C-Terminal Domain Swapping of SSB Changes the Size of · PDF fileC-Terminal Domain Swapping of...
-
Upload
truongquynh -
Category
Documents
-
view
226 -
download
1
Transcript of C-Terminal Domain Swapping of SSB Changes the Size of · PDF fileC-Terminal Domain Swapping of...
Research ArticleC-Terminal Domain Swapping of SSB Changes the Size ofthe ssDNA Binding Site
Yen-Hua Huang1 and Cheng-Yang Huang12
1 School of Biomedical Sciences Chung Shan Medical University No110 Sec1 Chien-Kuo N Rd Taichung City Taiwan2Department of Medical Research Chung Shan Medical University Hospital No110 Sec1 Chien-Kuo N Rd Taichung City Taiwan
Correspondence should be addressed to Cheng-Yang Huang cyhuangcsmuedutw
Received 26 May 2014 Accepted 9 July 2014 Published 4 August 2014
Academic Editor Huangen Ding
Copyright copy 2014 Y-H Huang and C-Y Huang This is an open access article distributed under the Creative CommonsAttribution License which permits unrestricted use distribution and reproduction in any medium provided the original work isproperly cited
Single-stranded DNA-binding protein (SSB) plays an important role in DNA metabolism including DNA replication repair andrecombination and is therefore essential for cell survival Bacterial SSB consists of an N-terminal ssDNA-bindingoligomerizationdomain and a flexible C-terminal protein-protein interaction domainWe characterized the ssDNA-binding properties ofKlebsiellapneumoniae SSB (KpSSB) Salmonella enterica Serovar TyphimuriumLT2 SSB (StSSB) Pseudomonas aeruginosa PAO1 SSB (PaSSB)and two chimeric KpSSB proteins namely KpSSBnStSSBc and KpSSBnPaSSBc The C-terminal domain of StSSB or PaSSB wasexchanged with that of KpSSB through protein chimeragenesis By using the electrophoretic mobility shift assay we characterizedthe stoichiometry of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSSBnPaSSBc complexed with a series of ssDNA homopolymersThe binding site sizes were determined to be 26plusmn2 21plusmn2 29plusmn2 21plusmn2 and 29plusmn2 nucleotides (nt) respectively Comparison of thebinding site sizes of KpSSB KpSSBnStSSBc and KpSSBnPaSSBc showed that the C-terminal domain swapping of SSB changes thesize of the binding site Our observations suggest that not only the conserved N-terminal domain but also the C-terminal domainof SSB is an important determinant for ssDNA binding
1 Introduction
Single-stranded DNA-binding protein (SSB) specificallybinds to single-stranded DNA (ssDNA) and is known tohave important functions in the DNA metabolic processessuch as DNA replication repair and recombination of bothprokaryotes and eukaryotes [1ndash4] During these reactionsSSB binds to and protects susceptible ssDNA fromnucleolyticdigestion and chemical attacks and also prevents secondarystructure formation [5] Many but not all bacterial andhuman mitochondrial SSBs are active as homotetramers [5ndash7] in which four oligonucleotideoligosaccharide-bindingfolds (OB folds) form a DNA-binding domain [8ndash12] How-ever SSB from the bacterial phylum Deinococcus-Thermusfunctions as a homodimer in which each monomer containstwo OB folds linked by a conserved spacer sequence [13ndash20]SSB from Sulfolobus solfataricus is a monomer that includesoneOB fold which differentiates SSB from the bacterial formand is likely to be a more ancestral ldquosimplerdquo SSB [21ndash25] The
DdrB protein from Deinococcus radiodurans is an alternativeSSB and functions as a pentamer [26] Recent studies foundthat a distinct SSB from hyperthermophilic Crenarchaeatermed ThermoDBP has ssDNA-binding domains that aremarkedly different from the classicalOB folds of bacterial SSB[27 28]
Bacterial SSBs consist of two domains namely an N-terminal ssDNA-bindingoligomerization domain and a flex-ible C-terminal protein-protein interaction domainwithout adefined tertiary structure [3 29] Tyrosine phosphorylation ofSSB increases binding to ssDNA by almost 200-fold in vitro[30 31] The N-terminal domain is separated from the highlyconserved acidic tail of the last 10 C-terminal amino acidresidues of SSB by a long proline- or glycine-rich hinge [3 32]SSB interacts with other auxiliary proteins that are essentialfor cell survival [33] The C-terminal acidic tail of SSB suchas ldquoDDDIPFrdquo has been shown to bind to more than a dozendifferent proteins and the activity of some of these proteins isstimulated by their interactions with ssDNA-bound SSB [3]
Hindawi Publishing CorporationBioMed Research InternationalVolume 2014 Article ID 573936 16 pageshttpdxdoiorg1011552014573936
2 BioMed Research International
The binding of SSB to ssDNA makes the glycine-rich regionmore easily accessible to other proteins such as proteases andDNA polymerase III [33 34]The C-terminus in SSB can alsointeract with the OB fold and regulate the ssDNA-bindingactivity of SSB itself [35 36]
Studies on SSB from different organisms have grownrapidly during the past few years and knowledge on howSSBs interact with ssDNA has increased [22 32 37ndash46]The most thoroughly studied SSB is that of Escherichia coli(EcSSB) which binds cooperatively to ssDNA [47] The esti-mated binding site size of EcSSB is dependent on the saltconcentration in fluorescence titrations with poly(dT) [47]EcSSB mainly binds to 35- and 65-nucleotide- (nt) longssDNA via the (SSB)
35- and (SSB)
65-binding modes respec-
tively In the (SSB)35-binding mode two subunits of the
EcSSB tetramer interact with ssDNA whereas in the (SSB)65-
bindingmode all four subunits participate in ssDNAbindingThese different binding modes may be required during dif-ferent stages of DNA metabolism for the in vivo function ofSSB [48ndash50] Although SSB binds to ssDNA via the highlyconserved ssDNA-binding domain the reason that the bind-ing site sizes of SSBs from different organisms differ remainsunclear For example differences are found among the bind-ing site sizes ofMethanococcus jannaschii SSB [51] the Gono-coccal Genetic Island-encoded SSB from Neisseria gonorr-hoeae [39] the thermostableThermotoga maritima andTher-motoga neapolitana SSBs [32] and the psychrophilic bacterialSSBs [37] In addition the (SSB)
35- and (SSB)
65-binding
modes are not found in some SSBs [32 39 42]Previously we have examined the electrophoretic mobil-
ity shift patterns of a His-tagged Klebsiella pneumoniae SSB(KpSSB) [40] a His-tagged Salmonella enterica serovar Typh-imurium LT2 SSB (StSSB) [43] and a His-tagged Pseu-domonas aeruginosa PAO1 SSB (PaSSB) [42] bound to differ-ent lengths of ssDNA We also determined their correspond-ing binding site sizes that is 26 22 and 29 nt per tetramerrespectively The electrophoretic mobility shift assay (EMSA)is a well-established approach in studies of molecular biology[52] and the use of radioactive tracer in this assay allows visu-alization of the actual formation of the distinct protein-DNAcomplex(es)[53] The expected result of EMSA is that whenthe length of the nucleotides is sufficient for the binding oftwo or more SSB molecules the electrophoretic mobility ofthe higher SSB oligomer complex will be lower than that ofthe smaller SSB oligomer complex [52 54] Recent studieson SSB binding also reveal that determination of the ssDNA-binding site size by using EMSA is significantly consistentwith that of the cocrystal structure of SSB with ssDNA [27]
KpSSB StSSB and PaSSB are similar proteins whoseN-terminal ssDNA-binding domains are almost identicalexcept for different ssDNA-binding site sizes [40 42 43]Thus we should assess whether the glycine-rich hinge whichis not conserved among SSBs is involved in the determina-tion of the binding site size of SSB In this study we swappedthe C-terminal domains of StSSB and PaSSB into that ofKpSSB through protein chimeragenesis Chimeras are pro-teins that contain segments from two ormore different parentproteins and serve as valuable tools to understand enzyme
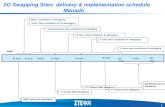
mechanism and protein function [55] The EMSA behav-ior (patterns) of the resultant chimeric proteins namelyKpSSBnStSSBc and KpSSBnPaSSBc was characterized andcomparedwith untaggedKpSSB StSSB and PaSSB (Figure 1)On the basis of the chimeragenesis results the flexible C-ter-minal domain of SSBwas found to be involved in determiningthe ssDNA-binding site sizes
2 Materials and Methods
21 Materials All restriction enzymes and DNA-modifyingenzymes were purchased from New England Biolabs(Ipswich MA USA) unless explicitly stated otherwise Allchemicals were purchased from Sigma-Aldrich (St LouisMO USA) unless explicitly stated otherwise The E colistrains TOP10F1015840 (Invitrogen USA) and BL21(DE3)pLysS(Novagen UK) were used for genetic construction and pro-tein expression respectively
22 Construction of Plasmids for KpSSB StSSB and PaSSBExpression The KpSSB [40] StSSB [43] and PaSSB [42]expression plasmids were constructed by the protocolsdescribed previously with minor modification to avoidhaving aHis tag fusedwith the gene product A fragment con-taining the coding sequence of KpSSB (KPN04446) StSSB(STM4256) and PaSSB (PA4232) (with the stop codon) wasdirectly amplified by PCR by using the genomic DNA ofK pneumoniae subsp pneumoniae MGH 78578 S entericaserovar Typhimurium LT2 or P aeruginosa PAO1 (Primers1 to 6 resp) During the process NdeI and XhoI restrictionsites were introduced at the 51015840-end and the 31015840-end of thesegenes after which they were ligated into the pET21b vector(Novagen Inc Madison WI USA) for protein expression inE coli BL21 The expected gene product expressed by theseplasmids does not contain any artificial residue including aHis tag Primers used for construction of these plasmids aresummarized in Table 1
23 Construction of Plasmids for KpSSBnStSSBc and KpSSBn-PaSSBc Expression through Protein Chimeragenesis To inves-tigate the effect of the C-terminal domain of SSB on thesize of the ssDNA-binding site the C-terminal domain ofKpSSB was replaced by that of StSSB and PaSSB pET21b-KpSSB (Primers 7 and 8) pET21b-StSSB (Primers 9 and10) and pET21b-PaSSB (Primers 11 and 12) vectors weremutated to create a desired SacI site and to obtain thevectors for expression of the chimeric proteins KpSSB-nStSSBc and KpSSBnPaSSBc The D91EQ92L-engineeredpET21b-KpSSB vector the D91EQ92L-engineered pET21b-StSSB vector and the G90EQ91L-engineered pET21b-PaSSBvector were cut at NdeI and SacI sites Subsequently theKpSSBn StSSBc-pET21b and PaSSBc-pET21b fragmentswere purified KpSSBn was ligated with StSSBc-pET21band PaSSBc-pET21b fragments to generate the engineeredpET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc vectorsTo avoid artificial residues positions 91 and 92 of the twoplasmids were mutated back (Primers 13 to 16) to obtainpET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc vectors
BioMed Research International 3
StSSB KpSSB PaSSB91 92
91 92 91 92 90 91
90 9191 92
91 92 91 92
91 92 91 92
E L
G Q
E L E L
E LE L
Mutation
N- N- N-
CAT CAG CAT CAA GGC CAG
GAG CTC GAG CTC GAG CTC
- C -C-C
Mutation Mutation
GAG CTC GAG CTC
CAT CAG CAT CAG
1
1 1
1
176
176
166
166
KpSSBnStSSBc
KpSSBnStSSBc
KpSSBnPaSSBc
KpSSBnPaSSBc
NdeISacISacI SacISacI
NdeISacINdeISacI NdeISacI
fragment purification and ligation
KpSSBn fragment purificationand ligation fragment purification
and ligation
Mutation Mutation
NdeI NdeI NdeIXhoI XhoI XhoI
StSSBc-pET21 PaSSBc-pET21
D
D D
Q
Q Q
D Q
Figure 1 Construction of plasmids for expression of the chimeric KpSSBnStSSBc and KpSSBnPaSSBc proteins To investigate the effect of theC-terminal domain of SSB on the size of the ssDNA-binding site the C-terminal domain of KpSSB was replaced by that of StSSB and PaSSBpET21b-KpSSB (Primers 7 and 8) pET21b-StSSB (Primers 9 and 10) and pET21b-PaSSB (Primers 11 and 12) vectors were mutated to createa desired SacI site and to obtain the vectors for expression of the chimeric proteins KpSSBnStSSBc and KpSSBnPaSSBc The D91EQ92L-engineered pET21b-KpSSB vector the D91EQ92L-engineered pET21b-StSSB vector and the G90EQ91L-engineered pET21b-PaSSB vectorwere cut at NdeI and SacI sites Subsequently the KpSSBn StSSBc-pET21b and PaSSBc-pET21b fragments were purified KpSSBn was ligatedwith StSSBc-pET21b and PaSSBc-pET21b fragments to generate the engineered pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc vectorsTo avoid artificial residues positions 91 and 92 of the two plasmids were mutated back (Primers 13 to 16) to obtain pET21b-KpSSBnStSSBcand pET21b-KpSSBnPaSSBc vectors Thus pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc will express KpSSB1-91 fused StSSB92-176and PaSSB91-165 respectively Note that KpSSBnPaSSBc will have 166 amino acid residues
Thus pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBcwill express KpSSB1-91 fused StSSB92-176 and PaSSB91-165respectively Note that KpSSBnPaSSBc will have 166 aminoacid residues Plasmids were verified by DNA sequencingUnderlined nucleotides indicate the designated site for muta-tion or the restriction site (Table 1)
24 Protein Expression and Purification The recombinantSSBs were expressed using the protocol described previously[9 40 42 43 56ndash60] Purification of these recombinant SSBswas carried out as described previously with the followingmodifications [61 62] Briefly E coli BL21(DE3) cells wereindividually transformed with the expression vector andgrown to OD
600of 09 at 37∘C in Luria-Bertani medium
containing 250 120583gmL ampicillin with rapid shaking Over-expression of the expression plasmids was induced by incu-bating with 1mM isopropyl thiogalactoside (IPTG) for 3 h at37∘CThe cells overexpressing the protein were chilled on iceharvested by centrifugation resuspended in Buffer A (20mMTris-HCl 5mM imidazole and 02M ammonium sulfate pH79) and disrupted by sonicationwith ice coolingThe proteinsolution (50mL) was precipitated from the supernatant ofthe cell lysate by incubation with 027 gmL of ammoniumsulfate for 30min and centrifugation at 20000 g for 10minThepelletswerewashed twicewith 20mLof Buffer B (20mM
Tris-HCl 5mM imidazole and 12M ammonium sulfatepH 79) After dialysis against Buffer C (20mM Tris-HCl5mM imidazole 1mMEDTA and 100mMNaCl pH 79) theprotein solution applied to theQ column (GEHealthcare Bio-Sciences Piscataway NJ USA) was eluted with a linear NaClgradient from 01 to 06M with Buffer C using the AKTA-FPLC system (GE Healthcare Bio-Sciences Piscataway NJUSA) The peak fractions with the ssDNA-binding activitywere collected and dialyzed against Buffer D (20mM potas-sium phosphate 1mM EDTA and 100mM NaCl pH 70)The protein solution was then applied to the Heparin HPcolumn (GE Healthcare Bio-Sciences Piscataway NJ USA)and eluted with a linear NaCl gradient from 01 to 10Mwith Buffer DThe peak fractions from this chromatographicstep with the ssDNA-binding activity were collected andconcentrated and the purity of these SSBs was checkedby Coomassie-stained SDS-PAGE (Mini-PROTEAN TetraSystem Bio-Rad CA USA Figure 3)
25 Protein Concentration The protein concentration of thesolutions was determined by the Bio-Rad Protein Assay usingbovine serum albumin as a standard (Bio-Rad CA USA)The Bio-Rad Protein Assay is a dye-binding assay in which adifferential color change of a dye occurs in response to variousconcentrations of protein
4 BioMed Research International
Table 1 Primers used for construction of plasmids
Oligonucleotide Primer1 KpSSB-NdeI-N GGGCATATGGCCAGCAGAGGCGTAAAC2 KpSSB-XhoI-C GGGCTCGAGTTAGAACGGGATGTCGTC3 StSSB-NdeI-N CTGAACATATGGCCAGCAGAGGCGTAA4 StSSB-XhoI-C TGGAACTCGAGTTAGAACGGAATGTCG5 PaSSB-NdeI-N TTGCTCATATGGCCCGTGGGGTTAACA6 PaSSB-XhoI-C TTGCACTCGAGTTAGAACGGAATGTCG7 KpSSB(D91EQ92L-SacI)-N AAGTGGACCGAGCTCTCCGGTCAGGACA8 KpSSB(D91EQ92L-SacI)-C GTCCTGACCGGAGAGCTCGGTCCACTT9 StSSB(D91EQ92L-SacI)-N AAGTGGACCGAGCTCAGTGGCCAGGAA10 StSSB(D91EQ92L-SacI)-C TTCCTGGCCACTGAGCTCGGTCCACTT11 PaSSB(G90EQ91L-SacI)-N AAGTGGCAGGAGCTCGACGGTCAGGAT12 PaSSB(G90EQ91L-SacI)-C ATCCTGACCGTCGAGCTCCTGCCACTT13 KpSSBnStSSBc(E91DL92Q)-N AAGTGGACCGATCAGAGTGGCCAGGAA14 KpSSBnStSSBc(E91DL92Q)-C TTCCTGGCCACTCTGATCGGTCCACTT15 KpSSBnPaSSBc(E91DL92Q)-N AAGTGGACCGATCAGGACGGTCAGGAT16 KpSSBnPaSSBc(E91DL92Q)-C ATCCTGACCGTCCTGATCGGTCCACTTA fragment containing the coding sequence of KpSSB StSSB and PaSSB (with the stop codon) was cloned into the pET21b vector (using Primers 1ndash6) Duringthe process NdeI and XhoI restriction sites were introduced at the 51015840-end and the 31015840-end of these genes after which they were ligated into the pET21b vectorTo obtain the vectors for expression of the chimeric proteins KpSSBnStSSBc and KpSSBnPaSSBc pET21b-KpSSB (Primers 7 and 8) pET21b-StSSB (Primers9 and 10) and pET21b-PaSSB (Primers 11 and 12) vectors were mutated to create a desired SacI site The D91EQ92L-engineered pET21b-KpSSB vectorthe D91EQ92L-engineered pET21b-StSSB vector and the G90EQ91L-engineered pET21b-PaSSB vector were cut at NdeI and SacI sites Subsequently theKpSSBn StSSBc-pET21b and PaSSBc-pET21b fragments were purified KpSSBn was ligated with StSSBc-pET21b and PaSSBc-pET21b fragments to generatethe engineered pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc vectors To avoid artificial residues positions 91 and 92 of the two plasmids were mutatedback (Primers 13 to 16) to obtain pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc vectors Thus pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc willexpress KpSSB1-91 fused StSSB92-176 and PaSSB91-165 respectively These plasmids were verified by DNA sequencing Underlined nucleotides indicate thedesignated site for mutation or the restriction site
26 Gel-Filtration Chromatography Gel-filtration chroma-tography was carried out by the AKTA-FPLC system (GEHealthcare Bio-Sciences Piscataway NJ USA) Briefly puri-fied protein (2mgmL) was applied to a Superdex 200HR1030 column (GE Healthcare Bio-Sciences Piscataway NJUSA) equilibrated with Buffer D The column was operatedat a flow rate of 05mLmin and 05mL fractions were col-lected The proteins were detected by measuring the absorb-ance at 280 nm The column was calibrated with proteinsof known molecular weight thyroglobulin (670 kDa) 120574-globulin (158 kDa) ovalbumin (44 kDa) and myoglobin(17 kDa) The 119870av values for the standard proteins and theSSB variants were calculated from the equation 119870av = (119881119890 minus119881119900)(119881119888minus 119881119900) where 119881
119900is column void volume 119881
119890is elution
volume and 119881119888is geometric column volume
27 Electrophoretic Mobility Shift Assay (EMSA) EMSA [52]for these SSBs was carried out by the protocol describedpreviously for DnaB [63] PriB [59 64ndash66] DnaT [57 67]and SSB proteins [40 42 43 52] Briefly radiolabeling ofvarious lengths of ssDNA oligonucleotides was carried outwith [12057432P]ATP (6000Cimmol PerkinElmer Life SciencesWaltham MA) and T4 polynucleotide kinase (PromegaMadison WI USA) The protein (0 19 37 77 155 310 6301250 2500 and 5000 nM) was incubated for 30min at 25∘Cwith 17 nM DNA substrates (dT15ndash65) in a total volume of10 120583L in 20mMTris-HCl pH 80 and 100mMNaCl Aliquots(5 120583L) were removed from each of the reaction solutions and
added to 2 120583L of gel-loading solution (025 bromophenolblue and 40 sucrose) The resulting samples were resolvedon a native 8 polyacrylamide gel at 4∘C in TBE buffer(89mMTris borate and 1mMEDTA) for 1 h at 100V andwerevisualized by autoradiography Complexed and free DNAbands were scanned and quantified
28 DNA-Binding Ability The ssDNA-binding ability([Protein]
50 119870119889app) for the protein was estimated from the
protein concentration that binds 50 of the input ssDNA[52] Each [Protein]
50is calculated as the average of three
measurements plusmn SD
29 Bioinformatics Sequence alignment of KpSSB StSSBandPaSSBwas generated byCLUSTALW2 [68]The structureof the C-terminal domain of these SSBs was modeled by(PS)2 (http140113239111simps2v2docsphp) The struc-tures were visualized by using the program PyMol
3 Results
31 Sequence Analysis Based on the nucleotide sequencefound using a database search through the National Centerfor Biotechnology Information (NCBI) we predicted thatKpSSB StSSB and PaSSB monomer proteins have lengths of174 176 and 165 amino acid residues respectively The sizeof the ssDNA-binding site of His-tagged KpSSB [40] StSSB
BioMed Research International 5
M A S R G V N K V I L V G N L G Q D P E V R Y M P S G G A V A N F T L A T S E S W R D K Q T G
M A S R G V N K V I L V G N L G Q D P E V R Y M P S G G A V A N L T L A T S E S W R D K Q T G
M A - R G V N K V I L V G N V G G D P E T R Y M P N G N A V T N I T L A T S E S W K D K Q T G
K L A E V A G E Y L R K G S Q V Y I E G Q L R T R K W T D Q S G Q D K Y T T E V - V V N V G G
K L A E V A G E Y L R K G S Q V Y I E G Q L R T R K W T D Q S G Q E R Y T T E I N V P Q I G G
R L A E I A G E Y L R K G S Q V Y V E G S L R T R K W Q G Q D G Q D R Y T T E I - V V D I N G
G G G Q Q Q G G W G Q P Q Q P Q - - - G G N Q F S G G A Q S R P Q Q Q A P A A P S N E P P M D
G - G Q Q Q G G W G Q P Q Q P Q Q P Q G G N Q F S G G A Q S R P Q Q S A P - A P S N E P P M D
G - D D S Q R A P R E P M Q R P - - - - - - Q Q A P Q Q Q S R P A P Q Q Q P A P Q P A Q D Y D
E M K E Q T E W H R V V L F G
E M K E Q T E W H R V V M F G
Q Q Q E R T E W H R V V F F G
T M Q M L G G R Q G G G A P A
V M Q M L G G R Q G G G A P A
N M Q L L G G R - - - - - P S
- F D D D I P F
- F D D D I P F
S F D D D I P F
62
62
61
123
124
120
174176
165
KpSSB
StSSB
PaSSB
KpSSB
StSSB
PaSSB
KpSSB
StSSB
PaSSB
Figure 2 Multiple amino acid sequence alignment of SSB proteins Sequence alignment of KpSSB StSSB and PaSSB was generated byCLUSTALW2 Identical amino acid residues are colored in red Gly and Gln residues are shaded in cyan and gray The N-terminal domainsof these SSBs are significantly conserved
(kDa)
40-
35-
25-
15-
M 1 2 3 4 5
Figure 3 Protein purity Coomassie Blue-stained SDS-PAGE (15)of the purified KpSSB (lane 1) StSSB (lane 2) PaSSB (lane 3)KpSSBnStSSBc (lane 4) KpSSBnPaSSBc (lane 5) and molecularmass standards (M) are shown The sizes of the standard proteinsfrom the top down are as follows 55 40 35 25 15 and 10 kDa Thepurified SSBs migrated between the 25 and 15 kDa standards on theSDS-PAGE
[43] and PaSSB [42] was determined to be 26plusmn 1 22plusmn 1 and29plusmn1 nt respectivelyThe longer the length of the polypeptidechain the smaller the size for ssDNA binding Analysis ofthe primary structures of KpSSB StSSB and PaSSB by RPS-BLAST revealed the presence of a putative OB-fold domainthat is common to all known SSBs Figure 2 shows that thealignments of the amino acid sequences of KpSSB StSSB andPaSSB amino acid residues in their N-terminal domains arehighly conserved (colored in red) In the E coli SSB-ssDNAcomplex [11] four essential aromatic residues namely Trp40Trp54 Phe60 and Trp88 participate in ssDNA binding viastacking interactions [11] These residues are conserved inmost SSB families including KpSSB StSSB and PaSSB Theimportantmotif in the C-terminal tail of E coli SSB DDDIPFresidues is also conserved in KpSSB StSSB and PaSSB By
contrast to those motifs the residues found in the glycine-rich hinge of E coli SSB are not conserved in KpSSB StSSBand PaSSB (Figure 2) Thus the length and composition ofthe amino acid residues in the glycine-rich hinge may beresponsible for the different ssDNA-binding site sizes of SSBs
32 Expression and Purification of KpSSB StSSB and PaSSBThe N-terminal ssDNA-binding domain of SSB has beenwell-established to be highly conserved However SSBspossessing different ssDNA-binding site sizes have beenreported The reason that SSBs have similar ssDNA-bindingdomains but possess varying ssDNA-binding site sizesremains unclear Although the ssDNA-binding site sizes ofKpSSB StSSB and PaSSB have been reported we reinves-tigated the ssDNA-binding properties of KpSSB StSSB andPaSSB in the absence of a His tag to avoid the unknown effectof a His tag (hexahistidine) on the ssDNA binding of SSB
33 KpSSB Bound to ssDNA To investigate the length ofnucleotides sufficient for the formation of the KpSSB-ssDNAcomplex and the ssDNA-binding ability of KpSSB westudied the binding of KpSSB to dT20 (Figure 4(a)) dT25(Figure 4(b)) dT35 (Figure 4(c)) dT45 (Figure 4(d)) dT50(Figure 4(e)) dT55 (Figure 4(f)) and dT60 (Figure 4(g))with different protein concentrations As shown inFigure 4(a) no band shift was observed when KpSSBwas incubated with dT20 indicating that KpSSB couldnot form a stable complex with this homopolymer Bycontrast to dT20 longer dT homopolymers which includedT25ndash50 produced a significant band shift (C complex)that is formation of a stable protein-DNA complex insolution Furthermore two different complexes for dT55were formed by KpSSB (Figure 4(f)) At lower proteinconcentrations KpSSB formed a single complex (C1) withdT55 similar to that observed with dT50 (Figure 4(e))However when the KpSSB concentration was increasedanother slower migrating complex (C2) was observed
6 BioMed Research International
[KpSSB]
-dT20
mdash
(a)
-dT25
-C
mdash[KpSSB]
(b)
-dT35
-C
mdash[KpSSB]
(c)
-dT45
-C
mdash[KpSSB]
(d)
-dT50
-C
[KpSSB]
mdash
(e)
-dT55
-C1-C2
[KpSSB]mdash
(f)
-dT60
-C1-C2
[KpSSB]mdash
(g)
Figure 4 Binding of KpSSB to dT20ndash60 KpSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT20 (b) dT25 (c) dT35 (d) dT45 (e) dT50 (f) dT55 or (g) dT60 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
Two different complexes of KpSSB were also observedto bind to dT60 (Figure 4(g)) The appearance of thesecond complex resulted from the increased KpSSB con-centration suggesting that two KpSSB proteins maybe present per oligonucleotide Although dT55 is only 5 ntlonger thandT50 is the presence of an extra 5 nt in dT55 com-pared with that of dT50 provides enough interaction spacefor the binding of two KpSSB proteins Therefore one KpSSB
occupies 25 (502 = 25) nt to 275 (552 = 275) nt of thessDNA The EMSA results suggest that the length of anssDNA (or the binding site size) [52] required for KpSSBbinding is 26 plusmn 2 nt
34 StSSB Bound to ssDNA The binding of StSSB to dT15(Figure 5(a)) dT20 (Figure 5(b)) dT30 (Figure 5(c)) dT40(Figure 5(d)) dT45 (Figure 5(e)) and dT50 (Figure 5(f)) was
BioMed Research International 7
[StSSB]
-dT15
-C
mdash
(a)
-dT20
-C
---
-
mdash[StSSB]
(b)
-dT30
-C
[StSSB]mdash
(c)
-dT40
-C
mdash[StSSB]
(d)
-dT45
-C1-C2
mdash[StSSB]
(e)
-dT50
-C1-C2
mdash
[StSSB]
(f)
Figure 5 Binding of StSSB to dT15ndash50 StSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT15 (b) dT20 (c) dT30 (d) dT40 (e) dT45 or (f) dT50 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and 100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blue and40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
examined using EMSA StSSB can bind and form a singlecomplex with dT15 (Figure 5(a)) and dT20 (Figure 5(b)) butKpSSB cannot (Figure 4(a)) StSSB bound to dT15ndash40 andformed a single complex For dT45 and dT50 two differentcomplexes of StSSB appeared at high protein concentrations(Figures 5(e) and 5(f)) Therefore one StSSB occupies 20(402 = 20) nt to 225 (452 = 225) nt of the ssDNA TheEMSA results suggest that the length of an ssDNA (or thebinding site size) [52] required for StSSB binding is 21 plusmn 2 nt
35 PaSSB Bound to ssDNA The binding of PaSSB to dT20(Figure 6(a)) dT25 (Figure 6(b)) dT35 (Figure 6(c)) dT45(Figure 6(d)) dT55 (Figure 6(e)) dT60 (Figure 6(f)) anddT65 (Figure 6(g)) was studied by EMSA Unlike StSSB nocomplexwas observedwhen PaSSBwas incubatedwith dT20Some smears were observed indicating that PaSSB interactswith dT20 However the ssDNA may be too short to befully wrapped by PaSSB PaSSB could form a single complexwith dT25ndash55 and form two distinct complexes with dT60
and dT65 (Figures 6(f) and 6(g)) respectivelyTherefore onePaSSB occupies 275 (552 = 275) nt to 30 (602 = 30) ntof the ssDNA These results from EMSA suggest that thelength of an ssDNA (or the binding site size) [52] required forPaSSB binding is 29plusmn2 nt Although the SSBs that is KpSSBStSSB and PaSSB have significantly similar ssDNA-bindingdomains their binding site sizes are different and range from19 (21 plusmn 2 StSSB) to 31 (29 plusmn 2 PaSSB) nt The obtainedEMSA results (Figures 4ndash6) also show that the binding sitesizes of the untagged SSBs (KpSSB StSSB and PaSSB) werefound to be almost identical to those of the His-tagged ones[40 42 43]
36 Design of the Chimeric KpSSB Proteins KpSSBnStSSBc andKpSSBnPaSSBc The N-terminal ssDNA-binding domain ofKpSSB StSSB and PaSSB is highly conserved (Figure 2) buttheir binding site sizes are different (Figures 4ndash6) and rangefrom 19 nt to 31 nt The C-terminal acidic tails DDDIPFare conserved (Figure 2) and these features led us to assess
8 BioMed Research International
[PaSSB]
-dT20
mdash
(a)
-dT25
-C
[PaSSB]mdash
(b)
-dT35
-C
-
-
[PaSSB]
(c)
-dT45
-C
[PaSSB]
(d)
-dT55
-C
[PaSSB]
(e)
-dT60
-C1-C2
[PaSSB]
(f)
-dT65
-C1-C2
[PaSSB]
(g)
Figure 6 Binding of PaSSB to dT20ndash65 PaSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT20 (b) dT25 (c) dT35 (d) dT45 (e) dT55 (f) dT60 or (g) dT65 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
whether the flexible glycine-rich hinge in the C-terminaldomain which is not conserved among SSBs is involved inthe determination of the binding site size of SSB Thus theC-terminal domains of StSSB and PaSSB were swapped withKpSSB through protein chimeragenesis
37 KpSSBnStSSBc Bound to ssDNA The binding ofKpSSBnStSSBc to dT15 (Figure 7(a)) dT20 (Figure 7(b))dT40 (Figure 7(c)) and dT45 (Figure 7(d)) was examinedusing EMSA KpSSBnStSSBc exhibited significantly different
ssDNA-binding properties from those of KpSSB UnlikeKpSSB (Figure 4) both KpSSBnStSSBc (Figure 8) and StSSB(Figure 5) can bind and form a single complex with dT15 anddT20 Similar to StSSB KpSSBnStSSBc binds to dT15ndash40 andforms a single complex For dT45 two different complexesof KpSSBnStSSBc appeared at high protein concentrations(Figure 8(d)) this EMSA feature was also similar to that ofStSSB One KpSSBnStSSBc occupies 20 (402 = 20) nt to225 (452 = 225) nt of the ssDNA These EMSA resultssuggest that the length of an ssDNA (or the binding site
BioMed Research International 9
[KpSSBnStSSBc]
-dT15
-C
(a)
[KpSSBnStSSBc]
-dT20
-C
(b)
[KpSSBnStSSBc]
-dT40
-C
(c)
[KpSSBnStSSBc]
-dT45
-C1-C2
(d)
Figure 7 Binding of KpSSBnStSSBc to dT15ndash45 KpSSBnStSSBc (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for30min at 25∘C with 17 nM of (a) dT15 (b) dT20 (c) dT40 or (d) dT45 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and 100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blue and40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
size) [52] required for KpSSBnStSSBc binding is 21 plusmn 2 nt avalue identical to that for StSSB (Figure 5) Swapping of theC-terminal domain of StSSB with KpSSB changes the size ofthe ssDNA-binding site from 26 nt to 21 nt
38 KpSSBnPaSSBc Bound to ssDNA The binding fea-tures of KpSSBnPaSSBc with dT20 (Figure 8(a)) dT25(Figure 8(b)) dT40 (Figure 8(c)) dT55 (Figure 8(d)) anddT60 (Figure 8(e)) were studied by EMSA Similar to thecases of KpSSB and PaSSB no complex was observed whenKpSSBnPaSSBc was incubated with dT20 However KpSSB-nPaSSBc still exhibited dramatically different ssDNA-bindingproperties from those of KpSSB KpSSB can form two distinctcomplexes with dT55 (Figure 4(f)) but both KpSSBnPaSSBc(Figure 9) and PaSSB (Figure 6) cannot One KpSSBnPaSSBcoccupies 275 (552 = 275) nt to 30 (602 = 30) nt ofthe ssDNA The above EMSA results suggest that the lengthof an ssDNA (or the binding site size) [52] required forKpSSBnPaSSBc binding is 29 plusmn 2 nt a value identical to thatof PaSSB Swapping of the C-terminal domain of PaSSB toKpSSB changes the size of the ssDNA-binding site from 26 ntto 29 nt Although these SSBs namely KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc have nearly identicalssDNA-binding domains their binding site sizes are different(Table 2) Thus the size of the ssDNA-binding site requiredfor second SSB binding is likely to be dependent on the C-terminal domain of SSB
39 Binding Constants of the SSB-ssDNA Complexes Deter-mined from EMSA To compare the ssDNA-binding abil-ities of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc the midpoint values for input ssDNA bind-ing calculated from the titration curves of EMSA andreferred to as [Protein]
50(monomer) were quantified and
are summarized in Table 2 Although the N-terminal ssDNA-binding domains of these SSB proteins are highly similar(Figure 2) their ssDNA-binding activities and binding sitesizes are different (Table 2) [KpSSB]
50values ranged from
100 nM to 220 nM [StSSB]50
values ranged from 420 nM to650 nM [PaSSB]
50values ranged from 550 nM to 1700 nM
[KpSSBnStSSBc]50values ranged from 110 nM to 260 nM and
[KpSSBnPaSSBc]50
values ranged from 220 nM to 390 nMThe ssDNA-binding ability is as follows in the order ofdecreasing affinity KpSSB gt KpSSBnStSSBc gt KpSSBn-PaSSBc gt StSSB gt PaSSB Results from the above analysesindicate that the exchange of the C-terminal domain inSSB significantly changed the ssDNA-binding ability and theDNA-binding behavior (complex number) The reason asto why swapping of the C-terminal domain can affect thessDNA-binding activity of SSB remains unclear The C-ter-minal domain of SSB is suggested to be involved in ssDNAbinding However this relation is not evident in the results ofthe cocrystal structure
310 Oligomeric State of KpSSBnStSSBc and KpSSBnPaSSBc inSolution Gel-filtration chromatography was used to confirm
10 BioMed Research International
[KpSSBnPaSSBc]
-dT20
(a)
[KpSSBnPaSSBc]
-dT25
-C
(b)
[KpSSBnPaSSBc]
-C
-dT40
(c)
-C
[KpSSBnPaSSBc]
-dT55
(d)
-dT60
-C1-C2
[KpSSBnPaSSBc]
(e)
Figure 8 Binding of KpSSBnPaSSBc to dT20ndash60 KpSSBnPaSSBc (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for30min at 25∘C with 17 nM of (a) dT20 (b) dT25 (c) dT40 (d) dT55 or (e) dT60 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
that the oligomeric state of KpSSBnStSSBc and KpSSBn-PaSSBc remains as tetramers after chimeragenesisThe analy-sis of purified KpSSBnStSSBc and KpSSBnPaSSBc (2mgmL)using a Superdex 200HR 1030 column revealed a singlepeak with elution volumes of 786 and 789mL respectivelyAssuming that KpSSBnStSSBc and KpSSBnPaSSBc both haveshapes and partial specific volumes similar to the standardproteins the native molecular masses of KpSSBnStSSBc andKpSSBnPaSSBc were estimated to be 76641 and 74827Da ascalculated from a standard linear regression equation 119870av =minus03684(logMw) + 22707 (Figure 9) The native molecularmasses for KpSSBnStSSBc and KpSSBnPaSSBc are approx-imately four times the mass of the monomer (sim19 kDa)Therefore KpSSBnStSSBc and KpSSBnPaSSBc under theabove chromatographic conditions are stable tetramers insolution Although the exchange of the C-terminal domainin SSB significantly changed the ssDNA-binding ability andDNA-binding behavior (complex number) protein chimera-genesis did not cause any change in the oligomeric state ofSSB
311 Summary of Gly Gln and Pro Number in SSBs To ana-lyze the C-terminal amino acid composition of SSBs wefurther counted the number of Gly Gln and Pro residuesin different SSB segments SSB is abundant in Gly Glnand Pro (GQP) (Table 3) The GQP contents of KpSSB1ndash91 StSSB1ndash91 and PaSSB1ndash90 are similar However the Glynumber of PaSSB116ndash165 is significantly lower than that ofKpSSB116ndash174 and StSSB117ndash176 PaSSB116ndash165 contains only1 Gly but KpSSB116ndash174 and StSSB117ndash176 contain 11 and 12Gly respectively In addition we found different distributionpatterns among KpSSB StSSB and PaSSB Although theycontain similar number of Gln (Q) the QQQ pattern isfrequently found in PaSSB (Table 3)
312 Structural Modeling of SSBs Given its disordered C-ter-minal domain the crystal structure of the full-length SSB islacking even when SSB can be crystallized with DNA [69]We attempted to model the structure by homology mod-eling using the bioinformatics program (PS)2 to obtain an
BioMed Research International 11
Table 2 ssDNA binding properties of KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc as analyzed by EMSA
Protein DNA [Protein]50 (nM) Complex number
KpSSB
dT20 ND 0dT25 200 plusmn 20 1dT35 220 plusmn 30 1dT45 100 plusmn 10 1dT50 110 plusmn 20 1dT55 100 plusmn 20 2dT60 100 plusmn 10 2
StSSB
dT15 650 plusmn 120 1dT20 450 plusmn 80 1dT30 420 plusmn 60 1dT40 420 plusmn 80 1dT45 440 plusmn 60 2dT50 440 plusmn 50 2
PaSSB
dT20 ND 0dT25 1700 plusmn 250 1dT35 950 plusmn 180 1dT45 780 plusmn 160 1dT55 820 plusmn 90 1dT60 810 plusmn 110 2dT65 550 plusmn 70 2
KpSSBnStSSBc
dT15 260 plusmn 60 1dT20 110 plusmn 20 1dT40 120 plusmn 20 1dT45 160 plusmn 20 2
KpSSBnPaSSBc
dT20 ND 0dT25 390 plusmn 60 1dT40 220 plusmn 30 1dT55 230 plusmn 30 1dT60 230 plusmn 30 2
[Protein]50 was calculated from the titration curves of EMSA by determiningthe concentration of the protein (120583M) needed to achieve the midpointvalue for input ssDNA binding For some oligonucleotides input ssDNAbinding was the sum of the intensities from the two separate ssDNA-proteincomplexes Errors are standard deviations determined by three independenttitration experiments
Table 3 Summary of Gly Gln and Pro number in SSB
SSB segment G Q PKpSSB1ndash91 10 5 2StSSB1ndash91 10 5 2PaSSB1ndash90 11 6 2KpSSB92ndash174 18 16 9StSSB92ndash176 17 18 11PaSSB91ndash165 5 15 11KpSSB116ndash174 11 12 9StSSB117ndash176 12 13 10PaSSB116ndash165 1 12 11
in-depth understanding of the structure-function relation-ship of the C-terminal domains of these SSBs [70 71]
40 45 50 55
Kav
00
02
04
06
08
10
KpSSBnStSSBcKpSSBnPaSSBc
Y = minus03684X + 22707
R2= 0998
log Mw
Figure 9Gel-filtration chromatographic analyses of KpSSBnStSSBcand KpSSBnPaSSBc Purified protein (2mgmL) was applied toa Superdex 200HR 1030 column (GE Healthcare Bio-SciencesPiscataway NJ USA) equilibrated with Buffer D The column wasoperated at a flow rate of 05mLmin and 05mL fractions werecollected The proteins were detected by measuring the absorbanceat 280 nm The column was calibrated with proteins of knownmolecular weight thyroglobulin (670 kDa) 120574-globulin (158 kDa)ovalbumin (44 kDa) and myoglobin (17 kDa) The 119870av values forthe standard proteins and the SSB variants were calculated from theequation119870av = (119881119890minus119881119900)(119881119888minus119881119900) where119881119900 is column void volume119881119890is elution volume and 119881
119888is geometric column volume
(PS)2 (http140113239111simps2v2docsphp) is an auto-matic homology modeling server that combines bothsequence and secondary structure information to detect thehomologous proteins with remote similarity and the target-template alignment After pasting the amino acid sequenceto the website of (PS)2 only one hit (Protein Data Bankentry 1QVC EcSSB) for the C-terminal domains of KpSSBand StSSB was suggested For the C-terminal domain ofPaSSB only one hit that is CstF-77 (ProteinData Bank entry2OOE cleavage stimulation factor CstF) but not EcSSB wassuggested as the template for modeling Figure 10 shows thatmodeled structures of these SSB C-terminal domains arehighly disordered but that of PaSSB is more ordered than thatof other domains
4 Discussion
In this study we examined the sizes of the binding site ofthe untagged SSB and the chimeric SSB from the ubiquitousopportunistic pathogens K pneumoniae S enterica serovarTyphimurium LT2 and P aeruginosa PAO1 Many clinicalstrains of the abovementioned bacteria are highly resistantto antibiotics [72ndash75] The development of clinically usefulsmall-molecule antibiotics has been a seminal event in thefield of infectious diseases [48] Nucleic acid metabolismis one of the most basic biological functions and shouldbe a prime target in antibiotic development [76ndash78] Manybacterial SSBs form conserved protein interaction ldquohubsrdquothat are essential to recruit many proteins involved in DNA
12 BioMed Research International
Figure 10 Structure modeling of SSB The structures of KpSSB1ndash115 StSSB1ndash115 and PaSSB1ndash115 (the N-terminal domain of SSB)were modeled by SWISS-MODEL The structures of KpSSB116ndash142 StSSB116ndash142 and PaSSB121ndash160 (the C-terminal domain ofSSB) were modeled by (PS)2 Other regions of SSBs could not bemodeled by these two programs The structures of the N-terminaldomain and the C-terminal domain of these SSBs were manuallylinked (KpSSB1ndash142 blue StSSB1ndash142 pink PaSSB1ndash160 green) andsuperimposed with the crystal structure of EcSSB1ndash142 (orange)(PDB entry 1QVC) for comparison For clarity only one subunitof the tetramer was shown for each SSB
replication recombination and repair SSBDNA nucleopro-tein substrates [79] Thus SSBs may be promising targets inantibiotic development [80] As a first step toward achievingthis goal we investigated why SSBs possess highly conservedN-terminal ssDNA-binding domain but exhibit varying bind-ing site sizes One significant clue is that their flexible hingesand the length at the C-terminus are different as revealed bysequence alignment (Figure 2)
The interactions of various SSBs with ssDNA have beenanalyzed using a variety of techniques such as tryptophan-fluorescence quenching [47] filter binding [81] EMSA [5282] analytical ultracentrifugation [83] electron microscopy[84] nuclease digestion [44] single-molecule fluorescencemicroscopy [48] and crystallographic analyses [11] In thisstudy we have examined the electrophoretic mobility shiftpatterns of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc bound to different lengths of ssDNA and deter-mined the corresponding binding site sizes to be 26 2129 22 and 29 nt per tetramer respectively (Figures 4ndash8) PaSSB and KpSSBnPaSSBc have the largest sizes forssDNA binding among the SSBs studied We also identifiedHis-tagged and untagged SSBs that have similar ssDNA-binding site sizes [40 42 43] EMSA is a well-establishedapproach in studies of molecular biology [52] and the use ofradioactive tracer in this assay allows detection of the actualformation of the distinct protein-DNA complex(es) [53] For
exampleDNase protection assay and footprinting assay usingradioactive tracer can determine the specific DNA sequencecomplexed by a protein In EMSA when the length of thenucleotides is sufficient for the binding of two or more SSBmolecules the electrophoretic mobility of the higher SSBoligomer complex will be lower than that of the smaller SSBoligomer complex [52 54] In addition results of the ssDNA-binding site size from EMSA and cocrystal structure of SSBwere consistent [27] Thus throughout this paper we deter-mined the ssDNA-binding site sizes of SSB from the EMSAbehavior
Many SSBs bind to ssDNA with some degree of positivecooperativity Cooperativity can result from direct protein-protein interactions between the nearest neighbors such asthe LAST motif in the T4 gene-32 protein [85] and thearginine-mediated interaction motif in Thermus SSB [8687] Cooperativity can also result from the protein-induceddistortion of adjacent DNA as demonstrated in SulfolobusSSB PriB and FOXK1a proteins [23 60 88] In the cases ofKpSSB StSSB and PaSSB (Figures 4ndash6) binding appeared tobe nearly noncooperative for several DNAs because all DNAmainly shifts into the first complex (C1) before the appearanceof the second complex (C2) when subjected to increasingprotein concentrationsThe length dependence of the [SSB]
50
values suggests that the amount of spacing is optimum forsteric considerations (Table 2)
Because bacteria have varying genomic DNA sizes theirSSBs may need to evolve to have different binding site sizesfor DNA metabolism Results from protein chimeragenesisshowed the C-terminal domain dependence of the bindingsite sizes of SSB (Figure 11) The experimental data showedthat the binding site size of KpSSBnStSSBc was similar to thatof StSSB and the size of the binding site of KpSSBnPaSSBcwas similar to that of PaSSB The reason for which the bind-ing site size of SSB changed followed by swapping of theC-terminal domain remains unclear Flexibility numberof glycine residues andor different QQQ patterns of theC-terminal domain of SSB (Figure 2 and Table 3) may beimportant factors for determining the ssDNA-binding sitesize In fact the C-terminal domain of PaSSB that isPaSSB116ndash165 has only 1 Gly residue which is significantlyless than that of KpSSB (11 Gly) and StSSB (12Gly) Gly (andPro) is an important component of the flexible region aprotein that contains low Gly content is predicted to havelow flexibility Unlike typical SSB [35 69] PaSSB116ndash165 hasa partial structure (Figure 10) Although KpSSB StSSB andPaSSB contain similar number of Gln (Q) the QQQ patternis frequently found in PaSSB (Figure 2 and Table 3) PolyQand repeated sequences GAGAG are commonly found inthe structures of amyloids silk fibers and neurodegradationproteins [89ndash92] Considering that the simple coil polyQ theheptapeptide GNNQQNY and the hexapeptide NNQQNYcan cause protein aggregation and nucleation [93ndash95] thedistribution of Gln in the C-terminal domain of a tetramericSSB may also be an important determinant of the ssDNA-binding site size of SSB by some steric hindrances (Figure 11)However the above speculationmust be confirmed by furtherbiochemical experiments
BioMed Research International 13
PaSSBStSSB KpSSB
C-terC-ter C-ter
sim 21 nt sim 26 nt sim 29 nt
Figure 11 Possiblemodels for explainingwhy SSBs arewith different binding site sizes Twomodeled structures of KpSSB1ndash142 (blue) StSSB1ndash142 (pink) and PaSSB1ndash160 (green) complexed with ssDNA (gold) are shown For clarity only one C-terminal domain was shown for eachSSB tetramer By using the electrophoretic mobility shift assay and the protein chimeragenesis we characterized that the binding site sizes ofKpSSB StSSB PaSSB KpSSBnStSSBc and KpSSBnPaSSBc were 26 21 29 21 and 29 nt per tetramer respectively KpSSB StSSB and PaSSBare similar proteins whose N-terminal ssDNA-binding domains are almost identical Thus the C-terminal domain of SSB may indirectlycontribute to ssDNA binding and wrapping and affects the binding site size by the steric hindrance
5 Conclusion
In this study we characterized the ssDNA-binding propertiesof untagged SSBs from K pneumoniae S enterica sero-var Typhimurium LT2 and P aeruginosa PAO1 and proposeda role of the C-terminal flexible domain for ssDNA bind-ing from the protein chimeragenesis and EMSA resultsThe amino acid sequence of the N-terminal ssDNA-bind-ingoligomerization domain in these pathogenic SSBs ishighly conserved but their apparent binding site sizes aredifferent This finding indicates that the C-terminal protein-protein interaction domain may also indirectly contribute tossDNA binding and wrapping
Conflict of Interests
The authors declare that there is no conflict of interestsregarding the publication of this paper
Acknowledgment
This researchwas supported by aGrant from theNational Sci-ence Council Taiwan (NSC 102-2320-B-040-019 to Cheng-Yang Huang)
References
[1] R Reyes-Lamothe D J Sherratt and M C Leake ldquoStoichiom-etry and architecture of active DNA replication machinery inescherichia colirdquo Science vol 328 no 5977 pp 498ndash501 2010
[2] D J Richard E Bolderson andK K Khanna ldquoMultiple humansingle-stranded DNA binding proteins function in genomemaintenance structural biochemical and functional analysisrdquo
Critical Reviews in Biochemistry and Molecular Biology vol 44no 2-3 pp 98ndash116 2009
[3] R D Shereda A G Kozlov T M LohmanMM Cox and J LKeck ldquoSSB as an organizermobilizer of genome maintenancecomplexesrdquo Critical Reviews in Biochemistry and MolecularBiology vol 43 no 5 pp 289ndash318 2008
[4] D J Richard E Bolderson L Cubeddu et al ldquoSingle-strandedDNA-binding protein hSSB1 is critical for genomic stabilityrdquoNature vol 453 no 7195 pp 677ndash681 2008
[5] R R Meyer and P S Laine ldquoThe single-stranded DNA-bindingprotein of Escherichia colirdquoMicrobiological Reviews vol 54 no4 pp 342ndash380 1990
[6] C Yang U Curth C Urbanke and C Kang ldquoCrystal structureof human mitochondrial single-stranded DNA binding proteinat 24 A resolutionrdquo Nature Structural Biology vol 4 no 2 pp153ndash157 1997
[7] G Webster J Genschel U Curth C Urbanke C Kang and RHilgenfeld ldquoA common core for binding single-stranded DNAstructural comparison of the single-stranded DNA-bindingproteins (SSB) from E coli and human mitochondriardquo FEBSLetters vol 411 pp 313ndash316 1997
[8] R L Flynn and L Zou ldquoOligonucleotideoligosaccharide-bind-ing fold proteins a growing family of genome guardiansrdquo Crit-ical Reviews in Biochemistry and Molecular Biology vol 45 no4 pp 266ndash275 2010
[9] K Chan Y Lee CWangHHuang andY Sun ldquoSingle-Strand-ed DNA-Binding Protein Complex from Helicobacter pyloriSuggests an ssDNA-Binding Surfacerdquo Journal of MolecularBiology vol 388 no 3 pp 508ndash519 2009
[10] D L Theobald R M Mitton-Fry and D S Wuttke ldquoNucleicacid recognition by OB-fold proteinsrdquo Annual Review of Bio-physics and Biomolecular Structure vol 32 pp 115ndash133 2003
[11] S Raghunathan A G Kozlov TM Lohman andGWaksmanldquoStructure of the DNA binding domain of E coli SSB bound to
14 BioMed Research International
ssDNArdquo Nature Structural Biology vol 7 no 8 pp 648ndash6522000
[12] A G Murzin ldquoOB(oligonucleotideoligosaccharide binding)-fold common structural and functional solution for non-homologous sequencesrdquo EMBO Journal vol 12 no 3 pp 861ndash867 1993
[13] N P George K V Ngo S Chitteni-Pattu et al ldquoStructure andcellular dynamics of deinococcus radiodurans single-strandedDNA (ssDNA)-binding protein (SSB)-DNA complexesrdquo TheJournal of Biological Chemistry vol 287 no 26 pp 22123ndash221322012
[14] P Filipkowski and J Kur ldquoIdentification and propertiesof the Deinococcus grandis and Deinococcus proteolyticussingle-stranded DNA binding proteins (SSB)rdquo Acta BiochimicaPolonica vol 54 no 1 pp 79ndash87 2007
[15] P Filipkowski A Duraj-Thatte and J Kur ldquoIdentificationcloning expression and characterization of a highly ther-mostable single-stranded-DNA-binding protein (SSB) fromDeinococcus murrayirdquo Protein Expression and Purification vol53 no 1 pp 201ndash208 2007
[16] P Filipkowski M Koziatek and J Kur ldquoA highly ther-mostable homodimeric single-stranded DNA-binding proteinfrom Deinococcus radiopugnansrdquo Extremophiles vol 10 no 6pp 607ndash614 2006
[17] P Filipkowski A Duraj-Thatte and J Kur ldquoNovel thermostablesingle-stranded DNA-binding protein (SSB) fromDeinococcusgeothermalisrdquo Archives of Microbiology vol 186 no 2 pp 129ndash137 2006
[18] G Witte C Urbanke and U Curth ldquoSingle-stranded DNA-binding protein of Deinococcus radiodurans a biophysicalcharacterizationrdquoNucleic Acids Research vol 33 no 5 pp 1662ndash1670 2005
[19] D A Bernstein J M Eggingtont M P Killoran A M MisicMMCox and J L Keck ldquoCrystal structure of theDeinococcusradiodurans single-stranded DNA-binding protein suggests amechanism for coping with DNA damagerdquo Proceedings of theNational Academy of Sciences of the United States of Americavol 101 no 23 pp 8575ndash8580 2004
[20] S Dabrowski M Olszewski R Piatek A Brillowska-Dabrowska G Konopa and J Kur ldquoIdentification and charac-terization of single-stranded-DNA-binding proteins fromTher-mus thermophilus and Thermus aquaticusmdashnew arrange-ment of binding domainsrdquo Microbiology vol 148 no 10 pp3307ndash3315 2002
[21] R Gamsjaeger R Kariawasam C Touma A H Kwan M FWhite and L Cubeddu ldquoBackbone and side-chain 1H 13C and15N resonance assignments of the OB domain of the singlestranded DNA binding protein from Sulfolobus solfataricusand chemical shift mapping of the DNA-binding interfacerdquoBiomolecular NMR Assignments 2013
[22] M L Rolfsmeier and C A Haseltine ldquoThe single-strandedDNA binding protein of Sulfolobus solfataricus acts in the pre-synaptic step of homologous recombinationrdquo Journal of Molec-ular Biology vol 397 no 1 pp 31ndash45 2010
[23] I D Kerr R I M Wadsworth L Cubeddu W Blankenfeldt JHNaismith andM FWhite ldquoInsights into ssDNA recognitionby the OB fold from a structural and thermodynamic study ofSulfolobus SSB proteinrdquo EMBO Journal vol 22 no 11 pp 2561ndash2570 2003
[24] C A Haseltine and S C Kowalczykowski ldquoA distinctive single-stranded DNA-binding protein from the Archaeon Sulfolobussolfataricusrdquo Molecular Microbiology vol 43 no 6 pp 1505ndash1515 2002
[25] R I M Wadsworth and M F White ldquoIdentification andproperties of the crenarchaeal single-stranded DNA bindingprotein from Sulfolobus solfataricusrdquo Nucleic Acids Researchvol 29 no 4 pp 914ndash920 2001
[26] S Sugiman-Marangos and M S Junop ldquoThe structure of DdrBfrom Deinococcus a new fold for single-stranded DNA bindingproteinsrdquo Nucleic Acids Research vol 38 no 10 Article IDgkq036 pp 3432ndash3440 2010
[27] H Ghalei H V Moeller D Eppers et al ldquoEntrapment of DNAin an intersubunit tunnel system of a single-stranded DNA-binding proteinrdquo Nucleic Acids Research vol 42 no 10 pp6698ndash6708 2014
[28] S Paytubi S A McMahon S Graham et al ldquoDisplacement ofthe canonical single-strandedDNA-binding protein in the ther-moprotealesrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 109 no 7 pp E398ndashE405 2012
[29] T H Dickey S E Altschuler and D S Wuttke ldquoSingle-stranded DNA-binding proteins multiple domains for multiplefunctionsrdquo Structure vol 21 no 7 pp 1074ndash1084 2013
[30] D Vujaklija and BMacek ldquoDetecting posttranslational modifi-cations of bacterial SSB proteinsrdquoMethods inMolecular Biologyvol 922 pp 205ndash218 2012
[31] I Mijakovic D Petranovic B Macek et al ldquoBacterial single-stranded DNA-binding proteins are phosphorylated on tyro-sinerdquoNucleic Acids Research vol 34 no 5 pp 1588ndash1596 2006
[32] M Olszewski A Grot M Wojciechowski M Nowak MMickiewicz and J Kur ldquoCharacterization of exceptionally ther-mostable single-strandedDNA-binding proteins fromThermo-toga maritima and Thermotoga neapolitanardquo BMC Microbiol-ogy vol 10 article 260 2010
[33] U Curth J Genschel C Urbanke and J Greipel ldquoIn vitro andin vivo function of the C-terminus of Escherichia coil single-stranded DNA binding proteinrdquoNucleic Acids Research vol 24no 14 pp 2706ndash2711 1996
[34] G Witte C Urbanke and U Curth ldquoDNA polymerase IIIchi subunit ties single-stranded DNA binding protein to thebacterial replication machineryrdquoNucleic Acids Research vol 31pp 4434ndash4440 2003
[35] D Shishmarev YWang C EMason et al ldquoOtting Intramolec-ular binding mode of the C-terminus of Escherichia coli single-strandedDNAbinding protein determined by nuclearmagneticresonance spectroscopyrdquo Nucleic Acids Research vol 42 pp2750ndash2757 2014
[36] A G Kozlov M M Cox and T M Lohman ldquoRegulation ofsingle-stranded DNA binding by the C termini of Escherichiacoli single-stranded DNA-binding (SSB) proteinrdquo Journal ofBiological Chemistry vol 285 no 22 pp 17246ndash17252 2010
[37] M Nowak M Olszewski M Spibida and J Kur ldquoChar-acterization of single-stranded DNA-binding proteins fromthe psychrophilic bacteria Desulfotalea psychrophila Flavobac-terium psychrophilum Psychrobacter arcticus Psychrobactercryohalolentis Psychromonas ingrahamii Psychroflexus torquisand Photobacterium profundumrdquo BMC Microbiology vol 14article 91 2014
BioMed Research International 15
[38] T Paradzik N Ivic Z Filic et al ldquoStructure-function relation-ships of two paralogous single-stranded DNA-binding proteinsfrom Streptomyces coelicolor implication of SsbB in chromo-some segregation during sporulationrdquo Nucleic Acids Researchvol 41 no 6 pp 3659ndash3672 2013
[39] S JainM Zweig E Peeters et al ldquoCharacterization of the singlestrandedDNA binding protein SsbB encoded in the gonoccocalgenetic islandrdquo PLoS ONE vol 7 no 4 Article ID e35285 2012
[40] Y H Huang and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from Klebsiella pneumoniaemutation at either Arg73 or Ser76 causes a less cooperativecomplex onDNArdquoGenes to Cells vol 17 no 2 pp 146ndash157 2012
[41] E Antony E A Weiland S Korolev and T M LohmanldquoPlasmodium falciparum SSB tetramer wraps single-strandedDNAwith similar topology but opposite polarity to E Coli SSBrdquoJournal ofMolecular Biology vol 420 no 4-5 pp 269ndash283 2012
[42] H Jan Y L Lee and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from pseudomonas aeruginosaPAO1rdquo Protein Journal vol 30 no 1 pp 20ndash26 2011
[43] Y-H Huang Y-L Lee and C-Y Huang ldquoCharacterization of asingle-stranded DNA binding protein from Salmonella entericaserovar typhimurium LT2rdquo Protein Journal vol 30 no 2 pp102ndash108 2011
[44] S K Bharti K Rex P Sreedhar N Krishnan and U VarshneyldquoChimeras of Escherichia coli and Mycobacterium tuberculosissingle-stranded DNA binding proteins characterization andfunction in Escherichia colirdquo PLoS ONE vol 6 no 12 ArticleID e27216 2011
[45] M T Oliveira and L S Kaguni ldquoFunctional roles of the N-and C-terminal regions of the human mitochondrial single-stranded DNA-binding proteinrdquo PLoS ONE vol 5 no 10Article ID e15379 2010
[46] A G Kozlov J M Eggington M M Cox and T MLohman ldquoBinding of the dimeric Deinococcus radioduranssingle-strandedDNA binding protein to single-strandedDNArdquoBiochemistry vol 49 no 38 pp 8266ndash8275 2010
[47] T M Lohman and M E Ferrari ldquoEscherichia coli single-stranded DNA-binding protein multiple DNA-binding modesand cooperativesrdquo Annual Review of Biochemistry vol 63 pp527ndash570 1994
[48] R Zhou A G Kozlov R Roy et al ldquoSSB functions as a slidingplatform that migrates on DNA via reptationrdquo Cell vol 146 pp222ndash232 2011
[49] R Roy A G Kozlov T M Lohman and T Ha ldquoSSB proteindiffusion on single-stranded DNA stimulates RecA filamentformationrdquo Nature vol 461 pp 1092ndash1097 2009
[50] R Roy A G Kozlov T M Lohman and T Ha ldquoDynamicstructural rearrangements between DNA binding modes of Ecoli SSB proteinrdquo Journal of Molecular Biology vol 369 no 5pp 1244ndash1257 2007
[51] T J Kelly P Simancek and G S Brush ldquoIdentification andcharacterization of a single-stranded DNA-binding proteinfrom the archaeon Methanococcus jannaschiirdquo Proceedings ofthe National Academy of Sciences of the United States of Americavol 95 no 25 pp 14634ndash14639 1998
[52] C Y Huang Determination of the Binding Site-Size of theProtein-DNA Complex by Use of the Electrophoretic MobilityShift Assay InTech Press Rijeka Croatia 2012
[53] A Kornberg ldquoTen commandments of enzymology amendedrdquoTrends in Biochemical Sciences vol 28 no 10 pp 515ndash517 2003
[54] W Zhang X Lu and J Shen ldquoEMSA and single-moleculeforce spectroscopy study of interactions between bacillus sub-tilis single-stranded DNA-binding protein and single-strandedDNArdquo Langmuir vol 27 no 24 pp 15008ndash15015 2011
[55] M Ostermeier and S J Benkovic ldquoEvolution of protein func-tion by domain swappingrdquo Advances in Protein Chemistry vol55 pp 29ndash77 2000
[56] W F Peng and C Y Huang ldquoAllantoinase and dihydroorotasebinding and inhibition by flavonols and the substrates of cyclicamidohydrolasesrdquo Biochimie vol 101 pp 113ndash122 2014
[57] Y H Huang M J Lin and C Y Huang ldquoDnaT is a single-stranded DNA binding proteinrdquo Genes to Cells vol 18 no 11pp 1007ndash1019 2013
[58] Y Y Ho Y H Huang and C Y Huang ldquoChemical rescue of thepost-translationally carboxylated lysine mutant of allantoinaseand dihydroorotase by metal ions and short-chain carboxylicacidsrdquo Amino Acids vol 44 no 4 pp 1181ndash1191 2013
[59] Y H Huang Y Lo W Huang and C Y Huang ldquoCrystalstructure and DNA-binding mode of Klebsiella pneumoniaeprimosomal PriB proteinrdquoGenes to Cells vol 17 no 10 pp 837ndash849 2012
[60] C Y Huang C Y Hsu Y Sun H Wu and C Hsiao ldquoCom-plexed crystal structure of replication restart primosome pro-tein PriB reveals a novel single-stranded DNA-binding moderdquoNucleic Acids Research vol 34 no 14 pp 3878ndash3886 2006
[61] T Kinebuchi H Shindo H Nagai N Shimamoto andM Shimizu ldquoFunctional domains of Escherichia coli single-stranded DNA binding protein as assessed by analyses of thedeletion mutantsrdquo Biochemistry vol 36 no 22 pp 6732ndash67381997
[62] N Shimamoto N Ikushima H Utiyama H Tachibana andK Horie ldquoSpecific and cooperative binding of E coli single-stranded DNA binding protein to mRNArdquo Nucleic AcidsResearch vol 15 no 13 pp 5241ndash5250 1987
[63] H Lin andC YHuang ldquoCharacterization of flavonol inhibitionof DnaB helicase Real-time monitoring structural modelingand proposed mechanismrdquo Journal of Biomedicine and Biotech-nology vol 2012 Article ID 735368 2012
[64] Y H Huang H H Lin and C Y Huang ldquoA single residuedetermines the cooperative binding property of a primosomalDNA replication protein PriB to single-stranded DNArdquo Bio-science Biotechnology and Biochemistry vol 76 no 6 pp 1110ndash1115 2012
[65] H C Hsieh and C Y Huang ldquoIdentification of a novel proteinPriB in Klebsiella pneumoniaerdquo Biochemical and BiophysicalResearch Communications vol 404 no 1 pp 546ndash551 2011
[66] J H Liu T W Chang C Y Huang et al ldquoCrystal structure ofPriB a primosomalDNA replication protein ofEscherichia colirdquoThe Journal of Biological Chemistry vol 279 no 48 pp 50465ndash50471 2004
[67] Y H Huang and C Y Huang ldquoThe N-terminal domain ofDnaT a primosomalDNA replication protein is crucial for PriBbinding and self-trimerizationrdquo Biochemical and BiophysicalResearch Communications vol 442 pp 147ndash152 2013
[68] M A Larkin G Blackshields N P Brown et al ldquoClustalW andClustal X version 20rdquo Bioinformatics vol 23 no 21 pp 2947ndash2948 2007
16 BioMed Research International
[69] S N Savvides S Raghunathan K Futterer A G Kozlov T MLohman and G Waksman ldquoThe C-terminal domain of full-length E coli SSB is disordered even when bound to DNArdquoProtein Science vol 13 no 7 pp 1942ndash1947 2004
[70] C-C Chen J-K Hwang and J-M Yang ldquo(PS)2-v2 template-based protein structure prediction serverrdquo BMC Bioinformaticsvol 10 article 366 2009
[71] C Chen J Hwang and J Yang ldquo(PS)2 protein structure pre-diction serverrdquoNucleic Acids Research vol 34 pp W152ndashW1572006
[72] K Bush and J F Fisher ldquoEpidemiological expansion structuralstudies and clinical challenges of new 120573-lactamases from gram-negative bacteriardquo Annual Review of Microbiology vol 65 pp455ndash478 2011
[73] K K Kumarasamy M A Toleman T R Walsh et al ldquoEmer-gence of a new antibiotic resistance mechanism in India Pak-istan and the UK a molecular biological and epidemiologicalstudyrdquoThe Lancet Infectious Diseases vol 10 no 9 pp 597ndash6022010
[74] K Bush and M J MacIelag ldquoNew 120573-lactam antibiotics and 120573-lactamase inhibitorsrdquo Expert Opinion on Therapeutic Patentsvol 20 no 10 pp 1277ndash1293 2010
[75] K Bush ldquoAlarming 120573-lactamase-mediated resistance in multi-drug-resistant Enterobacteriaceaerdquo Current Opinion in Microbi-ology vol 13 no 5 pp 558ndash564 2010
[76] A Srivastava M Talaue S Liu et al ldquoNew target for inhibitionof bacterial RNA polymerase ldquoswitch regionrdquordquo Current Opinionin Microbiology vol 14 no 5 pp 532ndash543 2011
[77] K Chono K Katsumata T Kontani et al ldquoASP2151 a novelhelicase-primase inhibitor possesses antiviral activity againstvaricella-zoster virus and herpes simplex virus types 1 and 2rdquoJournal of Antimicrobial Chemotherapy vol 65 no 8 pp 1733ndash1741 2010
[78] M T Black and K Coleman ldquoNew inhibitors of bacterial topoi-somerase GyrAParC subunitsrdquo Current Opinion in Investiga-tional Drugs vol 10 no 8 pp 804ndash810 2009
[79] A H Marceau D A Bernstein B W Walsh W Shapiro LA Simmons and J L Keck ldquoProtein interactions in genomemaintenance as novel antibacterial targetsrdquo PLoS ONE vol 8no 3 Article ID e58765 2013
[80] D Lu D A Bernstein K A Satyshur and J L Keck ldquoSmall-molecule tools for dissecting the roles of SSBprotein inter-actions in genome maintenancerdquo Proceedings of the NationalAcademy of Sciences of the United States of America vol 107 no2 pp 633ndash638 2010
[81] I Wong and T M Lohman ldquoA double-filter method fornitrocellulose-filter binding application to protein-nucleic acidinteractionsrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 90 no 12 pp 5428ndash5432 1993
[82] M Mitas J Y Chock and M Christy ldquoThe binding-site sizesof Escherichia coli single-stranded-DNA-binding protein andmammalian replication protein A are 65 and ge 54 nucleotidesrespectivelyrdquo Biochemical Journal vol 324 no 3 pp 957ndash9611997
[83] C Urbanke G Witte and U Curth ldquoSedimentation velocitymethod in the analytical ultracentrifuge for the study of protein-protein interactionsrdquoMethods inMolecular Biology vol 305 pp101ndash114 2005
[84] S Chrysogelos and J Griffith ldquoEscherichia coli single-strandbinding protein organizes single-strandedDNA innucleosome-like unitsrdquo Proceedings of the National Academy of Sciences of theUnited States of America vol 79 no 19 pp 5803ndash5807 1982
[85] J R Casas-Finet K R Fischer and R L Karpel ldquoStructuralbasis for the nucleic acid binding cooperativity of bacteriophageT4 gene 32 protein the (LysArg)3(SerThr)2 (LAST) motifrdquoProceedings of the National Academy of Sciences of the UnitedStates of America vol 89 pp 1050ndash1054 1992
[86] G Witte R Fedorov and U Curth ldquoBiophysical analysis ofThermus aquaticus single-stranded DNA binding proteinrdquo Bio-physical Journal vol 94 no 6 pp 2269ndash2279 2008
[87] R Fedorov G Witte C Urbanke D J Manstein and UCurth ldquo3D structure of Thermus aquaticus single-strandedDNA-binding protein gives insight into the functioning of SSBproteinsrdquo Nucleic Acids Research vol 34 no 22 pp 6708ndash67172006
[88] K L Tsai C Y Huang C H Chang Y J Sun W J ChuangandC DHsiao ldquoCrystal structure of the human FOXK1a-DNAcomplex and its implications on the diverse binding specificityof winged helixforkhead proteinsrdquo Journal of Biological Chem-istry vol 281 no 25 pp 17400ndash17409 2006
[89] A H Mao N Lyle and R V Pappu ldquoDescribing sequence-ensemble relationships for intrinsically disordered proteinsrdquoBiochemical Journal vol 449 no 2 pp 307ndash318 2013
[90] R Wetzel ldquoPhysical chemistry of polyglutamine Intriguingtales of a monotonous sequencerdquo Journal of Molecular Biologyvol 421 no 4-5 pp 466ndash490 2012
[91] H T Orr ldquoPolyglutamine neurodegeneration expanded glu-tamines enhance native functionsrdquo Current Opinion in Geneticsand Development vol 22 no 3 pp 251ndash255 2012
[92] M Figiel W J Szlachcic P M Switonski A Gabka and W JKrzyzosiak ldquoMouse models of polyglutamine diseases reviewand data table Part Irdquo Molecular Neurobiology vol 46 no 2pp 393ndash429 2012
[93] J Nasica-Labouze and N Mousseau ldquoKinetics of amyloidaggregation a study of the GNNQQNY prion sequencerdquo PLoSComputational Biology vol 8 no 11 Article ID e1002782 2012
[94] J Nasica-Labouze M Meli P Derreumaux G Colombo andN Mousseau ldquoA multiscale approach to characterize the earlyaggregation steps of the amyloid-forming peptide gnnqqnyfrom the yeast prion sup-35rdquo PLoS Computational Biology vol7 no 5 Article ID e1002051 2011
[95] J R Lewandowski P C A van der Wel M Rigney N Grig-orieff and R G Griffin ldquoStructural complexity of a compositeamyloid fibrilrdquo Journal of the American Chemical Society vol133 no 37 pp 14686ndash14698 2011
2 BioMed Research International
The binding of SSB to ssDNA makes the glycine-rich regionmore easily accessible to other proteins such as proteases andDNA polymerase III [33 34]The C-terminus in SSB can alsointeract with the OB fold and regulate the ssDNA-bindingactivity of SSB itself [35 36]
Studies on SSB from different organisms have grownrapidly during the past few years and knowledge on howSSBs interact with ssDNA has increased [22 32 37ndash46]The most thoroughly studied SSB is that of Escherichia coli(EcSSB) which binds cooperatively to ssDNA [47] The esti-mated binding site size of EcSSB is dependent on the saltconcentration in fluorescence titrations with poly(dT) [47]EcSSB mainly binds to 35- and 65-nucleotide- (nt) longssDNA via the (SSB)
35- and (SSB)
65-binding modes respec-
tively In the (SSB)35-binding mode two subunits of the
EcSSB tetramer interact with ssDNA whereas in the (SSB)65-
bindingmode all four subunits participate in ssDNAbindingThese different binding modes may be required during dif-ferent stages of DNA metabolism for the in vivo function ofSSB [48ndash50] Although SSB binds to ssDNA via the highlyconserved ssDNA-binding domain the reason that the bind-ing site sizes of SSBs from different organisms differ remainsunclear For example differences are found among the bind-ing site sizes ofMethanococcus jannaschii SSB [51] the Gono-coccal Genetic Island-encoded SSB from Neisseria gonorr-hoeae [39] the thermostableThermotoga maritima andTher-motoga neapolitana SSBs [32] and the psychrophilic bacterialSSBs [37] In addition the (SSB)
35- and (SSB)
65-binding
modes are not found in some SSBs [32 39 42]Previously we have examined the electrophoretic mobil-
ity shift patterns of a His-tagged Klebsiella pneumoniae SSB(KpSSB) [40] a His-tagged Salmonella enterica serovar Typh-imurium LT2 SSB (StSSB) [43] and a His-tagged Pseu-domonas aeruginosa PAO1 SSB (PaSSB) [42] bound to differ-ent lengths of ssDNA We also determined their correspond-ing binding site sizes that is 26 22 and 29 nt per tetramerrespectively The electrophoretic mobility shift assay (EMSA)is a well-established approach in studies of molecular biology[52] and the use of radioactive tracer in this assay allows visu-alization of the actual formation of the distinct protein-DNAcomplex(es)[53] The expected result of EMSA is that whenthe length of the nucleotides is sufficient for the binding oftwo or more SSB molecules the electrophoretic mobility ofthe higher SSB oligomer complex will be lower than that ofthe smaller SSB oligomer complex [52 54] Recent studieson SSB binding also reveal that determination of the ssDNA-binding site size by using EMSA is significantly consistentwith that of the cocrystal structure of SSB with ssDNA [27]
KpSSB StSSB and PaSSB are similar proteins whoseN-terminal ssDNA-binding domains are almost identicalexcept for different ssDNA-binding site sizes [40 42 43]Thus we should assess whether the glycine-rich hinge whichis not conserved among SSBs is involved in the determina-tion of the binding site size of SSB In this study we swappedthe C-terminal domains of StSSB and PaSSB into that ofKpSSB through protein chimeragenesis Chimeras are pro-teins that contain segments from two ormore different parentproteins and serve as valuable tools to understand enzyme
mechanism and protein function [55] The EMSA behav-ior (patterns) of the resultant chimeric proteins namelyKpSSBnStSSBc and KpSSBnPaSSBc was characterized andcomparedwith untaggedKpSSB StSSB and PaSSB (Figure 1)On the basis of the chimeragenesis results the flexible C-ter-minal domain of SSBwas found to be involved in determiningthe ssDNA-binding site sizes
2 Materials and Methods
21 Materials All restriction enzymes and DNA-modifyingenzymes were purchased from New England Biolabs(Ipswich MA USA) unless explicitly stated otherwise Allchemicals were purchased from Sigma-Aldrich (St LouisMO USA) unless explicitly stated otherwise The E colistrains TOP10F1015840 (Invitrogen USA) and BL21(DE3)pLysS(Novagen UK) were used for genetic construction and pro-tein expression respectively
22 Construction of Plasmids for KpSSB StSSB and PaSSBExpression The KpSSB [40] StSSB [43] and PaSSB [42]expression plasmids were constructed by the protocolsdescribed previously with minor modification to avoidhaving aHis tag fusedwith the gene product A fragment con-taining the coding sequence of KpSSB (KPN04446) StSSB(STM4256) and PaSSB (PA4232) (with the stop codon) wasdirectly amplified by PCR by using the genomic DNA ofK pneumoniae subsp pneumoniae MGH 78578 S entericaserovar Typhimurium LT2 or P aeruginosa PAO1 (Primers1 to 6 resp) During the process NdeI and XhoI restrictionsites were introduced at the 51015840-end and the 31015840-end of thesegenes after which they were ligated into the pET21b vector(Novagen Inc Madison WI USA) for protein expression inE coli BL21 The expected gene product expressed by theseplasmids does not contain any artificial residue including aHis tag Primers used for construction of these plasmids aresummarized in Table 1
23 Construction of Plasmids for KpSSBnStSSBc and KpSSBn-PaSSBc Expression through Protein Chimeragenesis To inves-tigate the effect of the C-terminal domain of SSB on thesize of the ssDNA-binding site the C-terminal domain ofKpSSB was replaced by that of StSSB and PaSSB pET21b-KpSSB (Primers 7 and 8) pET21b-StSSB (Primers 9 and10) and pET21b-PaSSB (Primers 11 and 12) vectors weremutated to create a desired SacI site and to obtain thevectors for expression of the chimeric proteins KpSSB-nStSSBc and KpSSBnPaSSBc The D91EQ92L-engineeredpET21b-KpSSB vector the D91EQ92L-engineered pET21b-StSSB vector and the G90EQ91L-engineered pET21b-PaSSBvector were cut at NdeI and SacI sites Subsequently theKpSSBn StSSBc-pET21b and PaSSBc-pET21b fragmentswere purified KpSSBn was ligated with StSSBc-pET21band PaSSBc-pET21b fragments to generate the engineeredpET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc vectorsTo avoid artificial residues positions 91 and 92 of the twoplasmids were mutated back (Primers 13 to 16) to obtainpET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc vectors
BioMed Research International 3
StSSB KpSSB PaSSB91 92
91 92 91 92 90 91
90 9191 92
91 92 91 92
91 92 91 92
E L
G Q
E L E L
E LE L
Mutation
N- N- N-
CAT CAG CAT CAA GGC CAG
GAG CTC GAG CTC GAG CTC
- C -C-C
Mutation Mutation
GAG CTC GAG CTC
CAT CAG CAT CAG
1
1 1
1
176
176
166
166
KpSSBnStSSBc
KpSSBnStSSBc
KpSSBnPaSSBc
KpSSBnPaSSBc
NdeISacISacI SacISacI
