Chemistry in your cupboard: Finish
Transcript of Chemistry in your cupboard: Finish
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
Chemistryinyourcupboard:Finish
Introduction
Machinedishwashingisacomplexprocesswhichhastorelyonchemistryratherthanmanual effort to clean dishes. Dishwasher cleaning products contain a huge range ofchemicals including surfactants or detergents, alkalis, bleaches, biosubstances andbuilders.Eachof thesehasadistinct role in thecleaningofdishesbut thedifficulty isgetting all these working together when they have different optimum conditions.Finish™ is abrandwith a rangeofproducts available indifferent formulationsbut allbasedonthesamebroadprinciples.
Linkstothecurriculum
This section contains information relevant to the following areas of your chemistrycurriculum:
Ratesofreactions Group7elements Group1and2elements Biochemistry(proteins,fatsandcarbohydrates) Acidsandbases
FINISH
AccordingtotheOfficeofNationalStatistics,in2008almost40%ofhomesintheUKhadadishwasher,upfromaround25%in1998.Itistemptingtothinkofmachinedishwashingascomparabletohandwashingofdishesbutinfactitisamuchmorecomplexprocessandinvolvesagreatdealmorechemistry.
Handdishwashingreliesmostlyonmechanicaleffort‐continuingtobrushorsponge(withthehelpofhotwaterandadetergent),untilthedishesappearclean.Withmachinedishwashing,mechanicaleffortcountsforless,andthechemistryhastodothecleaning.However,theconditionsformachinewashingcanbebothhotterandmorealkalinethatcouldbewithstoodbyhumanhands.
Herewewilllookatsomeofthechemistrythatgoesoninsidethedishwasherandrelateittochemistrythatyoualreadyknow.Thescienceofdishwasherproductsiscomplexandwecanonlycoverthebasicshere.
Didyouknow?JosephineCochraneinventedthemoderndishwasherin1886.
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
Cochranewasawealthysocialitewhoseservantskeptchippingherfinechinawhilehandwashingit.Shedevelopedarackandwaterjetsystemthatwasfirstdemonstratedatthe1893ChicagoWorldFair.
Figure1:AproductfromtheFinishrange
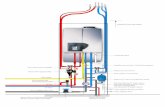
Howdoesadishwasherwork?(1of2)Beforeweconsiderthechemistry,weneedtounderstandthemechanicsofadishwasher,Figure2.Thisisessentiallyverysimple.
Figure2:Thebasiclayoutofadishwasher(courtesyofhowstuffworks.com)
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
Apumpdrawswaterthroughawatersoftenerunit/ionexchangerandintothebaseofthemachine.Hereaheaterheatsthewatertoasuitabletemperatureandthedishwasherproductdispenseropenssothatthedishwashertabletorpowdercontainingamixofchemicalsfallsintothewaterafterapproximately5‐10minutes,whenthewaterisstillcold.
Apumpthenforcesthewaterthroughtworotatingsprayarmswhichspraythewaterontothedishesarrangedintwobaskets,Figure3.
Figure3:Thewashingcycle(courtesyofhowstuffworks.com)
Thedirtywaterisremovedfromthebaseofthemachine,Figure4.
Figure4:Thedrainingcycle(courtesyofhowstuffworks.com)
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
Thisisthenrefilledwithcleanwaterwhichissprayedonthedishestorinsethem.
Youcanfindmoredetailfromthelinksbelow.
http://home.howstuffworks.com/dishwasher.htm
http://www.dishwasher‐care.org.uk/best.html
Howdoesadishwasherwork?(2of2)Atvariouspointsinthecycle,thewaterisheatedtoasuitabletemperaturefortheprocesstakingplace.TheprofileofatypicalwashingcycleisshowninFigure5inwhichtemperatureisplottedverticallyandtimehorizontally.
Hotwaterisshowninred,cold(ieroomtemperature)inblueandsectionsinvolvingtheionexchangeresininwhite.
