechanical microenvironments within the fracture site. For example, a compression plate applied across a transverse osteotomy generates high pressure and therefore improved contact in the cortex located directly under the plate. On the other hand, the far cortex becomes a tension side and is therefore predisposed to gap healing [7, 46]. Both gap and contact healing differ from indirect bone healing by the absence of resorption of the fracture ends.
2.1 Contact healing
Contact healing occurs across contact areas, where the defect between bone ends is less than 0.01 mm and interfragmentary strain is less than 2% [27, 47] . In this situation, primary osteonal reconstruction results in direct formation
of lamellar bone, oriented in the normal axial direction (Fig 3-3) [7, 27]. The process is initiated by the formation of cutting cones at the ends of the os-te ons closest to the fracture site [8]. Osteoclasts line the spearhead of the cutting cone, while osteoblasts form the rear so that bony union and Haversian remodeling occur simultaneously [8, 46].
Osteoclasts advance across the fracture site, creat-ing longitudinally oriented cavities. The capillary loops within these cavities are accompanied by perivascular osteoblastic precursors that differenti-ate into osteoblasts and produce osteoid [27]. In one study, osteons were seen to traverse canine ra-dial osteotomies stabilized with a plate 3 weeks after surgery [23, 46]. These cutting cones prog-ress across the fracture site from one frag -ment to the other at a rate of 50–100 µm/day [8, 23]. Bridging osteons mature by fi lling with
Fig 3-3 Primary bone healing after rigid fracture fixation. Contact heal-ing occurs in the cortex underlying the plate, by direct Haversian re-modeling. In the far cortex, a small gap heals in two stages: layers of bone are first laid down perpendic-ular to the long axis of the bone, and are then replaced by longitudi-nally oriented osteons by cutting cones progressing across the gap.
Direct bone healing occurs without callus formation in stable fracture conditions.
Direct bone healing takes two forms, contact healing and gap healing, depending on the proximity of the fracture ends. In contact healing, bone union and remodeling occur simultaneously, while in gap healing they are sequential steps.

80
osteonal lamellar bone and become the “spot welds” that unite the two fragments, without formation of periosteal callus. If the new lamellar bone is im-mediately aligned parallel to the long axis of the bone, it is less dense than intact cortex during the fi rst few months. The fracture area therefore remains visible on radiographs until complete re-modeling, which takes from a few months to a few years depending on the species, is achieved [7].
2.2 Gap healing
A different process of direct bone healing has been observed in gaps less than 800 µm to 1 mm, again when the interfragmentary deformation is less than 2%. In “gap healing”, bony union and Haversian remodeling are separate sequential steps (Fig 3-3) [46]. The fracture site fi lls directly by intramem-branous bone formation, but the newly formed lamellar bone is oriented perpendicular to the long
axis. Later it undergoes secondary osteonal recon-struction. This type of healing is similar to that described by Schenk in unicortical transverse burr holes [23]. In this experiment, 200 µm diameter holes created in one cortex of the tibia of rabbits initially contained a well-vascularized granulation tissue. Subsequently, osteoblasts formed a continu-ous layer and deposited lamellar bone in a concen-tric manner. This bone was gradually replaced by longitudinally oriented osteons over the next months. A vascular loop from the medullary vas-culature grew into the gap and loose connective tissue fi lled the site [47]. Osteoprogenitor cells accompany the vascular loop and differentiate into osteoblasts. After two weeks, the blood supply is well established and osteoblasts deposit layer upon layer of lamellar bone on each surface of the gap, until the two fragment ends are united [8]. In larger gaps, woven bone may form initially in larger gaps to subdivide the area into smaller com-partments, to subsequently fi ll with lamellar bone (Fig 3-4) [46]. Although the fragment ends are united by lamellar bone, this area remains me-chanically weak, as the bone is oriented perpen-dicular to the long axis and is poorly connected to adjacent intact cortex. Haversian remodeling starts somewhere between 3 and 8 weeks, when osteo-clasts form longitudinally oriented resorption cavi-ties [8, 48]. These cutting cones are formed by new osteons within the fracture gap and by osteons originating from the surrounding intact bone. They advance across the fracture plane to unite the new lamellar bone deposited in the gap to each fragment end. The resorption cavities mature into longitudinally oriented lamellar bone, so that, with time, the anatomical and mechanical integ-rity of the cortex is reestablished (Fig 3-4).
Fig 3-4 Cross-section of a diaphyseal unicortical defect undergoing Haversian remodeling: irregularly oriented osteons (white arrows) are gradually replaced by lamellar bone oriented in the longitudinal axis of the long bone (to the right of the black arrows).

3 Fracture healing
81
3 Stimulation of fracture healing
The concept of “biological osteosynthesis” em-phasizes the role of soft-tissue integrity in bone healing and a “less than rigid” fi xation of the frac-ture [41, 49]. This concept contrasts with the “bio-mechanical approach” to fracture management, aiming for anatomical reduction and rigid fi xation. The goals of biological fracture fi xation are to re-store the overall length and alignment of the bone while limiting surgical approach and manipulation of fragments. Fracture reduction is performed in a closed fashion or through a limited exposure to minimize disruption of the fracture hematoma and surrounding soft tissues. Implants, such as exter-nal fi xators and locked intramedullary nails, which minimize interference with the healing fracture site are preferred for stabilization. Circular ring fi xators are consequently gaining in popular-ity for fracture management because of their versa-tility and the use of small wires, decreasing soft-tissue trauma even further [50–52].
The principles of biological fracture fi xation are especially applicable to the distal limbs. However, humeral and femoral fractures can be repaired using a “biological plating technique” with indirect fracture reduction and an “open-but-do-not-touch” approach, prior to bone grafting. A plate, generally combined with an intramedullary pin, bridges the entire bone and transfers the weight-bearing load until healing proceeds.
Implant designs have also been modifi ed to limit contact with underlying bone and preserve the extraosseous blood supply. Such examples include the limited contact dynamic compression plate (LC-DCP) and a point contact fi xator (PC-Fix) [53, 54]. Research efforts are currently striving to
Biological techniques for fracture fi xation include in direct reduction of frag ments and bridging fi xation application.
improve the percutaneous placement of internal fi xation devices, in terms of both ease of applica-tion and biomechanical strength.
Biological fracture fi xation is especially relevant in the management of comminuted fractures. Ana-tomical reduction is frequently diffi cult and time-consuming and these high-energy fractures are often associated with signifi cant soft-tissue trau-ma. Unsuccessful attempts at anatomical reduction may further compromise the local blood supply and increase interfragmentary strain between malapposed fragments. On the other hand, biolo-gical strategies applied to highly comminuted fractures of long bones have been found to ac -celerate healing and return of biomechanical strength (see 3 Fracture healing; 4 Ra dio graphic evaluation of fracture healing). Overall, this ap-proach has lowered complication rates associated with the treatment of comminuted fractures in humans and small animals [38, 55].
Biological osteosynthesis also emphasizes the use of fresh autogenous grafts to stimulate healing of fracture gaps. Cancellous bone grafting is generally indicated in cases with low mechanical and/or bio-logical fracture assessment scores [48]. Indeed, autogenous cancellous bone is the most effec-tive material in promoting rapid healing and is still considered the “gold standard” when evalu-ating bone graft substitutes (Fig 3-5) [56, 57]. It not only offers a scaffold for osteoprogenitor cells but, in contrast to other materials, it also provides viable cells, while avoiding immune reactions and disease transmission. Although cancellous bone con tains local growth factors within its extracellular matrix, its osteoinductive properties remain controversial. Indeed, ectopic implantation of intact cancellous bone (without demineralization) does not induce bone formation [58, 59].
Autogenous cancellous bone graft is indicated when rapid bone formation is de -sired. It is particularly effec ti ve in complex fractures.