NdeISacINdeISacI NdeISacI
fragment purification and ligation
KpSSBn fragment purificationand ligation fragment purification
and ligation
Mutation Mutation
NdeI NdeI NdeIXhoI XhoI XhoI
StSSBc-pET21 PaSSBc-pET21
D
D D
Q
Q Q
D Q
Figure 1 Construction of plasmids for expression of the chimeric KpSSBnStSSBc and KpSSBnPaSSBc proteins To investigate the effect of theC-terminal domain of SSB on the size of the ssDNA-binding site the C-terminal domain of KpSSB was replaced by that of StSSB and PaSSBpET21b-KpSSB (Primers 7 and 8) pET21b-StSSB (Primers 9 and 10) and pET21b-PaSSB (Primers 11 and 12) vectors were mutated to createa desired SacI site and to obtain the vectors for expression of the chimeric proteins KpSSBnStSSBc and KpSSBnPaSSBc The D91EQ92L-engineered pET21b-KpSSB vector the D91EQ92L-engineered pET21b-StSSB vector and the G90EQ91L-engineered pET21b-PaSSB vectorwere cut at NdeI and SacI sites Subsequently the KpSSBn StSSBc-pET21b and PaSSBc-pET21b fragments were purified KpSSBn was ligatedwith StSSBc-pET21b and PaSSBc-pET21b fragments to generate the engineered pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc vectorsTo avoid artificial residues positions 91 and 92 of the two plasmids were mutated back (Primers 13 to 16) to obtain pET21b-KpSSBnStSSBcand pET21b-KpSSBnPaSSBc vectors Thus pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc will express KpSSB1-91 fused StSSB92-176and PaSSB91-165 respectively Note that KpSSBnPaSSBc will have 166 amino acid residues
Thus pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBcwill express KpSSB1-91 fused StSSB92-176 and PaSSB91-165respectively Note that KpSSBnPaSSBc will have 166 aminoacid residues Plasmids were verified by DNA sequencingUnderlined nucleotides indicate the designated site for muta-tion or the restriction site (Table 1)
24 Protein Expression and Purification The recombinantSSBs were expressed using the protocol described previously[9 40 42 43 56ndash60] Purification of these recombinant SSBswas carried out as described previously with the followingmodifications [61 62] Briefly E coli BL21(DE3) cells wereindividually transformed with the expression vector andgrown to OD
600of 09 at 37∘C in Luria-Bertani medium
containing 250 120583gmL ampicillin with rapid shaking Over-expression of the expression plasmids was induced by incu-bating with 1mM isopropyl thiogalactoside (IPTG) for 3 h at37∘CThe cells overexpressing the protein were chilled on iceharvested by centrifugation resuspended in Buffer A (20mMTris-HCl 5mM imidazole and 02M ammonium sulfate pH79) and disrupted by sonicationwith ice coolingThe proteinsolution (50mL) was precipitated from the supernatant ofthe cell lysate by incubation with 027 gmL of ammoniumsulfate for 30min and centrifugation at 20000 g for 10minThepelletswerewashed twicewith 20mLof Buffer B (20mM
Tris-HCl 5mM imidazole and 12M ammonium sulfatepH 79) After dialysis against Buffer C (20mM Tris-HCl5mM imidazole 1mMEDTA and 100mMNaCl pH 79) theprotein solution applied to theQ column (GEHealthcare Bio-Sciences Piscataway NJ USA) was eluted with a linear NaClgradient from 01 to 06M with Buffer C using the AKTA-FPLC system (GE Healthcare Bio-Sciences Piscataway NJUSA) The peak fractions with the ssDNA-binding activitywere collected and dialyzed against Buffer D (20mM potas-sium phosphate 1mM EDTA and 100mM NaCl pH 70)The protein solution was then applied to the Heparin HPcolumn (GE Healthcare Bio-Sciences Piscataway NJ USA)and eluted with a linear NaCl gradient from 01 to 10Mwith Buffer DThe peak fractions from this chromatographicstep with the ssDNA-binding activity were collected andconcentrated and the purity of these SSBs was checkedby Coomassie-stained SDS-PAGE (Mini-PROTEAN TetraSystem Bio-Rad CA USA Figure 3)
25 Protein Concentration The protein concentration of thesolutions was determined by the Bio-Rad Protein Assay usingbovine serum albumin as a standard (Bio-Rad CA USA)The Bio-Rad Protein Assay is a dye-binding assay in which adifferential color change of a dye occurs in response to variousconcentrations of protein
4 BioMed Research International
Table 1 Primers used for construction of plasmids
Oligonucleotide Primer1 KpSSB-NdeI-N GGGCATATGGCCAGCAGAGGCGTAAAC2 KpSSB-XhoI-C GGGCTCGAGTTAGAACGGGATGTCGTC3 StSSB-NdeI-N CTGAACATATGGCCAGCAGAGGCGTAA4 StSSB-XhoI-C TGGAACTCGAGTTAGAACGGAATGTCG5 PaSSB-NdeI-N TTGCTCATATGGCCCGTGGGGTTAACA6 PaSSB-XhoI-C TTGCACTCGAGTTAGAACGGAATGTCG7 KpSSB(D91EQ92L-SacI)-N AAGTGGACCGAGCTCTCCGGTCAGGACA8 KpSSB(D91EQ92L-SacI)-C GTCCTGACCGGAGAGCTCGGTCCACTT9 StSSB(D91EQ92L-SacI)-N AAGTGGACCGAGCTCAGTGGCCAGGAA10 StSSB(D91EQ92L-SacI)-C TTCCTGGCCACTGAGCTCGGTCCACTT11 PaSSB(G90EQ91L-SacI)-N AAGTGGCAGGAGCTCGACGGTCAGGAT12 PaSSB(G90EQ91L-SacI)-C ATCCTGACCGTCGAGCTCCTGCCACTT13 KpSSBnStSSBc(E91DL92Q)-N AAGTGGACCGATCAGAGTGGCCAGGAA14 KpSSBnStSSBc(E91DL92Q)-C TTCCTGGCCACTCTGATCGGTCCACTT15 KpSSBnPaSSBc(E91DL92Q)-N AAGTGGACCGATCAGGACGGTCAGGAT16 KpSSBnPaSSBc(E91DL92Q)-C ATCCTGACCGTCCTGATCGGTCCACTTA fragment containing the coding sequence of KpSSB StSSB and PaSSB (with the stop codon) was cloned into the pET21b vector (using Primers 1ndash6) Duringthe process NdeI and XhoI restriction sites were introduced at the 51015840-end and the 31015840-end of these genes after which they were ligated into the pET21b vectorTo obtain the vectors for expression of the chimeric proteins KpSSBnStSSBc and KpSSBnPaSSBc pET21b-KpSSB (Primers 7 and 8) pET21b-StSSB (Primers9 and 10) and pET21b-PaSSB (Primers 11 and 12) vectors were mutated to create a desired SacI site The D91EQ92L-engineered pET21b-KpSSB vectorthe D91EQ92L-engineered pET21b-StSSB vector and the G90EQ91L-engineered pET21b-PaSSB vector were cut at NdeI and SacI sites Subsequently theKpSSBn StSSBc-pET21b and PaSSBc-pET21b fragments were purified KpSSBn was ligated with StSSBc-pET21b and PaSSBc-pET21b fragments to generatethe engineered pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc vectors To avoid artificial residues positions 91 and 92 of the two plasmids were mutatedback (Primers 13 to 16) to obtain pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc vectors Thus pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc willexpress KpSSB1-91 fused StSSB92-176 and PaSSB91-165 respectively These plasmids were verified by DNA sequencing Underlined nucleotides indicate thedesignated site for mutation or the restriction site
26 Gel-Filtration Chromatography Gel-filtration chroma-tography was carried out by the AKTA-FPLC system (GEHealthcare Bio-Sciences Piscataway NJ USA) Briefly puri-fied protein (2mgmL) was applied to a Superdex 200HR1030 column (GE Healthcare Bio-Sciences Piscataway NJUSA) equilibrated with Buffer D The column was operatedat a flow rate of 05mLmin and 05mL fractions were col-lected The proteins were detected by measuring the absorb-ance at 280 nm The column was calibrated with proteinsof known molecular weight thyroglobulin (670 kDa) 120574-globulin (158 kDa) ovalbumin (44 kDa) and myoglobin(17 kDa) The 119870av values for the standard proteins and theSSB variants were calculated from the equation 119870av = (119881119890 minus119881119900)(119881119888minus 119881119900) where 119881
119900is column void volume 119881
119890is elution
volume and 119881119888is geometric column volume
27 Electrophoretic Mobility Shift Assay (EMSA) EMSA [52]for these SSBs was carried out by the protocol describedpreviously for DnaB [63] PriB [59 64ndash66] DnaT [57 67]and SSB proteins [40 42 43 52] Briefly radiolabeling ofvarious lengths of ssDNA oligonucleotides was carried outwith [12057432P]ATP (6000Cimmol PerkinElmer Life SciencesWaltham MA) and T4 polynucleotide kinase (PromegaMadison WI USA) The protein (0 19 37 77 155 310 6301250 2500 and 5000 nM) was incubated for 30min at 25∘Cwith 17 nM DNA substrates (dT15ndash65) in a total volume of10 120583L in 20mMTris-HCl pH 80 and 100mMNaCl Aliquots(5 120583L) were removed from each of the reaction solutions and
added to 2 120583L of gel-loading solution (025 bromophenolblue and 40 sucrose) The resulting samples were resolvedon a native 8 polyacrylamide gel at 4∘C in TBE buffer(89mMTris borate and 1mMEDTA) for 1 h at 100V andwerevisualized by autoradiography Complexed and free DNAbands were scanned and quantified
28 DNA-Binding Ability The ssDNA-binding ability([Protein]
50 119870119889app) for the protein was estimated from the
protein concentration that binds 50 of the input ssDNA[52] Each [Protein]
50is calculated as the average of three
measurements plusmn SD
29 Bioinformatics Sequence alignment of KpSSB StSSBandPaSSBwas generated byCLUSTALW2 [68]The structureof the C-terminal domain of these SSBs was modeled by(PS)2 (http140113239111simps2v2docsphp) The struc-tures were visualized by using the program PyMol
3 Results
31 Sequence Analysis Based on the nucleotide sequencefound using a database search through the National Centerfor Biotechnology Information (NCBI) we predicted thatKpSSB StSSB and PaSSB monomer proteins have lengths of174 176 and 165 amino acid residues respectively The sizeof the ssDNA-binding site of His-tagged KpSSB [40] StSSB
BioMed Research International 5
M A S R G V N K V I L V G N L G Q D P E V R Y M P S G G A V A N F T L A T S E S W R D K Q T G
M A S R G V N K V I L V G N L G Q D P E V R Y M P S G G A V A N L T L A T S E S W R D K Q T G
M A - R G V N K V I L V G N V G G D P E T R Y M P N G N A V T N I T L A T S E S W K D K Q T G
K L A E V A G E Y L R K G S Q V Y I E G Q L R T R K W T D Q S G Q D K Y T T E V - V V N V G G
K L A E V A G E Y L R K G S Q V Y I E G Q L R T R K W T D Q S G Q E R Y T T E I N V P Q I G G
R L A E I A G E Y L R K G S Q V Y V E G S L R T R K W Q G Q D G Q D R Y T T E I - V V D I N G
G G G Q Q Q G G W G Q P Q Q P Q - - - G G N Q F S G G A Q S R P Q Q Q A P A A P S N E P P M D
G - G Q Q Q G G W G Q P Q Q P Q Q P Q G G N Q F S G G A Q S R P Q Q S A P - A P S N E P P M D
G - D D S Q R A P R E P M Q R P - - - - - - Q Q A P Q Q Q S R P A P Q Q Q P A P Q P A Q D Y D
E M K E Q T E W H R V V L F G
E M K E Q T E W H R V V M F G
Q Q Q E R T E W H R V V F F G
T M Q M L G G R Q G G G A P A
V M Q M L G G R Q G G G A P A
N M Q L L G G R - - - - - P S
- F D D D I P F
- F D D D I P F
S F D D D I P F
62
62
61
123
124
120
174176
165
KpSSB
StSSB
PaSSB
KpSSB
StSSB
PaSSB
KpSSB
StSSB
PaSSB
Figure 2 Multiple amino acid sequence alignment of SSB proteins Sequence alignment of KpSSB StSSB and PaSSB was generated byCLUSTALW2 Identical amino acid residues are colored in red Gly and Gln residues are shaded in cyan and gray The N-terminal domainsof these SSBs are significantly conserved
(kDa)
40-
35-
25-
15-
M 1 2 3 4 5
Figure 3 Protein purity Coomassie Blue-stained SDS-PAGE (15)of the purified KpSSB (lane 1) StSSB (lane 2) PaSSB (lane 3)KpSSBnStSSBc (lane 4) KpSSBnPaSSBc (lane 5) and molecularmass standards (M) are shown The sizes of the standard proteinsfrom the top down are as follows 55 40 35 25 15 and 10 kDa Thepurified SSBs migrated between the 25 and 15 kDa standards on theSDS-PAGE
[43] and PaSSB [42] was determined to be 26plusmn 1 22plusmn 1 and29plusmn1 nt respectivelyThe longer the length of the polypeptidechain the smaller the size for ssDNA binding Analysis ofthe primary structures of KpSSB StSSB and PaSSB by RPS-BLAST revealed the presence of a putative OB-fold domainthat is common to all known SSBs Figure 2 shows that thealignments of the amino acid sequences of KpSSB StSSB andPaSSB amino acid residues in their N-terminal domains arehighly conserved (colored in red) In the E coli SSB-ssDNAcomplex [11] four essential aromatic residues namely Trp40Trp54 Phe60 and Trp88 participate in ssDNA binding viastacking interactions [11] These residues are conserved inmost SSB families including KpSSB StSSB and PaSSB Theimportantmotif in the C-terminal tail of E coli SSB DDDIPFresidues is also conserved in KpSSB StSSB and PaSSB By
contrast to those motifs the residues found in the glycine-rich hinge of E coli SSB are not conserved in KpSSB StSSBand PaSSB (Figure 2) Thus the length and composition ofthe amino acid residues in the glycine-rich hinge may beresponsible for the different ssDNA-binding site sizes of SSBs
32 Expression and Purification of KpSSB StSSB and PaSSBThe N-terminal ssDNA-binding domain of SSB has beenwell-established to be highly conserved However SSBspossessing different ssDNA-binding site sizes have beenreported The reason that SSBs have similar ssDNA-bindingdomains but possess varying ssDNA-binding site sizesremains unclear Although the ssDNA-binding site sizes ofKpSSB StSSB and PaSSB have been reported we reinves-tigated the ssDNA-binding properties of KpSSB StSSB andPaSSB in the absence of a His tag to avoid the unknown effectof a His tag (hexahistidine) on the ssDNA binding of SSB
33 KpSSB Bound to ssDNA To investigate the length ofnucleotides sufficient for the formation of the KpSSB-ssDNAcomplex and the ssDNA-binding ability of KpSSB westudied the binding of KpSSB to dT20 (Figure 4(a)) dT25(Figure 4(b)) dT35 (Figure 4(c)) dT45 (Figure 4(d)) dT50(Figure 4(e)) dT55 (Figure 4(f)) and dT60 (Figure 4(g))with different protein concentrations As shown inFigure 4(a) no band shift was observed when KpSSBwas incubated with dT20 indicating that KpSSB couldnot form a stable complex with this homopolymer Bycontrast to dT20 longer dT homopolymers which includedT25ndash50 produced a significant band shift (C complex)that is formation of a stable protein-DNA complex insolution Furthermore two different complexes for dT55were formed by KpSSB (Figure 4(f)) At lower proteinconcentrations KpSSB formed a single complex (C1) withdT55 similar to that observed with dT50 (Figure 4(e))However when the KpSSB concentration was increasedanother slower migrating complex (C2) was observed
6 BioMed Research International
[KpSSB]
-dT20
mdash
(a)
-dT25
-C
mdash[KpSSB]
(b)
-dT35
-C
mdash[KpSSB]
(c)
-dT45
-C
mdash[KpSSB]
(d)
-dT50
-C
[KpSSB]
mdash
(e)
-dT55
-C1-C2
[KpSSB]mdash
(f)
-dT60
-C1-C2
[KpSSB]mdash
(g)
Figure 4 Binding of KpSSB to dT20ndash60 KpSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT20 (b) dT25 (c) dT35 (d) dT45 (e) dT50 (f) dT55 or (g) dT60 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
Two different complexes of KpSSB were also observedto bind to dT60 (Figure 4(g)) The appearance of thesecond complex resulted from the increased KpSSB con-centration suggesting that two KpSSB proteins maybe present per oligonucleotide Although dT55 is only 5 ntlonger thandT50 is the presence of an extra 5 nt in dT55 com-pared with that of dT50 provides enough interaction spacefor the binding of two KpSSB proteins Therefore one KpSSB
occupies 25 (502 = 25) nt to 275 (552 = 275) nt of thessDNA The EMSA results suggest that the length of anssDNA (or the binding site size) [52] required for KpSSBbinding is 26 plusmn 2 nt
34 StSSB Bound to ssDNA The binding of StSSB to dT15(Figure 5(a)) dT20 (Figure 5(b)) dT30 (Figure 5(c)) dT40(Figure 5(d)) dT45 (Figure 5(e)) and dT50 (Figure 5(f)) was
BioMed Research International 7
[StSSB]
-dT15
-C
mdash
(a)
-dT20
-C
---
-
mdash[StSSB]
(b)
-dT30
-C
[StSSB]mdash
(c)
-dT40
-C
mdash[StSSB]
(d)
-dT45
-C1-C2
mdash[StSSB]
(e)
-dT50
-C1-C2
mdash
[StSSB]
(f)
Figure 5 Binding of StSSB to dT15ndash50 StSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT15 (b) dT20 (c) dT30 (d) dT40 (e) dT45 or (f) dT50 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and 100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blue and40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
examined using EMSA StSSB can bind and form a singlecomplex with dT15 (Figure 5(a)) and dT20 (Figure 5(b)) butKpSSB cannot (Figure 4(a)) StSSB bound to dT15ndash40 andformed a single complex For dT45 and dT50 two differentcomplexes of StSSB appeared at high protein concentrations(Figures 5(e) and 5(f)) Therefore one StSSB occupies 20(402 = 20) nt to 225 (452 = 225) nt of the ssDNA TheEMSA results suggest that the length of an ssDNA (or thebinding site size) [52] required for StSSB binding is 21 plusmn 2 nt
35 PaSSB Bound to ssDNA The binding of PaSSB to dT20(Figure 6(a)) dT25 (Figure 6(b)) dT35 (Figure 6(c)) dT45(Figure 6(d)) dT55 (Figure 6(e)) dT60 (Figure 6(f)) anddT65 (Figure 6(g)) was studied by EMSA Unlike StSSB nocomplexwas observedwhen PaSSBwas incubatedwith dT20Some smears were observed indicating that PaSSB interactswith dT20 However the ssDNA may be too short to befully wrapped by PaSSB PaSSB could form a single complexwith dT25ndash55 and form two distinct complexes with dT60
and dT65 (Figures 6(f) and 6(g)) respectivelyTherefore onePaSSB occupies 275 (552 = 275) nt to 30 (602 = 30) ntof the ssDNA These results from EMSA suggest that thelength of an ssDNA (or the binding site size) [52] required forPaSSB binding is 29plusmn2 nt Although the SSBs that is KpSSBStSSB and PaSSB have significantly similar ssDNA-bindingdomains their binding site sizes are different and range from19 (21 plusmn 2 StSSB) to 31 (29 plusmn 2 PaSSB) nt The obtainedEMSA results (Figures 4ndash6) also show that the binding sitesizes of the untagged SSBs (KpSSB StSSB and PaSSB) werefound to be almost identical to those of the His-tagged ones[40 42 43]
36 Design of the Chimeric KpSSB Proteins KpSSBnStSSBc andKpSSBnPaSSBc The N-terminal ssDNA-binding domain ofKpSSB StSSB and PaSSB is highly conserved (Figure 2) buttheir binding site sizes are different (Figures 4ndash6) and rangefrom 19 nt to 31 nt The C-terminal acidic tails DDDIPFare conserved (Figure 2) and these features led us to assess
8 BioMed Research International
[PaSSB]
-dT20
mdash
(a)
-dT25
-C
[PaSSB]mdash
(b)
-dT35
-C
-
-
[PaSSB]
(c)
-dT45
-C
[PaSSB]
(d)
-dT55
-C
[PaSSB]
(e)
-dT60
-C1-C2
[PaSSB]
(f)
-dT65
-C1-C2
[PaSSB]
(g)
Figure 6 Binding of PaSSB to dT20ndash65 PaSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT20 (b) dT25 (c) dT35 (d) dT45 (e) dT55 (f) dT60 or (g) dT65 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
whether the flexible glycine-rich hinge in the C-terminaldomain which is not conserved among SSBs is involved inthe determination of the binding site size of SSB Thus theC-terminal domains of StSSB and PaSSB were swapped withKpSSB through protein chimeragenesis
37 KpSSBnStSSBc Bound to ssDNA The binding ofKpSSBnStSSBc to dT15 (Figure 7(a)) dT20 (Figure 7(b))dT40 (Figure 7(c)) and dT45 (Figure 7(d)) was examinedusing EMSA KpSSBnStSSBc exhibited significantly different
ssDNA-binding properties from those of KpSSB UnlikeKpSSB (Figure 4) both KpSSBnStSSBc (Figure 8) and StSSB(Figure 5) can bind and form a single complex with dT15 anddT20 Similar to StSSB KpSSBnStSSBc binds to dT15ndash40 andforms a single complex For dT45 two different complexesof KpSSBnStSSBc appeared at high protein concentrations(Figure 8(d)) this EMSA feature was also similar to that ofStSSB One KpSSBnStSSBc occupies 20 (402 = 20) nt to225 (452 = 225) nt of the ssDNA These EMSA resultssuggest that the length of an ssDNA (or the binding site
BioMed Research International 9
[KpSSBnStSSBc]
-dT15
-C
(a)
[KpSSBnStSSBc]
-dT20
-C
(b)
[KpSSBnStSSBc]
-dT40
-C
(c)
[KpSSBnStSSBc]
-dT45
-C1-C2
(d)
Figure 7 Binding of KpSSBnStSSBc to dT15ndash45 KpSSBnStSSBc (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for30min at 25∘C with 17 nM of (a) dT15 (b) dT20 (c) dT40 or (d) dT45 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and 100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blue and40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
size) [52] required for KpSSBnStSSBc binding is 21 plusmn 2 nt avalue identical to that for StSSB (Figure 5) Swapping of theC-terminal domain of StSSB with KpSSB changes the size ofthe ssDNA-binding site from 26 nt to 21 nt
38 KpSSBnPaSSBc Bound to ssDNA The binding fea-tures of KpSSBnPaSSBc with dT20 (Figure 8(a)) dT25(Figure 8(b)) dT40 (Figure 8(c)) dT55 (Figure 8(d)) anddT60 (Figure 8(e)) were studied by EMSA Similar to thecases of KpSSB and PaSSB no complex was observed whenKpSSBnPaSSBc was incubated with dT20 However KpSSB-nPaSSBc still exhibited dramatically different ssDNA-bindingproperties from those of KpSSB KpSSB can form two distinctcomplexes with dT55 (Figure 4(f)) but both KpSSBnPaSSBc(Figure 9) and PaSSB (Figure 6) cannot One KpSSBnPaSSBcoccupies 275 (552 = 275) nt to 30 (602 = 30) nt ofthe ssDNA The above EMSA results suggest that the lengthof an ssDNA (or the binding site size) [52] required forKpSSBnPaSSBc binding is 29 plusmn 2 nt a value identical to thatof PaSSB Swapping of the C-terminal domain of PaSSB toKpSSB changes the size of the ssDNA-binding site from 26 ntto 29 nt Although these SSBs namely KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc have nearly identicalssDNA-binding domains their binding site sizes are different(Table 2) Thus the size of the ssDNA-binding site requiredfor second SSB binding is likely to be dependent on the C-terminal domain of SSB
39 Binding Constants of the SSB-ssDNA Complexes Deter-mined from EMSA To compare the ssDNA-binding abil-ities of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc the midpoint values for input ssDNA bind-ing calculated from the titration curves of EMSA andreferred to as [Protein]
50(monomer) were quantified and
are summarized in Table 2 Although the N-terminal ssDNA-binding domains of these SSB proteins are highly similar(Figure 2) their ssDNA-binding activities and binding sitesizes are different (Table 2) [KpSSB]
50values ranged from
100 nM to 220 nM [StSSB]50
values ranged from 420 nM to650 nM [PaSSB]
50values ranged from 550 nM to 1700 nM
[KpSSBnStSSBc]50values ranged from 110 nM to 260 nM and
[KpSSBnPaSSBc]50
values ranged from 220 nM to 390 nMThe ssDNA-binding ability is as follows in the order ofdecreasing affinity KpSSB gt KpSSBnStSSBc gt KpSSBn-PaSSBc gt StSSB gt PaSSB Results from the above analysesindicate that the exchange of the C-terminal domain inSSB significantly changed the ssDNA-binding ability and theDNA-binding behavior (complex number) The reason asto why swapping of the C-terminal domain can affect thessDNA-binding activity of SSB remains unclear The C-ter-minal domain of SSB is suggested to be involved in ssDNAbinding However this relation is not evident in the results ofthe cocrystal structure
310 Oligomeric State of KpSSBnStSSBc and KpSSBnPaSSBc inSolution Gel-filtration chromatography was used to confirm
10 BioMed Research International
[KpSSBnPaSSBc]
-dT20
(a)
[KpSSBnPaSSBc]
-dT25
-C
(b)
[KpSSBnPaSSBc]
-C
-dT40
(c)
-C
[KpSSBnPaSSBc]
-dT55
(d)
-dT60
-C1-C2
[KpSSBnPaSSBc]
(e)
Figure 8 Binding of KpSSBnPaSSBc to dT20ndash60 KpSSBnPaSSBc (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for30min at 25∘C with 17 nM of (a) dT20 (b) dT25 (c) dT40 (d) dT55 or (e) dT60 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
that the oligomeric state of KpSSBnStSSBc and KpSSBn-PaSSBc remains as tetramers after chimeragenesisThe analy-sis of purified KpSSBnStSSBc and KpSSBnPaSSBc (2mgmL)using a Superdex 200HR 1030 column revealed a singlepeak with elution volumes of 786 and 789mL respectivelyAssuming that KpSSBnStSSBc and KpSSBnPaSSBc both haveshapes and partial specific volumes similar to the standardproteins the native molecular masses of KpSSBnStSSBc andKpSSBnPaSSBc were estimated to be 76641 and 74827Da ascalculated from a standard linear regression equation 119870av =minus03684(logMw) + 22707 (Figure 9) The native molecularmasses for KpSSBnStSSBc and KpSSBnPaSSBc are approx-imately four times the mass of the monomer (sim19 kDa)Therefore KpSSBnStSSBc and KpSSBnPaSSBc under theabove chromatographic conditions are stable tetramers insolution Although the exchange of the C-terminal domainin SSB significantly changed the ssDNA-binding ability andDNA-binding behavior (complex number) protein chimera-genesis did not cause any change in the oligomeric state ofSSB
311 Summary of Gly Gln and Pro Number in SSBs To ana-lyze the C-terminal amino acid composition of SSBs wefurther counted the number of Gly Gln and Pro residuesin different SSB segments SSB is abundant in Gly Glnand Pro (GQP) (Table 3) The GQP contents of KpSSB1ndash91 StSSB1ndash91 and PaSSB1ndash90 are similar However the Glynumber of PaSSB116ndash165 is significantly lower than that ofKpSSB116ndash174 and StSSB117ndash176 PaSSB116ndash165 contains only1 Gly but KpSSB116ndash174 and StSSB117ndash176 contain 11 and 12Gly respectively In addition we found different distributionpatterns among KpSSB StSSB and PaSSB Although theycontain similar number of Gln (Q) the QQQ pattern isfrequently found in PaSSB (Table 3)
312 Structural Modeling of SSBs Given its disordered C-ter-minal domain the crystal structure of the full-length SSB islacking even when SSB can be crystallized with DNA [69]We attempted to model the structure by homology mod-eling using the bioinformatics program (PS)2 to obtain an
BioMed Research International 11
Table 2 ssDNA binding properties of KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc as analyzed by EMSA
Protein DNA [Protein]50 (nM) Complex number
KpSSB
dT20 ND 0dT25 200 plusmn 20 1dT35 220 plusmn 30 1dT45 100 plusmn 10 1dT50 110 plusmn 20 1dT55 100 plusmn 20 2dT60 100 plusmn 10 2
StSSB
dT15 650 plusmn 120 1dT20 450 plusmn 80 1dT30 420 plusmn 60 1dT40 420 plusmn 80 1dT45 440 plusmn 60 2dT50 440 plusmn 50 2
PaSSB
dT20 ND 0dT25 1700 plusmn 250 1dT35 950 plusmn 180 1dT45 780 plusmn 160 1dT55 820 plusmn 90 1dT60 810 plusmn 110 2dT65 550 plusmn 70 2
KpSSBnStSSBc
dT15 260 plusmn 60 1dT20 110 plusmn 20 1dT40 120 plusmn 20 1dT45 160 plusmn 20 2
KpSSBnPaSSBc
dT20 ND 0dT25 390 plusmn 60 1dT40 220 plusmn 30 1dT55 230 plusmn 30 1dT60 230 plusmn 30 2
[Protein]50 was calculated from the titration curves of EMSA by determiningthe concentration of the protein (120583M) needed to achieve the midpointvalue for input ssDNA binding For some oligonucleotides input ssDNAbinding was the sum of the intensities from the two separate ssDNA-proteincomplexes Errors are standard deviations determined by three independenttitration experiments
Table 3 Summary of Gly Gln and Pro number in SSB
SSB segment G Q PKpSSB1ndash91 10 5 2StSSB1ndash91 10 5 2PaSSB1ndash90 11 6 2KpSSB92ndash174 18 16 9StSSB92ndash176 17 18 11PaSSB91ndash165 5 15 11KpSSB116ndash174 11 12 9StSSB117ndash176 12 13 10PaSSB116ndash165 1 12 11
in-depth understanding of the structure-function relation-ship of the C-terminal domains of these SSBs [70 71]
40 45 50 55
Kav
00
02
04
06
08
10
KpSSBnStSSBcKpSSBnPaSSBc
Y = minus03684X + 22707
R2= 0998
log Mw
Figure 9Gel-filtration chromatographic analyses of KpSSBnStSSBcand KpSSBnPaSSBc Purified protein (2mgmL) was applied toa Superdex 200HR 1030 column (GE Healthcare Bio-SciencesPiscataway NJ USA) equilibrated with Buffer D The column wasoperated at a flow rate of 05mLmin and 05mL fractions werecollected The proteins were detected by measuring the absorbanceat 280 nm The column was calibrated with proteins of knownmolecular weight thyroglobulin (670 kDa) 120574-globulin (158 kDa)ovalbumin (44 kDa) and myoglobin (17 kDa) The 119870av values forthe standard proteins and the SSB variants were calculated from theequation119870av = (119881119890minus119881119900)(119881119888minus119881119900) where119881119900 is column void volume119881119890is elution volume and 119881
119888is geometric column volume
(PS)2 (http140113239111simps2v2docsphp) is an auto-matic homology modeling server that combines bothsequence and secondary structure information to detect thehomologous proteins with remote similarity and the target-template alignment After pasting the amino acid sequenceto the website of (PS)2 only one hit (Protein Data Bankentry 1QVC EcSSB) for the C-terminal domains of KpSSBand StSSB was suggested For the C-terminal domain ofPaSSB only one hit that is CstF-77 (ProteinData Bank entry2OOE cleavage stimulation factor CstF) but not EcSSB wassuggested as the template for modeling Figure 10 shows thatmodeled structures of these SSB C-terminal domains arehighly disordered but that of PaSSB is more ordered than thatof other domains
4 Discussion
In this study we examined the sizes of the binding site ofthe untagged SSB and the chimeric SSB from the ubiquitousopportunistic pathogens K pneumoniae S enterica serovarTyphimurium LT2 and P aeruginosa PAO1 Many clinicalstrains of the abovementioned bacteria are highly resistantto antibiotics [72ndash75] The development of clinically usefulsmall-molecule antibiotics has been a seminal event in thefield of infectious diseases [48] Nucleic acid metabolismis one of the most basic biological functions and shouldbe a prime target in antibiotic development [76ndash78] Manybacterial SSBs form conserved protein interaction ldquohubsrdquothat are essential to recruit many proteins involved in DNA
12 BioMed Research International
Figure 10 Structure modeling of SSB The structures of KpSSB1ndash115 StSSB1ndash115 and PaSSB1ndash115 (the N-terminal domain of SSB)were modeled by SWISS-MODEL The structures of KpSSB116ndash142 StSSB116ndash142 and PaSSB121ndash160 (the C-terminal domain ofSSB) were modeled by (PS)2 Other regions of SSBs could not bemodeled by these two programs The structures of the N-terminaldomain and the C-terminal domain of these SSBs were manuallylinked (KpSSB1ndash142 blue StSSB1ndash142 pink PaSSB1ndash160 green) andsuperimposed with the crystal structure of EcSSB1ndash142 (orange)(PDB entry 1QVC) for comparison For clarity only one subunitof the tetramer was shown for each SSB
replication recombination and repair SSBDNA nucleopro-tein substrates [79] Thus SSBs may be promising targets inantibiotic development [80] As a first step toward achievingthis goal we investigated why SSBs possess highly conservedN-terminal ssDNA-binding domain but exhibit varying bind-ing site sizes One significant clue is that their flexible hingesand the length at the C-terminus are different as revealed bysequence alignment (Figure 2)
The interactions of various SSBs with ssDNA have beenanalyzed using a variety of techniques such as tryptophan-fluorescence quenching [47] filter binding [81] EMSA [5282] analytical ultracentrifugation [83] electron microscopy[84] nuclease digestion [44] single-molecule fluorescencemicroscopy [48] and crystallographic analyses [11] In thisstudy we have examined the electrophoretic mobility shiftpatterns of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc bound to different lengths of ssDNA and deter-mined the corresponding binding site sizes to be 26 2129 22 and 29 nt per tetramer respectively (Figures 4ndash8) PaSSB and KpSSBnPaSSBc have the largest sizes forssDNA binding among the SSBs studied We also identifiedHis-tagged and untagged SSBs that have similar ssDNA-binding site sizes [40 42 43] EMSA is a well-establishedapproach in studies of molecular biology [52] and the use ofradioactive tracer in this assay allows detection of the actualformation of the distinct protein-DNA complex(es) [53] For
exampleDNase protection assay and footprinting assay usingradioactive tracer can determine the specific DNA sequencecomplexed by a protein In EMSA when the length of thenucleotides is sufficient for the binding of two or more SSBmolecules the electrophoretic mobility of the higher SSBoligomer complex will be lower than that of the smaller SSBoligomer complex [52 54] In addition results of the ssDNA-binding site size from EMSA and cocrystal structure of SSBwere consistent [27] Thus throughout this paper we deter-mined the ssDNA-binding site sizes of SSB from the EMSAbehavior
Many SSBs bind to ssDNA with some degree of positivecooperativity Cooperativity can result from direct protein-protein interactions between the nearest neighbors such asthe LAST motif in the T4 gene-32 protein [85] and thearginine-mediated interaction motif in Thermus SSB [8687] Cooperativity can also result from the protein-induceddistortion of adjacent DNA as demonstrated in SulfolobusSSB PriB and FOXK1a proteins [23 60 88] In the cases ofKpSSB StSSB and PaSSB (Figures 4ndash6) binding appeared tobe nearly noncooperative for several DNAs because all DNAmainly shifts into the first complex (C1) before the appearanceof the second complex (C2) when subjected to increasingprotein concentrationsThe length dependence of the [SSB]
50
values suggests that the amount of spacing is optimum forsteric considerations (Table 2)
Because bacteria have varying genomic DNA sizes theirSSBs may need to evolve to have different binding site sizesfor DNA metabolism Results from protein chimeragenesisshowed the C-terminal domain dependence of the bindingsite sizes of SSB (Figure 11) The experimental data showedthat the binding site size of KpSSBnStSSBc was similar to thatof StSSB and the size of the binding site of KpSSBnPaSSBcwas similar to that of PaSSB The reason for which the bind-ing site size of SSB changed followed by swapping of theC-terminal domain remains unclear Flexibility numberof glycine residues andor different QQQ patterns of theC-terminal domain of SSB (Figure 2 and Table 3) may beimportant factors for determining the ssDNA-binding sitesize In fact the C-terminal domain of PaSSB that isPaSSB116ndash165 has only 1 Gly residue which is significantlyless than that of KpSSB (11 Gly) and StSSB (12Gly) Gly (andPro) is an important component of the flexible region aprotein that contains low Gly content is predicted to havelow flexibility Unlike typical SSB [35 69] PaSSB116ndash165 hasa partial structure (Figure 10) Although KpSSB StSSB andPaSSB contain similar number of Gln (Q) the QQQ patternis frequently found in PaSSB (Figure 2 and Table 3) PolyQand repeated sequences GAGAG are commonly found inthe structures of amyloids silk fibers and neurodegradationproteins [89ndash92] Considering that the simple coil polyQ theheptapeptide GNNQQNY and the hexapeptide NNQQNYcan cause protein aggregation and nucleation [93ndash95] thedistribution of Gln in the C-terminal domain of a tetramericSSB may also be an important determinant of the ssDNA-binding site size of SSB by some steric hindrances (Figure 11)However the above speculationmust be confirmed by furtherbiochemical experiments
BioMed Research International 13
PaSSBStSSB KpSSB
C-terC-ter C-ter
sim 21 nt sim 26 nt sim 29 nt
Figure 11 Possiblemodels for explainingwhy SSBs arewith different binding site sizes Twomodeled structures of KpSSB1ndash142 (blue) StSSB1ndash142 (pink) and PaSSB1ndash160 (green) complexed with ssDNA (gold) are shown For clarity only one C-terminal domain was shown for eachSSB tetramer By using the electrophoretic mobility shift assay and the protein chimeragenesis we characterized that the binding site sizes ofKpSSB StSSB PaSSB KpSSBnStSSBc and KpSSBnPaSSBc were 26 21 29 21 and 29 nt per tetramer respectively KpSSB StSSB and PaSSBare similar proteins whose N-terminal ssDNA-binding domains are almost identical Thus the C-terminal domain of SSB may indirectlycontribute to ssDNA binding and wrapping and affects the binding site size by the steric hindrance
5 Conclusion
In this study we characterized the ssDNA-binding propertiesof untagged SSBs from K pneumoniae S enterica sero-var Typhimurium LT2 and P aeruginosa PAO1 and proposeda role of the C-terminal flexible domain for ssDNA bind-ing from the protein chimeragenesis and EMSA resultsThe amino acid sequence of the N-terminal ssDNA-bind-ingoligomerization domain in these pathogenic SSBs ishighly conserved but their apparent binding site sizes aredifferent This finding indicates that the C-terminal protein-protein interaction domain may also indirectly contribute tossDNA binding and wrapping
Conflict of Interests
The authors declare that there is no conflict of interestsregarding the publication of this paper
Acknowledgment
This researchwas supported by aGrant from theNational Sci-ence Council Taiwan (NSC 102-2320-B-040-019 to Cheng-Yang Huang)
References
[1] R Reyes-Lamothe D J Sherratt and M C Leake ldquoStoichiom-etry and architecture of active DNA replication machinery inescherichia colirdquo Science vol 328 no 5977 pp 498ndash501 2010
[2] D J Richard E Bolderson andK K Khanna ldquoMultiple humansingle-stranded DNA binding proteins function in genomemaintenance structural biochemical and functional analysisrdquo
Critical Reviews in Biochemistry and Molecular Biology vol 44no 2-3 pp 98ndash116 2009
[3] R D Shereda A G Kozlov T M LohmanMM Cox and J LKeck ldquoSSB as an organizermobilizer of genome maintenancecomplexesrdquo Critical Reviews in Biochemistry and MolecularBiology vol 43 no 5 pp 289ndash318 2008
[4] D J Richard E Bolderson L Cubeddu et al ldquoSingle-strandedDNA-binding protein hSSB1 is critical for genomic stabilityrdquoNature vol 453 no 7195 pp 677ndash681 2008
[5] R R Meyer and P S Laine ldquoThe single-stranded DNA-bindingprotein of Escherichia colirdquoMicrobiological Reviews vol 54 no4 pp 342ndash380 1990
[6] C Yang U Curth C Urbanke and C Kang ldquoCrystal structureof human mitochondrial single-stranded DNA binding proteinat 24 A resolutionrdquo Nature Structural Biology vol 4 no 2 pp153ndash157 1997
[7] G Webster J Genschel U Curth C Urbanke C Kang and RHilgenfeld ldquoA common core for binding single-stranded DNAstructural comparison of the single-stranded DNA-bindingproteins (SSB) from E coli and human mitochondriardquo FEBSLetters vol 411 pp 313ndash316 1997
[8] R L Flynn and L Zou ldquoOligonucleotideoligosaccharide-bind-ing fold proteins a growing family of genome guardiansrdquo Crit-ical Reviews in Biochemistry and Molecular Biology vol 45 no4 pp 266ndash275 2010
[9] K Chan Y Lee CWangHHuang andY Sun ldquoSingle-Strand-ed DNA-Binding Protein Complex from Helicobacter pyloriSuggests an ssDNA-Binding Surfacerdquo Journal of MolecularBiology vol 388 no 3 pp 508ndash519 2009
[10] D L Theobald R M Mitton-Fry and D S Wuttke ldquoNucleicacid recognition by OB-fold proteinsrdquo Annual Review of Bio-physics and Biomolecular Structure vol 32 pp 115ndash133 2003
[11] S Raghunathan A G Kozlov TM Lohman andGWaksmanldquoStructure of the DNA binding domain of E coli SSB bound to
14 BioMed Research International
ssDNArdquo Nature Structural Biology vol 7 no 8 pp 648ndash6522000
[12] A G Murzin ldquoOB(oligonucleotideoligosaccharide binding)-fold common structural and functional solution for non-homologous sequencesrdquo EMBO Journal vol 12 no 3 pp 861ndash867 1993
[13] N P George K V Ngo S Chitteni-Pattu et al ldquoStructure andcellular dynamics of deinococcus radiodurans single-strandedDNA (ssDNA)-binding protein (SSB)-DNA complexesrdquo TheJournal of Biological Chemistry vol 287 no 26 pp 22123ndash221322012
[14] P Filipkowski and J Kur ldquoIdentification and propertiesof the Deinococcus grandis and Deinococcus proteolyticussingle-stranded DNA binding proteins (SSB)rdquo Acta BiochimicaPolonica vol 54 no 1 pp 79ndash87 2007
[15] P Filipkowski A Duraj-Thatte and J Kur ldquoIdentificationcloning expression and characterization of a highly ther-mostable single-stranded-DNA-binding protein (SSB) fromDeinococcus murrayirdquo Protein Expression and Purification vol53 no 1 pp 201ndash208 2007
[16] P Filipkowski M Koziatek and J Kur ldquoA highly ther-mostable homodimeric single-stranded DNA-binding proteinfrom Deinococcus radiopugnansrdquo Extremophiles vol 10 no 6pp 607ndash614 2006
[17] P Filipkowski A Duraj-Thatte and J Kur ldquoNovel thermostablesingle-stranded DNA-binding protein (SSB) fromDeinococcusgeothermalisrdquo Archives of Microbiology vol 186 no 2 pp 129ndash137 2006
[18] G Witte C Urbanke and U Curth ldquoSingle-stranded DNA-binding protein of Deinococcus radiodurans a biophysicalcharacterizationrdquoNucleic Acids Research vol 33 no 5 pp 1662ndash1670 2005
[19] D A Bernstein J M Eggingtont M P Killoran A M MisicMMCox and J L Keck ldquoCrystal structure of theDeinococcusradiodurans single-stranded DNA-binding protein suggests amechanism for coping with DNA damagerdquo Proceedings of theNational Academy of Sciences of the United States of Americavol 101 no 23 pp 8575ndash8580 2004
[20] S Dabrowski M Olszewski R Piatek A Brillowska-Dabrowska G Konopa and J Kur ldquoIdentification and charac-terization of single-stranded-DNA-binding proteins fromTher-mus thermophilus and Thermus aquaticusmdashnew arrange-ment of binding domainsrdquo Microbiology vol 148 no 10 pp3307ndash3315 2002
[21] R Gamsjaeger R Kariawasam C Touma A H Kwan M FWhite and L Cubeddu ldquoBackbone and side-chain 1H 13C and15N resonance assignments of the OB domain of the singlestranded DNA binding protein from Sulfolobus solfataricusand chemical shift mapping of the DNA-binding interfacerdquoBiomolecular NMR Assignments 2013
[22] M L Rolfsmeier and C A Haseltine ldquoThe single-strandedDNA binding protein of Sulfolobus solfataricus acts in the pre-synaptic step of homologous recombinationrdquo Journal of Molec-ular Biology vol 397 no 1 pp 31ndash45 2010
[23] I D Kerr R I M Wadsworth L Cubeddu W Blankenfeldt JHNaismith andM FWhite ldquoInsights into ssDNA recognitionby the OB fold from a structural and thermodynamic study ofSulfolobus SSB proteinrdquo EMBO Journal vol 22 no 11 pp 2561ndash2570 2003
[24] C A Haseltine and S C Kowalczykowski ldquoA distinctive single-stranded DNA-binding protein from the Archaeon Sulfolobussolfataricusrdquo Molecular Microbiology vol 43 no 6 pp 1505ndash1515 2002
[25] R I M Wadsworth and M F White ldquoIdentification andproperties of the crenarchaeal single-stranded DNA bindingprotein from Sulfolobus solfataricusrdquo Nucleic Acids Researchvol 29 no 4 pp 914ndash920 2001
[26] S Sugiman-Marangos and M S Junop ldquoThe structure of DdrBfrom Deinococcus a new fold for single-stranded DNA bindingproteinsrdquo Nucleic Acids Research vol 38 no 10 Article IDgkq036 pp 3432ndash3440 2010
[27] H Ghalei H V Moeller D Eppers et al ldquoEntrapment of DNAin an intersubunit tunnel system of a single-stranded DNA-binding proteinrdquo Nucleic Acids Research vol 42 no 10 pp6698ndash6708 2014
[28] S Paytubi S A McMahon S Graham et al ldquoDisplacement ofthe canonical single-strandedDNA-binding protein in the ther-moprotealesrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 109 no 7 pp E398ndashE405 2012
[29] T H Dickey S E Altschuler and D S Wuttke ldquoSingle-stranded DNA-binding proteins multiple domains for multiplefunctionsrdquo Structure vol 21 no 7 pp 1074ndash1084 2013
[30] D Vujaklija and BMacek ldquoDetecting posttranslational modifi-cations of bacterial SSB proteinsrdquoMethods inMolecular Biologyvol 922 pp 205ndash218 2012
[31] I Mijakovic D Petranovic B Macek et al ldquoBacterial single-stranded DNA-binding proteins are phosphorylated on tyro-sinerdquoNucleic Acids Research vol 34 no 5 pp 1588ndash1596 2006
[32] M Olszewski A Grot M Wojciechowski M Nowak MMickiewicz and J Kur ldquoCharacterization of exceptionally ther-mostable single-strandedDNA-binding proteins fromThermo-toga maritima and Thermotoga neapolitanardquo BMC Microbiol-ogy vol 10 article 260 2010
[33] U Curth J Genschel C Urbanke and J Greipel ldquoIn vitro andin vivo function of the C-terminus of Escherichia coil single-stranded DNA binding proteinrdquoNucleic Acids Research vol 24no 14 pp 2706ndash2711 1996
[34] G Witte C Urbanke and U Curth ldquoDNA polymerase IIIchi subunit ties single-stranded DNA binding protein to thebacterial replication machineryrdquoNucleic Acids Research vol 31pp 4434ndash4440 2003
[35] D Shishmarev YWang C EMason et al ldquoOtting Intramolec-ular binding mode of the C-terminus of Escherichia coli single-strandedDNAbinding protein determined by nuclearmagneticresonance spectroscopyrdquo Nucleic Acids Research vol 42 pp2750ndash2757 2014
[36] A G Kozlov M M Cox and T M Lohman ldquoRegulation ofsingle-stranded DNA binding by the C termini of Escherichiacoli single-stranded DNA-binding (SSB) proteinrdquo Journal ofBiological Chemistry vol 285 no 22 pp 17246ndash17252 2010
[37] M Nowak M Olszewski M Spibida and J Kur ldquoChar-acterization of single-stranded DNA-binding proteins fromthe psychrophilic bacteria Desulfotalea psychrophila Flavobac-terium psychrophilum Psychrobacter arcticus Psychrobactercryohalolentis Psychromonas ingrahamii Psychroflexus torquisand Photobacterium profundumrdquo BMC Microbiology vol 14article 91 2014
BioMed Research International 15
[38] T Paradzik N Ivic Z Filic et al ldquoStructure-function relation-ships of two paralogous single-stranded DNA-binding proteinsfrom Streptomyces coelicolor implication of SsbB in chromo-some segregation during sporulationrdquo Nucleic Acids Researchvol 41 no 6 pp 3659ndash3672 2013
[39] S JainM Zweig E Peeters et al ldquoCharacterization of the singlestrandedDNA binding protein SsbB encoded in the gonoccocalgenetic islandrdquo PLoS ONE vol 7 no 4 Article ID e35285 2012
[40] Y H Huang and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from Klebsiella pneumoniaemutation at either Arg73 or Ser76 causes a less cooperativecomplex onDNArdquoGenes to Cells vol 17 no 2 pp 146ndash157 2012
[41] E Antony E A Weiland S Korolev and T M LohmanldquoPlasmodium falciparum SSB tetramer wraps single-strandedDNAwith similar topology but opposite polarity to E Coli SSBrdquoJournal ofMolecular Biology vol 420 no 4-5 pp 269ndash283 2012
[42] H Jan Y L Lee and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from pseudomonas aeruginosaPAO1rdquo Protein Journal vol 30 no 1 pp 20ndash26 2011
[43] Y-H Huang Y-L Lee and C-Y Huang ldquoCharacterization of asingle-stranded DNA binding protein from Salmonella entericaserovar typhimurium LT2rdquo Protein Journal vol 30 no 2 pp102ndash108 2011
[44] S K Bharti K Rex P Sreedhar N Krishnan and U VarshneyldquoChimeras of Escherichia coli and Mycobacterium tuberculosissingle-stranded DNA binding proteins characterization andfunction in Escherichia colirdquo PLoS ONE vol 6 no 12 ArticleID e27216 2011
[45] M T Oliveira and L S Kaguni ldquoFunctional roles of the N-and C-terminal regions of the human mitochondrial single-stranded DNA-binding proteinrdquo PLoS ONE vol 5 no 10Article ID e15379 2010
[46] A G Kozlov J M Eggington M M Cox and T MLohman ldquoBinding of the dimeric Deinococcus radioduranssingle-strandedDNA binding protein to single-strandedDNArdquoBiochemistry vol 49 no 38 pp 8266ndash8275 2010
[47] T M Lohman and M E Ferrari ldquoEscherichia coli single-stranded DNA-binding protein multiple DNA-binding modesand cooperativesrdquo Annual Review of Biochemistry vol 63 pp527ndash570 1994
[48] R Zhou A G Kozlov R Roy et al ldquoSSB functions as a slidingplatform that migrates on DNA via reptationrdquo Cell vol 146 pp222ndash232 2011
[49] R Roy A G Kozlov T M Lohman and T Ha ldquoSSB proteindiffusion on single-stranded DNA stimulates RecA filamentformationrdquo Nature vol 461 pp 1092ndash1097 2009
[50] R Roy A G Kozlov T M Lohman and T Ha ldquoDynamicstructural rearrangements between DNA binding modes of Ecoli SSB proteinrdquo Journal of Molecular Biology vol 369 no 5pp 1244ndash1257 2007
[51] T J Kelly P Simancek and G S Brush ldquoIdentification andcharacterization of a single-stranded DNA-binding proteinfrom the archaeon Methanococcus jannaschiirdquo Proceedings ofthe National Academy of Sciences of the United States of Americavol 95 no 25 pp 14634ndash14639 1998
[52] C Y Huang Determination of the Binding Site-Size of theProtein-DNA Complex by Use of the Electrophoretic MobilityShift Assay InTech Press Rijeka Croatia 2012
[53] A Kornberg ldquoTen commandments of enzymology amendedrdquoTrends in Biochemical Sciences vol 28 no 10 pp 515ndash517 2003
[54] W Zhang X Lu and J Shen ldquoEMSA and single-moleculeforce spectroscopy study of interactions between bacillus sub-tilis single-stranded DNA-binding protein and single-strandedDNArdquo Langmuir vol 27 no 24 pp 15008ndash15015 2011
[55] M Ostermeier and S J Benkovic ldquoEvolution of protein func-tion by domain swappingrdquo Advances in Protein Chemistry vol55 pp 29ndash77 2000
[56] W F Peng and C Y Huang ldquoAllantoinase and dihydroorotasebinding and inhibition by flavonols and the substrates of cyclicamidohydrolasesrdquo Biochimie vol 101 pp 113ndash122 2014
[57] Y H Huang M J Lin and C Y Huang ldquoDnaT is a single-stranded DNA binding proteinrdquo Genes to Cells vol 18 no 11pp 1007ndash1019 2013
[58] Y Y Ho Y H Huang and C Y Huang ldquoChemical rescue of thepost-translationally carboxylated lysine mutant of allantoinaseand dihydroorotase by metal ions and short-chain carboxylicacidsrdquo Amino Acids vol 44 no 4 pp 1181ndash1191 2013
[59] Y H Huang Y Lo W Huang and C Y Huang ldquoCrystalstructure and DNA-binding mode of Klebsiella pneumoniaeprimosomal PriB proteinrdquoGenes to Cells vol 17 no 10 pp 837ndash849 2012
[60] C Y Huang C Y Hsu Y Sun H Wu and C Hsiao ldquoCom-plexed crystal structure of replication restart primosome pro-tein PriB reveals a novel single-stranded DNA-binding moderdquoNucleic Acids Research vol 34 no 14 pp 3878ndash3886 2006
[61] T Kinebuchi H Shindo H Nagai N Shimamoto andM Shimizu ldquoFunctional domains of Escherichia coli single-stranded DNA binding protein as assessed by analyses of thedeletion mutantsrdquo Biochemistry vol 36 no 22 pp 6732ndash67381997
[62] N Shimamoto N Ikushima H Utiyama H Tachibana andK Horie ldquoSpecific and cooperative binding of E coli single-stranded DNA binding protein to mRNArdquo Nucleic AcidsResearch vol 15 no 13 pp 5241ndash5250 1987
[63] H Lin andC YHuang ldquoCharacterization of flavonol inhibitionof DnaB helicase Real-time monitoring structural modelingand proposed mechanismrdquo Journal of Biomedicine and Biotech-nology vol 2012 Article ID 735368 2012
[64] Y H Huang H H Lin and C Y Huang ldquoA single residuedetermines the cooperative binding property of a primosomalDNA replication protein PriB to single-stranded DNArdquo Bio-science Biotechnology and Biochemistry vol 76 no 6 pp 1110ndash1115 2012
[65] H C Hsieh and C Y Huang ldquoIdentification of a novel proteinPriB in Klebsiella pneumoniaerdquo Biochemical and BiophysicalResearch Communications vol 404 no 1 pp 546ndash551 2011
[66] J H Liu T W Chang C Y Huang et al ldquoCrystal structure ofPriB a primosomalDNA replication protein ofEscherichia colirdquoThe Journal of Biological Chemistry vol 279 no 48 pp 50465ndash50471 2004
[67] Y H Huang and C Y Huang ldquoThe N-terminal domain ofDnaT a primosomalDNA replication protein is crucial for PriBbinding and self-trimerizationrdquo Biochemical and BiophysicalResearch Communications vol 442 pp 147ndash152 2013
[68] M A Larkin G Blackshields N P Brown et al ldquoClustalW andClustal X version 20rdquo Bioinformatics vol 23 no 21 pp 2947ndash2948 2007
16 BioMed Research International
[69] S N Savvides S Raghunathan K Futterer A G Kozlov T MLohman and G Waksman ldquoThe C-terminal domain of full-length E coli SSB is disordered even when bound to DNArdquoProtein Science vol 13 no 7 pp 1942ndash1947 2004
[70] C-C Chen J-K Hwang and J-M Yang ldquo(PS)2-v2 template-based protein structure prediction serverrdquo BMC Bioinformaticsvol 10 article 366 2009
[71] C Chen J Hwang and J Yang ldquo(PS)2 protein structure pre-diction serverrdquoNucleic Acids Research vol 34 pp W152ndashW1572006
[72] K Bush and J F Fisher ldquoEpidemiological expansion structuralstudies and clinical challenges of new 120573-lactamases from gram-negative bacteriardquo Annual Review of Microbiology vol 65 pp455ndash478 2011
[73] K K Kumarasamy M A Toleman T R Walsh et al ldquoEmer-gence of a new antibiotic resistance mechanism in India Pak-istan and the UK a molecular biological and epidemiologicalstudyrdquoThe Lancet Infectious Diseases vol 10 no 9 pp 597ndash6022010
[74] K Bush and M J MacIelag ldquoNew 120573-lactam antibiotics and 120573-lactamase inhibitorsrdquo Expert Opinion on Therapeutic Patentsvol 20 no 10 pp 1277ndash1293 2010
[75] K Bush ldquoAlarming 120573-lactamase-mediated resistance in multi-drug-resistant Enterobacteriaceaerdquo Current Opinion in Microbi-ology vol 13 no 5 pp 558ndash564 2010
[76] A Srivastava M Talaue S Liu et al ldquoNew target for inhibitionof bacterial RNA polymerase ldquoswitch regionrdquordquo Current Opinionin Microbiology vol 14 no 5 pp 532ndash543 2011
[77] K Chono K Katsumata T Kontani et al ldquoASP2151 a novelhelicase-primase inhibitor possesses antiviral activity againstvaricella-zoster virus and herpes simplex virus types 1 and 2rdquoJournal of Antimicrobial Chemotherapy vol 65 no 8 pp 1733ndash1741 2010
[78] M T Black and K Coleman ldquoNew inhibitors of bacterial topoi-somerase GyrAParC subunitsrdquo Current Opinion in Investiga-tional Drugs vol 10 no 8 pp 804ndash810 2009
[79] A H Marceau D A Bernstein B W Walsh W Shapiro LA Simmons and J L Keck ldquoProtein interactions in genomemaintenance as novel antibacterial targetsrdquo PLoS ONE vol 8no 3 Article ID e58765 2013
[80] D Lu D A Bernstein K A Satyshur and J L Keck ldquoSmall-molecule tools for dissecting the roles of SSBprotein inter-actions in genome maintenancerdquo Proceedings of the NationalAcademy of Sciences of the United States of America vol 107 no2 pp 633ndash638 2010
[81] I Wong and T M Lohman ldquoA double-filter method fornitrocellulose-filter binding application to protein-nucleic acidinteractionsrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 90 no 12 pp 5428ndash5432 1993
[82] M Mitas J Y Chock and M Christy ldquoThe binding-site sizesof Escherichia coli single-stranded-DNA-binding protein andmammalian replication protein A are 65 and ge 54 nucleotidesrespectivelyrdquo Biochemical Journal vol 324 no 3 pp 957ndash9611997
[83] C Urbanke G Witte and U Curth ldquoSedimentation velocitymethod in the analytical ultracentrifuge for the study of protein-protein interactionsrdquoMethods inMolecular Biology vol 305 pp101ndash114 2005
[84] S Chrysogelos and J Griffith ldquoEscherichia coli single-strandbinding protein organizes single-strandedDNA innucleosome-like unitsrdquo Proceedings of the National Academy of Sciences of theUnited States of America vol 79 no 19 pp 5803ndash5807 1982
[85] J R Casas-Finet K R Fischer and R L Karpel ldquoStructuralbasis for the nucleic acid binding cooperativity of bacteriophageT4 gene 32 protein the (LysArg)3(SerThr)2 (LAST) motifrdquoProceedings of the National Academy of Sciences of the UnitedStates of America vol 89 pp 1050ndash1054 1992
[86] G Witte R Fedorov and U Curth ldquoBiophysical analysis ofThermus aquaticus single-stranded DNA binding proteinrdquo Bio-physical Journal vol 94 no 6 pp 2269ndash2279 2008
[87] R Fedorov G Witte C Urbanke D J Manstein and UCurth ldquo3D structure of Thermus aquaticus single-strandedDNA-binding protein gives insight into the functioning of SSBproteinsrdquo Nucleic Acids Research vol 34 no 22 pp 6708ndash67172006
[88] K L Tsai C Y Huang C H Chang Y J Sun W J ChuangandC DHsiao ldquoCrystal structure of the human FOXK1a-DNAcomplex and its implications on the diverse binding specificityof winged helixforkhead proteinsrdquo Journal of Biological Chem-istry vol 281 no 25 pp 17400ndash17409 2006
[89] A H Mao N Lyle and R V Pappu ldquoDescribing sequence-ensemble relationships for intrinsically disordered proteinsrdquoBiochemical Journal vol 449 no 2 pp 307ndash318 2013
[90] R Wetzel ldquoPhysical chemistry of polyglutamine Intriguingtales of a monotonous sequencerdquo Journal of Molecular Biologyvol 421 no 4-5 pp 466ndash490 2012
[91] H T Orr ldquoPolyglutamine neurodegeneration expanded glu-tamines enhance native functionsrdquo Current Opinion in Geneticsand Development vol 22 no 3 pp 251ndash255 2012
[92] M Figiel W J Szlachcic P M Switonski A Gabka and W JKrzyzosiak ldquoMouse models of polyglutamine diseases reviewand data table Part Irdquo Molecular Neurobiology vol 46 no 2pp 393ndash429 2012
[93] J Nasica-Labouze and N Mousseau ldquoKinetics of amyloidaggregation a study of the GNNQQNY prion sequencerdquo PLoSComputational Biology vol 8 no 11 Article ID e1002782 2012
[94] J Nasica-Labouze M Meli P Derreumaux G Colombo andN Mousseau ldquoA multiscale approach to characterize the earlyaggregation steps of the amyloid-forming peptide gnnqqnyfrom the yeast prion sup-35rdquo PLoS Computational Biology vol7 no 5 Article ID e1002051 2011
[95] J R Lewandowski P C A van der Wel M Rigney N Grig-orieff and R G Griffin ldquoStructural complexity of a compositeamyloid fibrilrdquo Journal of the American Chemical Society vol133 no 37 pp 14686ndash14698 2011
BioMed Research International 3
StSSB KpSSB PaSSB91 92
91 92 91 92 90 91
90 9191 92
91 92 91 92
91 92 91 92
E L
G Q
E L E L
E LE L
Mutation
N- N- N-
CAT CAG CAT CAA GGC CAG
GAG CTC GAG CTC GAG CTC
- C -C-C
Mutation Mutation
GAG CTC GAG CTC
CAT CAG CAT CAG
1
1 1
1
176
176
166
166
KpSSBnStSSBc
KpSSBnStSSBc
KpSSBnPaSSBc
KpSSBnPaSSBc
NdeISacISacI SacISacI
NdeISacINdeISacI NdeISacI
fragment purification and ligation
KpSSBn fragment purificationand ligation fragment purification
and ligation
Mutation Mutation
NdeI NdeI NdeIXhoI XhoI XhoI
StSSBc-pET21 PaSSBc-pET21
D
D D
Q
Q Q
D Q
Figure 1 Construction of plasmids for expression of the chimeric KpSSBnStSSBc and KpSSBnPaSSBc proteins To investigate the effect of theC-terminal domain of SSB on the size of the ssDNA-binding site the C-terminal domain of KpSSB was replaced by that of StSSB and PaSSBpET21b-KpSSB (Primers 7 and 8) pET21b-StSSB (Primers 9 and 10) and pET21b-PaSSB (Primers 11 and 12) vectors were mutated to createa desired SacI site and to obtain the vectors for expression of the chimeric proteins KpSSBnStSSBc and KpSSBnPaSSBc The D91EQ92L-engineered pET21b-KpSSB vector the D91EQ92L-engineered pET21b-StSSB vector and the G90EQ91L-engineered pET21b-PaSSB vectorwere cut at NdeI and SacI sites Subsequently the KpSSBn StSSBc-pET21b and PaSSBc-pET21b fragments were purified KpSSBn was ligatedwith StSSBc-pET21b and PaSSBc-pET21b fragments to generate the engineered pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc vectorsTo avoid artificial residues positions 91 and 92 of the two plasmids were mutated back (Primers 13 to 16) to obtain pET21b-KpSSBnStSSBcand pET21b-KpSSBnPaSSBc vectors Thus pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc will express KpSSB1-91 fused StSSB92-176and PaSSB91-165 respectively Note that KpSSBnPaSSBc will have 166 amino acid residues
Thus pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBcwill express KpSSB1-91 fused StSSB92-176 and PaSSB91-165respectively Note that KpSSBnPaSSBc will have 166 aminoacid residues Plasmids were verified by DNA sequencingUnderlined nucleotides indicate the designated site for muta-tion or the restriction site (Table 1)
24 Protein Expression and Purification The recombinantSSBs were expressed using the protocol described previously[9 40 42 43 56ndash60] Purification of these recombinant SSBswas carried out as described previously with the followingmodifications [61 62] Briefly E coli BL21(DE3) cells wereindividually transformed with the expression vector andgrown to OD
600of 09 at 37∘C in Luria-Bertani medium
containing 250 120583gmL ampicillin with rapid shaking Over-expression of the expression plasmids was induced by incu-bating with 1mM isopropyl thiogalactoside (IPTG) for 3 h at37∘CThe cells overexpressing the protein were chilled on iceharvested by centrifugation resuspended in Buffer A (20mMTris-HCl 5mM imidazole and 02M ammonium sulfate pH79) and disrupted by sonicationwith ice coolingThe proteinsolution (50mL) was precipitated from the supernatant ofthe cell lysate by incubation with 027 gmL of ammoniumsulfate for 30min and centrifugation at 20000 g for 10minThepelletswerewashed twicewith 20mLof Buffer B (20mM
Tris-HCl 5mM imidazole and 12M ammonium sulfatepH 79) After dialysis against Buffer C (20mM Tris-HCl5mM imidazole 1mMEDTA and 100mMNaCl pH 79) theprotein solution applied to theQ column (GEHealthcare Bio-Sciences Piscataway NJ USA) was eluted with a linear NaClgradient from 01 to 06M with Buffer C using the AKTA-FPLC system (GE Healthcare Bio-Sciences Piscataway NJUSA) The peak fractions with the ssDNA-binding activitywere collected and dialyzed against Buffer D (20mM potas-sium phosphate 1mM EDTA and 100mM NaCl pH 70)The protein solution was then applied to the Heparin HPcolumn (GE Healthcare Bio-Sciences Piscataway NJ USA)and eluted with a linear NaCl gradient from 01 to 10Mwith Buffer DThe peak fractions from this chromatographicstep with the ssDNA-binding activity were collected andconcentrated and the purity of these SSBs was checkedby Coomassie-stained SDS-PAGE (Mini-PROTEAN TetraSystem Bio-Rad CA USA Figure 3)
25 Protein Concentration The protein concentration of thesolutions was determined by the Bio-Rad Protein Assay usingbovine serum albumin as a standard (Bio-Rad CA USA)The Bio-Rad Protein Assay is a dye-binding assay in which adifferential color change of a dye occurs in response to variousconcentrations of protein
4 BioMed Research International
Table 1 Primers used for construction of plasmids
Oligonucleotide Primer1 KpSSB-NdeI-N GGGCATATGGCCAGCAGAGGCGTAAAC2 KpSSB-XhoI-C GGGCTCGAGTTAGAACGGGATGTCGTC3 StSSB-NdeI-N CTGAACATATGGCCAGCAGAGGCGTAA4 StSSB-XhoI-C TGGAACTCGAGTTAGAACGGAATGTCG5 PaSSB-NdeI-N TTGCTCATATGGCCCGTGGGGTTAACA6 PaSSB-XhoI-C TTGCACTCGAGTTAGAACGGAATGTCG7 KpSSB(D91EQ92L-SacI)-N AAGTGGACCGAGCTCTCCGGTCAGGACA8 KpSSB(D91EQ92L-SacI)-C GTCCTGACCGGAGAGCTCGGTCCACTT9 StSSB(D91EQ92L-SacI)-N AAGTGGACCGAGCTCAGTGGCCAGGAA10 StSSB(D91EQ92L-SacI)-C TTCCTGGCCACTGAGCTCGGTCCACTT11 PaSSB(G90EQ91L-SacI)-N AAGTGGCAGGAGCTCGACGGTCAGGAT12 PaSSB(G90EQ91L-SacI)-C ATCCTGACCGTCGAGCTCCTGCCACTT13 KpSSBnStSSBc(E91DL92Q)-N AAGTGGACCGATCAGAGTGGCCAGGAA14 KpSSBnStSSBc(E91DL92Q)-C TTCCTGGCCACTCTGATCGGTCCACTT15 KpSSBnPaSSBc(E91DL92Q)-N AAGTGGACCGATCAGGACGGTCAGGAT16 KpSSBnPaSSBc(E91DL92Q)-C ATCCTGACCGTCCTGATCGGTCCACTTA fragment containing the coding sequence of KpSSB StSSB and PaSSB (with the stop codon) was cloned into the pET21b vector (using Primers 1ndash6) Duringthe process NdeI and XhoI restriction sites were introduced at the 51015840-end and the 31015840-end of these genes after which they were ligated into the pET21b vectorTo obtain the vectors for expression of the chimeric proteins KpSSBnStSSBc and KpSSBnPaSSBc pET21b-KpSSB (Primers 7 and 8) pET21b-StSSB (Primers9 and 10) and pET21b-PaSSB (Primers 11 and 12) vectors were mutated to create a desired SacI site The D91EQ92L-engineered pET21b-KpSSB vectorthe D91EQ92L-engineered pET21b-StSSB vector and the G90EQ91L-engineered pET21b-PaSSB vector were cut at NdeI and SacI sites Subsequently theKpSSBn StSSBc-pET21b and PaSSBc-pET21b fragments were purified KpSSBn was ligated with StSSBc-pET21b and PaSSBc-pET21b fragments to generatethe engineered pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc vectors To avoid artificial residues positions 91 and 92 of the two plasmids were mutatedback (Primers 13 to 16) to obtain pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc vectors Thus pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc willexpress KpSSB1-91 fused StSSB92-176 and PaSSB91-165 respectively These plasmids were verified by DNA sequencing Underlined nucleotides indicate thedesignated site for mutation or the restriction site
26 Gel-Filtration Chromatography Gel-filtration chroma-tography was carried out by the AKTA-FPLC system (GEHealthcare Bio-Sciences Piscataway NJ USA) Briefly puri-fied protein (2mgmL) was applied to a Superdex 200HR1030 column (GE Healthcare Bio-Sciences Piscataway NJUSA) equilibrated with Buffer D The column was operatedat a flow rate of 05mLmin and 05mL fractions were col-lected The proteins were detected by measuring the absorb-ance at 280 nm The column was calibrated with proteinsof known molecular weight thyroglobulin (670 kDa) 120574-globulin (158 kDa) ovalbumin (44 kDa) and myoglobin(17 kDa) The 119870av values for the standard proteins and theSSB variants were calculated from the equation 119870av = (119881119890 minus119881119900)(119881119888minus 119881119900) where 119881
119900is column void volume 119881
119890is elution
volume and 119881119888is geometric column volume
27 Electrophoretic Mobility Shift Assay (EMSA) EMSA [52]for these SSBs was carried out by the protocol describedpreviously for DnaB [63] PriB [59 64ndash66] DnaT [57 67]and SSB proteins [40 42 43 52] Briefly radiolabeling ofvarious lengths of ssDNA oligonucleotides was carried outwith [12057432P]ATP (6000Cimmol PerkinElmer Life SciencesWaltham MA) and T4 polynucleotide kinase (PromegaMadison WI USA) The protein (0 19 37 77 155 310 6301250 2500 and 5000 nM) was incubated for 30min at 25∘Cwith 17 nM DNA substrates (dT15ndash65) in a total volume of10 120583L in 20mMTris-HCl pH 80 and 100mMNaCl Aliquots(5 120583L) were removed from each of the reaction solutions and
added to 2 120583L of gel-loading solution (025 bromophenolblue and 40 sucrose) The resulting samples were resolvedon a native 8 polyacrylamide gel at 4∘C in TBE buffer(89mMTris borate and 1mMEDTA) for 1 h at 100V andwerevisualized by autoradiography Complexed and free DNAbands were scanned and quantified
28 DNA-Binding Ability The ssDNA-binding ability([Protein]
50 119870119889app) for the protein was estimated from the
protein concentration that binds 50 of the input ssDNA[52] Each [Protein]
50is calculated as the average of three
measurements plusmn SD
29 Bioinformatics Sequence alignment of KpSSB StSSBandPaSSBwas generated byCLUSTALW2 [68]The structureof the C-terminal domain of these SSBs was modeled by(PS)2 (http140113239111simps2v2docsphp) The struc-tures were visualized by using the program PyMol
3 Results
31 Sequence Analysis Based on the nucleotide sequencefound using a database search through the National Centerfor Biotechnology Information (NCBI) we predicted thatKpSSB StSSB and PaSSB monomer proteins have lengths of174 176 and 165 amino acid residues respectively The sizeof the ssDNA-binding site of His-tagged KpSSB [40] StSSB
BioMed Research International 5
M A S R G V N K V I L V G N L G Q D P E V R Y M P S G G A V A N F T L A T S E S W R D K Q T G
M A S R G V N K V I L V G N L G Q D P E V R Y M P S G G A V A N L T L A T S E S W R D K Q T G
M A - R G V N K V I L V G N V G G D P E T R Y M P N G N A V T N I T L A T S E S W K D K Q T G
K L A E V A G E Y L R K G S Q V Y I E G Q L R T R K W T D Q S G Q D K Y T T E V - V V N V G G
K L A E V A G E Y L R K G S Q V Y I E G Q L R T R K W T D Q S G Q E R Y T T E I N V P Q I G G
R L A E I A G E Y L R K G S Q V Y V E G S L R T R K W Q G Q D G Q D R Y T T E I - V V D I N G
G G G Q Q Q G G W G Q P Q Q P Q - - - G G N Q F S G G A Q S R P Q Q Q A P A A P S N E P P M D
G - G Q Q Q G G W G Q P Q Q P Q Q P Q G G N Q F S G G A Q S R P Q Q S A P - A P S N E P P M D
G - D D S Q R A P R E P M Q R P - - - - - - Q Q A P Q Q Q S R P A P Q Q Q P A P Q P A Q D Y D
E M K E Q T E W H R V V L F G
E M K E Q T E W H R V V M F G
Q Q Q E R T E W H R V V F F G
T M Q M L G G R Q G G G A P A
V M Q M L G G R Q G G G A P A
N M Q L L G G R - - - - - P S
- F D D D I P F
- F D D D I P F
S F D D D I P F
62
62
61
123
124
120
174176
165
KpSSB
StSSB
PaSSB
KpSSB
StSSB
PaSSB
KpSSB
StSSB
PaSSB
Figure 2 Multiple amino acid sequence alignment of SSB proteins Sequence alignment of KpSSB StSSB and PaSSB was generated byCLUSTALW2 Identical amino acid residues are colored in red Gly and Gln residues are shaded in cyan and gray The N-terminal domainsof these SSBs are significantly conserved
(kDa)
40-
35-
25-
15-
M 1 2 3 4 5
Figure 3 Protein purity Coomassie Blue-stained SDS-PAGE (15)of the purified KpSSB (lane 1) StSSB (lane 2) PaSSB (lane 3)KpSSBnStSSBc (lane 4) KpSSBnPaSSBc (lane 5) and molecularmass standards (M) are shown The sizes of the standard proteinsfrom the top down are as follows 55 40 35 25 15 and 10 kDa Thepurified SSBs migrated between the 25 and 15 kDa standards on theSDS-PAGE
[43] and PaSSB [42] was determined to be 26plusmn 1 22plusmn 1 and29plusmn1 nt respectivelyThe longer the length of the polypeptidechain the smaller the size for ssDNA binding Analysis ofthe primary structures of KpSSB StSSB and PaSSB by RPS-BLAST revealed the presence of a putative OB-fold domainthat is common to all known SSBs Figure 2 shows that thealignments of the amino acid sequences of KpSSB StSSB andPaSSB amino acid residues in their N-terminal domains arehighly conserved (colored in red) In the E coli SSB-ssDNAcomplex [11] four essential aromatic residues namely Trp40Trp54 Phe60 and Trp88 participate in ssDNA binding viastacking interactions [11] These residues are conserved inmost SSB families including KpSSB StSSB and PaSSB Theimportantmotif in the C-terminal tail of E coli SSB DDDIPFresidues is also conserved in KpSSB StSSB and PaSSB By
contrast to those motifs the residues found in the glycine-rich hinge of E coli SSB are not conserved in KpSSB StSSBand PaSSB (Figure 2) Thus the length and composition ofthe amino acid residues in the glycine-rich hinge may beresponsible for the different ssDNA-binding site sizes of SSBs
32 Expression and Purification of KpSSB StSSB and PaSSBThe N-terminal ssDNA-binding domain of SSB has beenwell-established to be highly conserved However SSBspossessing different ssDNA-binding site sizes have beenreported The reason that SSBs have similar ssDNA-bindingdomains but possess varying ssDNA-binding site sizesremains unclear Although the ssDNA-binding site sizes ofKpSSB StSSB and PaSSB have been reported we reinves-tigated the ssDNA-binding properties of KpSSB StSSB andPaSSB in the absence of a His tag to avoid the unknown effectof a His tag (hexahistidine) on the ssDNA binding of SSB
33 KpSSB Bound to ssDNA To investigate the length ofnucleotides sufficient for the formation of the KpSSB-ssDNAcomplex and the ssDNA-binding ability of KpSSB westudied the binding of KpSSB to dT20 (Figure 4(a)) dT25(Figure 4(b)) dT35 (Figure 4(c)) dT45 (Figure 4(d)) dT50(Figure 4(e)) dT55 (Figure 4(f)) and dT60 (Figure 4(g))with different protein concentrations As shown inFigure 4(a) no band shift was observed when KpSSBwas incubated with dT20 indicating that KpSSB couldnot form a stable complex with this homopolymer Bycontrast to dT20 longer dT homopolymers which includedT25ndash50 produced a significant band shift (C complex)that is formation of a stable protein-DNA complex insolution Furthermore two different complexes for dT55were formed by KpSSB (Figure 4(f)) At lower proteinconcentrations KpSSB formed a single complex (C1) withdT55 similar to that observed with dT50 (Figure 4(e))However when the KpSSB concentration was increasedanother slower migrating complex (C2) was observed
6 BioMed Research International
[KpSSB]
-dT20
mdash
(a)
-dT25
-C
mdash[KpSSB]
(b)
-dT35
-C
mdash[KpSSB]
(c)
-dT45
-C
mdash[KpSSB]
(d)
-dT50
-C
[KpSSB]
mdash
(e)
-dT55
-C1-C2
[KpSSB]mdash
(f)
-dT60
-C1-C2
[KpSSB]mdash
(g)
Figure 4 Binding of KpSSB to dT20ndash60 KpSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT20 (b) dT25 (c) dT35 (d) dT45 (e) dT50 (f) dT55 or (g) dT60 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
Two different complexes of KpSSB were also observedto bind to dT60 (Figure 4(g)) The appearance of thesecond complex resulted from the increased KpSSB con-centration suggesting that two KpSSB proteins maybe present per oligonucleotide Although dT55 is only 5 ntlonger thandT50 is the presence of an extra 5 nt in dT55 com-pared with that of dT50 provides enough interaction spacefor the binding of two KpSSB proteins Therefore one KpSSB
occupies 25 (502 = 25) nt to 275 (552 = 275) nt of thessDNA The EMSA results suggest that the length of anssDNA (or the binding site size) [52] required for KpSSBbinding is 26 plusmn 2 nt
34 StSSB Bound to ssDNA The binding of StSSB to dT15(Figure 5(a)) dT20 (Figure 5(b)) dT30 (Figure 5(c)) dT40(Figure 5(d)) dT45 (Figure 5(e)) and dT50 (Figure 5(f)) was
BioMed Research International 7
[StSSB]
-dT15
-C
mdash
(a)
-dT20
-C
---
-
mdash[StSSB]
(b)
-dT30
-C
[StSSB]mdash
(c)
-dT40
-C
mdash[StSSB]
(d)
-dT45
-C1-C2
mdash[StSSB]
(e)
-dT50
-C1-C2
mdash
[StSSB]
(f)
Figure 5 Binding of StSSB to dT15ndash50 StSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT15 (b) dT20 (c) dT30 (d) dT40 (e) dT45 or (f) dT50 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and 100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blue and40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
examined using EMSA StSSB can bind and form a singlecomplex with dT15 (Figure 5(a)) and dT20 (Figure 5(b)) butKpSSB cannot (Figure 4(a)) StSSB bound to dT15ndash40 andformed a single complex For dT45 and dT50 two differentcomplexes of StSSB appeared at high protein concentrations(Figures 5(e) and 5(f)) Therefore one StSSB occupies 20(402 = 20) nt to 225 (452 = 225) nt of the ssDNA TheEMSA results suggest that the length of an ssDNA (or thebinding site size) [52] required for StSSB binding is 21 plusmn 2 nt
35 PaSSB Bound to ssDNA The binding of PaSSB to dT20(Figure 6(a)) dT25 (Figure 6(b)) dT35 (Figure 6(c)) dT45(Figure 6(d)) dT55 (Figure 6(e)) dT60 (Figure 6(f)) anddT65 (Figure 6(g)) was studied by EMSA Unlike StSSB nocomplexwas observedwhen PaSSBwas incubatedwith dT20Some smears were observed indicating that PaSSB interactswith dT20 However the ssDNA may be too short to befully wrapped by PaSSB PaSSB could form a single complexwith dT25ndash55 and form two distinct complexes with dT60
and dT65 (Figures 6(f) and 6(g)) respectivelyTherefore onePaSSB occupies 275 (552 = 275) nt to 30 (602 = 30) ntof the ssDNA These results from EMSA suggest that thelength of an ssDNA (or the binding site size) [52] required forPaSSB binding is 29plusmn2 nt Although the SSBs that is KpSSBStSSB and PaSSB have significantly similar ssDNA-bindingdomains their binding site sizes are different and range from19 (21 plusmn 2 StSSB) to 31 (29 plusmn 2 PaSSB) nt The obtainedEMSA results (Figures 4ndash6) also show that the binding sitesizes of the untagged SSBs (KpSSB StSSB and PaSSB) werefound to be almost identical to those of the His-tagged ones[40 42 43]