Figure5:Atypicaldishwashercleaningcycle.Onlyintensiveprogramsuseprewashcycles.NormalorEcoprogramsimmediatelystartwiththemaincycle.Thedetergentisonlydosedinthemain
wash.
SofteningthewaterThefirstchemicalprocessindishwashingissofteningthewater.WaterinmostareasoftheUKcontainssignificantlevelsofCa2+andMg2+(andinsomecasesFe3+)ions.
Thesearisefromthefactthatinmanyareaswatersoaksthroughlimestonerocksanddissolvessomecompoundscontainingtheseions.Thisisassistedbythefactthatrainwaterisnaturallyacidicduetodissolvedcarbondioxide.Sotapwatercontainsdissolvedcompoundssuchascalciumhydrogencarbonateandmagnesiumsulfate.
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
Figure6:HardnessofwaterintheUK.Theunitsaremg/l,iemgdm‐3,ofcalciumcarbonateequivalent(CourtesyofCaterchemUK)
Themap,Figure6,indicatesgenerallythehardnessofwaterinvariouspartsoftheUK.Thesemetalionsinhardwatercaninterferewithanumberofthechemicalprocessesinvolvedindishwashing,whichareexplainedbelow,anditisthereforebettertoremovethem.Theymayalsocauselimescaletobuildupinsidethemachine.TherearemoredetailsofthechemistryofhardwateranditseffectoncleaningprocessesinCalgon.
Dishwasherssoftenwaterbypassingitthroughanionexchangeresinbeforeitentersthemachineitself.Theresiniswell‐named–itliterallyexchangesdoublyandtriplypositively‐chargedmetalionsforsinglypositively‐chargedsodiumionswhichdonotaffectthewashingprocesses.
Question1Explainwhymetalsalwaysformpositiveions.Thiscannotgoonforever,ofcourse‐theresineventuallyrunsoutofsodiumionstoexchange.Topreventthissituationtheresinisregeneratedbyflushingaconcentratedsolutionofsodiumchloridethroughit.Thisreplacesthecalciumandmagnesiumionsontheresinforsodiumionsagainandthecalciumandmagnesiumionsareflushedaway.Fromtimetotime,puresalt(sodiumchloride)mustbeaddedtothereservoirinthedishwasherwherethesaltsolutionismade.Tablesaltshouldnotbeused,asmostbrandsofthiscontainmagnesiumsulfateasananti‐cakingagent‐thelastthingrequiredinawatersoftener.
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
Thisprocessisessentiallyareversiblereactionandcanberepresentedbyanequation,whereRrepresentstheresin:
RNa+Na+(s)+Ca2+(aq) RCa2+(s)+2Na+(aq)
Althoughitisareversiblereaction,itneverreachesequilibriumasinbothsofteningandregenerationthewaterisconstantlypassingoverthesolidresin.
Whatdoesadishwashercleaningagentcontain?Thepurposeofadishwashercleaningagentistoremovedirt(oftencalled‘soil’)fromthearticlesinsidebydissolvingitormakingitintoasuspensioninthewashingwater.Itmustalsopreventitsre‐depositiononthearticlessothatthesoilcanbecarriedawaybythewashingwater.
Itmustbechemicallycompatiblewithallthematerialsbeingwashedandalsowiththematerialsfromwhichthedishwasheritselfismade.
Dishwashertabletsandpowderscontainasurprisingmixofchemicals,farmorethanjustthedetergentyoumightexpect.
Theseareasfollows:
Surfactants(detergents)‐thesepromotemixingbetweenoil‐andfat‐basedsoilandwater.