82
In dogs, the largest amounts of autogenous cancel-lous bone can be collected from the iliac crest, followed by the proximal humerus and medial proximal tibia [60]. Ribs and proximal femur have been used less commonly as donor sites [61, 62]. Healing of donor sites varies somewhat according to location but a second collection can be obtained from the same humeral or femoral site 12 weeks after the fi rst harvest [60, 61]. Defects in the proxi-mal tibia heal slower than those located in the humerus and fi ll with more fi brous tissue [61].
The decision to use an autogenous cancellous bone graft must be made prior to performing the fracture stabilization procedure, and an appropriate donor site prepared. In general, autogenous cancellous bone graft is harvested after the fracture is stabilized to minimize graft storage time. Instrumentation is simple, consisting of an intra-medullary pin or drill bit to penetrate the cortex
Fig 3-5a–ba Autogenous cancellous graft harvested
immediately prior to implantation is still considered the “gold standard” with which other grafting agents are com-pared. It provides a support for cell pro-liferation, contains growth factors, and osteoprogenitor cells.
b Banked demineralized bone matrix is osteoconductive and osteoinductive. It can be expanded with a bone marrow aspirate to provide viable cells.
a
The most common donor sites for autogenous cancel-lous bone grafts are the proximal humerus, the iliac crest, and the proximal tibia.
and a bone curette to harvest the trabecular bone from the proximal humerus or tibia. An osteotome is used to resect a wedge of the iliac crest and ex-pose the ilial wing for trabecular bone harvest. The crest may be morselized with rongeurs for addition-al bone graft. The trabecular bone may be mixed with whole blood obtained from the donor site to improve handling characteristics for implantation.
Cell viability in fresh autografts ranges from 85 to 100% and decreases with storage time [63]. Every effort should therefore be made to harvest the graft immediately prior to placing it in the recipient bed. Intraoperative storage at room tem-perature in blood-soaked swabs has traditionally been advocated [62] , but cell viability decreased to 57% in a study testing 3-hour-storage tech-niques of cancellous bone in rabbits [63]. In the same experiment, viability was worse (about 46%) after storage in a cold isotonic saline solution and
b

3 Fracture healing
83
best (70–73%) after storage on ice and in solu -tions such as phosphate-buffered sucrose and EuroCollins designed to prevent adverse effects of hypothermia [63]. Packing of the graft within and around the fracture gap has generally been recom-mended [62, 64]. Compression increases the amount of graft that may be placed between two fragments, and may therefore improve the out -come of the grafting procedure. However, for an equal mass, compression does not affect the osteo-productive properties of cancellous autografts [65].
Although the incidence of complications associated with collection of autograft in small animals has not
been reported, the morbidity rate approaches 25% in humans, with major complications in 3–4% of patients [66, 67]. Complications include pain, sep-sis, stress fractures, intraoperative blood loss, in-creased surgical time, and limited supply [67, 68]. Another shortcoming of the autogenous cancellous graft is its lack of biomechanical strength. This pre-cludes its use as a structural graft.
Frozen segments of allogenic bone are cur-rently the only osteoconductive implants with biomechanical properties suitable for use as structural grafts in large bone defects (Fig 3-6).
Fig 3-6a–b Segments of allogenic bone are the only structural grafts available for reconstruction of large bone defects in veterinary orthopedics. a Intraoperative view of an allograft used to repair a severely comminuted fracture of the femur.b Nonunion of the host-graft junctions: radiographs immediately, 2 months, and 6 months after a limb sparing
technique (left to right).
a b

84
Although banking techniques have been investi-gated in small animals, establishing a reliable bone bank remains impractical for most clinics and therefore limits the application of allografting techniques in veterinary orthopedics [56, 69, 70]. However, these tissues are commercially available in the United States in a variety of presentations and shapes. Chips, struts, wedges, dowels, and whole canine and feline allogenic bone can be pur-chased to optimize the match between graft and donor site. The usual type of cortical graft used in veterinary orthopedics is the segmental graft em-ployed in limb salvage procedures after tumor exci-sion [56, 71]. The main complications of these large allograft segments are associated with their incomplete resorption, leading to fa -ti gue failure and infections (Fig 3-6) [72–74] .
To overcome these limitations and address public concerns over use of allografts in humans, the development of bone graft substitutes has gained
tremendous popularity over the last two decades. Osteoconductive materials act as scaffolds onto which osteoprogenitor cells can lay new bone, and include a variety of agents such as bone products, ceramics, bioactive glasses, collagen, polymers, and composites. The properties and clinical appli-cations of each material depend upon its composi-tion and physical presentation as well as other factors, such as manufacturing technique, gran ulo-metry, and interconnective porosity.
Calcium sulfate, also known as plaster of Paris, has been used since the 1950s to treat bone defects. It is produced by calcining natural gypsum at tem-peratures of 110–130°C, which removes 75% of the water and results in crystals of irregular shape. Because of its natural origin, concerns were raised about potential toxicity of impurities such as iron, magnesium, strontium, lead, and other heavy metals prior to processing [67]. Medical grade calcium sulfate (Fig 3-7) is now available in an
Fig 3-7a–c a Calcium sulfate is manufactured as
medical grade pellets to slow its other wise rapid degradation rate and antibiotic release rate.
b Tobramycin-impregnated pellets have been packed with autogenous can-cellous graft after debridment of an infected tibial plateau leveling osteo-tomy.
c Radiographic appearance of the pel-lets immediately after implantation.
bc
a