36 Design of the Chimeric KpSSB Proteins KpSSBnStSSBc andKpSSBnPaSSBc The N-terminal ssDNA-binding domain ofKpSSB StSSB and PaSSB is highly conserved (Figure 2) buttheir binding site sizes are different (Figures 4ndash6) and rangefrom 19 nt to 31 nt The C-terminal acidic tails DDDIPFare conserved (Figure 2) and these features led us to assess
8 BioMed Research International
[PaSSB]
-dT20
mdash
(a)
-dT25
-C
[PaSSB]mdash
(b)
-dT35
-C
-
-
[PaSSB]
(c)
-dT45
-C
[PaSSB]
(d)
-dT55
-C
[PaSSB]
(e)
-dT60
-C1-C2
[PaSSB]
(f)
-dT65
-C1-C2
[PaSSB]
(g)
Figure 6 Binding of PaSSB to dT20ndash65 PaSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT20 (b) dT25 (c) dT35 (d) dT45 (e) dT55 (f) dT60 or (g) dT65 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
whether the flexible glycine-rich hinge in the C-terminaldomain which is not conserved among SSBs is involved inthe determination of the binding site size of SSB Thus theC-terminal domains of StSSB and PaSSB were swapped withKpSSB through protein chimeragenesis
37 KpSSBnStSSBc Bound to ssDNA The binding ofKpSSBnStSSBc to dT15 (Figure 7(a)) dT20 (Figure 7(b))dT40 (Figure 7(c)) and dT45 (Figure 7(d)) was examinedusing EMSA KpSSBnStSSBc exhibited significantly different
ssDNA-binding properties from those of KpSSB UnlikeKpSSB (Figure 4) both KpSSBnStSSBc (Figure 8) and StSSB(Figure 5) can bind and form a single complex with dT15 anddT20 Similar to StSSB KpSSBnStSSBc binds to dT15ndash40 andforms a single complex For dT45 two different complexesof KpSSBnStSSBc appeared at high protein concentrations(Figure 8(d)) this EMSA feature was also similar to that ofStSSB One KpSSBnStSSBc occupies 20 (402 = 20) nt to225 (452 = 225) nt of the ssDNA These EMSA resultssuggest that the length of an ssDNA (or the binding site
BioMed Research International 9
[KpSSBnStSSBc]
-dT15
-C
(a)
[KpSSBnStSSBc]
-dT20
-C
(b)
[KpSSBnStSSBc]
-dT40
-C
(c)
[KpSSBnStSSBc]
-dT45
-C1-C2
(d)
Figure 7 Binding of KpSSBnStSSBc to dT15ndash45 KpSSBnStSSBc (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for30min at 25∘C with 17 nM of (a) dT15 (b) dT20 (c) dT40 or (d) dT45 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and 100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blue and40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
size) [52] required for KpSSBnStSSBc binding is 21 plusmn 2 nt avalue identical to that for StSSB (Figure 5) Swapping of theC-terminal domain of StSSB with KpSSB changes the size ofthe ssDNA-binding site from 26 nt to 21 nt
38 KpSSBnPaSSBc Bound to ssDNA The binding fea-tures of KpSSBnPaSSBc with dT20 (Figure 8(a)) dT25(Figure 8(b)) dT40 (Figure 8(c)) dT55 (Figure 8(d)) anddT60 (Figure 8(e)) were studied by EMSA Similar to thecases of KpSSB and PaSSB no complex was observed whenKpSSBnPaSSBc was incubated with dT20 However KpSSB-nPaSSBc still exhibited dramatically different ssDNA-bindingproperties from those of KpSSB KpSSB can form two distinctcomplexes with dT55 (Figure 4(f)) but both KpSSBnPaSSBc(Figure 9) and PaSSB (Figure 6) cannot One KpSSBnPaSSBcoccupies 275 (552 = 275) nt to 30 (602 = 30) nt ofthe ssDNA The above EMSA results suggest that the lengthof an ssDNA (or the binding site size) [52] required forKpSSBnPaSSBc binding is 29 plusmn 2 nt a value identical to thatof PaSSB Swapping of the C-terminal domain of PaSSB toKpSSB changes the size of the ssDNA-binding site from 26 ntto 29 nt Although these SSBs namely KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc have nearly identicalssDNA-binding domains their binding site sizes are different(Table 2) Thus the size of the ssDNA-binding site requiredfor second SSB binding is likely to be dependent on the C-terminal domain of SSB
39 Binding Constants of the SSB-ssDNA Complexes Deter-mined from EMSA To compare the ssDNA-binding abil-ities of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc the midpoint values for input ssDNA bind-ing calculated from the titration curves of EMSA andreferred to as [Protein]
50(monomer) were quantified and
are summarized in Table 2 Although the N-terminal ssDNA-binding domains of these SSB proteins are highly similar(Figure 2) their ssDNA-binding activities and binding sitesizes are different (Table 2) [KpSSB]
50values ranged from
100 nM to 220 nM [StSSB]50
values ranged from 420 nM to650 nM [PaSSB]
50values ranged from 550 nM to 1700 nM
[KpSSBnStSSBc]50values ranged from 110 nM to 260 nM and
[KpSSBnPaSSBc]50
values ranged from 220 nM to 390 nMThe ssDNA-binding ability is as follows in the order ofdecreasing affinity KpSSB gt KpSSBnStSSBc gt KpSSBn-PaSSBc gt StSSB gt PaSSB Results from the above analysesindicate that the exchange of the C-terminal domain inSSB significantly changed the ssDNA-binding ability and theDNA-binding behavior (complex number) The reason asto why swapping of the C-terminal domain can affect thessDNA-binding activity of SSB remains unclear The C-ter-minal domain of SSB is suggested to be involved in ssDNAbinding However this relation is not evident in the results ofthe cocrystal structure
310 Oligomeric State of KpSSBnStSSBc and KpSSBnPaSSBc inSolution Gel-filtration chromatography was used to confirm
10 BioMed Research International
[KpSSBnPaSSBc]
-dT20
(a)
[KpSSBnPaSSBc]
-dT25
-C
(b)
[KpSSBnPaSSBc]
-C
-dT40
(c)
-C
[KpSSBnPaSSBc]
-dT55
(d)
-dT60
-C1-C2
[KpSSBnPaSSBc]
(e)
Figure 8 Binding of KpSSBnPaSSBc to dT20ndash60 KpSSBnPaSSBc (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for30min at 25∘C with 17 nM of (a) dT20 (b) dT25 (c) dT40 (d) dT55 or (e) dT60 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
that the oligomeric state of KpSSBnStSSBc and KpSSBn-PaSSBc remains as tetramers after chimeragenesisThe analy-sis of purified KpSSBnStSSBc and KpSSBnPaSSBc (2mgmL)using a Superdex 200HR 1030 column revealed a singlepeak with elution volumes of 786 and 789mL respectivelyAssuming that KpSSBnStSSBc and KpSSBnPaSSBc both haveshapes and partial specific volumes similar to the standardproteins the native molecular masses of KpSSBnStSSBc andKpSSBnPaSSBc were estimated to be 76641 and 74827Da ascalculated from a standard linear regression equation 119870av =minus03684(logMw) + 22707 (Figure 9) The native molecularmasses for KpSSBnStSSBc and KpSSBnPaSSBc are approx-imately four times the mass of the monomer (sim19 kDa)Therefore KpSSBnStSSBc and KpSSBnPaSSBc under theabove chromatographic conditions are stable tetramers insolution Although the exchange of the C-terminal domainin SSB significantly changed the ssDNA-binding ability andDNA-binding behavior (complex number) protein chimera-genesis did not cause any change in the oligomeric state ofSSB
311 Summary of Gly Gln and Pro Number in SSBs To ana-lyze the C-terminal amino acid composition of SSBs wefurther counted the number of Gly Gln and Pro residuesin different SSB segments SSB is abundant in Gly Glnand Pro (GQP) (Table 3) The GQP contents of KpSSB1ndash91 StSSB1ndash91 and PaSSB1ndash90 are similar However the Glynumber of PaSSB116ndash165 is significantly lower than that ofKpSSB116ndash174 and StSSB117ndash176 PaSSB116ndash165 contains only1 Gly but KpSSB116ndash174 and StSSB117ndash176 contain 11 and 12Gly respectively In addition we found different distributionpatterns among KpSSB StSSB and PaSSB Although theycontain similar number of Gln (Q) the QQQ pattern isfrequently found in PaSSB (Table 3)
312 Structural Modeling of SSBs Given its disordered C-ter-minal domain the crystal structure of the full-length SSB islacking even when SSB can be crystallized with DNA [69]We attempted to model the structure by homology mod-eling using the bioinformatics program (PS)2 to obtain an
BioMed Research International 11
Table 2 ssDNA binding properties of KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc as analyzed by EMSA
Protein DNA [Protein]50 (nM) Complex number
KpSSB
dT20 ND 0dT25 200 plusmn 20 1dT35 220 plusmn 30 1dT45 100 plusmn 10 1dT50 110 plusmn 20 1dT55 100 plusmn 20 2dT60 100 plusmn 10 2
StSSB
dT15 650 plusmn 120 1dT20 450 plusmn 80 1dT30 420 plusmn 60 1dT40 420 plusmn 80 1dT45 440 plusmn 60 2dT50 440 plusmn 50 2
PaSSB
dT20 ND 0dT25 1700 plusmn 250 1dT35 950 plusmn 180 1dT45 780 plusmn 160 1dT55 820 plusmn 90 1dT60 810 plusmn 110 2dT65 550 plusmn 70 2
KpSSBnStSSBc
dT15 260 plusmn 60 1dT20 110 plusmn 20 1dT40 120 plusmn 20 1dT45 160 plusmn 20 2
KpSSBnPaSSBc
dT20 ND 0dT25 390 plusmn 60 1dT40 220 plusmn 30 1dT55 230 plusmn 30 1dT60 230 plusmn 30 2
[Protein]50 was calculated from the titration curves of EMSA by determiningthe concentration of the protein (120583M) needed to achieve the midpointvalue for input ssDNA binding For some oligonucleotides input ssDNAbinding was the sum of the intensities from the two separate ssDNA-proteincomplexes Errors are standard deviations determined by three independenttitration experiments
Table 3 Summary of Gly Gln and Pro number in SSB
SSB segment G Q PKpSSB1ndash91 10 5 2StSSB1ndash91 10 5 2PaSSB1ndash90 11 6 2KpSSB92ndash174 18 16 9StSSB92ndash176 17 18 11PaSSB91ndash165 5 15 11KpSSB116ndash174 11 12 9StSSB117ndash176 12 13 10PaSSB116ndash165 1 12 11
in-depth understanding of the structure-function relation-ship of the C-terminal domains of these SSBs [70 71]
40 45 50 55
Kav
00
02
04
06
08
10
KpSSBnStSSBcKpSSBnPaSSBc
Y = minus03684X + 22707
R2= 0998
log Mw
Figure 9Gel-filtration chromatographic analyses of KpSSBnStSSBcand KpSSBnPaSSBc Purified protein (2mgmL) was applied toa Superdex 200HR 1030 column (GE Healthcare Bio-SciencesPiscataway NJ USA) equilibrated with Buffer D The column wasoperated at a flow rate of 05mLmin and 05mL fractions werecollected The proteins were detected by measuring the absorbanceat 280 nm The column was calibrated with proteins of knownmolecular weight thyroglobulin (670 kDa) 120574-globulin (158 kDa)ovalbumin (44 kDa) and myoglobin (17 kDa) The 119870av values forthe standard proteins and the SSB variants were calculated from theequation119870av = (119881119890minus119881119900)(119881119888minus119881119900) where119881119900 is column void volume119881119890is elution volume and 119881
119888is geometric column volume
(PS)2 (http140113239111simps2v2docsphp) is an auto-matic homology modeling server that combines bothsequence and secondary structure information to detect thehomologous proteins with remote similarity and the target-template alignment After pasting the amino acid sequenceto the website of (PS)2 only one hit (Protein Data Bankentry 1QVC EcSSB) for the C-terminal domains of KpSSBand StSSB was suggested For the C-terminal domain ofPaSSB only one hit that is CstF-77 (ProteinData Bank entry2OOE cleavage stimulation factor CstF) but not EcSSB wassuggested as the template for modeling Figure 10 shows thatmodeled structures of these SSB C-terminal domains arehighly disordered but that of PaSSB is more ordered than thatof other domains
4 Discussion
In this study we examined the sizes of the binding site ofthe untagged SSB and the chimeric SSB from the ubiquitousopportunistic pathogens K pneumoniae S enterica serovarTyphimurium LT2 and P aeruginosa PAO1 Many clinicalstrains of the abovementioned bacteria are highly resistantto antibiotics [72ndash75] The development of clinically usefulsmall-molecule antibiotics has been a seminal event in thefield of infectious diseases [48] Nucleic acid metabolismis one of the most basic biological functions and shouldbe a prime target in antibiotic development [76ndash78] Manybacterial SSBs form conserved protein interaction ldquohubsrdquothat are essential to recruit many proteins involved in DNA
12 BioMed Research International
Figure 10 Structure modeling of SSB The structures of KpSSB1ndash115 StSSB1ndash115 and PaSSB1ndash115 (the N-terminal domain of SSB)were modeled by SWISS-MODEL The structures of KpSSB116ndash142 StSSB116ndash142 and PaSSB121ndash160 (the C-terminal domain ofSSB) were modeled by (PS)2 Other regions of SSBs could not bemodeled by these two programs The structures of the N-terminaldomain and the C-terminal domain of these SSBs were manuallylinked (KpSSB1ndash142 blue StSSB1ndash142 pink PaSSB1ndash160 green) andsuperimposed with the crystal structure of EcSSB1ndash142 (orange)(PDB entry 1QVC) for comparison For clarity only one subunitof the tetramer was shown for each SSB
replication recombination and repair SSBDNA nucleopro-tein substrates [79] Thus SSBs may be promising targets inantibiotic development [80] As a first step toward achievingthis goal we investigated why SSBs possess highly conservedN-terminal ssDNA-binding domain but exhibit varying bind-ing site sizes One significant clue is that their flexible hingesand the length at the C-terminus are different as revealed bysequence alignment (Figure 2)
The interactions of various SSBs with ssDNA have beenanalyzed using a variety of techniques such as tryptophan-fluorescence quenching [47] filter binding [81] EMSA [5282] analytical ultracentrifugation [83] electron microscopy[84] nuclease digestion [44] single-molecule fluorescencemicroscopy [48] and crystallographic analyses [11] In thisstudy we have examined the electrophoretic mobility shiftpatterns of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc bound to different lengths of ssDNA and deter-mined the corresponding binding site sizes to be 26 2129 22 and 29 nt per tetramer respectively (Figures 4ndash8) PaSSB and KpSSBnPaSSBc have the largest sizes forssDNA binding among the SSBs studied We also identifiedHis-tagged and untagged SSBs that have similar ssDNA-binding site sizes [40 42 43] EMSA is a well-establishedapproach in studies of molecular biology [52] and the use ofradioactive tracer in this assay allows detection of the actualformation of the distinct protein-DNA complex(es) [53] For
exampleDNase protection assay and footprinting assay usingradioactive tracer can determine the specific DNA sequencecomplexed by a protein In EMSA when the length of thenucleotides is sufficient for the binding of two or more SSBmolecules the electrophoretic mobility of the higher SSBoligomer complex will be lower than that of the smaller SSBoligomer complex [52 54] In addition results of the ssDNA-binding site size from EMSA and cocrystal structure of SSBwere consistent [27] Thus throughout this paper we deter-mined the ssDNA-binding site sizes of SSB from the EMSAbehavior
Many SSBs bind to ssDNA with some degree of positivecooperativity Cooperativity can result from direct protein-protein interactions between the nearest neighbors such asthe LAST motif in the T4 gene-32 protein [85] and thearginine-mediated interaction motif in Thermus SSB [8687] Cooperativity can also result from the protein-induceddistortion of adjacent DNA as demonstrated in SulfolobusSSB PriB and FOXK1a proteins [23 60 88] In the cases ofKpSSB StSSB and PaSSB (Figures 4ndash6) binding appeared tobe nearly noncooperative for several DNAs because all DNAmainly shifts into the first complex (C1) before the appearanceof the second complex (C2) when subjected to increasingprotein concentrationsThe length dependence of the [SSB]
50
values suggests that the amount of spacing is optimum forsteric considerations (Table 2)
Because bacteria have varying genomic DNA sizes theirSSBs may need to evolve to have different binding site sizesfor DNA metabolism Results from protein chimeragenesisshowed the C-terminal domain dependence of the bindingsite sizes of SSB (Figure 11) The experimental data showedthat the binding site size of KpSSBnStSSBc was similar to thatof StSSB and the size of the binding site of KpSSBnPaSSBcwas similar to that of PaSSB The reason for which the bind-ing site size of SSB changed followed by swapping of theC-terminal domain remains unclear Flexibility numberof glycine residues andor different QQQ patterns of theC-terminal domain of SSB (Figure 2 and Table 3) may beimportant factors for determining the ssDNA-binding sitesize In fact the C-terminal domain of PaSSB that isPaSSB116ndash165 has only 1 Gly residue which is significantlyless than that of KpSSB (11 Gly) and StSSB (12Gly) Gly (andPro) is an important component of the flexible region aprotein that contains low Gly content is predicted to havelow flexibility Unlike typical SSB [35 69] PaSSB116ndash165 hasa partial structure (Figure 10) Although KpSSB StSSB andPaSSB contain similar number of Gln (Q) the QQQ patternis frequently found in PaSSB (Figure 2 and Table 3) PolyQand repeated sequences GAGAG are commonly found inthe structures of amyloids silk fibers and neurodegradationproteins [89ndash92] Considering that the simple coil polyQ theheptapeptide GNNQQNY and the hexapeptide NNQQNYcan cause protein aggregation and nucleation [93ndash95] thedistribution of Gln in the C-terminal domain of a tetramericSSB may also be an important determinant of the ssDNA-binding site size of SSB by some steric hindrances (Figure 11)However the above speculationmust be confirmed by furtherbiochemical experiments
BioMed Research International 13
PaSSBStSSB KpSSB
C-terC-ter C-ter
sim 21 nt sim 26 nt sim 29 nt
Figure 11 Possiblemodels for explainingwhy SSBs arewith different binding site sizes Twomodeled structures of KpSSB1ndash142 (blue) StSSB1ndash142 (pink) and PaSSB1ndash160 (green) complexed with ssDNA (gold) are shown For clarity only one C-terminal domain was shown for eachSSB tetramer By using the electrophoretic mobility shift assay and the protein chimeragenesis we characterized that the binding site sizes ofKpSSB StSSB PaSSB KpSSBnStSSBc and KpSSBnPaSSBc were 26 21 29 21 and 29 nt per tetramer respectively KpSSB StSSB and PaSSBare similar proteins whose N-terminal ssDNA-binding domains are almost identical Thus the C-terminal domain of SSB may indirectlycontribute to ssDNA binding and wrapping and affects the binding site size by the steric hindrance
5 Conclusion
In this study we characterized the ssDNA-binding propertiesof untagged SSBs from K pneumoniae S enterica sero-var Typhimurium LT2 and P aeruginosa PAO1 and proposeda role of the C-terminal flexible domain for ssDNA bind-ing from the protein chimeragenesis and EMSA resultsThe amino acid sequence of the N-terminal ssDNA-bind-ingoligomerization domain in these pathogenic SSBs ishighly conserved but their apparent binding site sizes aredifferent This finding indicates that the C-terminal protein-protein interaction domain may also indirectly contribute tossDNA binding and wrapping
Conflict of Interests
The authors declare that there is no conflict of interestsregarding the publication of this paper
Acknowledgment
This researchwas supported by aGrant from theNational Sci-ence Council Taiwan (NSC 102-2320-B-040-019 to Cheng-Yang Huang)
References
[1] R Reyes-Lamothe D J Sherratt and M C Leake ldquoStoichiom-etry and architecture of active DNA replication machinery inescherichia colirdquo Science vol 328 no 5977 pp 498ndash501 2010
[2] D J Richard E Bolderson andK K Khanna ldquoMultiple humansingle-stranded DNA binding proteins function in genomemaintenance structural biochemical and functional analysisrdquo
Critical Reviews in Biochemistry and Molecular Biology vol 44no 2-3 pp 98ndash116 2009
[3] R D Shereda A G Kozlov T M LohmanMM Cox and J LKeck ldquoSSB as an organizermobilizer of genome maintenancecomplexesrdquo Critical Reviews in Biochemistry and MolecularBiology vol 43 no 5 pp 289ndash318 2008
[4] D J Richard E Bolderson L Cubeddu et al ldquoSingle-strandedDNA-binding protein hSSB1 is critical for genomic stabilityrdquoNature vol 453 no 7195 pp 677ndash681 2008
[5] R R Meyer and P S Laine ldquoThe single-stranded DNA-bindingprotein of Escherichia colirdquoMicrobiological Reviews vol 54 no4 pp 342ndash380 1990
[6] C Yang U Curth C Urbanke and C Kang ldquoCrystal structureof human mitochondrial single-stranded DNA binding proteinat 24 A resolutionrdquo Nature Structural Biology vol 4 no 2 pp153ndash157 1997
[7] G Webster J Genschel U Curth C Urbanke C Kang and RHilgenfeld ldquoA common core for binding single-stranded DNAstructural comparison of the single-stranded DNA-bindingproteins (SSB) from E coli and human mitochondriardquo FEBSLetters vol 411 pp 313ndash316 1997
[8] R L Flynn and L Zou ldquoOligonucleotideoligosaccharide-bind-ing fold proteins a growing family of genome guardiansrdquo Crit-ical Reviews in Biochemistry and Molecular Biology vol 45 no4 pp 266ndash275 2010
[9] K Chan Y Lee CWangHHuang andY Sun ldquoSingle-Strand-ed DNA-Binding Protein Complex from Helicobacter pyloriSuggests an ssDNA-Binding Surfacerdquo Journal of MolecularBiology vol 388 no 3 pp 508ndash519 2009
[10] D L Theobald R M Mitton-Fry and D S Wuttke ldquoNucleicacid recognition by OB-fold proteinsrdquo Annual Review of Bio-physics and Biomolecular Structure vol 32 pp 115ndash133 2003
[11] S Raghunathan A G Kozlov TM Lohman andGWaksmanldquoStructure of the DNA binding domain of E coli SSB bound to
14 BioMed Research International
ssDNArdquo Nature Structural Biology vol 7 no 8 pp 648ndash6522000
[12] A G Murzin ldquoOB(oligonucleotideoligosaccharide binding)-fold common structural and functional solution for non-homologous sequencesrdquo EMBO Journal vol 12 no 3 pp 861ndash867 1993
[13] N P George K V Ngo S Chitteni-Pattu et al ldquoStructure andcellular dynamics of deinococcus radiodurans single-strandedDNA (ssDNA)-binding protein (SSB)-DNA complexesrdquo TheJournal of Biological Chemistry vol 287 no 26 pp 22123ndash221322012
[14] P Filipkowski and J Kur ldquoIdentification and propertiesof the Deinococcus grandis and Deinococcus proteolyticussingle-stranded DNA binding proteins (SSB)rdquo Acta BiochimicaPolonica vol 54 no 1 pp 79ndash87 2007
[15] P Filipkowski A Duraj-Thatte and J Kur ldquoIdentificationcloning expression and characterization of a highly ther-mostable single-stranded-DNA-binding protein (SSB) fromDeinococcus murrayirdquo Protein Expression and Purification vol53 no 1 pp 201ndash208 2007
[16] P Filipkowski M Koziatek and J Kur ldquoA highly ther-mostable homodimeric single-stranded DNA-binding proteinfrom Deinococcus radiopugnansrdquo Extremophiles vol 10 no 6pp 607ndash614 2006
[17] P Filipkowski A Duraj-Thatte and J Kur ldquoNovel thermostablesingle-stranded DNA-binding protein (SSB) fromDeinococcusgeothermalisrdquo Archives of Microbiology vol 186 no 2 pp 129ndash137 2006
[18] G Witte C Urbanke and U Curth ldquoSingle-stranded DNA-binding protein of Deinococcus radiodurans a biophysicalcharacterizationrdquoNucleic Acids Research vol 33 no 5 pp 1662ndash1670 2005
[19] D A Bernstein J M Eggingtont M P Killoran A M MisicMMCox and J L Keck ldquoCrystal structure of theDeinococcusradiodurans single-stranded DNA-binding protein suggests amechanism for coping with DNA damagerdquo Proceedings of theNational Academy of Sciences of the United States of Americavol 101 no 23 pp 8575ndash8580 2004
[20] S Dabrowski M Olszewski R Piatek A Brillowska-Dabrowska G Konopa and J Kur ldquoIdentification and charac-terization of single-stranded-DNA-binding proteins fromTher-mus thermophilus and Thermus aquaticusmdashnew arrange-ment of binding domainsrdquo Microbiology vol 148 no 10 pp3307ndash3315 2002
[21] R Gamsjaeger R Kariawasam C Touma A H Kwan M FWhite and L Cubeddu ldquoBackbone and side-chain 1H 13C and15N resonance assignments of the OB domain of the singlestranded DNA binding protein from Sulfolobus solfataricusand chemical shift mapping of the DNA-binding interfacerdquoBiomolecular NMR Assignments 2013
[22] M L Rolfsmeier and C A Haseltine ldquoThe single-strandedDNA binding protein of Sulfolobus solfataricus acts in the pre-synaptic step of homologous recombinationrdquo Journal of Molec-ular Biology vol 397 no 1 pp 31ndash45 2010
[23] I D Kerr R I M Wadsworth L Cubeddu W Blankenfeldt JHNaismith andM FWhite ldquoInsights into ssDNA recognitionby the OB fold from a structural and thermodynamic study ofSulfolobus SSB proteinrdquo EMBO Journal vol 22 no 11 pp 2561ndash2570 2003
[24] C A Haseltine and S C Kowalczykowski ldquoA distinctive single-stranded DNA-binding protein from the Archaeon Sulfolobussolfataricusrdquo Molecular Microbiology vol 43 no 6 pp 1505ndash1515 2002
[25] R I M Wadsworth and M F White ldquoIdentification andproperties of the crenarchaeal single-stranded DNA bindingprotein from Sulfolobus solfataricusrdquo Nucleic Acids Researchvol 29 no 4 pp 914ndash920 2001
[26] S Sugiman-Marangos and M S Junop ldquoThe structure of DdrBfrom Deinococcus a new fold for single-stranded DNA bindingproteinsrdquo Nucleic Acids Research vol 38 no 10 Article IDgkq036 pp 3432ndash3440 2010
[27] H Ghalei H V Moeller D Eppers et al ldquoEntrapment of DNAin an intersubunit tunnel system of a single-stranded DNA-binding proteinrdquo Nucleic Acids Research vol 42 no 10 pp6698ndash6708 2014
[28] S Paytubi S A McMahon S Graham et al ldquoDisplacement ofthe canonical single-strandedDNA-binding protein in the ther-moprotealesrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 109 no 7 pp E398ndashE405 2012
[29] T H Dickey S E Altschuler and D S Wuttke ldquoSingle-stranded DNA-binding proteins multiple domains for multiplefunctionsrdquo Structure vol 21 no 7 pp 1074ndash1084 2013
[30] D Vujaklija and BMacek ldquoDetecting posttranslational modifi-cations of bacterial SSB proteinsrdquoMethods inMolecular Biologyvol 922 pp 205ndash218 2012
[31] I Mijakovic D Petranovic B Macek et al ldquoBacterial single-stranded DNA-binding proteins are phosphorylated on tyro-sinerdquoNucleic Acids Research vol 34 no 5 pp 1588ndash1596 2006
[32] M Olszewski A Grot M Wojciechowski M Nowak MMickiewicz and J Kur ldquoCharacterization of exceptionally ther-mostable single-strandedDNA-binding proteins fromThermo-toga maritima and Thermotoga neapolitanardquo BMC Microbiol-ogy vol 10 article 260 2010
[33] U Curth J Genschel C Urbanke and J Greipel ldquoIn vitro andin vivo function of the C-terminus of Escherichia coil single-stranded DNA binding proteinrdquoNucleic Acids Research vol 24no 14 pp 2706ndash2711 1996
[34] G Witte C Urbanke and U Curth ldquoDNA polymerase IIIchi subunit ties single-stranded DNA binding protein to thebacterial replication machineryrdquoNucleic Acids Research vol 31pp 4434ndash4440 2003
[35] D Shishmarev YWang C EMason et al ldquoOtting Intramolec-ular binding mode of the C-terminus of Escherichia coli single-strandedDNAbinding protein determined by nuclearmagneticresonance spectroscopyrdquo Nucleic Acids Research vol 42 pp2750ndash2757 2014
[36] A G Kozlov M M Cox and T M Lohman ldquoRegulation ofsingle-stranded DNA binding by the C termini of Escherichiacoli single-stranded DNA-binding (SSB) proteinrdquo Journal ofBiological Chemistry vol 285 no 22 pp 17246ndash17252 2010
[37] M Nowak M Olszewski M Spibida and J Kur ldquoChar-acterization of single-stranded DNA-binding proteins fromthe psychrophilic bacteria Desulfotalea psychrophila Flavobac-terium psychrophilum Psychrobacter arcticus Psychrobactercryohalolentis Psychromonas ingrahamii Psychroflexus torquisand Photobacterium profundumrdquo BMC Microbiology vol 14article 91 2014
BioMed Research International 15
[38] T Paradzik N Ivic Z Filic et al ldquoStructure-function relation-ships of two paralogous single-stranded DNA-binding proteinsfrom Streptomyces coelicolor implication of SsbB in chromo-some segregation during sporulationrdquo Nucleic Acids Researchvol 41 no 6 pp 3659ndash3672 2013
[39] S JainM Zweig E Peeters et al ldquoCharacterization of the singlestrandedDNA binding protein SsbB encoded in the gonoccocalgenetic islandrdquo PLoS ONE vol 7 no 4 Article ID e35285 2012
[40] Y H Huang and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from Klebsiella pneumoniaemutation at either Arg73 or Ser76 causes a less cooperativecomplex onDNArdquoGenes to Cells vol 17 no 2 pp 146ndash157 2012
[41] E Antony E A Weiland S Korolev and T M LohmanldquoPlasmodium falciparum SSB tetramer wraps single-strandedDNAwith similar topology but opposite polarity to E Coli SSBrdquoJournal ofMolecular Biology vol 420 no 4-5 pp 269ndash283 2012
[42] H Jan Y L Lee and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from pseudomonas aeruginosaPAO1rdquo Protein Journal vol 30 no 1 pp 20ndash26 2011
[43] Y-H Huang Y-L Lee and C-Y Huang ldquoCharacterization of asingle-stranded DNA binding protein from Salmonella entericaserovar typhimurium LT2rdquo Protein Journal vol 30 no 2 pp102ndash108 2011
[44] S K Bharti K Rex P Sreedhar N Krishnan and U VarshneyldquoChimeras of Escherichia coli and Mycobacterium tuberculosissingle-stranded DNA binding proteins characterization andfunction in Escherichia colirdquo PLoS ONE vol 6 no 12 ArticleID e27216 2011
[45] M T Oliveira and L S Kaguni ldquoFunctional roles of the N-and C-terminal regions of the human mitochondrial single-stranded DNA-binding proteinrdquo PLoS ONE vol 5 no 10Article ID e15379 2010
[46] A G Kozlov J M Eggington M M Cox and T MLohman ldquoBinding of the dimeric Deinococcus radioduranssingle-strandedDNA binding protein to single-strandedDNArdquoBiochemistry vol 49 no 38 pp 8266ndash8275 2010
[47] T M Lohman and M E Ferrari ldquoEscherichia coli single-stranded DNA-binding protein multiple DNA-binding modesand cooperativesrdquo Annual Review of Biochemistry vol 63 pp527ndash570 1994
[48] R Zhou A G Kozlov R Roy et al ldquoSSB functions as a slidingplatform that migrates on DNA via reptationrdquo Cell vol 146 pp222ndash232 2011
[49] R Roy A G Kozlov T M Lohman and T Ha ldquoSSB proteindiffusion on single-stranded DNA stimulates RecA filamentformationrdquo Nature vol 461 pp 1092ndash1097 2009
[50] R Roy A G Kozlov T M Lohman and T Ha ldquoDynamicstructural rearrangements between DNA binding modes of Ecoli SSB proteinrdquo Journal of Molecular Biology vol 369 no 5pp 1244ndash1257 2007
[51] T J Kelly P Simancek and G S Brush ldquoIdentification andcharacterization of a single-stranded DNA-binding proteinfrom the archaeon Methanococcus jannaschiirdquo Proceedings ofthe National Academy of Sciences of the United States of Americavol 95 no 25 pp 14634ndash14639 1998
[52] C Y Huang Determination of the Binding Site-Size of theProtein-DNA Complex by Use of the Electrophoretic MobilityShift Assay InTech Press Rijeka Croatia 2012
[53] A Kornberg ldquoTen commandments of enzymology amendedrdquoTrends in Biochemical Sciences vol 28 no 10 pp 515ndash517 2003
[54] W Zhang X Lu and J Shen ldquoEMSA and single-moleculeforce spectroscopy study of interactions between bacillus sub-tilis single-stranded DNA-binding protein and single-strandedDNArdquo Langmuir vol 27 no 24 pp 15008ndash15015 2011
[55] M Ostermeier and S J Benkovic ldquoEvolution of protein func-tion by domain swappingrdquo Advances in Protein Chemistry vol55 pp 29ndash77 2000
[56] W F Peng and C Y Huang ldquoAllantoinase and dihydroorotasebinding and inhibition by flavonols and the substrates of cyclicamidohydrolasesrdquo Biochimie vol 101 pp 113ndash122 2014
[57] Y H Huang M J Lin and C Y Huang ldquoDnaT is a single-stranded DNA binding proteinrdquo Genes to Cells vol 18 no 11pp 1007ndash1019 2013
[58] Y Y Ho Y H Huang and C Y Huang ldquoChemical rescue of thepost-translationally carboxylated lysine mutant of allantoinaseand dihydroorotase by metal ions and short-chain carboxylicacidsrdquo Amino Acids vol 44 no 4 pp 1181ndash1191 2013
[59] Y H Huang Y Lo W Huang and C Y Huang ldquoCrystalstructure and DNA-binding mode of Klebsiella pneumoniaeprimosomal PriB proteinrdquoGenes to Cells vol 17 no 10 pp 837ndash849 2012
[60] C Y Huang C Y Hsu Y Sun H Wu and C Hsiao ldquoCom-plexed crystal structure of replication restart primosome pro-tein PriB reveals a novel single-stranded DNA-binding moderdquoNucleic Acids Research vol 34 no 14 pp 3878ndash3886 2006
[61] T Kinebuchi H Shindo H Nagai N Shimamoto andM Shimizu ldquoFunctional domains of Escherichia coli single-stranded DNA binding protein as assessed by analyses of thedeletion mutantsrdquo Biochemistry vol 36 no 22 pp 6732ndash67381997
[62] N Shimamoto N Ikushima H Utiyama H Tachibana andK Horie ldquoSpecific and cooperative binding of E coli single-stranded DNA binding protein to mRNArdquo Nucleic AcidsResearch vol 15 no 13 pp 5241ndash5250 1987
[63] H Lin andC YHuang ldquoCharacterization of flavonol inhibitionof DnaB helicase Real-time monitoring structural modelingand proposed mechanismrdquo Journal of Biomedicine and Biotech-nology vol 2012 Article ID 735368 2012
[64] Y H Huang H H Lin and C Y Huang ldquoA single residuedetermines the cooperative binding property of a primosomalDNA replication protein PriB to single-stranded DNArdquo Bio-science Biotechnology and Biochemistry vol 76 no 6 pp 1110ndash1115 2012
[65] H C Hsieh and C Y Huang ldquoIdentification of a novel proteinPriB in Klebsiella pneumoniaerdquo Biochemical and BiophysicalResearch Communications vol 404 no 1 pp 546ndash551 2011
[66] J H Liu T W Chang C Y Huang et al ldquoCrystal structure ofPriB a primosomalDNA replication protein ofEscherichia colirdquoThe Journal of Biological Chemistry vol 279 no 48 pp 50465ndash50471 2004
[67] Y H Huang and C Y Huang ldquoThe N-terminal domain ofDnaT a primosomalDNA replication protein is crucial for PriBbinding and self-trimerizationrdquo Biochemical and BiophysicalResearch Communications vol 442 pp 147ndash152 2013
[68] M A Larkin G Blackshields N P Brown et al ldquoClustalW andClustal X version 20rdquo Bioinformatics vol 23 no 21 pp 2947ndash2948 2007
16 BioMed Research International
[69] S N Savvides S Raghunathan K Futterer A G Kozlov T MLohman and G Waksman ldquoThe C-terminal domain of full-length E coli SSB is disordered even when bound to DNArdquoProtein Science vol 13 no 7 pp 1942ndash1947 2004
[70] C-C Chen J-K Hwang and J-M Yang ldquo(PS)2-v2 template-based protein structure prediction serverrdquo BMC Bioinformaticsvol 10 article 366 2009
[71] C Chen J Hwang and J Yang ldquo(PS)2 protein structure pre-diction serverrdquoNucleic Acids Research vol 34 pp W152ndashW1572006
[72] K Bush and J F Fisher ldquoEpidemiological expansion structuralstudies and clinical challenges of new 120573-lactamases from gram-negative bacteriardquo Annual Review of Microbiology vol 65 pp455ndash478 2011
[73] K K Kumarasamy M A Toleman T R Walsh et al ldquoEmer-gence of a new antibiotic resistance mechanism in India Pak-istan and the UK a molecular biological and epidemiologicalstudyrdquoThe Lancet Infectious Diseases vol 10 no 9 pp 597ndash6022010
[74] K Bush and M J MacIelag ldquoNew 120573-lactam antibiotics and 120573-lactamase inhibitorsrdquo Expert Opinion on Therapeutic Patentsvol 20 no 10 pp 1277ndash1293 2010
[75] K Bush ldquoAlarming 120573-lactamase-mediated resistance in multi-drug-resistant Enterobacteriaceaerdquo Current Opinion in Microbi-ology vol 13 no 5 pp 558ndash564 2010
[76] A Srivastava M Talaue S Liu et al ldquoNew target for inhibitionof bacterial RNA polymerase ldquoswitch regionrdquordquo Current Opinionin Microbiology vol 14 no 5 pp 532ndash543 2011
[77] K Chono K Katsumata T Kontani et al ldquoASP2151 a novelhelicase-primase inhibitor possesses antiviral activity againstvaricella-zoster virus and herpes simplex virus types 1 and 2rdquoJournal of Antimicrobial Chemotherapy vol 65 no 8 pp 1733ndash1741 2010
[78] M T Black and K Coleman ldquoNew inhibitors of bacterial topoi-somerase GyrAParC subunitsrdquo Current Opinion in Investiga-tional Drugs vol 10 no 8 pp 804ndash810 2009
[79] A H Marceau D A Bernstein B W Walsh W Shapiro LA Simmons and J L Keck ldquoProtein interactions in genomemaintenance as novel antibacterial targetsrdquo PLoS ONE vol 8no 3 Article ID e58765 2013
[80] D Lu D A Bernstein K A Satyshur and J L Keck ldquoSmall-molecule tools for dissecting the roles of SSBprotein inter-actions in genome maintenancerdquo Proceedings of the NationalAcademy of Sciences of the United States of America vol 107 no2 pp 633ndash638 2010
[81] I Wong and T M Lohman ldquoA double-filter method fornitrocellulose-filter binding application to protein-nucleic acidinteractionsrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 90 no 12 pp 5428ndash5432 1993
[82] M Mitas J Y Chock and M Christy ldquoThe binding-site sizesof Escherichia coli single-stranded-DNA-binding protein andmammalian replication protein A are 65 and ge 54 nucleotidesrespectivelyrdquo Biochemical Journal vol 324 no 3 pp 957ndash9611997
[83] C Urbanke G Witte and U Curth ldquoSedimentation velocitymethod in the analytical ultracentrifuge for the study of protein-protein interactionsrdquoMethods inMolecular Biology vol 305 pp101ndash114 2005
[84] S Chrysogelos and J Griffith ldquoEscherichia coli single-strandbinding protein organizes single-strandedDNA innucleosome-like unitsrdquo Proceedings of the National Academy of Sciences of theUnited States of America vol 79 no 19 pp 5803ndash5807 1982
[85] J R Casas-Finet K R Fischer and R L Karpel ldquoStructuralbasis for the nucleic acid binding cooperativity of bacteriophageT4 gene 32 protein the (LysArg)3(SerThr)2 (LAST) motifrdquoProceedings of the National Academy of Sciences of the UnitedStates of America vol 89 pp 1050ndash1054 1992
[86] G Witte R Fedorov and U Curth ldquoBiophysical analysis ofThermus aquaticus single-stranded DNA binding proteinrdquo Bio-physical Journal vol 94 no 6 pp 2269ndash2279 2008
[87] R Fedorov G Witte C Urbanke D J Manstein and UCurth ldquo3D structure of Thermus aquaticus single-strandedDNA-binding protein gives insight into the functioning of SSBproteinsrdquo Nucleic Acids Research vol 34 no 22 pp 6708ndash67172006
[88] K L Tsai C Y Huang C H Chang Y J Sun W J ChuangandC DHsiao ldquoCrystal structure of the human FOXK1a-DNAcomplex and its implications on the diverse binding specificityof winged helixforkhead proteinsrdquo Journal of Biological Chem-istry vol 281 no 25 pp 17400ndash17409 2006
[89] A H Mao N Lyle and R V Pappu ldquoDescribing sequence-ensemble relationships for intrinsically disordered proteinsrdquoBiochemical Journal vol 449 no 2 pp 307ndash318 2013
[90] R Wetzel ldquoPhysical chemistry of polyglutamine Intriguingtales of a monotonous sequencerdquo Journal of Molecular Biologyvol 421 no 4-5 pp 466ndash490 2012
[91] H T Orr ldquoPolyglutamine neurodegeneration expanded glu-tamines enhance native functionsrdquo Current Opinion in Geneticsand Development vol 22 no 3 pp 251ndash255 2012
[92] M Figiel W J Szlachcic P M Switonski A Gabka and W JKrzyzosiak ldquoMouse models of polyglutamine diseases reviewand data table Part Irdquo Molecular Neurobiology vol 46 no 2pp 393ndash429 2012
[93] J Nasica-Labouze and N Mousseau ldquoKinetics of amyloidaggregation a study of the GNNQQNY prion sequencerdquo PLoSComputational Biology vol 8 no 11 Article ID e1002782 2012
[94] J Nasica-Labouze M Meli P Derreumaux G Colombo andN Mousseau ldquoA multiscale approach to characterize the earlyaggregation steps of the amyloid-forming peptide gnnqqnyfrom the yeast prion sup-35rdquo PLoS Computational Biology vol7 no 5 Article ID e1002051 2011
[95] J R Lewandowski P C A van der Wel M Rigney N Grig-orieff and R G Griffin ldquoStructural complexity of a compositeamyloid fibrilrdquo Journal of the American Chemical Society vol133 no 37 pp 14686ndash14698 2011
4 BioMed Research International
Table 1 Primers used for construction of plasmids
Oligonucleotide Primer1 KpSSB-NdeI-N GGGCATATGGCCAGCAGAGGCGTAAAC2 KpSSB-XhoI-C GGGCTCGAGTTAGAACGGGATGTCGTC3 StSSB-NdeI-N CTGAACATATGGCCAGCAGAGGCGTAA4 StSSB-XhoI-C TGGAACTCGAGTTAGAACGGAATGTCG5 PaSSB-NdeI-N TTGCTCATATGGCCCGTGGGGTTAACA6 PaSSB-XhoI-C TTGCACTCGAGTTAGAACGGAATGTCG7 KpSSB(D91EQ92L-SacI)-N AAGTGGACCGAGCTCTCCGGTCAGGACA8 KpSSB(D91EQ92L-SacI)-C GTCCTGACCGGAGAGCTCGGTCCACTT9 StSSB(D91EQ92L-SacI)-N AAGTGGACCGAGCTCAGTGGCCAGGAA10 StSSB(D91EQ92L-SacI)-C TTCCTGGCCACTGAGCTCGGTCCACTT11 PaSSB(G90EQ91L-SacI)-N AAGTGGCAGGAGCTCGACGGTCAGGAT12 PaSSB(G90EQ91L-SacI)-C ATCCTGACCGTCGAGCTCCTGCCACTT13 KpSSBnStSSBc(E91DL92Q)-N AAGTGGACCGATCAGAGTGGCCAGGAA14 KpSSBnStSSBc(E91DL92Q)-C TTCCTGGCCACTCTGATCGGTCCACTT15 KpSSBnPaSSBc(E91DL92Q)-N AAGTGGACCGATCAGGACGGTCAGGAT16 KpSSBnPaSSBc(E91DL92Q)-C ATCCTGACCGTCCTGATCGGTCCACTTA fragment containing the coding sequence of KpSSB StSSB and PaSSB (with the stop codon) was cloned into the pET21b vector (using Primers 1ndash6) Duringthe process NdeI and XhoI restriction sites were introduced at the 51015840-end and the 31015840-end of these genes after which they were ligated into the pET21b vectorTo obtain the vectors for expression of the chimeric proteins KpSSBnStSSBc and KpSSBnPaSSBc pET21b-KpSSB (Primers 7 and 8) pET21b-StSSB (Primers9 and 10) and pET21b-PaSSB (Primers 11 and 12) vectors were mutated to create a desired SacI site The D91EQ92L-engineered pET21b-KpSSB vectorthe D91EQ92L-engineered pET21b-StSSB vector and the G90EQ91L-engineered pET21b-PaSSB vector were cut at NdeI and SacI sites Subsequently theKpSSBn StSSBc-pET21b and PaSSBc-pET21b fragments were purified KpSSBn was ligated with StSSBc-pET21b and PaSSBc-pET21b fragments to generatethe engineered pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc vectors To avoid artificial residues positions 91 and 92 of the two plasmids were mutatedback (Primers 13 to 16) to obtain pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc vectors Thus pET21b-KpSSBnStSSBc and pET21b-KpSSBnPaSSBc willexpress KpSSB1-91 fused StSSB92-176 and PaSSB91-165 respectively These plasmids were verified by DNA sequencing Underlined nucleotides indicate thedesignated site for mutation or the restriction site
26 Gel-Filtration Chromatography Gel-filtration chroma-tography was carried out by the AKTA-FPLC system (GEHealthcare Bio-Sciences Piscataway NJ USA) Briefly puri-fied protein (2mgmL) was applied to a Superdex 200HR1030 column (GE Healthcare Bio-Sciences Piscataway NJUSA) equilibrated with Buffer D The column was operatedat a flow rate of 05mLmin and 05mL fractions were col-lected The proteins were detected by measuring the absorb-ance at 280 nm The column was calibrated with proteinsof known molecular weight thyroglobulin (670 kDa) 120574-globulin (158 kDa) ovalbumin (44 kDa) and myoglobin(17 kDa) The 119870av values for the standard proteins and theSSB variants were calculated from the equation 119870av = (119881119890 minus119881119900)(119881119888minus 119881119900) where 119881
119900is column void volume 119881
119890is elution
volume and 119881119888is geometric column volume
27 Electrophoretic Mobility Shift Assay (EMSA) EMSA [52]for these SSBs was carried out by the protocol describedpreviously for DnaB [63] PriB [59 64ndash66] DnaT [57 67]and SSB proteins [40 42 43 52] Briefly radiolabeling ofvarious lengths of ssDNA oligonucleotides was carried outwith [12057432P]ATP (6000Cimmol PerkinElmer Life SciencesWaltham MA) and T4 polynucleotide kinase (PromegaMadison WI USA) The protein (0 19 37 77 155 310 6301250 2500 and 5000 nM) was incubated for 30min at 25∘Cwith 17 nM DNA substrates (dT15ndash65) in a total volume of10 120583L in 20mMTris-HCl pH 80 and 100mMNaCl Aliquots(5 120583L) were removed from each of the reaction solutions and
added to 2 120583L of gel-loading solution (025 bromophenolblue and 40 sucrose) The resulting samples were resolvedon a native 8 polyacrylamide gel at 4∘C in TBE buffer(89mMTris borate and 1mMEDTA) for 1 h at 100V andwerevisualized by autoradiography Complexed and free DNAbands were scanned and quantified
28 DNA-Binding Ability The ssDNA-binding ability([Protein]
50 119870119889app) for the protein was estimated from the
protein concentration that binds 50 of the input ssDNA[52] Each [Protein]
50is calculated as the average of three
measurements plusmn SD
29 Bioinformatics Sequence alignment of KpSSB StSSBandPaSSBwas generated byCLUSTALW2 [68]The structureof the C-terminal domain of these SSBs was modeled by(PS)2 (http140113239111simps2v2docsphp) The struc-tures were visualized by using the program PyMol
3 Results
31 Sequence Analysis Based on the nucleotide sequencefound using a database search through the National Centerfor Biotechnology Information (NCBI) we predicted thatKpSSB StSSB and PaSSB monomer proteins have lengths of174 176 and 165 amino acid residues respectively The sizeof the ssDNA-binding site of His-tagged KpSSB [40] StSSB
BioMed Research International 5
M A S R G V N K V I L V G N L G Q D P E V R Y M P S G G A V A N F T L A T S E S W R D K Q T G
M A S R G V N K V I L V G N L G Q D P E V R Y M P S G G A V A N L T L A T S E S W R D K Q T G
M A - R G V N K V I L V G N V G G D P E T R Y M P N G N A V T N I T L A T S E S W K D K Q T G
K L A E V A G E Y L R K G S Q V Y I E G Q L R T R K W T D Q S G Q D K Y T T E V - V V N V G G
K L A E V A G E Y L R K G S Q V Y I E G Q L R T R K W T D Q S G Q E R Y T T E I N V P Q I G G
R L A E I A G E Y L R K G S Q V Y V E G S L R T R K W Q G Q D G Q D R Y T T E I - V V D I N G
G G G Q Q Q G G W G Q P Q Q P Q - - - G G N Q F S G G A Q S R P Q Q Q A P A A P S N E P P M D
G - G Q Q Q G G W G Q P Q Q P Q Q P Q G G N Q F S G G A Q S R P Q Q S A P - A P S N E P P M D
G - D D S Q R A P R E P M Q R P - - - - - - Q Q A P Q Q Q S R P A P Q Q Q P A P Q P A Q D Y D
E M K E Q T E W H R V V L F G
E M K E Q T E W H R V V M F G
Q Q Q E R T E W H R V V F F G
T M Q M L G G R Q G G G A P A
V M Q M L G G R Q G G G A P A
N M Q L L G G R - - - - - P S
- F D D D I P F
- F D D D I P F
S F D D D I P F
62
62
61
123
124
120
174176
165
KpSSB
StSSB
PaSSB
KpSSB
StSSB
PaSSB
KpSSB
StSSB
PaSSB
Figure 2 Multiple amino acid sequence alignment of SSB proteins Sequence alignment of KpSSB StSSB and PaSSB was generated byCLUSTALW2 Identical amino acid residues are colored in red Gly and Gln residues are shaded in cyan and gray The N-terminal domainsof these SSBs are significantly conserved
(kDa)
40-
35-
25-
15-
M 1 2 3 4 5
Figure 3 Protein purity Coomassie Blue-stained SDS-PAGE (15)of the purified KpSSB (lane 1) StSSB (lane 2) PaSSB (lane 3)KpSSBnStSSBc (lane 4) KpSSBnPaSSBc (lane 5) and molecularmass standards (M) are shown The sizes of the standard proteinsfrom the top down are as follows 55 40 35 25 15 and 10 kDa Thepurified SSBs migrated between the 25 and 15 kDa standards on theSDS-PAGE
[43] and PaSSB [42] was determined to be 26plusmn 1 22plusmn 1 and29plusmn1 nt respectivelyThe longer the length of the polypeptidechain the smaller the size for ssDNA binding Analysis ofthe primary structures of KpSSB StSSB and PaSSB by RPS-BLAST revealed the presence of a putative OB-fold domainthat is common to all known SSBs Figure 2 shows that thealignments of the amino acid sequences of KpSSB StSSB andPaSSB amino acid residues in their N-terminal domains arehighly conserved (colored in red) In the E coli SSB-ssDNAcomplex [11] four essential aromatic residues namely Trp40Trp54 Phe60 and Trp88 participate in ssDNA binding viastacking interactions [11] These residues are conserved inmost SSB families including KpSSB StSSB and PaSSB Theimportantmotif in the C-terminal tail of E coli SSB DDDIPFresidues is also conserved in KpSSB StSSB and PaSSB By
contrast to those motifs the residues found in the glycine-rich hinge of E coli SSB are not conserved in KpSSB StSSBand PaSSB (Figure 2) Thus the length and composition ofthe amino acid residues in the glycine-rich hinge may beresponsible for the different ssDNA-binding site sizes of SSBs
32 Expression and Purification of KpSSB StSSB and PaSSBThe N-terminal ssDNA-binding domain of SSB has beenwell-established to be highly conserved However SSBspossessing different ssDNA-binding site sizes have beenreported The reason that SSBs have similar ssDNA-bindingdomains but possess varying ssDNA-binding site sizesremains unclear Although the ssDNA-binding site sizes ofKpSSB StSSB and PaSSB have been reported we reinves-tigated the ssDNA-binding properties of KpSSB StSSB andPaSSB in the absence of a His tag to avoid the unknown effectof a His tag (hexahistidine) on the ssDNA binding of SSB
33 KpSSB Bound to ssDNA To investigate the length ofnucleotides sufficient for the formation of the KpSSB-ssDNAcomplex and the ssDNA-binding ability of KpSSB westudied the binding of KpSSB to dT20 (Figure 4(a)) dT25(Figure 4(b)) dT35 (Figure 4(c)) dT45 (Figure 4(d)) dT50(Figure 4(e)) dT55 (Figure 4(f)) and dT60 (Figure 4(g))with different protein concentrations As shown inFigure 4(a) no band shift was observed when KpSSBwas incubated with dT20 indicating that KpSSB couldnot form a stable complex with this homopolymer Bycontrast to dT20 longer dT homopolymers which includedT25ndash50 produced a significant band shift (C complex)that is formation of a stable protein-DNA complex insolution Furthermore two different complexes for dT55were formed by KpSSB (Figure 4(f)) At lower proteinconcentrations KpSSB formed a single complex (C1) withdT55 similar to that observed with dT50 (Figure 4(e))However when the KpSSB concentration was increasedanother slower migrating complex (C2) was observed
6 BioMed Research International
[KpSSB]
-dT20
mdash
(a)
-dT25
-C
mdash[KpSSB]
(b)
-dT35
-C
mdash[KpSSB]
(c)
-dT45
-C
mdash[KpSSB]
(d)
-dT50
-C
[KpSSB]
mdash
(e)
-dT55
-C1-C2
[KpSSB]mdash
(f)
-dT60
-C1-C2
[KpSSB]mdash
(g)
Figure 4 Binding of KpSSB to dT20ndash60 KpSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT20 (b) dT25 (c) dT35 (d) dT45 (e) dT50 (f) dT55 or (g) dT60 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
Two different complexes of KpSSB were also observedto bind to dT60 (Figure 4(g)) The appearance of thesecond complex resulted from the increased KpSSB con-centration suggesting that two KpSSB proteins maybe present per oligonucleotide Although dT55 is only 5 ntlonger thandT50 is the presence of an extra 5 nt in dT55 com-pared with that of dT50 provides enough interaction spacefor the binding of two KpSSB proteins Therefore one KpSSB
occupies 25 (502 = 25) nt to 275 (552 = 275) nt of thessDNA The EMSA results suggest that the length of anssDNA (or the binding site size) [52] required for KpSSBbinding is 26 plusmn 2 nt
34 StSSB Bound to ssDNA The binding of StSSB to dT15(Figure 5(a)) dT20 (Figure 5(b)) dT30 (Figure 5(c)) dT40(Figure 5(d)) dT45 (Figure 5(e)) and dT50 (Figure 5(f)) was
BioMed Research International 7
[StSSB]
-dT15
-C
mdash
(a)
-dT20
-C
---
-
mdash[StSSB]
(b)
-dT30
-C
[StSSB]mdash
(c)
-dT40
-C
mdash[StSSB]
(d)
-dT45
-C1-C2
mdash[StSSB]
(e)
-dT50
-C1-C2
mdash
[StSSB]
(f)
Figure 5 Binding of StSSB to dT15ndash50 StSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT15 (b) dT20 (c) dT30 (d) dT40 (e) dT45 or (f) dT50 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and 100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blue and40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
examined using EMSA StSSB can bind and form a singlecomplex with dT15 (Figure 5(a)) and dT20 (Figure 5(b)) butKpSSB cannot (Figure 4(a)) StSSB bound to dT15ndash40 andformed a single complex For dT45 and dT50 two differentcomplexes of StSSB appeared at high protein concentrations(Figures 5(e) and 5(f)) Therefore one StSSB occupies 20(402 = 20) nt to 225 (452 = 225) nt of the ssDNA TheEMSA results suggest that the length of an ssDNA (or thebinding site size) [52] required for StSSB binding is 21 plusmn 2 nt
35 PaSSB Bound to ssDNA The binding of PaSSB to dT20(Figure 6(a)) dT25 (Figure 6(b)) dT35 (Figure 6(c)) dT45(Figure 6(d)) dT55 (Figure 6(e)) dT60 (Figure 6(f)) anddT65 (Figure 6(g)) was studied by EMSA Unlike StSSB nocomplexwas observedwhen PaSSBwas incubatedwith dT20Some smears were observed indicating that PaSSB interactswith dT20 However the ssDNA may be too short to befully wrapped by PaSSB PaSSB could form a single complexwith dT25ndash55 and form two distinct complexes with dT60
and dT65 (Figures 6(f) and 6(g)) respectivelyTherefore onePaSSB occupies 275 (552 = 275) nt to 30 (602 = 30) ntof the ssDNA These results from EMSA suggest that thelength of an ssDNA (or the binding site size) [52] required forPaSSB binding is 29plusmn2 nt Although the SSBs that is KpSSBStSSB and PaSSB have significantly similar ssDNA-bindingdomains their binding site sizes are different and range from19 (21 plusmn 2 StSSB) to 31 (29 plusmn 2 PaSSB) nt The obtainedEMSA results (Figures 4ndash6) also show that the binding sitesizes of the untagged SSBs (KpSSB StSSB and PaSSB) werefound to be almost identical to those of the His-tagged ones[40 42 43]
36 Design of the Chimeric KpSSB Proteins KpSSBnStSSBc andKpSSBnPaSSBc The N-terminal ssDNA-binding domain ofKpSSB StSSB and PaSSB is highly conserved (Figure 2) buttheir binding site sizes are different (Figures 4ndash6) and rangefrom 19 nt to 31 nt The C-terminal acidic tails DDDIPFare conserved (Figure 2) and these features led us to assess
8 BioMed Research International
[PaSSB]
-dT20
mdash
(a)
-dT25
-C
[PaSSB]mdash
(b)
-dT35
-C
-
-
[PaSSB]
(c)
-dT45
-C
[PaSSB]
(d)
-dT55
-C
[PaSSB]
(e)
-dT60
-C1-C2
[PaSSB]
(f)
-dT65
-C1-C2
[PaSSB]
(g)
Figure 6 Binding of PaSSB to dT20ndash65 PaSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT20 (b) dT25 (c) dT35 (d) dT45 (e) dT55 (f) dT60 or (g) dT65 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
whether the flexible glycine-rich hinge in the C-terminaldomain which is not conserved among SSBs is involved inthe determination of the binding site size of SSB Thus theC-terminal domains of StSSB and PaSSB were swapped withKpSSB through protein chimeragenesis
37 KpSSBnStSSBc Bound to ssDNA The binding ofKpSSBnStSSBc to dT15 (Figure 7(a)) dT20 (Figure 7(b))dT40 (Figure 7(c)) and dT45 (Figure 7(d)) was examinedusing EMSA KpSSBnStSSBc exhibited significantly different
ssDNA-binding properties from those of KpSSB UnlikeKpSSB (Figure 4) both KpSSBnStSSBc (Figure 8) and StSSB(Figure 5) can bind and form a single complex with dT15 anddT20 Similar to StSSB KpSSBnStSSBc binds to dT15ndash40 andforms a single complex For dT45 two different complexesof KpSSBnStSSBc appeared at high protein concentrations(Figure 8(d)) this EMSA feature was also similar to that ofStSSB One KpSSBnStSSBc occupies 20 (402 = 20) nt to225 (452 = 225) nt of the ssDNA These EMSA resultssuggest that the length of an ssDNA (or the binding site
BioMed Research International 9
[KpSSBnStSSBc]
-dT15
-C
(a)
[KpSSBnStSSBc]
-dT20
-C
(b)
[KpSSBnStSSBc]
-dT40
-C
(c)
[KpSSBnStSSBc]
-dT45
-C1-C2
(d)
Figure 7 Binding of KpSSBnStSSBc to dT15ndash45 KpSSBnStSSBc (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for30min at 25∘C with 17 nM of (a) dT15 (b) dT20 (c) dT40 or (d) dT45 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and 100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blue and40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
size) [52] required for KpSSBnStSSBc binding is 21 plusmn 2 nt avalue identical to that for StSSB (Figure 5) Swapping of theC-terminal domain of StSSB with KpSSB changes the size ofthe ssDNA-binding site from 26 nt to 21 nt
38 KpSSBnPaSSBc Bound to ssDNA The binding fea-tures of KpSSBnPaSSBc with dT20 (Figure 8(a)) dT25(Figure 8(b)) dT40 (Figure 8(c)) dT55 (Figure 8(d)) anddT60 (Figure 8(e)) were studied by EMSA Similar to thecases of KpSSB and PaSSB no complex was observed whenKpSSBnPaSSBc was incubated with dT20 However KpSSB-nPaSSBc still exhibited dramatically different ssDNA-bindingproperties from those of KpSSB KpSSB can form two distinctcomplexes with dT55 (Figure 4(f)) but both KpSSBnPaSSBc(Figure 9) and PaSSB (Figure 6) cannot One KpSSBnPaSSBcoccupies 275 (552 = 275) nt to 30 (602 = 30) nt ofthe ssDNA The above EMSA results suggest that the lengthof an ssDNA (or the binding site size) [52] required forKpSSBnPaSSBc binding is 29 plusmn 2 nt a value identical to thatof PaSSB Swapping of the C-terminal domain of PaSSB toKpSSB changes the size of the ssDNA-binding site from 26 ntto 29 nt Although these SSBs namely KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc have nearly identicalssDNA-binding domains their binding site sizes are different(Table 2) Thus the size of the ssDNA-binding site requiredfor second SSB binding is likely to be dependent on the C-terminal domain of SSB
39 Binding Constants of the SSB-ssDNA Complexes Deter-mined from EMSA To compare the ssDNA-binding abil-ities of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc the midpoint values for input ssDNA bind-ing calculated from the titration curves of EMSA andreferred to as [Protein]
50(monomer) were quantified and
are summarized in Table 2 Although the N-terminal ssDNA-binding domains of these SSB proteins are highly similar(Figure 2) their ssDNA-binding activities and binding sitesizes are different (Table 2) [KpSSB]
50values ranged from
100 nM to 220 nM [StSSB]50
values ranged from 420 nM to650 nM [PaSSB]
50values ranged from 550 nM to 1700 nM
[KpSSBnStSSBc]50values ranged from 110 nM to 260 nM and
[KpSSBnPaSSBc]50
values ranged from 220 nM to 390 nMThe ssDNA-binding ability is as follows in the order ofdecreasing affinity KpSSB gt KpSSBnStSSBc gt KpSSBn-PaSSBc gt StSSB gt PaSSB Results from the above analysesindicate that the exchange of the C-terminal domain inSSB significantly changed the ssDNA-binding ability and theDNA-binding behavior (complex number) The reason asto why swapping of the C-terminal domain can affect thessDNA-binding activity of SSB remains unclear The C-ter-minal domain of SSB is suggested to be involved in ssDNAbinding However this relation is not evident in the results ofthe cocrystal structure
310 Oligomeric State of KpSSBnStSSBc and KpSSBnPaSSBc inSolution Gel-filtration chromatography was used to confirm
10 BioMed Research International
[KpSSBnPaSSBc]
-dT20
(a)
[KpSSBnPaSSBc]
-dT25
-C
(b)
[KpSSBnPaSSBc]
-C
-dT40
(c)
-C
[KpSSBnPaSSBc]
-dT55
(d)
-dT60
-C1-C2
[KpSSBnPaSSBc]
(e)
Figure 8 Binding of KpSSBnPaSSBc to dT20ndash60 KpSSBnPaSSBc (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for30min at 25∘C with 17 nM of (a) dT20 (b) dT25 (c) dT40 (d) dT55 or (e) dT60 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
that the oligomeric state of KpSSBnStSSBc and KpSSBn-PaSSBc remains as tetramers after chimeragenesisThe analy-sis of purified KpSSBnStSSBc and KpSSBnPaSSBc (2mgmL)using a Superdex 200HR 1030 column revealed a singlepeak with elution volumes of 786 and 789mL respectivelyAssuming that KpSSBnStSSBc and KpSSBnPaSSBc both haveshapes and partial specific volumes similar to the standardproteins the native molecular masses of KpSSBnStSSBc andKpSSBnPaSSBc were estimated to be 76641 and 74827Da ascalculated from a standard linear regression equation 119870av =minus03684(logMw) + 22707 (Figure 9) The native molecularmasses for KpSSBnStSSBc and KpSSBnPaSSBc are approx-imately four times the mass of the monomer (sim19 kDa)Therefore KpSSBnStSSBc and KpSSBnPaSSBc under theabove chromatographic conditions are stable tetramers insolution Although the exchange of the C-terminal domainin SSB significantly changed the ssDNA-binding ability andDNA-binding behavior (complex number) protein chimera-genesis did not cause any change in the oligomeric state ofSSB
311 Summary of Gly Gln and Pro Number in SSBs To ana-lyze the C-terminal amino acid composition of SSBs wefurther counted the number of Gly Gln and Pro residuesin different SSB segments SSB is abundant in Gly Glnand Pro (GQP) (Table 3) The GQP contents of KpSSB1ndash91 StSSB1ndash91 and PaSSB1ndash90 are similar However the Glynumber of PaSSB116ndash165 is significantly lower than that ofKpSSB116ndash174 and StSSB117ndash176 PaSSB116ndash165 contains only1 Gly but KpSSB116ndash174 and StSSB117ndash176 contain 11 and 12Gly respectively In addition we found different distributionpatterns among KpSSB StSSB and PaSSB Although theycontain similar number of Gln (Q) the QQQ pattern isfrequently found in PaSSB (Table 3)
312 Structural Modeling of SSBs Given its disordered C-ter-minal domain the crystal structure of the full-length SSB islacking even when SSB can be crystallized with DNA [69]We attempted to model the structure by homology mod-eling using the bioinformatics program (PS)2 to obtain an
BioMed Research International 11
Table 2 ssDNA binding properties of KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc as analyzed by EMSA
Protein DNA [Protein]50 (nM) Complex number
KpSSB
dT20 ND 0dT25 200 plusmn 20 1dT35 220 plusmn 30 1dT45 100 plusmn 10 1dT50 110 plusmn 20 1dT55 100 plusmn 20 2dT60 100 plusmn 10 2
StSSB
dT15 650 plusmn 120 1dT20 450 plusmn 80 1dT30 420 plusmn 60 1dT40 420 plusmn 80 1dT45 440 plusmn 60 2dT50 440 plusmn 50 2
PaSSB
dT20 ND 0dT25 1700 plusmn 250 1dT35 950 plusmn 180 1dT45 780 plusmn 160 1dT55 820 plusmn 90 1dT60 810 plusmn 110 2dT65 550 plusmn 70 2
KpSSBnStSSBc
dT15 260 plusmn 60 1dT20 110 plusmn 20 1dT40 120 plusmn 20 1dT45 160 plusmn 20 2
KpSSBnPaSSBc
dT20 ND 0dT25 390 plusmn 60 1dT40 220 plusmn 30 1dT55 230 plusmn 30 1dT60 230 plusmn 30 2
[Protein]50 was calculated from the titration curves of EMSA by determiningthe concentration of the protein (120583M) needed to achieve the midpointvalue for input ssDNA binding For some oligonucleotides input ssDNAbinding was the sum of the intensities from the two separate ssDNA-proteincomplexes Errors are standard deviations determined by three independenttitration experiments
Table 3 Summary of Gly Gln and Pro number in SSB
SSB segment G Q PKpSSB1ndash91 10 5 2StSSB1ndash91 10 5 2PaSSB1ndash90 11 6 2KpSSB92ndash174 18 16 9StSSB92ndash176 17 18 11PaSSB91ndash165 5 15 11KpSSB116ndash174 11 12 9StSSB117ndash176 12 13 10PaSSB116ndash165 1 12 11
in-depth understanding of the structure-function relation-ship of the C-terminal domains of these SSBs [70 71]
40 45 50 55
Kav
00
02
04
06
08
10
KpSSBnStSSBcKpSSBnPaSSBc
Y = minus03684X + 22707
R2= 0998
log Mw
Figure 9Gel-filtration chromatographic analyses of KpSSBnStSSBcand KpSSBnPaSSBc Purified protein (2mgmL) was applied toa Superdex 200HR 1030 column (GE Healthcare Bio-SciencesPiscataway NJ USA) equilibrated with Buffer D The column wasoperated at a flow rate of 05mLmin and 05mL fractions werecollected The proteins were detected by measuring the absorbanceat 280 nm The column was calibrated with proteins of knownmolecular weight thyroglobulin (670 kDa) 120574-globulin (158 kDa)ovalbumin (44 kDa) and myoglobin (17 kDa) The 119870av values forthe standard proteins and the SSB variants were calculated from theequation119870av = (119881119890minus119881119900)(119881119888minus119881119900) where119881119900 is column void volume119881119890is elution volume and 119881
119888is geometric column volume
(PS)2 (http140113239111simps2v2docsphp) is an auto-matic homology modeling server that combines bothsequence and secondary structure information to detect thehomologous proteins with remote similarity and the target-template alignment After pasting the amino acid sequenceto the website of (PS)2 only one hit (Protein Data Bankentry 1QVC EcSSB) for the C-terminal domains of KpSSBand StSSB was suggested For the C-terminal domain ofPaSSB only one hit that is CstF-77 (ProteinData Bank entry2OOE cleavage stimulation factor CstF) but not EcSSB wassuggested as the template for modeling Figure 10 shows thatmodeled structures of these SSB C-terminal domains arehighly disordered but that of PaSSB is more ordered than thatof other domains
4 Discussion
In this study we examined the sizes of the binding site ofthe untagged SSB and the chimeric SSB from the ubiquitousopportunistic pathogens K pneumoniae S enterica serovarTyphimurium LT2 and P aeruginosa PAO1 Many clinicalstrains of the abovementioned bacteria are highly resistantto antibiotics [72ndash75] The development of clinically usefulsmall-molecule antibiotics has been a seminal event in thefield of infectious diseases [48] Nucleic acid metabolismis one of the most basic biological functions and shouldbe a prime target in antibiotic development [76ndash78] Manybacterial SSBs form conserved protein interaction ldquohubsrdquothat are essential to recruit many proteins involved in DNA
12 BioMed Research International
Figure 10 Structure modeling of SSB The structures of KpSSB1ndash115 StSSB1ndash115 and PaSSB1ndash115 (the N-terminal domain of SSB)were modeled by SWISS-MODEL The structures of KpSSB116ndash142 StSSB116ndash142 and PaSSB121ndash160 (the C-terminal domain ofSSB) were modeled by (PS)2 Other regions of SSBs could not bemodeled by these two programs The structures of the N-terminaldomain and the C-terminal domain of these SSBs were manuallylinked (KpSSB1ndash142 blue StSSB1ndash142 pink PaSSB1ndash160 green) andsuperimposed with the crystal structure of EcSSB1ndash142 (orange)(PDB entry 1QVC) for comparison For clarity only one subunitof the tetramer was shown for each SSB
replication recombination and repair SSBDNA nucleopro-tein substrates [79] Thus SSBs may be promising targets inantibiotic development [80] As a first step toward achievingthis goal we investigated why SSBs possess highly conservedN-terminal ssDNA-binding domain but exhibit varying bind-ing site sizes One significant clue is that their flexible hingesand the length at the C-terminus are different as revealed bysequence alignment (Figure 2)
The interactions of various SSBs with ssDNA have beenanalyzed using a variety of techniques such as tryptophan-fluorescence quenching [47] filter binding [81] EMSA [5282] analytical ultracentrifugation [83] electron microscopy[84] nuclease digestion [44] single-molecule fluorescencemicroscopy [48] and crystallographic analyses [11] In thisstudy we have examined the electrophoretic mobility shiftpatterns of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc bound to different lengths of ssDNA and deter-mined the corresponding binding site sizes to be 26 2129 22 and 29 nt per tetramer respectively (Figures 4ndash8) PaSSB and KpSSBnPaSSBc have the largest sizes forssDNA binding among the SSBs studied We also identifiedHis-tagged and untagged SSBs that have similar ssDNA-binding site sizes [40 42 43] EMSA is a well-establishedapproach in studies of molecular biology [52] and the use ofradioactive tracer in this assay allows detection of the actualformation of the distinct protein-DNA complex(es) [53] For
exampleDNase protection assay and footprinting assay usingradioactive tracer can determine the specific DNA sequencecomplexed by a protein In EMSA when the length of thenucleotides is sufficient for the binding of two or more SSBmolecules the electrophoretic mobility of the higher SSBoligomer complex will be lower than that of the smaller SSBoligomer complex [52 54] In addition results of the ssDNA-binding site size from EMSA and cocrystal structure of SSBwere consistent [27] Thus throughout this paper we deter-mined the ssDNA-binding site sizes of SSB from the EMSAbehavior
Many SSBs bind to ssDNA with some degree of positivecooperativity Cooperativity can result from direct protein-protein interactions between the nearest neighbors such asthe LAST motif in the T4 gene-32 protein [85] and thearginine-mediated interaction motif in Thermus SSB [8687] Cooperativity can also result from the protein-induceddistortion of adjacent DNA as demonstrated in SulfolobusSSB PriB and FOXK1a proteins [23 60 88] In the cases ofKpSSB StSSB and PaSSB (Figures 4ndash6) binding appeared tobe nearly noncooperative for several DNAs because all DNAmainly shifts into the first complex (C1) before the appearanceof the second complex (C2) when subjected to increasingprotein concentrationsThe length dependence of the [SSB]
50
values suggests that the amount of spacing is optimum forsteric considerations (Table 2)
Because bacteria have varying genomic DNA sizes theirSSBs may need to evolve to have different binding site sizesfor DNA metabolism Results from protein chimeragenesisshowed the C-terminal domain dependence of the bindingsite sizes of SSB (Figure 11) The experimental data showedthat the binding site size of KpSSBnStSSBc was similar to thatof StSSB and the size of the binding site of KpSSBnPaSSBcwas similar to that of PaSSB The reason for which the bind-ing site size of SSB changed followed by swapping of theC-terminal domain remains unclear Flexibility numberof glycine residues andor different QQQ patterns of theC-terminal domain of SSB (Figure 2 and Table 3) may beimportant factors for determining the ssDNA-binding sitesize In fact the C-terminal domain of PaSSB that isPaSSB116ndash165 has only 1 Gly residue which is significantlyless than that of KpSSB (11 Gly) and StSSB (12Gly) Gly (andPro) is an important component of the flexible region aprotein that contains low Gly content is predicted to havelow flexibility Unlike typical SSB [35 69] PaSSB116ndash165 hasa partial structure (Figure 10) Although KpSSB StSSB andPaSSB contain similar number of Gln (Q) the QQQ patternis frequently found in PaSSB (Figure 2 and Table 3) PolyQand repeated sequences GAGAG are commonly found inthe structures of amyloids silk fibers and neurodegradationproteins [89ndash92] Considering that the simple coil polyQ theheptapeptide GNNQQNY and the hexapeptide NNQQNYcan cause protein aggregation and nucleation [93ndash95] thedistribution of Gln in the C-terminal domain of a tetramericSSB may also be an important determinant of the ssDNA-binding site size of SSB by some steric hindrances (Figure 11)However the above speculationmust be confirmed by furtherbiochemical experiments
BioMed Research International 13
PaSSBStSSB KpSSB
C-terC-ter C-ter
sim 21 nt sim 26 nt sim 29 nt
Figure 11 Possiblemodels for explainingwhy SSBs arewith different binding site sizes Twomodeled structures of KpSSB1ndash142 (blue) StSSB1ndash142 (pink) and PaSSB1ndash160 (green) complexed with ssDNA (gold) are shown For clarity only one C-terminal domain was shown for eachSSB tetramer By using the electrophoretic mobility shift assay and the protein chimeragenesis we characterized that the binding site sizes ofKpSSB StSSB PaSSB KpSSBnStSSBc and KpSSBnPaSSBc were 26 21 29 21 and 29 nt per tetramer respectively KpSSB StSSB and PaSSBare similar proteins whose N-terminal ssDNA-binding domains are almost identical Thus the C-terminal domain of SSB may indirectlycontribute to ssDNA binding and wrapping and affects the binding site size by the steric hindrance
5 Conclusion
In this study we characterized the ssDNA-binding propertiesof untagged SSBs from K pneumoniae S enterica sero-var Typhimurium LT2 and P aeruginosa PAO1 and proposeda role of the C-terminal flexible domain for ssDNA bind-ing from the protein chimeragenesis and EMSA resultsThe amino acid sequence of the N-terminal ssDNA-bind-ingoligomerization domain in these pathogenic SSBs ishighly conserved but their apparent binding site sizes aredifferent This finding indicates that the C-terminal protein-protein interaction domain may also indirectly contribute tossDNA binding and wrapping
Conflict of Interests
The authors declare that there is no conflict of interestsregarding the publication of this paper
Acknowledgment
This researchwas supported by aGrant from theNational Sci-ence Council Taiwan (NSC 102-2320-B-040-019 to Cheng-Yang Huang)
References
[1] R Reyes-Lamothe D J Sherratt and M C Leake ldquoStoichiom-etry and architecture of active DNA replication machinery inescherichia colirdquo Science vol 328 no 5977 pp 498ndash501 2010
[2] D J Richard E Bolderson andK K Khanna ldquoMultiple humansingle-stranded DNA binding proteins function in genomemaintenance structural biochemical and functional analysisrdquo
Critical Reviews in Biochemistry and Molecular Biology vol 44no 2-3 pp 98ndash116 2009
[3] R D Shereda A G Kozlov T M LohmanMM Cox and J LKeck ldquoSSB as an organizermobilizer of genome maintenancecomplexesrdquo Critical Reviews in Biochemistry and MolecularBiology vol 43 no 5 pp 289ndash318 2008
[4] D J Richard E Bolderson L Cubeddu et al ldquoSingle-strandedDNA-binding protein hSSB1 is critical for genomic stabilityrdquoNature vol 453 no 7195 pp 677ndash681 2008
[5] R R Meyer and P S Laine ldquoThe single-stranded DNA-bindingprotein of Escherichia colirdquoMicrobiological Reviews vol 54 no4 pp 342ndash380 1990
[6] C Yang U Curth C Urbanke and C Kang ldquoCrystal structureof human mitochondrial single-stranded DNA binding proteinat 24 A resolutionrdquo Nature Structural Biology vol 4 no 2 pp153ndash157 1997
[7] G Webster J Genschel U Curth C Urbanke C Kang and RHilgenfeld ldquoA common core for binding single-stranded DNAstructural comparison of the single-stranded DNA-bindingproteins (SSB) from E coli and human mitochondriardquo FEBSLetters vol 411 pp 313ndash316 1997
[8] R L Flynn and L Zou ldquoOligonucleotideoligosaccharide-bind-ing fold proteins a growing family of genome guardiansrdquo Crit-ical Reviews in Biochemistry and Molecular Biology vol 45 no4 pp 266ndash275 2010
[9] K Chan Y Lee CWangHHuang andY Sun ldquoSingle-Strand-ed DNA-Binding Protein Complex from Helicobacter pyloriSuggests an ssDNA-Binding Surfacerdquo Journal of MolecularBiology vol 388 no 3 pp 508ndash519 2009
[10] D L Theobald R M Mitton-Fry and D S Wuttke ldquoNucleicacid recognition by OB-fold proteinsrdquo Annual Review of Bio-physics and Biomolecular Structure vol 32 pp 115ndash133 2003
[11] S Raghunathan A G Kozlov TM Lohman andGWaksmanldquoStructure of the DNA binding domain of E coli SSB bound to
14 BioMed Research International
ssDNArdquo Nature Structural Biology vol 7 no 8 pp 648ndash6522000
[12] A G Murzin ldquoOB(oligonucleotideoligosaccharide binding)-fold common structural and functional solution for non-homologous sequencesrdquo EMBO Journal vol 12 no 3 pp 861ndash867 1993
[13] N P George K V Ngo S Chitteni-Pattu et al ldquoStructure andcellular dynamics of deinococcus radiodurans single-strandedDNA (ssDNA)-binding protein (SSB)-DNA complexesrdquo TheJournal of Biological Chemistry vol 287 no 26 pp 22123ndash221322012
[14] P Filipkowski and J Kur ldquoIdentification and propertiesof the Deinococcus grandis and Deinococcus proteolyticussingle-stranded DNA binding proteins (SSB)rdquo Acta BiochimicaPolonica vol 54 no 1 pp 79ndash87 2007
[15] P Filipkowski A Duraj-Thatte and J Kur ldquoIdentificationcloning expression and characterization of a highly ther-mostable single-stranded-DNA-binding protein (SSB) fromDeinococcus murrayirdquo Protein Expression and Purification vol53 no 1 pp 201ndash208 2007