Alkalis‐theseemulsifygreaseandadjustthepHofthewatertotheoptimumfortheothercomponentstowork
Bleaches‐theseoxidisecolouredsubstancestocolourlessones Biosubstances‐theseareenzymesthatbreakdownstarch‐andprotein‐based
soils Builders‐thesehelptosoftenwaterandtrapmetalionsthatwouldinterfere
withthecleaningprocessandholddirtinsolution Auxiliaries‐theseincludesubstancesusedtomakeanddisintegratethetablet
aswellascoloursandperfumes
Oneproblemthatmaybeencounteredisgettingallthesedifferentingredientstoworktogetherwhentheyhavedifferentoptimumconditions.Enzymes,forexample,workbestatmoderatetemperaturesaround50°C‐seeFigure7.Theyaredenaturedandwillnolongerworkiftheyhavebeenexposedtotemperaturesmuchabovethisforanylengthoftime.
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
Figure7:Thetemperaturedependenceofenzymeactivity.Proteasesdecomposeproteinsandamylasesdecomposestarch‐basedsoils
Ontheotherhand,greaseremovalwillworkbestathightemperatureswhenfatsmelttooilsmakingthemeasiertoremove.And,ofcourse,allchemicalreactionsgofasterathighertemperatures‐twiceasfastforevery10°Criseisausefulruleofthumb.
ThereisasimilarissuewithpH.CleaningtakesplacebestatalkalinepHsbuttheoptimumpHformostenzymesisneutraltomildlyalkaline‐theyaredenaturedinstronglyacidicoralkalineenvironments.TheoptimumpHforthebleach,however,isaround10.
Onefurthercomplicationforthedesignersofdishwashersandtheirassociatedcleaningproductsistodowithuserbehaviour;itisusualtowashtogetheralargevarietyofmaterialse.g.glass,porcelain,plastics,anddifferentmetals.Thisisincontrastwithclotheswashing,whereusersseempreparedtoseparateoutdifferenttypesofmaterial‐wool,synthetics,cottonetc.Theoptimumconditionsformanyofthematerialsinthedishwasherwilldiffer.
Surfactantsordetergents(1of2)Greasystainsdonotmixwithwaterbecausethemaininteractionsbetweenwatermoleculesarehydrogenbondingandthosebetweenmoleculesofoilsandfats(whichconstitutegrease)arevanderWaalsforces.
Togetwaterandgreasetomixweusemoleculescalledsurfactantsordetergents.Thesetwotermsrefertoessentiallythesamething‐moleculesthatare‘tadpoleshaped’inthattheyhaveanon‐polar‘tail’andapolarorionic‘head’.The‘tail’canformvanderWaalsbondswithnon‐polargreasemoleculeswhilstthe‘head’canformhydrogenbondswithwater,Figure8.Thisisanexampleofthe‘likedissolveslike’rule.
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
Figure8:Thenon‐polartails(inyellow)of‘tadpoleshaped’detergentmoleculesmixwithgrease,whilethepolarheads(inred)mixwithwater,thusforcingthegreaseandwatertomix
Thereareessentiallythreetypesofsurfactants–anionic,cationicandnon‐ionic.
Anionicsurfactantshaveanegativelychargedhead.Commontypesincludesoaps,Figure9,andalkylbenzenesulfonates,Figure10.
Figure9:Sodiumstearate(asoap)–ananionicsurfactant
Figure10:Sodiumdodecylbenzenesulfonate–ananionicsurfactant
Cationicsurfactantshaveapositivelychargedhead.Commontypesincludealkylammoniumchlorides,Figure11.
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
Figure11:Trimethylhexadecylammoniumchloride–acationicsurfactant
Nonionicsurfactantshaveapolar,butuncharged,head.Commontypesincludepolyethyleneethoxylates,Figure12.
Figure12:Apolyethyleneethoxylate–anonionicdetergent
Surfactantsordetergents(2of2)Surfactantsareprobablytheingredientsoneexpectsinadishwasherproduct‐weoftencalltheproductsimplyadishwasherdetergent.However,theyplayarelativelyminorroleintheproduct.Non‐ionicpolyethyleneethoxylatesarechosenasthemainsurfactantsinFinishastheyproducerelativelylittlefoam.