3 Fracture healing
85
injectable form of cement [75] , and in pellets for-mulated to slow its otherwise very rapid resorption rate. It is manufactured for use as a void fi ller in nonweight-bearing applications, although it is completely resorbed in 2–5 weeks in animals and 4–8 weeks in humans [76]. Pellets must be intact to slow the resorption rate and do not conform to bone defects. However, they can be impregnated with antibiotics for local management of osteo-myelitis. Calcium sulfate remains more affordable than bioceramics and may be applicable to the treatment of bone infections in small animals (Fig 3-7) [77].
Ceramics have gained much popularity in human orthopedics because of their excellent biocompati-bility and improved osteoconductive properties conferred by a composition and 3-D structure resembling that of bone (Fig 3-7). Hydroxyapatite (HA), tricalcium phosphate (TCP), and combina-tions of the two, are the most popular agents com-
mercially available (Fig 3-8) [78, 79]. After in vivo implantation, they undergo cellular degradation at a rate varying with their chemical composition (HA is much slower to resorb than TCP), porosity (interconnective pores should measure at least 100 µm to allow bone ingrowth) and presentation (smaller granules are absorbed faster) [78–81]. Therefore, resorption of dense hydroxyapatite is very slow (years) and incomplete compared to macro porous forms of HA. One method of obtaining macroporous HA was inspired by scleractinian corals, such as Porites and Gonoporia, whose macro scopic porous architecture approaches that of human cancellous bone [82]. A replamineform technique (meaning replicated life forms) pro -duces ceramic replicas of the coral structure with complete resorption within 6–18 months. Another alternative to control the degradation rate of bio-ceramics consists of combining HA and TCP in dense or macroporous, particulate forms (Fig 3-8) [57]. In a recent study, an ultraporous scaffold
a b
c d
Fig 3-8a–d a Granules of macroporous tricalcium
phosphate-hydroxyapatite have been impacted in a 50% mixture of allo-genic bone in metaphyseal defects in sheep.
b Remnants appear radiopaque on com-puted tomography 14 weeks after im-plantation.
c Newly formed bone. d At that time, newly formed bone is in
direct contact with ceramic particles (arrows) undergoing cellular degra-dation.

86
composed of β-TCP was almost entirely (82%) replaced by bone 12 weeks after implantation in canine metaphyseal defects [83]. However, in-creased porosity leads to biomechanical weakness, and these composites are too brittle for use as struc-tural implants. Their indications are restricted, but they may be used as void fi llers in non- to limited weight-bearing situations with normal regenerative capacities, such as bone cysts and fracture gaps in healthy patients. They are also radiopaque and may impede radiographic assessment of bone healing.
Calcium phosphate cements have improved han-dling characteristics as they can be molded or injected into defects. Some have signifi cant com-pressive strength and are approved for augmenta-tion of specifi c fracture repairs in man [84]. These cements harden at body temperature in 10–30 minutes and generate negligible heat. However, their bending and shear strengths restrict their use as structural grafts [85]. Injection of cement prior to insertion of 4.0 mm, and larger, screws has been found to increase their pull-out strength [86, 87]. Although augmentation of smaller screws has not been evaluated, this application would be attrac-tive to veterinary surgeons. Moreover, it would im-prove the management of those metaphyseal frac-tures where only two screws can be placed within soft cancellous bone. The use of this simple and versatile augmentation technique could also be ex-tended to any situation where screws are stripped or are at increased risk of loosening, such as in pelvic fractures in immature dogs or luxations of the sacroiliac joint.
Osteoinductive bone graft substitutes initiate and stimulate the differentiation of undifferentiated mesenchymal cells into osteoprogenitor cells [88].
The fi rst strategy for developing osteoinductive im-plants is derived from Urist’s work in 1965, when ectopic implantation of demineralized bone in rodents induced bone formation. The goal of these strategies is to identify an optimal mixture of bone growth agents, either concocted from purifi ed agents, or generated biologically as the product of a tissue or a cultured cell. Following this concept, demineralized bone matrix (DBM) remains the most common type of osteoinductive agent used in human patients [89–91]. To prevent destruction of osteoinductive properties, allogenic bone is steril-ized by chemical sterilization (typicially combined with the demineralization process) rather than irradiation. Commercial preparations differ in manufacturing process and carrier, which, along with their natural origin, results in a somewhat variable effi cacy. They are available for human use in a variety of presentations including fi bers, fl ex, moldable gel, and putty as well as an injectable version. However, canine, feline, and equine de-mineralized bone matrix is only commercially available as a powder or as a mixture with cancel-lous chips, preventing percutaneous applications (Fig 3-5). Demineralized bone lacks structural properties, and can only be used as a gap fi ller in nonweight-bearing areas. Compared with osteo-conductive agents, osteoinductive implants are especially attractive in cases with compromised healing capacities, such as nonunions. All of these products may be immunogenic, carry a potential risk for disease transmission, and limited data are available regarding their clinical use, especially in veterinary medicine.
The second strategy for developing a bone-inducing agent is to identify a single purifi ed molecule that could ultimately be confi rmed as the