[16] P Filipkowski M Koziatek and J Kur ldquoA highly ther-mostable homodimeric single-stranded DNA-binding proteinfrom Deinococcus radiopugnansrdquo Extremophiles vol 10 no 6pp 607ndash614 2006
[17] P Filipkowski A Duraj-Thatte and J Kur ldquoNovel thermostablesingle-stranded DNA-binding protein (SSB) fromDeinococcusgeothermalisrdquo Archives of Microbiology vol 186 no 2 pp 129ndash137 2006
[18] G Witte C Urbanke and U Curth ldquoSingle-stranded DNA-binding protein of Deinococcus radiodurans a biophysicalcharacterizationrdquoNucleic Acids Research vol 33 no 5 pp 1662ndash1670 2005
[19] D A Bernstein J M Eggingtont M P Killoran A M MisicMMCox and J L Keck ldquoCrystal structure of theDeinococcusradiodurans single-stranded DNA-binding protein suggests amechanism for coping with DNA damagerdquo Proceedings of theNational Academy of Sciences of the United States of Americavol 101 no 23 pp 8575ndash8580 2004
[20] S Dabrowski M Olszewski R Piatek A Brillowska-Dabrowska G Konopa and J Kur ldquoIdentification and charac-terization of single-stranded-DNA-binding proteins fromTher-mus thermophilus and Thermus aquaticusmdashnew arrange-ment of binding domainsrdquo Microbiology vol 148 no 10 pp3307ndash3315 2002
[21] R Gamsjaeger R Kariawasam C Touma A H Kwan M FWhite and L Cubeddu ldquoBackbone and side-chain 1H 13C and15N resonance assignments of the OB domain of the singlestranded DNA binding protein from Sulfolobus solfataricusand chemical shift mapping of the DNA-binding interfacerdquoBiomolecular NMR Assignments 2013
[22] M L Rolfsmeier and C A Haseltine ldquoThe single-strandedDNA binding protein of Sulfolobus solfataricus acts in the pre-synaptic step of homologous recombinationrdquo Journal of Molec-ular Biology vol 397 no 1 pp 31ndash45 2010
[23] I D Kerr R I M Wadsworth L Cubeddu W Blankenfeldt JHNaismith andM FWhite ldquoInsights into ssDNA recognitionby the OB fold from a structural and thermodynamic study ofSulfolobus SSB proteinrdquo EMBO Journal vol 22 no 11 pp 2561ndash2570 2003
[24] C A Haseltine and S C Kowalczykowski ldquoA distinctive single-stranded DNA-binding protein from the Archaeon Sulfolobussolfataricusrdquo Molecular Microbiology vol 43 no 6 pp 1505ndash1515 2002
[25] R I M Wadsworth and M F White ldquoIdentification andproperties of the crenarchaeal single-stranded DNA bindingprotein from Sulfolobus solfataricusrdquo Nucleic Acids Researchvol 29 no 4 pp 914ndash920 2001
[26] S Sugiman-Marangos and M S Junop ldquoThe structure of DdrBfrom Deinococcus a new fold for single-stranded DNA bindingproteinsrdquo Nucleic Acids Research vol 38 no 10 Article IDgkq036 pp 3432ndash3440 2010
[27] H Ghalei H V Moeller D Eppers et al ldquoEntrapment of DNAin an intersubunit tunnel system of a single-stranded DNA-binding proteinrdquo Nucleic Acids Research vol 42 no 10 pp6698ndash6708 2014
[28] S Paytubi S A McMahon S Graham et al ldquoDisplacement ofthe canonical single-strandedDNA-binding protein in the ther-moprotealesrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 109 no 7 pp E398ndashE405 2012
[29] T H Dickey S E Altschuler and D S Wuttke ldquoSingle-stranded DNA-binding proteins multiple domains for multiplefunctionsrdquo Structure vol 21 no 7 pp 1074ndash1084 2013
[30] D Vujaklija and BMacek ldquoDetecting posttranslational modifi-cations of bacterial SSB proteinsrdquoMethods inMolecular Biologyvol 922 pp 205ndash218 2012
[31] I Mijakovic D Petranovic B Macek et al ldquoBacterial single-stranded DNA-binding proteins are phosphorylated on tyro-sinerdquoNucleic Acids Research vol 34 no 5 pp 1588ndash1596 2006
[32] M Olszewski A Grot M Wojciechowski M Nowak MMickiewicz and J Kur ldquoCharacterization of exceptionally ther-mostable single-strandedDNA-binding proteins fromThermo-toga maritima and Thermotoga neapolitanardquo BMC Microbiol-ogy vol 10 article 260 2010
[33] U Curth J Genschel C Urbanke and J Greipel ldquoIn vitro andin vivo function of the C-terminus of Escherichia coil single-stranded DNA binding proteinrdquoNucleic Acids Research vol 24no 14 pp 2706ndash2711 1996
[34] G Witte C Urbanke and U Curth ldquoDNA polymerase IIIchi subunit ties single-stranded DNA binding protein to thebacterial replication machineryrdquoNucleic Acids Research vol 31pp 4434ndash4440 2003
[35] D Shishmarev YWang C EMason et al ldquoOtting Intramolec-ular binding mode of the C-terminus of Escherichia coli single-strandedDNAbinding protein determined by nuclearmagneticresonance spectroscopyrdquo Nucleic Acids Research vol 42 pp2750ndash2757 2014
[36] A G Kozlov M M Cox and T M Lohman ldquoRegulation ofsingle-stranded DNA binding by the C termini of Escherichiacoli single-stranded DNA-binding (SSB) proteinrdquo Journal ofBiological Chemistry vol 285 no 22 pp 17246ndash17252 2010
[37] M Nowak M Olszewski M Spibida and J Kur ldquoChar-acterization of single-stranded DNA-binding proteins fromthe psychrophilic bacteria Desulfotalea psychrophila Flavobac-terium psychrophilum Psychrobacter arcticus Psychrobactercryohalolentis Psychromonas ingrahamii Psychroflexus torquisand Photobacterium profundumrdquo BMC Microbiology vol 14article 91 2014
BioMed Research International 15
[38] T Paradzik N Ivic Z Filic et al ldquoStructure-function relation-ships of two paralogous single-stranded DNA-binding proteinsfrom Streptomyces coelicolor implication of SsbB in chromo-some segregation during sporulationrdquo Nucleic Acids Researchvol 41 no 6 pp 3659ndash3672 2013
[39] S JainM Zweig E Peeters et al ldquoCharacterization of the singlestrandedDNA binding protein SsbB encoded in the gonoccocalgenetic islandrdquo PLoS ONE vol 7 no 4 Article ID e35285 2012
[40] Y H Huang and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from Klebsiella pneumoniaemutation at either Arg73 or Ser76 causes a less cooperativecomplex onDNArdquoGenes to Cells vol 17 no 2 pp 146ndash157 2012
[41] E Antony E A Weiland S Korolev and T M LohmanldquoPlasmodium falciparum SSB tetramer wraps single-strandedDNAwith similar topology but opposite polarity to E Coli SSBrdquoJournal ofMolecular Biology vol 420 no 4-5 pp 269ndash283 2012
[42] H Jan Y L Lee and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from pseudomonas aeruginosaPAO1rdquo Protein Journal vol 30 no 1 pp 20ndash26 2011
[43] Y-H Huang Y-L Lee and C-Y Huang ldquoCharacterization of asingle-stranded DNA binding protein from Salmonella entericaserovar typhimurium LT2rdquo Protein Journal vol 30 no 2 pp102ndash108 2011
[44] S K Bharti K Rex P Sreedhar N Krishnan and U VarshneyldquoChimeras of Escherichia coli and Mycobacterium tuberculosissingle-stranded DNA binding proteins characterization andfunction in Escherichia colirdquo PLoS ONE vol 6 no 12 ArticleID e27216 2011
[45] M T Oliveira and L S Kaguni ldquoFunctional roles of the N-and C-terminal regions of the human mitochondrial single-stranded DNA-binding proteinrdquo PLoS ONE vol 5 no 10Article ID e15379 2010
[46] A G Kozlov J M Eggington M M Cox and T MLohman ldquoBinding of the dimeric Deinococcus radioduranssingle-strandedDNA binding protein to single-strandedDNArdquoBiochemistry vol 49 no 38 pp 8266ndash8275 2010
[47] T M Lohman and M E Ferrari ldquoEscherichia coli single-stranded DNA-binding protein multiple DNA-binding modesand cooperativesrdquo Annual Review of Biochemistry vol 63 pp527ndash570 1994
[48] R Zhou A G Kozlov R Roy et al ldquoSSB functions as a slidingplatform that migrates on DNA via reptationrdquo Cell vol 146 pp222ndash232 2011
[49] R Roy A G Kozlov T M Lohman and T Ha ldquoSSB proteindiffusion on single-stranded DNA stimulates RecA filamentformationrdquo Nature vol 461 pp 1092ndash1097 2009
[50] R Roy A G Kozlov T M Lohman and T Ha ldquoDynamicstructural rearrangements between DNA binding modes of Ecoli SSB proteinrdquo Journal of Molecular Biology vol 369 no 5pp 1244ndash1257 2007
[51] T J Kelly P Simancek and G S Brush ldquoIdentification andcharacterization of a single-stranded DNA-binding proteinfrom the archaeon Methanococcus jannaschiirdquo Proceedings ofthe National Academy of Sciences of the United States of Americavol 95 no 25 pp 14634ndash14639 1998
[52] C Y Huang Determination of the Binding Site-Size of theProtein-DNA Complex by Use of the Electrophoretic MobilityShift Assay InTech Press Rijeka Croatia 2012
[53] A Kornberg ldquoTen commandments of enzymology amendedrdquoTrends in Biochemical Sciences vol 28 no 10 pp 515ndash517 2003
[54] W Zhang X Lu and J Shen ldquoEMSA and single-moleculeforce spectroscopy study of interactions between bacillus sub-tilis single-stranded DNA-binding protein and single-strandedDNArdquo Langmuir vol 27 no 24 pp 15008ndash15015 2011
[55] M Ostermeier and S J Benkovic ldquoEvolution of protein func-tion by domain swappingrdquo Advances in Protein Chemistry vol55 pp 29ndash77 2000
[56] W F Peng and C Y Huang ldquoAllantoinase and dihydroorotasebinding and inhibition by flavonols and the substrates of cyclicamidohydrolasesrdquo Biochimie vol 101 pp 113ndash122 2014
[57] Y H Huang M J Lin and C Y Huang ldquoDnaT is a single-stranded DNA binding proteinrdquo Genes to Cells vol 18 no 11pp 1007ndash1019 2013
[58] Y Y Ho Y H Huang and C Y Huang ldquoChemical rescue of thepost-translationally carboxylated lysine mutant of allantoinaseand dihydroorotase by metal ions and short-chain carboxylicacidsrdquo Amino Acids vol 44 no 4 pp 1181ndash1191 2013
[59] Y H Huang Y Lo W Huang and C Y Huang ldquoCrystalstructure and DNA-binding mode of Klebsiella pneumoniaeprimosomal PriB proteinrdquoGenes to Cells vol 17 no 10 pp 837ndash849 2012
[60] C Y Huang C Y Hsu Y Sun H Wu and C Hsiao ldquoCom-plexed crystal structure of replication restart primosome pro-tein PriB reveals a novel single-stranded DNA-binding moderdquoNucleic Acids Research vol 34 no 14 pp 3878ndash3886 2006
[61] T Kinebuchi H Shindo H Nagai N Shimamoto andM Shimizu ldquoFunctional domains of Escherichia coli single-stranded DNA binding protein as assessed by analyses of thedeletion mutantsrdquo Biochemistry vol 36 no 22 pp 6732ndash67381997
[62] N Shimamoto N Ikushima H Utiyama H Tachibana andK Horie ldquoSpecific and cooperative binding of E coli single-stranded DNA binding protein to mRNArdquo Nucleic AcidsResearch vol 15 no 13 pp 5241ndash5250 1987
[63] H Lin andC YHuang ldquoCharacterization of flavonol inhibitionof DnaB helicase Real-time monitoring structural modelingand proposed mechanismrdquo Journal of Biomedicine and Biotech-nology vol 2012 Article ID 735368 2012
[64] Y H Huang H H Lin and C Y Huang ldquoA single residuedetermines the cooperative binding property of a primosomalDNA replication protein PriB to single-stranded DNArdquo Bio-science Biotechnology and Biochemistry vol 76 no 6 pp 1110ndash1115 2012
[65] H C Hsieh and C Y Huang ldquoIdentification of a novel proteinPriB in Klebsiella pneumoniaerdquo Biochemical and BiophysicalResearch Communications vol 404 no 1 pp 546ndash551 2011
[66] J H Liu T W Chang C Y Huang et al ldquoCrystal structure ofPriB a primosomalDNA replication protein ofEscherichia colirdquoThe Journal of Biological Chemistry vol 279 no 48 pp 50465ndash50471 2004
[67] Y H Huang and C Y Huang ldquoThe N-terminal domain ofDnaT a primosomalDNA replication protein is crucial for PriBbinding and self-trimerizationrdquo Biochemical and BiophysicalResearch Communications vol 442 pp 147ndash152 2013
[68] M A Larkin G Blackshields N P Brown et al ldquoClustalW andClustal X version 20rdquo Bioinformatics vol 23 no 21 pp 2947ndash2948 2007
16 BioMed Research International
[69] S N Savvides S Raghunathan K Futterer A G Kozlov T MLohman and G Waksman ldquoThe C-terminal domain of full-length E coli SSB is disordered even when bound to DNArdquoProtein Science vol 13 no 7 pp 1942ndash1947 2004
[70] C-C Chen J-K Hwang and J-M Yang ldquo(PS)2-v2 template-based protein structure prediction serverrdquo BMC Bioinformaticsvol 10 article 366 2009
[71] C Chen J Hwang and J Yang ldquo(PS)2 protein structure pre-diction serverrdquoNucleic Acids Research vol 34 pp W152ndashW1572006
[72] K Bush and J F Fisher ldquoEpidemiological expansion structuralstudies and clinical challenges of new 120573-lactamases from gram-negative bacteriardquo Annual Review of Microbiology vol 65 pp455ndash478 2011
[73] K K Kumarasamy M A Toleman T R Walsh et al ldquoEmer-gence of a new antibiotic resistance mechanism in India Pak-istan and the UK a molecular biological and epidemiologicalstudyrdquoThe Lancet Infectious Diseases vol 10 no 9 pp 597ndash6022010
[74] K Bush and M J MacIelag ldquoNew 120573-lactam antibiotics and 120573-lactamase inhibitorsrdquo Expert Opinion on Therapeutic Patentsvol 20 no 10 pp 1277ndash1293 2010
[75] K Bush ldquoAlarming 120573-lactamase-mediated resistance in multi-drug-resistant Enterobacteriaceaerdquo Current Opinion in Microbi-ology vol 13 no 5 pp 558ndash564 2010
[76] A Srivastava M Talaue S Liu et al ldquoNew target for inhibitionof bacterial RNA polymerase ldquoswitch regionrdquordquo Current Opinionin Microbiology vol 14 no 5 pp 532ndash543 2011
[77] K Chono K Katsumata T Kontani et al ldquoASP2151 a novelhelicase-primase inhibitor possesses antiviral activity againstvaricella-zoster virus and herpes simplex virus types 1 and 2rdquoJournal of Antimicrobial Chemotherapy vol 65 no 8 pp 1733ndash1741 2010
[78] M T Black and K Coleman ldquoNew inhibitors of bacterial topoi-somerase GyrAParC subunitsrdquo Current Opinion in Investiga-tional Drugs vol 10 no 8 pp 804ndash810 2009
[79] A H Marceau D A Bernstein B W Walsh W Shapiro LA Simmons and J L Keck ldquoProtein interactions in genomemaintenance as novel antibacterial targetsrdquo PLoS ONE vol 8no 3 Article ID e58765 2013
[80] D Lu D A Bernstein K A Satyshur and J L Keck ldquoSmall-molecule tools for dissecting the roles of SSBprotein inter-actions in genome maintenancerdquo Proceedings of the NationalAcademy of Sciences of the United States of America vol 107 no2 pp 633ndash638 2010
[81] I Wong and T M Lohman ldquoA double-filter method fornitrocellulose-filter binding application to protein-nucleic acidinteractionsrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 90 no 12 pp 5428ndash5432 1993
[82] M Mitas J Y Chock and M Christy ldquoThe binding-site sizesof Escherichia coli single-stranded-DNA-binding protein andmammalian replication protein A are 65 and ge 54 nucleotidesrespectivelyrdquo Biochemical Journal vol 324 no 3 pp 957ndash9611997
[83] C Urbanke G Witte and U Curth ldquoSedimentation velocitymethod in the analytical ultracentrifuge for the study of protein-protein interactionsrdquoMethods inMolecular Biology vol 305 pp101ndash114 2005
[84] S Chrysogelos and J Griffith ldquoEscherichia coli single-strandbinding protein organizes single-strandedDNA innucleosome-like unitsrdquo Proceedings of the National Academy of Sciences of theUnited States of America vol 79 no 19 pp 5803ndash5807 1982
[85] J R Casas-Finet K R Fischer and R L Karpel ldquoStructuralbasis for the nucleic acid binding cooperativity of bacteriophageT4 gene 32 protein the (LysArg)3(SerThr)2 (LAST) motifrdquoProceedings of the National Academy of Sciences of the UnitedStates of America vol 89 pp 1050ndash1054 1992
[86] G Witte R Fedorov and U Curth ldquoBiophysical analysis ofThermus aquaticus single-stranded DNA binding proteinrdquo Bio-physical Journal vol 94 no 6 pp 2269ndash2279 2008
[87] R Fedorov G Witte C Urbanke D J Manstein and UCurth ldquo3D structure of Thermus aquaticus single-strandedDNA-binding protein gives insight into the functioning of SSBproteinsrdquo Nucleic Acids Research vol 34 no 22 pp 6708ndash67172006
[88] K L Tsai C Y Huang C H Chang Y J Sun W J ChuangandC DHsiao ldquoCrystal structure of the human FOXK1a-DNAcomplex and its implications on the diverse binding specificityof winged helixforkhead proteinsrdquo Journal of Biological Chem-istry vol 281 no 25 pp 17400ndash17409 2006
[89] A H Mao N Lyle and R V Pappu ldquoDescribing sequence-ensemble relationships for intrinsically disordered proteinsrdquoBiochemical Journal vol 449 no 2 pp 307ndash318 2013
[90] R Wetzel ldquoPhysical chemistry of polyglutamine Intriguingtales of a monotonous sequencerdquo Journal of Molecular Biologyvol 421 no 4-5 pp 466ndash490 2012
[91] H T Orr ldquoPolyglutamine neurodegeneration expanded glu-tamines enhance native functionsrdquo Current Opinion in Geneticsand Development vol 22 no 3 pp 251ndash255 2012
[92] M Figiel W J Szlachcic P M Switonski A Gabka and W JKrzyzosiak ldquoMouse models of polyglutamine diseases reviewand data table Part Irdquo Molecular Neurobiology vol 46 no 2pp 393ndash429 2012
[93] J Nasica-Labouze and N Mousseau ldquoKinetics of amyloidaggregation a study of the GNNQQNY prion sequencerdquo PLoSComputational Biology vol 8 no 11 Article ID e1002782 2012
[94] J Nasica-Labouze M Meli P Derreumaux G Colombo andN Mousseau ldquoA multiscale approach to characterize the earlyaggregation steps of the amyloid-forming peptide gnnqqnyfrom the yeast prion sup-35rdquo PLoS Computational Biology vol7 no 5 Article ID e1002051 2011
[95] J R Lewandowski P C A van der Wel M Rigney N Grig-orieff and R G Griffin ldquoStructural complexity of a compositeamyloid fibrilrdquo Journal of the American Chemical Society vol133 no 37 pp 14686ndash14698 2011
BioMed Research International 5
M A S R G V N K V I L V G N L G Q D P E V R Y M P S G G A V A N F T L A T S E S W R D K Q T G
M A S R G V N K V I L V G N L G Q D P E V R Y M P S G G A V A N L T L A T S E S W R D K Q T G
M A - R G V N K V I L V G N V G G D P E T R Y M P N G N A V T N I T L A T S E S W K D K Q T G
K L A E V A G E Y L R K G S Q V Y I E G Q L R T R K W T D Q S G Q D K Y T T E V - V V N V G G
K L A E V A G E Y L R K G S Q V Y I E G Q L R T R K W T D Q S G Q E R Y T T E I N V P Q I G G
R L A E I A G E Y L R K G S Q V Y V E G S L R T R K W Q G Q D G Q D R Y T T E I - V V D I N G
G G G Q Q Q G G W G Q P Q Q P Q - - - G G N Q F S G G A Q S R P Q Q Q A P A A P S N E P P M D
G - G Q Q Q G G W G Q P Q Q P Q Q P Q G G N Q F S G G A Q S R P Q Q S A P - A P S N E P P M D
G - D D S Q R A P R E P M Q R P - - - - - - Q Q A P Q Q Q S R P A P Q Q Q P A P Q P A Q D Y D
E M K E Q T E W H R V V L F G
E M K E Q T E W H R V V M F G
Q Q Q E R T E W H R V V F F G
T M Q M L G G R Q G G G A P A
V M Q M L G G R Q G G G A P A
N M Q L L G G R - - - - - P S
- F D D D I P F
- F D D D I P F
S F D D D I P F
62
62
61
123
124
120
174176
165
KpSSB
StSSB
PaSSB
KpSSB
StSSB
PaSSB
KpSSB
StSSB
PaSSB
Figure 2 Multiple amino acid sequence alignment of SSB proteins Sequence alignment of KpSSB StSSB and PaSSB was generated byCLUSTALW2 Identical amino acid residues are colored in red Gly and Gln residues are shaded in cyan and gray The N-terminal domainsof these SSBs are significantly conserved
(kDa)
40-
35-
25-
15-
M 1 2 3 4 5
Figure 3 Protein purity Coomassie Blue-stained SDS-PAGE (15)of the purified KpSSB (lane 1) StSSB (lane 2) PaSSB (lane 3)KpSSBnStSSBc (lane 4) KpSSBnPaSSBc (lane 5) and molecularmass standards (M) are shown The sizes of the standard proteinsfrom the top down are as follows 55 40 35 25 15 and 10 kDa Thepurified SSBs migrated between the 25 and 15 kDa standards on theSDS-PAGE
[43] and PaSSB [42] was determined to be 26plusmn 1 22plusmn 1 and29plusmn1 nt respectivelyThe longer the length of the polypeptidechain the smaller the size for ssDNA binding Analysis ofthe primary structures of KpSSB StSSB and PaSSB by RPS-BLAST revealed the presence of a putative OB-fold domainthat is common to all known SSBs Figure 2 shows that thealignments of the amino acid sequences of KpSSB StSSB andPaSSB amino acid residues in their N-terminal domains arehighly conserved (colored in red) In the E coli SSB-ssDNAcomplex [11] four essential aromatic residues namely Trp40Trp54 Phe60 and Trp88 participate in ssDNA binding viastacking interactions [11] These residues are conserved inmost SSB families including KpSSB StSSB and PaSSB Theimportantmotif in the C-terminal tail of E coli SSB DDDIPFresidues is also conserved in KpSSB StSSB and PaSSB By
contrast to those motifs the residues found in the glycine-rich hinge of E coli SSB are not conserved in KpSSB StSSBand PaSSB (Figure 2) Thus the length and composition ofthe amino acid residues in the glycine-rich hinge may beresponsible for the different ssDNA-binding site sizes of SSBs
32 Expression and Purification of KpSSB StSSB and PaSSBThe N-terminal ssDNA-binding domain of SSB has beenwell-established to be highly conserved However SSBspossessing different ssDNA-binding site sizes have beenreported The reason that SSBs have similar ssDNA-bindingdomains but possess varying ssDNA-binding site sizesremains unclear Although the ssDNA-binding site sizes ofKpSSB StSSB and PaSSB have been reported we reinves-tigated the ssDNA-binding properties of KpSSB StSSB andPaSSB in the absence of a His tag to avoid the unknown effectof a His tag (hexahistidine) on the ssDNA binding of SSB
33 KpSSB Bound to ssDNA To investigate the length ofnucleotides sufficient for the formation of the KpSSB-ssDNAcomplex and the ssDNA-binding ability of KpSSB westudied the binding of KpSSB to dT20 (Figure 4(a)) dT25(Figure 4(b)) dT35 (Figure 4(c)) dT45 (Figure 4(d)) dT50(Figure 4(e)) dT55 (Figure 4(f)) and dT60 (Figure 4(g))with different protein concentrations As shown inFigure 4(a) no band shift was observed when KpSSBwas incubated with dT20 indicating that KpSSB couldnot form a stable complex with this homopolymer Bycontrast to dT20 longer dT homopolymers which includedT25ndash50 produced a significant band shift (C complex)that is formation of a stable protein-DNA complex insolution Furthermore two different complexes for dT55were formed by KpSSB (Figure 4(f)) At lower proteinconcentrations KpSSB formed a single complex (C1) withdT55 similar to that observed with dT50 (Figure 4(e))However when the KpSSB concentration was increasedanother slower migrating complex (C2) was observed
6 BioMed Research International
[KpSSB]
-dT20
mdash
(a)
-dT25
-C
mdash[KpSSB]
(b)
-dT35
-C
mdash[KpSSB]
(c)
-dT45
-C
mdash[KpSSB]
(d)
-dT50
-C
[KpSSB]
mdash
(e)
-dT55
-C1-C2
[KpSSB]mdash
(f)
-dT60
-C1-C2
[KpSSB]mdash
(g)
Figure 4 Binding of KpSSB to dT20ndash60 KpSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT20 (b) dT25 (c) dT35 (d) dT45 (e) dT50 (f) dT55 or (g) dT60 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
Two different complexes of KpSSB were also observedto bind to dT60 (Figure 4(g)) The appearance of thesecond complex resulted from the increased KpSSB con-centration suggesting that two KpSSB proteins maybe present per oligonucleotide Although dT55 is only 5 ntlonger thandT50 is the presence of an extra 5 nt in dT55 com-pared with that of dT50 provides enough interaction spacefor the binding of two KpSSB proteins Therefore one KpSSB
occupies 25 (502 = 25) nt to 275 (552 = 275) nt of thessDNA The EMSA results suggest that the length of anssDNA (or the binding site size) [52] required for KpSSBbinding is 26 plusmn 2 nt
34 StSSB Bound to ssDNA The binding of StSSB to dT15(Figure 5(a)) dT20 (Figure 5(b)) dT30 (Figure 5(c)) dT40(Figure 5(d)) dT45 (Figure 5(e)) and dT50 (Figure 5(f)) was
BioMed Research International 7
[StSSB]
-dT15
-C
mdash
(a)
-dT20
-C
---
-
mdash[StSSB]
(b)
-dT30
-C
[StSSB]mdash
(c)
-dT40
-C
mdash[StSSB]
(d)
-dT45
-C1-C2
mdash[StSSB]
(e)
-dT50
-C1-C2
mdash
[StSSB]
(f)
Figure 5 Binding of StSSB to dT15ndash50 StSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT15 (b) dT20 (c) dT30 (d) dT40 (e) dT45 or (f) dT50 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and 100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blue and40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
examined using EMSA StSSB can bind and form a singlecomplex with dT15 (Figure 5(a)) and dT20 (Figure 5(b)) butKpSSB cannot (Figure 4(a)) StSSB bound to dT15ndash40 andformed a single complex For dT45 and dT50 two differentcomplexes of StSSB appeared at high protein concentrations(Figures 5(e) and 5(f)) Therefore one StSSB occupies 20(402 = 20) nt to 225 (452 = 225) nt of the ssDNA TheEMSA results suggest that the length of an ssDNA (or thebinding site size) [52] required for StSSB binding is 21 plusmn 2 nt
35 PaSSB Bound to ssDNA The binding of PaSSB to dT20(Figure 6(a)) dT25 (Figure 6(b)) dT35 (Figure 6(c)) dT45(Figure 6(d)) dT55 (Figure 6(e)) dT60 (Figure 6(f)) anddT65 (Figure 6(g)) was studied by EMSA Unlike StSSB nocomplexwas observedwhen PaSSBwas incubatedwith dT20Some smears were observed indicating that PaSSB interactswith dT20 However the ssDNA may be too short to befully wrapped by PaSSB PaSSB could form a single complexwith dT25ndash55 and form two distinct complexes with dT60
and dT65 (Figures 6(f) and 6(g)) respectivelyTherefore onePaSSB occupies 275 (552 = 275) nt to 30 (602 = 30) ntof the ssDNA These results from EMSA suggest that thelength of an ssDNA (or the binding site size) [52] required forPaSSB binding is 29plusmn2 nt Although the SSBs that is KpSSBStSSB and PaSSB have significantly similar ssDNA-bindingdomains their binding site sizes are different and range from19 (21 plusmn 2 StSSB) to 31 (29 plusmn 2 PaSSB) nt The obtainedEMSA results (Figures 4ndash6) also show that the binding sitesizes of the untagged SSBs (KpSSB StSSB and PaSSB) werefound to be almost identical to those of the His-tagged ones[40 42 43]
36 Design of the Chimeric KpSSB Proteins KpSSBnStSSBc andKpSSBnPaSSBc The N-terminal ssDNA-binding domain ofKpSSB StSSB and PaSSB is highly conserved (Figure 2) buttheir binding site sizes are different (Figures 4ndash6) and rangefrom 19 nt to 31 nt The C-terminal acidic tails DDDIPFare conserved (Figure 2) and these features led us to assess
8 BioMed Research International
[PaSSB]
-dT20
mdash
(a)
-dT25
-C
[PaSSB]mdash
(b)
-dT35
-C
-
-
[PaSSB]
(c)
-dT45
-C
[PaSSB]
(d)
-dT55
-C
[PaSSB]
(e)
-dT60
-C1-C2
[PaSSB]
(f)
-dT65
-C1-C2
[PaSSB]
(g)
Figure 6 Binding of PaSSB to dT20ndash65 PaSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT20 (b) dT25 (c) dT35 (d) dT45 (e) dT55 (f) dT60 or (g) dT65 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
whether the flexible glycine-rich hinge in the C-terminaldomain which is not conserved among SSBs is involved inthe determination of the binding site size of SSB Thus theC-terminal domains of StSSB and PaSSB were swapped withKpSSB through protein chimeragenesis
37 KpSSBnStSSBc Bound to ssDNA The binding ofKpSSBnStSSBc to dT15 (Figure 7(a)) dT20 (Figure 7(b))dT40 (Figure 7(c)) and dT45 (Figure 7(d)) was examinedusing EMSA KpSSBnStSSBc exhibited significantly different
ssDNA-binding properties from those of KpSSB UnlikeKpSSB (Figure 4) both KpSSBnStSSBc (Figure 8) and StSSB(Figure 5) can bind and form a single complex with dT15 anddT20 Similar to StSSB KpSSBnStSSBc binds to dT15ndash40 andforms a single complex For dT45 two different complexesof KpSSBnStSSBc appeared at high protein concentrations(Figure 8(d)) this EMSA feature was also similar to that ofStSSB One KpSSBnStSSBc occupies 20 (402 = 20) nt to225 (452 = 225) nt of the ssDNA These EMSA resultssuggest that the length of an ssDNA (or the binding site
BioMed Research International 9
[KpSSBnStSSBc]
-dT15
-C
(a)
[KpSSBnStSSBc]
-dT20
-C
(b)
[KpSSBnStSSBc]
-dT40
-C
(c)
[KpSSBnStSSBc]
-dT45
-C1-C2
(d)
Figure 7 Binding of KpSSBnStSSBc to dT15ndash45 KpSSBnStSSBc (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for30min at 25∘C with 17 nM of (a) dT15 (b) dT20 (c) dT40 or (d) dT45 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and 100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blue and40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
size) [52] required for KpSSBnStSSBc binding is 21 plusmn 2 nt avalue identical to that for StSSB (Figure 5) Swapping of theC-terminal domain of StSSB with KpSSB changes the size ofthe ssDNA-binding site from 26 nt to 21 nt
38 KpSSBnPaSSBc Bound to ssDNA The binding fea-tures of KpSSBnPaSSBc with dT20 (Figure 8(a)) dT25(Figure 8(b)) dT40 (Figure 8(c)) dT55 (Figure 8(d)) anddT60 (Figure 8(e)) were studied by EMSA Similar to thecases of KpSSB and PaSSB no complex was observed whenKpSSBnPaSSBc was incubated with dT20 However KpSSB-nPaSSBc still exhibited dramatically different ssDNA-bindingproperties from those of KpSSB KpSSB can form two distinctcomplexes with dT55 (Figure 4(f)) but both KpSSBnPaSSBc(Figure 9) and PaSSB (Figure 6) cannot One KpSSBnPaSSBcoccupies 275 (552 = 275) nt to 30 (602 = 30) nt ofthe ssDNA The above EMSA results suggest that the lengthof an ssDNA (or the binding site size) [52] required forKpSSBnPaSSBc binding is 29 plusmn 2 nt a value identical to thatof PaSSB Swapping of the C-terminal domain of PaSSB toKpSSB changes the size of the ssDNA-binding site from 26 ntto 29 nt Although these SSBs namely KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc have nearly identicalssDNA-binding domains their binding site sizes are different(Table 2) Thus the size of the ssDNA-binding site requiredfor second SSB binding is likely to be dependent on the C-terminal domain of SSB
39 Binding Constants of the SSB-ssDNA Complexes Deter-mined from EMSA To compare the ssDNA-binding abil-ities of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc the midpoint values for input ssDNA bind-ing calculated from the titration curves of EMSA andreferred to as [Protein]
50(monomer) were quantified and
are summarized in Table 2 Although the N-terminal ssDNA-binding domains of these SSB proteins are highly similar(Figure 2) their ssDNA-binding activities and binding sitesizes are different (Table 2) [KpSSB]
50values ranged from
100 nM to 220 nM [StSSB]50
values ranged from 420 nM to650 nM [PaSSB]
50values ranged from 550 nM to 1700 nM
[KpSSBnStSSBc]50values ranged from 110 nM to 260 nM and
[KpSSBnPaSSBc]50
values ranged from 220 nM to 390 nMThe ssDNA-binding ability is as follows in the order ofdecreasing affinity KpSSB gt KpSSBnStSSBc gt KpSSBn-PaSSBc gt StSSB gt PaSSB Results from the above analysesindicate that the exchange of the C-terminal domain inSSB significantly changed the ssDNA-binding ability and theDNA-binding behavior (complex number) The reason asto why swapping of the C-terminal domain can affect thessDNA-binding activity of SSB remains unclear The C-ter-minal domain of SSB is suggested to be involved in ssDNAbinding However this relation is not evident in the results ofthe cocrystal structure
310 Oligomeric State of KpSSBnStSSBc and KpSSBnPaSSBc inSolution Gel-filtration chromatography was used to confirm
10 BioMed Research International
[KpSSBnPaSSBc]
-dT20
(a)
[KpSSBnPaSSBc]
-dT25
-C
(b)
[KpSSBnPaSSBc]
-C
-dT40
(c)
-C
[KpSSBnPaSSBc]
-dT55
(d)
-dT60
-C1-C2
[KpSSBnPaSSBc]
(e)
Figure 8 Binding of KpSSBnPaSSBc to dT20ndash60 KpSSBnPaSSBc (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for30min at 25∘C with 17 nM of (a) dT20 (b) dT25 (c) dT40 (d) dT55 or (e) dT60 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
that the oligomeric state of KpSSBnStSSBc and KpSSBn-PaSSBc remains as tetramers after chimeragenesisThe analy-sis of purified KpSSBnStSSBc and KpSSBnPaSSBc (2mgmL)using a Superdex 200HR 1030 column revealed a singlepeak with elution volumes of 786 and 789mL respectivelyAssuming that KpSSBnStSSBc and KpSSBnPaSSBc both haveshapes and partial specific volumes similar to the standardproteins the native molecular masses of KpSSBnStSSBc andKpSSBnPaSSBc were estimated to be 76641 and 74827Da ascalculated from a standard linear regression equation 119870av =minus03684(logMw) + 22707 (Figure 9) The native molecularmasses for KpSSBnStSSBc and KpSSBnPaSSBc are approx-imately four times the mass of the monomer (sim19 kDa)Therefore KpSSBnStSSBc and KpSSBnPaSSBc under theabove chromatographic conditions are stable tetramers insolution Although the exchange of the C-terminal domainin SSB significantly changed the ssDNA-binding ability andDNA-binding behavior (complex number) protein chimera-genesis did not cause any change in the oligomeric state ofSSB
311 Summary of Gly Gln and Pro Number in SSBs To ana-lyze the C-terminal amino acid composition of SSBs wefurther counted the number of Gly Gln and Pro residuesin different SSB segments SSB is abundant in Gly Glnand Pro (GQP) (Table 3) The GQP contents of KpSSB1ndash91 StSSB1ndash91 and PaSSB1ndash90 are similar However the Glynumber of PaSSB116ndash165 is significantly lower than that ofKpSSB116ndash174 and StSSB117ndash176 PaSSB116ndash165 contains only1 Gly but KpSSB116ndash174 and StSSB117ndash176 contain 11 and 12Gly respectively In addition we found different distributionpatterns among KpSSB StSSB and PaSSB Although theycontain similar number of Gln (Q) the QQQ pattern isfrequently found in PaSSB (Table 3)
312 Structural Modeling of SSBs Given its disordered C-ter-minal domain the crystal structure of the full-length SSB islacking even when SSB can be crystallized with DNA [69]We attempted to model the structure by homology mod-eling using the bioinformatics program (PS)2 to obtain an
BioMed Research International 11
Table 2 ssDNA binding properties of KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc as analyzed by EMSA
Protein DNA [Protein]50 (nM) Complex number
KpSSB
dT20 ND 0dT25 200 plusmn 20 1dT35 220 plusmn 30 1dT45 100 plusmn 10 1dT50 110 plusmn 20 1dT55 100 plusmn 20 2dT60 100 plusmn 10 2
StSSB
dT15 650 plusmn 120 1dT20 450 plusmn 80 1dT30 420 plusmn 60 1dT40 420 plusmn 80 1dT45 440 plusmn 60 2dT50 440 plusmn 50 2
PaSSB
dT20 ND 0dT25 1700 plusmn 250 1dT35 950 plusmn 180 1dT45 780 plusmn 160 1dT55 820 plusmn 90 1dT60 810 plusmn 110 2dT65 550 plusmn 70 2
KpSSBnStSSBc
dT15 260 plusmn 60 1dT20 110 plusmn 20 1dT40 120 plusmn 20 1dT45 160 plusmn 20 2
KpSSBnPaSSBc
dT20 ND 0dT25 390 plusmn 60 1dT40 220 plusmn 30 1dT55 230 plusmn 30 1dT60 230 plusmn 30 2
[Protein]50 was calculated from the titration curves of EMSA by determiningthe concentration of the protein (120583M) needed to achieve the midpointvalue for input ssDNA binding For some oligonucleotides input ssDNAbinding was the sum of the intensities from the two separate ssDNA-proteincomplexes Errors are standard deviations determined by three independenttitration experiments
Table 3 Summary of Gly Gln and Pro number in SSB
SSB segment G Q PKpSSB1ndash91 10 5 2StSSB1ndash91 10 5 2PaSSB1ndash90 11 6 2KpSSB92ndash174 18 16 9StSSB92ndash176 17 18 11PaSSB91ndash165 5 15 11KpSSB116ndash174 11 12 9StSSB117ndash176 12 13 10PaSSB116ndash165 1 12 11
in-depth understanding of the structure-function relation-ship of the C-terminal domains of these SSBs [70 71]
40 45 50 55
Kav
00
02
04
06
08
10
KpSSBnStSSBcKpSSBnPaSSBc
Y = minus03684X + 22707
R2= 0998
log Mw
Figure 9Gel-filtration chromatographic analyses of KpSSBnStSSBcand KpSSBnPaSSBc Purified protein (2mgmL) was applied toa Superdex 200HR 1030 column (GE Healthcare Bio-SciencesPiscataway NJ USA) equilibrated with Buffer D The column wasoperated at a flow rate of 05mLmin and 05mL fractions werecollected The proteins were detected by measuring the absorbanceat 280 nm The column was calibrated with proteins of knownmolecular weight thyroglobulin (670 kDa) 120574-globulin (158 kDa)ovalbumin (44 kDa) and myoglobin (17 kDa) The 119870av values forthe standard proteins and the SSB variants were calculated from theequation119870av = (119881119890minus119881119900)(119881119888minus119881119900) where119881119900 is column void volume119881119890is elution volume and 119881
119888is geometric column volume
(PS)2 (http140113239111simps2v2docsphp) is an auto-matic homology modeling server that combines bothsequence and secondary structure information to detect thehomologous proteins with remote similarity and the target-template alignment After pasting the amino acid sequenceto the website of (PS)2 only one hit (Protein Data Bankentry 1QVC EcSSB) for the C-terminal domains of KpSSBand StSSB was suggested For the C-terminal domain ofPaSSB only one hit that is CstF-77 (ProteinData Bank entry2OOE cleavage stimulation factor CstF) but not EcSSB wassuggested as the template for modeling Figure 10 shows thatmodeled structures of these SSB C-terminal domains arehighly disordered but that of PaSSB is more ordered than thatof other domains
4 Discussion
In this study we examined the sizes of the binding site ofthe untagged SSB and the chimeric SSB from the ubiquitousopportunistic pathogens K pneumoniae S enterica serovarTyphimurium LT2 and P aeruginosa PAO1 Many clinicalstrains of the abovementioned bacteria are highly resistantto antibiotics [72ndash75] The development of clinically usefulsmall-molecule antibiotics has been a seminal event in thefield of infectious diseases [48] Nucleic acid metabolismis one of the most basic biological functions and shouldbe a prime target in antibiotic development [76ndash78] Manybacterial SSBs form conserved protein interaction ldquohubsrdquothat are essential to recruit many proteins involved in DNA
12 BioMed Research International
Figure 10 Structure modeling of SSB The structures of KpSSB1ndash115 StSSB1ndash115 and PaSSB1ndash115 (the N-terminal domain of SSB)were modeled by SWISS-MODEL The structures of KpSSB116ndash142 StSSB116ndash142 and PaSSB121ndash160 (the C-terminal domain ofSSB) were modeled by (PS)2 Other regions of SSBs could not bemodeled by these two programs The structures of the N-terminaldomain and the C-terminal domain of these SSBs were manuallylinked (KpSSB1ndash142 blue StSSB1ndash142 pink PaSSB1ndash160 green) andsuperimposed with the crystal structure of EcSSB1ndash142 (orange)(PDB entry 1QVC) for comparison For clarity only one subunitof the tetramer was shown for each SSB
replication recombination and repair SSBDNA nucleopro-tein substrates [79] Thus SSBs may be promising targets inantibiotic development [80] As a first step toward achievingthis goal we investigated why SSBs possess highly conservedN-terminal ssDNA-binding domain but exhibit varying bind-ing site sizes One significant clue is that their flexible hingesand the length at the C-terminus are different as revealed bysequence alignment (Figure 2)
The interactions of various SSBs with ssDNA have beenanalyzed using a variety of techniques such as tryptophan-fluorescence quenching [47] filter binding [81] EMSA [5282] analytical ultracentrifugation [83] electron microscopy[84] nuclease digestion [44] single-molecule fluorescencemicroscopy [48] and crystallographic analyses [11] In thisstudy we have examined the electrophoretic mobility shiftpatterns of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc bound to different lengths of ssDNA and deter-mined the corresponding binding site sizes to be 26 2129 22 and 29 nt per tetramer respectively (Figures 4ndash8) PaSSB and KpSSBnPaSSBc have the largest sizes forssDNA binding among the SSBs studied We also identifiedHis-tagged and untagged SSBs that have similar ssDNA-binding site sizes [40 42 43] EMSA is a well-establishedapproach in studies of molecular biology [52] and the use ofradioactive tracer in this assay allows detection of the actualformation of the distinct protein-DNA complex(es) [53] For
exampleDNase protection assay and footprinting assay usingradioactive tracer can determine the specific DNA sequencecomplexed by a protein In EMSA when the length of thenucleotides is sufficient for the binding of two or more SSBmolecules the electrophoretic mobility of the higher SSBoligomer complex will be lower than that of the smaller SSBoligomer complex [52 54] In addition results of the ssDNA-binding site size from EMSA and cocrystal structure of SSBwere consistent [27] Thus throughout this paper we deter-mined the ssDNA-binding site sizes of SSB from the EMSAbehavior
Many SSBs bind to ssDNA with some degree of positivecooperativity Cooperativity can result from direct protein-protein interactions between the nearest neighbors such asthe LAST motif in the T4 gene-32 protein [85] and thearginine-mediated interaction motif in Thermus SSB [8687] Cooperativity can also result from the protein-induceddistortion of adjacent DNA as demonstrated in SulfolobusSSB PriB and FOXK1a proteins [23 60 88] In the cases ofKpSSB StSSB and PaSSB (Figures 4ndash6) binding appeared tobe nearly noncooperative for several DNAs because all DNAmainly shifts into the first complex (C1) before the appearanceof the second complex (C2) when subjected to increasingprotein concentrationsThe length dependence of the [SSB]
50
values suggests that the amount of spacing is optimum forsteric considerations (Table 2)
Because bacteria have varying genomic DNA sizes theirSSBs may need to evolve to have different binding site sizesfor DNA metabolism Results from protein chimeragenesisshowed the C-terminal domain dependence of the bindingsite sizes of SSB (Figure 11) The experimental data showedthat the binding site size of KpSSBnStSSBc was similar to thatof StSSB and the size of the binding site of KpSSBnPaSSBcwas similar to that of PaSSB The reason for which the bind-ing site size of SSB changed followed by swapping of theC-terminal domain remains unclear Flexibility numberof glycine residues andor different QQQ patterns of theC-terminal domain of SSB (Figure 2 and Table 3) may beimportant factors for determining the ssDNA-binding sitesize In fact the C-terminal domain of PaSSB that isPaSSB116ndash165 has only 1 Gly residue which is significantlyless than that of KpSSB (11 Gly) and StSSB (12Gly) Gly (andPro) is an important component of the flexible region aprotein that contains low Gly content is predicted to havelow flexibility Unlike typical SSB [35 69] PaSSB116ndash165 hasa partial structure (Figure 10) Although KpSSB StSSB andPaSSB contain similar number of Gln (Q) the QQQ patternis frequently found in PaSSB (Figure 2 and Table 3) PolyQand repeated sequences GAGAG are commonly found inthe structures of amyloids silk fibers and neurodegradationproteins [89ndash92] Considering that the simple coil polyQ theheptapeptide GNNQQNY and the hexapeptide NNQQNYcan cause protein aggregation and nucleation [93ndash95] thedistribution of Gln in the C-terminal domain of a tetramericSSB may also be an important determinant of the ssDNA-binding site size of SSB by some steric hindrances (Figure 11)However the above speculationmust be confirmed by furtherbiochemical experiments
BioMed Research International 13
PaSSBStSSB KpSSB
C-terC-ter C-ter
sim 21 nt sim 26 nt sim 29 nt
Figure 11 Possiblemodels for explainingwhy SSBs arewith different binding site sizes Twomodeled structures of KpSSB1ndash142 (blue) StSSB1ndash142 (pink) and PaSSB1ndash160 (green) complexed with ssDNA (gold) are shown For clarity only one C-terminal domain was shown for eachSSB tetramer By using the electrophoretic mobility shift assay and the protein chimeragenesis we characterized that the binding site sizes ofKpSSB StSSB PaSSB KpSSBnStSSBc and KpSSBnPaSSBc were 26 21 29 21 and 29 nt per tetramer respectively KpSSB StSSB and PaSSBare similar proteins whose N-terminal ssDNA-binding domains are almost identical Thus the C-terminal domain of SSB may indirectlycontribute to ssDNA binding and wrapping and affects the binding site size by the steric hindrance
5 Conclusion
In this study we characterized the ssDNA-binding propertiesof untagged SSBs from K pneumoniae S enterica sero-var Typhimurium LT2 and P aeruginosa PAO1 and proposeda role of the C-terminal flexible domain for ssDNA bind-ing from the protein chimeragenesis and EMSA resultsThe amino acid sequence of the N-terminal ssDNA-bind-ingoligomerization domain in these pathogenic SSBs ishighly conserved but their apparent binding site sizes aredifferent This finding indicates that the C-terminal protein-protein interaction domain may also indirectly contribute tossDNA binding and wrapping
Conflict of Interests
The authors declare that there is no conflict of interestsregarding the publication of this paper
Acknowledgment
This researchwas supported by aGrant from theNational Sci-ence Council Taiwan (NSC 102-2320-B-040-019 to Cheng-Yang Huang)
References
[1] R Reyes-Lamothe D J Sherratt and M C Leake ldquoStoichiom-etry and architecture of active DNA replication machinery inescherichia colirdquo Science vol 328 no 5977 pp 498ndash501 2010
[2] D J Richard E Bolderson andK K Khanna ldquoMultiple humansingle-stranded DNA binding proteins function in genomemaintenance structural biochemical and functional analysisrdquo
Critical Reviews in Biochemistry and Molecular Biology vol 44no 2-3 pp 98ndash116 2009
[3] R D Shereda A G Kozlov T M LohmanMM Cox and J LKeck ldquoSSB as an organizermobilizer of genome maintenancecomplexesrdquo Critical Reviews in Biochemistry and MolecularBiology vol 43 no 5 pp 289ndash318 2008
[4] D J Richard E Bolderson L Cubeddu et al ldquoSingle-strandedDNA-binding protein hSSB1 is critical for genomic stabilityrdquoNature vol 453 no 7195 pp 677ndash681 2008
[5] R R Meyer and P S Laine ldquoThe single-stranded DNA-bindingprotein of Escherichia colirdquoMicrobiological Reviews vol 54 no4 pp 342ndash380 1990
[6] C Yang U Curth C Urbanke and C Kang ldquoCrystal structureof human mitochondrial single-stranded DNA binding proteinat 24 A resolutionrdquo Nature Structural Biology vol 4 no 2 pp153ndash157 1997
[7] G Webster J Genschel U Curth C Urbanke C Kang and RHilgenfeld ldquoA common core for binding single-stranded DNAstructural comparison of the single-stranded DNA-bindingproteins (SSB) from E coli and human mitochondriardquo FEBSLetters vol 411 pp 313ndash316 1997
[8] R L Flynn and L Zou ldquoOligonucleotideoligosaccharide-bind-ing fold proteins a growing family of genome guardiansrdquo Crit-ical Reviews in Biochemistry and Molecular Biology vol 45 no4 pp 266ndash275 2010
[9] K Chan Y Lee CWangHHuang andY Sun ldquoSingle-Strand-ed DNA-Binding Protein Complex from Helicobacter pyloriSuggests an ssDNA-Binding Surfacerdquo Journal of MolecularBiology vol 388 no 3 pp 508ndash519 2009
[10] D L Theobald R M Mitton-Fry and D S Wuttke ldquoNucleicacid recognition by OB-fold proteinsrdquo Annual Review of Bio-physics and Biomolecular Structure vol 32 pp 115ndash133 2003
[11] S Raghunathan A G Kozlov TM Lohman andGWaksmanldquoStructure of the DNA binding domain of E coli SSB bound to
14 BioMed Research International
ssDNArdquo Nature Structural Biology vol 7 no 8 pp 648ndash6522000
[12] A G Murzin ldquoOB(oligonucleotideoligosaccharide binding)-fold common structural and functional solution for non-homologous sequencesrdquo EMBO Journal vol 12 no 3 pp 861ndash867 1993
[13] N P George K V Ngo S Chitteni-Pattu et al ldquoStructure andcellular dynamics of deinococcus radiodurans single-strandedDNA (ssDNA)-binding protein (SSB)-DNA complexesrdquo TheJournal of Biological Chemistry vol 287 no 26 pp 22123ndash221322012
[14] P Filipkowski and J Kur ldquoIdentification and propertiesof the Deinococcus grandis and Deinococcus proteolyticussingle-stranded DNA binding proteins (SSB)rdquo Acta BiochimicaPolonica vol 54 no 1 pp 79ndash87 2007
[15] P Filipkowski A Duraj-Thatte and J Kur ldquoIdentificationcloning expression and characterization of a highly ther-mostable single-stranded-DNA-binding protein (SSB) fromDeinococcus murrayirdquo Protein Expression and Purification vol53 no 1 pp 201ndash208 2007
[16] P Filipkowski M Koziatek and J Kur ldquoA highly ther-mostable homodimeric single-stranded DNA-binding proteinfrom Deinococcus radiopugnansrdquo Extremophiles vol 10 no 6pp 607ndash614 2006
[17] P Filipkowski A Duraj-Thatte and J Kur ldquoNovel thermostablesingle-stranded DNA-binding protein (SSB) fromDeinococcusgeothermalisrdquo Archives of Microbiology vol 186 no 2 pp 129ndash137 2006
[18] G Witte C Urbanke and U Curth ldquoSingle-stranded DNA-binding protein of Deinococcus radiodurans a biophysicalcharacterizationrdquoNucleic Acids Research vol 33 no 5 pp 1662ndash1670 2005
[19] D A Bernstein J M Eggingtont M P Killoran A M MisicMMCox and J L Keck ldquoCrystal structure of theDeinococcusradiodurans single-stranded DNA-binding protein suggests amechanism for coping with DNA damagerdquo Proceedings of theNational Academy of Sciences of the United States of Americavol 101 no 23 pp 8575ndash8580 2004
[20] S Dabrowski M Olszewski R Piatek A Brillowska-Dabrowska G Konopa and J Kur ldquoIdentification and charac-terization of single-stranded-DNA-binding proteins fromTher-mus thermophilus and Thermus aquaticusmdashnew arrange-ment of binding domainsrdquo Microbiology vol 148 no 10 pp3307ndash3315 2002
[21] R Gamsjaeger R Kariawasam C Touma A H Kwan M FWhite and L Cubeddu ldquoBackbone and side-chain 1H 13C and15N resonance assignments of the OB domain of the singlestranded DNA binding protein from Sulfolobus solfataricusand chemical shift mapping of the DNA-binding interfacerdquoBiomolecular NMR Assignments 2013
[22] M L Rolfsmeier and C A Haseltine ldquoThe single-strandedDNA binding protein of Sulfolobus solfataricus acts in the pre-synaptic step of homologous recombinationrdquo Journal of Molec-ular Biology vol 397 no 1 pp 31ndash45 2010
[23] I D Kerr R I M Wadsworth L Cubeddu W Blankenfeldt JHNaismith andM FWhite ldquoInsights into ssDNA recognitionby the OB fold from a structural and thermodynamic study ofSulfolobus SSB proteinrdquo EMBO Journal vol 22 no 11 pp 2561ndash2570 2003
[24] C A Haseltine and S C Kowalczykowski ldquoA distinctive single-stranded DNA-binding protein from the Archaeon Sulfolobussolfataricusrdquo Molecular Microbiology vol 43 no 6 pp 1505ndash1515 2002
[25] R I M Wadsworth and M F White ldquoIdentification andproperties of the crenarchaeal single-stranded DNA bindingprotein from Sulfolobus solfataricusrdquo Nucleic Acids Researchvol 29 no 4 pp 914ndash920 2001
[26] S Sugiman-Marangos and M S Junop ldquoThe structure of DdrBfrom Deinococcus a new fold for single-stranded DNA bindingproteinsrdquo Nucleic Acids Research vol 38 no 10 Article IDgkq036 pp 3432ndash3440 2010
[27] H Ghalei H V Moeller D Eppers et al ldquoEntrapment of DNAin an intersubunit tunnel system of a single-stranded DNA-binding proteinrdquo Nucleic Acids Research vol 42 no 10 pp6698ndash6708 2014
[28] S Paytubi S A McMahon S Graham et al ldquoDisplacement ofthe canonical single-strandedDNA-binding protein in the ther-moprotealesrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 109 no 7 pp E398ndashE405 2012
[29] T H Dickey S E Altschuler and D S Wuttke ldquoSingle-stranded DNA-binding proteins multiple domains for multiplefunctionsrdquo Structure vol 21 no 7 pp 1074ndash1084 2013
[30] D Vujaklija and BMacek ldquoDetecting posttranslational modifi-cations of bacterial SSB proteinsrdquoMethods inMolecular Biologyvol 922 pp 205ndash218 2012
[31] I Mijakovic D Petranovic B Macek et al ldquoBacterial single-stranded DNA-binding proteins are phosphorylated on tyro-sinerdquoNucleic Acids Research vol 34 no 5 pp 1588ndash1596 2006
[32] M Olszewski A Grot M Wojciechowski M Nowak MMickiewicz and J Kur ldquoCharacterization of exceptionally ther-mostable single-strandedDNA-binding proteins fromThermo-toga maritima and Thermotoga neapolitanardquo BMC Microbiol-ogy vol 10 article 260 2010
[33] U Curth J Genschel C Urbanke and J Greipel ldquoIn vitro andin vivo function of the C-terminus of Escherichia coil single-stranded DNA binding proteinrdquoNucleic Acids Research vol 24no 14 pp 2706ndash2711 1996
[34] G Witte C Urbanke and U Curth ldquoDNA polymerase IIIchi subunit ties single-stranded DNA binding protein to thebacterial replication machineryrdquoNucleic Acids Research vol 31pp 4434ndash4440 2003
[35] D Shishmarev YWang C EMason et al ldquoOtting Intramolec-ular binding mode of the C-terminus of Escherichia coli single-strandedDNAbinding protein determined by nuclearmagneticresonance spectroscopyrdquo Nucleic Acids Research vol 42 pp2750ndash2757 2014
[36] A G Kozlov M M Cox and T M Lohman ldquoRegulation ofsingle-stranded DNA binding by the C termini of Escherichiacoli single-stranded DNA-binding (SSB) proteinrdquo Journal ofBiological Chemistry vol 285 no 22 pp 17246ndash17252 2010
[37] M Nowak M Olszewski M Spibida and J Kur ldquoChar-acterization of single-stranded DNA-binding proteins fromthe psychrophilic bacteria Desulfotalea psychrophila Flavobac-terium psychrophilum Psychrobacter arcticus Psychrobactercryohalolentis Psychromonas ingrahamii Psychroflexus torquisand Photobacterium profundumrdquo BMC Microbiology vol 14article 91 2014
BioMed Research International 15
[38] T Paradzik N Ivic Z Filic et al ldquoStructure-function relation-ships of two paralogous single-stranded DNA-binding proteinsfrom Streptomyces coelicolor implication of SsbB in chromo-some segregation during sporulationrdquo Nucleic Acids Researchvol 41 no 6 pp 3659ndash3672 2013
[39] S JainM Zweig E Peeters et al ldquoCharacterization of the singlestrandedDNA binding protein SsbB encoded in the gonoccocalgenetic islandrdquo PLoS ONE vol 7 no 4 Article ID e35285 2012
[40] Y H Huang and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from Klebsiella pneumoniaemutation at either Arg73 or Ser76 causes a less cooperativecomplex onDNArdquoGenes to Cells vol 17 no 2 pp 146ndash157 2012
[41] E Antony E A Weiland S Korolev and T M LohmanldquoPlasmodium falciparum SSB tetramer wraps single-strandedDNAwith similar topology but opposite polarity to E Coli SSBrdquoJournal ofMolecular Biology vol 420 no 4-5 pp 269ndash283 2012
[42] H Jan Y L Lee and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from pseudomonas aeruginosaPAO1rdquo Protein Journal vol 30 no 1 pp 20ndash26 2011
[43] Y-H Huang Y-L Lee and C-Y Huang ldquoCharacterization of asingle-stranded DNA binding protein from Salmonella entericaserovar typhimurium LT2rdquo Protein Journal vol 30 no 2 pp102ndash108 2011
[44] S K Bharti K Rex P Sreedhar N Krishnan and U VarshneyldquoChimeras of Escherichia coli and Mycobacterium tuberculosissingle-stranded DNA binding proteins characterization andfunction in Escherichia colirdquo PLoS ONE vol 6 no 12 ArticleID e27216 2011
[45] M T Oliveira and L S Kaguni ldquoFunctional roles of the N-and C-terminal regions of the human mitochondrial single-stranded DNA-binding proteinrdquo PLoS ONE vol 5 no 10Article ID e15379 2010
[46] A G Kozlov J M Eggington M M Cox and T MLohman ldquoBinding of the dimeric Deinococcus radioduranssingle-strandedDNA binding protein to single-strandedDNArdquoBiochemistry vol 49 no 38 pp 8266ndash8275 2010
[47] T M Lohman and M E Ferrari ldquoEscherichia coli single-stranded DNA-binding protein multiple DNA-binding modesand cooperativesrdquo Annual Review of Biochemistry vol 63 pp527ndash570 1994
[48] R Zhou A G Kozlov R Roy et al ldquoSSB functions as a slidingplatform that migrates on DNA via reptationrdquo Cell vol 146 pp222ndash232 2011
[49] R Roy A G Kozlov T M Lohman and T Ha ldquoSSB proteindiffusion on single-stranded DNA stimulates RecA filamentformationrdquo Nature vol 461 pp 1092ndash1097 2009
[50] R Roy A G Kozlov T M Lohman and T Ha ldquoDynamicstructural rearrangements between DNA binding modes of Ecoli SSB proteinrdquo Journal of Molecular Biology vol 369 no 5pp 1244ndash1257 2007
[51] T J Kelly P Simancek and G S Brush ldquoIdentification andcharacterization of a single-stranded DNA-binding proteinfrom the archaeon Methanococcus jannaschiirdquo Proceedings ofthe National Academy of Sciences of the United States of Americavol 95 no 25 pp 14634ndash14639 1998
[52] C Y Huang Determination of the Binding Site-Size of theProtein-DNA Complex by Use of the Electrophoretic MobilityShift Assay InTech Press Rijeka Croatia 2012
[53] A Kornberg ldquoTen commandments of enzymology amendedrdquoTrends in Biochemical Sciences vol 28 no 10 pp 515ndash517 2003
[54] W Zhang X Lu and J Shen ldquoEMSA and single-moleculeforce spectroscopy study of interactions between bacillus sub-tilis single-stranded DNA-binding protein and single-strandedDNArdquo Langmuir vol 27 no 24 pp 15008ndash15015 2011
[55] M Ostermeier and S J Benkovic ldquoEvolution of protein func-tion by domain swappingrdquo Advances in Protein Chemistry vol55 pp 29ndash77 2000
[56] W F Peng and C Y Huang ldquoAllantoinase and dihydroorotasebinding and inhibition by flavonols and the substrates of cyclicamidohydrolasesrdquo Biochimie vol 101 pp 113ndash122 2014
[57] Y H Huang M J Lin and C Y Huang ldquoDnaT is a single-stranded DNA binding proteinrdquo Genes to Cells vol 18 no 11pp 1007ndash1019 2013
[58] Y Y Ho Y H Huang and C Y Huang ldquoChemical rescue of thepost-translationally carboxylated lysine mutant of allantoinaseand dihydroorotase by metal ions and short-chain carboxylicacidsrdquo Amino Acids vol 44 no 4 pp 1181ndash1191 2013
[59] Y H Huang Y Lo W Huang and C Y Huang ldquoCrystalstructure and DNA-binding mode of Klebsiella pneumoniaeprimosomal PriB proteinrdquoGenes to Cells vol 17 no 10 pp 837ndash849 2012
[60] C Y Huang C Y Hsu Y Sun H Wu and C Hsiao ldquoCom-plexed crystal structure of replication restart primosome pro-tein PriB reveals a novel single-stranded DNA-binding moderdquoNucleic Acids Research vol 34 no 14 pp 3878ndash3886 2006
[61] T Kinebuchi H Shindo H Nagai N Shimamoto andM Shimizu ldquoFunctional domains of Escherichia coli single-stranded DNA binding protein as assessed by analyses of thedeletion mutantsrdquo Biochemistry vol 36 no 22 pp 6732ndash67381997
[62] N Shimamoto N Ikushima H Utiyama H Tachibana andK Horie ldquoSpecific and cooperative binding of E coli single-stranded DNA binding protein to mRNArdquo Nucleic AcidsResearch vol 15 no 13 pp 5241ndash5250 1987
[63] H Lin andC YHuang ldquoCharacterization of flavonol inhibitionof DnaB helicase Real-time monitoring structural modelingand proposed mechanismrdquo Journal of Biomedicine and Biotech-nology vol 2012 Article ID 735368 2012
[64] Y H Huang H H Lin and C Y Huang ldquoA single residuedetermines the cooperative binding property of a primosomalDNA replication protein PriB to single-stranded DNArdquo Bio-science Biotechnology and Biochemistry vol 76 no 6 pp 1110ndash1115 2012
[65] H C Hsieh and C Y Huang ldquoIdentification of a novel proteinPriB in Klebsiella pneumoniaerdquo Biochemical and BiophysicalResearch Communications vol 404 no 1 pp 546ndash551 2011
[66] J H Liu T W Chang C Y Huang et al ldquoCrystal structure ofPriB a primosomalDNA replication protein ofEscherichia colirdquoThe Journal of Biological Chemistry vol 279 no 48 pp 50465ndash50471 2004
[67] Y H Huang and C Y Huang ldquoThe N-terminal domain ofDnaT a primosomalDNA replication protein is crucial for PriBbinding and self-trimerizationrdquo Biochemical and BiophysicalResearch Communications vol 442 pp 147ndash152 2013
[68] M A Larkin G Blackshields N P Brown et al ldquoClustalW andClustal X version 20rdquo Bioinformatics vol 23 no 21 pp 2947ndash2948 2007
16 BioMed Research International
[69] S N Savvides S Raghunathan K Futterer A G Kozlov T MLohman and G Waksman ldquoThe C-terminal domain of full-length E coli SSB is disordered even when bound to DNArdquoProtein Science vol 13 no 7 pp 1942ndash1947 2004
[70] C-C Chen J-K Hwang and J-M Yang ldquo(PS)2-v2 template-based protein structure prediction serverrdquo BMC Bioinformaticsvol 10 article 366 2009
[71] C Chen J Hwang and J Yang ldquo(PS)2 protein structure pre-diction serverrdquoNucleic Acids Research vol 34 pp W152ndashW1572006
[72] K Bush and J F Fisher ldquoEpidemiological expansion structuralstudies and clinical challenges of new 120573-lactamases from gram-negative bacteriardquo Annual Review of Microbiology vol 65 pp455ndash478 2011
[73] K K Kumarasamy M A Toleman T R Walsh et al ldquoEmer-gence of a new antibiotic resistance mechanism in India Pak-istan and the UK a molecular biological and epidemiologicalstudyrdquoThe Lancet Infectious Diseases vol 10 no 9 pp 597ndash6022010
[74] K Bush and M J MacIelag ldquoNew 120573-lactam antibiotics and 120573-lactamase inhibitorsrdquo Expert Opinion on Therapeutic Patentsvol 20 no 10 pp 1277ndash1293 2010
[75] K Bush ldquoAlarming 120573-lactamase-mediated resistance in multi-drug-resistant Enterobacteriaceaerdquo Current Opinion in Microbi-ology vol 13 no 5 pp 558ndash564 2010
[76] A Srivastava M Talaue S Liu et al ldquoNew target for inhibitionof bacterial RNA polymerase ldquoswitch regionrdquordquo Current Opinionin Microbiology vol 14 no 5 pp 532ndash543 2011
[77] K Chono K Katsumata T Kontani et al ldquoASP2151 a novelhelicase-primase inhibitor possesses antiviral activity againstvaricella-zoster virus and herpes simplex virus types 1 and 2rdquoJournal of Antimicrobial Chemotherapy vol 65 no 8 pp 1733ndash1741 2010
[78] M T Black and K Coleman ldquoNew inhibitors of bacterial topoi-somerase GyrAParC subunitsrdquo Current Opinion in Investiga-tional Drugs vol 10 no 8 pp 804ndash810 2009
[79] A H Marceau D A Bernstein B W Walsh W Shapiro LA Simmons and J L Keck ldquoProtein interactions in genomemaintenance as novel antibacterial targetsrdquo PLoS ONE vol 8no 3 Article ID e58765 2013
[80] D Lu D A Bernstein K A Satyshur and J L Keck ldquoSmall-molecule tools for dissecting the roles of SSBprotein inter-actions in genome maintenancerdquo Proceedings of the NationalAcademy of Sciences of the United States of America vol 107 no2 pp 633ndash638 2010
[81] I Wong and T M Lohman ldquoA double-filter method fornitrocellulose-filter binding application to protein-nucleic acidinteractionsrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 90 no 12 pp 5428ndash5432 1993
[82] M Mitas J Y Chock and M Christy ldquoThe binding-site sizesof Escherichia coli single-stranded-DNA-binding protein andmammalian replication protein A are 65 and ge 54 nucleotidesrespectivelyrdquo Biochemical Journal vol 324 no 3 pp 957ndash9611997
[83] C Urbanke G Witte and U Curth ldquoSedimentation velocitymethod in the analytical ultracentrifuge for the study of protein-protein interactionsrdquoMethods inMolecular Biology vol 305 pp101ndash114 2005
[84] S Chrysogelos and J Griffith ldquoEscherichia coli single-strandbinding protein organizes single-strandedDNA innucleosome-like unitsrdquo Proceedings of the National Academy of Sciences of theUnited States of America vol 79 no 19 pp 5803ndash5807 1982
[85] J R Casas-Finet K R Fischer and R L Karpel ldquoStructuralbasis for the nucleic acid binding cooperativity of bacteriophageT4 gene 32 protein the (LysArg)3(SerThr)2 (LAST) motifrdquoProceedings of the National Academy of Sciences of the UnitedStates of America vol 89 pp 1050ndash1054 1992
[86] G Witte R Fedorov and U Curth ldquoBiophysical analysis ofThermus aquaticus single-stranded DNA binding proteinrdquo Bio-physical Journal vol 94 no 6 pp 2269ndash2279 2008
[87] R Fedorov G Witte C Urbanke D J Manstein and UCurth ldquo3D structure of Thermus aquaticus single-strandedDNA-binding protein gives insight into the functioning of SSBproteinsrdquo Nucleic Acids Research vol 34 no 22 pp 6708ndash67172006
[88] K L Tsai C Y Huang C H Chang Y J Sun W J ChuangandC DHsiao ldquoCrystal structure of the human FOXK1a-DNAcomplex and its implications on the diverse binding specificityof winged helixforkhead proteinsrdquo Journal of Biological Chem-istry vol 281 no 25 pp 17400ndash17409 2006
[89] A H Mao N Lyle and R V Pappu ldquoDescribing sequence-ensemble relationships for intrinsically disordered proteinsrdquoBiochemical Journal vol 449 no 2 pp 307ndash318 2013
[90] R Wetzel ldquoPhysical chemistry of polyglutamine Intriguingtales of a monotonous sequencerdquo Journal of Molecular Biologyvol 421 no 4-5 pp 466ndash490 2012
[91] H T Orr ldquoPolyglutamine neurodegeneration expanded glu-tamines enhance native functionsrdquo Current Opinion in Geneticsand Development vol 22 no 3 pp 251ndash255 2012
[92] M Figiel W J Szlachcic P M Switonski A Gabka and W JKrzyzosiak ldquoMouse models of polyglutamine diseases reviewand data table Part Irdquo Molecular Neurobiology vol 46 no 2pp 393ndash429 2012
[93] J Nasica-Labouze and N Mousseau ldquoKinetics of amyloidaggregation a study of the GNNQQNY prion sequencerdquo PLoSComputational Biology vol 8 no 11 Article ID e1002782 2012
[94] J Nasica-Labouze M Meli P Derreumaux G Colombo andN Mousseau ldquoA multiscale approach to characterize the earlyaggregation steps of the amyloid-forming peptide gnnqqnyfrom the yeast prion sup-35rdquo PLoS Computational Biology vol7 no 5 Article ID e1002051 2011
[95] J R Lewandowski P C A van der Wel M Rigney N Grig-orieff and R G Griffin ldquoStructural complexity of a compositeamyloid fibrilrdquo Journal of the American Chemical Society vol133 no 37 pp 14686ndash14698 2011
6 BioMed Research International
[KpSSB]
-dT20
mdash
(a)
-dT25
-C
mdash[KpSSB]
(b)
-dT35
-C
mdash[KpSSB]
(c)
-dT45
-C
mdash[KpSSB]
(d)
-dT50
-C
[KpSSB]
mdash
(e)
-dT55
-C1-C2
[KpSSB]mdash
(f)
-dT60
-C1-C2
[KpSSB]mdash
(g)
Figure 4 Binding of KpSSB to dT20ndash60 KpSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT20 (b) dT25 (c) dT35 (d) dT45 (e) dT50 (f) dT55 or (g) dT60 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
Two different complexes of KpSSB were also observedto bind to dT60 (Figure 4(g)) The appearance of thesecond complex resulted from the increased KpSSB con-centration suggesting that two KpSSB proteins maybe present per oligonucleotide Although dT55 is only 5 ntlonger thandT50 is the presence of an extra 5 nt in dT55 com-pared with that of dT50 provides enough interaction spacefor the binding of two KpSSB proteins Therefore one KpSSB
occupies 25 (502 = 25) nt to 275 (552 = 275) nt of thessDNA The EMSA results suggest that the length of anssDNA (or the binding site size) [52] required for KpSSBbinding is 26 plusmn 2 nt
34 StSSB Bound to ssDNA The binding of StSSB to dT15(Figure 5(a)) dT20 (Figure 5(b)) dT30 (Figure 5(c)) dT40(Figure 5(d)) dT45 (Figure 5(e)) and dT50 (Figure 5(f)) was
BioMed Research International 7
[StSSB]
-dT15
-C
mdash
(a)
-dT20
-C
---
-
mdash[StSSB]
(b)
-dT30
-C
[StSSB]mdash
(c)
-dT40
-C
mdash[StSSB]
(d)
-dT45
-C1-C2
mdash[StSSB]
(e)
-dT50
-C1-C2
mdash
[StSSB]
(f)
Figure 5 Binding of StSSB to dT15ndash50 StSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT15 (b) dT20 (c) dT30 (d) dT40 (e) dT45 or (f) dT50 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and 100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blue and40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
examined using EMSA StSSB can bind and form a singlecomplex with dT15 (Figure 5(a)) and dT20 (Figure 5(b)) butKpSSB cannot (Figure 4(a)) StSSB bound to dT15ndash40 andformed a single complex For dT45 and dT50 two differentcomplexes of StSSB appeared at high protein concentrations(Figures 5(e) and 5(f)) Therefore one StSSB occupies 20(402 = 20) nt to 225 (452 = 225) nt of the ssDNA TheEMSA results suggest that the length of an ssDNA (or thebinding site size) [52] required for StSSB binding is 21 plusmn 2 nt
35 PaSSB Bound to ssDNA The binding of PaSSB to dT20(Figure 6(a)) dT25 (Figure 6(b)) dT35 (Figure 6(c)) dT45(Figure 6(d)) dT55 (Figure 6(e)) dT60 (Figure 6(f)) anddT65 (Figure 6(g)) was studied by EMSA Unlike StSSB nocomplexwas observedwhen PaSSBwas incubatedwith dT20Some smears were observed indicating that PaSSB interactswith dT20 However the ssDNA may be too short to befully wrapped by PaSSB PaSSB could form a single complexwith dT25ndash55 and form two distinct complexes with dT60
and dT65 (Figures 6(f) and 6(g)) respectivelyTherefore onePaSSB occupies 275 (552 = 275) nt to 30 (602 = 30) ntof the ssDNA These results from EMSA suggest that thelength of an ssDNA (or the binding site size) [52] required forPaSSB binding is 29plusmn2 nt Although the SSBs that is KpSSBStSSB and PaSSB have significantly similar ssDNA-bindingdomains their binding site sizes are different and range from19 (21 plusmn 2 StSSB) to 31 (29 plusmn 2 PaSSB) nt The obtainedEMSA results (Figures 4ndash6) also show that the binding sitesizes of the untagged SSBs (KpSSB StSSB and PaSSB) werefound to be almost identical to those of the His-tagged ones[40 42 43]
36 Design of the Chimeric KpSSB Proteins KpSSBnStSSBc andKpSSBnPaSSBc The N-terminal ssDNA-binding domain ofKpSSB StSSB and PaSSB is highly conserved (Figure 2) buttheir binding site sizes are different (Figures 4ndash6) and rangefrom 19 nt to 31 nt The C-terminal acidic tails DDDIPFare conserved (Figure 2) and these features led us to assess
8 BioMed Research International
[PaSSB]
-dT20
mdash
(a)
-dT25
-C
[PaSSB]mdash
(b)
-dT35
-C
-
-
[PaSSB]
(c)
-dT45
-C
[PaSSB]
(d)
-dT55
-C
[PaSSB]
(e)
-dT60
-C1-C2
[PaSSB]
(f)
-dT65
-C1-C2
[PaSSB]
(g)
Figure 6 Binding of PaSSB to dT20ndash65 PaSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT20 (b) dT25 (c) dT35 (d) dT45 (e) dT55 (f) dT60 or (g) dT65 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
whether the flexible glycine-rich hinge in the C-terminaldomain which is not conserved among SSBs is involved inthe determination of the binding site size of SSB Thus theC-terminal domains of StSSB and PaSSB were swapped withKpSSB through protein chimeragenesis
37 KpSSBnStSSBc Bound to ssDNA The binding ofKpSSBnStSSBc to dT15 (Figure 7(a)) dT20 (Figure 7(b))dT40 (Figure 7(c)) and dT45 (Figure 7(d)) was examinedusing EMSA KpSSBnStSSBc exhibited significantly different
ssDNA-binding properties from those of KpSSB UnlikeKpSSB (Figure 4) both KpSSBnStSSBc (Figure 8) and StSSB(Figure 5) can bind and form a single complex with dT15 anddT20 Similar to StSSB KpSSBnStSSBc binds to dT15ndash40 andforms a single complex For dT45 two different complexesof KpSSBnStSSBc appeared at high protein concentrations(Figure 8(d)) this EMSA feature was also similar to that ofStSSB One KpSSBnStSSBc occupies 20 (402 = 20) nt to225 (452 = 225) nt of the ssDNA These EMSA resultssuggest that the length of an ssDNA (or the binding site
BioMed Research International 9
[KpSSBnStSSBc]
-dT15
-C
(a)
[KpSSBnStSSBc]
-dT20
-C
(b)
[KpSSBnStSSBc]
-dT40
-C
(c)
[KpSSBnStSSBc]
-dT45
-C1-C2
(d)
Figure 7 Binding of KpSSBnStSSBc to dT15ndash45 KpSSBnStSSBc (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for30min at 25∘C with 17 nM of (a) dT15 (b) dT20 (c) dT40 or (d) dT45 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and 100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blue and40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
size) [52] required for KpSSBnStSSBc binding is 21 plusmn 2 nt avalue identical to that for StSSB (Figure 5) Swapping of theC-terminal domain of StSSB with KpSSB changes the size ofthe ssDNA-binding site from 26 nt to 21 nt
38 KpSSBnPaSSBc Bound to ssDNA The binding fea-tures of KpSSBnPaSSBc with dT20 (Figure 8(a)) dT25(Figure 8(b)) dT40 (Figure 8(c)) dT55 (Figure 8(d)) anddT60 (Figure 8(e)) were studied by EMSA Similar to thecases of KpSSB and PaSSB no complex was observed whenKpSSBnPaSSBc was incubated with dT20 However KpSSB-nPaSSBc still exhibited dramatically different ssDNA-bindingproperties from those of KpSSB KpSSB can form two distinctcomplexes with dT55 (Figure 4(f)) but both KpSSBnPaSSBc(Figure 9) and PaSSB (Figure 6) cannot One KpSSBnPaSSBcoccupies 275 (552 = 275) nt to 30 (602 = 30) nt ofthe ssDNA The above EMSA results suggest that the lengthof an ssDNA (or the binding site size) [52] required forKpSSBnPaSSBc binding is 29 plusmn 2 nt a value identical to thatof PaSSB Swapping of the C-terminal domain of PaSSB toKpSSB changes the size of the ssDNA-binding site from 26 ntto 29 nt Although these SSBs namely KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc have nearly identicalssDNA-binding domains their binding site sizes are different(Table 2) Thus the size of the ssDNA-binding site requiredfor second SSB binding is likely to be dependent on the C-terminal domain of SSB
39 Binding Constants of the SSB-ssDNA Complexes Deter-mined from EMSA To compare the ssDNA-binding abil-ities of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc the midpoint values for input ssDNA bind-ing calculated from the titration curves of EMSA andreferred to as [Protein]
50(monomer) were quantified and
are summarized in Table 2 Although the N-terminal ssDNA-binding domains of these SSB proteins are highly similar(Figure 2) their ssDNA-binding activities and binding sitesizes are different (Table 2) [KpSSB]
50values ranged from
100 nM to 220 nM [StSSB]50
values ranged from 420 nM to650 nM [PaSSB]
50values ranged from 550 nM to 1700 nM
[KpSSBnStSSBc]50values ranged from 110 nM to 260 nM and
[KpSSBnPaSSBc]50
values ranged from 220 nM to 390 nMThe ssDNA-binding ability is as follows in the order ofdecreasing affinity KpSSB gt KpSSBnStSSBc gt KpSSBn-PaSSBc gt StSSB gt PaSSB Results from the above analysesindicate that the exchange of the C-terminal domain inSSB significantly changed the ssDNA-binding ability and theDNA-binding behavior (complex number) The reason asto why swapping of the C-terminal domain can affect thessDNA-binding activity of SSB remains unclear The C-ter-minal domain of SSB is suggested to be involved in ssDNAbinding However this relation is not evident in the results ofthe cocrystal structure
310 Oligomeric State of KpSSBnStSSBc and KpSSBnPaSSBc inSolution Gel-filtration chromatography was used to confirm
10 BioMed Research International
[KpSSBnPaSSBc]
-dT20
(a)
[KpSSBnPaSSBc]
-dT25
-C
(b)
[KpSSBnPaSSBc]
-C
-dT40
(c)
-C
[KpSSBnPaSSBc]
-dT55
(d)
-dT60
-C1-C2
[KpSSBnPaSSBc]
(e)
Figure 8 Binding of KpSSBnPaSSBc to dT20ndash60 KpSSBnPaSSBc (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for30min at 25∘C with 17 nM of (a) dT20 (b) dT25 (c) dT40 (d) dT55 or (e) dT60 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