Theirmainfunctionistoenablegreasysoilstomixwithwater.Muchofthesoilondishesisheldtherebygrease.Greasesandoilsarechemicallysimilar;theyconsistlargelyofestersoffattyacids‐longchaincarboxylicacids‐withglycerol,seeFigure13.Thesecontainlonghydrocarbonchainswhichareessentiallynon‐polar,andthereforedonotformhydrogenbondsordipole‐dipolebondswithwatermolecules.
Figure13:Atypicalfatoroil‐whichitisdependsonthechainlengthsofthethreefattyacidsandwhetherornottheirhydrocarbonchainsarebranched
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
Whendissolvedinwater,surfactantstendtoclusteratthesurface(hencethename),sothattheirnon‐polartailscanstickoutofthewater.Surfactantmoleculescanformstructurescalledmicelles:thesearesmallspheresmadeofsurfactantthattrapoilmoleculesandenablethemtodissolveinthewater.
Thedifferencebetweenfatsandoilsisoneofmeltingpoint‐atroomtemperaturefatsaresolidandoilsareliquidduetotheirshorterhydrocarbonchains.Solidgreasesadheretosurfacesbetterthanliquidoilswhichjusttendtorolloff.Soahightemperaturewhichturnsfatsintooilsishelpfulforcleaning.
AlkalisManycleaningproductsactbestinsomewhatalkalinesolution.
Thisisbecausealkalis
emulsifygreasebyreactingwithinsolublefattyacidstoformionicsaltswhicharesoluble
protectthemetalofwashingmachinesanddishwashersfromacidcorrosion helptoreducere‐depositionofdirtthathasbeenremoved,bycoatingparticlesof
itwithnegativelychargedhydroxideions–thismeansthedirtparticlesrepeleachotherandremaininsuspensionratherthanclumpingtogethertoformlargeaggregateswhichwouldtendtoprecipitateoutontodishes
percarbonate‐basedbleachesworkbestinsomewhatalkalinesolutionThedishwasherwateriskeptataboutpH10–significantlyalkaline.Atthehightemperatureofthewash,hydroxideionsreactwiththemoleculesofgreaseandbreakthemupintosaltsoffattyacidsandglycerol.Thisisthemainmechanismforremovinggrease.
ThereactionisshowninFigure14andisthesameasthatformakingsoapfromfatsandoils–saponification.
Figure14:Thesaponificationofgrease
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
Question2a)Explainwhytheproductsoftheabovereactionaremoresolublethantheoriginalfatmolecules.b)Givethesystematicnameofglycerol.
Sodiumcarbonate,Na2CO3,isusedinthedishwasherproducttomakethesolutionalkaline.Itisthesaltofastrongalkali(sodiumhydroxide)andaweakacid(carbonicacid)andisthereforealkaline.Itdissociatesinsolutiontoformcarbonateions,CO32‐,whichhelptomaintainthepHofthewashingwaterataround10.
Question3Explainhowthecarbonateioncanmakeanaqueoussolutionalkaline.Bleaches(1of2)Bleachesareusedtooxidisecolouredsubstancestocolourlessones.
Thebleachusedissodiumpercarbonate.ThisisawhitegranularpowderofformulaNa2CO3.3H2O2.
Figure15:Structureofsodiumpercarbonate
Inwateritbreaksdownintosodiumcarbonateandhydrogenperoxide.
2Na2CO3.3H2O2(aq) 2Na2CO3(aq)+3H2O2(aq)
Hydrogenperoxideistheactiveoxidisingagentasitinturnbreaksdowntooxygenandwater.Thebeautyofthissystemisthatthestartingmaterialisarelativelystablepowder(althoughitobviouslymustbekeptdry)andtheby‐products(sodiumcarbonateandwater)areinnocuous.
Question4a)Writeanequationforhydrogenperoxidedecomposingtooxygenandwaterb)Usethisandtheequationabovetoworkouthowmanymolesofoxygencanbeobtainedfrom2molesofsodiumpercarbonate.