3 Fracture healing
87
agent of choice for clinical promotion of bone healing and regeneration [92]. This concept is de-rived from previous work by Wozney and co-workers, who isolated human complementary DNA clones corresponding to bovine BMP-1, BMP-2A in 1988 [93]. Since then, at least ten BMPs have been identifi ed but two of these proteins have been particularly well described in humans: recombinant BMP-2 and BMP-7 (OP-1) [93–97]. Their properties have been studied in several ani-mal experiments, and the most advanced human clinical trials have focused on these two growth factors [95, 98–101].
Synthetic agents do not carry any risk of disease but do not contain the optimal mixture of growth factors naturally present in bone-derived products. Because osteoinductive proteins are water soluble and readily diffusible, they must be combined with a biocompatible carrier to be effective. Encour -ag ing results were reported in preliminary clini -cal trials evaluating combinations of collagen and recombinant bone morphogenetic proteins (rhBMP-2, rhBMP-7) for the treatment of human tibial nonunions [100]. However, the bovine origin of collagen affects its future as a delivery system for growth factors in man and the cost of these prepara-tions remains prohibitive for the veterinary market.
Biological stimulation of bone healing constitutes only one aspect of therapies developed for enhanc-ing bone regeneration. Much progress has been made in the elaboration of techniques targeting various factors involved in bone regeneration (Fig 3-9). The wide therapeutic range now offered may pose a dilemma for clinicians, for while it pro-vides more freedom of choice it also complicates the decision-making process. A rational attitude
towards solving this issue should be based on an assessment of the factors affecting bone healing. Understanding which factors are defi cient in a given patient determines the criteria for selecting a therapeutic strategy. If grafting is the answer, a good knowledge of the grafting agents available will guide the ultimate choice. Most often, several contributing factors can be identifi ed and a combi-nation of therapies required. Internal fi xation and autogenous grafting for treatment of nonunions is a classic example combining biomechanical and
Dynamization, D O*surgical stabilization
FRACTURE MOVEMENT
ANGIOGENESIS
Grafting techniques:cells, growth factors,
support**
BONE REGENERATION
Ú
ÚÚ
Û
ÛÛ
Healthy soft-tissue bed,biological fixation, grafting techniques
* Distraction osteogenesis ** Osteoconductive agents
Fig 3-9 Factors affecting fracture healing and current strategies for en-hancing bone formation. Diagram derived from Marsh and Li’s triad of interrelationships in the regenerative repair of fractures.

88
biological strategies to enhance bone formation. Highly comminuted fractures in human diabetics may justify the use of allograft as an osteoconduc-tive gap fi ller, mixed with a gel of demineralized bone matrix to provide growth factors. In post-irradiation human patients, this combination can be further augmented by an autogenous bone mar-row aspirate, to increase the local population of mesenchymal stem cells [102]. The next goal for biological enhancement of bone formation will be to produce a safe, synthetic osteogenic material, delivering growth factors in a cascade mimicking the physiological sequence of bone healing. Im-provement in the delivery characteristics of osteo-conductive materials, tissue engineering tech-niques and gene therapy may provide the answer.
4 Radiographic evaluation of fracture healing
Direct fracture healing is initially characterized radiographically by a gradual disappearance of the fracture line without the formation of an external callus (Fig 3-10). Although there is no resorption of the fragment ends with contact healing, the pro-gression of cutting cones across the gap causes the zone around the fracture to lose radiopacity [6, 7]. The fracture line slowly disappears without forma-tion of a periosteal callus. This type of healing is occasionally encountered in clinical cases after compression and rigid stabilization of a simple frac-ture. Depending on the species, complete remod-eling takes a few months to a few years, during
Direct fracture healing is characterized radiographically by a gradual disappearance of the fracture line without the formation of an external callus.
Fig 3-10a–c Radiographic appearance of primary bone healing. a A distal radial fracture in a 5-month-old
Italian greyhound. b It has been stabilized with a plate. In an
attempt to pre vent the development of an angular limb deformity because of concur-rent trauma to the distal ulnar growth plate, a fat pad has been placed at the ulnectomy site.
c 6 weeks later, the radial fracture is healing without callus formation, whereas callus is bridging the ulnectomy site.
a b c

3 Fracture healing
89
which the fracture site will remain radiolucent compared with the intact cortex [7].
Indirect or secondary bone healing is the type of healing expected after external coaptation or semi-rigid internal fi xation of fractures (Fig 3-11). Initial resorption of fragment ends can be recognized radiographically as a local loss of radiopacity and widening of the gap [6]. The edges of the fracture become less defi ned and sharp 5–7 days after injury. While the repair phase is initiated within the fi rst
week following trauma, callus formation does not become apparent radiographically until mineraliza-tion proceeds. The periosteal component of the cal-lus develops fi rst and appears as a collar around the fracture site. The smaller internal callus forming within the medullary cavity is harder to see radio-graphically due to superimposition of the external callus. A calcifi ed callus may be seen 10–12 days after repair, and occasionally sooner, in young pa-tients with simple fractures and minimum soft- tissue trauma [6].
Indirect bone healing is characterized radiographically by early bone resorption and subsequent callus formation.
c d
Fig 3-11a–d Secondary bone healing of a fracture under restricted motion. a A comminuted femoral fracture in a cat. b It has been stabilized with two cerclage wires placed around a large fragment and an intramedullary nail.
The site of comminution was not manipulated. c–d Prominent callus 6 weeks later.
a b

90
The age of the patient affects not only the speed at which callus is formed but also its appearance. Periosteal stripping in immature animals results in production of a callus away from the bone as osteoprogenitor cells get pulled with the periosteum. In mature patients, the peri-osteum has a tendency to tear rather than strip. Thus the osteoprogenitor cells remain attached to the bone and produce a callus in contact with the fracture site. Interfragmentary strain, local blood supply, and tissue oxygenation also affect the size of the callus. Local hypoxia encourages mesenchymal cells to differentiate into chondrocytes rather than osteoblasts [22–24]. In these cases, the full extent of the bridging callus is underestimated on radio-graphs, since the large cartilaginous portion is not visible. With maturation of the fi brocartilaginous callus into a “hard callus”, the fracture line disap-pears and the fracture gap gains a radiopacity simi-lar to that of adjacent bone. Remodeling can then proceed and the external callus assumes a fusiform shape [6]. Little of the remodeling of the internal callus can be appreciated radiographically. How-ever, its osteoclastic resorption allows restoration of the medullary blood fl ow. Thinning of the exter-nal callus eventually results in restoration of the original shape of the bone. This event is slow and occurs so late within the healing phase that it has little clinical signifi cance.
In clinical cases, serial radiographs are typically obtained every 4–6 weeks to assess implant stability, verify alignment of the bone, con-fi rm the absence of complications, and mon-itor bone healing. Although the radiographic
signs of fracture healing are well de scribed, their interpretation remains subjective. In a recent sur-vey of 444 human orthopedic surgeons, cortical continuity was the radiographic sign most com-monly used to assess fracture healing [103]. Other radiographic signs included size of the callus and progressive loss of the fracture line. This study emphasized the lack of consensus among surgeons in terms of both acceptable bone alignment and normal healing time [103]. Defi ning normal healing time and, therefore, early diagnosis of delayed union and nonunion is even more problematic.
Numerous factors affect fracture healing and should be taken into consideration when pre -dicting healing time. The timeline for radiographic signs indicating normal secondary healing of a simple fracture in dogs has been described as:
• widening of the fracture gap and “smudging” of the fracture edges at 5–7 days after trauma,
• appearance of a bony callus at 10–12 days,• disappearance of the fracture line within 30
days, and • complete remodeling of the callus 90 days after
repair [104].
However, slower healing should be expected in older animals, complicated fractures, and patients with concurrent disease or injuries. Clinical union will occur faster in sites containing abundant cancellous bone and highly vascu-larized marrow, such as in metaphyseal frac-tures. In contrast, fractures of compact bone, such as middiaphyseal fractures, especially if the