that the oligomeric state of KpSSBnStSSBc and KpSSBn-PaSSBc remains as tetramers after chimeragenesisThe analy-sis of purified KpSSBnStSSBc and KpSSBnPaSSBc (2mgmL)using a Superdex 200HR 1030 column revealed a singlepeak with elution volumes of 786 and 789mL respectivelyAssuming that KpSSBnStSSBc and KpSSBnPaSSBc both haveshapes and partial specific volumes similar to the standardproteins the native molecular masses of KpSSBnStSSBc andKpSSBnPaSSBc were estimated to be 76641 and 74827Da ascalculated from a standard linear regression equation 119870av =minus03684(logMw) + 22707 (Figure 9) The native molecularmasses for KpSSBnStSSBc and KpSSBnPaSSBc are approx-imately four times the mass of the monomer (sim19 kDa)Therefore KpSSBnStSSBc and KpSSBnPaSSBc under theabove chromatographic conditions are stable tetramers insolution Although the exchange of the C-terminal domainin SSB significantly changed the ssDNA-binding ability andDNA-binding behavior (complex number) protein chimera-genesis did not cause any change in the oligomeric state ofSSB
311 Summary of Gly Gln and Pro Number in SSBs To ana-lyze the C-terminal amino acid composition of SSBs wefurther counted the number of Gly Gln and Pro residuesin different SSB segments SSB is abundant in Gly Glnand Pro (GQP) (Table 3) The GQP contents of KpSSB1ndash91 StSSB1ndash91 and PaSSB1ndash90 are similar However the Glynumber of PaSSB116ndash165 is significantly lower than that ofKpSSB116ndash174 and StSSB117ndash176 PaSSB116ndash165 contains only1 Gly but KpSSB116ndash174 and StSSB117ndash176 contain 11 and 12Gly respectively In addition we found different distributionpatterns among KpSSB StSSB and PaSSB Although theycontain similar number of Gln (Q) the QQQ pattern isfrequently found in PaSSB (Table 3)
312 Structural Modeling of SSBs Given its disordered C-ter-minal domain the crystal structure of the full-length SSB islacking even when SSB can be crystallized with DNA [69]We attempted to model the structure by homology mod-eling using the bioinformatics program (PS)2 to obtain an
BioMed Research International 11
Table 2 ssDNA binding properties of KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc as analyzed by EMSA
Protein DNA [Protein]50 (nM) Complex number
KpSSB
dT20 ND 0dT25 200 plusmn 20 1dT35 220 plusmn 30 1dT45 100 plusmn 10 1dT50 110 plusmn 20 1dT55 100 plusmn 20 2dT60 100 plusmn 10 2
StSSB
dT15 650 plusmn 120 1dT20 450 plusmn 80 1dT30 420 plusmn 60 1dT40 420 plusmn 80 1dT45 440 plusmn 60 2dT50 440 plusmn 50 2
PaSSB
dT20 ND 0dT25 1700 plusmn 250 1dT35 950 plusmn 180 1dT45 780 plusmn 160 1dT55 820 plusmn 90 1dT60 810 plusmn 110 2dT65 550 plusmn 70 2
KpSSBnStSSBc
dT15 260 plusmn 60 1dT20 110 plusmn 20 1dT40 120 plusmn 20 1dT45 160 plusmn 20 2
KpSSBnPaSSBc
dT20 ND 0dT25 390 plusmn 60 1dT40 220 plusmn 30 1dT55 230 plusmn 30 1dT60 230 plusmn 30 2
[Protein]50 was calculated from the titration curves of EMSA by determiningthe concentration of the protein (120583M) needed to achieve the midpointvalue for input ssDNA binding For some oligonucleotides input ssDNAbinding was the sum of the intensities from the two separate ssDNA-proteincomplexes Errors are standard deviations determined by three independenttitration experiments
Table 3 Summary of Gly Gln and Pro number in SSB
SSB segment G Q PKpSSB1ndash91 10 5 2StSSB1ndash91 10 5 2PaSSB1ndash90 11 6 2KpSSB92ndash174 18 16 9StSSB92ndash176 17 18 11PaSSB91ndash165 5 15 11KpSSB116ndash174 11 12 9StSSB117ndash176 12 13 10PaSSB116ndash165 1 12 11
in-depth understanding of the structure-function relation-ship of the C-terminal domains of these SSBs [70 71]
40 45 50 55
Kav
00
02
04
06
08
10
KpSSBnStSSBcKpSSBnPaSSBc
Y = minus03684X + 22707
R2= 0998
log Mw
Figure 9Gel-filtration chromatographic analyses of KpSSBnStSSBcand KpSSBnPaSSBc Purified protein (2mgmL) was applied toa Superdex 200HR 1030 column (GE Healthcare Bio-SciencesPiscataway NJ USA) equilibrated with Buffer D The column wasoperated at a flow rate of 05mLmin and 05mL fractions werecollected The proteins were detected by measuring the absorbanceat 280 nm The column was calibrated with proteins of knownmolecular weight thyroglobulin (670 kDa) 120574-globulin (158 kDa)ovalbumin (44 kDa) and myoglobin (17 kDa) The 119870av values forthe standard proteins and the SSB variants were calculated from theequation119870av = (119881119890minus119881119900)(119881119888minus119881119900) where119881119900 is column void volume119881119890is elution volume and 119881
119888is geometric column volume
(PS)2 (http140113239111simps2v2docsphp) is an auto-matic homology modeling server that combines bothsequence and secondary structure information to detect thehomologous proteins with remote similarity and the target-template alignment After pasting the amino acid sequenceto the website of (PS)2 only one hit (Protein Data Bankentry 1QVC EcSSB) for the C-terminal domains of KpSSBand StSSB was suggested For the C-terminal domain ofPaSSB only one hit that is CstF-77 (ProteinData Bank entry2OOE cleavage stimulation factor CstF) but not EcSSB wassuggested as the template for modeling Figure 10 shows thatmodeled structures of these SSB C-terminal domains arehighly disordered but that of PaSSB is more ordered than thatof other domains
4 Discussion
In this study we examined the sizes of the binding site ofthe untagged SSB and the chimeric SSB from the ubiquitousopportunistic pathogens K pneumoniae S enterica serovarTyphimurium LT2 and P aeruginosa PAO1 Many clinicalstrains of the abovementioned bacteria are highly resistantto antibiotics [72ndash75] The development of clinically usefulsmall-molecule antibiotics has been a seminal event in thefield of infectious diseases [48] Nucleic acid metabolismis one of the most basic biological functions and shouldbe a prime target in antibiotic development [76ndash78] Manybacterial SSBs form conserved protein interaction ldquohubsrdquothat are essential to recruit many proteins involved in DNA
12 BioMed Research International
Figure 10 Structure modeling of SSB The structures of KpSSB1ndash115 StSSB1ndash115 and PaSSB1ndash115 (the N-terminal domain of SSB)were modeled by SWISS-MODEL The structures of KpSSB116ndash142 StSSB116ndash142 and PaSSB121ndash160 (the C-terminal domain ofSSB) were modeled by (PS)2 Other regions of SSBs could not bemodeled by these two programs The structures of the N-terminaldomain and the C-terminal domain of these SSBs were manuallylinked (KpSSB1ndash142 blue StSSB1ndash142 pink PaSSB1ndash160 green) andsuperimposed with the crystal structure of EcSSB1ndash142 (orange)(PDB entry 1QVC) for comparison For clarity only one subunitof the tetramer was shown for each SSB
replication recombination and repair SSBDNA nucleopro-tein substrates [79] Thus SSBs may be promising targets inantibiotic development [80] As a first step toward achievingthis goal we investigated why SSBs possess highly conservedN-terminal ssDNA-binding domain but exhibit varying bind-ing site sizes One significant clue is that their flexible hingesand the length at the C-terminus are different as revealed bysequence alignment (Figure 2)
The interactions of various SSBs with ssDNA have beenanalyzed using a variety of techniques such as tryptophan-fluorescence quenching [47] filter binding [81] EMSA [5282] analytical ultracentrifugation [83] electron microscopy[84] nuclease digestion [44] single-molecule fluorescencemicroscopy [48] and crystallographic analyses [11] In thisstudy we have examined the electrophoretic mobility shiftpatterns of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc bound to different lengths of ssDNA and deter-mined the corresponding binding site sizes to be 26 2129 22 and 29 nt per tetramer respectively (Figures 4ndash8) PaSSB and KpSSBnPaSSBc have the largest sizes forssDNA binding among the SSBs studied We also identifiedHis-tagged and untagged SSBs that have similar ssDNA-binding site sizes [40 42 43] EMSA is a well-establishedapproach in studies of molecular biology [52] and the use ofradioactive tracer in this assay allows detection of the actualformation of the distinct protein-DNA complex(es) [53] For
exampleDNase protection assay and footprinting assay usingradioactive tracer can determine the specific DNA sequencecomplexed by a protein In EMSA when the length of thenucleotides is sufficient for the binding of two or more SSBmolecules the electrophoretic mobility of the higher SSBoligomer complex will be lower than that of the smaller SSBoligomer complex [52 54] In addition results of the ssDNA-binding site size from EMSA and cocrystal structure of SSBwere consistent [27] Thus throughout this paper we deter-mined the ssDNA-binding site sizes of SSB from the EMSAbehavior
Many SSBs bind to ssDNA with some degree of positivecooperativity Cooperativity can result from direct protein-protein interactions between the nearest neighbors such asthe LAST motif in the T4 gene-32 protein [85] and thearginine-mediated interaction motif in Thermus SSB [8687] Cooperativity can also result from the protein-induceddistortion of adjacent DNA as demonstrated in SulfolobusSSB PriB and FOXK1a proteins [23 60 88] In the cases ofKpSSB StSSB and PaSSB (Figures 4ndash6) binding appeared tobe nearly noncooperative for several DNAs because all DNAmainly shifts into the first complex (C1) before the appearanceof the second complex (C2) when subjected to increasingprotein concentrationsThe length dependence of the [SSB]
50
values suggests that the amount of spacing is optimum forsteric considerations (Table 2)
Because bacteria have varying genomic DNA sizes theirSSBs may need to evolve to have different binding site sizesfor DNA metabolism Results from protein chimeragenesisshowed the C-terminal domain dependence of the bindingsite sizes of SSB (Figure 11) The experimental data showedthat the binding site size of KpSSBnStSSBc was similar to thatof StSSB and the size of the binding site of KpSSBnPaSSBcwas similar to that of PaSSB The reason for which the bind-ing site size of SSB changed followed by swapping of theC-terminal domain remains unclear Flexibility numberof glycine residues andor different QQQ patterns of theC-terminal domain of SSB (Figure 2 and Table 3) may beimportant factors for determining the ssDNA-binding sitesize In fact the C-terminal domain of PaSSB that isPaSSB116ndash165 has only 1 Gly residue which is significantlyless than that of KpSSB (11 Gly) and StSSB (12Gly) Gly (andPro) is an important component of the flexible region aprotein that contains low Gly content is predicted to havelow flexibility Unlike typical SSB [35 69] PaSSB116ndash165 hasa partial structure (Figure 10) Although KpSSB StSSB andPaSSB contain similar number of Gln (Q) the QQQ patternis frequently found in PaSSB (Figure 2 and Table 3) PolyQand repeated sequences GAGAG are commonly found inthe structures of amyloids silk fibers and neurodegradationproteins [89ndash92] Considering that the simple coil polyQ theheptapeptide GNNQQNY and the hexapeptide NNQQNYcan cause protein aggregation and nucleation [93ndash95] thedistribution of Gln in the C-terminal domain of a tetramericSSB may also be an important determinant of the ssDNA-binding site size of SSB by some steric hindrances (Figure 11)However the above speculationmust be confirmed by furtherbiochemical experiments
BioMed Research International 13
PaSSBStSSB KpSSB
C-terC-ter C-ter
sim 21 nt sim 26 nt sim 29 nt
Figure 11 Possiblemodels for explainingwhy SSBs arewith different binding site sizes Twomodeled structures of KpSSB1ndash142 (blue) StSSB1ndash142 (pink) and PaSSB1ndash160 (green) complexed with ssDNA (gold) are shown For clarity only one C-terminal domain was shown for eachSSB tetramer By using the electrophoretic mobility shift assay and the protein chimeragenesis we characterized that the binding site sizes ofKpSSB StSSB PaSSB KpSSBnStSSBc and KpSSBnPaSSBc were 26 21 29 21 and 29 nt per tetramer respectively KpSSB StSSB and PaSSBare similar proteins whose N-terminal ssDNA-binding domains are almost identical Thus the C-terminal domain of SSB may indirectlycontribute to ssDNA binding and wrapping and affects the binding site size by the steric hindrance
5 Conclusion
In this study we characterized the ssDNA-binding propertiesof untagged SSBs from K pneumoniae S enterica sero-var Typhimurium LT2 and P aeruginosa PAO1 and proposeda role of the C-terminal flexible domain for ssDNA bind-ing from the protein chimeragenesis and EMSA resultsThe amino acid sequence of the N-terminal ssDNA-bind-ingoligomerization domain in these pathogenic SSBs ishighly conserved but their apparent binding site sizes aredifferent This finding indicates that the C-terminal protein-protein interaction domain may also indirectly contribute tossDNA binding and wrapping
Conflict of Interests
The authors declare that there is no conflict of interestsregarding the publication of this paper
Acknowledgment
This researchwas supported by aGrant from theNational Sci-ence Council Taiwan (NSC 102-2320-B-040-019 to Cheng-Yang Huang)
References
[1] R Reyes-Lamothe D J Sherratt and M C Leake ldquoStoichiom-etry and architecture of active DNA replication machinery inescherichia colirdquo Science vol 328 no 5977 pp 498ndash501 2010
[2] D J Richard E Bolderson andK K Khanna ldquoMultiple humansingle-stranded DNA binding proteins function in genomemaintenance structural biochemical and functional analysisrdquo
Critical Reviews in Biochemistry and Molecular Biology vol 44no 2-3 pp 98ndash116 2009
[3] R D Shereda A G Kozlov T M LohmanMM Cox and J LKeck ldquoSSB as an organizermobilizer of genome maintenancecomplexesrdquo Critical Reviews in Biochemistry and MolecularBiology vol 43 no 5 pp 289ndash318 2008
[4] D J Richard E Bolderson L Cubeddu et al ldquoSingle-strandedDNA-binding protein hSSB1 is critical for genomic stabilityrdquoNature vol 453 no 7195 pp 677ndash681 2008
[5] R R Meyer and P S Laine ldquoThe single-stranded DNA-bindingprotein of Escherichia colirdquoMicrobiological Reviews vol 54 no4 pp 342ndash380 1990
[6] C Yang U Curth C Urbanke and C Kang ldquoCrystal structureof human mitochondrial single-stranded DNA binding proteinat 24 A resolutionrdquo Nature Structural Biology vol 4 no 2 pp153ndash157 1997
[7] G Webster J Genschel U Curth C Urbanke C Kang and RHilgenfeld ldquoA common core for binding single-stranded DNAstructural comparison of the single-stranded DNA-bindingproteins (SSB) from E coli and human mitochondriardquo FEBSLetters vol 411 pp 313ndash316 1997
[8] R L Flynn and L Zou ldquoOligonucleotideoligosaccharide-bind-ing fold proteins a growing family of genome guardiansrdquo Crit-ical Reviews in Biochemistry and Molecular Biology vol 45 no4 pp 266ndash275 2010
[9] K Chan Y Lee CWangHHuang andY Sun ldquoSingle-Strand-ed DNA-Binding Protein Complex from Helicobacter pyloriSuggests an ssDNA-Binding Surfacerdquo Journal of MolecularBiology vol 388 no 3 pp 508ndash519 2009
[10] D L Theobald R M Mitton-Fry and D S Wuttke ldquoNucleicacid recognition by OB-fold proteinsrdquo Annual Review of Bio-physics and Biomolecular Structure vol 32 pp 115ndash133 2003
[11] S Raghunathan A G Kozlov TM Lohman andGWaksmanldquoStructure of the DNA binding domain of E coli SSB bound to
14 BioMed Research International
ssDNArdquo Nature Structural Biology vol 7 no 8 pp 648ndash6522000
[12] A G Murzin ldquoOB(oligonucleotideoligosaccharide binding)-fold common structural and functional solution for non-homologous sequencesrdquo EMBO Journal vol 12 no 3 pp 861ndash867 1993
[13] N P George K V Ngo S Chitteni-Pattu et al ldquoStructure andcellular dynamics of deinococcus radiodurans single-strandedDNA (ssDNA)-binding protein (SSB)-DNA complexesrdquo TheJournal of Biological Chemistry vol 287 no 26 pp 22123ndash221322012
[14] P Filipkowski and J Kur ldquoIdentification and propertiesof the Deinococcus grandis and Deinococcus proteolyticussingle-stranded DNA binding proteins (SSB)rdquo Acta BiochimicaPolonica vol 54 no 1 pp 79ndash87 2007
[15] P Filipkowski A Duraj-Thatte and J Kur ldquoIdentificationcloning expression and characterization of a highly ther-mostable single-stranded-DNA-binding protein (SSB) fromDeinococcus murrayirdquo Protein Expression and Purification vol53 no 1 pp 201ndash208 2007
[16] P Filipkowski M Koziatek and J Kur ldquoA highly ther-mostable homodimeric single-stranded DNA-binding proteinfrom Deinococcus radiopugnansrdquo Extremophiles vol 10 no 6pp 607ndash614 2006
[17] P Filipkowski A Duraj-Thatte and J Kur ldquoNovel thermostablesingle-stranded DNA-binding protein (SSB) fromDeinococcusgeothermalisrdquo Archives of Microbiology vol 186 no 2 pp 129ndash137 2006
[18] G Witte C Urbanke and U Curth ldquoSingle-stranded DNA-binding protein of Deinococcus radiodurans a biophysicalcharacterizationrdquoNucleic Acids Research vol 33 no 5 pp 1662ndash1670 2005
[19] D A Bernstein J M Eggingtont M P Killoran A M MisicMMCox and J L Keck ldquoCrystal structure of theDeinococcusradiodurans single-stranded DNA-binding protein suggests amechanism for coping with DNA damagerdquo Proceedings of theNational Academy of Sciences of the United States of Americavol 101 no 23 pp 8575ndash8580 2004
[20] S Dabrowski M Olszewski R Piatek A Brillowska-Dabrowska G Konopa and J Kur ldquoIdentification and charac-terization of single-stranded-DNA-binding proteins fromTher-mus thermophilus and Thermus aquaticusmdashnew arrange-ment of binding domainsrdquo Microbiology vol 148 no 10 pp3307ndash3315 2002
[21] R Gamsjaeger R Kariawasam C Touma A H Kwan M FWhite and L Cubeddu ldquoBackbone and side-chain 1H 13C and15N resonance assignments of the OB domain of the singlestranded DNA binding protein from Sulfolobus solfataricusand chemical shift mapping of the DNA-binding interfacerdquoBiomolecular NMR Assignments 2013
[22] M L Rolfsmeier and C A Haseltine ldquoThe single-strandedDNA binding protein of Sulfolobus solfataricus acts in the pre-synaptic step of homologous recombinationrdquo Journal of Molec-ular Biology vol 397 no 1 pp 31ndash45 2010
[23] I D Kerr R I M Wadsworth L Cubeddu W Blankenfeldt JHNaismith andM FWhite ldquoInsights into ssDNA recognitionby the OB fold from a structural and thermodynamic study ofSulfolobus SSB proteinrdquo EMBO Journal vol 22 no 11 pp 2561ndash2570 2003
[24] C A Haseltine and S C Kowalczykowski ldquoA distinctive single-stranded DNA-binding protein from the Archaeon Sulfolobussolfataricusrdquo Molecular Microbiology vol 43 no 6 pp 1505ndash1515 2002
[25] R I M Wadsworth and M F White ldquoIdentification andproperties of the crenarchaeal single-stranded DNA bindingprotein from Sulfolobus solfataricusrdquo Nucleic Acids Researchvol 29 no 4 pp 914ndash920 2001
[26] S Sugiman-Marangos and M S Junop ldquoThe structure of DdrBfrom Deinococcus a new fold for single-stranded DNA bindingproteinsrdquo Nucleic Acids Research vol 38 no 10 Article IDgkq036 pp 3432ndash3440 2010
[27] H Ghalei H V Moeller D Eppers et al ldquoEntrapment of DNAin an intersubunit tunnel system of a single-stranded DNA-binding proteinrdquo Nucleic Acids Research vol 42 no 10 pp6698ndash6708 2014
[28] S Paytubi S A McMahon S Graham et al ldquoDisplacement ofthe canonical single-strandedDNA-binding protein in the ther-moprotealesrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 109 no 7 pp E398ndashE405 2012
[29] T H Dickey S E Altschuler and D S Wuttke ldquoSingle-stranded DNA-binding proteins multiple domains for multiplefunctionsrdquo Structure vol 21 no 7 pp 1074ndash1084 2013
[30] D Vujaklija and BMacek ldquoDetecting posttranslational modifi-cations of bacterial SSB proteinsrdquoMethods inMolecular Biologyvol 922 pp 205ndash218 2012
[31] I Mijakovic D Petranovic B Macek et al ldquoBacterial single-stranded DNA-binding proteins are phosphorylated on tyro-sinerdquoNucleic Acids Research vol 34 no 5 pp 1588ndash1596 2006
[32] M Olszewski A Grot M Wojciechowski M Nowak MMickiewicz and J Kur ldquoCharacterization of exceptionally ther-mostable single-strandedDNA-binding proteins fromThermo-toga maritima and Thermotoga neapolitanardquo BMC Microbiol-ogy vol 10 article 260 2010
[33] U Curth J Genschel C Urbanke and J Greipel ldquoIn vitro andin vivo function of the C-terminus of Escherichia coil single-stranded DNA binding proteinrdquoNucleic Acids Research vol 24no 14 pp 2706ndash2711 1996
[34] G Witte C Urbanke and U Curth ldquoDNA polymerase IIIchi subunit ties single-stranded DNA binding protein to thebacterial replication machineryrdquoNucleic Acids Research vol 31pp 4434ndash4440 2003
[35] D Shishmarev YWang C EMason et al ldquoOtting Intramolec-ular binding mode of the C-terminus of Escherichia coli single-strandedDNAbinding protein determined by nuclearmagneticresonance spectroscopyrdquo Nucleic Acids Research vol 42 pp2750ndash2757 2014
[36] A G Kozlov M M Cox and T M Lohman ldquoRegulation ofsingle-stranded DNA binding by the C termini of Escherichiacoli single-stranded DNA-binding (SSB) proteinrdquo Journal ofBiological Chemistry vol 285 no 22 pp 17246ndash17252 2010
[37] M Nowak M Olszewski M Spibida and J Kur ldquoChar-acterization of single-stranded DNA-binding proteins fromthe psychrophilic bacteria Desulfotalea psychrophila Flavobac-terium psychrophilum Psychrobacter arcticus Psychrobactercryohalolentis Psychromonas ingrahamii Psychroflexus torquisand Photobacterium profundumrdquo BMC Microbiology vol 14article 91 2014
BioMed Research International 15
[38] T Paradzik N Ivic Z Filic et al ldquoStructure-function relation-ships of two paralogous single-stranded DNA-binding proteinsfrom Streptomyces coelicolor implication of SsbB in chromo-some segregation during sporulationrdquo Nucleic Acids Researchvol 41 no 6 pp 3659ndash3672 2013
[39] S JainM Zweig E Peeters et al ldquoCharacterization of the singlestrandedDNA binding protein SsbB encoded in the gonoccocalgenetic islandrdquo PLoS ONE vol 7 no 4 Article ID e35285 2012
[40] Y H Huang and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from Klebsiella pneumoniaemutation at either Arg73 or Ser76 causes a less cooperativecomplex onDNArdquoGenes to Cells vol 17 no 2 pp 146ndash157 2012
[41] E Antony E A Weiland S Korolev and T M LohmanldquoPlasmodium falciparum SSB tetramer wraps single-strandedDNAwith similar topology but opposite polarity to E Coli SSBrdquoJournal ofMolecular Biology vol 420 no 4-5 pp 269ndash283 2012
[42] H Jan Y L Lee and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from pseudomonas aeruginosaPAO1rdquo Protein Journal vol 30 no 1 pp 20ndash26 2011
[43] Y-H Huang Y-L Lee and C-Y Huang ldquoCharacterization of asingle-stranded DNA binding protein from Salmonella entericaserovar typhimurium LT2rdquo Protein Journal vol 30 no 2 pp102ndash108 2011
[44] S K Bharti K Rex P Sreedhar N Krishnan and U VarshneyldquoChimeras of Escherichia coli and Mycobacterium tuberculosissingle-stranded DNA binding proteins characterization andfunction in Escherichia colirdquo PLoS ONE vol 6 no 12 ArticleID e27216 2011
[45] M T Oliveira and L S Kaguni ldquoFunctional roles of the N-and C-terminal regions of the human mitochondrial single-stranded DNA-binding proteinrdquo PLoS ONE vol 5 no 10Article ID e15379 2010
[46] A G Kozlov J M Eggington M M Cox and T MLohman ldquoBinding of the dimeric Deinococcus radioduranssingle-strandedDNA binding protein to single-strandedDNArdquoBiochemistry vol 49 no 38 pp 8266ndash8275 2010
[47] T M Lohman and M E Ferrari ldquoEscherichia coli single-stranded DNA-binding protein multiple DNA-binding modesand cooperativesrdquo Annual Review of Biochemistry vol 63 pp527ndash570 1994
[48] R Zhou A G Kozlov R Roy et al ldquoSSB functions as a slidingplatform that migrates on DNA via reptationrdquo Cell vol 146 pp222ndash232 2011
[49] R Roy A G Kozlov T M Lohman and T Ha ldquoSSB proteindiffusion on single-stranded DNA stimulates RecA filamentformationrdquo Nature vol 461 pp 1092ndash1097 2009
[50] R Roy A G Kozlov T M Lohman and T Ha ldquoDynamicstructural rearrangements between DNA binding modes of Ecoli SSB proteinrdquo Journal of Molecular Biology vol 369 no 5pp 1244ndash1257 2007
[51] T J Kelly P Simancek and G S Brush ldquoIdentification andcharacterization of a single-stranded DNA-binding proteinfrom the archaeon Methanococcus jannaschiirdquo Proceedings ofthe National Academy of Sciences of the United States of Americavol 95 no 25 pp 14634ndash14639 1998
[52] C Y Huang Determination of the Binding Site-Size of theProtein-DNA Complex by Use of the Electrophoretic MobilityShift Assay InTech Press Rijeka Croatia 2012
[53] A Kornberg ldquoTen commandments of enzymology amendedrdquoTrends in Biochemical Sciences vol 28 no 10 pp 515ndash517 2003
[54] W Zhang X Lu and J Shen ldquoEMSA and single-moleculeforce spectroscopy study of interactions between bacillus sub-tilis single-stranded DNA-binding protein and single-strandedDNArdquo Langmuir vol 27 no 24 pp 15008ndash15015 2011
[55] M Ostermeier and S J Benkovic ldquoEvolution of protein func-tion by domain swappingrdquo Advances in Protein Chemistry vol55 pp 29ndash77 2000
[56] W F Peng and C Y Huang ldquoAllantoinase and dihydroorotasebinding and inhibition by flavonols and the substrates of cyclicamidohydrolasesrdquo Biochimie vol 101 pp 113ndash122 2014
[57] Y H Huang M J Lin and C Y Huang ldquoDnaT is a single-stranded DNA binding proteinrdquo Genes to Cells vol 18 no 11pp 1007ndash1019 2013
[58] Y Y Ho Y H Huang and C Y Huang ldquoChemical rescue of thepost-translationally carboxylated lysine mutant of allantoinaseand dihydroorotase by metal ions and short-chain carboxylicacidsrdquo Amino Acids vol 44 no 4 pp 1181ndash1191 2013
[59] Y H Huang Y Lo W Huang and C Y Huang ldquoCrystalstructure and DNA-binding mode of Klebsiella pneumoniaeprimosomal PriB proteinrdquoGenes to Cells vol 17 no 10 pp 837ndash849 2012
[60] C Y Huang C Y Hsu Y Sun H Wu and C Hsiao ldquoCom-plexed crystal structure of replication restart primosome pro-tein PriB reveals a novel single-stranded DNA-binding moderdquoNucleic Acids Research vol 34 no 14 pp 3878ndash3886 2006
[61] T Kinebuchi H Shindo H Nagai N Shimamoto andM Shimizu ldquoFunctional domains of Escherichia coli single-stranded DNA binding protein as assessed by analyses of thedeletion mutantsrdquo Biochemistry vol 36 no 22 pp 6732ndash67381997
[62] N Shimamoto N Ikushima H Utiyama H Tachibana andK Horie ldquoSpecific and cooperative binding of E coli single-stranded DNA binding protein to mRNArdquo Nucleic AcidsResearch vol 15 no 13 pp 5241ndash5250 1987
[63] H Lin andC YHuang ldquoCharacterization of flavonol inhibitionof DnaB helicase Real-time monitoring structural modelingand proposed mechanismrdquo Journal of Biomedicine and Biotech-nology vol 2012 Article ID 735368 2012
[64] Y H Huang H H Lin and C Y Huang ldquoA single residuedetermines the cooperative binding property of a primosomalDNA replication protein PriB to single-stranded DNArdquo Bio-science Biotechnology and Biochemistry vol 76 no 6 pp 1110ndash1115 2012
[65] H C Hsieh and C Y Huang ldquoIdentification of a novel proteinPriB in Klebsiella pneumoniaerdquo Biochemical and BiophysicalResearch Communications vol 404 no 1 pp 546ndash551 2011
[66] J H Liu T W Chang C Y Huang et al ldquoCrystal structure ofPriB a primosomalDNA replication protein ofEscherichia colirdquoThe Journal of Biological Chemistry vol 279 no 48 pp 50465ndash50471 2004
[67] Y H Huang and C Y Huang ldquoThe N-terminal domain ofDnaT a primosomalDNA replication protein is crucial for PriBbinding and self-trimerizationrdquo Biochemical and BiophysicalResearch Communications vol 442 pp 147ndash152 2013
[68] M A Larkin G Blackshields N P Brown et al ldquoClustalW andClustal X version 20rdquo Bioinformatics vol 23 no 21 pp 2947ndash2948 2007
16 BioMed Research International
[69] S N Savvides S Raghunathan K Futterer A G Kozlov T MLohman and G Waksman ldquoThe C-terminal domain of full-length E coli SSB is disordered even when bound to DNArdquoProtein Science vol 13 no 7 pp 1942ndash1947 2004
[70] C-C Chen J-K Hwang and J-M Yang ldquo(PS)2-v2 template-based protein structure prediction serverrdquo BMC Bioinformaticsvol 10 article 366 2009
[71] C Chen J Hwang and J Yang ldquo(PS)2 protein structure pre-diction serverrdquoNucleic Acids Research vol 34 pp W152ndashW1572006
[72] K Bush and J F Fisher ldquoEpidemiological expansion structuralstudies and clinical challenges of new 120573-lactamases from gram-negative bacteriardquo Annual Review of Microbiology vol 65 pp455ndash478 2011
[73] K K Kumarasamy M A Toleman T R Walsh et al ldquoEmer-gence of a new antibiotic resistance mechanism in India Pak-istan and the UK a molecular biological and epidemiologicalstudyrdquoThe Lancet Infectious Diseases vol 10 no 9 pp 597ndash6022010
[74] K Bush and M J MacIelag ldquoNew 120573-lactam antibiotics and 120573-lactamase inhibitorsrdquo Expert Opinion on Therapeutic Patentsvol 20 no 10 pp 1277ndash1293 2010
[75] K Bush ldquoAlarming 120573-lactamase-mediated resistance in multi-drug-resistant Enterobacteriaceaerdquo Current Opinion in Microbi-ology vol 13 no 5 pp 558ndash564 2010
[76] A Srivastava M Talaue S Liu et al ldquoNew target for inhibitionof bacterial RNA polymerase ldquoswitch regionrdquordquo Current Opinionin Microbiology vol 14 no 5 pp 532ndash543 2011
[77] K Chono K Katsumata T Kontani et al ldquoASP2151 a novelhelicase-primase inhibitor possesses antiviral activity againstvaricella-zoster virus and herpes simplex virus types 1 and 2rdquoJournal of Antimicrobial Chemotherapy vol 65 no 8 pp 1733ndash1741 2010
[78] M T Black and K Coleman ldquoNew inhibitors of bacterial topoi-somerase GyrAParC subunitsrdquo Current Opinion in Investiga-tional Drugs vol 10 no 8 pp 804ndash810 2009
[79] A H Marceau D A Bernstein B W Walsh W Shapiro LA Simmons and J L Keck ldquoProtein interactions in genomemaintenance as novel antibacterial targetsrdquo PLoS ONE vol 8no 3 Article ID e58765 2013
[80] D Lu D A Bernstein K A Satyshur and J L Keck ldquoSmall-molecule tools for dissecting the roles of SSBprotein inter-actions in genome maintenancerdquo Proceedings of the NationalAcademy of Sciences of the United States of America vol 107 no2 pp 633ndash638 2010
[81] I Wong and T M Lohman ldquoA double-filter method fornitrocellulose-filter binding application to protein-nucleic acidinteractionsrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 90 no 12 pp 5428ndash5432 1993
[82] M Mitas J Y Chock and M Christy ldquoThe binding-site sizesof Escherichia coli single-stranded-DNA-binding protein andmammalian replication protein A are 65 and ge 54 nucleotidesrespectivelyrdquo Biochemical Journal vol 324 no 3 pp 957ndash9611997
[83] C Urbanke G Witte and U Curth ldquoSedimentation velocitymethod in the analytical ultracentrifuge for the study of protein-protein interactionsrdquoMethods inMolecular Biology vol 305 pp101ndash114 2005
[84] S Chrysogelos and J Griffith ldquoEscherichia coli single-strandbinding protein organizes single-strandedDNA innucleosome-like unitsrdquo Proceedings of the National Academy of Sciences of theUnited States of America vol 79 no 19 pp 5803ndash5807 1982
[85] J R Casas-Finet K R Fischer and R L Karpel ldquoStructuralbasis for the nucleic acid binding cooperativity of bacteriophageT4 gene 32 protein the (LysArg)3(SerThr)2 (LAST) motifrdquoProceedings of the National Academy of Sciences of the UnitedStates of America vol 89 pp 1050ndash1054 1992
[86] G Witte R Fedorov and U Curth ldquoBiophysical analysis ofThermus aquaticus single-stranded DNA binding proteinrdquo Bio-physical Journal vol 94 no 6 pp 2269ndash2279 2008
[87] R Fedorov G Witte C Urbanke D J Manstein and UCurth ldquo3D structure of Thermus aquaticus single-strandedDNA-binding protein gives insight into the functioning of SSBproteinsrdquo Nucleic Acids Research vol 34 no 22 pp 6708ndash67172006
[88] K L Tsai C Y Huang C H Chang Y J Sun W J ChuangandC DHsiao ldquoCrystal structure of the human FOXK1a-DNAcomplex and its implications on the diverse binding specificityof winged helixforkhead proteinsrdquo Journal of Biological Chem-istry vol 281 no 25 pp 17400ndash17409 2006
[89] A H Mao N Lyle and R V Pappu ldquoDescribing sequence-ensemble relationships for intrinsically disordered proteinsrdquoBiochemical Journal vol 449 no 2 pp 307ndash318 2013
[90] R Wetzel ldquoPhysical chemistry of polyglutamine Intriguingtales of a monotonous sequencerdquo Journal of Molecular Biologyvol 421 no 4-5 pp 466ndash490 2012
[91] H T Orr ldquoPolyglutamine neurodegeneration expanded glu-tamines enhance native functionsrdquo Current Opinion in Geneticsand Development vol 22 no 3 pp 251ndash255 2012
[92] M Figiel W J Szlachcic P M Switonski A Gabka and W JKrzyzosiak ldquoMouse models of polyglutamine diseases reviewand data table Part Irdquo Molecular Neurobiology vol 46 no 2pp 393ndash429 2012
[93] J Nasica-Labouze and N Mousseau ldquoKinetics of amyloidaggregation a study of the GNNQQNY prion sequencerdquo PLoSComputational Biology vol 8 no 11 Article ID e1002782 2012
[94] J Nasica-Labouze M Meli P Derreumaux G Colombo andN Mousseau ldquoA multiscale approach to characterize the earlyaggregation steps of the amyloid-forming peptide gnnqqnyfrom the yeast prion sup-35rdquo PLoS Computational Biology vol7 no 5 Article ID e1002051 2011
[95] J R Lewandowski P C A van der Wel M Rigney N Grig-orieff and R G Griffin ldquoStructural complexity of a compositeamyloid fibrilrdquo Journal of the American Chemical Society vol133 no 37 pp 14686ndash14698 2011
BioMed Research International 7
[StSSB]
-dT15
-C
mdash
(a)
-dT20
-C
---
-
mdash[StSSB]
(b)
-dT30
-C
[StSSB]mdash
(c)
-dT40
-C
mdash[StSSB]
(d)
-dT45
-C1-C2
mdash[StSSB]
(e)
-dT50
-C1-C2
mdash
[StSSB]
(f)
Figure 5 Binding of StSSB to dT15ndash50 StSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT15 (b) dT20 (c) dT30 (d) dT40 (e) dT45 or (f) dT50 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and 100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blue and40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
examined using EMSA StSSB can bind and form a singlecomplex with dT15 (Figure 5(a)) and dT20 (Figure 5(b)) butKpSSB cannot (Figure 4(a)) StSSB bound to dT15ndash40 andformed a single complex For dT45 and dT50 two differentcomplexes of StSSB appeared at high protein concentrations(Figures 5(e) and 5(f)) Therefore one StSSB occupies 20(402 = 20) nt to 225 (452 = 225) nt of the ssDNA TheEMSA results suggest that the length of an ssDNA (or thebinding site size) [52] required for StSSB binding is 21 plusmn 2 nt
35 PaSSB Bound to ssDNA The binding of PaSSB to dT20(Figure 6(a)) dT25 (Figure 6(b)) dT35 (Figure 6(c)) dT45(Figure 6(d)) dT55 (Figure 6(e)) dT60 (Figure 6(f)) anddT65 (Figure 6(g)) was studied by EMSA Unlike StSSB nocomplexwas observedwhen PaSSBwas incubatedwith dT20Some smears were observed indicating that PaSSB interactswith dT20 However the ssDNA may be too short to befully wrapped by PaSSB PaSSB could form a single complexwith dT25ndash55 and form two distinct complexes with dT60
and dT65 (Figures 6(f) and 6(g)) respectivelyTherefore onePaSSB occupies 275 (552 = 275) nt to 30 (602 = 30) ntof the ssDNA These results from EMSA suggest that thelength of an ssDNA (or the binding site size) [52] required forPaSSB binding is 29plusmn2 nt Although the SSBs that is KpSSBStSSB and PaSSB have significantly similar ssDNA-bindingdomains their binding site sizes are different and range from19 (21 plusmn 2 StSSB) to 31 (29 plusmn 2 PaSSB) nt The obtainedEMSA results (Figures 4ndash6) also show that the binding sitesizes of the untagged SSBs (KpSSB StSSB and PaSSB) werefound to be almost identical to those of the His-tagged ones[40 42 43]
36 Design of the Chimeric KpSSB Proteins KpSSBnStSSBc andKpSSBnPaSSBc The N-terminal ssDNA-binding domain ofKpSSB StSSB and PaSSB is highly conserved (Figure 2) buttheir binding site sizes are different (Figures 4ndash6) and rangefrom 19 nt to 31 nt The C-terminal acidic tails DDDIPFare conserved (Figure 2) and these features led us to assess
8 BioMed Research International
[PaSSB]
-dT20
mdash
(a)
-dT25
-C
[PaSSB]mdash
(b)
-dT35
-C
-
-
[PaSSB]
(c)
-dT45
-C
[PaSSB]
(d)
-dT55
-C
[PaSSB]
(e)
-dT60
-C1-C2
[PaSSB]
(f)
-dT65
-C1-C2
[PaSSB]
(g)
Figure 6 Binding of PaSSB to dT20ndash65 PaSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT20 (b) dT25 (c) dT35 (d) dT45 (e) dT55 (f) dT60 or (g) dT65 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
whether the flexible glycine-rich hinge in the C-terminaldomain which is not conserved among SSBs is involved inthe determination of the binding site size of SSB Thus theC-terminal domains of StSSB and PaSSB were swapped withKpSSB through protein chimeragenesis
37 KpSSBnStSSBc Bound to ssDNA The binding ofKpSSBnStSSBc to dT15 (Figure 7(a)) dT20 (Figure 7(b))dT40 (Figure 7(c)) and dT45 (Figure 7(d)) was examinedusing EMSA KpSSBnStSSBc exhibited significantly different
ssDNA-binding properties from those of KpSSB UnlikeKpSSB (Figure 4) both KpSSBnStSSBc (Figure 8) and StSSB(Figure 5) can bind and form a single complex with dT15 anddT20 Similar to StSSB KpSSBnStSSBc binds to dT15ndash40 andforms a single complex For dT45 two different complexesof KpSSBnStSSBc appeared at high protein concentrations(Figure 8(d)) this EMSA feature was also similar to that ofStSSB One KpSSBnStSSBc occupies 20 (402 = 20) nt to225 (452 = 225) nt of the ssDNA These EMSA resultssuggest that the length of an ssDNA (or the binding site
BioMed Research International 9
[KpSSBnStSSBc]
-dT15
-C
(a)
[KpSSBnStSSBc]
-dT20
-C
(b)
[KpSSBnStSSBc]
-dT40
-C
(c)
[KpSSBnStSSBc]
-dT45
-C1-C2
(d)
Figure 7 Binding of KpSSBnStSSBc to dT15ndash45 KpSSBnStSSBc (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for30min at 25∘C with 17 nM of (a) dT15 (b) dT20 (c) dT40 or (d) dT45 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and 100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blue and40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
size) [52] required for KpSSBnStSSBc binding is 21 plusmn 2 nt avalue identical to that for StSSB (Figure 5) Swapping of theC-terminal domain of StSSB with KpSSB changes the size ofthe ssDNA-binding site from 26 nt to 21 nt
38 KpSSBnPaSSBc Bound to ssDNA The binding fea-tures of KpSSBnPaSSBc with dT20 (Figure 8(a)) dT25(Figure 8(b)) dT40 (Figure 8(c)) dT55 (Figure 8(d)) anddT60 (Figure 8(e)) were studied by EMSA Similar to thecases of KpSSB and PaSSB no complex was observed whenKpSSBnPaSSBc was incubated with dT20 However KpSSB-nPaSSBc still exhibited dramatically different ssDNA-bindingproperties from those of KpSSB KpSSB can form two distinctcomplexes with dT55 (Figure 4(f)) but both KpSSBnPaSSBc(Figure 9) and PaSSB (Figure 6) cannot One KpSSBnPaSSBcoccupies 275 (552 = 275) nt to 30 (602 = 30) nt ofthe ssDNA The above EMSA results suggest that the lengthof an ssDNA (or the binding site size) [52] required forKpSSBnPaSSBc binding is 29 plusmn 2 nt a value identical to thatof PaSSB Swapping of the C-terminal domain of PaSSB toKpSSB changes the size of the ssDNA-binding site from 26 ntto 29 nt Although these SSBs namely KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc have nearly identicalssDNA-binding domains their binding site sizes are different(Table 2) Thus the size of the ssDNA-binding site requiredfor second SSB binding is likely to be dependent on the C-terminal domain of SSB
39 Binding Constants of the SSB-ssDNA Complexes Deter-mined from EMSA To compare the ssDNA-binding abil-ities of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc the midpoint values for input ssDNA bind-ing calculated from the titration curves of EMSA andreferred to as [Protein]
50(monomer) were quantified and
are summarized in Table 2 Although the N-terminal ssDNA-binding domains of these SSB proteins are highly similar(Figure 2) their ssDNA-binding activities and binding sitesizes are different (Table 2) [KpSSB]
50values ranged from
100 nM to 220 nM [StSSB]50
values ranged from 420 nM to650 nM [PaSSB]
50values ranged from 550 nM to 1700 nM
[KpSSBnStSSBc]50values ranged from 110 nM to 260 nM and
[KpSSBnPaSSBc]50
values ranged from 220 nM to 390 nMThe ssDNA-binding ability is as follows in the order ofdecreasing affinity KpSSB gt KpSSBnStSSBc gt KpSSBn-PaSSBc gt StSSB gt PaSSB Results from the above analysesindicate that the exchange of the C-terminal domain inSSB significantly changed the ssDNA-binding ability and theDNA-binding behavior (complex number) The reason asto why swapping of the C-terminal domain can affect thessDNA-binding activity of SSB remains unclear The C-ter-minal domain of SSB is suggested to be involved in ssDNAbinding However this relation is not evident in the results ofthe cocrystal structure
310 Oligomeric State of KpSSBnStSSBc and KpSSBnPaSSBc inSolution Gel-filtration chromatography was used to confirm
10 BioMed Research International
[KpSSBnPaSSBc]
-dT20
(a)
[KpSSBnPaSSBc]
-dT25
-C
(b)
[KpSSBnPaSSBc]
-C
-dT40
(c)
-C
[KpSSBnPaSSBc]
-dT55
(d)
-dT60
-C1-C2
[KpSSBnPaSSBc]
(e)
Figure 8 Binding of KpSSBnPaSSBc to dT20ndash60 KpSSBnPaSSBc (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for30min at 25∘C with 17 nM of (a) dT20 (b) dT25 (c) dT40 (d) dT55 or (e) dT60 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
that the oligomeric state of KpSSBnStSSBc and KpSSBn-PaSSBc remains as tetramers after chimeragenesisThe analy-sis of purified KpSSBnStSSBc and KpSSBnPaSSBc (2mgmL)using a Superdex 200HR 1030 column revealed a singlepeak with elution volumes of 786 and 789mL respectivelyAssuming that KpSSBnStSSBc and KpSSBnPaSSBc both haveshapes and partial specific volumes similar to the standardproteins the native molecular masses of KpSSBnStSSBc andKpSSBnPaSSBc were estimated to be 76641 and 74827Da ascalculated from a standard linear regression equation 119870av =minus03684(logMw) + 22707 (Figure 9) The native molecularmasses for KpSSBnStSSBc and KpSSBnPaSSBc are approx-imately four times the mass of the monomer (sim19 kDa)Therefore KpSSBnStSSBc and KpSSBnPaSSBc under theabove chromatographic conditions are stable tetramers insolution Although the exchange of the C-terminal domainin SSB significantly changed the ssDNA-binding ability andDNA-binding behavior (complex number) protein chimera-genesis did not cause any change in the oligomeric state ofSSB
311 Summary of Gly Gln and Pro Number in SSBs To ana-lyze the C-terminal amino acid composition of SSBs wefurther counted the number of Gly Gln and Pro residuesin different SSB segments SSB is abundant in Gly Glnand Pro (GQP) (Table 3) The GQP contents of KpSSB1ndash91 StSSB1ndash91 and PaSSB1ndash90 are similar However the Glynumber of PaSSB116ndash165 is significantly lower than that ofKpSSB116ndash174 and StSSB117ndash176 PaSSB116ndash165 contains only1 Gly but KpSSB116ndash174 and StSSB117ndash176 contain 11 and 12Gly respectively In addition we found different distributionpatterns among KpSSB StSSB and PaSSB Although theycontain similar number of Gln (Q) the QQQ pattern isfrequently found in PaSSB (Table 3)
312 Structural Modeling of SSBs Given its disordered C-ter-minal domain the crystal structure of the full-length SSB islacking even when SSB can be crystallized with DNA [69]We attempted to model the structure by homology mod-eling using the bioinformatics program (PS)2 to obtain an
BioMed Research International 11
Table 2 ssDNA binding properties of KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc as analyzed by EMSA
Protein DNA [Protein]50 (nM) Complex number
KpSSB
dT20 ND 0dT25 200 plusmn 20 1dT35 220 plusmn 30 1dT45 100 plusmn 10 1dT50 110 plusmn 20 1dT55 100 plusmn 20 2dT60 100 plusmn 10 2
StSSB
dT15 650 plusmn 120 1dT20 450 plusmn 80 1dT30 420 plusmn 60 1dT40 420 plusmn 80 1dT45 440 plusmn 60 2dT50 440 plusmn 50 2
PaSSB
dT20 ND 0dT25 1700 plusmn 250 1dT35 950 plusmn 180 1dT45 780 plusmn 160 1dT55 820 plusmn 90 1dT60 810 plusmn 110 2dT65 550 plusmn 70 2
KpSSBnStSSBc
dT15 260 plusmn 60 1dT20 110 plusmn 20 1dT40 120 plusmn 20 1dT45 160 plusmn 20 2
KpSSBnPaSSBc
dT20 ND 0dT25 390 plusmn 60 1dT40 220 plusmn 30 1dT55 230 plusmn 30 1dT60 230 plusmn 30 2
[Protein]50 was calculated from the titration curves of EMSA by determiningthe concentration of the protein (120583M) needed to achieve the midpointvalue for input ssDNA binding For some oligonucleotides input ssDNAbinding was the sum of the intensities from the two separate ssDNA-proteincomplexes Errors are standard deviations determined by three independenttitration experiments
Table 3 Summary of Gly Gln and Pro number in SSB
SSB segment G Q PKpSSB1ndash91 10 5 2StSSB1ndash91 10 5 2PaSSB1ndash90 11 6 2KpSSB92ndash174 18 16 9StSSB92ndash176 17 18 11PaSSB91ndash165 5 15 11KpSSB116ndash174 11 12 9StSSB117ndash176 12 13 10PaSSB116ndash165 1 12 11
in-depth understanding of the structure-function relation-ship of the C-terminal domains of these SSBs [70 71]
40 45 50 55
Kav
00
02
04
06
08
10
KpSSBnStSSBcKpSSBnPaSSBc
Y = minus03684X + 22707
R2= 0998
log Mw
Figure 9Gel-filtration chromatographic analyses of KpSSBnStSSBcand KpSSBnPaSSBc Purified protein (2mgmL) was applied toa Superdex 200HR 1030 column (GE Healthcare Bio-SciencesPiscataway NJ USA) equilibrated with Buffer D The column wasoperated at a flow rate of 05mLmin and 05mL fractions werecollected The proteins were detected by measuring the absorbanceat 280 nm The column was calibrated with proteins of knownmolecular weight thyroglobulin (670 kDa) 120574-globulin (158 kDa)ovalbumin (44 kDa) and myoglobin (17 kDa) The 119870av values forthe standard proteins and the SSB variants were calculated from theequation119870av = (119881119890minus119881119900)(119881119888minus119881119900) where119881119900 is column void volume119881119890is elution volume and 119881
119888is geometric column volume
(PS)2 (http140113239111simps2v2docsphp) is an auto-matic homology modeling server that combines bothsequence and secondary structure information to detect thehomologous proteins with remote similarity and the target-template alignment After pasting the amino acid sequenceto the website of (PS)2 only one hit (Protein Data Bankentry 1QVC EcSSB) for the C-terminal domains of KpSSBand StSSB was suggested For the C-terminal domain ofPaSSB only one hit that is CstF-77 (ProteinData Bank entry2OOE cleavage stimulation factor CstF) but not EcSSB wassuggested as the template for modeling Figure 10 shows thatmodeled structures of these SSB C-terminal domains arehighly disordered but that of PaSSB is more ordered than thatof other domains
4 Discussion
In this study we examined the sizes of the binding site ofthe untagged SSB and the chimeric SSB from the ubiquitousopportunistic pathogens K pneumoniae S enterica serovarTyphimurium LT2 and P aeruginosa PAO1 Many clinicalstrains of the abovementioned bacteria are highly resistantto antibiotics [72ndash75] The development of clinically usefulsmall-molecule antibiotics has been a seminal event in thefield of infectious diseases [48] Nucleic acid metabolismis one of the most basic biological functions and shouldbe a prime target in antibiotic development [76ndash78] Manybacterial SSBs form conserved protein interaction ldquohubsrdquothat are essential to recruit many proteins involved in DNA
12 BioMed Research International
Figure 10 Structure modeling of SSB The structures of KpSSB1ndash115 StSSB1ndash115 and PaSSB1ndash115 (the N-terminal domain of SSB)were modeled by SWISS-MODEL The structures of KpSSB116ndash142 StSSB116ndash142 and PaSSB121ndash160 (the C-terminal domain ofSSB) were modeled by (PS)2 Other regions of SSBs could not bemodeled by these two programs The structures of the N-terminaldomain and the C-terminal domain of these SSBs were manuallylinked (KpSSB1ndash142 blue StSSB1ndash142 pink PaSSB1ndash160 green) andsuperimposed with the crystal structure of EcSSB1ndash142 (orange)(PDB entry 1QVC) for comparison For clarity only one subunitof the tetramer was shown for each SSB
replication recombination and repair SSBDNA nucleopro-tein substrates [79] Thus SSBs may be promising targets inantibiotic development [80] As a first step toward achievingthis goal we investigated why SSBs possess highly conservedN-terminal ssDNA-binding domain but exhibit varying bind-ing site sizes One significant clue is that their flexible hingesand the length at the C-terminus are different as revealed bysequence alignment (Figure 2)
The interactions of various SSBs with ssDNA have beenanalyzed using a variety of techniques such as tryptophan-fluorescence quenching [47] filter binding [81] EMSA [5282] analytical ultracentrifugation [83] electron microscopy[84] nuclease digestion [44] single-molecule fluorescencemicroscopy [48] and crystallographic analyses [11] In thisstudy we have examined the electrophoretic mobility shiftpatterns of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc bound to different lengths of ssDNA and deter-mined the corresponding binding site sizes to be 26 2129 22 and 29 nt per tetramer respectively (Figures 4ndash8) PaSSB and KpSSBnPaSSBc have the largest sizes forssDNA binding among the SSBs studied We also identifiedHis-tagged and untagged SSBs that have similar ssDNA-binding site sizes [40 42 43] EMSA is a well-establishedapproach in studies of molecular biology [52] and the use ofradioactive tracer in this assay allows detection of the actualformation of the distinct protein-DNA complex(es) [53] For
exampleDNase protection assay and footprinting assay usingradioactive tracer can determine the specific DNA sequencecomplexed by a protein In EMSA when the length of thenucleotides is sufficient for the binding of two or more SSBmolecules the electrophoretic mobility of the higher SSBoligomer complex will be lower than that of the smaller SSBoligomer complex [52 54] In addition results of the ssDNA-binding site size from EMSA and cocrystal structure of SSBwere consistent [27] Thus throughout this paper we deter-mined the ssDNA-binding site sizes of SSB from the EMSAbehavior
Many SSBs bind to ssDNA with some degree of positivecooperativity Cooperativity can result from direct protein-protein interactions between the nearest neighbors such asthe LAST motif in the T4 gene-32 protein [85] and thearginine-mediated interaction motif in Thermus SSB [8687] Cooperativity can also result from the protein-induceddistortion of adjacent DNA as demonstrated in SulfolobusSSB PriB and FOXK1a proteins [23 60 88] In the cases ofKpSSB StSSB and PaSSB (Figures 4ndash6) binding appeared tobe nearly noncooperative for several DNAs because all DNAmainly shifts into the first complex (C1) before the appearanceof the second complex (C2) when subjected to increasingprotein concentrationsThe length dependence of the [SSB]
50
values suggests that the amount of spacing is optimum forsteric considerations (Table 2)
Because bacteria have varying genomic DNA sizes theirSSBs may need to evolve to have different binding site sizesfor DNA metabolism Results from protein chimeragenesisshowed the C-terminal domain dependence of the bindingsite sizes of SSB (Figure 11) The experimental data showedthat the binding site size of KpSSBnStSSBc was similar to thatof StSSB and the size of the binding site of KpSSBnPaSSBcwas similar to that of PaSSB The reason for which the bind-ing site size of SSB changed followed by swapping of theC-terminal domain remains unclear Flexibility numberof glycine residues andor different QQQ patterns of theC-terminal domain of SSB (Figure 2 and Table 3) may beimportant factors for determining the ssDNA-binding sitesize In fact the C-terminal domain of PaSSB that isPaSSB116ndash165 has only 1 Gly residue which is significantlyless than that of KpSSB (11 Gly) and StSSB (12Gly) Gly (andPro) is an important component of the flexible region aprotein that contains low Gly content is predicted to havelow flexibility Unlike typical SSB [35 69] PaSSB116ndash165 hasa partial structure (Figure 10) Although KpSSB StSSB andPaSSB contain similar number of Gln (Q) the QQQ patternis frequently found in PaSSB (Figure 2 and Table 3) PolyQand repeated sequences GAGAG are commonly found inthe structures of amyloids silk fibers and neurodegradationproteins [89ndash92] Considering that the simple coil polyQ theheptapeptide GNNQQNY and the hexapeptide NNQQNYcan cause protein aggregation and nucleation [93ndash95] thedistribution of Gln in the C-terminal domain of a tetramericSSB may also be an important determinant of the ssDNA-binding site size of SSB by some steric hindrances (Figure 11)However the above speculationmust be confirmed by furtherbiochemical experiments
BioMed Research International 13
PaSSBStSSB KpSSB
C-terC-ter C-ter
sim 21 nt sim 26 nt sim 29 nt
Figure 11 Possiblemodels for explainingwhy SSBs arewith different binding site sizes Twomodeled structures of KpSSB1ndash142 (blue) StSSB1ndash142 (pink) and PaSSB1ndash160 (green) complexed with ssDNA (gold) are shown For clarity only one C-terminal domain was shown for eachSSB tetramer By using the electrophoretic mobility shift assay and the protein chimeragenesis we characterized that the binding site sizes ofKpSSB StSSB PaSSB KpSSBnStSSBc and KpSSBnPaSSBc were 26 21 29 21 and 29 nt per tetramer respectively KpSSB StSSB and PaSSBare similar proteins whose N-terminal ssDNA-binding domains are almost identical Thus the C-terminal domain of SSB may indirectlycontribute to ssDNA binding and wrapping and affects the binding site size by the steric hindrance
5 Conclusion
In this study we characterized the ssDNA-binding propertiesof untagged SSBs from K pneumoniae S enterica sero-var Typhimurium LT2 and P aeruginosa PAO1 and proposeda role of the C-terminal flexible domain for ssDNA bind-ing from the protein chimeragenesis and EMSA resultsThe amino acid sequence of the N-terminal ssDNA-bind-ingoligomerization domain in these pathogenic SSBs ishighly conserved but their apparent binding site sizes aredifferent This finding indicates that the C-terminal protein-protein interaction domain may also indirectly contribute tossDNA binding and wrapping
Conflict of Interests
The authors declare that there is no conflict of interestsregarding the publication of this paper
Acknowledgment
This researchwas supported by aGrant from theNational Sci-ence Council Taiwan (NSC 102-2320-B-040-019 to Cheng-Yang Huang)
References
[1] R Reyes-Lamothe D J Sherratt and M C Leake ldquoStoichiom-etry and architecture of active DNA replication machinery inescherichia colirdquo Science vol 328 no 5977 pp 498ndash501 2010
[2] D J Richard E Bolderson andK K Khanna ldquoMultiple humansingle-stranded DNA binding proteins function in genomemaintenance structural biochemical and functional analysisrdquo
Critical Reviews in Biochemistry and Molecular Biology vol 44no 2-3 pp 98ndash116 2009
[3] R D Shereda A G Kozlov T M LohmanMM Cox and J LKeck ldquoSSB as an organizermobilizer of genome maintenancecomplexesrdquo Critical Reviews in Biochemistry and MolecularBiology vol 43 no 5 pp 289ndash318 2008
[4] D J Richard E Bolderson L Cubeddu et al ldquoSingle-strandedDNA-binding protein hSSB1 is critical for genomic stabilityrdquoNature vol 453 no 7195 pp 677ndash681 2008
[5] R R Meyer and P S Laine ldquoThe single-stranded DNA-bindingprotein of Escherichia colirdquoMicrobiological Reviews vol 54 no4 pp 342ndash380 1990
[6] C Yang U Curth C Urbanke and C Kang ldquoCrystal structureof human mitochondrial single-stranded DNA binding proteinat 24 A resolutionrdquo Nature Structural Biology vol 4 no 2 pp153ndash157 1997
[7] G Webster J Genschel U Curth C Urbanke C Kang and RHilgenfeld ldquoA common core for binding single-stranded DNAstructural comparison of the single-stranded DNA-bindingproteins (SSB) from E coli and human mitochondriardquo FEBSLetters vol 411 pp 313ndash316 1997
[8] R L Flynn and L Zou ldquoOligonucleotideoligosaccharide-bind-ing fold proteins a growing family of genome guardiansrdquo Crit-ical Reviews in Biochemistry and Molecular Biology vol 45 no4 pp 266ndash275 2010
[9] K Chan Y Lee CWangHHuang andY Sun ldquoSingle-Strand-ed DNA-Binding Protein Complex from Helicobacter pyloriSuggests an ssDNA-Binding Surfacerdquo Journal of MolecularBiology vol 388 no 3 pp 508ndash519 2009
[10] D L Theobald R M Mitton-Fry and D S Wuttke ldquoNucleicacid recognition by OB-fold proteinsrdquo Annual Review of Bio-physics and Biomolecular Structure vol 32 pp 115ndash133 2003
[11] S Raghunathan A G Kozlov TM Lohman andGWaksmanldquoStructure of the DNA binding domain of E coli SSB bound to
14 BioMed Research International
ssDNArdquo Nature Structural Biology vol 7 no 8 pp 648ndash6522000
[12] A G Murzin ldquoOB(oligonucleotideoligosaccharide binding)-fold common structural and functional solution for non-homologous sequencesrdquo EMBO Journal vol 12 no 3 pp 861ndash867 1993
[13] N P George K V Ngo S Chitteni-Pattu et al ldquoStructure andcellular dynamics of deinococcus radiodurans single-strandedDNA (ssDNA)-binding protein (SSB)-DNA complexesrdquo TheJournal of Biological Chemistry vol 287 no 26 pp 22123ndash221322012
[14] P Filipkowski and J Kur ldquoIdentification and propertiesof the Deinococcus grandis and Deinococcus proteolyticussingle-stranded DNA binding proteins (SSB)rdquo Acta BiochimicaPolonica vol 54 no 1 pp 79ndash87 2007
[15] P Filipkowski A Duraj-Thatte and J Kur ldquoIdentificationcloning expression and characterization of a highly ther-mostable single-stranded-DNA-binding protein (SSB) fromDeinococcus murrayirdquo Protein Expression and Purification vol53 no 1 pp 201ndash208 2007
[16] P Filipkowski M Koziatek and J Kur ldquoA highly ther-mostable homodimeric single-stranded DNA-binding proteinfrom Deinococcus radiopugnansrdquo Extremophiles vol 10 no 6pp 607ndash614 2006
[17] P Filipkowski A Duraj-Thatte and J Kur ldquoNovel thermostablesingle-stranded DNA-binding protein (SSB) fromDeinococcusgeothermalisrdquo Archives of Microbiology vol 186 no 2 pp 129ndash137 2006
[18] G Witte C Urbanke and U Curth ldquoSingle-stranded DNA-binding protein of Deinococcus radiodurans a biophysicalcharacterizationrdquoNucleic Acids Research vol 33 no 5 pp 1662ndash1670 2005
[19] D A Bernstein J M Eggingtont M P Killoran A M MisicMMCox and J L Keck ldquoCrystal structure of theDeinococcusradiodurans single-stranded DNA-binding protein suggests amechanism for coping with DNA damagerdquo Proceedings of theNational Academy of Sciences of the United States of Americavol 101 no 23 pp 8575ndash8580 2004
[20] S Dabrowski M Olszewski R Piatek A Brillowska-Dabrowska G Konopa and J Kur ldquoIdentification and charac-terization of single-stranded-DNA-binding proteins fromTher-mus thermophilus and Thermus aquaticusmdashnew arrange-ment of binding domainsrdquo Microbiology vol 148 no 10 pp3307ndash3315 2002
[21] R Gamsjaeger R Kariawasam C Touma A H Kwan M FWhite and L Cubeddu ldquoBackbone and side-chain 1H 13C and15N resonance assignments of the OB domain of the singlestranded DNA binding protein from Sulfolobus solfataricusand chemical shift mapping of the DNA-binding interfacerdquoBiomolecular NMR Assignments 2013
[22] M L Rolfsmeier and C A Haseltine ldquoThe single-strandedDNA binding protein of Sulfolobus solfataricus acts in the pre-synaptic step of homologous recombinationrdquo Journal of Molec-ular Biology vol 397 no 1 pp 31ndash45 2010
[23] I D Kerr R I M Wadsworth L Cubeddu W Blankenfeldt JHNaismith andM FWhite ldquoInsights into ssDNA recognitionby the OB fold from a structural and thermodynamic study ofSulfolobus SSB proteinrdquo EMBO Journal vol 22 no 11 pp 2561ndash2570 2003
[24] C A Haseltine and S C Kowalczykowski ldquoA distinctive single-stranded DNA-binding protein from the Archaeon Sulfolobussolfataricusrdquo Molecular Microbiology vol 43 no 6 pp 1505ndash1515 2002
[25] R I M Wadsworth and M F White ldquoIdentification andproperties of the crenarchaeal single-stranded DNA bindingprotein from Sulfolobus solfataricusrdquo Nucleic Acids Researchvol 29 no 4 pp 914ndash920 2001
[26] S Sugiman-Marangos and M S Junop ldquoThe structure of DdrBfrom Deinococcus a new fold for single-stranded DNA bindingproteinsrdquo Nucleic Acids Research vol 38 no 10 Article IDgkq036 pp 3432ndash3440 2010
[27] H Ghalei H V Moeller D Eppers et al ldquoEntrapment of DNAin an intersubunit tunnel system of a single-stranded DNA-binding proteinrdquo Nucleic Acids Research vol 42 no 10 pp6698ndash6708 2014
[28] S Paytubi S A McMahon S Graham et al ldquoDisplacement ofthe canonical single-strandedDNA-binding protein in the ther-moprotealesrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 109 no 7 pp E398ndashE405 2012
[29] T H Dickey S E Altschuler and D S Wuttke ldquoSingle-stranded DNA-binding proteins multiple domains for multiplefunctionsrdquo Structure vol 21 no 7 pp 1074ndash1084 2013
[30] D Vujaklija and BMacek ldquoDetecting posttranslational modifi-cations of bacterial SSB proteinsrdquoMethods inMolecular Biologyvol 922 pp 205ndash218 2012
[31] I Mijakovic D Petranovic B Macek et al ldquoBacterial single-stranded DNA-binding proteins are phosphorylated on tyro-sinerdquoNucleic Acids Research vol 34 no 5 pp 1588ndash1596 2006
[32] M Olszewski A Grot M Wojciechowski M Nowak MMickiewicz and J Kur ldquoCharacterization of exceptionally ther-mostable single-strandedDNA-binding proteins fromThermo-toga maritima and Thermotoga neapolitanardquo BMC Microbiol-ogy vol 10 article 260 2010
[33] U Curth J Genschel C Urbanke and J Greipel ldquoIn vitro andin vivo function of the C-terminus of Escherichia coil single-stranded DNA binding proteinrdquoNucleic Acids Research vol 24no 14 pp 2706ndash2711 1996
[34] G Witte C Urbanke and U Curth ldquoDNA polymerase IIIchi subunit ties single-stranded DNA binding protein to thebacterial replication machineryrdquoNucleic Acids Research vol 31pp 4434ndash4440 2003
[35] D Shishmarev YWang C EMason et al ldquoOtting Intramolec-ular binding mode of the C-terminus of Escherichia coli single-strandedDNAbinding protein determined by nuclearmagneticresonance spectroscopyrdquo Nucleic Acids Research vol 42 pp2750ndash2757 2014
[36] A G Kozlov M M Cox and T M Lohman ldquoRegulation ofsingle-stranded DNA binding by the C termini of Escherichiacoli single-stranded DNA-binding (SSB) proteinrdquo Journal ofBiological Chemistry vol 285 no 22 pp 17246ndash17252 2010
[37] M Nowak M Olszewski M Spibida and J Kur ldquoChar-acterization of single-stranded DNA-binding proteins fromthe psychrophilic bacteria Desulfotalea psychrophila Flavobac-terium psychrophilum Psychrobacter arcticus Psychrobactercryohalolentis Psychromonas ingrahamii Psychroflexus torquisand Photobacterium profundumrdquo BMC Microbiology vol 14article 91 2014
BioMed Research International 15
[38] T Paradzik N Ivic Z Filic et al ldquoStructure-function relation-ships of two paralogous single-stranded DNA-binding proteinsfrom Streptomyces coelicolor implication of SsbB in chromo-some segregation during sporulationrdquo Nucleic Acids Researchvol 41 no 6 pp 3659ndash3672 2013
[39] S JainM Zweig E Peeters et al ldquoCharacterization of the singlestrandedDNA binding protein SsbB encoded in the gonoccocalgenetic islandrdquo PLoS ONE vol 7 no 4 Article ID e35285 2012
[40] Y H Huang and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from Klebsiella pneumoniaemutation at either Arg73 or Ser76 causes a less cooperativecomplex onDNArdquoGenes to Cells vol 17 no 2 pp 146ndash157 2012
[41] E Antony E A Weiland S Korolev and T M LohmanldquoPlasmodium falciparum SSB tetramer wraps single-strandedDNAwith similar topology but opposite polarity to E Coli SSBrdquoJournal ofMolecular Biology vol 420 no 4-5 pp 269ndash283 2012
[42] H Jan Y L Lee and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from pseudomonas aeruginosaPAO1rdquo Protein Journal vol 30 no 1 pp 20ndash26 2011
[43] Y-H Huang Y-L Lee and C-Y Huang ldquoCharacterization of asingle-stranded DNA binding protein from Salmonella entericaserovar typhimurium LT2rdquo Protein Journal vol 30 no 2 pp102ndash108 2011
[44] S K Bharti K Rex P Sreedhar N Krishnan and U VarshneyldquoChimeras of Escherichia coli and Mycobacterium tuberculosissingle-stranded DNA binding proteins characterization andfunction in Escherichia colirdquo PLoS ONE vol 6 no 12 ArticleID e27216 2011
[45] M T Oliveira and L S Kaguni ldquoFunctional roles of the N-and C-terminal regions of the human mitochondrial single-stranded DNA-binding proteinrdquo PLoS ONE vol 5 no 10Article ID e15379 2010
[46] A G Kozlov J M Eggington M M Cox and T MLohman ldquoBinding of the dimeric Deinococcus radioduranssingle-strandedDNA binding protein to single-strandedDNArdquoBiochemistry vol 49 no 38 pp 8266ndash8275 2010
[47] T M Lohman and M E Ferrari ldquoEscherichia coli single-stranded DNA-binding protein multiple DNA-binding modesand cooperativesrdquo Annual Review of Biochemistry vol 63 pp527ndash570 1994
[48] R Zhou A G Kozlov R Roy et al ldquoSSB functions as a slidingplatform that migrates on DNA via reptationrdquo Cell vol 146 pp222ndash232 2011
[49] R Roy A G Kozlov T M Lohman and T Ha ldquoSSB proteindiffusion on single-stranded DNA stimulates RecA filamentformationrdquo Nature vol 461 pp 1092ndash1097 2009
[50] R Roy A G Kozlov T M Lohman and T Ha ldquoDynamicstructural rearrangements between DNA binding modes of Ecoli SSB proteinrdquo Journal of Molecular Biology vol 369 no 5pp 1244ndash1257 2007
[51] T J Kelly P Simancek and G S Brush ldquoIdentification andcharacterization of a single-stranded DNA-binding proteinfrom the archaeon Methanococcus jannaschiirdquo Proceedings ofthe National Academy of Sciences of the United States of Americavol 95 no 25 pp 14634ndash14639 1998
[52] C Y Huang Determination of the Binding Site-Size of theProtein-DNA Complex by Use of the Electrophoretic MobilityShift Assay InTech Press Rijeka Croatia 2012
[53] A Kornberg ldquoTen commandments of enzymology amendedrdquoTrends in Biochemical Sciences vol 28 no 10 pp 515ndash517 2003
[54] W Zhang X Lu and J Shen ldquoEMSA and single-moleculeforce spectroscopy study of interactions between bacillus sub-tilis single-stranded DNA-binding protein and single-strandedDNArdquo Langmuir vol 27 no 24 pp 15008ndash15015 2011
[55] M Ostermeier and S J Benkovic ldquoEvolution of protein func-tion by domain swappingrdquo Advances in Protein Chemistry vol55 pp 29ndash77 2000
[56] W F Peng and C Y Huang ldquoAllantoinase and dihydroorotasebinding and inhibition by flavonols and the substrates of cyclicamidohydrolasesrdquo Biochimie vol 101 pp 113ndash122 2014
[57] Y H Huang M J Lin and C Y Huang ldquoDnaT is a single-stranded DNA binding proteinrdquo Genes to Cells vol 18 no 11pp 1007ndash1019 2013
[58] Y Y Ho Y H Huang and C Y Huang ldquoChemical rescue of thepost-translationally carboxylated lysine mutant of allantoinaseand dihydroorotase by metal ions and short-chain carboxylicacidsrdquo Amino Acids vol 44 no 4 pp 1181ndash1191 2013
[59] Y H Huang Y Lo W Huang and C Y Huang ldquoCrystalstructure and DNA-binding mode of Klebsiella pneumoniaeprimosomal PriB proteinrdquoGenes to Cells vol 17 no 10 pp 837ndash849 2012
[60] C Y Huang C Y Hsu Y Sun H Wu and C Hsiao ldquoCom-plexed crystal structure of replication restart primosome pro-tein PriB reveals a novel single-stranded DNA-binding moderdquoNucleic Acids Research vol 34 no 14 pp 3878ndash3886 2006
[61] T Kinebuchi H Shindo H Nagai N Shimamoto andM Shimizu ldquoFunctional domains of Escherichia coli single-stranded DNA binding protein as assessed by analyses of thedeletion mutantsrdquo Biochemistry vol 36 no 22 pp 6732ndash67381997
[62] N Shimamoto N Ikushima H Utiyama H Tachibana andK Horie ldquoSpecific and cooperative binding of E coli single-stranded DNA binding protein to mRNArdquo Nucleic AcidsResearch vol 15 no 13 pp 5241ndash5250 1987
[63] H Lin andC YHuang ldquoCharacterization of flavonol inhibitionof DnaB helicase Real-time monitoring structural modelingand proposed mechanismrdquo Journal of Biomedicine and Biotech-nology vol 2012 Article ID 735368 2012
[64] Y H Huang H H Lin and C Y Huang ldquoA single residuedetermines the cooperative binding property of a primosomalDNA replication protein PriB to single-stranded DNArdquo Bio-science Biotechnology and Biochemistry vol 76 no 6 pp 1110ndash1115 2012
[65] H C Hsieh and C Y Huang ldquoIdentification of a novel proteinPriB in Klebsiella pneumoniaerdquo Biochemical and BiophysicalResearch Communications vol 404 no 1 pp 546ndash551 2011
[66] J H Liu T W Chang C Y Huang et al ldquoCrystal structure ofPriB a primosomalDNA replication protein ofEscherichia colirdquoThe Journal of Biological Chemistry vol 279 no 48 pp 50465ndash50471 2004
[67] Y H Huang and C Y Huang ldquoThe N-terminal domain ofDnaT a primosomalDNA replication protein is crucial for PriBbinding and self-trimerizationrdquo Biochemical and BiophysicalResearch Communications vol 442 pp 147ndash152 2013
[68] M A Larkin G Blackshields N P Brown et al ldquoClustalW andClustal X version 20rdquo Bioinformatics vol 23 no 21 pp 2947ndash2948 2007
16 BioMed Research International
[69] S N Savvides S Raghunathan K Futterer A G Kozlov T MLohman and G Waksman ldquoThe C-terminal domain of full-length E coli SSB is disordered even when bound to DNArdquoProtein Science vol 13 no 7 pp 1942ndash1947 2004
[70] C-C Chen J-K Hwang and J-M Yang ldquo(PS)2-v2 template-based protein structure prediction serverrdquo BMC Bioinformaticsvol 10 article 366 2009
[71] C Chen J Hwang and J Yang ldquo(PS)2 protein structure pre-diction serverrdquoNucleic Acids Research vol 34 pp W152ndashW1572006
[72] K Bush and J F Fisher ldquoEpidemiological expansion structuralstudies and clinical challenges of new 120573-lactamases from gram-negative bacteriardquo Annual Review of Microbiology vol 65 pp455ndash478 2011
[73] K K Kumarasamy M A Toleman T R Walsh et al ldquoEmer-gence of a new antibiotic resistance mechanism in India Pak-istan and the UK a molecular biological and epidemiologicalstudyrdquoThe Lancet Infectious Diseases vol 10 no 9 pp 597ndash6022010
[74] K Bush and M J MacIelag ldquoNew 120573-lactam antibiotics and 120573-lactamase inhibitorsrdquo Expert Opinion on Therapeutic Patentsvol 20 no 10 pp 1277ndash1293 2010
[75] K Bush ldquoAlarming 120573-lactamase-mediated resistance in multi-drug-resistant Enterobacteriaceaerdquo Current Opinion in Microbi-ology vol 13 no 5 pp 558ndash564 2010
[76] A Srivastava M Talaue S Liu et al ldquoNew target for inhibitionof bacterial RNA polymerase ldquoswitch regionrdquordquo Current Opinionin Microbiology vol 14 no 5 pp 532ndash543 2011
[77] K Chono K Katsumata T Kontani et al ldquoASP2151 a novelhelicase-primase inhibitor possesses antiviral activity againstvaricella-zoster virus and herpes simplex virus types 1 and 2rdquoJournal of Antimicrobial Chemotherapy vol 65 no 8 pp 1733ndash1741 2010
[78] M T Black and K Coleman ldquoNew inhibitors of bacterial topoi-somerase GyrAParC subunitsrdquo Current Opinion in Investiga-tional Drugs vol 10 no 8 pp 804ndash810 2009
[79] A H Marceau D A Bernstein B W Walsh W Shapiro LA Simmons and J L Keck ldquoProtein interactions in genomemaintenance as novel antibacterial targetsrdquo PLoS ONE vol 8no 3 Article ID e58765 2013
[80] D Lu D A Bernstein K A Satyshur and J L Keck ldquoSmall-molecule tools for dissecting the roles of SSBprotein inter-actions in genome maintenancerdquo Proceedings of the NationalAcademy of Sciences of the United States of America vol 107 no2 pp 633ndash638 2010
[81] I Wong and T M Lohman ldquoA double-filter method fornitrocellulose-filter binding application to protein-nucleic acidinteractionsrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 90 no 12 pp 5428ndash5432 1993
[82] M Mitas J Y Chock and M Christy ldquoThe binding-site sizesof Escherichia coli single-stranded-DNA-binding protein andmammalian replication protein A are 65 and ge 54 nucleotidesrespectivelyrdquo Biochemical Journal vol 324 no 3 pp 957ndash9611997
[83] C Urbanke G Witte and U Curth ldquoSedimentation velocitymethod in the analytical ultracentrifuge for the study of protein-protein interactionsrdquoMethods inMolecular Biology vol 305 pp101ndash114 2005
[84] S Chrysogelos and J Griffith ldquoEscherichia coli single-strandbinding protein organizes single-strandedDNA innucleosome-like unitsrdquo Proceedings of the National Academy of Sciences of theUnited States of America vol 79 no 19 pp 5803ndash5807 1982
[85] J R Casas-Finet K R Fischer and R L Karpel ldquoStructuralbasis for the nucleic acid binding cooperativity of bacteriophageT4 gene 32 protein the (LysArg)3(SerThr)2 (LAST) motifrdquoProceedings of the National Academy of Sciences of the UnitedStates of America vol 89 pp 1050ndash1054 1992
[86] G Witte R Fedorov and U Curth ldquoBiophysical analysis ofThermus aquaticus single-stranded DNA binding proteinrdquo Bio-physical Journal vol 94 no 6 pp 2269ndash2279 2008
[87] R Fedorov G Witte C Urbanke D J Manstein and UCurth ldquo3D structure of Thermus aquaticus single-strandedDNA-binding protein gives insight into the functioning of SSBproteinsrdquo Nucleic Acids Research vol 34 no 22 pp 6708ndash67172006
[88] K L Tsai C Y Huang C H Chang Y J Sun W J ChuangandC DHsiao ldquoCrystal structure of the human FOXK1a-DNAcomplex and its implications on the diverse binding specificityof winged helixforkhead proteinsrdquo Journal of Biological Chem-istry vol 281 no 25 pp 17400ndash17409 2006
[89] A H Mao N Lyle and R V Pappu ldquoDescribing sequence-ensemble relationships for intrinsically disordered proteinsrdquoBiochemical Journal vol 449 no 2 pp 307ndash318 2013
[90] R Wetzel ldquoPhysical chemistry of polyglutamine Intriguingtales of a monotonous sequencerdquo Journal of Molecular Biologyvol 421 no 4-5 pp 466ndash490 2012
[91] H T Orr ldquoPolyglutamine neurodegeneration expanded glu-tamines enhance native functionsrdquo Current Opinion in Geneticsand Development vol 22 no 3 pp 251ndash255 2012
[92] M Figiel W J Szlachcic P M Switonski A Gabka and W JKrzyzosiak ldquoMouse models of polyglutamine diseases reviewand data table Part Irdquo Molecular Neurobiology vol 46 no 2pp 393ndash429 2012
[93] J Nasica-Labouze and N Mousseau ldquoKinetics of amyloidaggregation a study of the GNNQQNY prion sequencerdquo PLoSComputational Biology vol 8 no 11 Article ID e1002782 2012