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
c)Nowworkoutthesequantitiesintermsofgramsofsodiumpercarbonateandoxygen.d)Onecommercialspecificationforsodiumpercarbonateguarantees‘notlessthan13%activeoxygen.Fromyourcalculationabove,isthisrealistic?
Bleaches(2of2)Activatorsareaddedtohelpthebleacheswork.Theseareessentially,andactsimilarlyto,catalysts.Theyspeedupthereactionsothatitcantakeplaceeffectivelyatlowertemperaturesthanwithouttheactivator.However,activatorsareconsumedstoichiometricallywhilstcatalystsarenot.
Theuseofactivatorsmakesthebleachesmorecompatiblewiththeenzymesinthedishwasherformulation.
Question5Explainthephrase‘Thismakesthebleachesmorecompatiblewiththeenzymes.’
Figure16showbleachactivityandenzymeactivityatdifferentstagesofthewashcycle.
Figure16:Enzymeactivity(blue)andbleachactivity(red)atdifferentstagesofthewashcycle
BiosubstancesEssentially,theseareenzymes–
proteases(tohelpbreakdownproteins)
amylases(tohelpbreakdownstarches)
Question6Suggestfoodsthatarebasedona)proteinsb)starches
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
Proteasesdecomposebig,water‐insolubleproteinmoleculesintosmallsolubleunits.
C C
H
H
N C C
H
CH3
H O
N
H O
N
H
C
H
CH2SH
C
O
Figure17:Partofaproteinmolecule
Amylasesdecomposebig,water‐insolublestarchmoleculesintosmallsolubleunits.
O
OH
OH
CH2OH
O
O
OH
OH
CH2OH
O
O
OH
OH
CH2OH
O
O
OH
OH
CH2OH
O
Figure18:Partofastarchmolecule
Question7Suggestwhatarethe‘smallsolubleunits’producedbythebreakdownofa)proteinsb)starches
Inbothcases,thebreakdownprocessisahydrolysisreaction(reactionwithwater).
Question8a)LookatthestructuresinFigures17and18.Eachhasoneofthelinkageswherehydrolysistakesplacecircled.Drawtheproductsafterhydrolysishastakenplaceforpartofaproteinmoleculeb)LookatthestructuresinFigures17and18.Eachhasoneofthelinkageswherehydrolysistakesplacecircled.DrawtheproductsafterhydrolysishastakenplaceforpartofastarchmoleculeAsindicatedpreviously,enzymeshaveoptimumpHsandtemperatures,sothishastobebuiltintothedesignofthewashingcycle.Enzymeswillbedenaturedandceasetofunctionafterthemainwashcycleofthedishwasherprogram.
BuildersBuildershaveanumberoffunctionsincludingsoftening,bufferingandemulsifying.
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
Ca2+ionshavelargelybeenremovedfromthestartingwashingwaterbytheionexchangeresininthedishwasherbutmoremayoriginatefromthesoilitself.Thesecanbedealtwithbythebuilder.
Buildersusedincludephosphates,citratesandpolycarboxylates,Figure19.Noticethateachonehasoxygenatomswithanegativecharge.ThesecanformcomplexeswithCa2+ionsandeffectivelyremovethemfromsolution.
HO P O
O-
O
P O P OH
O- O-
OO
phosphate
H C C C
OH
COO-COO-COO-
H
HH
citrate
COO- COO- COO- COO-
polycarboxylate
Figure19:Structuresofsomecommonbuilders
Question9a)WhatfeaturedotheoxygenatomshavewhichenablesthemtoformabondwithCa2+ions?b)Whatnameisgiventothistypeofbonding?
TheCOO‐groupscanacceptprotonsfromacidicwaterandkeepitalkaline.
Itisoftenthoughtthatphosphatesarenolongerusedinhouseholdproductsbecausetheycangetintowatercoursesandcauseeutrophication‐theovergrowthofplantscausedbythefertilisingeffectofphosphates.InfactthereisnosuchblanketbanandphosphatesarestillusedindishwashingproductsasthecontributiontothetotalP‐loadislessthan5%.Themainquantityiscomingfromagriculturalland,humanmetabolismandindustrialwastewater.