3 Fracture healing
91
surrounding soft tissues are sparse or compro-mised, will heal slower. Interfragmentary motion and therefore fi xation technique also play a crucial role when determining the expected radiographic appearance of healing. For example, radiographic healing of comminuted femoral fractures in human patients was seen, on average, 36 weeks after reconstruction and compression plating com-pared to 23 weeks with bridging plates [41]. Shorter healing times have also been described with other bridging techniques such as locked intramedullary nails (14–19 weeks) and external fi xation (14–18 weeks) [38, 105]. Faster healing would be antici-pated in small animals since the regenerative ca-pacity of mammalian bone is inversely propor -tional to its position on the phylogenic scale [95, 106]. In a retrospective study of femoral comminuted fractures in dogs, fi rst radiographic evidence of bony bridging was noticed at a mean of 15 and 10 weeks after traditional plating and application of a bridging plate, respectively [42]. However, values ranged from 5 to 37 weeks, stressing the importance of considering individual factors when determining expected healing time. In the absence of good guidelines for predicting the healing timeline, deciding when to intervene because fractures appear “slow” to heal remains something of an art.
5 Implant removal
In the absence of irritation, implant removal has been recommended in younger patients to prevent changes associated with stress protection and to decrease the risk of fracture at the junction be-tween the plate and the bone [107]. Stainless steel plates have a greater coeffi cient of elasticity than bone and are more likely to induce bone atrophy than titanium alloy implants [108]. However bone loss associated with appropriately sized plates is usually not an issue in small animals. Indications for plate removal therefore include osteomyelitis, pain on palpation of the plated bone or radio -graphic evidence of osteopenia. Plating of long bones with limited soft-tissue coverage, such as the distal limbs, may occasionally result in lameness associated with cold transmission through the metal and warrants plate removal. By far the most common indication for implant removal in small animals is related to the use of external fi xators.
The timing of implant removal varies with the frac-ture assessment score and the type of healing ex-pected. With secondary healing, the spatial align-ment of the callus, away from the central axis of the bone, is mechanically advantageous. Time of fi xator removal ranged from 4 to 32 weeks in vari-ous studies, with a mean of about 15 weeks in com-minuted fractures of the tibia and radius [75, 109, 110]. Experimental data support the con-cept of gradual disassembly of external fi xators to encourage callus formation and remodeling [111].
Indications for plate removal include osteomyelitis, pain on palpation of the plated bone, or radiographic evidence of osteopenia.

92
6 Bibliography
1. Schiller AL (1988) Bones and joints. Rubin E,
Farber JL (eds), Pathology. Philadelphia: Lippincott,
1304–1393.
2. Perren SM, Cordey J (1980) The concept
of interfragmentary strain. Uhthoff HK (ed),
Current Concepts of Internal Fixation of Fractures.
Berlin Heidelberg New York: Springer-Verlag, 63.
3. Rahn BA (1982) Bone healing: histologic and
physiologic concepts. Sumner-Smith G (ed), Bone
in Clinical Orthopaedics. Philadelphia: WB Saunders,
335–386.
4. Ham AW (1969) Histology. Philadelphia: JB
Lippincott Co.
5. Harris WR (1990) Fracture healing. Wittick WG
(ed), Canine Orthopedics. 2nd ed. Section III:
Preparation, principles, and procedures for
surgery. Philadelphia: Lea & Febiger, 158–165.
6. Morgan JP, Leighton RL (1995) Radiographic
appearance of fracture healing. Morgan JP,
Leighton RL (eds), Radiology of Small Animal Fracture
Management. Philadelphia: WB Saunders, 34–43.
7. Rahn BA (2002) Bone healing: histologic and
physiologic concepts. Fackelman GE (ed), Bone in
Clinical Orthopaedics. Stuttgart New York: Thieme,
287–326.
8. Hulse D, Hyman B (1993) Fracture biology and
biomechanics. Slatter D (ed), Textbook of Small Animal
Surgery. Philadephia: WB Saunders, 1595–1603.
9. Mizuno K, Mineo K, Tachibana T, et al (1990)
The osteogenic potential of fracture hematoma.
Subperiosteal and intramuscular transplantation of
the hematoma. J Bone Joint Surg [Br]; 72(5):822–829.
10. Street J, Winter D, Wang JH, et al (2000) Is
human fracture hematoma inherently angiogenic?
Clin Orthop Rel Res; (378):224–237.
Based on these results, destabilization of external fi xators is indicated 6 weeks after fracture repair in healthy dogs.
On the other hand, primary bone healing is the biological response to anatomical reduction and stability. Callus does not form and fractures under-going primary healing are initially weaker than those united by callus. Implant removal may be considered once the cortex has recovered its inter-nal architecture and becomes as strong as bone healed by secondary healing. This process takes about 18 months in mature diaphyseal bone [107]. The remodeling phase may be prolonged by numer-ous factors, including the age and health of the patient, the degree of comminution and devitaliza-tion of the fracture, accuracy of the reduction, the stability of the fi xation, and adjuvant treatments. For example, bone grafts would act as positive adjuvants, whereas concurrent administration of immunosuppressive drugs or chemotherapy would have an adverse effect on bone healing.
Following removal of a plate, the ridges commonly found on each side of the plated area should not be excised as they contribute to the mechanical integ-rity of the treated bone. However, screw and pin holes act as stress risers. It is therefore recom-mended to restrict the amount of loading applied to a weight-bearing bone for about 6 weeks after removal of an implant [107]. Exercise should be restricted in all cases and occasionally splints are indicated to protect the weakened bone.
The plate functions to protect the bone during direct bone healing and should not be removed prematurely.