[94] J Nasica-Labouze M Meli P Derreumaux G Colombo andN Mousseau ldquoA multiscale approach to characterize the earlyaggregation steps of the amyloid-forming peptide gnnqqnyfrom the yeast prion sup-35rdquo PLoS Computational Biology vol7 no 5 Article ID e1002051 2011
[95] J R Lewandowski P C A van der Wel M Rigney N Grig-orieff and R G Griffin ldquoStructural complexity of a compositeamyloid fibrilrdquo Journal of the American Chemical Society vol133 no 37 pp 14686ndash14698 2011
8 BioMed Research International
[PaSSB]
-dT20
mdash
(a)
-dT25
-C
[PaSSB]mdash
(b)
-dT35
-C
-
-
[PaSSB]
(c)
-dT45
-C
[PaSSB]
(d)
-dT55
-C
[PaSSB]
(e)
-dT60
-C1-C2
[PaSSB]
(f)
-dT65
-C1-C2
[PaSSB]
(g)
Figure 6 Binding of PaSSB to dT20ndash65 PaSSB (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for 30min at 25∘C with17 nM of (a) dT20 (b) dT25 (c) dT35 (d) dT45 (e) dT55 (f) dT60 or (g) dT65 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
whether the flexible glycine-rich hinge in the C-terminaldomain which is not conserved among SSBs is involved inthe determination of the binding site size of SSB Thus theC-terminal domains of StSSB and PaSSB were swapped withKpSSB through protein chimeragenesis
37 KpSSBnStSSBc Bound to ssDNA The binding ofKpSSBnStSSBc to dT15 (Figure 7(a)) dT20 (Figure 7(b))dT40 (Figure 7(c)) and dT45 (Figure 7(d)) was examinedusing EMSA KpSSBnStSSBc exhibited significantly different
ssDNA-binding properties from those of KpSSB UnlikeKpSSB (Figure 4) both KpSSBnStSSBc (Figure 8) and StSSB(Figure 5) can bind and form a single complex with dT15 anddT20 Similar to StSSB KpSSBnStSSBc binds to dT15ndash40 andforms a single complex For dT45 two different complexesof KpSSBnStSSBc appeared at high protein concentrations(Figure 8(d)) this EMSA feature was also similar to that ofStSSB One KpSSBnStSSBc occupies 20 (402 = 20) nt to225 (452 = 225) nt of the ssDNA These EMSA resultssuggest that the length of an ssDNA (or the binding site
BioMed Research International 9
[KpSSBnStSSBc]
-dT15
-C
(a)
[KpSSBnStSSBc]
-dT20
-C
(b)
[KpSSBnStSSBc]
-dT40
-C
(c)
[KpSSBnStSSBc]
-dT45
-C1-C2
(d)
Figure 7 Binding of KpSSBnStSSBc to dT15ndash45 KpSSBnStSSBc (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for30min at 25∘C with 17 nM of (a) dT15 (b) dT20 (c) dT40 or (d) dT45 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and 100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blue and40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
size) [52] required for KpSSBnStSSBc binding is 21 plusmn 2 nt avalue identical to that for StSSB (Figure 5) Swapping of theC-terminal domain of StSSB with KpSSB changes the size ofthe ssDNA-binding site from 26 nt to 21 nt
38 KpSSBnPaSSBc Bound to ssDNA The binding fea-tures of KpSSBnPaSSBc with dT20 (Figure 8(a)) dT25(Figure 8(b)) dT40 (Figure 8(c)) dT55 (Figure 8(d)) anddT60 (Figure 8(e)) were studied by EMSA Similar to thecases of KpSSB and PaSSB no complex was observed whenKpSSBnPaSSBc was incubated with dT20 However KpSSB-nPaSSBc still exhibited dramatically different ssDNA-bindingproperties from those of KpSSB KpSSB can form two distinctcomplexes with dT55 (Figure 4(f)) but both KpSSBnPaSSBc(Figure 9) and PaSSB (Figure 6) cannot One KpSSBnPaSSBcoccupies 275 (552 = 275) nt to 30 (602 = 30) nt ofthe ssDNA The above EMSA results suggest that the lengthof an ssDNA (or the binding site size) [52] required forKpSSBnPaSSBc binding is 29 plusmn 2 nt a value identical to thatof PaSSB Swapping of the C-terminal domain of PaSSB toKpSSB changes the size of the ssDNA-binding site from 26 ntto 29 nt Although these SSBs namely KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc have nearly identicalssDNA-binding domains their binding site sizes are different(Table 2) Thus the size of the ssDNA-binding site requiredfor second SSB binding is likely to be dependent on the C-terminal domain of SSB
39 Binding Constants of the SSB-ssDNA Complexes Deter-mined from EMSA To compare the ssDNA-binding abil-ities of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc the midpoint values for input ssDNA bind-ing calculated from the titration curves of EMSA andreferred to as [Protein]
50(monomer) were quantified and
are summarized in Table 2 Although the N-terminal ssDNA-binding domains of these SSB proteins are highly similar(Figure 2) their ssDNA-binding activities and binding sitesizes are different (Table 2) [KpSSB]
50values ranged from
100 nM to 220 nM [StSSB]50
values ranged from 420 nM to650 nM [PaSSB]
50values ranged from 550 nM to 1700 nM
[KpSSBnStSSBc]50values ranged from 110 nM to 260 nM and
[KpSSBnPaSSBc]50
values ranged from 220 nM to 390 nMThe ssDNA-binding ability is as follows in the order ofdecreasing affinity KpSSB gt KpSSBnStSSBc gt KpSSBn-PaSSBc gt StSSB gt PaSSB Results from the above analysesindicate that the exchange of the C-terminal domain inSSB significantly changed the ssDNA-binding ability and theDNA-binding behavior (complex number) The reason asto why swapping of the C-terminal domain can affect thessDNA-binding activity of SSB remains unclear The C-ter-minal domain of SSB is suggested to be involved in ssDNAbinding However this relation is not evident in the results ofthe cocrystal structure
310 Oligomeric State of KpSSBnStSSBc and KpSSBnPaSSBc inSolution Gel-filtration chromatography was used to confirm
10 BioMed Research International
[KpSSBnPaSSBc]
-dT20
(a)
[KpSSBnPaSSBc]
-dT25
-C
(b)
[KpSSBnPaSSBc]
-C
-dT40
(c)
-C
[KpSSBnPaSSBc]
-dT55
(d)
-dT60
-C1-C2
[KpSSBnPaSSBc]
(e)
Figure 8 Binding of KpSSBnPaSSBc to dT20ndash60 KpSSBnPaSSBc (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for30min at 25∘C with 17 nM of (a) dT20 (b) dT25 (c) dT40 (d) dT55 or (e) dT60 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
that the oligomeric state of KpSSBnStSSBc and KpSSBn-PaSSBc remains as tetramers after chimeragenesisThe analy-sis of purified KpSSBnStSSBc and KpSSBnPaSSBc (2mgmL)using a Superdex 200HR 1030 column revealed a singlepeak with elution volumes of 786 and 789mL respectivelyAssuming that KpSSBnStSSBc and KpSSBnPaSSBc both haveshapes and partial specific volumes similar to the standardproteins the native molecular masses of KpSSBnStSSBc andKpSSBnPaSSBc were estimated to be 76641 and 74827Da ascalculated from a standard linear regression equation 119870av =minus03684(logMw) + 22707 (Figure 9) The native molecularmasses for KpSSBnStSSBc and KpSSBnPaSSBc are approx-imately four times the mass of the monomer (sim19 kDa)Therefore KpSSBnStSSBc and KpSSBnPaSSBc under theabove chromatographic conditions are stable tetramers insolution Although the exchange of the C-terminal domainin SSB significantly changed the ssDNA-binding ability andDNA-binding behavior (complex number) protein chimera-genesis did not cause any change in the oligomeric state ofSSB
311 Summary of Gly Gln and Pro Number in SSBs To ana-lyze the C-terminal amino acid composition of SSBs wefurther counted the number of Gly Gln and Pro residuesin different SSB segments SSB is abundant in Gly Glnand Pro (GQP) (Table 3) The GQP contents of KpSSB1ndash91 StSSB1ndash91 and PaSSB1ndash90 are similar However the Glynumber of PaSSB116ndash165 is significantly lower than that ofKpSSB116ndash174 and StSSB117ndash176 PaSSB116ndash165 contains only1 Gly but KpSSB116ndash174 and StSSB117ndash176 contain 11 and 12Gly respectively In addition we found different distributionpatterns among KpSSB StSSB and PaSSB Although theycontain similar number of Gln (Q) the QQQ pattern isfrequently found in PaSSB (Table 3)
312 Structural Modeling of SSBs Given its disordered C-ter-minal domain the crystal structure of the full-length SSB islacking even when SSB can be crystallized with DNA [69]We attempted to model the structure by homology mod-eling using the bioinformatics program (PS)2 to obtain an
BioMed Research International 11
Table 2 ssDNA binding properties of KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc as analyzed by EMSA
Protein DNA [Protein]50 (nM) Complex number
KpSSB
dT20 ND 0dT25 200 plusmn 20 1dT35 220 plusmn 30 1dT45 100 plusmn 10 1dT50 110 plusmn 20 1dT55 100 plusmn 20 2dT60 100 plusmn 10 2
StSSB
dT15 650 plusmn 120 1dT20 450 plusmn 80 1dT30 420 plusmn 60 1dT40 420 plusmn 80 1dT45 440 plusmn 60 2dT50 440 plusmn 50 2
PaSSB
dT20 ND 0dT25 1700 plusmn 250 1dT35 950 plusmn 180 1dT45 780 plusmn 160 1dT55 820 plusmn 90 1dT60 810 plusmn 110 2dT65 550 plusmn 70 2
KpSSBnStSSBc
dT15 260 plusmn 60 1dT20 110 plusmn 20 1dT40 120 plusmn 20 1dT45 160 plusmn 20 2
KpSSBnPaSSBc
dT20 ND 0dT25 390 plusmn 60 1dT40 220 plusmn 30 1dT55 230 plusmn 30 1dT60 230 plusmn 30 2
[Protein]50 was calculated from the titration curves of EMSA by determiningthe concentration of the protein (120583M) needed to achieve the midpointvalue for input ssDNA binding For some oligonucleotides input ssDNAbinding was the sum of the intensities from the two separate ssDNA-proteincomplexes Errors are standard deviations determined by three independenttitration experiments
Table 3 Summary of Gly Gln and Pro number in SSB
SSB segment G Q PKpSSB1ndash91 10 5 2StSSB1ndash91 10 5 2PaSSB1ndash90 11 6 2KpSSB92ndash174 18 16 9StSSB92ndash176 17 18 11PaSSB91ndash165 5 15 11KpSSB116ndash174 11 12 9StSSB117ndash176 12 13 10PaSSB116ndash165 1 12 11
in-depth understanding of the structure-function relation-ship of the C-terminal domains of these SSBs [70 71]
40 45 50 55
Kav
00
02
04
06
08
10
KpSSBnStSSBcKpSSBnPaSSBc
Y = minus03684X + 22707
R2= 0998
log Mw
Figure 9Gel-filtration chromatographic analyses of KpSSBnStSSBcand KpSSBnPaSSBc Purified protein (2mgmL) was applied toa Superdex 200HR 1030 column (GE Healthcare Bio-SciencesPiscataway NJ USA) equilibrated with Buffer D The column wasoperated at a flow rate of 05mLmin and 05mL fractions werecollected The proteins were detected by measuring the absorbanceat 280 nm The column was calibrated with proteins of knownmolecular weight thyroglobulin (670 kDa) 120574-globulin (158 kDa)ovalbumin (44 kDa) and myoglobin (17 kDa) The 119870av values forthe standard proteins and the SSB variants were calculated from theequation119870av = (119881119890minus119881119900)(119881119888minus119881119900) where119881119900 is column void volume119881119890is elution volume and 119881
119888is geometric column volume
(PS)2 (http140113239111simps2v2docsphp) is an auto-matic homology modeling server that combines bothsequence and secondary structure information to detect thehomologous proteins with remote similarity and the target-template alignment After pasting the amino acid sequenceto the website of (PS)2 only one hit (Protein Data Bankentry 1QVC EcSSB) for the C-terminal domains of KpSSBand StSSB was suggested For the C-terminal domain ofPaSSB only one hit that is CstF-77 (ProteinData Bank entry2OOE cleavage stimulation factor CstF) but not EcSSB wassuggested as the template for modeling Figure 10 shows thatmodeled structures of these SSB C-terminal domains arehighly disordered but that of PaSSB is more ordered than thatof other domains
4 Discussion
In this study we examined the sizes of the binding site ofthe untagged SSB and the chimeric SSB from the ubiquitousopportunistic pathogens K pneumoniae S enterica serovarTyphimurium LT2 and P aeruginosa PAO1 Many clinicalstrains of the abovementioned bacteria are highly resistantto antibiotics [72ndash75] The development of clinically usefulsmall-molecule antibiotics has been a seminal event in thefield of infectious diseases [48] Nucleic acid metabolismis one of the most basic biological functions and shouldbe a prime target in antibiotic development [76ndash78] Manybacterial SSBs form conserved protein interaction ldquohubsrdquothat are essential to recruit many proteins involved in DNA
12 BioMed Research International
Figure 10 Structure modeling of SSB The structures of KpSSB1ndash115 StSSB1ndash115 and PaSSB1ndash115 (the N-terminal domain of SSB)were modeled by SWISS-MODEL The structures of KpSSB116ndash142 StSSB116ndash142 and PaSSB121ndash160 (the C-terminal domain ofSSB) were modeled by (PS)2 Other regions of SSBs could not bemodeled by these two programs The structures of the N-terminaldomain and the C-terminal domain of these SSBs were manuallylinked (KpSSB1ndash142 blue StSSB1ndash142 pink PaSSB1ndash160 green) andsuperimposed with the crystal structure of EcSSB1ndash142 (orange)(PDB entry 1QVC) for comparison For clarity only one subunitof the tetramer was shown for each SSB
replication recombination and repair SSBDNA nucleopro-tein substrates [79] Thus SSBs may be promising targets inantibiotic development [80] As a first step toward achievingthis goal we investigated why SSBs possess highly conservedN-terminal ssDNA-binding domain but exhibit varying bind-ing site sizes One significant clue is that their flexible hingesand the length at the C-terminus are different as revealed bysequence alignment (Figure 2)
The interactions of various SSBs with ssDNA have beenanalyzed using a variety of techniques such as tryptophan-fluorescence quenching [47] filter binding [81] EMSA [5282] analytical ultracentrifugation [83] electron microscopy[84] nuclease digestion [44] single-molecule fluorescencemicroscopy [48] and crystallographic analyses [11] In thisstudy we have examined the electrophoretic mobility shiftpatterns of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc bound to different lengths of ssDNA and deter-mined the corresponding binding site sizes to be 26 2129 22 and 29 nt per tetramer respectively (Figures 4ndash8) PaSSB and KpSSBnPaSSBc have the largest sizes forssDNA binding among the SSBs studied We also identifiedHis-tagged and untagged SSBs that have similar ssDNA-binding site sizes [40 42 43] EMSA is a well-establishedapproach in studies of molecular biology [52] and the use ofradioactive tracer in this assay allows detection of the actualformation of the distinct protein-DNA complex(es) [53] For
exampleDNase protection assay and footprinting assay usingradioactive tracer can determine the specific DNA sequencecomplexed by a protein In EMSA when the length of thenucleotides is sufficient for the binding of two or more SSBmolecules the electrophoretic mobility of the higher SSBoligomer complex will be lower than that of the smaller SSBoligomer complex [52 54] In addition results of the ssDNA-binding site size from EMSA and cocrystal structure of SSBwere consistent [27] Thus throughout this paper we deter-mined the ssDNA-binding site sizes of SSB from the EMSAbehavior
Many SSBs bind to ssDNA with some degree of positivecooperativity Cooperativity can result from direct protein-protein interactions between the nearest neighbors such asthe LAST motif in the T4 gene-32 protein [85] and thearginine-mediated interaction motif in Thermus SSB [8687] Cooperativity can also result from the protein-induceddistortion of adjacent DNA as demonstrated in SulfolobusSSB PriB and FOXK1a proteins [23 60 88] In the cases ofKpSSB StSSB and PaSSB (Figures 4ndash6) binding appeared tobe nearly noncooperative for several DNAs because all DNAmainly shifts into the first complex (C1) before the appearanceof the second complex (C2) when subjected to increasingprotein concentrationsThe length dependence of the [SSB]
50
values suggests that the amount of spacing is optimum forsteric considerations (Table 2)
Because bacteria have varying genomic DNA sizes theirSSBs may need to evolve to have different binding site sizesfor DNA metabolism Results from protein chimeragenesisshowed the C-terminal domain dependence of the bindingsite sizes of SSB (Figure 11) The experimental data showedthat the binding site size of KpSSBnStSSBc was similar to thatof StSSB and the size of the binding site of KpSSBnPaSSBcwas similar to that of PaSSB The reason for which the bind-ing site size of SSB changed followed by swapping of theC-terminal domain remains unclear Flexibility numberof glycine residues andor different QQQ patterns of theC-terminal domain of SSB (Figure 2 and Table 3) may beimportant factors for determining the ssDNA-binding sitesize In fact the C-terminal domain of PaSSB that isPaSSB116ndash165 has only 1 Gly residue which is significantlyless than that of KpSSB (11 Gly) and StSSB (12Gly) Gly (andPro) is an important component of the flexible region aprotein that contains low Gly content is predicted to havelow flexibility Unlike typical SSB [35 69] PaSSB116ndash165 hasa partial structure (Figure 10) Although KpSSB StSSB andPaSSB contain similar number of Gln (Q) the QQQ patternis frequently found in PaSSB (Figure 2 and Table 3) PolyQand repeated sequences GAGAG are commonly found inthe structures of amyloids silk fibers and neurodegradationproteins [89ndash92] Considering that the simple coil polyQ theheptapeptide GNNQQNY and the hexapeptide NNQQNYcan cause protein aggregation and nucleation [93ndash95] thedistribution of Gln in the C-terminal domain of a tetramericSSB may also be an important determinant of the ssDNA-binding site size of SSB by some steric hindrances (Figure 11)However the above speculationmust be confirmed by furtherbiochemical experiments
BioMed Research International 13
PaSSBStSSB KpSSB
C-terC-ter C-ter
sim 21 nt sim 26 nt sim 29 nt
Figure 11 Possiblemodels for explainingwhy SSBs arewith different binding site sizes Twomodeled structures of KpSSB1ndash142 (blue) StSSB1ndash142 (pink) and PaSSB1ndash160 (green) complexed with ssDNA (gold) are shown For clarity only one C-terminal domain was shown for eachSSB tetramer By using the electrophoretic mobility shift assay and the protein chimeragenesis we characterized that the binding site sizes ofKpSSB StSSB PaSSB KpSSBnStSSBc and KpSSBnPaSSBc were 26 21 29 21 and 29 nt per tetramer respectively KpSSB StSSB and PaSSBare similar proteins whose N-terminal ssDNA-binding domains are almost identical Thus the C-terminal domain of SSB may indirectlycontribute to ssDNA binding and wrapping and affects the binding site size by the steric hindrance
5 Conclusion
In this study we characterized the ssDNA-binding propertiesof untagged SSBs from K pneumoniae S enterica sero-var Typhimurium LT2 and P aeruginosa PAO1 and proposeda role of the C-terminal flexible domain for ssDNA bind-ing from the protein chimeragenesis and EMSA resultsThe amino acid sequence of the N-terminal ssDNA-bind-ingoligomerization domain in these pathogenic SSBs ishighly conserved but their apparent binding site sizes aredifferent This finding indicates that the C-terminal protein-protein interaction domain may also indirectly contribute tossDNA binding and wrapping
Conflict of Interests
The authors declare that there is no conflict of interestsregarding the publication of this paper
Acknowledgment
This researchwas supported by aGrant from theNational Sci-ence Council Taiwan (NSC 102-2320-B-040-019 to Cheng-Yang Huang)
References
[1] R Reyes-Lamothe D J Sherratt and M C Leake ldquoStoichiom-etry and architecture of active DNA replication machinery inescherichia colirdquo Science vol 328 no 5977 pp 498ndash501 2010
[2] D J Richard E Bolderson andK K Khanna ldquoMultiple humansingle-stranded DNA binding proteins function in genomemaintenance structural biochemical and functional analysisrdquo
Critical Reviews in Biochemistry and Molecular Biology vol 44no 2-3 pp 98ndash116 2009
[3] R D Shereda A G Kozlov T M LohmanMM Cox and J LKeck ldquoSSB as an organizermobilizer of genome maintenancecomplexesrdquo Critical Reviews in Biochemistry and MolecularBiology vol 43 no 5 pp 289ndash318 2008
[4] D J Richard E Bolderson L Cubeddu et al ldquoSingle-strandedDNA-binding protein hSSB1 is critical for genomic stabilityrdquoNature vol 453 no 7195 pp 677ndash681 2008
[5] R R Meyer and P S Laine ldquoThe single-stranded DNA-bindingprotein of Escherichia colirdquoMicrobiological Reviews vol 54 no4 pp 342ndash380 1990
[6] C Yang U Curth C Urbanke and C Kang ldquoCrystal structureof human mitochondrial single-stranded DNA binding proteinat 24 A resolutionrdquo Nature Structural Biology vol 4 no 2 pp153ndash157 1997
[7] G Webster J Genschel U Curth C Urbanke C Kang and RHilgenfeld ldquoA common core for binding single-stranded DNAstructural comparison of the single-stranded DNA-bindingproteins (SSB) from E coli and human mitochondriardquo FEBSLetters vol 411 pp 313ndash316 1997
[8] R L Flynn and L Zou ldquoOligonucleotideoligosaccharide-bind-ing fold proteins a growing family of genome guardiansrdquo Crit-ical Reviews in Biochemistry and Molecular Biology vol 45 no4 pp 266ndash275 2010
[9] K Chan Y Lee CWangHHuang andY Sun ldquoSingle-Strand-ed DNA-Binding Protein Complex from Helicobacter pyloriSuggests an ssDNA-Binding Surfacerdquo Journal of MolecularBiology vol 388 no 3 pp 508ndash519 2009
[10] D L Theobald R M Mitton-Fry and D S Wuttke ldquoNucleicacid recognition by OB-fold proteinsrdquo Annual Review of Bio-physics and Biomolecular Structure vol 32 pp 115ndash133 2003
[11] S Raghunathan A G Kozlov TM Lohman andGWaksmanldquoStructure of the DNA binding domain of E coli SSB bound to
14 BioMed Research International
ssDNArdquo Nature Structural Biology vol 7 no 8 pp 648ndash6522000
[12] A G Murzin ldquoOB(oligonucleotideoligosaccharide binding)-fold common structural and functional solution for non-homologous sequencesrdquo EMBO Journal vol 12 no 3 pp 861ndash867 1993
[13] N P George K V Ngo S Chitteni-Pattu et al ldquoStructure andcellular dynamics of deinococcus radiodurans single-strandedDNA (ssDNA)-binding protein (SSB)-DNA complexesrdquo TheJournal of Biological Chemistry vol 287 no 26 pp 22123ndash221322012
[14] P Filipkowski and J Kur ldquoIdentification and propertiesof the Deinococcus grandis and Deinococcus proteolyticussingle-stranded DNA binding proteins (SSB)rdquo Acta BiochimicaPolonica vol 54 no 1 pp 79ndash87 2007
[15] P Filipkowski A Duraj-Thatte and J Kur ldquoIdentificationcloning expression and characterization of a highly ther-mostable single-stranded-DNA-binding protein (SSB) fromDeinococcus murrayirdquo Protein Expression and Purification vol53 no 1 pp 201ndash208 2007
[16] P Filipkowski M Koziatek and J Kur ldquoA highly ther-mostable homodimeric single-stranded DNA-binding proteinfrom Deinococcus radiopugnansrdquo Extremophiles vol 10 no 6pp 607ndash614 2006
[17] P Filipkowski A Duraj-Thatte and J Kur ldquoNovel thermostablesingle-stranded DNA-binding protein (SSB) fromDeinococcusgeothermalisrdquo Archives of Microbiology vol 186 no 2 pp 129ndash137 2006
[18] G Witte C Urbanke and U Curth ldquoSingle-stranded DNA-binding protein of Deinococcus radiodurans a biophysicalcharacterizationrdquoNucleic Acids Research vol 33 no 5 pp 1662ndash1670 2005
[19] D A Bernstein J M Eggingtont M P Killoran A M MisicMMCox and J L Keck ldquoCrystal structure of theDeinococcusradiodurans single-stranded DNA-binding protein suggests amechanism for coping with DNA damagerdquo Proceedings of theNational Academy of Sciences of the United States of Americavol 101 no 23 pp 8575ndash8580 2004
[20] S Dabrowski M Olszewski R Piatek A Brillowska-Dabrowska G Konopa and J Kur ldquoIdentification and charac-terization of single-stranded-DNA-binding proteins fromTher-mus thermophilus and Thermus aquaticusmdashnew arrange-ment of binding domainsrdquo Microbiology vol 148 no 10 pp3307ndash3315 2002
[21] R Gamsjaeger R Kariawasam C Touma A H Kwan M FWhite and L Cubeddu ldquoBackbone and side-chain 1H 13C and15N resonance assignments of the OB domain of the singlestranded DNA binding protein from Sulfolobus solfataricusand chemical shift mapping of the DNA-binding interfacerdquoBiomolecular NMR Assignments 2013
[22] M L Rolfsmeier and C A Haseltine ldquoThe single-strandedDNA binding protein of Sulfolobus solfataricus acts in the pre-synaptic step of homologous recombinationrdquo Journal of Molec-ular Biology vol 397 no 1 pp 31ndash45 2010
[23] I D Kerr R I M Wadsworth L Cubeddu W Blankenfeldt JHNaismith andM FWhite ldquoInsights into ssDNA recognitionby the OB fold from a structural and thermodynamic study ofSulfolobus SSB proteinrdquo EMBO Journal vol 22 no 11 pp 2561ndash2570 2003
[24] C A Haseltine and S C Kowalczykowski ldquoA distinctive single-stranded DNA-binding protein from the Archaeon Sulfolobussolfataricusrdquo Molecular Microbiology vol 43 no 6 pp 1505ndash1515 2002
[25] R I M Wadsworth and M F White ldquoIdentification andproperties of the crenarchaeal single-stranded DNA bindingprotein from Sulfolobus solfataricusrdquo Nucleic Acids Researchvol 29 no 4 pp 914ndash920 2001
[26] S Sugiman-Marangos and M S Junop ldquoThe structure of DdrBfrom Deinococcus a new fold for single-stranded DNA bindingproteinsrdquo Nucleic Acids Research vol 38 no 10 Article IDgkq036 pp 3432ndash3440 2010
[27] H Ghalei H V Moeller D Eppers et al ldquoEntrapment of DNAin an intersubunit tunnel system of a single-stranded DNA-binding proteinrdquo Nucleic Acids Research vol 42 no 10 pp6698ndash6708 2014
[28] S Paytubi S A McMahon S Graham et al ldquoDisplacement ofthe canonical single-strandedDNA-binding protein in the ther-moprotealesrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 109 no 7 pp E398ndashE405 2012
[29] T H Dickey S E Altschuler and D S Wuttke ldquoSingle-stranded DNA-binding proteins multiple domains for multiplefunctionsrdquo Structure vol 21 no 7 pp 1074ndash1084 2013
[30] D Vujaklija and BMacek ldquoDetecting posttranslational modifi-cations of bacterial SSB proteinsrdquoMethods inMolecular Biologyvol 922 pp 205ndash218 2012
[31] I Mijakovic D Petranovic B Macek et al ldquoBacterial single-stranded DNA-binding proteins are phosphorylated on tyro-sinerdquoNucleic Acids Research vol 34 no 5 pp 1588ndash1596 2006
[32] M Olszewski A Grot M Wojciechowski M Nowak MMickiewicz and J Kur ldquoCharacterization of exceptionally ther-mostable single-strandedDNA-binding proteins fromThermo-toga maritima and Thermotoga neapolitanardquo BMC Microbiol-ogy vol 10 article 260 2010
[33] U Curth J Genschel C Urbanke and J Greipel ldquoIn vitro andin vivo function of the C-terminus of Escherichia coil single-stranded DNA binding proteinrdquoNucleic Acids Research vol 24no 14 pp 2706ndash2711 1996
[34] G Witte C Urbanke and U Curth ldquoDNA polymerase IIIchi subunit ties single-stranded DNA binding protein to thebacterial replication machineryrdquoNucleic Acids Research vol 31pp 4434ndash4440 2003
[35] D Shishmarev YWang C EMason et al ldquoOtting Intramolec-ular binding mode of the C-terminus of Escherichia coli single-strandedDNAbinding protein determined by nuclearmagneticresonance spectroscopyrdquo Nucleic Acids Research vol 42 pp2750ndash2757 2014
[36] A G Kozlov M M Cox and T M Lohman ldquoRegulation ofsingle-stranded DNA binding by the C termini of Escherichiacoli single-stranded DNA-binding (SSB) proteinrdquo Journal ofBiological Chemistry vol 285 no 22 pp 17246ndash17252 2010
[37] M Nowak M Olszewski M Spibida and J Kur ldquoChar-acterization of single-stranded DNA-binding proteins fromthe psychrophilic bacteria Desulfotalea psychrophila Flavobac-terium psychrophilum Psychrobacter arcticus Psychrobactercryohalolentis Psychromonas ingrahamii Psychroflexus torquisand Photobacterium profundumrdquo BMC Microbiology vol 14article 91 2014
BioMed Research International 15
[38] T Paradzik N Ivic Z Filic et al ldquoStructure-function relation-ships of two paralogous single-stranded DNA-binding proteinsfrom Streptomyces coelicolor implication of SsbB in chromo-some segregation during sporulationrdquo Nucleic Acids Researchvol 41 no 6 pp 3659ndash3672 2013
[39] S JainM Zweig E Peeters et al ldquoCharacterization of the singlestrandedDNA binding protein SsbB encoded in the gonoccocalgenetic islandrdquo PLoS ONE vol 7 no 4 Article ID e35285 2012
[40] Y H Huang and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from Klebsiella pneumoniaemutation at either Arg73 or Ser76 causes a less cooperativecomplex onDNArdquoGenes to Cells vol 17 no 2 pp 146ndash157 2012
[41] E Antony E A Weiland S Korolev and T M LohmanldquoPlasmodium falciparum SSB tetramer wraps single-strandedDNAwith similar topology but opposite polarity to E Coli SSBrdquoJournal ofMolecular Biology vol 420 no 4-5 pp 269ndash283 2012
[42] H Jan Y L Lee and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from pseudomonas aeruginosaPAO1rdquo Protein Journal vol 30 no 1 pp 20ndash26 2011
[43] Y-H Huang Y-L Lee and C-Y Huang ldquoCharacterization of asingle-stranded DNA binding protein from Salmonella entericaserovar typhimurium LT2rdquo Protein Journal vol 30 no 2 pp102ndash108 2011
[44] S K Bharti K Rex P Sreedhar N Krishnan and U VarshneyldquoChimeras of Escherichia coli and Mycobacterium tuberculosissingle-stranded DNA binding proteins characterization andfunction in Escherichia colirdquo PLoS ONE vol 6 no 12 ArticleID e27216 2011
[45] M T Oliveira and L S Kaguni ldquoFunctional roles of the N-and C-terminal regions of the human mitochondrial single-stranded DNA-binding proteinrdquo PLoS ONE vol 5 no 10Article ID e15379 2010
[46] A G Kozlov J M Eggington M M Cox and T MLohman ldquoBinding of the dimeric Deinococcus radioduranssingle-strandedDNA binding protein to single-strandedDNArdquoBiochemistry vol 49 no 38 pp 8266ndash8275 2010
[47] T M Lohman and M E Ferrari ldquoEscherichia coli single-stranded DNA-binding protein multiple DNA-binding modesand cooperativesrdquo Annual Review of Biochemistry vol 63 pp527ndash570 1994
[48] R Zhou A G Kozlov R Roy et al ldquoSSB functions as a slidingplatform that migrates on DNA via reptationrdquo Cell vol 146 pp222ndash232 2011
[49] R Roy A G Kozlov T M Lohman and T Ha ldquoSSB proteindiffusion on single-stranded DNA stimulates RecA filamentformationrdquo Nature vol 461 pp 1092ndash1097 2009
[50] R Roy A G Kozlov T M Lohman and T Ha ldquoDynamicstructural rearrangements between DNA binding modes of Ecoli SSB proteinrdquo Journal of Molecular Biology vol 369 no 5pp 1244ndash1257 2007
[51] T J Kelly P Simancek and G S Brush ldquoIdentification andcharacterization of a single-stranded DNA-binding proteinfrom the archaeon Methanococcus jannaschiirdquo Proceedings ofthe National Academy of Sciences of the United States of Americavol 95 no 25 pp 14634ndash14639 1998
[52] C Y Huang Determination of the Binding Site-Size of theProtein-DNA Complex by Use of the Electrophoretic MobilityShift Assay InTech Press Rijeka Croatia 2012
[53] A Kornberg ldquoTen commandments of enzymology amendedrdquoTrends in Biochemical Sciences vol 28 no 10 pp 515ndash517 2003
[54] W Zhang X Lu and J Shen ldquoEMSA and single-moleculeforce spectroscopy study of interactions between bacillus sub-tilis single-stranded DNA-binding protein and single-strandedDNArdquo Langmuir vol 27 no 24 pp 15008ndash15015 2011
[55] M Ostermeier and S J Benkovic ldquoEvolution of protein func-tion by domain swappingrdquo Advances in Protein Chemistry vol55 pp 29ndash77 2000
[56] W F Peng and C Y Huang ldquoAllantoinase and dihydroorotasebinding and inhibition by flavonols and the substrates of cyclicamidohydrolasesrdquo Biochimie vol 101 pp 113ndash122 2014
[57] Y H Huang M J Lin and C Y Huang ldquoDnaT is a single-stranded DNA binding proteinrdquo Genes to Cells vol 18 no 11pp 1007ndash1019 2013
[58] Y Y Ho Y H Huang and C Y Huang ldquoChemical rescue of thepost-translationally carboxylated lysine mutant of allantoinaseand dihydroorotase by metal ions and short-chain carboxylicacidsrdquo Amino Acids vol 44 no 4 pp 1181ndash1191 2013
[59] Y H Huang Y Lo W Huang and C Y Huang ldquoCrystalstructure and DNA-binding mode of Klebsiella pneumoniaeprimosomal PriB proteinrdquoGenes to Cells vol 17 no 10 pp 837ndash849 2012
[60] C Y Huang C Y Hsu Y Sun H Wu and C Hsiao ldquoCom-plexed crystal structure of replication restart primosome pro-tein PriB reveals a novel single-stranded DNA-binding moderdquoNucleic Acids Research vol 34 no 14 pp 3878ndash3886 2006
[61] T Kinebuchi H Shindo H Nagai N Shimamoto andM Shimizu ldquoFunctional domains of Escherichia coli single-stranded DNA binding protein as assessed by analyses of thedeletion mutantsrdquo Biochemistry vol 36 no 22 pp 6732ndash67381997
[62] N Shimamoto N Ikushima H Utiyama H Tachibana andK Horie ldquoSpecific and cooperative binding of E coli single-stranded DNA binding protein to mRNArdquo Nucleic AcidsResearch vol 15 no 13 pp 5241ndash5250 1987
[63] H Lin andC YHuang ldquoCharacterization of flavonol inhibitionof DnaB helicase Real-time monitoring structural modelingand proposed mechanismrdquo Journal of Biomedicine and Biotech-nology vol 2012 Article ID 735368 2012
[64] Y H Huang H H Lin and C Y Huang ldquoA single residuedetermines the cooperative binding property of a primosomalDNA replication protein PriB to single-stranded DNArdquo Bio-science Biotechnology and Biochemistry vol 76 no 6 pp 1110ndash1115 2012
[65] H C Hsieh and C Y Huang ldquoIdentification of a novel proteinPriB in Klebsiella pneumoniaerdquo Biochemical and BiophysicalResearch Communications vol 404 no 1 pp 546ndash551 2011
[66] J H Liu T W Chang C Y Huang et al ldquoCrystal structure ofPriB a primosomalDNA replication protein ofEscherichia colirdquoThe Journal of Biological Chemistry vol 279 no 48 pp 50465ndash50471 2004
[67] Y H Huang and C Y Huang ldquoThe N-terminal domain ofDnaT a primosomalDNA replication protein is crucial for PriBbinding and self-trimerizationrdquo Biochemical and BiophysicalResearch Communications vol 442 pp 147ndash152 2013
[68] M A Larkin G Blackshields N P Brown et al ldquoClustalW andClustal X version 20rdquo Bioinformatics vol 23 no 21 pp 2947ndash2948 2007
16 BioMed Research International
[69] S N Savvides S Raghunathan K Futterer A G Kozlov T MLohman and G Waksman ldquoThe C-terminal domain of full-length E coli SSB is disordered even when bound to DNArdquoProtein Science vol 13 no 7 pp 1942ndash1947 2004
[70] C-C Chen J-K Hwang and J-M Yang ldquo(PS)2-v2 template-based protein structure prediction serverrdquo BMC Bioinformaticsvol 10 article 366 2009
[71] C Chen J Hwang and J Yang ldquo(PS)2 protein structure pre-diction serverrdquoNucleic Acids Research vol 34 pp W152ndashW1572006
[72] K Bush and J F Fisher ldquoEpidemiological expansion structuralstudies and clinical challenges of new 120573-lactamases from gram-negative bacteriardquo Annual Review of Microbiology vol 65 pp455ndash478 2011
[73] K K Kumarasamy M A Toleman T R Walsh et al ldquoEmer-gence of a new antibiotic resistance mechanism in India Pak-istan and the UK a molecular biological and epidemiologicalstudyrdquoThe Lancet Infectious Diseases vol 10 no 9 pp 597ndash6022010
[74] K Bush and M J MacIelag ldquoNew 120573-lactam antibiotics and 120573-lactamase inhibitorsrdquo Expert Opinion on Therapeutic Patentsvol 20 no 10 pp 1277ndash1293 2010
[75] K Bush ldquoAlarming 120573-lactamase-mediated resistance in multi-drug-resistant Enterobacteriaceaerdquo Current Opinion in Microbi-ology vol 13 no 5 pp 558ndash564 2010
[76] A Srivastava M Talaue S Liu et al ldquoNew target for inhibitionof bacterial RNA polymerase ldquoswitch regionrdquordquo Current Opinionin Microbiology vol 14 no 5 pp 532ndash543 2011
[77] K Chono K Katsumata T Kontani et al ldquoASP2151 a novelhelicase-primase inhibitor possesses antiviral activity againstvaricella-zoster virus and herpes simplex virus types 1 and 2rdquoJournal of Antimicrobial Chemotherapy vol 65 no 8 pp 1733ndash1741 2010
[78] M T Black and K Coleman ldquoNew inhibitors of bacterial topoi-somerase GyrAParC subunitsrdquo Current Opinion in Investiga-tional Drugs vol 10 no 8 pp 804ndash810 2009
[79] A H Marceau D A Bernstein B W Walsh W Shapiro LA Simmons and J L Keck ldquoProtein interactions in genomemaintenance as novel antibacterial targetsrdquo PLoS ONE vol 8no 3 Article ID e58765 2013
[80] D Lu D A Bernstein K A Satyshur and J L Keck ldquoSmall-molecule tools for dissecting the roles of SSBprotein inter-actions in genome maintenancerdquo Proceedings of the NationalAcademy of Sciences of the United States of America vol 107 no2 pp 633ndash638 2010
[81] I Wong and T M Lohman ldquoA double-filter method fornitrocellulose-filter binding application to protein-nucleic acidinteractionsrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 90 no 12 pp 5428ndash5432 1993
[82] M Mitas J Y Chock and M Christy ldquoThe binding-site sizesof Escherichia coli single-stranded-DNA-binding protein andmammalian replication protein A are 65 and ge 54 nucleotidesrespectivelyrdquo Biochemical Journal vol 324 no 3 pp 957ndash9611997
[83] C Urbanke G Witte and U Curth ldquoSedimentation velocitymethod in the analytical ultracentrifuge for the study of protein-protein interactionsrdquoMethods inMolecular Biology vol 305 pp101ndash114 2005
[84] S Chrysogelos and J Griffith ldquoEscherichia coli single-strandbinding protein organizes single-strandedDNA innucleosome-like unitsrdquo Proceedings of the National Academy of Sciences of theUnited States of America vol 79 no 19 pp 5803ndash5807 1982
[85] J R Casas-Finet K R Fischer and R L Karpel ldquoStructuralbasis for the nucleic acid binding cooperativity of bacteriophageT4 gene 32 protein the (LysArg)3(SerThr)2 (LAST) motifrdquoProceedings of the National Academy of Sciences of the UnitedStates of America vol 89 pp 1050ndash1054 1992
[86] G Witte R Fedorov and U Curth ldquoBiophysical analysis ofThermus aquaticus single-stranded DNA binding proteinrdquo Bio-physical Journal vol 94 no 6 pp 2269ndash2279 2008
[87] R Fedorov G Witte C Urbanke D J Manstein and UCurth ldquo3D structure of Thermus aquaticus single-strandedDNA-binding protein gives insight into the functioning of SSBproteinsrdquo Nucleic Acids Research vol 34 no 22 pp 6708ndash67172006
[88] K L Tsai C Y Huang C H Chang Y J Sun W J ChuangandC DHsiao ldquoCrystal structure of the human FOXK1a-DNAcomplex and its implications on the diverse binding specificityof winged helixforkhead proteinsrdquo Journal of Biological Chem-istry vol 281 no 25 pp 17400ndash17409 2006
[89] A H Mao N Lyle and R V Pappu ldquoDescribing sequence-ensemble relationships for intrinsically disordered proteinsrdquoBiochemical Journal vol 449 no 2 pp 307ndash318 2013
[90] R Wetzel ldquoPhysical chemistry of polyglutamine Intriguingtales of a monotonous sequencerdquo Journal of Molecular Biologyvol 421 no 4-5 pp 466ndash490 2012
[91] H T Orr ldquoPolyglutamine neurodegeneration expanded glu-tamines enhance native functionsrdquo Current Opinion in Geneticsand Development vol 22 no 3 pp 251ndash255 2012
[92] M Figiel W J Szlachcic P M Switonski A Gabka and W JKrzyzosiak ldquoMouse models of polyglutamine diseases reviewand data table Part Irdquo Molecular Neurobiology vol 46 no 2pp 393ndash429 2012
[93] J Nasica-Labouze and N Mousseau ldquoKinetics of amyloidaggregation a study of the GNNQQNY prion sequencerdquo PLoSComputational Biology vol 8 no 11 Article ID e1002782 2012
[94] J Nasica-Labouze M Meli P Derreumaux G Colombo andN Mousseau ldquoA multiscale approach to characterize the earlyaggregation steps of the amyloid-forming peptide gnnqqnyfrom the yeast prion sup-35rdquo PLoS Computational Biology vol7 no 5 Article ID e1002051 2011
[95] J R Lewandowski P C A van der Wel M Rigney N Grig-orieff and R G Griffin ldquoStructural complexity of a compositeamyloid fibrilrdquo Journal of the American Chemical Society vol133 no 37 pp 14686ndash14698 2011
BioMed Research International 9
[KpSSBnStSSBc]
-dT15
-C
(a)
[KpSSBnStSSBc]
-dT20
-C
(b)
[KpSSBnStSSBc]
-dT40
-C
(c)
[KpSSBnStSSBc]
-dT45
-C1-C2
(d)
Figure 7 Binding of KpSSBnStSSBc to dT15ndash45 KpSSBnStSSBc (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for30min at 25∘C with 17 nM of (a) dT15 (b) dT20 (c) dT40 or (d) dT45 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and 100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blue and40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
size) [52] required for KpSSBnStSSBc binding is 21 plusmn 2 nt avalue identical to that for StSSB (Figure 5) Swapping of theC-terminal domain of StSSB with KpSSB changes the size ofthe ssDNA-binding site from 26 nt to 21 nt
38 KpSSBnPaSSBc Bound to ssDNA The binding fea-tures of KpSSBnPaSSBc with dT20 (Figure 8(a)) dT25(Figure 8(b)) dT40 (Figure 8(c)) dT55 (Figure 8(d)) anddT60 (Figure 8(e)) were studied by EMSA Similar to thecases of KpSSB and PaSSB no complex was observed whenKpSSBnPaSSBc was incubated with dT20 However KpSSB-nPaSSBc still exhibited dramatically different ssDNA-bindingproperties from those of KpSSB KpSSB can form two distinctcomplexes with dT55 (Figure 4(f)) but both KpSSBnPaSSBc(Figure 9) and PaSSB (Figure 6) cannot One KpSSBnPaSSBcoccupies 275 (552 = 275) nt to 30 (602 = 30) nt ofthe ssDNA The above EMSA results suggest that the lengthof an ssDNA (or the binding site size) [52] required forKpSSBnPaSSBc binding is 29 plusmn 2 nt a value identical to thatof PaSSB Swapping of the C-terminal domain of PaSSB toKpSSB changes the size of the ssDNA-binding site from 26 ntto 29 nt Although these SSBs namely KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc have nearly identicalssDNA-binding domains their binding site sizes are different(Table 2) Thus the size of the ssDNA-binding site requiredfor second SSB binding is likely to be dependent on the C-terminal domain of SSB
39 Binding Constants of the SSB-ssDNA Complexes Deter-mined from EMSA To compare the ssDNA-binding abil-ities of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc the midpoint values for input ssDNA bind-ing calculated from the titration curves of EMSA andreferred to as [Protein]
50(monomer) were quantified and
are summarized in Table 2 Although the N-terminal ssDNA-binding domains of these SSB proteins are highly similar(Figure 2) their ssDNA-binding activities and binding sitesizes are different (Table 2) [KpSSB]
50values ranged from
100 nM to 220 nM [StSSB]50
values ranged from 420 nM to650 nM [PaSSB]
50values ranged from 550 nM to 1700 nM
[KpSSBnStSSBc]50values ranged from 110 nM to 260 nM and
[KpSSBnPaSSBc]50
values ranged from 220 nM to 390 nMThe ssDNA-binding ability is as follows in the order ofdecreasing affinity KpSSB gt KpSSBnStSSBc gt KpSSBn-PaSSBc gt StSSB gt PaSSB Results from the above analysesindicate that the exchange of the C-terminal domain inSSB significantly changed the ssDNA-binding ability and theDNA-binding behavior (complex number) The reason asto why swapping of the C-terminal domain can affect thessDNA-binding activity of SSB remains unclear The C-ter-minal domain of SSB is suggested to be involved in ssDNAbinding However this relation is not evident in the results ofthe cocrystal structure
310 Oligomeric State of KpSSBnStSSBc and KpSSBnPaSSBc inSolution Gel-filtration chromatography was used to confirm
10 BioMed Research International
[KpSSBnPaSSBc]
-dT20
(a)
[KpSSBnPaSSBc]
-dT25
-C
(b)
[KpSSBnPaSSBc]
-C
-dT40
(c)
-C
[KpSSBnPaSSBc]
-dT55
(d)
-dT60
-C1-C2
[KpSSBnPaSSBc]
(e)
Figure 8 Binding of KpSSBnPaSSBc to dT20ndash60 KpSSBnPaSSBc (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for30min at 25∘C with 17 nM of (a) dT20 (b) dT25 (c) dT40 (d) dT55 or (e) dT60 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
that the oligomeric state of KpSSBnStSSBc and KpSSBn-PaSSBc remains as tetramers after chimeragenesisThe analy-sis of purified KpSSBnStSSBc and KpSSBnPaSSBc (2mgmL)using a Superdex 200HR 1030 column revealed a singlepeak with elution volumes of 786 and 789mL respectivelyAssuming that KpSSBnStSSBc and KpSSBnPaSSBc both haveshapes and partial specific volumes similar to the standardproteins the native molecular masses of KpSSBnStSSBc andKpSSBnPaSSBc were estimated to be 76641 and 74827Da ascalculated from a standard linear regression equation 119870av =minus03684(logMw) + 22707 (Figure 9) The native molecularmasses for KpSSBnStSSBc and KpSSBnPaSSBc are approx-imately four times the mass of the monomer (sim19 kDa)Therefore KpSSBnStSSBc and KpSSBnPaSSBc under theabove chromatographic conditions are stable tetramers insolution Although the exchange of the C-terminal domainin SSB significantly changed the ssDNA-binding ability andDNA-binding behavior (complex number) protein chimera-genesis did not cause any change in the oligomeric state ofSSB
311 Summary of Gly Gln and Pro Number in SSBs To ana-lyze the C-terminal amino acid composition of SSBs wefurther counted the number of Gly Gln and Pro residuesin different SSB segments SSB is abundant in Gly Glnand Pro (GQP) (Table 3) The GQP contents of KpSSB1ndash91 StSSB1ndash91 and PaSSB1ndash90 are similar However the Glynumber of PaSSB116ndash165 is significantly lower than that ofKpSSB116ndash174 and StSSB117ndash176 PaSSB116ndash165 contains only1 Gly but KpSSB116ndash174 and StSSB117ndash176 contain 11 and 12Gly respectively In addition we found different distributionpatterns among KpSSB StSSB and PaSSB Although theycontain similar number of Gln (Q) the QQQ pattern isfrequently found in PaSSB (Table 3)
312 Structural Modeling of SSBs Given its disordered C-ter-minal domain the crystal structure of the full-length SSB islacking even when SSB can be crystallized with DNA [69]We attempted to model the structure by homology mod-eling using the bioinformatics program (PS)2 to obtain an
BioMed Research International 11
Table 2 ssDNA binding properties of KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc as analyzed by EMSA
Protein DNA [Protein]50 (nM) Complex number
KpSSB
dT20 ND 0dT25 200 plusmn 20 1dT35 220 plusmn 30 1dT45 100 plusmn 10 1dT50 110 plusmn 20 1dT55 100 plusmn 20 2dT60 100 plusmn 10 2
StSSB
dT15 650 plusmn 120 1dT20 450 plusmn 80 1dT30 420 plusmn 60 1dT40 420 plusmn 80 1dT45 440 plusmn 60 2dT50 440 plusmn 50 2
PaSSB
dT20 ND 0dT25 1700 plusmn 250 1dT35 950 plusmn 180 1dT45 780 plusmn 160 1dT55 820 plusmn 90 1dT60 810 plusmn 110 2dT65 550 plusmn 70 2
KpSSBnStSSBc
dT15 260 plusmn 60 1dT20 110 plusmn 20 1dT40 120 plusmn 20 1dT45 160 plusmn 20 2
KpSSBnPaSSBc
dT20 ND 0dT25 390 plusmn 60 1dT40 220 plusmn 30 1dT55 230 plusmn 30 1dT60 230 plusmn 30 2
[Protein]50 was calculated from the titration curves of EMSA by determiningthe concentration of the protein (120583M) needed to achieve the midpointvalue for input ssDNA binding For some oligonucleotides input ssDNAbinding was the sum of the intensities from the two separate ssDNA-proteincomplexes Errors are standard deviations determined by three independenttitration experiments
Table 3 Summary of Gly Gln and Pro number in SSB
SSB segment G Q PKpSSB1ndash91 10 5 2StSSB1ndash91 10 5 2PaSSB1ndash90 11 6 2KpSSB92ndash174 18 16 9StSSB92ndash176 17 18 11PaSSB91ndash165 5 15 11KpSSB116ndash174 11 12 9StSSB117ndash176 12 13 10PaSSB116ndash165 1 12 11
in-depth understanding of the structure-function relation-ship of the C-terminal domains of these SSBs [70 71]
40 45 50 55
Kav
00
02
04
06
08
10
KpSSBnStSSBcKpSSBnPaSSBc
Y = minus03684X + 22707
R2= 0998
log Mw
Figure 9Gel-filtration chromatographic analyses of KpSSBnStSSBcand KpSSBnPaSSBc Purified protein (2mgmL) was applied toa Superdex 200HR 1030 column (GE Healthcare Bio-SciencesPiscataway NJ USA) equilibrated with Buffer D The column wasoperated at a flow rate of 05mLmin and 05mL fractions werecollected The proteins were detected by measuring the absorbanceat 280 nm The column was calibrated with proteins of knownmolecular weight thyroglobulin (670 kDa) 120574-globulin (158 kDa)ovalbumin (44 kDa) and myoglobin (17 kDa) The 119870av values forthe standard proteins and the SSB variants were calculated from theequation119870av = (119881119890minus119881119900)(119881119888minus119881119900) where119881119900 is column void volume119881119890is elution volume and 119881
119888is geometric column volume
(PS)2 (http140113239111simps2v2docsphp) is an auto-matic homology modeling server that combines bothsequence and secondary structure information to detect thehomologous proteins with remote similarity and the target-template alignment After pasting the amino acid sequenceto the website of (PS)2 only one hit (Protein Data Bankentry 1QVC EcSSB) for the C-terminal domains of KpSSBand StSSB was suggested For the C-terminal domain ofPaSSB only one hit that is CstF-77 (ProteinData Bank entry2OOE cleavage stimulation factor CstF) but not EcSSB wassuggested as the template for modeling Figure 10 shows thatmodeled structures of these SSB C-terminal domains arehighly disordered but that of PaSSB is more ordered than thatof other domains
4 Discussion
In this study we examined the sizes of the binding site ofthe untagged SSB and the chimeric SSB from the ubiquitousopportunistic pathogens K pneumoniae S enterica serovarTyphimurium LT2 and P aeruginosa PAO1 Many clinicalstrains of the abovementioned bacteria are highly resistantto antibiotics [72ndash75] The development of clinically usefulsmall-molecule antibiotics has been a seminal event in thefield of infectious diseases [48] Nucleic acid metabolismis one of the most basic biological functions and shouldbe a prime target in antibiotic development [76ndash78] Manybacterial SSBs form conserved protein interaction ldquohubsrdquothat are essential to recruit many proteins involved in DNA
12 BioMed Research International
Figure 10 Structure modeling of SSB The structures of KpSSB1ndash115 StSSB1ndash115 and PaSSB1ndash115 (the N-terminal domain of SSB)were modeled by SWISS-MODEL The structures of KpSSB116ndash142 StSSB116ndash142 and PaSSB121ndash160 (the C-terminal domain ofSSB) were modeled by (PS)2 Other regions of SSBs could not bemodeled by these two programs The structures of the N-terminaldomain and the C-terminal domain of these SSBs were manuallylinked (KpSSB1ndash142 blue StSSB1ndash142 pink PaSSB1ndash160 green) andsuperimposed with the crystal structure of EcSSB1ndash142 (orange)(PDB entry 1QVC) for comparison For clarity only one subunitof the tetramer was shown for each SSB
replication recombination and repair SSBDNA nucleopro-tein substrates [79] Thus SSBs may be promising targets inantibiotic development [80] As a first step toward achievingthis goal we investigated why SSBs possess highly conservedN-terminal ssDNA-binding domain but exhibit varying bind-ing site sizes One significant clue is that their flexible hingesand the length at the C-terminus are different as revealed bysequence alignment (Figure 2)
The interactions of various SSBs with ssDNA have beenanalyzed using a variety of techniques such as tryptophan-fluorescence quenching [47] filter binding [81] EMSA [5282] analytical ultracentrifugation [83] electron microscopy[84] nuclease digestion [44] single-molecule fluorescencemicroscopy [48] and crystallographic analyses [11] In thisstudy we have examined the electrophoretic mobility shiftpatterns of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc bound to different lengths of ssDNA and deter-mined the corresponding binding site sizes to be 26 2129 22 and 29 nt per tetramer respectively (Figures 4ndash8) PaSSB and KpSSBnPaSSBc have the largest sizes forssDNA binding among the SSBs studied We also identifiedHis-tagged and untagged SSBs that have similar ssDNA-binding site sizes [40 42 43] EMSA is a well-establishedapproach in studies of molecular biology [52] and the use ofradioactive tracer in this assay allows detection of the actualformation of the distinct protein-DNA complex(es) [53] For
exampleDNase protection assay and footprinting assay usingradioactive tracer can determine the specific DNA sequencecomplexed by a protein In EMSA when the length of thenucleotides is sufficient for the binding of two or more SSBmolecules the electrophoretic mobility of the higher SSBoligomer complex will be lower than that of the smaller SSBoligomer complex [52 54] In addition results of the ssDNA-binding site size from EMSA and cocrystal structure of SSBwere consistent [27] Thus throughout this paper we deter-mined the ssDNA-binding site sizes of SSB from the EMSAbehavior
Many SSBs bind to ssDNA with some degree of positivecooperativity Cooperativity can result from direct protein-protein interactions between the nearest neighbors such asthe LAST motif in the T4 gene-32 protein [85] and thearginine-mediated interaction motif in Thermus SSB [8687] Cooperativity can also result from the protein-induceddistortion of adjacent DNA as demonstrated in SulfolobusSSB PriB and FOXK1a proteins [23 60 88] In the cases ofKpSSB StSSB and PaSSB (Figures 4ndash6) binding appeared tobe nearly noncooperative for several DNAs because all DNAmainly shifts into the first complex (C1) before the appearanceof the second complex (C2) when subjected to increasingprotein concentrationsThe length dependence of the [SSB]
50
values suggests that the amount of spacing is optimum forsteric considerations (Table 2)
Because bacteria have varying genomic DNA sizes theirSSBs may need to evolve to have different binding site sizesfor DNA metabolism Results from protein chimeragenesisshowed the C-terminal domain dependence of the bindingsite sizes of SSB (Figure 11) The experimental data showedthat the binding site size of KpSSBnStSSBc was similar to thatof StSSB and the size of the binding site of KpSSBnPaSSBcwas similar to that of PaSSB The reason for which the bind-ing site size of SSB changed followed by swapping of theC-terminal domain remains unclear Flexibility numberof glycine residues andor different QQQ patterns of theC-terminal domain of SSB (Figure 2 and Table 3) may beimportant factors for determining the ssDNA-binding sitesize In fact the C-terminal domain of PaSSB that isPaSSB116ndash165 has only 1 Gly residue which is significantlyless than that of KpSSB (11 Gly) and StSSB (12Gly) Gly (andPro) is an important component of the flexible region aprotein that contains low Gly content is predicted to havelow flexibility Unlike typical SSB [35 69] PaSSB116ndash165 hasa partial structure (Figure 10) Although KpSSB StSSB andPaSSB contain similar number of Gln (Q) the QQQ patternis frequently found in PaSSB (Figure 2 and Table 3) PolyQand repeated sequences GAGAG are commonly found inthe structures of amyloids silk fibers and neurodegradationproteins [89ndash92] Considering that the simple coil polyQ theheptapeptide GNNQQNY and the hexapeptide NNQQNYcan cause protein aggregation and nucleation [93ndash95] thedistribution of Gln in the C-terminal domain of a tetramericSSB may also be an important determinant of the ssDNA-binding site size of SSB by some steric hindrances (Figure 11)However the above speculationmust be confirmed by furtherbiochemical experiments
BioMed Research International 13
PaSSBStSSB KpSSB
C-terC-ter C-ter
sim 21 nt sim 26 nt sim 29 nt
Figure 11 Possiblemodels for explainingwhy SSBs arewith different binding site sizes Twomodeled structures of KpSSB1ndash142 (blue) StSSB1ndash142 (pink) and PaSSB1ndash160 (green) complexed with ssDNA (gold) are shown For clarity only one C-terminal domain was shown for eachSSB tetramer By using the electrophoretic mobility shift assay and the protein chimeragenesis we characterized that the binding site sizes ofKpSSB StSSB PaSSB KpSSBnStSSBc and KpSSBnPaSSBc were 26 21 29 21 and 29 nt per tetramer respectively KpSSB StSSB and PaSSBare similar proteins whose N-terminal ssDNA-binding domains are almost identical Thus the C-terminal domain of SSB may indirectlycontribute to ssDNA binding and wrapping and affects the binding site size by the steric hindrance
5 Conclusion
In this study we characterized the ssDNA-binding propertiesof untagged SSBs from K pneumoniae S enterica sero-var Typhimurium LT2 and P aeruginosa PAO1 and proposeda role of the C-terminal flexible domain for ssDNA bind-ing from the protein chimeragenesis and EMSA resultsThe amino acid sequence of the N-terminal ssDNA-bind-ingoligomerization domain in these pathogenic SSBs ishighly conserved but their apparent binding site sizes aredifferent This finding indicates that the C-terminal protein-protein interaction domain may also indirectly contribute tossDNA binding and wrapping
Conflict of Interests
The authors declare that there is no conflict of interestsregarding the publication of this paper
Acknowledgment
This researchwas supported by aGrant from theNational Sci-ence Council Taiwan (NSC 102-2320-B-040-019 to Cheng-Yang Huang)
References
[1] R Reyes-Lamothe D J Sherratt and M C Leake ldquoStoichiom-etry and architecture of active DNA replication machinery inescherichia colirdquo Science vol 328 no 5977 pp 498ndash501 2010
[2] D J Richard E Bolderson andK K Khanna ldquoMultiple humansingle-stranded DNA binding proteins function in genomemaintenance structural biochemical and functional analysisrdquo
Critical Reviews in Biochemistry and Molecular Biology vol 44no 2-3 pp 98ndash116 2009
[3] R D Shereda A G Kozlov T M LohmanMM Cox and J LKeck ldquoSSB as an organizermobilizer of genome maintenancecomplexesrdquo Critical Reviews in Biochemistry and MolecularBiology vol 43 no 5 pp 289ndash318 2008
[4] D J Richard E Bolderson L Cubeddu et al ldquoSingle-strandedDNA-binding protein hSSB1 is critical for genomic stabilityrdquoNature vol 453 no 7195 pp 677ndash681 2008
[5] R R Meyer and P S Laine ldquoThe single-stranded DNA-bindingprotein of Escherichia colirdquoMicrobiological Reviews vol 54 no4 pp 342ndash380 1990
[6] C Yang U Curth C Urbanke and C Kang ldquoCrystal structureof human mitochondrial single-stranded DNA binding proteinat 24 A resolutionrdquo Nature Structural Biology vol 4 no 2 pp153ndash157 1997
[7] G Webster J Genschel U Curth C Urbanke C Kang and RHilgenfeld ldquoA common core for binding single-stranded DNAstructural comparison of the single-stranded DNA-bindingproteins (SSB) from E coli and human mitochondriardquo FEBSLetters vol 411 pp 313ndash316 1997
[8] R L Flynn and L Zou ldquoOligonucleotideoligosaccharide-bind-ing fold proteins a growing family of genome guardiansrdquo Crit-ical Reviews in Biochemistry and Molecular Biology vol 45 no4 pp 266ndash275 2010
[9] K Chan Y Lee CWangHHuang andY Sun ldquoSingle-Strand-ed DNA-Binding Protein Complex from Helicobacter pyloriSuggests an ssDNA-Binding Surfacerdquo Journal of MolecularBiology vol 388 no 3 pp 508ndash519 2009
[10] D L Theobald R M Mitton-Fry and D S Wuttke ldquoNucleicacid recognition by OB-fold proteinsrdquo Annual Review of Bio-physics and Biomolecular Structure vol 32 pp 115ndash133 2003
[11] S Raghunathan A G Kozlov TM Lohman andGWaksmanldquoStructure of the DNA binding domain of E coli SSB bound to
14 BioMed Research International
ssDNArdquo Nature Structural Biology vol 7 no 8 pp 648ndash6522000
[12] A G Murzin ldquoOB(oligonucleotideoligosaccharide binding)-fold common structural and functional solution for non-homologous sequencesrdquo EMBO Journal vol 12 no 3 pp 861ndash867 1993
[13] N P George K V Ngo S Chitteni-Pattu et al ldquoStructure andcellular dynamics of deinococcus radiodurans single-strandedDNA (ssDNA)-binding protein (SSB)-DNA complexesrdquo TheJournal of Biological Chemistry vol 287 no 26 pp 22123ndash221322012
[14] P Filipkowski and J Kur ldquoIdentification and propertiesof the Deinococcus grandis and Deinococcus proteolyticussingle-stranded DNA binding proteins (SSB)rdquo Acta BiochimicaPolonica vol 54 no 1 pp 79ndash87 2007
[15] P Filipkowski A Duraj-Thatte and J Kur ldquoIdentificationcloning expression and characterization of a highly ther-mostable single-stranded-DNA-binding protein (SSB) fromDeinococcus murrayirdquo Protein Expression and Purification vol53 no 1 pp 201ndash208 2007
[16] P Filipkowski M Koziatek and J Kur ldquoA highly ther-mostable homodimeric single-stranded DNA-binding proteinfrom Deinococcus radiopugnansrdquo Extremophiles vol 10 no 6pp 607ndash614 2006
[17] P Filipkowski A Duraj-Thatte and J Kur ldquoNovel thermostablesingle-stranded DNA-binding protein (SSB) fromDeinococcusgeothermalisrdquo Archives of Microbiology vol 186 no 2 pp 129ndash137 2006
[18] G Witte C Urbanke and U Curth ldquoSingle-stranded DNA-binding protein of Deinococcus radiodurans a biophysicalcharacterizationrdquoNucleic Acids Research vol 33 no 5 pp 1662ndash1670 2005
[19] D A Bernstein J M Eggingtont M P Killoran A M MisicMMCox and J L Keck ldquoCrystal structure of theDeinococcusradiodurans single-stranded DNA-binding protein suggests amechanism for coping with DNA damagerdquo Proceedings of theNational Academy of Sciences of the United States of Americavol 101 no 23 pp 8575ndash8580 2004
[20] S Dabrowski M Olszewski R Piatek A Brillowska-Dabrowska G Konopa and J Kur ldquoIdentification and charac-terization of single-stranded-DNA-binding proteins fromTher-mus thermophilus and Thermus aquaticusmdashnew arrange-ment of binding domainsrdquo Microbiology vol 148 no 10 pp3307ndash3315 2002
[21] R Gamsjaeger R Kariawasam C Touma A H Kwan M FWhite and L Cubeddu ldquoBackbone and side-chain 1H 13C and15N resonance assignments of the OB domain of the singlestranded DNA binding protein from Sulfolobus solfataricusand chemical shift mapping of the DNA-binding interfacerdquoBiomolecular NMR Assignments 2013
[22] M L Rolfsmeier and C A Haseltine ldquoThe single-strandedDNA binding protein of Sulfolobus solfataricus acts in the pre-synaptic step of homologous recombinationrdquo Journal of Molec-ular Biology vol 397 no 1 pp 31ndash45 2010
[23] I D Kerr R I M Wadsworth L Cubeddu W Blankenfeldt JHNaismith andM FWhite ldquoInsights into ssDNA recognitionby the OB fold from a structural and thermodynamic study ofSulfolobus SSB proteinrdquo EMBO Journal vol 22 no 11 pp 2561ndash2570 2003
[24] C A Haseltine and S C Kowalczykowski ldquoA distinctive single-stranded DNA-binding protein from the Archaeon Sulfolobussolfataricusrdquo Molecular Microbiology vol 43 no 6 pp 1505ndash1515 2002
[25] R I M Wadsworth and M F White ldquoIdentification andproperties of the crenarchaeal single-stranded DNA bindingprotein from Sulfolobus solfataricusrdquo Nucleic Acids Researchvol 29 no 4 pp 914ndash920 2001
[26] S Sugiman-Marangos and M S Junop ldquoThe structure of DdrBfrom Deinococcus a new fold for single-stranded DNA bindingproteinsrdquo Nucleic Acids Research vol 38 no 10 Article IDgkq036 pp 3432ndash3440 2010
[27] H Ghalei H V Moeller D Eppers et al ldquoEntrapment of DNAin an intersubunit tunnel system of a single-stranded DNA-binding proteinrdquo Nucleic Acids Research vol 42 no 10 pp6698ndash6708 2014
[28] S Paytubi S A McMahon S Graham et al ldquoDisplacement ofthe canonical single-strandedDNA-binding protein in the ther-moprotealesrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 109 no 7 pp E398ndashE405 2012
[29] T H Dickey S E Altschuler and D S Wuttke ldquoSingle-stranded DNA-binding proteins multiple domains for multiplefunctionsrdquo Structure vol 21 no 7 pp 1074ndash1084 2013
[30] D Vujaklija and BMacek ldquoDetecting posttranslational modifi-cations of bacterial SSB proteinsrdquoMethods inMolecular Biologyvol 922 pp 205ndash218 2012
[31] I Mijakovic D Petranovic B Macek et al ldquoBacterial single-stranded DNA-binding proteins are phosphorylated on tyro-sinerdquoNucleic Acids Research vol 34 no 5 pp 1588ndash1596 2006
[32] M Olszewski A Grot M Wojciechowski M Nowak MMickiewicz and J Kur ldquoCharacterization of exceptionally ther-mostable single-strandedDNA-binding proteins fromThermo-toga maritima and Thermotoga neapolitanardquo BMC Microbiol-ogy vol 10 article 260 2010
[33] U Curth J Genschel C Urbanke and J Greipel ldquoIn vitro andin vivo function of the C-terminus of Escherichia coil single-stranded DNA binding proteinrdquoNucleic Acids Research vol 24no 14 pp 2706ndash2711 1996
[34] G Witte C Urbanke and U Curth ldquoDNA polymerase IIIchi subunit ties single-stranded DNA binding protein to thebacterial replication machineryrdquoNucleic Acids Research vol 31pp 4434ndash4440 2003
[35] D Shishmarev YWang C EMason et al ldquoOtting Intramolec-ular binding mode of the C-terminus of Escherichia coli single-strandedDNAbinding protein determined by nuclearmagneticresonance spectroscopyrdquo Nucleic Acids Research vol 42 pp2750ndash2757 2014
[36] A G Kozlov M M Cox and T M Lohman ldquoRegulation ofsingle-stranded DNA binding by the C termini of Escherichiacoli single-stranded DNA-binding (SSB) proteinrdquo Journal ofBiological Chemistry vol 285 no 22 pp 17246ndash17252 2010
[37] M Nowak M Olszewski M Spibida and J Kur ldquoChar-acterization of single-stranded DNA-binding proteins fromthe psychrophilic bacteria Desulfotalea psychrophila Flavobac-terium psychrophilum Psychrobacter arcticus Psychrobactercryohalolentis Psychromonas ingrahamii Psychroflexus torquisand Photobacterium profundumrdquo BMC Microbiology vol 14article 91 2014
BioMed Research International 15
[38] T Paradzik N Ivic Z Filic et al ldquoStructure-function relation-ships of two paralogous single-stranded DNA-binding proteinsfrom Streptomyces coelicolor implication of SsbB in chromo-some segregation during sporulationrdquo Nucleic Acids Researchvol 41 no 6 pp 3659ndash3672 2013
[39] S JainM Zweig E Peeters et al ldquoCharacterization of the singlestrandedDNA binding protein SsbB encoded in the gonoccocalgenetic islandrdquo PLoS ONE vol 7 no 4 Article ID e35285 2012
[40] Y H Huang and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from Klebsiella pneumoniaemutation at either Arg73 or Ser76 causes a less cooperativecomplex onDNArdquoGenes to Cells vol 17 no 2 pp 146ndash157 2012
[41] E Antony E A Weiland S Korolev and T M LohmanldquoPlasmodium falciparum SSB tetramer wraps single-strandedDNAwith similar topology but opposite polarity to E Coli SSBrdquoJournal ofMolecular Biology vol 420 no 4-5 pp 269ndash283 2012
[42] H Jan Y L Lee and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from pseudomonas aeruginosaPAO1rdquo Protein Journal vol 30 no 1 pp 20ndash26 2011
[43] Y-H Huang Y-L Lee and C-Y Huang ldquoCharacterization of asingle-stranded DNA binding protein from Salmonella entericaserovar typhimurium LT2rdquo Protein Journal vol 30 no 2 pp102ndash108 2011
[44] S K Bharti K Rex P Sreedhar N Krishnan and U VarshneyldquoChimeras of Escherichia coli and Mycobacterium tuberculosissingle-stranded DNA binding proteins characterization andfunction in Escherichia colirdquo PLoS ONE vol 6 no 12 ArticleID e27216 2011
[45] M T Oliveira and L S Kaguni ldquoFunctional roles of the N-and C-terminal regions of the human mitochondrial single-stranded DNA-binding proteinrdquo PLoS ONE vol 5 no 10Article ID e15379 2010
[46] A G Kozlov J M Eggington M M Cox and T MLohman ldquoBinding of the dimeric Deinococcus radioduranssingle-strandedDNA binding protein to single-strandedDNArdquoBiochemistry vol 49 no 38 pp 8266ndash8275 2010
[47] T M Lohman and M E Ferrari ldquoEscherichia coli single-stranded DNA-binding protein multiple DNA-binding modesand cooperativesrdquo Annual Review of Biochemistry vol 63 pp527ndash570 1994
[48] R Zhou A G Kozlov R Roy et al ldquoSSB functions as a slidingplatform that migrates on DNA via reptationrdquo Cell vol 146 pp222ndash232 2011
[49] R Roy A G Kozlov T M Lohman and T Ha ldquoSSB proteindiffusion on single-stranded DNA stimulates RecA filamentformationrdquo Nature vol 461 pp 1092ndash1097 2009
[50] R Roy A G Kozlov T M Lohman and T Ha ldquoDynamicstructural rearrangements between DNA binding modes of Ecoli SSB proteinrdquo Journal of Molecular Biology vol 369 no 5pp 1244ndash1257 2007
[51] T J Kelly P Simancek and G S Brush ldquoIdentification andcharacterization of a single-stranded DNA-binding proteinfrom the archaeon Methanococcus jannaschiirdquo Proceedings ofthe National Academy of Sciences of the United States of Americavol 95 no 25 pp 14634ndash14639 1998
[52] C Y Huang Determination of the Binding Site-Size of theProtein-DNA Complex by Use of the Electrophoretic MobilityShift Assay InTech Press Rijeka Croatia 2012
[53] A Kornberg ldquoTen commandments of enzymology amendedrdquoTrends in Biochemical Sciences vol 28 no 10 pp 515ndash517 2003
[54] W Zhang X Lu and J Shen ldquoEMSA and single-moleculeforce spectroscopy study of interactions between bacillus sub-tilis single-stranded DNA-binding protein and single-strandedDNArdquo Langmuir vol 27 no 24 pp 15008ndash15015 2011
[55] M Ostermeier and S J Benkovic ldquoEvolution of protein func-tion by domain swappingrdquo Advances in Protein Chemistry vol55 pp 29ndash77 2000
[56] W F Peng and C Y Huang ldquoAllantoinase and dihydroorotasebinding and inhibition by flavonols and the substrates of cyclicamidohydrolasesrdquo Biochimie vol 101 pp 113ndash122 2014
[57] Y H Huang M J Lin and C Y Huang ldquoDnaT is a single-stranded DNA binding proteinrdquo Genes to Cells vol 18 no 11pp 1007ndash1019 2013
[58] Y Y Ho Y H Huang and C Y Huang ldquoChemical rescue of thepost-translationally carboxylated lysine mutant of allantoinaseand dihydroorotase by metal ions and short-chain carboxylicacidsrdquo Amino Acids vol 44 no 4 pp 1181ndash1191 2013
[59] Y H Huang Y Lo W Huang and C Y Huang ldquoCrystalstructure and DNA-binding mode of Klebsiella pneumoniaeprimosomal PriB proteinrdquoGenes to Cells vol 17 no 10 pp 837ndash849 2012
[60] C Y Huang C Y Hsu Y Sun H Wu and C Hsiao ldquoCom-plexed crystal structure of replication restart primosome pro-tein PriB reveals a novel single-stranded DNA-binding moderdquoNucleic Acids Research vol 34 no 14 pp 3878ndash3886 2006
[61] T Kinebuchi H Shindo H Nagai N Shimamoto andM Shimizu ldquoFunctional domains of Escherichia coli single-stranded DNA binding protein as assessed by analyses of thedeletion mutantsrdquo Biochemistry vol 36 no 22 pp 6732ndash67381997
[62] N Shimamoto N Ikushima H Utiyama H Tachibana andK Horie ldquoSpecific and cooperative binding of E coli single-stranded DNA binding protein to mRNArdquo Nucleic AcidsResearch vol 15 no 13 pp 5241ndash5250 1987
[63] H Lin andC YHuang ldquoCharacterization of flavonol inhibitionof DnaB helicase Real-time monitoring structural modelingand proposed mechanismrdquo Journal of Biomedicine and Biotech-nology vol 2012 Article ID 735368 2012
[64] Y H Huang H H Lin and C Y Huang ldquoA single residuedetermines the cooperative binding property of a primosomalDNA replication protein PriB to single-stranded DNArdquo Bio-science Biotechnology and Biochemistry vol 76 no 6 pp 1110ndash1115 2012
[65] H C Hsieh and C Y Huang ldquoIdentification of a novel proteinPriB in Klebsiella pneumoniaerdquo Biochemical and BiophysicalResearch Communications vol 404 no 1 pp 546ndash551 2011
[66] J H Liu T W Chang C Y Huang et al ldquoCrystal structure ofPriB a primosomalDNA replication protein ofEscherichia colirdquoThe Journal of Biological Chemistry vol 279 no 48 pp 50465ndash50471 2004
[67] Y H Huang and C Y Huang ldquoThe N-terminal domain ofDnaT a primosomalDNA replication protein is crucial for PriBbinding and self-trimerizationrdquo Biochemical and BiophysicalResearch Communications vol 442 pp 147ndash152 2013
[68] M A Larkin G Blackshields N P Brown et al ldquoClustalW andClustal X version 20rdquo Bioinformatics vol 23 no 21 pp 2947ndash2948 2007
16 BioMed Research International
[69] S N Savvides S Raghunathan K Futterer A G Kozlov T MLohman and G Waksman ldquoThe C-terminal domain of full-length E coli SSB is disordered even when bound to DNArdquoProtein Science vol 13 no 7 pp 1942ndash1947 2004
[70] C-C Chen J-K Hwang and J-M Yang ldquo(PS)2-v2 template-based protein structure prediction serverrdquo BMC Bioinformaticsvol 10 article 366 2009
[71] C Chen J Hwang and J Yang ldquo(PS)2 protein structure pre-diction serverrdquoNucleic Acids Research vol 34 pp W152ndashW1572006
[72] K Bush and J F Fisher ldquoEpidemiological expansion structuralstudies and clinical challenges of new 120573-lactamases from gram-negative bacteriardquo Annual Review of Microbiology vol 65 pp455ndash478 2011
[73] K K Kumarasamy M A Toleman T R Walsh et al ldquoEmer-gence of a new antibiotic resistance mechanism in India Pak-istan and the UK a molecular biological and epidemiologicalstudyrdquoThe Lancet Infectious Diseases vol 10 no 9 pp 597ndash6022010
[74] K Bush and M J MacIelag ldquoNew 120573-lactam antibiotics and 120573-lactamase inhibitorsrdquo Expert Opinion on Therapeutic Patentsvol 20 no 10 pp 1277ndash1293 2010
[75] K Bush ldquoAlarming 120573-lactamase-mediated resistance in multi-drug-resistant Enterobacteriaceaerdquo Current Opinion in Microbi-ology vol 13 no 5 pp 558ndash564 2010
[76] A Srivastava M Talaue S Liu et al ldquoNew target for inhibitionof bacterial RNA polymerase ldquoswitch regionrdquordquo Current Opinionin Microbiology vol 14 no 5 pp 532ndash543 2011
[77] K Chono K Katsumata T Kontani et al ldquoASP2151 a novelhelicase-primase inhibitor possesses antiviral activity againstvaricella-zoster virus and herpes simplex virus types 1 and 2rdquoJournal of Antimicrobial Chemotherapy vol 65 no 8 pp 1733ndash1741 2010
[78] M T Black and K Coleman ldquoNew inhibitors of bacterial topoi-somerase GyrAParC subunitsrdquo Current Opinion in Investiga-tional Drugs vol 10 no 8 pp 804ndash810 2009
[79] A H Marceau D A Bernstein B W Walsh W Shapiro LA Simmons and J L Keck ldquoProtein interactions in genomemaintenance as novel antibacterial targetsrdquo PLoS ONE vol 8no 3 Article ID e58765 2013
[80] D Lu D A Bernstein K A Satyshur and J L Keck ldquoSmall-molecule tools for dissecting the roles of SSBprotein inter-actions in genome maintenancerdquo Proceedings of the NationalAcademy of Sciences of the United States of America vol 107 no2 pp 633ndash638 2010
[81] I Wong and T M Lohman ldquoA double-filter method fornitrocellulose-filter binding application to protein-nucleic acidinteractionsrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 90 no 12 pp 5428ndash5432 1993
[82] M Mitas J Y Chock and M Christy ldquoThe binding-site sizesof Escherichia coli single-stranded-DNA-binding protein andmammalian replication protein A are 65 and ge 54 nucleotidesrespectivelyrdquo Biochemical Journal vol 324 no 3 pp 957ndash9611997
[83] C Urbanke G Witte and U Curth ldquoSedimentation velocitymethod in the analytical ultracentrifuge for the study of protein-protein interactionsrdquoMethods inMolecular Biology vol 305 pp101ndash114 2005