RinseaidThefinalpartofthewashingcycleisarinsewithcleanwaterthathasbeensoftenedbytheionexchangeresin.Inmostmachinesitispossibletoaddarinseaidtothiscyclefromaseparatecompartmenttothemaindishwashingproduct.
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
Rinseaidisasurfactantthatreducesthesurfacetensionofwater.Surfacetensionisthe‘skin’effectonthesurfaceofwaterthatmakesit‘curlup’intodropletsoncleansurfaces,seeFigure20,ratherthanspreadinathinlayeroverthewholesurface.
Figure20:Thesurfacetensionofwatercausestheformationofdroplets
Surfacetensioniscausedbyhydrogenbondsbetweenwatermoleculesatthesurfaceofliquidwater.Surfactantmoleculesclusteratthesurfaceofawaterdropletbecausetheir‘tails’donotformhydrogenbondswithwaterandthesurfaceistheonlyplacewherethiscanoccur.Thisdisruptsthehydrogenbonding,loweringthesurfacetensionandallowingthewatertospreadout,leavingagreatersurfaceareawhichspeedsevaporationandhencedrying.
Italsoallowsthewatertodrainmoreeasilyfromthesurfacesofthedishesasshownbelow.
Figure21:Rinseaidproducesdropletswithagreatersurfaceareatospeeddryinganddraining
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
RecentdevelopmentsDishwashertechnologycontinuestoimprove;newermachinesmayhavesensorswhichcanbeusedtodeterminethebestprogramforawash.Theseinclude:
determinationoftheamountofsoilinthewashingwater
measurementofwaterhardness
determinationofdishloadQuestion10Suggesthowthehardnessofthewaterenteringadishwashermightbemeasured.Remembertheresultmustbeobtainedquicklyand‘electronically’sothatitcanbefedbacktocontrolthemachine,soatitration,forexample,wouldnotdo.Hint,hardnessiscausedbyaqueousCa2+andMg2+ions.Forexampletheamountofsoilinthewatercanbemeasuredbyshiningalightthroughasampleofwaterandnotinghowmuchisabsorbed–themorelightabsorbed,thedirtierthewater.
Figure22:Dishwashinginaction(courtesyofhowstuffworks.com)
DifferentformulationsFinishbrandedproductsaresoldinanumberofformswithdifferentformulations,Figure23.
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
Figure23:DifferentformsandformulationsofFinish
Theingredientsofjustoneformulation,FinishPowerballclassictabletsregular,arelistedinFigure24.
Figure24:IngredientsfoundinFinishPowerballclassictabletsregular
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
Someofthesehavebeendiscussedabovebutthereareanumberofothersthatarebeyondthescopeofthispiece.Allofthemcontributetothefinalproductinsomeway.
FurtherinformationSeveralvarietiesofFinish™aresoldintheUKbyReckittBenckiser(http://www.finish.co.uk/).Thereareotherbrandedproductswhichworkinasimilarway.
Teachersandstudentswhoareinterestedinmoredetailsabouthouseholdproductsmightwishtoconsultthefollowingbook:
G.Wagner etal, HouseholdCleaning,CareandMaintenance‐Products‐Chemistry,Application,EcologyandConsumerSafety, VerlagfürchemischeIndustrie ISBN3‐87846‐241‐7
AcknowledgementsTheRoyalSocietyofChemistrywishestothankChrisJones,AlexandraHaryandGunnarNoedingofReckittBenckiserforhelpinpreparingthismaterial.
TheRoyalSocietyofChemistrygratefullyacknowledgesthatthisprojectwasinitiallysupportedbyReckittBenckiserin2007.ReckittBenckiserconductedafinalreviewin2013sopleasenotethatcertaininformationmaybeoutofdate.