3 Fracture healing
93
11. Bolander ME (1994) Regulation of fracture repair
and synthesis of matrix macromolecules. Brighton
CT, Friedlander GE, Lane JM (eds), Bone Formation
and Repair. Rosemont: American Academy of
Orthopaedic Surgeons, 117–141.
12. Lieberman JR, Daluiski A, Einhorn TA (2002)
The role of growth factors in the repair of bone.
J Bone Joint Surg [Am]; 84(6):1032–1044.
13. Millis DL (1999) Bone and non-bone-derived
growth factors and effects on bone healing. Vet Clin
North Am Small Anim Pract; 29(5):1221–1246.
14. Heppenstall RB (1980) Fracture healing.
Heppenstall RB (ed), Fracture Treatment and Healing.
Philadelphia: WB Saunders, 35–64.
15. Marks RM, Roche WR, Czerniecki M, et al
(1986) Mast cell granules cause proliferation of
human microvascular endothelial cells. Lab Invest;
55(3):289–294.
16. McLaughlin RM (1991) The evolution of the
understanding of bone healing. Vet Comp Orthop
Traumatol; 4:16–20.
17. Wilson JW (2002) Blood supply to developing,
mature, and healing bone. Fackelman GE (ed),
Bone in Clinical Orthopaedics. Stuttgart New York:
Thieme, 23–117.
18. Leibovich SJ, Ross R (1975) The role of the
macrophage in wound repair: A study with
hydrocortisone and antimacrophage serum.
Am J Pathol; 78(1):71–100.
19. Richards RR, Schemitsch EH (1989) Effect of
muscle fl ap coverage on bone blood fl ow following
devascularization of a segment of tibia: an
experimental investigation in the dog. J Orthop Res;
7(4):550–558.
20. Perren SM, Boitzy A (1978) Cellular differentiation
and bone biomechanics during the consolidation of a
fracture. Anat Clin; 1:13.
21. Lane JM, Boskey AL, Li WKP, et al (1979) A
temporal study of collagen, proteoglycans, lipids and
mineral constituents in a model of endochondral
osseous repair. Metab Bone Dis Rel Res; 1:319.
22. Remedios A (1999) Bone and bone healing. Vet Clin
North Am Small Anim Pract; 29(5):1029–1044.
23. Schenk RK, Hunziker EB (1994) Histologic
and ulstrastructural features of fracture healing.
Brighton CT, Friedlander GE, Lane JM (eds), Bone
Formation and Repair. Rosemont: American Academy
of Orthopaedic Surgeons, 117–145.
24. Carter DR, Beaupré GS, Giori NJ, et al (1998)
Mechanobiology of skeletal regeneration. Clin Orthop;
355(Suppl):41–55.
25. Binnington AG (1990) Bone remodeling and trans-
plantation. Wittick WG (ed), Canine Orthopedics. 2nd
ed. Section III: Preparation, principles, and procedu-
res for surgery. Philadelphia: Lea & Febiger, 166–189.
26. Rhinelander FW, Wilson JW (1982) Blood
supply to developing, mature and healing bone.
Sumner-Smith G (ed), Bone in Clinical Orthopaedics.
Philadelphia: WB Saunders, 81–159.
27. Mann FA, Payne JT (1989) Bone healing. Semin Vet
Med Surg (Small Anim); 4(4):312–321.
28. Perren SM (1979) Physical and biological aspects of
fracture healing with special reference to internal
fi xation. Clin Orthop; (138):175–196.
29. Ketenjian AY, Arsenis C (1975) Morphological
and biochemical studies during differentiation and
calcifi cation of fracture callus cartilage. Clin Orthop;
(107):266–273.
30. Frost HM (1989) The biology of fracture healing.
An overview for clinicians. Part I. Clin Orthop;
(248):283–293.
31. Wendeberg B (1961) Mineral metabolism of
fractures of the tibia in man studied with external
counting of strontium 85. Acta Orthop Scand;
52(Suppl):1–79.

94
43. Danis R (1949) Theorie et Pratique de l’Osteosynthese.
Paris: Masson.
44. Schenk RK, Willenegger H (1963) [On the
histological picture of so-called primary healing of
pressure osteosynthesis in experimental osteotomies
in the dog]. Experentia; 19:593–596.
45. O’Sullivan ME, Chao EYS, Kelly PJ (1989) The
effects of fi xation on fracture healing. J Bone Joint
Surg [Am]; 71(2):306–310.
46. Kaderly RE (1991) Primary bone healing. Semin Vet
Med Surg (Small Anim); 6(1):21–25.
47. Shapiro F (1988) Cortical bone repair. The
relationship of the lacunar-canalicular system and
intercellular gap junctions to the repair process.
J Bone Joint Surg [Am]; 70(7):1067–1081.
48. Johnson AL, Hulse DA (2002) Fundamentals
of orthopedic surgery and fracture management.
Fossum TW (ed), Small Animal Surgery. 2nd ed.
St Louis: Mosby, 855–859.
49. Aron DN, Palmer RH, Johnson AL (1995)
Biologic strategies and a balanced concept for repair
of highly comminuted long bone fractures. Compend
Contin Educ Pract Vet; 17:35–49.
50. Farese JP, Lewis DD, Cross AR, et al (2002)
Use of IMEX SK-circular external fi xator hybrid
constructs for fracture stabilization in dogs and cats.
J Am Anim Hosp Assoc; 38(3):279–289.
51. Lewis DDR, Beale RM, Stallings JT, et al (1999)
Initial clinical experience with the IMEX circular
external skeletal fi xation system. Part I: Use in
fractures and arthrodeses. Vet Comp Orthop Traumatol;
12:108–117.
52. Marcellin-Little DJ (1999) Fracture treatment
with circular external fi xation. Vet Clin North Am
Small Anim Pract; 29(5):1153–1170.
53. Perren SM (1991) The concept of biological plating
under limited contact-dynamic compression plate
(LC-DCP). Scientifi c background, design and
application. Injury; 22(Suppl 1):1–41.
32. Bassett CAL (1971) Biophysical principles affecting
bone structure. Bourne GH (ed), Biochemistry and
Physiology of Bone. 2nd ed. New York: Academic Press,
341–376.
33. Cruess RL, Dumont J (1985) Basic fracture
healing. Newton CD, Nunamaker DM (eds), Textbook
of small animal orthopaedics. Philadelphia: Lippincott,
35–38.
34. Burwell RG (1969) The fate of bone grafts. Apley
AG (ed), Recent Advances in Orthopedics. Baltimore:
Williams & Wilkins, 115–207.
35. Pape HC, Giannoudis PV, Grimme K, et al
(2002) Effects of intramedullary femoral fracture
fi xation: what is the impact of experimental studies
in regards to the clinical knowledge?
Shock; 18(4):291–300.
36. Kessler S, Hallfeldt K, Perren SM, et al (1986)
The effects of reaming and intramedullary nailing
on fracture healing. Clin Orthop; (212):18–25.
37. Runkel M, Wenda K, Ritter G, et al (1994) [Bone
healing after unreamed intramedullary nailing].
Unfallchirurg; 97(1):1–7.
38. Claes L, Heitemeyer U, Krischak G, et al (1999)
Fixation technique infl uences osteogenesis of
comminuted fractures. Clin Orthop; (365):221–229.
39. Hutzschenreuter P, Perren SM, Steinemann S
(1980) Some effects of rigidity of internal fi xation on
the healing pattern of osteotomies. Injury; 1:77–85.
40. Perren SM (2002) Evolution of the internal
fi xation of long bone fractures. J Bone Joint Surg [Br];
84(8):1093–1110.
41. Heitemeyer U, Kemper F, Hierholzer G, et al
(1987) Severely comminuted femoral fractures:
treatment by bridging-plate osteosynthesis. Arch
Orthop Trauma Surg; 106(5):327–330.
42. Johnson AL, Smith CW, Schaeffer DJ (1998)
Fragment reconstruction and bone plate fi xation
versus bridging plate fi xation for treating highly
comminuted femoral fractures in dogs: 35 cases
(1987–1997). J Am Vet Med Assoc; 213(8):1157–1161.