[84] S Chrysogelos and J Griffith ldquoEscherichia coli single-strandbinding protein organizes single-strandedDNA innucleosome-like unitsrdquo Proceedings of the National Academy of Sciences of theUnited States of America vol 79 no 19 pp 5803ndash5807 1982
[85] J R Casas-Finet K R Fischer and R L Karpel ldquoStructuralbasis for the nucleic acid binding cooperativity of bacteriophageT4 gene 32 protein the (LysArg)3(SerThr)2 (LAST) motifrdquoProceedings of the National Academy of Sciences of the UnitedStates of America vol 89 pp 1050ndash1054 1992
[86] G Witte R Fedorov and U Curth ldquoBiophysical analysis ofThermus aquaticus single-stranded DNA binding proteinrdquo Bio-physical Journal vol 94 no 6 pp 2269ndash2279 2008
[87] R Fedorov G Witte C Urbanke D J Manstein and UCurth ldquo3D structure of Thermus aquaticus single-strandedDNA-binding protein gives insight into the functioning of SSBproteinsrdquo Nucleic Acids Research vol 34 no 22 pp 6708ndash67172006
[88] K L Tsai C Y Huang C H Chang Y J Sun W J ChuangandC DHsiao ldquoCrystal structure of the human FOXK1a-DNAcomplex and its implications on the diverse binding specificityof winged helixforkhead proteinsrdquo Journal of Biological Chem-istry vol 281 no 25 pp 17400ndash17409 2006
[89] A H Mao N Lyle and R V Pappu ldquoDescribing sequence-ensemble relationships for intrinsically disordered proteinsrdquoBiochemical Journal vol 449 no 2 pp 307ndash318 2013
[90] R Wetzel ldquoPhysical chemistry of polyglutamine Intriguingtales of a monotonous sequencerdquo Journal of Molecular Biologyvol 421 no 4-5 pp 466ndash490 2012
[91] H T Orr ldquoPolyglutamine neurodegeneration expanded glu-tamines enhance native functionsrdquo Current Opinion in Geneticsand Development vol 22 no 3 pp 251ndash255 2012
[92] M Figiel W J Szlachcic P M Switonski A Gabka and W JKrzyzosiak ldquoMouse models of polyglutamine diseases reviewand data table Part Irdquo Molecular Neurobiology vol 46 no 2pp 393ndash429 2012
[93] J Nasica-Labouze and N Mousseau ldquoKinetics of amyloidaggregation a study of the GNNQQNY prion sequencerdquo PLoSComputational Biology vol 8 no 11 Article ID e1002782 2012
[94] J Nasica-Labouze M Meli P Derreumaux G Colombo andN Mousseau ldquoA multiscale approach to characterize the earlyaggregation steps of the amyloid-forming peptide gnnqqnyfrom the yeast prion sup-35rdquo PLoS Computational Biology vol7 no 5 Article ID e1002051 2011
[95] J R Lewandowski P C A van der Wel M Rigney N Grig-orieff and R G Griffin ldquoStructural complexity of a compositeamyloid fibrilrdquo Journal of the American Chemical Society vol133 no 37 pp 14686ndash14698 2011
10 BioMed Research International
[KpSSBnPaSSBc]
-dT20
(a)
[KpSSBnPaSSBc]
-dT25
-C
(b)
[KpSSBnPaSSBc]
-C
-dT40
(c)
-C
[KpSSBnPaSSBc]
-dT55
(d)
-dT60
-C1-C2
[KpSSBnPaSSBc]
(e)
Figure 8 Binding of KpSSBnPaSSBc to dT20ndash60 KpSSBnPaSSBc (0 19 37 77 155 310 630 1250 2500 and 5000 nM) was incubated for30min at 25∘C with 17 nM of (a) dT20 (b) dT25 (c) dT40 (d) dT55 or (e) dT60 in a total volume of 10 120583L in 20mM Tris-HCl pH 80 and100mMNaCl Aliquots (5 120583L) were removed from each reaction solution and added to 2 120583L of gel-loading solution (025 bromophenol blueand 40 sucrose) The resulting samples were resolved on a native 8 polyacrylamide gel at 4∘C in TBE buffer (89mM Tris borate and 1mMEDTA) for 1 h at 100V and visualized by autoradiography Complexed and free DNA bands were scanned and quantified
that the oligomeric state of KpSSBnStSSBc and KpSSBn-PaSSBc remains as tetramers after chimeragenesisThe analy-sis of purified KpSSBnStSSBc and KpSSBnPaSSBc (2mgmL)using a Superdex 200HR 1030 column revealed a singlepeak with elution volumes of 786 and 789mL respectivelyAssuming that KpSSBnStSSBc and KpSSBnPaSSBc both haveshapes and partial specific volumes similar to the standardproteins the native molecular masses of KpSSBnStSSBc andKpSSBnPaSSBc were estimated to be 76641 and 74827Da ascalculated from a standard linear regression equation 119870av =minus03684(logMw) + 22707 (Figure 9) The native molecularmasses for KpSSBnStSSBc and KpSSBnPaSSBc are approx-imately four times the mass of the monomer (sim19 kDa)Therefore KpSSBnStSSBc and KpSSBnPaSSBc under theabove chromatographic conditions are stable tetramers insolution Although the exchange of the C-terminal domainin SSB significantly changed the ssDNA-binding ability andDNA-binding behavior (complex number) protein chimera-genesis did not cause any change in the oligomeric state ofSSB
311 Summary of Gly Gln and Pro Number in SSBs To ana-lyze the C-terminal amino acid composition of SSBs wefurther counted the number of Gly Gln and Pro residuesin different SSB segments SSB is abundant in Gly Glnand Pro (GQP) (Table 3) The GQP contents of KpSSB1ndash91 StSSB1ndash91 and PaSSB1ndash90 are similar However the Glynumber of PaSSB116ndash165 is significantly lower than that ofKpSSB116ndash174 and StSSB117ndash176 PaSSB116ndash165 contains only1 Gly but KpSSB116ndash174 and StSSB117ndash176 contain 11 and 12Gly respectively In addition we found different distributionpatterns among KpSSB StSSB and PaSSB Although theycontain similar number of Gln (Q) the QQQ pattern isfrequently found in PaSSB (Table 3)
312 Structural Modeling of SSBs Given its disordered C-ter-minal domain the crystal structure of the full-length SSB islacking even when SSB can be crystallized with DNA [69]We attempted to model the structure by homology mod-eling using the bioinformatics program (PS)2 to obtain an
BioMed Research International 11
Table 2 ssDNA binding properties of KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc as analyzed by EMSA
Protein DNA [Protein]50 (nM) Complex number
KpSSB
dT20 ND 0dT25 200 plusmn 20 1dT35 220 plusmn 30 1dT45 100 plusmn 10 1dT50 110 plusmn 20 1dT55 100 plusmn 20 2dT60 100 plusmn 10 2
StSSB
dT15 650 plusmn 120 1dT20 450 plusmn 80 1dT30 420 plusmn 60 1dT40 420 plusmn 80 1dT45 440 plusmn 60 2dT50 440 plusmn 50 2
PaSSB
dT20 ND 0dT25 1700 plusmn 250 1dT35 950 plusmn 180 1dT45 780 plusmn 160 1dT55 820 plusmn 90 1dT60 810 plusmn 110 2dT65 550 plusmn 70 2
KpSSBnStSSBc
dT15 260 plusmn 60 1dT20 110 plusmn 20 1dT40 120 plusmn 20 1dT45 160 plusmn 20 2
KpSSBnPaSSBc
dT20 ND 0dT25 390 plusmn 60 1dT40 220 plusmn 30 1dT55 230 plusmn 30 1dT60 230 plusmn 30 2
[Protein]50 was calculated from the titration curves of EMSA by determiningthe concentration of the protein (120583M) needed to achieve the midpointvalue for input ssDNA binding For some oligonucleotides input ssDNAbinding was the sum of the intensities from the two separate ssDNA-proteincomplexes Errors are standard deviations determined by three independenttitration experiments
Table 3 Summary of Gly Gln and Pro number in SSB
SSB segment G Q PKpSSB1ndash91 10 5 2StSSB1ndash91 10 5 2PaSSB1ndash90 11 6 2KpSSB92ndash174 18 16 9StSSB92ndash176 17 18 11PaSSB91ndash165 5 15 11KpSSB116ndash174 11 12 9StSSB117ndash176 12 13 10PaSSB116ndash165 1 12 11
in-depth understanding of the structure-function relation-ship of the C-terminal domains of these SSBs [70 71]
40 45 50 55
Kav
00
02
04
06
08
10
KpSSBnStSSBcKpSSBnPaSSBc
Y = minus03684X + 22707
R2= 0998
log Mw
Figure 9Gel-filtration chromatographic analyses of KpSSBnStSSBcand KpSSBnPaSSBc Purified protein (2mgmL) was applied toa Superdex 200HR 1030 column (GE Healthcare Bio-SciencesPiscataway NJ USA) equilibrated with Buffer D The column wasoperated at a flow rate of 05mLmin and 05mL fractions werecollected The proteins were detected by measuring the absorbanceat 280 nm The column was calibrated with proteins of knownmolecular weight thyroglobulin (670 kDa) 120574-globulin (158 kDa)ovalbumin (44 kDa) and myoglobin (17 kDa) The 119870av values forthe standard proteins and the SSB variants were calculated from theequation119870av = (119881119890minus119881119900)(119881119888minus119881119900) where119881119900 is column void volume119881119890is elution volume and 119881
119888is geometric column volume
(PS)2 (http140113239111simps2v2docsphp) is an auto-matic homology modeling server that combines bothsequence and secondary structure information to detect thehomologous proteins with remote similarity and the target-template alignment After pasting the amino acid sequenceto the website of (PS)2 only one hit (Protein Data Bankentry 1QVC EcSSB) for the C-terminal domains of KpSSBand StSSB was suggested For the C-terminal domain ofPaSSB only one hit that is CstF-77 (ProteinData Bank entry2OOE cleavage stimulation factor CstF) but not EcSSB wassuggested as the template for modeling Figure 10 shows thatmodeled structures of these SSB C-terminal domains arehighly disordered but that of PaSSB is more ordered than thatof other domains
4 Discussion
In this study we examined the sizes of the binding site ofthe untagged SSB and the chimeric SSB from the ubiquitousopportunistic pathogens K pneumoniae S enterica serovarTyphimurium LT2 and P aeruginosa PAO1 Many clinicalstrains of the abovementioned bacteria are highly resistantto antibiotics [72ndash75] The development of clinically usefulsmall-molecule antibiotics has been a seminal event in thefield of infectious diseases [48] Nucleic acid metabolismis one of the most basic biological functions and shouldbe a prime target in antibiotic development [76ndash78] Manybacterial SSBs form conserved protein interaction ldquohubsrdquothat are essential to recruit many proteins involved in DNA
12 BioMed Research International
Figure 10 Structure modeling of SSB The structures of KpSSB1ndash115 StSSB1ndash115 and PaSSB1ndash115 (the N-terminal domain of SSB)were modeled by SWISS-MODEL The structures of KpSSB116ndash142 StSSB116ndash142 and PaSSB121ndash160 (the C-terminal domain ofSSB) were modeled by (PS)2 Other regions of SSBs could not bemodeled by these two programs The structures of the N-terminaldomain and the C-terminal domain of these SSBs were manuallylinked (KpSSB1ndash142 blue StSSB1ndash142 pink PaSSB1ndash160 green) andsuperimposed with the crystal structure of EcSSB1ndash142 (orange)(PDB entry 1QVC) for comparison For clarity only one subunitof the tetramer was shown for each SSB
replication recombination and repair SSBDNA nucleopro-tein substrates [79] Thus SSBs may be promising targets inantibiotic development [80] As a first step toward achievingthis goal we investigated why SSBs possess highly conservedN-terminal ssDNA-binding domain but exhibit varying bind-ing site sizes One significant clue is that their flexible hingesand the length at the C-terminus are different as revealed bysequence alignment (Figure 2)
The interactions of various SSBs with ssDNA have beenanalyzed using a variety of techniques such as tryptophan-fluorescence quenching [47] filter binding [81] EMSA [5282] analytical ultracentrifugation [83] electron microscopy[84] nuclease digestion [44] single-molecule fluorescencemicroscopy [48] and crystallographic analyses [11] In thisstudy we have examined the electrophoretic mobility shiftpatterns of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc bound to different lengths of ssDNA and deter-mined the corresponding binding site sizes to be 26 2129 22 and 29 nt per tetramer respectively (Figures 4ndash8) PaSSB and KpSSBnPaSSBc have the largest sizes forssDNA binding among the SSBs studied We also identifiedHis-tagged and untagged SSBs that have similar ssDNA-binding site sizes [40 42 43] EMSA is a well-establishedapproach in studies of molecular biology [52] and the use ofradioactive tracer in this assay allows detection of the actualformation of the distinct protein-DNA complex(es) [53] For
exampleDNase protection assay and footprinting assay usingradioactive tracer can determine the specific DNA sequencecomplexed by a protein In EMSA when the length of thenucleotides is sufficient for the binding of two or more SSBmolecules the electrophoretic mobility of the higher SSBoligomer complex will be lower than that of the smaller SSBoligomer complex [52 54] In addition results of the ssDNA-binding site size from EMSA and cocrystal structure of SSBwere consistent [27] Thus throughout this paper we deter-mined the ssDNA-binding site sizes of SSB from the EMSAbehavior
Many SSBs bind to ssDNA with some degree of positivecooperativity Cooperativity can result from direct protein-protein interactions between the nearest neighbors such asthe LAST motif in the T4 gene-32 protein [85] and thearginine-mediated interaction motif in Thermus SSB [8687] Cooperativity can also result from the protein-induceddistortion of adjacent DNA as demonstrated in SulfolobusSSB PriB and FOXK1a proteins [23 60 88] In the cases ofKpSSB StSSB and PaSSB (Figures 4ndash6) binding appeared tobe nearly noncooperative for several DNAs because all DNAmainly shifts into the first complex (C1) before the appearanceof the second complex (C2) when subjected to increasingprotein concentrationsThe length dependence of the [SSB]
50
values suggests that the amount of spacing is optimum forsteric considerations (Table 2)
Because bacteria have varying genomic DNA sizes theirSSBs may need to evolve to have different binding site sizesfor DNA metabolism Results from protein chimeragenesisshowed the C-terminal domain dependence of the bindingsite sizes of SSB (Figure 11) The experimental data showedthat the binding site size of KpSSBnStSSBc was similar to thatof StSSB and the size of the binding site of KpSSBnPaSSBcwas similar to that of PaSSB The reason for which the bind-ing site size of SSB changed followed by swapping of theC-terminal domain remains unclear Flexibility numberof glycine residues andor different QQQ patterns of theC-terminal domain of SSB (Figure 2 and Table 3) may beimportant factors for determining the ssDNA-binding sitesize In fact the C-terminal domain of PaSSB that isPaSSB116ndash165 has only 1 Gly residue which is significantlyless than that of KpSSB (11 Gly) and StSSB (12Gly) Gly (andPro) is an important component of the flexible region aprotein that contains low Gly content is predicted to havelow flexibility Unlike typical SSB [35 69] PaSSB116ndash165 hasa partial structure (Figure 10) Although KpSSB StSSB andPaSSB contain similar number of Gln (Q) the QQQ patternis frequently found in PaSSB (Figure 2 and Table 3) PolyQand repeated sequences GAGAG are commonly found inthe structures of amyloids silk fibers and neurodegradationproteins [89ndash92] Considering that the simple coil polyQ theheptapeptide GNNQQNY and the hexapeptide NNQQNYcan cause protein aggregation and nucleation [93ndash95] thedistribution of Gln in the C-terminal domain of a tetramericSSB may also be an important determinant of the ssDNA-binding site size of SSB by some steric hindrances (Figure 11)However the above speculationmust be confirmed by furtherbiochemical experiments
BioMed Research International 13
PaSSBStSSB KpSSB
C-terC-ter C-ter
sim 21 nt sim 26 nt sim 29 nt
Figure 11 Possiblemodels for explainingwhy SSBs arewith different binding site sizes Twomodeled structures of KpSSB1ndash142 (blue) StSSB1ndash142 (pink) and PaSSB1ndash160 (green) complexed with ssDNA (gold) are shown For clarity only one C-terminal domain was shown for eachSSB tetramer By using the electrophoretic mobility shift assay and the protein chimeragenesis we characterized that the binding site sizes ofKpSSB StSSB PaSSB KpSSBnStSSBc and KpSSBnPaSSBc were 26 21 29 21 and 29 nt per tetramer respectively KpSSB StSSB and PaSSBare similar proteins whose N-terminal ssDNA-binding domains are almost identical Thus the C-terminal domain of SSB may indirectlycontribute to ssDNA binding and wrapping and affects the binding site size by the steric hindrance
5 Conclusion
In this study we characterized the ssDNA-binding propertiesof untagged SSBs from K pneumoniae S enterica sero-var Typhimurium LT2 and P aeruginosa PAO1 and proposeda role of the C-terminal flexible domain for ssDNA bind-ing from the protein chimeragenesis and EMSA resultsThe amino acid sequence of the N-terminal ssDNA-bind-ingoligomerization domain in these pathogenic SSBs ishighly conserved but their apparent binding site sizes aredifferent This finding indicates that the C-terminal protein-protein interaction domain may also indirectly contribute tossDNA binding and wrapping
Conflict of Interests
The authors declare that there is no conflict of interestsregarding the publication of this paper
Acknowledgment
This researchwas supported by aGrant from theNational Sci-ence Council Taiwan (NSC 102-2320-B-040-019 to Cheng-Yang Huang)
References
[1] R Reyes-Lamothe D J Sherratt and M C Leake ldquoStoichiom-etry and architecture of active DNA replication machinery inescherichia colirdquo Science vol 328 no 5977 pp 498ndash501 2010
[2] D J Richard E Bolderson andK K Khanna ldquoMultiple humansingle-stranded DNA binding proteins function in genomemaintenance structural biochemical and functional analysisrdquo
Critical Reviews in Biochemistry and Molecular Biology vol 44no 2-3 pp 98ndash116 2009
[3] R D Shereda A G Kozlov T M LohmanMM Cox and J LKeck ldquoSSB as an organizermobilizer of genome maintenancecomplexesrdquo Critical Reviews in Biochemistry and MolecularBiology vol 43 no 5 pp 289ndash318 2008
[4] D J Richard E Bolderson L Cubeddu et al ldquoSingle-strandedDNA-binding protein hSSB1 is critical for genomic stabilityrdquoNature vol 453 no 7195 pp 677ndash681 2008
[5] R R Meyer and P S Laine ldquoThe single-stranded DNA-bindingprotein of Escherichia colirdquoMicrobiological Reviews vol 54 no4 pp 342ndash380 1990
[6] C Yang U Curth C Urbanke and C Kang ldquoCrystal structureof human mitochondrial single-stranded DNA binding proteinat 24 A resolutionrdquo Nature Structural Biology vol 4 no 2 pp153ndash157 1997
[7] G Webster J Genschel U Curth C Urbanke C Kang and RHilgenfeld ldquoA common core for binding single-stranded DNAstructural comparison of the single-stranded DNA-bindingproteins (SSB) from E coli and human mitochondriardquo FEBSLetters vol 411 pp 313ndash316 1997
[8] R L Flynn and L Zou ldquoOligonucleotideoligosaccharide-bind-ing fold proteins a growing family of genome guardiansrdquo Crit-ical Reviews in Biochemistry and Molecular Biology vol 45 no4 pp 266ndash275 2010
[9] K Chan Y Lee CWangHHuang andY Sun ldquoSingle-Strand-ed DNA-Binding Protein Complex from Helicobacter pyloriSuggests an ssDNA-Binding Surfacerdquo Journal of MolecularBiology vol 388 no 3 pp 508ndash519 2009
[10] D L Theobald R M Mitton-Fry and D S Wuttke ldquoNucleicacid recognition by OB-fold proteinsrdquo Annual Review of Bio-physics and Biomolecular Structure vol 32 pp 115ndash133 2003
[11] S Raghunathan A G Kozlov TM Lohman andGWaksmanldquoStructure of the DNA binding domain of E coli SSB bound to
14 BioMed Research International
ssDNArdquo Nature Structural Biology vol 7 no 8 pp 648ndash6522000
[12] A G Murzin ldquoOB(oligonucleotideoligosaccharide binding)-fold common structural and functional solution for non-homologous sequencesrdquo EMBO Journal vol 12 no 3 pp 861ndash867 1993
[13] N P George K V Ngo S Chitteni-Pattu et al ldquoStructure andcellular dynamics of deinococcus radiodurans single-strandedDNA (ssDNA)-binding protein (SSB)-DNA complexesrdquo TheJournal of Biological Chemistry vol 287 no 26 pp 22123ndash221322012
[14] P Filipkowski and J Kur ldquoIdentification and propertiesof the Deinococcus grandis and Deinococcus proteolyticussingle-stranded DNA binding proteins (SSB)rdquo Acta BiochimicaPolonica vol 54 no 1 pp 79ndash87 2007
[15] P Filipkowski A Duraj-Thatte and J Kur ldquoIdentificationcloning expression and characterization of a highly ther-mostable single-stranded-DNA-binding protein (SSB) fromDeinococcus murrayirdquo Protein Expression and Purification vol53 no 1 pp 201ndash208 2007
[16] P Filipkowski M Koziatek and J Kur ldquoA highly ther-mostable homodimeric single-stranded DNA-binding proteinfrom Deinococcus radiopugnansrdquo Extremophiles vol 10 no 6pp 607ndash614 2006
[17] P Filipkowski A Duraj-Thatte and J Kur ldquoNovel thermostablesingle-stranded DNA-binding protein (SSB) fromDeinococcusgeothermalisrdquo Archives of Microbiology vol 186 no 2 pp 129ndash137 2006
[18] G Witte C Urbanke and U Curth ldquoSingle-stranded DNA-binding protein of Deinococcus radiodurans a biophysicalcharacterizationrdquoNucleic Acids Research vol 33 no 5 pp 1662ndash1670 2005
[19] D A Bernstein J M Eggingtont M P Killoran A M MisicMMCox and J L Keck ldquoCrystal structure of theDeinococcusradiodurans single-stranded DNA-binding protein suggests amechanism for coping with DNA damagerdquo Proceedings of theNational Academy of Sciences of the United States of Americavol 101 no 23 pp 8575ndash8580 2004
[20] S Dabrowski M Olszewski R Piatek A Brillowska-Dabrowska G Konopa and J Kur ldquoIdentification and charac-terization of single-stranded-DNA-binding proteins fromTher-mus thermophilus and Thermus aquaticusmdashnew arrange-ment of binding domainsrdquo Microbiology vol 148 no 10 pp3307ndash3315 2002
[21] R Gamsjaeger R Kariawasam C Touma A H Kwan M FWhite and L Cubeddu ldquoBackbone and side-chain 1H 13C and15N resonance assignments of the OB domain of the singlestranded DNA binding protein from Sulfolobus solfataricusand chemical shift mapping of the DNA-binding interfacerdquoBiomolecular NMR Assignments 2013
[22] M L Rolfsmeier and C A Haseltine ldquoThe single-strandedDNA binding protein of Sulfolobus solfataricus acts in the pre-synaptic step of homologous recombinationrdquo Journal of Molec-ular Biology vol 397 no 1 pp 31ndash45 2010
[23] I D Kerr R I M Wadsworth L Cubeddu W Blankenfeldt JHNaismith andM FWhite ldquoInsights into ssDNA recognitionby the OB fold from a structural and thermodynamic study ofSulfolobus SSB proteinrdquo EMBO Journal vol 22 no 11 pp 2561ndash2570 2003
[24] C A Haseltine and S C Kowalczykowski ldquoA distinctive single-stranded DNA-binding protein from the Archaeon Sulfolobussolfataricusrdquo Molecular Microbiology vol 43 no 6 pp 1505ndash1515 2002
[25] R I M Wadsworth and M F White ldquoIdentification andproperties of the crenarchaeal single-stranded DNA bindingprotein from Sulfolobus solfataricusrdquo Nucleic Acids Researchvol 29 no 4 pp 914ndash920 2001
[26] S Sugiman-Marangos and M S Junop ldquoThe structure of DdrBfrom Deinococcus a new fold for single-stranded DNA bindingproteinsrdquo Nucleic Acids Research vol 38 no 10 Article IDgkq036 pp 3432ndash3440 2010
[27] H Ghalei H V Moeller D Eppers et al ldquoEntrapment of DNAin an intersubunit tunnel system of a single-stranded DNA-binding proteinrdquo Nucleic Acids Research vol 42 no 10 pp6698ndash6708 2014
[28] S Paytubi S A McMahon S Graham et al ldquoDisplacement ofthe canonical single-strandedDNA-binding protein in the ther-moprotealesrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 109 no 7 pp E398ndashE405 2012
[29] T H Dickey S E Altschuler and D S Wuttke ldquoSingle-stranded DNA-binding proteins multiple domains for multiplefunctionsrdquo Structure vol 21 no 7 pp 1074ndash1084 2013
[30] D Vujaklija and BMacek ldquoDetecting posttranslational modifi-cations of bacterial SSB proteinsrdquoMethods inMolecular Biologyvol 922 pp 205ndash218 2012
[31] I Mijakovic D Petranovic B Macek et al ldquoBacterial single-stranded DNA-binding proteins are phosphorylated on tyro-sinerdquoNucleic Acids Research vol 34 no 5 pp 1588ndash1596 2006
[32] M Olszewski A Grot M Wojciechowski M Nowak MMickiewicz and J Kur ldquoCharacterization of exceptionally ther-mostable single-strandedDNA-binding proteins fromThermo-toga maritima and Thermotoga neapolitanardquo BMC Microbiol-ogy vol 10 article 260 2010
[33] U Curth J Genschel C Urbanke and J Greipel ldquoIn vitro andin vivo function of the C-terminus of Escherichia coil single-stranded DNA binding proteinrdquoNucleic Acids Research vol 24no 14 pp 2706ndash2711 1996
[34] G Witte C Urbanke and U Curth ldquoDNA polymerase IIIchi subunit ties single-stranded DNA binding protein to thebacterial replication machineryrdquoNucleic Acids Research vol 31pp 4434ndash4440 2003
[35] D Shishmarev YWang C EMason et al ldquoOtting Intramolec-ular binding mode of the C-terminus of Escherichia coli single-strandedDNAbinding protein determined by nuclearmagneticresonance spectroscopyrdquo Nucleic Acids Research vol 42 pp2750ndash2757 2014
[36] A G Kozlov M M Cox and T M Lohman ldquoRegulation ofsingle-stranded DNA binding by the C termini of Escherichiacoli single-stranded DNA-binding (SSB) proteinrdquo Journal ofBiological Chemistry vol 285 no 22 pp 17246ndash17252 2010
[37] M Nowak M Olszewski M Spibida and J Kur ldquoChar-acterization of single-stranded DNA-binding proteins fromthe psychrophilic bacteria Desulfotalea psychrophila Flavobac-terium psychrophilum Psychrobacter arcticus Psychrobactercryohalolentis Psychromonas ingrahamii Psychroflexus torquisand Photobacterium profundumrdquo BMC Microbiology vol 14article 91 2014
BioMed Research International 15
[38] T Paradzik N Ivic Z Filic et al ldquoStructure-function relation-ships of two paralogous single-stranded DNA-binding proteinsfrom Streptomyces coelicolor implication of SsbB in chromo-some segregation during sporulationrdquo Nucleic Acids Researchvol 41 no 6 pp 3659ndash3672 2013
[39] S JainM Zweig E Peeters et al ldquoCharacterization of the singlestrandedDNA binding protein SsbB encoded in the gonoccocalgenetic islandrdquo PLoS ONE vol 7 no 4 Article ID e35285 2012
[40] Y H Huang and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from Klebsiella pneumoniaemutation at either Arg73 or Ser76 causes a less cooperativecomplex onDNArdquoGenes to Cells vol 17 no 2 pp 146ndash157 2012
[41] E Antony E A Weiland S Korolev and T M LohmanldquoPlasmodium falciparum SSB tetramer wraps single-strandedDNAwith similar topology but opposite polarity to E Coli SSBrdquoJournal ofMolecular Biology vol 420 no 4-5 pp 269ndash283 2012
[42] H Jan Y L Lee and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from pseudomonas aeruginosaPAO1rdquo Protein Journal vol 30 no 1 pp 20ndash26 2011
[43] Y-H Huang Y-L Lee and C-Y Huang ldquoCharacterization of asingle-stranded DNA binding protein from Salmonella entericaserovar typhimurium LT2rdquo Protein Journal vol 30 no 2 pp102ndash108 2011
[44] S K Bharti K Rex P Sreedhar N Krishnan and U VarshneyldquoChimeras of Escherichia coli and Mycobacterium tuberculosissingle-stranded DNA binding proteins characterization andfunction in Escherichia colirdquo PLoS ONE vol 6 no 12 ArticleID e27216 2011
[45] M T Oliveira and L S Kaguni ldquoFunctional roles of the N-and C-terminal regions of the human mitochondrial single-stranded DNA-binding proteinrdquo PLoS ONE vol 5 no 10Article ID e15379 2010
[46] A G Kozlov J M Eggington M M Cox and T MLohman ldquoBinding of the dimeric Deinococcus radioduranssingle-strandedDNA binding protein to single-strandedDNArdquoBiochemistry vol 49 no 38 pp 8266ndash8275 2010
[47] T M Lohman and M E Ferrari ldquoEscherichia coli single-stranded DNA-binding protein multiple DNA-binding modesand cooperativesrdquo Annual Review of Biochemistry vol 63 pp527ndash570 1994
[48] R Zhou A G Kozlov R Roy et al ldquoSSB functions as a slidingplatform that migrates on DNA via reptationrdquo Cell vol 146 pp222ndash232 2011
[49] R Roy A G Kozlov T M Lohman and T Ha ldquoSSB proteindiffusion on single-stranded DNA stimulates RecA filamentformationrdquo Nature vol 461 pp 1092ndash1097 2009
[50] R Roy A G Kozlov T M Lohman and T Ha ldquoDynamicstructural rearrangements between DNA binding modes of Ecoli SSB proteinrdquo Journal of Molecular Biology vol 369 no 5pp 1244ndash1257 2007
[51] T J Kelly P Simancek and G S Brush ldquoIdentification andcharacterization of a single-stranded DNA-binding proteinfrom the archaeon Methanococcus jannaschiirdquo Proceedings ofthe National Academy of Sciences of the United States of Americavol 95 no 25 pp 14634ndash14639 1998
[52] C Y Huang Determination of the Binding Site-Size of theProtein-DNA Complex by Use of the Electrophoretic MobilityShift Assay InTech Press Rijeka Croatia 2012
[53] A Kornberg ldquoTen commandments of enzymology amendedrdquoTrends in Biochemical Sciences vol 28 no 10 pp 515ndash517 2003
[54] W Zhang X Lu and J Shen ldquoEMSA and single-moleculeforce spectroscopy study of interactions between bacillus sub-tilis single-stranded DNA-binding protein and single-strandedDNArdquo Langmuir vol 27 no 24 pp 15008ndash15015 2011
[55] M Ostermeier and S J Benkovic ldquoEvolution of protein func-tion by domain swappingrdquo Advances in Protein Chemistry vol55 pp 29ndash77 2000
[56] W F Peng and C Y Huang ldquoAllantoinase and dihydroorotasebinding and inhibition by flavonols and the substrates of cyclicamidohydrolasesrdquo Biochimie vol 101 pp 113ndash122 2014
[57] Y H Huang M J Lin and C Y Huang ldquoDnaT is a single-stranded DNA binding proteinrdquo Genes to Cells vol 18 no 11pp 1007ndash1019 2013
[58] Y Y Ho Y H Huang and C Y Huang ldquoChemical rescue of thepost-translationally carboxylated lysine mutant of allantoinaseand dihydroorotase by metal ions and short-chain carboxylicacidsrdquo Amino Acids vol 44 no 4 pp 1181ndash1191 2013
[59] Y H Huang Y Lo W Huang and C Y Huang ldquoCrystalstructure and DNA-binding mode of Klebsiella pneumoniaeprimosomal PriB proteinrdquoGenes to Cells vol 17 no 10 pp 837ndash849 2012
[60] C Y Huang C Y Hsu Y Sun H Wu and C Hsiao ldquoCom-plexed crystal structure of replication restart primosome pro-tein PriB reveals a novel single-stranded DNA-binding moderdquoNucleic Acids Research vol 34 no 14 pp 3878ndash3886 2006
[61] T Kinebuchi H Shindo H Nagai N Shimamoto andM Shimizu ldquoFunctional domains of Escherichia coli single-stranded DNA binding protein as assessed by analyses of thedeletion mutantsrdquo Biochemistry vol 36 no 22 pp 6732ndash67381997
[62] N Shimamoto N Ikushima H Utiyama H Tachibana andK Horie ldquoSpecific and cooperative binding of E coli single-stranded DNA binding protein to mRNArdquo Nucleic AcidsResearch vol 15 no 13 pp 5241ndash5250 1987
[63] H Lin andC YHuang ldquoCharacterization of flavonol inhibitionof DnaB helicase Real-time monitoring structural modelingand proposed mechanismrdquo Journal of Biomedicine and Biotech-nology vol 2012 Article ID 735368 2012
[64] Y H Huang H H Lin and C Y Huang ldquoA single residuedetermines the cooperative binding property of a primosomalDNA replication protein PriB to single-stranded DNArdquo Bio-science Biotechnology and Biochemistry vol 76 no 6 pp 1110ndash1115 2012
[65] H C Hsieh and C Y Huang ldquoIdentification of a novel proteinPriB in Klebsiella pneumoniaerdquo Biochemical and BiophysicalResearch Communications vol 404 no 1 pp 546ndash551 2011
[66] J H Liu T W Chang C Y Huang et al ldquoCrystal structure ofPriB a primosomalDNA replication protein ofEscherichia colirdquoThe Journal of Biological Chemistry vol 279 no 48 pp 50465ndash50471 2004
[67] Y H Huang and C Y Huang ldquoThe N-terminal domain ofDnaT a primosomalDNA replication protein is crucial for PriBbinding and self-trimerizationrdquo Biochemical and BiophysicalResearch Communications vol 442 pp 147ndash152 2013
[68] M A Larkin G Blackshields N P Brown et al ldquoClustalW andClustal X version 20rdquo Bioinformatics vol 23 no 21 pp 2947ndash2948 2007
16 BioMed Research International
[69] S N Savvides S Raghunathan K Futterer A G Kozlov T MLohman and G Waksman ldquoThe C-terminal domain of full-length E coli SSB is disordered even when bound to DNArdquoProtein Science vol 13 no 7 pp 1942ndash1947 2004
[70] C-C Chen J-K Hwang and J-M Yang ldquo(PS)2-v2 template-based protein structure prediction serverrdquo BMC Bioinformaticsvol 10 article 366 2009
[71] C Chen J Hwang and J Yang ldquo(PS)2 protein structure pre-diction serverrdquoNucleic Acids Research vol 34 pp W152ndashW1572006
[72] K Bush and J F Fisher ldquoEpidemiological expansion structuralstudies and clinical challenges of new 120573-lactamases from gram-negative bacteriardquo Annual Review of Microbiology vol 65 pp455ndash478 2011
[73] K K Kumarasamy M A Toleman T R Walsh et al ldquoEmer-gence of a new antibiotic resistance mechanism in India Pak-istan and the UK a molecular biological and epidemiologicalstudyrdquoThe Lancet Infectious Diseases vol 10 no 9 pp 597ndash6022010
[74] K Bush and M J MacIelag ldquoNew 120573-lactam antibiotics and 120573-lactamase inhibitorsrdquo Expert Opinion on Therapeutic Patentsvol 20 no 10 pp 1277ndash1293 2010
[75] K Bush ldquoAlarming 120573-lactamase-mediated resistance in multi-drug-resistant Enterobacteriaceaerdquo Current Opinion in Microbi-ology vol 13 no 5 pp 558ndash564 2010
[76] A Srivastava M Talaue S Liu et al ldquoNew target for inhibitionof bacterial RNA polymerase ldquoswitch regionrdquordquo Current Opinionin Microbiology vol 14 no 5 pp 532ndash543 2011
[77] K Chono K Katsumata T Kontani et al ldquoASP2151 a novelhelicase-primase inhibitor possesses antiviral activity againstvaricella-zoster virus and herpes simplex virus types 1 and 2rdquoJournal of Antimicrobial Chemotherapy vol 65 no 8 pp 1733ndash1741 2010
[78] M T Black and K Coleman ldquoNew inhibitors of bacterial topoi-somerase GyrAParC subunitsrdquo Current Opinion in Investiga-tional Drugs vol 10 no 8 pp 804ndash810 2009
[79] A H Marceau D A Bernstein B W Walsh W Shapiro LA Simmons and J L Keck ldquoProtein interactions in genomemaintenance as novel antibacterial targetsrdquo PLoS ONE vol 8no 3 Article ID e58765 2013
[80] D Lu D A Bernstein K A Satyshur and J L Keck ldquoSmall-molecule tools for dissecting the roles of SSBprotein inter-actions in genome maintenancerdquo Proceedings of the NationalAcademy of Sciences of the United States of America vol 107 no2 pp 633ndash638 2010
[81] I Wong and T M Lohman ldquoA double-filter method fornitrocellulose-filter binding application to protein-nucleic acidinteractionsrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 90 no 12 pp 5428ndash5432 1993
[82] M Mitas J Y Chock and M Christy ldquoThe binding-site sizesof Escherichia coli single-stranded-DNA-binding protein andmammalian replication protein A are 65 and ge 54 nucleotidesrespectivelyrdquo Biochemical Journal vol 324 no 3 pp 957ndash9611997
[83] C Urbanke G Witte and U Curth ldquoSedimentation velocitymethod in the analytical ultracentrifuge for the study of protein-protein interactionsrdquoMethods inMolecular Biology vol 305 pp101ndash114 2005
[84] S Chrysogelos and J Griffith ldquoEscherichia coli single-strandbinding protein organizes single-strandedDNA innucleosome-like unitsrdquo Proceedings of the National Academy of Sciences of theUnited States of America vol 79 no 19 pp 5803ndash5807 1982
[85] J R Casas-Finet K R Fischer and R L Karpel ldquoStructuralbasis for the nucleic acid binding cooperativity of bacteriophageT4 gene 32 protein the (LysArg)3(SerThr)2 (LAST) motifrdquoProceedings of the National Academy of Sciences of the UnitedStates of America vol 89 pp 1050ndash1054 1992
[86] G Witte R Fedorov and U Curth ldquoBiophysical analysis ofThermus aquaticus single-stranded DNA binding proteinrdquo Bio-physical Journal vol 94 no 6 pp 2269ndash2279 2008
[87] R Fedorov G Witte C Urbanke D J Manstein and UCurth ldquo3D structure of Thermus aquaticus single-strandedDNA-binding protein gives insight into the functioning of SSBproteinsrdquo Nucleic Acids Research vol 34 no 22 pp 6708ndash67172006
[88] K L Tsai C Y Huang C H Chang Y J Sun W J ChuangandC DHsiao ldquoCrystal structure of the human FOXK1a-DNAcomplex and its implications on the diverse binding specificityof winged helixforkhead proteinsrdquo Journal of Biological Chem-istry vol 281 no 25 pp 17400ndash17409 2006
[89] A H Mao N Lyle and R V Pappu ldquoDescribing sequence-ensemble relationships for intrinsically disordered proteinsrdquoBiochemical Journal vol 449 no 2 pp 307ndash318 2013
[90] R Wetzel ldquoPhysical chemistry of polyglutamine Intriguingtales of a monotonous sequencerdquo Journal of Molecular Biologyvol 421 no 4-5 pp 466ndash490 2012
[91] H T Orr ldquoPolyglutamine neurodegeneration expanded glu-tamines enhance native functionsrdquo Current Opinion in Geneticsand Development vol 22 no 3 pp 251ndash255 2012
[92] M Figiel W J Szlachcic P M Switonski A Gabka and W JKrzyzosiak ldquoMouse models of polyglutamine diseases reviewand data table Part Irdquo Molecular Neurobiology vol 46 no 2pp 393ndash429 2012
[93] J Nasica-Labouze and N Mousseau ldquoKinetics of amyloidaggregation a study of the GNNQQNY prion sequencerdquo PLoSComputational Biology vol 8 no 11 Article ID e1002782 2012
[94] J Nasica-Labouze M Meli P Derreumaux G Colombo andN Mousseau ldquoA multiscale approach to characterize the earlyaggregation steps of the amyloid-forming peptide gnnqqnyfrom the yeast prion sup-35rdquo PLoS Computational Biology vol7 no 5 Article ID e1002051 2011
[95] J R Lewandowski P C A van der Wel M Rigney N Grig-orieff and R G Griffin ldquoStructural complexity of a compositeamyloid fibrilrdquo Journal of the American Chemical Society vol133 no 37 pp 14686ndash14698 2011
BioMed Research International 11
Table 2 ssDNA binding properties of KpSSB StSSB PaSSBKpSSBnStSSBc and KpSSBnPaSSBc as analyzed by EMSA
Protein DNA [Protein]50 (nM) Complex number
KpSSB
dT20 ND 0dT25 200 plusmn 20 1dT35 220 plusmn 30 1dT45 100 plusmn 10 1dT50 110 plusmn 20 1dT55 100 plusmn 20 2dT60 100 plusmn 10 2
StSSB
dT15 650 plusmn 120 1dT20 450 plusmn 80 1dT30 420 plusmn 60 1dT40 420 plusmn 80 1dT45 440 plusmn 60 2dT50 440 plusmn 50 2
PaSSB
dT20 ND 0dT25 1700 plusmn 250 1dT35 950 plusmn 180 1dT45 780 plusmn 160 1dT55 820 plusmn 90 1dT60 810 plusmn 110 2dT65 550 plusmn 70 2
KpSSBnStSSBc
dT15 260 plusmn 60 1dT20 110 plusmn 20 1dT40 120 plusmn 20 1dT45 160 plusmn 20 2
KpSSBnPaSSBc
dT20 ND 0dT25 390 plusmn 60 1dT40 220 plusmn 30 1dT55 230 plusmn 30 1dT60 230 plusmn 30 2
[Protein]50 was calculated from the titration curves of EMSA by determiningthe concentration of the protein (120583M) needed to achieve the midpointvalue for input ssDNA binding For some oligonucleotides input ssDNAbinding was the sum of the intensities from the two separate ssDNA-proteincomplexes Errors are standard deviations determined by three independenttitration experiments
Table 3 Summary of Gly Gln and Pro number in SSB
SSB segment G Q PKpSSB1ndash91 10 5 2StSSB1ndash91 10 5 2PaSSB1ndash90 11 6 2KpSSB92ndash174 18 16 9StSSB92ndash176 17 18 11PaSSB91ndash165 5 15 11KpSSB116ndash174 11 12 9StSSB117ndash176 12 13 10PaSSB116ndash165 1 12 11
in-depth understanding of the structure-function relation-ship of the C-terminal domains of these SSBs [70 71]
40 45 50 55
Kav
00
02
04
06
08
10
KpSSBnStSSBcKpSSBnPaSSBc
Y = minus03684X + 22707
R2= 0998
log Mw
Figure 9Gel-filtration chromatographic analyses of KpSSBnStSSBcand KpSSBnPaSSBc Purified protein (2mgmL) was applied toa Superdex 200HR 1030 column (GE Healthcare Bio-SciencesPiscataway NJ USA) equilibrated with Buffer D The column wasoperated at a flow rate of 05mLmin and 05mL fractions werecollected The proteins were detected by measuring the absorbanceat 280 nm The column was calibrated with proteins of knownmolecular weight thyroglobulin (670 kDa) 120574-globulin (158 kDa)ovalbumin (44 kDa) and myoglobin (17 kDa) The 119870av values forthe standard proteins and the SSB variants were calculated from theequation119870av = (119881119890minus119881119900)(119881119888minus119881119900) where119881119900 is column void volume119881119890is elution volume and 119881
119888is geometric column volume
(PS)2 (http140113239111simps2v2docsphp) is an auto-matic homology modeling server that combines bothsequence and secondary structure information to detect thehomologous proteins with remote similarity and the target-template alignment After pasting the amino acid sequenceto the website of (PS)2 only one hit (Protein Data Bankentry 1QVC EcSSB) for the C-terminal domains of KpSSBand StSSB was suggested For the C-terminal domain ofPaSSB only one hit that is CstF-77 (ProteinData Bank entry2OOE cleavage stimulation factor CstF) but not EcSSB wassuggested as the template for modeling Figure 10 shows thatmodeled structures of these SSB C-terminal domains arehighly disordered but that of PaSSB is more ordered than thatof other domains
4 Discussion
In this study we examined the sizes of the binding site ofthe untagged SSB and the chimeric SSB from the ubiquitousopportunistic pathogens K pneumoniae S enterica serovarTyphimurium LT2 and P aeruginosa PAO1 Many clinicalstrains of the abovementioned bacteria are highly resistantto antibiotics [72ndash75] The development of clinically usefulsmall-molecule antibiotics has been a seminal event in thefield of infectious diseases [48] Nucleic acid metabolismis one of the most basic biological functions and shouldbe a prime target in antibiotic development [76ndash78] Manybacterial SSBs form conserved protein interaction ldquohubsrdquothat are essential to recruit many proteins involved in DNA
12 BioMed Research International
Figure 10 Structure modeling of SSB The structures of KpSSB1ndash115 StSSB1ndash115 and PaSSB1ndash115 (the N-terminal domain of SSB)were modeled by SWISS-MODEL The structures of KpSSB116ndash142 StSSB116ndash142 and PaSSB121ndash160 (the C-terminal domain ofSSB) were modeled by (PS)2 Other regions of SSBs could not bemodeled by these two programs The structures of the N-terminaldomain and the C-terminal domain of these SSBs were manuallylinked (KpSSB1ndash142 blue StSSB1ndash142 pink PaSSB1ndash160 green) andsuperimposed with the crystal structure of EcSSB1ndash142 (orange)(PDB entry 1QVC) for comparison For clarity only one subunitof the tetramer was shown for each SSB
replication recombination and repair SSBDNA nucleopro-tein substrates [79] Thus SSBs may be promising targets inantibiotic development [80] As a first step toward achievingthis goal we investigated why SSBs possess highly conservedN-terminal ssDNA-binding domain but exhibit varying bind-ing site sizes One significant clue is that their flexible hingesand the length at the C-terminus are different as revealed bysequence alignment (Figure 2)
The interactions of various SSBs with ssDNA have beenanalyzed using a variety of techniques such as tryptophan-fluorescence quenching [47] filter binding [81] EMSA [5282] analytical ultracentrifugation [83] electron microscopy[84] nuclease digestion [44] single-molecule fluorescencemicroscopy [48] and crystallographic analyses [11] In thisstudy we have examined the electrophoretic mobility shiftpatterns of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc bound to different lengths of ssDNA and deter-mined the corresponding binding site sizes to be 26 2129 22 and 29 nt per tetramer respectively (Figures 4ndash8) PaSSB and KpSSBnPaSSBc have the largest sizes forssDNA binding among the SSBs studied We also identifiedHis-tagged and untagged SSBs that have similar ssDNA-binding site sizes [40 42 43] EMSA is a well-establishedapproach in studies of molecular biology [52] and the use ofradioactive tracer in this assay allows detection of the actualformation of the distinct protein-DNA complex(es) [53] For
exampleDNase protection assay and footprinting assay usingradioactive tracer can determine the specific DNA sequencecomplexed by a protein In EMSA when the length of thenucleotides is sufficient for the binding of two or more SSBmolecules the electrophoretic mobility of the higher SSBoligomer complex will be lower than that of the smaller SSBoligomer complex [52 54] In addition results of the ssDNA-binding site size from EMSA and cocrystal structure of SSBwere consistent [27] Thus throughout this paper we deter-mined the ssDNA-binding site sizes of SSB from the EMSAbehavior
Many SSBs bind to ssDNA with some degree of positivecooperativity Cooperativity can result from direct protein-protein interactions between the nearest neighbors such asthe LAST motif in the T4 gene-32 protein [85] and thearginine-mediated interaction motif in Thermus SSB [8687] Cooperativity can also result from the protein-induceddistortion of adjacent DNA as demonstrated in SulfolobusSSB PriB and FOXK1a proteins [23 60 88] In the cases ofKpSSB StSSB and PaSSB (Figures 4ndash6) binding appeared tobe nearly noncooperative for several DNAs because all DNAmainly shifts into the first complex (C1) before the appearanceof the second complex (C2) when subjected to increasingprotein concentrationsThe length dependence of the [SSB]
50
values suggests that the amount of spacing is optimum forsteric considerations (Table 2)
Because bacteria have varying genomic DNA sizes theirSSBs may need to evolve to have different binding site sizesfor DNA metabolism Results from protein chimeragenesisshowed the C-terminal domain dependence of the bindingsite sizes of SSB (Figure 11) The experimental data showedthat the binding site size of KpSSBnStSSBc was similar to thatof StSSB and the size of the binding site of KpSSBnPaSSBcwas similar to that of PaSSB The reason for which the bind-ing site size of SSB changed followed by swapping of theC-terminal domain remains unclear Flexibility numberof glycine residues andor different QQQ patterns of theC-terminal domain of SSB (Figure 2 and Table 3) may beimportant factors for determining the ssDNA-binding sitesize In fact the C-terminal domain of PaSSB that isPaSSB116ndash165 has only 1 Gly residue which is significantlyless than that of KpSSB (11 Gly) and StSSB (12Gly) Gly (andPro) is an important component of the flexible region aprotein that contains low Gly content is predicted to havelow flexibility Unlike typical SSB [35 69] PaSSB116ndash165 hasa partial structure (Figure 10) Although KpSSB StSSB andPaSSB contain similar number of Gln (Q) the QQQ patternis frequently found in PaSSB (Figure 2 and Table 3) PolyQand repeated sequences GAGAG are commonly found inthe structures of amyloids silk fibers and neurodegradationproteins [89ndash92] Considering that the simple coil polyQ theheptapeptide GNNQQNY and the hexapeptide NNQQNYcan cause protein aggregation and nucleation [93ndash95] thedistribution of Gln in the C-terminal domain of a tetramericSSB may also be an important determinant of the ssDNA-binding site size of SSB by some steric hindrances (Figure 11)However the above speculationmust be confirmed by furtherbiochemical experiments
BioMed Research International 13
PaSSBStSSB KpSSB
C-terC-ter C-ter
sim 21 nt sim 26 nt sim 29 nt
Figure 11 Possiblemodels for explainingwhy SSBs arewith different binding site sizes Twomodeled structures of KpSSB1ndash142 (blue) StSSB1ndash142 (pink) and PaSSB1ndash160 (green) complexed with ssDNA (gold) are shown For clarity only one C-terminal domain was shown for eachSSB tetramer By using the electrophoretic mobility shift assay and the protein chimeragenesis we characterized that the binding site sizes ofKpSSB StSSB PaSSB KpSSBnStSSBc and KpSSBnPaSSBc were 26 21 29 21 and 29 nt per tetramer respectively KpSSB StSSB and PaSSBare similar proteins whose N-terminal ssDNA-binding domains are almost identical Thus the C-terminal domain of SSB may indirectlycontribute to ssDNA binding and wrapping and affects the binding site size by the steric hindrance
5 Conclusion
In this study we characterized the ssDNA-binding propertiesof untagged SSBs from K pneumoniae S enterica sero-var Typhimurium LT2 and P aeruginosa PAO1 and proposeda role of the C-terminal flexible domain for ssDNA bind-ing from the protein chimeragenesis and EMSA resultsThe amino acid sequence of the N-terminal ssDNA-bind-ingoligomerization domain in these pathogenic SSBs ishighly conserved but their apparent binding site sizes aredifferent This finding indicates that the C-terminal protein-protein interaction domain may also indirectly contribute tossDNA binding and wrapping
Conflict of Interests
The authors declare that there is no conflict of interestsregarding the publication of this paper
Acknowledgment
This researchwas supported by aGrant from theNational Sci-ence Council Taiwan (NSC 102-2320-B-040-019 to Cheng-Yang Huang)
References
[1] R Reyes-Lamothe D J Sherratt and M C Leake ldquoStoichiom-etry and architecture of active DNA replication machinery inescherichia colirdquo Science vol 328 no 5977 pp 498ndash501 2010
[2] D J Richard E Bolderson andK K Khanna ldquoMultiple humansingle-stranded DNA binding proteins function in genomemaintenance structural biochemical and functional analysisrdquo
Critical Reviews in Biochemistry and Molecular Biology vol 44no 2-3 pp 98ndash116 2009
[3] R D Shereda A G Kozlov T M LohmanMM Cox and J LKeck ldquoSSB as an organizermobilizer of genome maintenancecomplexesrdquo Critical Reviews in Biochemistry and MolecularBiology vol 43 no 5 pp 289ndash318 2008
[4] D J Richard E Bolderson L Cubeddu et al ldquoSingle-strandedDNA-binding protein hSSB1 is critical for genomic stabilityrdquoNature vol 453 no 7195 pp 677ndash681 2008
[5] R R Meyer and P S Laine ldquoThe single-stranded DNA-bindingprotein of Escherichia colirdquoMicrobiological Reviews vol 54 no4 pp 342ndash380 1990
[6] C Yang U Curth C Urbanke and C Kang ldquoCrystal structureof human mitochondrial single-stranded DNA binding proteinat 24 A resolutionrdquo Nature Structural Biology vol 4 no 2 pp153ndash157 1997
[7] G Webster J Genschel U Curth C Urbanke C Kang and RHilgenfeld ldquoA common core for binding single-stranded DNAstructural comparison of the single-stranded DNA-bindingproteins (SSB) from E coli and human mitochondriardquo FEBSLetters vol 411 pp 313ndash316 1997
[8] R L Flynn and L Zou ldquoOligonucleotideoligosaccharide-bind-ing fold proteins a growing family of genome guardiansrdquo Crit-ical Reviews in Biochemistry and Molecular Biology vol 45 no4 pp 266ndash275 2010
[9] K Chan Y Lee CWangHHuang andY Sun ldquoSingle-Strand-ed DNA-Binding Protein Complex from Helicobacter pyloriSuggests an ssDNA-Binding Surfacerdquo Journal of MolecularBiology vol 388 no 3 pp 508ndash519 2009
[10] D L Theobald R M Mitton-Fry and D S Wuttke ldquoNucleicacid recognition by OB-fold proteinsrdquo Annual Review of Bio-physics and Biomolecular Structure vol 32 pp 115ndash133 2003
[11] S Raghunathan A G Kozlov TM Lohman andGWaksmanldquoStructure of the DNA binding domain of E coli SSB bound to
14 BioMed Research International
ssDNArdquo Nature Structural Biology vol 7 no 8 pp 648ndash6522000
[12] A G Murzin ldquoOB(oligonucleotideoligosaccharide binding)-fold common structural and functional solution for non-homologous sequencesrdquo EMBO Journal vol 12 no 3 pp 861ndash867 1993
[13] N P George K V Ngo S Chitteni-Pattu et al ldquoStructure andcellular dynamics of deinococcus radiodurans single-strandedDNA (ssDNA)-binding protein (SSB)-DNA complexesrdquo TheJournal of Biological Chemistry vol 287 no 26 pp 22123ndash221322012
[14] P Filipkowski and J Kur ldquoIdentification and propertiesof the Deinococcus grandis and Deinococcus proteolyticussingle-stranded DNA binding proteins (SSB)rdquo Acta BiochimicaPolonica vol 54 no 1 pp 79ndash87 2007
[15] P Filipkowski A Duraj-Thatte and J Kur ldquoIdentificationcloning expression and characterization of a highly ther-mostable single-stranded-DNA-binding protein (SSB) fromDeinococcus murrayirdquo Protein Expression and Purification vol53 no 1 pp 201ndash208 2007
[16] P Filipkowski M Koziatek and J Kur ldquoA highly ther-mostable homodimeric single-stranded DNA-binding proteinfrom Deinococcus radiopugnansrdquo Extremophiles vol 10 no 6pp 607ndash614 2006
[17] P Filipkowski A Duraj-Thatte and J Kur ldquoNovel thermostablesingle-stranded DNA-binding protein (SSB) fromDeinococcusgeothermalisrdquo Archives of Microbiology vol 186 no 2 pp 129ndash137 2006
[18] G Witte C Urbanke and U Curth ldquoSingle-stranded DNA-binding protein of Deinococcus radiodurans a biophysicalcharacterizationrdquoNucleic Acids Research vol 33 no 5 pp 1662ndash1670 2005
[19] D A Bernstein J M Eggingtont M P Killoran A M MisicMMCox and J L Keck ldquoCrystal structure of theDeinococcusradiodurans single-stranded DNA-binding protein suggests amechanism for coping with DNA damagerdquo Proceedings of theNational Academy of Sciences of the United States of Americavol 101 no 23 pp 8575ndash8580 2004
[20] S Dabrowski M Olszewski R Piatek A Brillowska-Dabrowska G Konopa and J Kur ldquoIdentification and charac-terization of single-stranded-DNA-binding proteins fromTher-mus thermophilus and Thermus aquaticusmdashnew arrange-ment of binding domainsrdquo Microbiology vol 148 no 10 pp3307ndash3315 2002
[21] R Gamsjaeger R Kariawasam C Touma A H Kwan M FWhite and L Cubeddu ldquoBackbone and side-chain 1H 13C and15N resonance assignments of the OB domain of the singlestranded DNA binding protein from Sulfolobus solfataricusand chemical shift mapping of the DNA-binding interfacerdquoBiomolecular NMR Assignments 2013
[22] M L Rolfsmeier and C A Haseltine ldquoThe single-strandedDNA binding protein of Sulfolobus solfataricus acts in the pre-synaptic step of homologous recombinationrdquo Journal of Molec-ular Biology vol 397 no 1 pp 31ndash45 2010
[23] I D Kerr R I M Wadsworth L Cubeddu W Blankenfeldt JHNaismith andM FWhite ldquoInsights into ssDNA recognitionby the OB fold from a structural and thermodynamic study ofSulfolobus SSB proteinrdquo EMBO Journal vol 22 no 11 pp 2561ndash2570 2003
[24] C A Haseltine and S C Kowalczykowski ldquoA distinctive single-stranded DNA-binding protein from the Archaeon Sulfolobussolfataricusrdquo Molecular Microbiology vol 43 no 6 pp 1505ndash1515 2002
[25] R I M Wadsworth and M F White ldquoIdentification andproperties of the crenarchaeal single-stranded DNA bindingprotein from Sulfolobus solfataricusrdquo Nucleic Acids Researchvol 29 no 4 pp 914ndash920 2001
[26] S Sugiman-Marangos and M S Junop ldquoThe structure of DdrBfrom Deinococcus a new fold for single-stranded DNA bindingproteinsrdquo Nucleic Acids Research vol 38 no 10 Article IDgkq036 pp 3432ndash3440 2010
[27] H Ghalei H V Moeller D Eppers et al ldquoEntrapment of DNAin an intersubunit tunnel system of a single-stranded DNA-binding proteinrdquo Nucleic Acids Research vol 42 no 10 pp6698ndash6708 2014
[28] S Paytubi S A McMahon S Graham et al ldquoDisplacement ofthe canonical single-strandedDNA-binding protein in the ther-moprotealesrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 109 no 7 pp E398ndashE405 2012
[29] T H Dickey S E Altschuler and D S Wuttke ldquoSingle-stranded DNA-binding proteins multiple domains for multiplefunctionsrdquo Structure vol 21 no 7 pp 1074ndash1084 2013
[30] D Vujaklija and BMacek ldquoDetecting posttranslational modifi-cations of bacterial SSB proteinsrdquoMethods inMolecular Biologyvol 922 pp 205ndash218 2012
[31] I Mijakovic D Petranovic B Macek et al ldquoBacterial single-stranded DNA-binding proteins are phosphorylated on tyro-sinerdquoNucleic Acids Research vol 34 no 5 pp 1588ndash1596 2006
[32] M Olszewski A Grot M Wojciechowski M Nowak MMickiewicz and J Kur ldquoCharacterization of exceptionally ther-mostable single-strandedDNA-binding proteins fromThermo-toga maritima and Thermotoga neapolitanardquo BMC Microbiol-ogy vol 10 article 260 2010
[33] U Curth J Genschel C Urbanke and J Greipel ldquoIn vitro andin vivo function of the C-terminus of Escherichia coil single-stranded DNA binding proteinrdquoNucleic Acids Research vol 24no 14 pp 2706ndash2711 1996
[34] G Witte C Urbanke and U Curth ldquoDNA polymerase IIIchi subunit ties single-stranded DNA binding protein to thebacterial replication machineryrdquoNucleic Acids Research vol 31pp 4434ndash4440 2003
[35] D Shishmarev YWang C EMason et al ldquoOtting Intramolec-ular binding mode of the C-terminus of Escherichia coli single-strandedDNAbinding protein determined by nuclearmagneticresonance spectroscopyrdquo Nucleic Acids Research vol 42 pp2750ndash2757 2014
[36] A G Kozlov M M Cox and T M Lohman ldquoRegulation ofsingle-stranded DNA binding by the C termini of Escherichiacoli single-stranded DNA-binding (SSB) proteinrdquo Journal ofBiological Chemistry vol 285 no 22 pp 17246ndash17252 2010
[37] M Nowak M Olszewski M Spibida and J Kur ldquoChar-acterization of single-stranded DNA-binding proteins fromthe psychrophilic bacteria Desulfotalea psychrophila Flavobac-terium psychrophilum Psychrobacter arcticus Psychrobactercryohalolentis Psychromonas ingrahamii Psychroflexus torquisand Photobacterium profundumrdquo BMC Microbiology vol 14article 91 2014
BioMed Research International 15
[38] T Paradzik N Ivic Z Filic et al ldquoStructure-function relation-ships of two paralogous single-stranded DNA-binding proteinsfrom Streptomyces coelicolor implication of SsbB in chromo-some segregation during sporulationrdquo Nucleic Acids Researchvol 41 no 6 pp 3659ndash3672 2013
[39] S JainM Zweig E Peeters et al ldquoCharacterization of the singlestrandedDNA binding protein SsbB encoded in the gonoccocalgenetic islandrdquo PLoS ONE vol 7 no 4 Article ID e35285 2012
[40] Y H Huang and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from Klebsiella pneumoniaemutation at either Arg73 or Ser76 causes a less cooperativecomplex onDNArdquoGenes to Cells vol 17 no 2 pp 146ndash157 2012
[41] E Antony E A Weiland S Korolev and T M LohmanldquoPlasmodium falciparum SSB tetramer wraps single-strandedDNAwith similar topology but opposite polarity to E Coli SSBrdquoJournal ofMolecular Biology vol 420 no 4-5 pp 269ndash283 2012
[42] H Jan Y L Lee and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from pseudomonas aeruginosaPAO1rdquo Protein Journal vol 30 no 1 pp 20ndash26 2011
[43] Y-H Huang Y-L Lee and C-Y Huang ldquoCharacterization of asingle-stranded DNA binding protein from Salmonella entericaserovar typhimurium LT2rdquo Protein Journal vol 30 no 2 pp102ndash108 2011
[44] S K Bharti K Rex P Sreedhar N Krishnan and U VarshneyldquoChimeras of Escherichia coli and Mycobacterium tuberculosissingle-stranded DNA binding proteins characterization andfunction in Escherichia colirdquo PLoS ONE vol 6 no 12 ArticleID e27216 2011
[45] M T Oliveira and L S Kaguni ldquoFunctional roles of the N-and C-terminal regions of the human mitochondrial single-stranded DNA-binding proteinrdquo PLoS ONE vol 5 no 10Article ID e15379 2010
[46] A G Kozlov J M Eggington M M Cox and T MLohman ldquoBinding of the dimeric Deinococcus radioduranssingle-strandedDNA binding protein to single-strandedDNArdquoBiochemistry vol 49 no 38 pp 8266ndash8275 2010
[47] T M Lohman and M E Ferrari ldquoEscherichia coli single-stranded DNA-binding protein multiple DNA-binding modesand cooperativesrdquo Annual Review of Biochemistry vol 63 pp527ndash570 1994
[48] R Zhou A G Kozlov R Roy et al ldquoSSB functions as a slidingplatform that migrates on DNA via reptationrdquo Cell vol 146 pp222ndash232 2011
[49] R Roy A G Kozlov T M Lohman and T Ha ldquoSSB proteindiffusion on single-stranded DNA stimulates RecA filamentformationrdquo Nature vol 461 pp 1092ndash1097 2009
[50] R Roy A G Kozlov T M Lohman and T Ha ldquoDynamicstructural rearrangements between DNA binding modes of Ecoli SSB proteinrdquo Journal of Molecular Biology vol 369 no 5pp 1244ndash1257 2007
[51] T J Kelly P Simancek and G S Brush ldquoIdentification andcharacterization of a single-stranded DNA-binding proteinfrom the archaeon Methanococcus jannaschiirdquo Proceedings ofthe National Academy of Sciences of the United States of Americavol 95 no 25 pp 14634ndash14639 1998
[52] C Y Huang Determination of the Binding Site-Size of theProtein-DNA Complex by Use of the Electrophoretic MobilityShift Assay InTech Press Rijeka Croatia 2012
[53] A Kornberg ldquoTen commandments of enzymology amendedrdquoTrends in Biochemical Sciences vol 28 no 10 pp 515ndash517 2003
[54] W Zhang X Lu and J Shen ldquoEMSA and single-moleculeforce spectroscopy study of interactions between bacillus sub-tilis single-stranded DNA-binding protein and single-strandedDNArdquo Langmuir vol 27 no 24 pp 15008ndash15015 2011
[55] M Ostermeier and S J Benkovic ldquoEvolution of protein func-tion by domain swappingrdquo Advances in Protein Chemistry vol55 pp 29ndash77 2000
[56] W F Peng and C Y Huang ldquoAllantoinase and dihydroorotasebinding and inhibition by flavonols and the substrates of cyclicamidohydrolasesrdquo Biochimie vol 101 pp 113ndash122 2014
[57] Y H Huang M J Lin and C Y Huang ldquoDnaT is a single-stranded DNA binding proteinrdquo Genes to Cells vol 18 no 11pp 1007ndash1019 2013
[58] Y Y Ho Y H Huang and C Y Huang ldquoChemical rescue of thepost-translationally carboxylated lysine mutant of allantoinaseand dihydroorotase by metal ions and short-chain carboxylicacidsrdquo Amino Acids vol 44 no 4 pp 1181ndash1191 2013
[59] Y H Huang Y Lo W Huang and C Y Huang ldquoCrystalstructure and DNA-binding mode of Klebsiella pneumoniaeprimosomal PriB proteinrdquoGenes to Cells vol 17 no 10 pp 837ndash849 2012
[60] C Y Huang C Y Hsu Y Sun H Wu and C Hsiao ldquoCom-plexed crystal structure of replication restart primosome pro-tein PriB reveals a novel single-stranded DNA-binding moderdquoNucleic Acids Research vol 34 no 14 pp 3878ndash3886 2006
[61] T Kinebuchi H Shindo H Nagai N Shimamoto andM Shimizu ldquoFunctional domains of Escherichia coli single-stranded DNA binding protein as assessed by analyses of thedeletion mutantsrdquo Biochemistry vol 36 no 22 pp 6732ndash67381997
[62] N Shimamoto N Ikushima H Utiyama H Tachibana andK Horie ldquoSpecific and cooperative binding of E coli single-stranded DNA binding protein to mRNArdquo Nucleic AcidsResearch vol 15 no 13 pp 5241ndash5250 1987
[63] H Lin andC YHuang ldquoCharacterization of flavonol inhibitionof DnaB helicase Real-time monitoring structural modelingand proposed mechanismrdquo Journal of Biomedicine and Biotech-nology vol 2012 Article ID 735368 2012
[64] Y H Huang H H Lin and C Y Huang ldquoA single residuedetermines the cooperative binding property of a primosomalDNA replication protein PriB to single-stranded DNArdquo Bio-science Biotechnology and Biochemistry vol 76 no 6 pp 1110ndash1115 2012
[65] H C Hsieh and C Y Huang ldquoIdentification of a novel proteinPriB in Klebsiella pneumoniaerdquo Biochemical and BiophysicalResearch Communications vol 404 no 1 pp 546ndash551 2011
[66] J H Liu T W Chang C Y Huang et al ldquoCrystal structure ofPriB a primosomalDNA replication protein ofEscherichia colirdquoThe Journal of Biological Chemistry vol 279 no 48 pp 50465ndash50471 2004
[67] Y H Huang and C Y Huang ldquoThe N-terminal domain ofDnaT a primosomalDNA replication protein is crucial for PriBbinding and self-trimerizationrdquo Biochemical and BiophysicalResearch Communications vol 442 pp 147ndash152 2013
[68] M A Larkin G Blackshields N P Brown et al ldquoClustalW andClustal X version 20rdquo Bioinformatics vol 23 no 21 pp 2947ndash2948 2007
16 BioMed Research International
[69] S N Savvides S Raghunathan K Futterer A G Kozlov T MLohman and G Waksman ldquoThe C-terminal domain of full-length E coli SSB is disordered even when bound to DNArdquoProtein Science vol 13 no 7 pp 1942ndash1947 2004
[70] C-C Chen J-K Hwang and J-M Yang ldquo(PS)2-v2 template-based protein structure prediction serverrdquo BMC Bioinformaticsvol 10 article 366 2009
[71] C Chen J Hwang and J Yang ldquo(PS)2 protein structure pre-diction serverrdquoNucleic Acids Research vol 34 pp W152ndashW1572006
[72] K Bush and J F Fisher ldquoEpidemiological expansion structuralstudies and clinical challenges of new 120573-lactamases from gram-negative bacteriardquo Annual Review of Microbiology vol 65 pp455ndash478 2011
[73] K K Kumarasamy M A Toleman T R Walsh et al ldquoEmer-gence of a new antibiotic resistance mechanism in India Pak-istan and the UK a molecular biological and epidemiologicalstudyrdquoThe Lancet Infectious Diseases vol 10 no 9 pp 597ndash6022010
[74] K Bush and M J MacIelag ldquoNew 120573-lactam antibiotics and 120573-lactamase inhibitorsrdquo Expert Opinion on Therapeutic Patentsvol 20 no 10 pp 1277ndash1293 2010
[75] K Bush ldquoAlarming 120573-lactamase-mediated resistance in multi-drug-resistant Enterobacteriaceaerdquo Current Opinion in Microbi-ology vol 13 no 5 pp 558ndash564 2010
[76] A Srivastava M Talaue S Liu et al ldquoNew target for inhibitionof bacterial RNA polymerase ldquoswitch regionrdquordquo Current Opinionin Microbiology vol 14 no 5 pp 532ndash543 2011
[77] K Chono K Katsumata T Kontani et al ldquoASP2151 a novelhelicase-primase inhibitor possesses antiviral activity againstvaricella-zoster virus and herpes simplex virus types 1 and 2rdquoJournal of Antimicrobial Chemotherapy vol 65 no 8 pp 1733ndash1741 2010
[78] M T Black and K Coleman ldquoNew inhibitors of bacterial topoi-somerase GyrAParC subunitsrdquo Current Opinion in Investiga-tional Drugs vol 10 no 8 pp 804ndash810 2009
[79] A H Marceau D A Bernstein B W Walsh W Shapiro LA Simmons and J L Keck ldquoProtein interactions in genomemaintenance as novel antibacterial targetsrdquo PLoS ONE vol 8no 3 Article ID e58765 2013
[80] D Lu D A Bernstein K A Satyshur and J L Keck ldquoSmall-molecule tools for dissecting the roles of SSBprotein inter-actions in genome maintenancerdquo Proceedings of the NationalAcademy of Sciences of the United States of America vol 107 no2 pp 633ndash638 2010
[81] I Wong and T M Lohman ldquoA double-filter method fornitrocellulose-filter binding application to protein-nucleic acidinteractionsrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 90 no 12 pp 5428ndash5432 1993
[82] M Mitas J Y Chock and M Christy ldquoThe binding-site sizesof Escherichia coli single-stranded-DNA-binding protein andmammalian replication protein A are 65 and ge 54 nucleotidesrespectivelyrdquo Biochemical Journal vol 324 no 3 pp 957ndash9611997
[83] C Urbanke G Witte and U Curth ldquoSedimentation velocitymethod in the analytical ultracentrifuge for the study of protein-protein interactionsrdquoMethods inMolecular Biology vol 305 pp101ndash114 2005
[84] S Chrysogelos and J Griffith ldquoEscherichia coli single-strandbinding protein organizes single-strandedDNA innucleosome-like unitsrdquo Proceedings of the National Academy of Sciences of theUnited States of America vol 79 no 19 pp 5803ndash5807 1982
[85] J R Casas-Finet K R Fischer and R L Karpel ldquoStructuralbasis for the nucleic acid binding cooperativity of bacteriophageT4 gene 32 protein the (LysArg)3(SerThr)2 (LAST) motifrdquoProceedings of the National Academy of Sciences of the UnitedStates of America vol 89 pp 1050ndash1054 1992
[86] G Witte R Fedorov and U Curth ldquoBiophysical analysis ofThermus aquaticus single-stranded DNA binding proteinrdquo Bio-physical Journal vol 94 no 6 pp 2269ndash2279 2008
[87] R Fedorov G Witte C Urbanke D J Manstein and UCurth ldquo3D structure of Thermus aquaticus single-strandedDNA-binding protein gives insight into the functioning of SSBproteinsrdquo Nucleic Acids Research vol 34 no 22 pp 6708ndash67172006
[88] K L Tsai C Y Huang C H Chang Y J Sun W J ChuangandC DHsiao ldquoCrystal structure of the human FOXK1a-DNAcomplex and its implications on the diverse binding specificityof winged helixforkhead proteinsrdquo Journal of Biological Chem-istry vol 281 no 25 pp 17400ndash17409 2006
[89] A H Mao N Lyle and R V Pappu ldquoDescribing sequence-ensemble relationships for intrinsically disordered proteinsrdquoBiochemical Journal vol 449 no 2 pp 307ndash318 2013
[90] R Wetzel ldquoPhysical chemistry of polyglutamine Intriguingtales of a monotonous sequencerdquo Journal of Molecular Biologyvol 421 no 4-5 pp 466ndash490 2012
[91] H T Orr ldquoPolyglutamine neurodegeneration expanded glu-tamines enhance native functionsrdquo Current Opinion in Geneticsand Development vol 22 no 3 pp 251ndash255 2012
[92] M Figiel W J Szlachcic P M Switonski A Gabka and W JKrzyzosiak ldquoMouse models of polyglutamine diseases reviewand data table Part Irdquo Molecular Neurobiology vol 46 no 2pp 393ndash429 2012
[93] J Nasica-Labouze and N Mousseau ldquoKinetics of amyloidaggregation a study of the GNNQQNY prion sequencerdquo PLoSComputational Biology vol 8 no 11 Article ID e1002782 2012
[94] J Nasica-Labouze M Meli P Derreumaux G Colombo andN Mousseau ldquoA multiscale approach to characterize the earlyaggregation steps of the amyloid-forming peptide gnnqqnyfrom the yeast prion sup-35rdquo PLoS Computational Biology vol7 no 5 Article ID e1002051 2011
[95] J R Lewandowski P C A van der Wel M Rigney N Grig-orieff and R G Griffin ldquoStructural complexity of a compositeamyloid fibrilrdquo Journal of the American Chemical Society vol133 no 37 pp 14686ndash14698 2011
12 BioMed Research International
Figure 10 Structure modeling of SSB The structures of KpSSB1ndash115 StSSB1ndash115 and PaSSB1ndash115 (the N-terminal domain of SSB)were modeled by SWISS-MODEL The structures of KpSSB116ndash142 StSSB116ndash142 and PaSSB121ndash160 (the C-terminal domain ofSSB) were modeled by (PS)2 Other regions of SSBs could not bemodeled by these two programs The structures of the N-terminaldomain and the C-terminal domain of these SSBs were manuallylinked (KpSSB1ndash142 blue StSSB1ndash142 pink PaSSB1ndash160 green) andsuperimposed with the crystal structure of EcSSB1ndash142 (orange)(PDB entry 1QVC) for comparison For clarity only one subunitof the tetramer was shown for each SSB
replication recombination and repair SSBDNA nucleopro-tein substrates [79] Thus SSBs may be promising targets inantibiotic development [80] As a first step toward achievingthis goal we investigated why SSBs possess highly conservedN-terminal ssDNA-binding domain but exhibit varying bind-ing site sizes One significant clue is that their flexible hingesand the length at the C-terminus are different as revealed bysequence alignment (Figure 2)
The interactions of various SSBs with ssDNA have beenanalyzed using a variety of techniques such as tryptophan-fluorescence quenching [47] filter binding [81] EMSA [5282] analytical ultracentrifugation [83] electron microscopy[84] nuclease digestion [44] single-molecule fluorescencemicroscopy [48] and crystallographic analyses [11] In thisstudy we have examined the electrophoretic mobility shiftpatterns of KpSSB StSSB PaSSB KpSSBnStSSBc and KpSS-BnPaSSBc bound to different lengths of ssDNA and deter-mined the corresponding binding site sizes to be 26 2129 22 and 29 nt per tetramer respectively (Figures 4ndash8) PaSSB and KpSSBnPaSSBc have the largest sizes forssDNA binding among the SSBs studied We also identifiedHis-tagged and untagged SSBs that have similar ssDNA-binding site sizes [40 42 43] EMSA is a well-establishedapproach in studies of molecular biology [52] and the use ofradioactive tracer in this assay allows detection of the actualformation of the distinct protein-DNA complex(es) [53] For
exampleDNase protection assay and footprinting assay usingradioactive tracer can determine the specific DNA sequencecomplexed by a protein In EMSA when the length of thenucleotides is sufficient for the binding of two or more SSBmolecules the electrophoretic mobility of the higher SSBoligomer complex will be lower than that of the smaller SSBoligomer complex [52 54] In addition results of the ssDNA-binding site size from EMSA and cocrystal structure of SSBwere consistent [27] Thus throughout this paper we deter-mined the ssDNA-binding site sizes of SSB from the EMSAbehavior
Many SSBs bind to ssDNA with some degree of positivecooperativity Cooperativity can result from direct protein-protein interactions between the nearest neighbors such asthe LAST motif in the T4 gene-32 protein [85] and thearginine-mediated interaction motif in Thermus SSB [8687] Cooperativity can also result from the protein-induceddistortion of adjacent DNA as demonstrated in SulfolobusSSB PriB and FOXK1a proteins [23 60 88] In the cases ofKpSSB StSSB and PaSSB (Figures 4ndash6) binding appeared tobe nearly noncooperative for several DNAs because all DNAmainly shifts into the first complex (C1) before the appearanceof the second complex (C2) when subjected to increasingprotein concentrationsThe length dependence of the [SSB]
50
values suggests that the amount of spacing is optimum forsteric considerations (Table 2)
Because bacteria have varying genomic DNA sizes theirSSBs may need to evolve to have different binding site sizesfor DNA metabolism Results from protein chimeragenesisshowed the C-terminal domain dependence of the bindingsite sizes of SSB (Figure 11) The experimental data showedthat the binding site size of KpSSBnStSSBc was similar to thatof StSSB and the size of the binding site of KpSSBnPaSSBcwas similar to that of PaSSB The reason for which the bind-ing site size of SSB changed followed by swapping of theC-terminal domain remains unclear Flexibility numberof glycine residues andor different QQQ patterns of theC-terminal domain of SSB (Figure 2 and Table 3) may beimportant factors for determining the ssDNA-binding sitesize In fact the C-terminal domain of PaSSB that isPaSSB116ndash165 has only 1 Gly residue which is significantlyless than that of KpSSB (11 Gly) and StSSB (12Gly) Gly (andPro) is an important component of the flexible region aprotein that contains low Gly content is predicted to havelow flexibility Unlike typical SSB [35 69] PaSSB116ndash165 hasa partial structure (Figure 10) Although KpSSB StSSB andPaSSB contain similar number of Gln (Q) the QQQ patternis frequently found in PaSSB (Figure 2 and Table 3) PolyQand repeated sequences GAGAG are commonly found inthe structures of amyloids silk fibers and neurodegradationproteins [89ndash92] Considering that the simple coil polyQ theheptapeptide GNNQQNY and the hexapeptide NNQQNYcan cause protein aggregation and nucleation [93ndash95] thedistribution of Gln in the C-terminal domain of a tetramericSSB may also be an important determinant of the ssDNA-binding site size of SSB by some steric hindrances (Figure 11)However the above speculationmust be confirmed by furtherbiochemical experiments
BioMed Research International 13
PaSSBStSSB KpSSB
C-terC-ter C-ter
sim 21 nt sim 26 nt sim 29 nt