QUESTIONSANDANSWERSQuestion1Explainwhymetalsalwaysformpositiveions.Metalsallhaveone,twoorthreeelectronsintheiroutershells.Itiseasiertolosethesetoattainafulloutershellofelectrons(anoblegasarrangement)thantogainelectrons.Lossofnegatively‐chargedelectronsproducespositiveions.
Question2a)Explainwhytheproductsoftheabovereactionaremoresolublethantheoriginalfatmolecules.Oneproductisanegativeionwhichcaninteractwithwatermoleculesbetterthananeutralmolecule.Theotherisasmallmoleculewithahighproportionof–OHgroupsthatcanhydrogenbondwithwater.
b)Givethesystematicnameofglycerol.Propane‐1,2,3‐triol.
Question3Explainhowthecarbonateioncanmakeanaqueoussolutionalkaline.Itcanacceptprotons(H+ions)fromthesolutiontoformHCO3‐,leavinganexcessofOH‐ions.
Question4a)Writeanequationforhydrogenperoxidedecomposingtooxygenandwater
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
2H2O22H2O+O2
b)Usethisandtheequationabovetoworkouthowmanymolesofoxygencanbeobtainedfrom2molesofsodiumpercarbonate.2molsodiumpercarbonateproduces3molhydrogenperoxide,whichwouldproduce1.5moloxygenmolecules.
c)Nowworkoutthesequantitiesintermsofgramsofsodiumpercarbonateandoxygen.2molsodiumpercarbonatehasamassof314g
1.5moloxygenmoleculeshasamassof48g
d)Onecommercialspecificationforsodiumpercarbonateguarantees‘notlessthan13%activeoxygen.Fromyourcalculationabove,isthisrealistic?(48/314)x100=15.3%,sothespecificationisrealistic.
Question5Explainthephrase‘Thismakesthebleachesmorecompatiblewiththeenzymes.’Enzymeshavetheiroptimumtemperatureataround50oC.Abovethistemperaturetheproteinsfromwhichtheyaremadedenature.Thebleachingreaction,ontheotherhand,willcontinuetoworkfasterasthetemperatureincreasesandso,ideally,ahighertemperaturethan50oCwouldbebetter.
Question6Suggestfoodsthatarebasedona)proteinsMeats,cheese,eggetc
b)starchesBread,pasta,riceetc
Question7Suggestwhatarethe‘smallsolubleunits’producedbythebreakdownofa)proteinsAminoacids
b)starchesSugars
Question8a)LookatthestructuresinFigures17and18.Eachhasoneofthelinkageswherehydrolysistakesplacecircled.Drawtheproductsafterhydrolysishastakenplaceforpartofaproteinmolecule
C C
H
H
N C C
H
CH3
H O
N
H O
N
H
C
H
CH2SH
C
O
HOH
FinishisaregisteredtrademarkoftheReckittBenckisergroupofcompanies.LastreviewedbyReckittBenckiserin2013
b)LookatthestructuresinFigures17and18.Eachhasoneofthelinkageswherehydrolysistakesplacecircled.Drawtheproductsafterhydrolysishastakenplaceforpartofastarchmolecule
O
OH
OH
CH2OH
O
O
OH
OH
CH2OH
O
O
OH
OH
O
CH2OH
O
O
OH
OH
CH2OH
OH H
Question9a)WhatfeaturedotheoxygenatomshavewhichenablesthemtoformabondwithCa2+ions?Lonepairsofelectrons.
b)Whatnameisgiventothistypeofbonding?Dativeorco‐ordinatebonding.
Question10Suggesthowthehardnessofthewaterenteringadishwashermightbemeasured.Remembertheresultmustbeobtainedquicklyand‘electronically’sothatitcanbefedbacktocontrolthemachine,soatitration,forexample,wouldnotdo.Hint,hardnessiscausedbyaqueousCa2+andMg2+ions.Waterhardnesscouldbedeterminedbymeasuringtheelectricalconductivityofthewater–theharderthewater,themoreionsandthehighertheconductivity.(Othersuggestionsarepossible)