3 Fracture healing
95
54. Auer JA, Lischer C, Kaegi B, et al (1995)
Application of the point contact fi xator in large
animals. Injury; 26:B47–B50.
55. Dudley M, Johnson AL, Olmstead M, et al
(1997) Open reduction and bone plate stabilization,
compared with closed reduction and external
fi xation, for treatment of comminuted tibial
fractures: 47 cases (1980–1995) in dogs. J Am Vet Med
Assoc; 211(8):1008–1012.
56. Fitch R, Kerwin S, Newman-Gage H, et al
(1997) Bone autografts and allografts in dogs.
Compend Contin Educ Pract Vet; 19(5):558–578.
57. Griffon DJ (2002) Evaluation of Osteoproductive
Biomaterials. PhD Thesis, University of Helsinki Press,
Helsinki, Finland, 2002.
58. Griffon DJ, McLaughlin R, Hoskinson J (1996)
Effects of a bone inducing agent derived from
a cultured human osteosarcoma cell line after
orthotopic and heterotopic implantation in the dog.
Vet Comp Orthop Traumatol; 9:22–28.
59. Chalmers J, Gray DH, Rush J (1975) Observations
on the induction of bone in soft tissues. J Bone Joint
Surg [Br]; 57(1):36–45.
60. Penwick RC, Mosier DA, Clark DM (1991)
Healing of canine autogenous cancellous bone graft
donor sites. Vet Surg; 20(4):229–234.
61. Trevor PB, Smith MM, Stevenson S, et al (1992)
Evaluation of the proximal portion of the femur as
an autogenous cancellous bone donor site in dogs.
Am J Vet Res; 53(9):1599–1603.
62. Fox AM (1984) Cancellous bone grafting in the dog:
an overview. J Am Anim Hosp Assoc; 20:840–848.
63. MacAnulty JF (1999) Effect of various short-term
storage methods on viability of cancellous bone
fragments. Am J Vet Res; 60:63–67.
64. Meutsege FS (1984) Bone transplantation. Brinker
WO, Hohn RB, Prier WD (eds), Manual of Internal
Fixation in Small Animals. New York: Springer-Verlag,
273–280.
65. Martinez SA, Probst CW, Hauptman JG, et al
(1992) Effects of a fi xed compression load on the
osteogenic effect of cancellous bone grafts in dogs.
Am J Vet Res; 53(12):2381–2385.
66. Younger EM, Chapman MW (1989) Morbidity
at bone graft donor sites. J Orthop Trauma;
3(3):192–195.
67. Damien CJ, Parsons R (1991) Bone graft and bone
graft substitutes: a review of current technology and
applications. J Appl Biomater; 2(3):187–208.
68. Ferguson J (1996) Fracture of the humerus after
cancellous bone graft harvesting in a dog. J Small
Anim Pract; 37(5):232–234.
69. Johnson AL, Moutray M, Hoffmann WE (1987)
Effect of ethylene oxide sterilization and storage
conditions on canine cortical bone harvested for
banking. Vet Surg; 16(6):418–422.
70. Tshamala M, van Bree H, Mattheuws D (1994)
Biomechanical properties of ethylene oxide sterilized
and cryopreserserved cortical bone allografts.
Vet Comp Orthop Traumatol; 7:25–30.
71. LaRue SM, Withrow SJ, Powers BE, et al (1989)
Limb-sparing treatment for osteosarcoma in dogs.
J Am Vet Med Assoc; 195(12):1734–1744.
72. Thompson RC Jr., Pickvance EA, Garry D (1993)
Fractures in large-segment allografts. J Bone Joint
Surg [Am]; 75(11):1663–1673.
73. Dernell WS, Withrow SJ, Straw RC, et al
(1998) Clinical response to antibiotic impregnated
methylmethacrylate bead implantation of dog with
severe infections after limb sparing and allograft
replacement: 18 cases (1994–1996). Vet Comp Orthop
Traumatol; 11:94–99.
74. Straw RC, Withrow SJ (1996) Limb-sparing
surgery versus amputation for dogs with bone
tumors. Vet Clin North Am Small Anim Pract;
26(1):135–143.

96
84. Sanchez-Sotelo J, Munuera L, Madero R (2000)
Treatment of the distal radius with a remodellable
bone cement: a prospective randomized study using
Norian SRS. J Bone Joint Surg [Br]; 82(6):856–863.
85. Schmitz JP, Hollinger JO, Milam SB (1999)
Reconstruction of bone using calcium phosphate
cements: a critical review. J Oral Maxillofac Surg;
57(9):1122–1126.
86. Mermelstein LE, Chow LC, Friedman C, et al
(1996) The reinforcement of cancellous bone screws
with calcium phosphate cement. J Orthop Trauma;
10(1):15–20.
87. Stankewich CJ, Swiontkowski MF, Tencer AF,
et al (1996) Augmentation of femoral neck fracture
fi xation with an injectable calcium-phosphate bone
mineral cement. J Orthop Res; 14(5):786–793.
88. Urist MR, DeLange RJ, Finerman GAM (1983)
Bone cell differentiation and growth factors. Science;
220(4598):680–686.
89. Urist MR, Chang JJ, Lietze A, et al (1987)
Preparation and bioassay of bone morphogenetic
protein and polypeptides fragments. Methods
Enzymol; 146:294–312.
90. Bolander ME, Balian G (1986) The use of
demineralized bone matrix in the repair of
segmental defects. Augmentation with extracted
matrix proteins and a comparison with autologous
grafts. J Bone Joint Surg [Am]; 68(8):1264–1274.
91. Johnson EE, Urist MR, Finerman GA (1992)
Resistant nonunions and partial or complete
segmental defects of long bones. Treatment
with implants of a composite of human bone
morphogenetic protein (BMP) and autolyzed,
antigen-extracted, allogeneic (AAA) bone. Clin
Orthop; (277):229–237.
92. Anderson HC (1994) Recent advances in methods
for inducing bone formation. Curr Opin Ther Patents;
4(1):17–29.
75. Johnson AL, Seitz SE, Smith CW, et al (1996)
Closed reduction and type II external fi xation of
comminuted fractures of the radius and tibia in dogs:
23 cases (1990–1994). J Am Vet Med Assoc;
209:1445–1448.
76. Turner TM, Urban RM, Gitelis S, et al (2001)
Radiographic and histologic assessment of calcium
sulfate in experimental animal models and clinical
use as a resorbable bone-graft substitute, a bone-
graft expander, and a method for local antibiotic
delivery. J Bone Joint Surg [Am]; 83(Suppl 2,
Part 1):8–18.
77. Santschi EM, McGarvey L (2003) In vitro elution
of gentamicin from Plaster of Paris beads. Vet Surg;
32(2):128–133.
78. Behairy Y, Jasty M (1999) Bone grafts and bone
substitutes in hip and knee surgery. Orthop Clin North
Am; 30(4):661–671.
79. Tay BKB, Patel VV, Bradford DS (1999) Calcium
sulfate- and calcium phosphate-based bone
substitutes. Mimicry of the mineral phase of bone.
Orthop Clin North Am; 30(4):615–623.
80. Cornell CN (1999) Osteoconductive materials and
their role as substitutes for autogenous bone grafts.
Orthop Clin North Am; 30(4):591–598.
81. Burwell RG (1994) History of bone grafting and
bone substitutes with special reference to osteogenic
induction. Urist MR, O’Connor BT, Burwell
RG (eds), Bone Grafts, Derivatives and Substitutes.
Cambridge, Butterworth-Heinemann Ltd, 1–102.
82. Clayton Shors E (1999) Coralline bone graft
substitutes. Orthop Clin North Am; 30(4):599–613.
83. Erbe E, Clineff T, Lavagnino M, et al (2001)
Comparison of Vitoss and ProOsteon 500R in a
critical-sized defect at 1 year. Annual Meeting of the
Orthopedic Research Society. San Francisco; February
25–28: abstract 975.

3 Fracture healing
97
93. Wozney JM, Rosen V, Celeste AJ, et al (1988)
Novel regulators of bone formation: molecular clones
and activities. Science; 242(4885):1528–1534.
94. Wang EA, Rosen V, D’Allessandro JS, et al
(1990) Recombinant human bone morphogenetic
protein induces bone formation. Proc Natl Acad Sci
USA; 87(6):2220–2224.
95. Cook SD, Baffes GC, Wolfe MW, et al (1994)
Recombinant human bone morphogenetic protein-
7 induces healing in a canine long-bone segmental
defect model. Clin Orthop; (301):302–312.
96. Geesink RGT, Hoefnagels NHM, Bulstra SK
(1999) Osteogenic activity of OP-1 bone
morphogenetic protein (BMP-7) in a human fi bular
defect. J Bone Joint Surg [Br]; 81(4):710–718.
97. Mason DR, Renberg WC (2001) Postsurgical
enhancement of fracture repair: biological
alternatives to bone grafting. Compend Contin Educ
Pract Vet; 23(3):272–279.
98. Kirker-Head CA, Nevins M, Palmer R, et al
(1996) Maxillary sinus fl oor bone augmentation
using recombinant human bone morphogenetic
protein-2 and absorbable collagen sponge in goats.
Vet Surg; 25(5):431.
99. Rowley DI (2001) Enhancement of the healing of
fractures. Thorngren KG, Soucacos PN, Horan F, et al
(eds), European Instructional Course Lectures. London:
The British Editorial Society of Bone and Joint
Surgery; 5:24–30.
100. Valentin-Opran A, Wozney J, Csimma C, et al
(2002) Clinical evaluation of recombinant human
bone morphogenetic protein-2. Clin Orthop;
(395):110–120.
101. Lee FJ, Storer S, Hazan EJ, et al (2002) Repair
of bone allograft fracture using bone morphogenetic
protein-2. Clin Orthop; (397):119–126.
102. Redfern FC (2001) Osteoinductive bone grafting.
European Federation of National Associations
of Orthopaedics and Traumatology. Technical
symposium on “demineralized bone allografts and
osteoinduction“. Rhodes, June 4, 2001.
103. Bhandari M, Guyatt GH, Swiontkowski MF,
et al (2002) A lack of consensus in the assessment of
fracture healing among orthopaedic surgeons.
J Orthop Trauma; 16(8):562–566.
104. Sande R (1999) Radiography of orthopedic trauma
and fracture repair. Vet Clin North Am Small Anim
Pract; 29(5):1247–1260.
105. Kempf J, Grosse A, Beck G (1985) Closed locked
intramedullary nailing. J Bone Joint Surg [Am];
67(5):709–720.
106. Aspenberg P, Wang E, Thorngren KG (1992)
Bone morphogenetic protein induces bone in the
squirrel monkey, but bone matrix does not. Acta
Orthop Scand; 63(6):619–622.
107. Schatzker J [updated by Houlton JEF](2000)
Concepts of fracture stabilization. Sumner-Smith G
(ed), Bone in Clinical Orthopaedics, 2nd ed. Stuttgart:
Thieme, 327 –347.
108. Conzemius M, Swainman S (1999) Fracture
fi xation with screws and bone plates. Vet Clin North
Am Small Anim Pract; 29(5):1117–1134.
109. Johnson AL, Egger EL, Eurell JA, et al (1998)
Biomechanics and biology of fracture healing with
external skeletal fi xation. Compend Contin Educ Pract
Vet; 20:487–502.
110. Foland MA, Egger EL (1991) Application of type
III external fi xators: a review of 23 clinical fractures
in 20 dogs and two cats. J Am Anim Hosp Assoc;
27:193–202.
111. Egger EL, Histand MB, Norrdin RW, et al (1993)
Canine osteotomy healing when stabilized with
decreasingly rigid fi xation compared to constantly
rigid fi xation. Vet Comp Orthop Traumatol; 6:182–187.