Figure 11 Possiblemodels for explainingwhy SSBs arewith different binding site sizes Twomodeled structures of KpSSB1ndash142 (blue) StSSB1ndash142 (pink) and PaSSB1ndash160 (green) complexed with ssDNA (gold) are shown For clarity only one C-terminal domain was shown for eachSSB tetramer By using the electrophoretic mobility shift assay and the protein chimeragenesis we characterized that the binding site sizes ofKpSSB StSSB PaSSB KpSSBnStSSBc and KpSSBnPaSSBc were 26 21 29 21 and 29 nt per tetramer respectively KpSSB StSSB and PaSSBare similar proteins whose N-terminal ssDNA-binding domains are almost identical Thus the C-terminal domain of SSB may indirectlycontribute to ssDNA binding and wrapping and affects the binding site size by the steric hindrance
5 Conclusion
In this study we characterized the ssDNA-binding propertiesof untagged SSBs from K pneumoniae S enterica sero-var Typhimurium LT2 and P aeruginosa PAO1 and proposeda role of the C-terminal flexible domain for ssDNA bind-ing from the protein chimeragenesis and EMSA resultsThe amino acid sequence of the N-terminal ssDNA-bind-ingoligomerization domain in these pathogenic SSBs ishighly conserved but their apparent binding site sizes aredifferent This finding indicates that the C-terminal protein-protein interaction domain may also indirectly contribute tossDNA binding and wrapping
Conflict of Interests
The authors declare that there is no conflict of interestsregarding the publication of this paper
Acknowledgment
This researchwas supported by aGrant from theNational Sci-ence Council Taiwan (NSC 102-2320-B-040-019 to Cheng-Yang Huang)
References
[1] R Reyes-Lamothe D J Sherratt and M C Leake ldquoStoichiom-etry and architecture of active DNA replication machinery inescherichia colirdquo Science vol 328 no 5977 pp 498ndash501 2010
[2] D J Richard E Bolderson andK K Khanna ldquoMultiple humansingle-stranded DNA binding proteins function in genomemaintenance structural biochemical and functional analysisrdquo
Critical Reviews in Biochemistry and Molecular Biology vol 44no 2-3 pp 98ndash116 2009
[3] R D Shereda A G Kozlov T M LohmanMM Cox and J LKeck ldquoSSB as an organizermobilizer of genome maintenancecomplexesrdquo Critical Reviews in Biochemistry and MolecularBiology vol 43 no 5 pp 289ndash318 2008
[4] D J Richard E Bolderson L Cubeddu et al ldquoSingle-strandedDNA-binding protein hSSB1 is critical for genomic stabilityrdquoNature vol 453 no 7195 pp 677ndash681 2008
[5] R R Meyer and P S Laine ldquoThe single-stranded DNA-bindingprotein of Escherichia colirdquoMicrobiological Reviews vol 54 no4 pp 342ndash380 1990
[6] C Yang U Curth C Urbanke and C Kang ldquoCrystal structureof human mitochondrial single-stranded DNA binding proteinat 24 A resolutionrdquo Nature Structural Biology vol 4 no 2 pp153ndash157 1997
[7] G Webster J Genschel U Curth C Urbanke C Kang and RHilgenfeld ldquoA common core for binding single-stranded DNAstructural comparison of the single-stranded DNA-bindingproteins (SSB) from E coli and human mitochondriardquo FEBSLetters vol 411 pp 313ndash316 1997
[8] R L Flynn and L Zou ldquoOligonucleotideoligosaccharide-bind-ing fold proteins a growing family of genome guardiansrdquo Crit-ical Reviews in Biochemistry and Molecular Biology vol 45 no4 pp 266ndash275 2010
[9] K Chan Y Lee CWangHHuang andY Sun ldquoSingle-Strand-ed DNA-Binding Protein Complex from Helicobacter pyloriSuggests an ssDNA-Binding Surfacerdquo Journal of MolecularBiology vol 388 no 3 pp 508ndash519 2009
[10] D L Theobald R M Mitton-Fry and D S Wuttke ldquoNucleicacid recognition by OB-fold proteinsrdquo Annual Review of Bio-physics and Biomolecular Structure vol 32 pp 115ndash133 2003
[11] S Raghunathan A G Kozlov TM Lohman andGWaksmanldquoStructure of the DNA binding domain of E coli SSB bound to
14 BioMed Research International
ssDNArdquo Nature Structural Biology vol 7 no 8 pp 648ndash6522000
[12] A G Murzin ldquoOB(oligonucleotideoligosaccharide binding)-fold common structural and functional solution for non-homologous sequencesrdquo EMBO Journal vol 12 no 3 pp 861ndash867 1993
[13] N P George K V Ngo S Chitteni-Pattu et al ldquoStructure andcellular dynamics of deinococcus radiodurans single-strandedDNA (ssDNA)-binding protein (SSB)-DNA complexesrdquo TheJournal of Biological Chemistry vol 287 no 26 pp 22123ndash221322012
[14] P Filipkowski and J Kur ldquoIdentification and propertiesof the Deinococcus grandis and Deinococcus proteolyticussingle-stranded DNA binding proteins (SSB)rdquo Acta BiochimicaPolonica vol 54 no 1 pp 79ndash87 2007
[15] P Filipkowski A Duraj-Thatte and J Kur ldquoIdentificationcloning expression and characterization of a highly ther-mostable single-stranded-DNA-binding protein (SSB) fromDeinococcus murrayirdquo Protein Expression and Purification vol53 no 1 pp 201ndash208 2007
[16] P Filipkowski M Koziatek and J Kur ldquoA highly ther-mostable homodimeric single-stranded DNA-binding proteinfrom Deinococcus radiopugnansrdquo Extremophiles vol 10 no 6pp 607ndash614 2006
[17] P Filipkowski A Duraj-Thatte and J Kur ldquoNovel thermostablesingle-stranded DNA-binding protein (SSB) fromDeinococcusgeothermalisrdquo Archives of Microbiology vol 186 no 2 pp 129ndash137 2006
[18] G Witte C Urbanke and U Curth ldquoSingle-stranded DNA-binding protein of Deinococcus radiodurans a biophysicalcharacterizationrdquoNucleic Acids Research vol 33 no 5 pp 1662ndash1670 2005
[19] D A Bernstein J M Eggingtont M P Killoran A M MisicMMCox and J L Keck ldquoCrystal structure of theDeinococcusradiodurans single-stranded DNA-binding protein suggests amechanism for coping with DNA damagerdquo Proceedings of theNational Academy of Sciences of the United States of Americavol 101 no 23 pp 8575ndash8580 2004
[20] S Dabrowski M Olszewski R Piatek A Brillowska-Dabrowska G Konopa and J Kur ldquoIdentification and charac-terization of single-stranded-DNA-binding proteins fromTher-mus thermophilus and Thermus aquaticusmdashnew arrange-ment of binding domainsrdquo Microbiology vol 148 no 10 pp3307ndash3315 2002
[21] R Gamsjaeger R Kariawasam C Touma A H Kwan M FWhite and L Cubeddu ldquoBackbone and side-chain 1H 13C and15N resonance assignments of the OB domain of the singlestranded DNA binding protein from Sulfolobus solfataricusand chemical shift mapping of the DNA-binding interfacerdquoBiomolecular NMR Assignments 2013
[22] M L Rolfsmeier and C A Haseltine ldquoThe single-strandedDNA binding protein of Sulfolobus solfataricus acts in the pre-synaptic step of homologous recombinationrdquo Journal of Molec-ular Biology vol 397 no 1 pp 31ndash45 2010
[23] I D Kerr R I M Wadsworth L Cubeddu W Blankenfeldt JHNaismith andM FWhite ldquoInsights into ssDNA recognitionby the OB fold from a structural and thermodynamic study ofSulfolobus SSB proteinrdquo EMBO Journal vol 22 no 11 pp 2561ndash2570 2003
[24] C A Haseltine and S C Kowalczykowski ldquoA distinctive single-stranded DNA-binding protein from the Archaeon Sulfolobussolfataricusrdquo Molecular Microbiology vol 43 no 6 pp 1505ndash1515 2002
[25] R I M Wadsworth and M F White ldquoIdentification andproperties of the crenarchaeal single-stranded DNA bindingprotein from Sulfolobus solfataricusrdquo Nucleic Acids Researchvol 29 no 4 pp 914ndash920 2001
[26] S Sugiman-Marangos and M S Junop ldquoThe structure of DdrBfrom Deinococcus a new fold for single-stranded DNA bindingproteinsrdquo Nucleic Acids Research vol 38 no 10 Article IDgkq036 pp 3432ndash3440 2010
[27] H Ghalei H V Moeller D Eppers et al ldquoEntrapment of DNAin an intersubunit tunnel system of a single-stranded DNA-binding proteinrdquo Nucleic Acids Research vol 42 no 10 pp6698ndash6708 2014
[28] S Paytubi S A McMahon S Graham et al ldquoDisplacement ofthe canonical single-strandedDNA-binding protein in the ther-moprotealesrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 109 no 7 pp E398ndashE405 2012
[29] T H Dickey S E Altschuler and D S Wuttke ldquoSingle-stranded DNA-binding proteins multiple domains for multiplefunctionsrdquo Structure vol 21 no 7 pp 1074ndash1084 2013
[30] D Vujaklija and BMacek ldquoDetecting posttranslational modifi-cations of bacterial SSB proteinsrdquoMethods inMolecular Biologyvol 922 pp 205ndash218 2012
[31] I Mijakovic D Petranovic B Macek et al ldquoBacterial single-stranded DNA-binding proteins are phosphorylated on tyro-sinerdquoNucleic Acids Research vol 34 no 5 pp 1588ndash1596 2006
[32] M Olszewski A Grot M Wojciechowski M Nowak MMickiewicz and J Kur ldquoCharacterization of exceptionally ther-mostable single-strandedDNA-binding proteins fromThermo-toga maritima and Thermotoga neapolitanardquo BMC Microbiol-ogy vol 10 article 260 2010
[33] U Curth J Genschel C Urbanke and J Greipel ldquoIn vitro andin vivo function of the C-terminus of Escherichia coil single-stranded DNA binding proteinrdquoNucleic Acids Research vol 24no 14 pp 2706ndash2711 1996
[34] G Witte C Urbanke and U Curth ldquoDNA polymerase IIIchi subunit ties single-stranded DNA binding protein to thebacterial replication machineryrdquoNucleic Acids Research vol 31pp 4434ndash4440 2003
[35] D Shishmarev YWang C EMason et al ldquoOtting Intramolec-ular binding mode of the C-terminus of Escherichia coli single-strandedDNAbinding protein determined by nuclearmagneticresonance spectroscopyrdquo Nucleic Acids Research vol 42 pp2750ndash2757 2014
[36] A G Kozlov M M Cox and T M Lohman ldquoRegulation ofsingle-stranded DNA binding by the C termini of Escherichiacoli single-stranded DNA-binding (SSB) proteinrdquo Journal ofBiological Chemistry vol 285 no 22 pp 17246ndash17252 2010
[37] M Nowak M Olszewski M Spibida and J Kur ldquoChar-acterization of single-stranded DNA-binding proteins fromthe psychrophilic bacteria Desulfotalea psychrophila Flavobac-terium psychrophilum Psychrobacter arcticus Psychrobactercryohalolentis Psychromonas ingrahamii Psychroflexus torquisand Photobacterium profundumrdquo BMC Microbiology vol 14article 91 2014
BioMed Research International 15
[38] T Paradzik N Ivic Z Filic et al ldquoStructure-function relation-ships of two paralogous single-stranded DNA-binding proteinsfrom Streptomyces coelicolor implication of SsbB in chromo-some segregation during sporulationrdquo Nucleic Acids Researchvol 41 no 6 pp 3659ndash3672 2013
[39] S JainM Zweig E Peeters et al ldquoCharacterization of the singlestrandedDNA binding protein SsbB encoded in the gonoccocalgenetic islandrdquo PLoS ONE vol 7 no 4 Article ID e35285 2012
[40] Y H Huang and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from Klebsiella pneumoniaemutation at either Arg73 or Ser76 causes a less cooperativecomplex onDNArdquoGenes to Cells vol 17 no 2 pp 146ndash157 2012
[41] E Antony E A Weiland S Korolev and T M LohmanldquoPlasmodium falciparum SSB tetramer wraps single-strandedDNAwith similar topology but opposite polarity to E Coli SSBrdquoJournal ofMolecular Biology vol 420 no 4-5 pp 269ndash283 2012
[42] H Jan Y L Lee and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from pseudomonas aeruginosaPAO1rdquo Protein Journal vol 30 no 1 pp 20ndash26 2011
[43] Y-H Huang Y-L Lee and C-Y Huang ldquoCharacterization of asingle-stranded DNA binding protein from Salmonella entericaserovar typhimurium LT2rdquo Protein Journal vol 30 no 2 pp102ndash108 2011
[44] S K Bharti K Rex P Sreedhar N Krishnan and U VarshneyldquoChimeras of Escherichia coli and Mycobacterium tuberculosissingle-stranded DNA binding proteins characterization andfunction in Escherichia colirdquo PLoS ONE vol 6 no 12 ArticleID e27216 2011
[45] M T Oliveira and L S Kaguni ldquoFunctional roles of the N-and C-terminal regions of the human mitochondrial single-stranded DNA-binding proteinrdquo PLoS ONE vol 5 no 10Article ID e15379 2010
[46] A G Kozlov J M Eggington M M Cox and T MLohman ldquoBinding of the dimeric Deinococcus radioduranssingle-strandedDNA binding protein to single-strandedDNArdquoBiochemistry vol 49 no 38 pp 8266ndash8275 2010
[47] T M Lohman and M E Ferrari ldquoEscherichia coli single-stranded DNA-binding protein multiple DNA-binding modesand cooperativesrdquo Annual Review of Biochemistry vol 63 pp527ndash570 1994
[48] R Zhou A G Kozlov R Roy et al ldquoSSB functions as a slidingplatform that migrates on DNA via reptationrdquo Cell vol 146 pp222ndash232 2011
[49] R Roy A G Kozlov T M Lohman and T Ha ldquoSSB proteindiffusion on single-stranded DNA stimulates RecA filamentformationrdquo Nature vol 461 pp 1092ndash1097 2009
[50] R Roy A G Kozlov T M Lohman and T Ha ldquoDynamicstructural rearrangements between DNA binding modes of Ecoli SSB proteinrdquo Journal of Molecular Biology vol 369 no 5pp 1244ndash1257 2007
[51] T J Kelly P Simancek and G S Brush ldquoIdentification andcharacterization of a single-stranded DNA-binding proteinfrom the archaeon Methanococcus jannaschiirdquo Proceedings ofthe National Academy of Sciences of the United States of Americavol 95 no 25 pp 14634ndash14639 1998
[52] C Y Huang Determination of the Binding Site-Size of theProtein-DNA Complex by Use of the Electrophoretic MobilityShift Assay InTech Press Rijeka Croatia 2012
[53] A Kornberg ldquoTen commandments of enzymology amendedrdquoTrends in Biochemical Sciences vol 28 no 10 pp 515ndash517 2003
[54] W Zhang X Lu and J Shen ldquoEMSA and single-moleculeforce spectroscopy study of interactions between bacillus sub-tilis single-stranded DNA-binding protein and single-strandedDNArdquo Langmuir vol 27 no 24 pp 15008ndash15015 2011
[55] M Ostermeier and S J Benkovic ldquoEvolution of protein func-tion by domain swappingrdquo Advances in Protein Chemistry vol55 pp 29ndash77 2000
[56] W F Peng and C Y Huang ldquoAllantoinase and dihydroorotasebinding and inhibition by flavonols and the substrates of cyclicamidohydrolasesrdquo Biochimie vol 101 pp 113ndash122 2014
[57] Y H Huang M J Lin and C Y Huang ldquoDnaT is a single-stranded DNA binding proteinrdquo Genes to Cells vol 18 no 11pp 1007ndash1019 2013
[58] Y Y Ho Y H Huang and C Y Huang ldquoChemical rescue of thepost-translationally carboxylated lysine mutant of allantoinaseand dihydroorotase by metal ions and short-chain carboxylicacidsrdquo Amino Acids vol 44 no 4 pp 1181ndash1191 2013
[59] Y H Huang Y Lo W Huang and C Y Huang ldquoCrystalstructure and DNA-binding mode of Klebsiella pneumoniaeprimosomal PriB proteinrdquoGenes to Cells vol 17 no 10 pp 837ndash849 2012
[60] C Y Huang C Y Hsu Y Sun H Wu and C Hsiao ldquoCom-plexed crystal structure of replication restart primosome pro-tein PriB reveals a novel single-stranded DNA-binding moderdquoNucleic Acids Research vol 34 no 14 pp 3878ndash3886 2006
[61] T Kinebuchi H Shindo H Nagai N Shimamoto andM Shimizu ldquoFunctional domains of Escherichia coli single-stranded DNA binding protein as assessed by analyses of thedeletion mutantsrdquo Biochemistry vol 36 no 22 pp 6732ndash67381997
[62] N Shimamoto N Ikushima H Utiyama H Tachibana andK Horie ldquoSpecific and cooperative binding of E coli single-stranded DNA binding protein to mRNArdquo Nucleic AcidsResearch vol 15 no 13 pp 5241ndash5250 1987
[63] H Lin andC YHuang ldquoCharacterization of flavonol inhibitionof DnaB helicase Real-time monitoring structural modelingand proposed mechanismrdquo Journal of Biomedicine and Biotech-nology vol 2012 Article ID 735368 2012
[64] Y H Huang H H Lin and C Y Huang ldquoA single residuedetermines the cooperative binding property of a primosomalDNA replication protein PriB to single-stranded DNArdquo Bio-science Biotechnology and Biochemistry vol 76 no 6 pp 1110ndash1115 2012
[65] H C Hsieh and C Y Huang ldquoIdentification of a novel proteinPriB in Klebsiella pneumoniaerdquo Biochemical and BiophysicalResearch Communications vol 404 no 1 pp 546ndash551 2011
[66] J H Liu T W Chang C Y Huang et al ldquoCrystal structure ofPriB a primosomalDNA replication protein ofEscherichia colirdquoThe Journal of Biological Chemistry vol 279 no 48 pp 50465ndash50471 2004
[67] Y H Huang and C Y Huang ldquoThe N-terminal domain ofDnaT a primosomalDNA replication protein is crucial for PriBbinding and self-trimerizationrdquo Biochemical and BiophysicalResearch Communications vol 442 pp 147ndash152 2013
[68] M A Larkin G Blackshields N P Brown et al ldquoClustalW andClustal X version 20rdquo Bioinformatics vol 23 no 21 pp 2947ndash2948 2007
16 BioMed Research International
[69] S N Savvides S Raghunathan K Futterer A G Kozlov T MLohman and G Waksman ldquoThe C-terminal domain of full-length E coli SSB is disordered even when bound to DNArdquoProtein Science vol 13 no 7 pp 1942ndash1947 2004
[70] C-C Chen J-K Hwang and J-M Yang ldquo(PS)2-v2 template-based protein structure prediction serverrdquo BMC Bioinformaticsvol 10 article 366 2009
[71] C Chen J Hwang and J Yang ldquo(PS)2 protein structure pre-diction serverrdquoNucleic Acids Research vol 34 pp W152ndashW1572006
[72] K Bush and J F Fisher ldquoEpidemiological expansion structuralstudies and clinical challenges of new 120573-lactamases from gram-negative bacteriardquo Annual Review of Microbiology vol 65 pp455ndash478 2011
[73] K K Kumarasamy M A Toleman T R Walsh et al ldquoEmer-gence of a new antibiotic resistance mechanism in India Pak-istan and the UK a molecular biological and epidemiologicalstudyrdquoThe Lancet Infectious Diseases vol 10 no 9 pp 597ndash6022010
[74] K Bush and M J MacIelag ldquoNew 120573-lactam antibiotics and 120573-lactamase inhibitorsrdquo Expert Opinion on Therapeutic Patentsvol 20 no 10 pp 1277ndash1293 2010
[75] K Bush ldquoAlarming 120573-lactamase-mediated resistance in multi-drug-resistant Enterobacteriaceaerdquo Current Opinion in Microbi-ology vol 13 no 5 pp 558ndash564 2010
[76] A Srivastava M Talaue S Liu et al ldquoNew target for inhibitionof bacterial RNA polymerase ldquoswitch regionrdquordquo Current Opinionin Microbiology vol 14 no 5 pp 532ndash543 2011
[77] K Chono K Katsumata T Kontani et al ldquoASP2151 a novelhelicase-primase inhibitor possesses antiviral activity againstvaricella-zoster virus and herpes simplex virus types 1 and 2rdquoJournal of Antimicrobial Chemotherapy vol 65 no 8 pp 1733ndash1741 2010
[78] M T Black and K Coleman ldquoNew inhibitors of bacterial topoi-somerase GyrAParC subunitsrdquo Current Opinion in Investiga-tional Drugs vol 10 no 8 pp 804ndash810 2009
[79] A H Marceau D A Bernstein B W Walsh W Shapiro LA Simmons and J L Keck ldquoProtein interactions in genomemaintenance as novel antibacterial targetsrdquo PLoS ONE vol 8no 3 Article ID e58765 2013
[80] D Lu D A Bernstein K A Satyshur and J L Keck ldquoSmall-molecule tools for dissecting the roles of SSBprotein inter-actions in genome maintenancerdquo Proceedings of the NationalAcademy of Sciences of the United States of America vol 107 no2 pp 633ndash638 2010
[81] I Wong and T M Lohman ldquoA double-filter method fornitrocellulose-filter binding application to protein-nucleic acidinteractionsrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 90 no 12 pp 5428ndash5432 1993
[82] M Mitas J Y Chock and M Christy ldquoThe binding-site sizesof Escherichia coli single-stranded-DNA-binding protein andmammalian replication protein A are 65 and ge 54 nucleotidesrespectivelyrdquo Biochemical Journal vol 324 no 3 pp 957ndash9611997
[83] C Urbanke G Witte and U Curth ldquoSedimentation velocitymethod in the analytical ultracentrifuge for the study of protein-protein interactionsrdquoMethods inMolecular Biology vol 305 pp101ndash114 2005
[84] S Chrysogelos and J Griffith ldquoEscherichia coli single-strandbinding protein organizes single-strandedDNA innucleosome-like unitsrdquo Proceedings of the National Academy of Sciences of theUnited States of America vol 79 no 19 pp 5803ndash5807 1982
[85] J R Casas-Finet K R Fischer and R L Karpel ldquoStructuralbasis for the nucleic acid binding cooperativity of bacteriophageT4 gene 32 protein the (LysArg)3(SerThr)2 (LAST) motifrdquoProceedings of the National Academy of Sciences of the UnitedStates of America vol 89 pp 1050ndash1054 1992
[86] G Witte R Fedorov and U Curth ldquoBiophysical analysis ofThermus aquaticus single-stranded DNA binding proteinrdquo Bio-physical Journal vol 94 no 6 pp 2269ndash2279 2008
[87] R Fedorov G Witte C Urbanke D J Manstein and UCurth ldquo3D structure of Thermus aquaticus single-strandedDNA-binding protein gives insight into the functioning of SSBproteinsrdquo Nucleic Acids Research vol 34 no 22 pp 6708ndash67172006
[88] K L Tsai C Y Huang C H Chang Y J Sun W J ChuangandC DHsiao ldquoCrystal structure of the human FOXK1a-DNAcomplex and its implications on the diverse binding specificityof winged helixforkhead proteinsrdquo Journal of Biological Chem-istry vol 281 no 25 pp 17400ndash17409 2006
[89] A H Mao N Lyle and R V Pappu ldquoDescribing sequence-ensemble relationships for intrinsically disordered proteinsrdquoBiochemical Journal vol 449 no 2 pp 307ndash318 2013
[90] R Wetzel ldquoPhysical chemistry of polyglutamine Intriguingtales of a monotonous sequencerdquo Journal of Molecular Biologyvol 421 no 4-5 pp 466ndash490 2012
[91] H T Orr ldquoPolyglutamine neurodegeneration expanded glu-tamines enhance native functionsrdquo Current Opinion in Geneticsand Development vol 22 no 3 pp 251ndash255 2012
[92] M Figiel W J Szlachcic P M Switonski A Gabka and W JKrzyzosiak ldquoMouse models of polyglutamine diseases reviewand data table Part Irdquo Molecular Neurobiology vol 46 no 2pp 393ndash429 2012
[93] J Nasica-Labouze and N Mousseau ldquoKinetics of amyloidaggregation a study of the GNNQQNY prion sequencerdquo PLoSComputational Biology vol 8 no 11 Article ID e1002782 2012
[94] J Nasica-Labouze M Meli P Derreumaux G Colombo andN Mousseau ldquoA multiscale approach to characterize the earlyaggregation steps of the amyloid-forming peptide gnnqqnyfrom the yeast prion sup-35rdquo PLoS Computational Biology vol7 no 5 Article ID e1002051 2011
[95] J R Lewandowski P C A van der Wel M Rigney N Grig-orieff and R G Griffin ldquoStructural complexity of a compositeamyloid fibrilrdquo Journal of the American Chemical Society vol133 no 37 pp 14686ndash14698 2011
BioMed Research International 13
PaSSBStSSB KpSSB
C-terC-ter C-ter
sim 21 nt sim 26 nt sim 29 nt
Figure 11 Possiblemodels for explainingwhy SSBs arewith different binding site sizes Twomodeled structures of KpSSB1ndash142 (blue) StSSB1ndash142 (pink) and PaSSB1ndash160 (green) complexed with ssDNA (gold) are shown For clarity only one C-terminal domain was shown for eachSSB tetramer By using the electrophoretic mobility shift assay and the protein chimeragenesis we characterized that the binding site sizes ofKpSSB StSSB PaSSB KpSSBnStSSBc and KpSSBnPaSSBc were 26 21 29 21 and 29 nt per tetramer respectively KpSSB StSSB and PaSSBare similar proteins whose N-terminal ssDNA-binding domains are almost identical Thus the C-terminal domain of SSB may indirectlycontribute to ssDNA binding and wrapping and affects the binding site size by the steric hindrance
5 Conclusion
In this study we characterized the ssDNA-binding propertiesof untagged SSBs from K pneumoniae S enterica sero-var Typhimurium LT2 and P aeruginosa PAO1 and proposeda role of the C-terminal flexible domain for ssDNA bind-ing from the protein chimeragenesis and EMSA resultsThe amino acid sequence of the N-terminal ssDNA-bind-ingoligomerization domain in these pathogenic SSBs ishighly conserved but their apparent binding site sizes aredifferent This finding indicates that the C-terminal protein-protein interaction domain may also indirectly contribute tossDNA binding and wrapping
Conflict of Interests
The authors declare that there is no conflict of interestsregarding the publication of this paper
Acknowledgment
This researchwas supported by aGrant from theNational Sci-ence Council Taiwan (NSC 102-2320-B-040-019 to Cheng-Yang Huang)
References
[1] R Reyes-Lamothe D J Sherratt and M C Leake ldquoStoichiom-etry and architecture of active DNA replication machinery inescherichia colirdquo Science vol 328 no 5977 pp 498ndash501 2010
[2] D J Richard E Bolderson andK K Khanna ldquoMultiple humansingle-stranded DNA binding proteins function in genomemaintenance structural biochemical and functional analysisrdquo
Critical Reviews in Biochemistry and Molecular Biology vol 44no 2-3 pp 98ndash116 2009
[3] R D Shereda A G Kozlov T M LohmanMM Cox and J LKeck ldquoSSB as an organizermobilizer of genome maintenancecomplexesrdquo Critical Reviews in Biochemistry and MolecularBiology vol 43 no 5 pp 289ndash318 2008
[4] D J Richard E Bolderson L Cubeddu et al ldquoSingle-strandedDNA-binding protein hSSB1 is critical for genomic stabilityrdquoNature vol 453 no 7195 pp 677ndash681 2008
[5] R R Meyer and P S Laine ldquoThe single-stranded DNA-bindingprotein of Escherichia colirdquoMicrobiological Reviews vol 54 no4 pp 342ndash380 1990
[6] C Yang U Curth C Urbanke and C Kang ldquoCrystal structureof human mitochondrial single-stranded DNA binding proteinat 24 A resolutionrdquo Nature Structural Biology vol 4 no 2 pp153ndash157 1997
[7] G Webster J Genschel U Curth C Urbanke C Kang and RHilgenfeld ldquoA common core for binding single-stranded DNAstructural comparison of the single-stranded DNA-bindingproteins (SSB) from E coli and human mitochondriardquo FEBSLetters vol 411 pp 313ndash316 1997
[8] R L Flynn and L Zou ldquoOligonucleotideoligosaccharide-bind-ing fold proteins a growing family of genome guardiansrdquo Crit-ical Reviews in Biochemistry and Molecular Biology vol 45 no4 pp 266ndash275 2010
[9] K Chan Y Lee CWangHHuang andY Sun ldquoSingle-Strand-ed DNA-Binding Protein Complex from Helicobacter pyloriSuggests an ssDNA-Binding Surfacerdquo Journal of MolecularBiology vol 388 no 3 pp 508ndash519 2009
[10] D L Theobald R M Mitton-Fry and D S Wuttke ldquoNucleicacid recognition by OB-fold proteinsrdquo Annual Review of Bio-physics and Biomolecular Structure vol 32 pp 115ndash133 2003
[11] S Raghunathan A G Kozlov TM Lohman andGWaksmanldquoStructure of the DNA binding domain of E coli SSB bound to
14 BioMed Research International
ssDNArdquo Nature Structural Biology vol 7 no 8 pp 648ndash6522000
[12] A G Murzin ldquoOB(oligonucleotideoligosaccharide binding)-fold common structural and functional solution for non-homologous sequencesrdquo EMBO Journal vol 12 no 3 pp 861ndash867 1993
[13] N P George K V Ngo S Chitteni-Pattu et al ldquoStructure andcellular dynamics of deinococcus radiodurans single-strandedDNA (ssDNA)-binding protein (SSB)-DNA complexesrdquo TheJournal of Biological Chemistry vol 287 no 26 pp 22123ndash221322012
[14] P Filipkowski and J Kur ldquoIdentification and propertiesof the Deinococcus grandis and Deinococcus proteolyticussingle-stranded DNA binding proteins (SSB)rdquo Acta BiochimicaPolonica vol 54 no 1 pp 79ndash87 2007
[15] P Filipkowski A Duraj-Thatte and J Kur ldquoIdentificationcloning expression and characterization of a highly ther-mostable single-stranded-DNA-binding protein (SSB) fromDeinococcus murrayirdquo Protein Expression and Purification vol53 no 1 pp 201ndash208 2007
[16] P Filipkowski M Koziatek and J Kur ldquoA highly ther-mostable homodimeric single-stranded DNA-binding proteinfrom Deinococcus radiopugnansrdquo Extremophiles vol 10 no 6pp 607ndash614 2006
[17] P Filipkowski A Duraj-Thatte and J Kur ldquoNovel thermostablesingle-stranded DNA-binding protein (SSB) fromDeinococcusgeothermalisrdquo Archives of Microbiology vol 186 no 2 pp 129ndash137 2006
[18] G Witte C Urbanke and U Curth ldquoSingle-stranded DNA-binding protein of Deinococcus radiodurans a biophysicalcharacterizationrdquoNucleic Acids Research vol 33 no 5 pp 1662ndash1670 2005
[19] D A Bernstein J M Eggingtont M P Killoran A M MisicMMCox and J L Keck ldquoCrystal structure of theDeinococcusradiodurans single-stranded DNA-binding protein suggests amechanism for coping with DNA damagerdquo Proceedings of theNational Academy of Sciences of the United States of Americavol 101 no 23 pp 8575ndash8580 2004
[20] S Dabrowski M Olszewski R Piatek A Brillowska-Dabrowska G Konopa and J Kur ldquoIdentification and charac-terization of single-stranded-DNA-binding proteins fromTher-mus thermophilus and Thermus aquaticusmdashnew arrange-ment of binding domainsrdquo Microbiology vol 148 no 10 pp3307ndash3315 2002
[21] R Gamsjaeger R Kariawasam C Touma A H Kwan M FWhite and L Cubeddu ldquoBackbone and side-chain 1H 13C and15N resonance assignments of the OB domain of the singlestranded DNA binding protein from Sulfolobus solfataricusand chemical shift mapping of the DNA-binding interfacerdquoBiomolecular NMR Assignments 2013
[22] M L Rolfsmeier and C A Haseltine ldquoThe single-strandedDNA binding protein of Sulfolobus solfataricus acts in the pre-synaptic step of homologous recombinationrdquo Journal of Molec-ular Biology vol 397 no 1 pp 31ndash45 2010
[23] I D Kerr R I M Wadsworth L Cubeddu W Blankenfeldt JHNaismith andM FWhite ldquoInsights into ssDNA recognitionby the OB fold from a structural and thermodynamic study ofSulfolobus SSB proteinrdquo EMBO Journal vol 22 no 11 pp 2561ndash2570 2003
[24] C A Haseltine and S C Kowalczykowski ldquoA distinctive single-stranded DNA-binding protein from the Archaeon Sulfolobussolfataricusrdquo Molecular Microbiology vol 43 no 6 pp 1505ndash1515 2002
[25] R I M Wadsworth and M F White ldquoIdentification andproperties of the crenarchaeal single-stranded DNA bindingprotein from Sulfolobus solfataricusrdquo Nucleic Acids Researchvol 29 no 4 pp 914ndash920 2001
[26] S Sugiman-Marangos and M S Junop ldquoThe structure of DdrBfrom Deinococcus a new fold for single-stranded DNA bindingproteinsrdquo Nucleic Acids Research vol 38 no 10 Article IDgkq036 pp 3432ndash3440 2010
[27] H Ghalei H V Moeller D Eppers et al ldquoEntrapment of DNAin an intersubunit tunnel system of a single-stranded DNA-binding proteinrdquo Nucleic Acids Research vol 42 no 10 pp6698ndash6708 2014
[28] S Paytubi S A McMahon S Graham et al ldquoDisplacement ofthe canonical single-strandedDNA-binding protein in the ther-moprotealesrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 109 no 7 pp E398ndashE405 2012
[29] T H Dickey S E Altschuler and D S Wuttke ldquoSingle-stranded DNA-binding proteins multiple domains for multiplefunctionsrdquo Structure vol 21 no 7 pp 1074ndash1084 2013
[30] D Vujaklija and BMacek ldquoDetecting posttranslational modifi-cations of bacterial SSB proteinsrdquoMethods inMolecular Biologyvol 922 pp 205ndash218 2012
[31] I Mijakovic D Petranovic B Macek et al ldquoBacterial single-stranded DNA-binding proteins are phosphorylated on tyro-sinerdquoNucleic Acids Research vol 34 no 5 pp 1588ndash1596 2006
[32] M Olszewski A Grot M Wojciechowski M Nowak MMickiewicz and J Kur ldquoCharacterization of exceptionally ther-mostable single-strandedDNA-binding proteins fromThermo-toga maritima and Thermotoga neapolitanardquo BMC Microbiol-ogy vol 10 article 260 2010
[33] U Curth J Genschel C Urbanke and J Greipel ldquoIn vitro andin vivo function of the C-terminus of Escherichia coil single-stranded DNA binding proteinrdquoNucleic Acids Research vol 24no 14 pp 2706ndash2711 1996
[34] G Witte C Urbanke and U Curth ldquoDNA polymerase IIIchi subunit ties single-stranded DNA binding protein to thebacterial replication machineryrdquoNucleic Acids Research vol 31pp 4434ndash4440 2003
[35] D Shishmarev YWang C EMason et al ldquoOtting Intramolec-ular binding mode of the C-terminus of Escherichia coli single-strandedDNAbinding protein determined by nuclearmagneticresonance spectroscopyrdquo Nucleic Acids Research vol 42 pp2750ndash2757 2014
[36] A G Kozlov M M Cox and T M Lohman ldquoRegulation ofsingle-stranded DNA binding by the C termini of Escherichiacoli single-stranded DNA-binding (SSB) proteinrdquo Journal ofBiological Chemistry vol 285 no 22 pp 17246ndash17252 2010
[37] M Nowak M Olszewski M Spibida and J Kur ldquoChar-acterization of single-stranded DNA-binding proteins fromthe psychrophilic bacteria Desulfotalea psychrophila Flavobac-terium psychrophilum Psychrobacter arcticus Psychrobactercryohalolentis Psychromonas ingrahamii Psychroflexus torquisand Photobacterium profundumrdquo BMC Microbiology vol 14article 91 2014
BioMed Research International 15
[38] T Paradzik N Ivic Z Filic et al ldquoStructure-function relation-ships of two paralogous single-stranded DNA-binding proteinsfrom Streptomyces coelicolor implication of SsbB in chromo-some segregation during sporulationrdquo Nucleic Acids Researchvol 41 no 6 pp 3659ndash3672 2013
[39] S JainM Zweig E Peeters et al ldquoCharacterization of the singlestrandedDNA binding protein SsbB encoded in the gonoccocalgenetic islandrdquo PLoS ONE vol 7 no 4 Article ID e35285 2012
[40] Y H Huang and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from Klebsiella pneumoniaemutation at either Arg73 or Ser76 causes a less cooperativecomplex onDNArdquoGenes to Cells vol 17 no 2 pp 146ndash157 2012
[41] E Antony E A Weiland S Korolev and T M LohmanldquoPlasmodium falciparum SSB tetramer wraps single-strandedDNAwith similar topology but opposite polarity to E Coli SSBrdquoJournal ofMolecular Biology vol 420 no 4-5 pp 269ndash283 2012
[42] H Jan Y L Lee and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from pseudomonas aeruginosaPAO1rdquo Protein Journal vol 30 no 1 pp 20ndash26 2011
[43] Y-H Huang Y-L Lee and C-Y Huang ldquoCharacterization of asingle-stranded DNA binding protein from Salmonella entericaserovar typhimurium LT2rdquo Protein Journal vol 30 no 2 pp102ndash108 2011
[44] S K Bharti K Rex P Sreedhar N Krishnan and U VarshneyldquoChimeras of Escherichia coli and Mycobacterium tuberculosissingle-stranded DNA binding proteins characterization andfunction in Escherichia colirdquo PLoS ONE vol 6 no 12 ArticleID e27216 2011
[45] M T Oliveira and L S Kaguni ldquoFunctional roles of the N-and C-terminal regions of the human mitochondrial single-stranded DNA-binding proteinrdquo PLoS ONE vol 5 no 10Article ID e15379 2010
[46] A G Kozlov J M Eggington M M Cox and T MLohman ldquoBinding of the dimeric Deinococcus radioduranssingle-strandedDNA binding protein to single-strandedDNArdquoBiochemistry vol 49 no 38 pp 8266ndash8275 2010
[47] T M Lohman and M E Ferrari ldquoEscherichia coli single-stranded DNA-binding protein multiple DNA-binding modesand cooperativesrdquo Annual Review of Biochemistry vol 63 pp527ndash570 1994
[48] R Zhou A G Kozlov R Roy et al ldquoSSB functions as a slidingplatform that migrates on DNA via reptationrdquo Cell vol 146 pp222ndash232 2011
[49] R Roy A G Kozlov T M Lohman and T Ha ldquoSSB proteindiffusion on single-stranded DNA stimulates RecA filamentformationrdquo Nature vol 461 pp 1092ndash1097 2009
[50] R Roy A G Kozlov T M Lohman and T Ha ldquoDynamicstructural rearrangements between DNA binding modes of Ecoli SSB proteinrdquo Journal of Molecular Biology vol 369 no 5pp 1244ndash1257 2007
[51] T J Kelly P Simancek and G S Brush ldquoIdentification andcharacterization of a single-stranded DNA-binding proteinfrom the archaeon Methanococcus jannaschiirdquo Proceedings ofthe National Academy of Sciences of the United States of Americavol 95 no 25 pp 14634ndash14639 1998
[52] C Y Huang Determination of the Binding Site-Size of theProtein-DNA Complex by Use of the Electrophoretic MobilityShift Assay InTech Press Rijeka Croatia 2012
[53] A Kornberg ldquoTen commandments of enzymology amendedrdquoTrends in Biochemical Sciences vol 28 no 10 pp 515ndash517 2003
[54] W Zhang X Lu and J Shen ldquoEMSA and single-moleculeforce spectroscopy study of interactions between bacillus sub-tilis single-stranded DNA-binding protein and single-strandedDNArdquo Langmuir vol 27 no 24 pp 15008ndash15015 2011
[55] M Ostermeier and S J Benkovic ldquoEvolution of protein func-tion by domain swappingrdquo Advances in Protein Chemistry vol55 pp 29ndash77 2000
[56] W F Peng and C Y Huang ldquoAllantoinase and dihydroorotasebinding and inhibition by flavonols and the substrates of cyclicamidohydrolasesrdquo Biochimie vol 101 pp 113ndash122 2014
[57] Y H Huang M J Lin and C Y Huang ldquoDnaT is a single-stranded DNA binding proteinrdquo Genes to Cells vol 18 no 11pp 1007ndash1019 2013
[58] Y Y Ho Y H Huang and C Y Huang ldquoChemical rescue of thepost-translationally carboxylated lysine mutant of allantoinaseand dihydroorotase by metal ions and short-chain carboxylicacidsrdquo Amino Acids vol 44 no 4 pp 1181ndash1191 2013
[59] Y H Huang Y Lo W Huang and C Y Huang ldquoCrystalstructure and DNA-binding mode of Klebsiella pneumoniaeprimosomal PriB proteinrdquoGenes to Cells vol 17 no 10 pp 837ndash849 2012
[60] C Y Huang C Y Hsu Y Sun H Wu and C Hsiao ldquoCom-plexed crystal structure of replication restart primosome pro-tein PriB reveals a novel single-stranded DNA-binding moderdquoNucleic Acids Research vol 34 no 14 pp 3878ndash3886 2006
[61] T Kinebuchi H Shindo H Nagai N Shimamoto andM Shimizu ldquoFunctional domains of Escherichia coli single-stranded DNA binding protein as assessed by analyses of thedeletion mutantsrdquo Biochemistry vol 36 no 22 pp 6732ndash67381997
[62] N Shimamoto N Ikushima H Utiyama H Tachibana andK Horie ldquoSpecific and cooperative binding of E coli single-stranded DNA binding protein to mRNArdquo Nucleic AcidsResearch vol 15 no 13 pp 5241ndash5250 1987
[63] H Lin andC YHuang ldquoCharacterization of flavonol inhibitionof DnaB helicase Real-time monitoring structural modelingand proposed mechanismrdquo Journal of Biomedicine and Biotech-nology vol 2012 Article ID 735368 2012
[64] Y H Huang H H Lin and C Y Huang ldquoA single residuedetermines the cooperative binding property of a primosomalDNA replication protein PriB to single-stranded DNArdquo Bio-science Biotechnology and Biochemistry vol 76 no 6 pp 1110ndash1115 2012
[65] H C Hsieh and C Y Huang ldquoIdentification of a novel proteinPriB in Klebsiella pneumoniaerdquo Biochemical and BiophysicalResearch Communications vol 404 no 1 pp 546ndash551 2011
[66] J H Liu T W Chang C Y Huang et al ldquoCrystal structure ofPriB a primosomalDNA replication protein ofEscherichia colirdquoThe Journal of Biological Chemistry vol 279 no 48 pp 50465ndash50471 2004
[67] Y H Huang and C Y Huang ldquoThe N-terminal domain ofDnaT a primosomalDNA replication protein is crucial for PriBbinding and self-trimerizationrdquo Biochemical and BiophysicalResearch Communications vol 442 pp 147ndash152 2013
[68] M A Larkin G Blackshields N P Brown et al ldquoClustalW andClustal X version 20rdquo Bioinformatics vol 23 no 21 pp 2947ndash2948 2007
16 BioMed Research International
[69] S N Savvides S Raghunathan K Futterer A G Kozlov T MLohman and G Waksman ldquoThe C-terminal domain of full-length E coli SSB is disordered even when bound to DNArdquoProtein Science vol 13 no 7 pp 1942ndash1947 2004
[70] C-C Chen J-K Hwang and J-M Yang ldquo(PS)2-v2 template-based protein structure prediction serverrdquo BMC Bioinformaticsvol 10 article 366 2009
[71] C Chen J Hwang and J Yang ldquo(PS)2 protein structure pre-diction serverrdquoNucleic Acids Research vol 34 pp W152ndashW1572006
[72] K Bush and J F Fisher ldquoEpidemiological expansion structuralstudies and clinical challenges of new 120573-lactamases from gram-negative bacteriardquo Annual Review of Microbiology vol 65 pp455ndash478 2011
[73] K K Kumarasamy M A Toleman T R Walsh et al ldquoEmer-gence of a new antibiotic resistance mechanism in India Pak-istan and the UK a molecular biological and epidemiologicalstudyrdquoThe Lancet Infectious Diseases vol 10 no 9 pp 597ndash6022010
[74] K Bush and M J MacIelag ldquoNew 120573-lactam antibiotics and 120573-lactamase inhibitorsrdquo Expert Opinion on Therapeutic Patentsvol 20 no 10 pp 1277ndash1293 2010
[75] K Bush ldquoAlarming 120573-lactamase-mediated resistance in multi-drug-resistant Enterobacteriaceaerdquo Current Opinion in Microbi-ology vol 13 no 5 pp 558ndash564 2010
[76] A Srivastava M Talaue S Liu et al ldquoNew target for inhibitionof bacterial RNA polymerase ldquoswitch regionrdquordquo Current Opinionin Microbiology vol 14 no 5 pp 532ndash543 2011
[77] K Chono K Katsumata T Kontani et al ldquoASP2151 a novelhelicase-primase inhibitor possesses antiviral activity againstvaricella-zoster virus and herpes simplex virus types 1 and 2rdquoJournal of Antimicrobial Chemotherapy vol 65 no 8 pp 1733ndash1741 2010
[78] M T Black and K Coleman ldquoNew inhibitors of bacterial topoi-somerase GyrAParC subunitsrdquo Current Opinion in Investiga-tional Drugs vol 10 no 8 pp 804ndash810 2009
[79] A H Marceau D A Bernstein B W Walsh W Shapiro LA Simmons and J L Keck ldquoProtein interactions in genomemaintenance as novel antibacterial targetsrdquo PLoS ONE vol 8no 3 Article ID e58765 2013
[80] D Lu D A Bernstein K A Satyshur and J L Keck ldquoSmall-molecule tools for dissecting the roles of SSBprotein inter-actions in genome maintenancerdquo Proceedings of the NationalAcademy of Sciences of the United States of America vol 107 no2 pp 633ndash638 2010
[81] I Wong and T M Lohman ldquoA double-filter method fornitrocellulose-filter binding application to protein-nucleic acidinteractionsrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 90 no 12 pp 5428ndash5432 1993
[82] M Mitas J Y Chock and M Christy ldquoThe binding-site sizesof Escherichia coli single-stranded-DNA-binding protein andmammalian replication protein A are 65 and ge 54 nucleotidesrespectivelyrdquo Biochemical Journal vol 324 no 3 pp 957ndash9611997
[83] C Urbanke G Witte and U Curth ldquoSedimentation velocitymethod in the analytical ultracentrifuge for the study of protein-protein interactionsrdquoMethods inMolecular Biology vol 305 pp101ndash114 2005
[84] S Chrysogelos and J Griffith ldquoEscherichia coli single-strandbinding protein organizes single-strandedDNA innucleosome-like unitsrdquo Proceedings of the National Academy of Sciences of theUnited States of America vol 79 no 19 pp 5803ndash5807 1982
[85] J R Casas-Finet K R Fischer and R L Karpel ldquoStructuralbasis for the nucleic acid binding cooperativity of bacteriophageT4 gene 32 protein the (LysArg)3(SerThr)2 (LAST) motifrdquoProceedings of the National Academy of Sciences of the UnitedStates of America vol 89 pp 1050ndash1054 1992
[86] G Witte R Fedorov and U Curth ldquoBiophysical analysis ofThermus aquaticus single-stranded DNA binding proteinrdquo Bio-physical Journal vol 94 no 6 pp 2269ndash2279 2008
[87] R Fedorov G Witte C Urbanke D J Manstein and UCurth ldquo3D structure of Thermus aquaticus single-strandedDNA-binding protein gives insight into the functioning of SSBproteinsrdquo Nucleic Acids Research vol 34 no 22 pp 6708ndash67172006
[88] K L Tsai C Y Huang C H Chang Y J Sun W J ChuangandC DHsiao ldquoCrystal structure of the human FOXK1a-DNAcomplex and its implications on the diverse binding specificityof winged helixforkhead proteinsrdquo Journal of Biological Chem-istry vol 281 no 25 pp 17400ndash17409 2006
[89] A H Mao N Lyle and R V Pappu ldquoDescribing sequence-ensemble relationships for intrinsically disordered proteinsrdquoBiochemical Journal vol 449 no 2 pp 307ndash318 2013
[90] R Wetzel ldquoPhysical chemistry of polyglutamine Intriguingtales of a monotonous sequencerdquo Journal of Molecular Biologyvol 421 no 4-5 pp 466ndash490 2012
[91] H T Orr ldquoPolyglutamine neurodegeneration expanded glu-tamines enhance native functionsrdquo Current Opinion in Geneticsand Development vol 22 no 3 pp 251ndash255 2012
[92] M Figiel W J Szlachcic P M Switonski A Gabka and W JKrzyzosiak ldquoMouse models of polyglutamine diseases reviewand data table Part Irdquo Molecular Neurobiology vol 46 no 2pp 393ndash429 2012
[93] J Nasica-Labouze and N Mousseau ldquoKinetics of amyloidaggregation a study of the GNNQQNY prion sequencerdquo PLoSComputational Biology vol 8 no 11 Article ID e1002782 2012
[94] J Nasica-Labouze M Meli P Derreumaux G Colombo andN Mousseau ldquoA multiscale approach to characterize the earlyaggregation steps of the amyloid-forming peptide gnnqqnyfrom the yeast prion sup-35rdquo PLoS Computational Biology vol7 no 5 Article ID e1002051 2011
[95] J R Lewandowski P C A van der Wel M Rigney N Grig-orieff and R G Griffin ldquoStructural complexity of a compositeamyloid fibrilrdquo Journal of the American Chemical Society vol133 no 37 pp 14686ndash14698 2011
14 BioMed Research International
ssDNArdquo Nature Structural Biology vol 7 no 8 pp 648ndash6522000
[12] A G Murzin ldquoOB(oligonucleotideoligosaccharide binding)-fold common structural and functional solution for non-homologous sequencesrdquo EMBO Journal vol 12 no 3 pp 861ndash867 1993
[13] N P George K V Ngo S Chitteni-Pattu et al ldquoStructure andcellular dynamics of deinococcus radiodurans single-strandedDNA (ssDNA)-binding protein (SSB)-DNA complexesrdquo TheJournal of Biological Chemistry vol 287 no 26 pp 22123ndash221322012
[14] P Filipkowski and J Kur ldquoIdentification and propertiesof the Deinococcus grandis and Deinococcus proteolyticussingle-stranded DNA binding proteins (SSB)rdquo Acta BiochimicaPolonica vol 54 no 1 pp 79ndash87 2007
[15] P Filipkowski A Duraj-Thatte and J Kur ldquoIdentificationcloning expression and characterization of a highly ther-mostable single-stranded-DNA-binding protein (SSB) fromDeinococcus murrayirdquo Protein Expression and Purification vol53 no 1 pp 201ndash208 2007
[16] P Filipkowski M Koziatek and J Kur ldquoA highly ther-mostable homodimeric single-stranded DNA-binding proteinfrom Deinococcus radiopugnansrdquo Extremophiles vol 10 no 6pp 607ndash614 2006
[17] P Filipkowski A Duraj-Thatte and J Kur ldquoNovel thermostablesingle-stranded DNA-binding protein (SSB) fromDeinococcusgeothermalisrdquo Archives of Microbiology vol 186 no 2 pp 129ndash137 2006
[18] G Witte C Urbanke and U Curth ldquoSingle-stranded DNA-binding protein of Deinococcus radiodurans a biophysicalcharacterizationrdquoNucleic Acids Research vol 33 no 5 pp 1662ndash1670 2005
[19] D A Bernstein J M Eggingtont M P Killoran A M MisicMMCox and J L Keck ldquoCrystal structure of theDeinococcusradiodurans single-stranded DNA-binding protein suggests amechanism for coping with DNA damagerdquo Proceedings of theNational Academy of Sciences of the United States of Americavol 101 no 23 pp 8575ndash8580 2004
[20] S Dabrowski M Olszewski R Piatek A Brillowska-Dabrowska G Konopa and J Kur ldquoIdentification and charac-terization of single-stranded-DNA-binding proteins fromTher-mus thermophilus and Thermus aquaticusmdashnew arrange-ment of binding domainsrdquo Microbiology vol 148 no 10 pp3307ndash3315 2002
[21] R Gamsjaeger R Kariawasam C Touma A H Kwan M FWhite and L Cubeddu ldquoBackbone and side-chain 1H 13C and15N resonance assignments of the OB domain of the singlestranded DNA binding protein from Sulfolobus solfataricusand chemical shift mapping of the DNA-binding interfacerdquoBiomolecular NMR Assignments 2013
[22] M L Rolfsmeier and C A Haseltine ldquoThe single-strandedDNA binding protein of Sulfolobus solfataricus acts in the pre-synaptic step of homologous recombinationrdquo Journal of Molec-ular Biology vol 397 no 1 pp 31ndash45 2010
[23] I D Kerr R I M Wadsworth L Cubeddu W Blankenfeldt JHNaismith andM FWhite ldquoInsights into ssDNA recognitionby the OB fold from a structural and thermodynamic study ofSulfolobus SSB proteinrdquo EMBO Journal vol 22 no 11 pp 2561ndash2570 2003
[24] C A Haseltine and S C Kowalczykowski ldquoA distinctive single-stranded DNA-binding protein from the Archaeon Sulfolobussolfataricusrdquo Molecular Microbiology vol 43 no 6 pp 1505ndash1515 2002
[25] R I M Wadsworth and M F White ldquoIdentification andproperties of the crenarchaeal single-stranded DNA bindingprotein from Sulfolobus solfataricusrdquo Nucleic Acids Researchvol 29 no 4 pp 914ndash920 2001
[26] S Sugiman-Marangos and M S Junop ldquoThe structure of DdrBfrom Deinococcus a new fold for single-stranded DNA bindingproteinsrdquo Nucleic Acids Research vol 38 no 10 Article IDgkq036 pp 3432ndash3440 2010
[27] H Ghalei H V Moeller D Eppers et al ldquoEntrapment of DNAin an intersubunit tunnel system of a single-stranded DNA-binding proteinrdquo Nucleic Acids Research vol 42 no 10 pp6698ndash6708 2014
[28] S Paytubi S A McMahon S Graham et al ldquoDisplacement ofthe canonical single-strandedDNA-binding protein in the ther-moprotealesrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 109 no 7 pp E398ndashE405 2012
[29] T H Dickey S E Altschuler and D S Wuttke ldquoSingle-stranded DNA-binding proteins multiple domains for multiplefunctionsrdquo Structure vol 21 no 7 pp 1074ndash1084 2013
[30] D Vujaklija and BMacek ldquoDetecting posttranslational modifi-cations of bacterial SSB proteinsrdquoMethods inMolecular Biologyvol 922 pp 205ndash218 2012
[31] I Mijakovic D Petranovic B Macek et al ldquoBacterial single-stranded DNA-binding proteins are phosphorylated on tyro-sinerdquoNucleic Acids Research vol 34 no 5 pp 1588ndash1596 2006
[32] M Olszewski A Grot M Wojciechowski M Nowak MMickiewicz and J Kur ldquoCharacterization of exceptionally ther-mostable single-strandedDNA-binding proteins fromThermo-toga maritima and Thermotoga neapolitanardquo BMC Microbiol-ogy vol 10 article 260 2010
[33] U Curth J Genschel C Urbanke and J Greipel ldquoIn vitro andin vivo function of the C-terminus of Escherichia coil single-stranded DNA binding proteinrdquoNucleic Acids Research vol 24no 14 pp 2706ndash2711 1996
[34] G Witte C Urbanke and U Curth ldquoDNA polymerase IIIchi subunit ties single-stranded DNA binding protein to thebacterial replication machineryrdquoNucleic Acids Research vol 31pp 4434ndash4440 2003
[35] D Shishmarev YWang C EMason et al ldquoOtting Intramolec-ular binding mode of the C-terminus of Escherichia coli single-strandedDNAbinding protein determined by nuclearmagneticresonance spectroscopyrdquo Nucleic Acids Research vol 42 pp2750ndash2757 2014
[36] A G Kozlov M M Cox and T M Lohman ldquoRegulation ofsingle-stranded DNA binding by the C termini of Escherichiacoli single-stranded DNA-binding (SSB) proteinrdquo Journal ofBiological Chemistry vol 285 no 22 pp 17246ndash17252 2010
[37] M Nowak M Olszewski M Spibida and J Kur ldquoChar-acterization of single-stranded DNA-binding proteins fromthe psychrophilic bacteria Desulfotalea psychrophila Flavobac-terium psychrophilum Psychrobacter arcticus Psychrobactercryohalolentis Psychromonas ingrahamii Psychroflexus torquisand Photobacterium profundumrdquo BMC Microbiology vol 14article 91 2014
BioMed Research International 15
[38] T Paradzik N Ivic Z Filic et al ldquoStructure-function relation-ships of two paralogous single-stranded DNA-binding proteinsfrom Streptomyces coelicolor implication of SsbB in chromo-some segregation during sporulationrdquo Nucleic Acids Researchvol 41 no 6 pp 3659ndash3672 2013
[39] S JainM Zweig E Peeters et al ldquoCharacterization of the singlestrandedDNA binding protein SsbB encoded in the gonoccocalgenetic islandrdquo PLoS ONE vol 7 no 4 Article ID e35285 2012
[40] Y H Huang and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from Klebsiella pneumoniaemutation at either Arg73 or Ser76 causes a less cooperativecomplex onDNArdquoGenes to Cells vol 17 no 2 pp 146ndash157 2012
[41] E Antony E A Weiland S Korolev and T M LohmanldquoPlasmodium falciparum SSB tetramer wraps single-strandedDNAwith similar topology but opposite polarity to E Coli SSBrdquoJournal ofMolecular Biology vol 420 no 4-5 pp 269ndash283 2012
[42] H Jan Y L Lee and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from pseudomonas aeruginosaPAO1rdquo Protein Journal vol 30 no 1 pp 20ndash26 2011
[43] Y-H Huang Y-L Lee and C-Y Huang ldquoCharacterization of asingle-stranded DNA binding protein from Salmonella entericaserovar typhimurium LT2rdquo Protein Journal vol 30 no 2 pp102ndash108 2011
[44] S K Bharti K Rex P Sreedhar N Krishnan and U VarshneyldquoChimeras of Escherichia coli and Mycobacterium tuberculosissingle-stranded DNA binding proteins characterization andfunction in Escherichia colirdquo PLoS ONE vol 6 no 12 ArticleID e27216 2011
[45] M T Oliveira and L S Kaguni ldquoFunctional roles of the N-and C-terminal regions of the human mitochondrial single-stranded DNA-binding proteinrdquo PLoS ONE vol 5 no 10Article ID e15379 2010
[46] A G Kozlov J M Eggington M M Cox and T MLohman ldquoBinding of the dimeric Deinococcus radioduranssingle-strandedDNA binding protein to single-strandedDNArdquoBiochemistry vol 49 no 38 pp 8266ndash8275 2010
[47] T M Lohman and M E Ferrari ldquoEscherichia coli single-stranded DNA-binding protein multiple DNA-binding modesand cooperativesrdquo Annual Review of Biochemistry vol 63 pp527ndash570 1994
[48] R Zhou A G Kozlov R Roy et al ldquoSSB functions as a slidingplatform that migrates on DNA via reptationrdquo Cell vol 146 pp222ndash232 2011
[49] R Roy A G Kozlov T M Lohman and T Ha ldquoSSB proteindiffusion on single-stranded DNA stimulates RecA filamentformationrdquo Nature vol 461 pp 1092ndash1097 2009
[50] R Roy A G Kozlov T M Lohman and T Ha ldquoDynamicstructural rearrangements between DNA binding modes of Ecoli SSB proteinrdquo Journal of Molecular Biology vol 369 no 5pp 1244ndash1257 2007
[51] T J Kelly P Simancek and G S Brush ldquoIdentification andcharacterization of a single-stranded DNA-binding proteinfrom the archaeon Methanococcus jannaschiirdquo Proceedings ofthe National Academy of Sciences of the United States of Americavol 95 no 25 pp 14634ndash14639 1998
[52] C Y Huang Determination of the Binding Site-Size of theProtein-DNA Complex by Use of the Electrophoretic MobilityShift Assay InTech Press Rijeka Croatia 2012
[53] A Kornberg ldquoTen commandments of enzymology amendedrdquoTrends in Biochemical Sciences vol 28 no 10 pp 515ndash517 2003
[54] W Zhang X Lu and J Shen ldquoEMSA and single-moleculeforce spectroscopy study of interactions between bacillus sub-tilis single-stranded DNA-binding protein and single-strandedDNArdquo Langmuir vol 27 no 24 pp 15008ndash15015 2011
[55] M Ostermeier and S J Benkovic ldquoEvolution of protein func-tion by domain swappingrdquo Advances in Protein Chemistry vol55 pp 29ndash77 2000
[56] W F Peng and C Y Huang ldquoAllantoinase and dihydroorotasebinding and inhibition by flavonols and the substrates of cyclicamidohydrolasesrdquo Biochimie vol 101 pp 113ndash122 2014
[57] Y H Huang M J Lin and C Y Huang ldquoDnaT is a single-stranded DNA binding proteinrdquo Genes to Cells vol 18 no 11pp 1007ndash1019 2013
[58] Y Y Ho Y H Huang and C Y Huang ldquoChemical rescue of thepost-translationally carboxylated lysine mutant of allantoinaseand dihydroorotase by metal ions and short-chain carboxylicacidsrdquo Amino Acids vol 44 no 4 pp 1181ndash1191 2013
[59] Y H Huang Y Lo W Huang and C Y Huang ldquoCrystalstructure and DNA-binding mode of Klebsiella pneumoniaeprimosomal PriB proteinrdquoGenes to Cells vol 17 no 10 pp 837ndash849 2012
[60] C Y Huang C Y Hsu Y Sun H Wu and C Hsiao ldquoCom-plexed crystal structure of replication restart primosome pro-tein PriB reveals a novel single-stranded DNA-binding moderdquoNucleic Acids Research vol 34 no 14 pp 3878ndash3886 2006
[61] T Kinebuchi H Shindo H Nagai N Shimamoto andM Shimizu ldquoFunctional domains of Escherichia coli single-stranded DNA binding protein as assessed by analyses of thedeletion mutantsrdquo Biochemistry vol 36 no 22 pp 6732ndash67381997
[62] N Shimamoto N Ikushima H Utiyama H Tachibana andK Horie ldquoSpecific and cooperative binding of E coli single-stranded DNA binding protein to mRNArdquo Nucleic AcidsResearch vol 15 no 13 pp 5241ndash5250 1987
[63] H Lin andC YHuang ldquoCharacterization of flavonol inhibitionof DnaB helicase Real-time monitoring structural modelingand proposed mechanismrdquo Journal of Biomedicine and Biotech-nology vol 2012 Article ID 735368 2012
[64] Y H Huang H H Lin and C Y Huang ldquoA single residuedetermines the cooperative binding property of a primosomalDNA replication protein PriB to single-stranded DNArdquo Bio-science Biotechnology and Biochemistry vol 76 no 6 pp 1110ndash1115 2012
[65] H C Hsieh and C Y Huang ldquoIdentification of a novel proteinPriB in Klebsiella pneumoniaerdquo Biochemical and BiophysicalResearch Communications vol 404 no 1 pp 546ndash551 2011
[66] J H Liu T W Chang C Y Huang et al ldquoCrystal structure ofPriB a primosomalDNA replication protein ofEscherichia colirdquoThe Journal of Biological Chemistry vol 279 no 48 pp 50465ndash50471 2004
[67] Y H Huang and C Y Huang ldquoThe N-terminal domain ofDnaT a primosomalDNA replication protein is crucial for PriBbinding and self-trimerizationrdquo Biochemical and BiophysicalResearch Communications vol 442 pp 147ndash152 2013
[68] M A Larkin G Blackshields N P Brown et al ldquoClustalW andClustal X version 20rdquo Bioinformatics vol 23 no 21 pp 2947ndash2948 2007
16 BioMed Research International
[69] S N Savvides S Raghunathan K Futterer A G Kozlov T MLohman and G Waksman ldquoThe C-terminal domain of full-length E coli SSB is disordered even when bound to DNArdquoProtein Science vol 13 no 7 pp 1942ndash1947 2004
[70] C-C Chen J-K Hwang and J-M Yang ldquo(PS)2-v2 template-based protein structure prediction serverrdquo BMC Bioinformaticsvol 10 article 366 2009
[71] C Chen J Hwang and J Yang ldquo(PS)2 protein structure pre-diction serverrdquoNucleic Acids Research vol 34 pp W152ndashW1572006
[72] K Bush and J F Fisher ldquoEpidemiological expansion structuralstudies and clinical challenges of new 120573-lactamases from gram-negative bacteriardquo Annual Review of Microbiology vol 65 pp455ndash478 2011
[73] K K Kumarasamy M A Toleman T R Walsh et al ldquoEmer-gence of a new antibiotic resistance mechanism in India Pak-istan and the UK a molecular biological and epidemiologicalstudyrdquoThe Lancet Infectious Diseases vol 10 no 9 pp 597ndash6022010
[74] K Bush and M J MacIelag ldquoNew 120573-lactam antibiotics and 120573-lactamase inhibitorsrdquo Expert Opinion on Therapeutic Patentsvol 20 no 10 pp 1277ndash1293 2010
[75] K Bush ldquoAlarming 120573-lactamase-mediated resistance in multi-drug-resistant Enterobacteriaceaerdquo Current Opinion in Microbi-ology vol 13 no 5 pp 558ndash564 2010
[76] A Srivastava M Talaue S Liu et al ldquoNew target for inhibitionof bacterial RNA polymerase ldquoswitch regionrdquordquo Current Opinionin Microbiology vol 14 no 5 pp 532ndash543 2011
[77] K Chono K Katsumata T Kontani et al ldquoASP2151 a novelhelicase-primase inhibitor possesses antiviral activity againstvaricella-zoster virus and herpes simplex virus types 1 and 2rdquoJournal of Antimicrobial Chemotherapy vol 65 no 8 pp 1733ndash1741 2010
[78] M T Black and K Coleman ldquoNew inhibitors of bacterial topoi-somerase GyrAParC subunitsrdquo Current Opinion in Investiga-tional Drugs vol 10 no 8 pp 804ndash810 2009
[79] A H Marceau D A Bernstein B W Walsh W Shapiro LA Simmons and J L Keck ldquoProtein interactions in genomemaintenance as novel antibacterial targetsrdquo PLoS ONE vol 8no 3 Article ID e58765 2013
[80] D Lu D A Bernstein K A Satyshur and J L Keck ldquoSmall-molecule tools for dissecting the roles of SSBprotein inter-actions in genome maintenancerdquo Proceedings of the NationalAcademy of Sciences of the United States of America vol 107 no2 pp 633ndash638 2010
[81] I Wong and T M Lohman ldquoA double-filter method fornitrocellulose-filter binding application to protein-nucleic acidinteractionsrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 90 no 12 pp 5428ndash5432 1993
[82] M Mitas J Y Chock and M Christy ldquoThe binding-site sizesof Escherichia coli single-stranded-DNA-binding protein andmammalian replication protein A are 65 and ge 54 nucleotidesrespectivelyrdquo Biochemical Journal vol 324 no 3 pp 957ndash9611997
[83] C Urbanke G Witte and U Curth ldquoSedimentation velocitymethod in the analytical ultracentrifuge for the study of protein-protein interactionsrdquoMethods inMolecular Biology vol 305 pp101ndash114 2005
[84] S Chrysogelos and J Griffith ldquoEscherichia coli single-strandbinding protein organizes single-strandedDNA innucleosome-like unitsrdquo Proceedings of the National Academy of Sciences of theUnited States of America vol 79 no 19 pp 5803ndash5807 1982
[85] J R Casas-Finet K R Fischer and R L Karpel ldquoStructuralbasis for the nucleic acid binding cooperativity of bacteriophageT4 gene 32 protein the (LysArg)3(SerThr)2 (LAST) motifrdquoProceedings of the National Academy of Sciences of the UnitedStates of America vol 89 pp 1050ndash1054 1992
[86] G Witte R Fedorov and U Curth ldquoBiophysical analysis ofThermus aquaticus single-stranded DNA binding proteinrdquo Bio-physical Journal vol 94 no 6 pp 2269ndash2279 2008
[87] R Fedorov G Witte C Urbanke D J Manstein and UCurth ldquo3D structure of Thermus aquaticus single-strandedDNA-binding protein gives insight into the functioning of SSBproteinsrdquo Nucleic Acids Research vol 34 no 22 pp 6708ndash67172006
[88] K L Tsai C Y Huang C H Chang Y J Sun W J ChuangandC DHsiao ldquoCrystal structure of the human FOXK1a-DNAcomplex and its implications on the diverse binding specificityof winged helixforkhead proteinsrdquo Journal of Biological Chem-istry vol 281 no 25 pp 17400ndash17409 2006
[89] A H Mao N Lyle and R V Pappu ldquoDescribing sequence-ensemble relationships for intrinsically disordered proteinsrdquoBiochemical Journal vol 449 no 2 pp 307ndash318 2013
[90] R Wetzel ldquoPhysical chemistry of polyglutamine Intriguingtales of a monotonous sequencerdquo Journal of Molecular Biologyvol 421 no 4-5 pp 466ndash490 2012
[91] H T Orr ldquoPolyglutamine neurodegeneration expanded glu-tamines enhance native functionsrdquo Current Opinion in Geneticsand Development vol 22 no 3 pp 251ndash255 2012
[92] M Figiel W J Szlachcic P M Switonski A Gabka and W JKrzyzosiak ldquoMouse models of polyglutamine diseases reviewand data table Part Irdquo Molecular Neurobiology vol 46 no 2pp 393ndash429 2012
[93] J Nasica-Labouze and N Mousseau ldquoKinetics of amyloidaggregation a study of the GNNQQNY prion sequencerdquo PLoSComputational Biology vol 8 no 11 Article ID e1002782 2012
[94] J Nasica-Labouze M Meli P Derreumaux G Colombo andN Mousseau ldquoA multiscale approach to characterize the earlyaggregation steps of the amyloid-forming peptide gnnqqnyfrom the yeast prion sup-35rdquo PLoS Computational Biology vol7 no 5 Article ID e1002051 2011
[95] J R Lewandowski P C A van der Wel M Rigney N Grig-orieff and R G Griffin ldquoStructural complexity of a compositeamyloid fibrilrdquo Journal of the American Chemical Society vol133 no 37 pp 14686ndash14698 2011
BioMed Research International 15
[38] T Paradzik N Ivic Z Filic et al ldquoStructure-function relation-ships of two paralogous single-stranded DNA-binding proteinsfrom Streptomyces coelicolor implication of SsbB in chromo-some segregation during sporulationrdquo Nucleic Acids Researchvol 41 no 6 pp 3659ndash3672 2013
[39] S JainM Zweig E Peeters et al ldquoCharacterization of the singlestrandedDNA binding protein SsbB encoded in the gonoccocalgenetic islandrdquo PLoS ONE vol 7 no 4 Article ID e35285 2012
[40] Y H Huang and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from Klebsiella pneumoniaemutation at either Arg73 or Ser76 causes a less cooperativecomplex onDNArdquoGenes to Cells vol 17 no 2 pp 146ndash157 2012
[41] E Antony E A Weiland S Korolev and T M LohmanldquoPlasmodium falciparum SSB tetramer wraps single-strandedDNAwith similar topology but opposite polarity to E Coli SSBrdquoJournal ofMolecular Biology vol 420 no 4-5 pp 269ndash283 2012
[42] H Jan Y L Lee and C Y Huang ldquoCharacterization of a single-stranded DNA-binding protein from pseudomonas aeruginosaPAO1rdquo Protein Journal vol 30 no 1 pp 20ndash26 2011
[43] Y-H Huang Y-L Lee and C-Y Huang ldquoCharacterization of asingle-stranded DNA binding protein from Salmonella entericaserovar typhimurium LT2rdquo Protein Journal vol 30 no 2 pp102ndash108 2011
[44] S K Bharti K Rex P Sreedhar N Krishnan and U VarshneyldquoChimeras of Escherichia coli and Mycobacterium tuberculosissingle-stranded DNA binding proteins characterization andfunction in Escherichia colirdquo PLoS ONE vol 6 no 12 ArticleID e27216 2011
[45] M T Oliveira and L S Kaguni ldquoFunctional roles of the N-and C-terminal regions of the human mitochondrial single-stranded DNA-binding proteinrdquo PLoS ONE vol 5 no 10Article ID e15379 2010
[46] A G Kozlov J M Eggington M M Cox and T MLohman ldquoBinding of the dimeric Deinococcus radioduranssingle-strandedDNA binding protein to single-strandedDNArdquoBiochemistry vol 49 no 38 pp 8266ndash8275 2010
[47] T M Lohman and M E Ferrari ldquoEscherichia coli single-stranded DNA-binding protein multiple DNA-binding modesand cooperativesrdquo Annual Review of Biochemistry vol 63 pp527ndash570 1994
[48] R Zhou A G Kozlov R Roy et al ldquoSSB functions as a slidingplatform that migrates on DNA via reptationrdquo Cell vol 146 pp222ndash232 2011
[49] R Roy A G Kozlov T M Lohman and T Ha ldquoSSB proteindiffusion on single-stranded DNA stimulates RecA filamentformationrdquo Nature vol 461 pp 1092ndash1097 2009
[50] R Roy A G Kozlov T M Lohman and T Ha ldquoDynamicstructural rearrangements between DNA binding modes of Ecoli SSB proteinrdquo Journal of Molecular Biology vol 369 no 5pp 1244ndash1257 2007
[51] T J Kelly P Simancek and G S Brush ldquoIdentification andcharacterization of a single-stranded DNA-binding proteinfrom the archaeon Methanococcus jannaschiirdquo Proceedings ofthe National Academy of Sciences of the United States of Americavol 95 no 25 pp 14634ndash14639 1998
[52] C Y Huang Determination of the Binding Site-Size of theProtein-DNA Complex by Use of the Electrophoretic MobilityShift Assay InTech Press Rijeka Croatia 2012
[53] A Kornberg ldquoTen commandments of enzymology amendedrdquoTrends in Biochemical Sciences vol 28 no 10 pp 515ndash517 2003
[54] W Zhang X Lu and J Shen ldquoEMSA and single-moleculeforce spectroscopy study of interactions between bacillus sub-tilis single-stranded DNA-binding protein and single-strandedDNArdquo Langmuir vol 27 no 24 pp 15008ndash15015 2011
[55] M Ostermeier and S J Benkovic ldquoEvolution of protein func-tion by domain swappingrdquo Advances in Protein Chemistry vol55 pp 29ndash77 2000
[56] W F Peng and C Y Huang ldquoAllantoinase and dihydroorotasebinding and inhibition by flavonols and the substrates of cyclicamidohydrolasesrdquo Biochimie vol 101 pp 113ndash122 2014
[57] Y H Huang M J Lin and C Y Huang ldquoDnaT is a single-stranded DNA binding proteinrdquo Genes to Cells vol 18 no 11pp 1007ndash1019 2013
[58] Y Y Ho Y H Huang and C Y Huang ldquoChemical rescue of thepost-translationally carboxylated lysine mutant of allantoinaseand dihydroorotase by metal ions and short-chain carboxylicacidsrdquo Amino Acids vol 44 no 4 pp 1181ndash1191 2013
[59] Y H Huang Y Lo W Huang and C Y Huang ldquoCrystalstructure and DNA-binding mode of Klebsiella pneumoniaeprimosomal PriB proteinrdquoGenes to Cells vol 17 no 10 pp 837ndash849 2012
[60] C Y Huang C Y Hsu Y Sun H Wu and C Hsiao ldquoCom-plexed crystal structure of replication restart primosome pro-tein PriB reveals a novel single-stranded DNA-binding moderdquoNucleic Acids Research vol 34 no 14 pp 3878ndash3886 2006
[61] T Kinebuchi H Shindo H Nagai N Shimamoto andM Shimizu ldquoFunctional domains of Escherichia coli single-stranded DNA binding protein as assessed by analyses of thedeletion mutantsrdquo Biochemistry vol 36 no 22 pp 6732ndash67381997
[62] N Shimamoto N Ikushima H Utiyama H Tachibana andK Horie ldquoSpecific and cooperative binding of E coli single-stranded DNA binding protein to mRNArdquo Nucleic AcidsResearch vol 15 no 13 pp 5241ndash5250 1987
[63] H Lin andC YHuang ldquoCharacterization of flavonol inhibitionof DnaB helicase Real-time monitoring structural modelingand proposed mechanismrdquo Journal of Biomedicine and Biotech-nology vol 2012 Article ID 735368 2012
[64] Y H Huang H H Lin and C Y Huang ldquoA single residuedetermines the cooperative binding property of a primosomalDNA replication protein PriB to single-stranded DNArdquo Bio-science Biotechnology and Biochemistry vol 76 no 6 pp 1110ndash1115 2012
[65] H C Hsieh and C Y Huang ldquoIdentification of a novel proteinPriB in Klebsiella pneumoniaerdquo Biochemical and BiophysicalResearch Communications vol 404 no 1 pp 546ndash551 2011
[66] J H Liu T W Chang C Y Huang et al ldquoCrystal structure ofPriB a primosomalDNA replication protein ofEscherichia colirdquoThe Journal of Biological Chemistry vol 279 no 48 pp 50465ndash50471 2004
[67] Y H Huang and C Y Huang ldquoThe N-terminal domain ofDnaT a primosomalDNA replication protein is crucial for PriBbinding and self-trimerizationrdquo Biochemical and BiophysicalResearch Communications vol 442 pp 147ndash152 2013
[68] M A Larkin G Blackshields N P Brown et al ldquoClustalW andClustal X version 20rdquo Bioinformatics vol 23 no 21 pp 2947ndash2948 2007
16 BioMed Research International
[69] S N Savvides S Raghunathan K Futterer A G Kozlov T MLohman and G Waksman ldquoThe C-terminal domain of full-length E coli SSB is disordered even when bound to DNArdquoProtein Science vol 13 no 7 pp 1942ndash1947 2004
[70] C-C Chen J-K Hwang and J-M Yang ldquo(PS)2-v2 template-based protein structure prediction serverrdquo BMC Bioinformaticsvol 10 article 366 2009
[71] C Chen J Hwang and J Yang ldquo(PS)2 protein structure pre-diction serverrdquoNucleic Acids Research vol 34 pp W152ndashW1572006
[72] K Bush and J F Fisher ldquoEpidemiological expansion structuralstudies and clinical challenges of new 120573-lactamases from gram-negative bacteriardquo Annual Review of Microbiology vol 65 pp455ndash478 2011
[73] K K Kumarasamy M A Toleman T R Walsh et al ldquoEmer-gence of a new antibiotic resistance mechanism in India Pak-istan and the UK a molecular biological and epidemiologicalstudyrdquoThe Lancet Infectious Diseases vol 10 no 9 pp 597ndash6022010
[74] K Bush and M J MacIelag ldquoNew 120573-lactam antibiotics and 120573-lactamase inhibitorsrdquo Expert Opinion on Therapeutic Patentsvol 20 no 10 pp 1277ndash1293 2010
[75] K Bush ldquoAlarming 120573-lactamase-mediated resistance in multi-drug-resistant Enterobacteriaceaerdquo Current Opinion in Microbi-ology vol 13 no 5 pp 558ndash564 2010
[76] A Srivastava M Talaue S Liu et al ldquoNew target for inhibitionof bacterial RNA polymerase ldquoswitch regionrdquordquo Current Opinionin Microbiology vol 14 no 5 pp 532ndash543 2011
[77] K Chono K Katsumata T Kontani et al ldquoASP2151 a novelhelicase-primase inhibitor possesses antiviral activity againstvaricella-zoster virus and herpes simplex virus types 1 and 2rdquoJournal of Antimicrobial Chemotherapy vol 65 no 8 pp 1733ndash1741 2010
[78] M T Black and K Coleman ldquoNew inhibitors of bacterial topoi-somerase GyrAParC subunitsrdquo Current Opinion in Investiga-tional Drugs vol 10 no 8 pp 804ndash810 2009
[79] A H Marceau D A Bernstein B W Walsh W Shapiro LA Simmons and J L Keck ldquoProtein interactions in genomemaintenance as novel antibacterial targetsrdquo PLoS ONE vol 8no 3 Article ID e58765 2013
[80] D Lu D A Bernstein K A Satyshur and J L Keck ldquoSmall-molecule tools for dissecting the roles of SSBprotein inter-actions in genome maintenancerdquo Proceedings of the NationalAcademy of Sciences of the United States of America vol 107 no2 pp 633ndash638 2010
[81] I Wong and T M Lohman ldquoA double-filter method fornitrocellulose-filter binding application to protein-nucleic acidinteractionsrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 90 no 12 pp 5428ndash5432 1993
[82] M Mitas J Y Chock and M Christy ldquoThe binding-site sizesof Escherichia coli single-stranded-DNA-binding protein andmammalian replication protein A are 65 and ge 54 nucleotidesrespectivelyrdquo Biochemical Journal vol 324 no 3 pp 957ndash9611997
[83] C Urbanke G Witte and U Curth ldquoSedimentation velocitymethod in the analytical ultracentrifuge for the study of protein-protein interactionsrdquoMethods inMolecular Biology vol 305 pp101ndash114 2005
[84] S Chrysogelos and J Griffith ldquoEscherichia coli single-strandbinding protein organizes single-strandedDNA innucleosome-like unitsrdquo Proceedings of the National Academy of Sciences of theUnited States of America vol 79 no 19 pp 5803ndash5807 1982
[85] J R Casas-Finet K R Fischer and R L Karpel ldquoStructuralbasis for the nucleic acid binding cooperativity of bacteriophageT4 gene 32 protein the (LysArg)3(SerThr)2 (LAST) motifrdquoProceedings of the National Academy of Sciences of the UnitedStates of America vol 89 pp 1050ndash1054 1992
[86] G Witte R Fedorov and U Curth ldquoBiophysical analysis ofThermus aquaticus single-stranded DNA binding proteinrdquo Bio-physical Journal vol 94 no 6 pp 2269ndash2279 2008
[87] R Fedorov G Witte C Urbanke D J Manstein and UCurth ldquo3D structure of Thermus aquaticus single-strandedDNA-binding protein gives insight into the functioning of SSBproteinsrdquo Nucleic Acids Research vol 34 no 22 pp 6708ndash67172006
[88] K L Tsai C Y Huang C H Chang Y J Sun W J ChuangandC DHsiao ldquoCrystal structure of the human FOXK1a-DNAcomplex and its implications on the diverse binding specificityof winged helixforkhead proteinsrdquo Journal of Biological Chem-istry vol 281 no 25 pp 17400ndash17409 2006
[89] A H Mao N Lyle and R V Pappu ldquoDescribing sequence-ensemble relationships for intrinsically disordered proteinsrdquoBiochemical Journal vol 449 no 2 pp 307ndash318 2013
[90] R Wetzel ldquoPhysical chemistry of polyglutamine Intriguingtales of a monotonous sequencerdquo Journal of Molecular Biologyvol 421 no 4-5 pp 466ndash490 2012
[91] H T Orr ldquoPolyglutamine neurodegeneration expanded glu-tamines enhance native functionsrdquo Current Opinion in Geneticsand Development vol 22 no 3 pp 251ndash255 2012
[92] M Figiel W J Szlachcic P M Switonski A Gabka and W JKrzyzosiak ldquoMouse models of polyglutamine diseases reviewand data table Part Irdquo Molecular Neurobiology vol 46 no 2pp 393ndash429 2012
[93] J Nasica-Labouze and N Mousseau ldquoKinetics of amyloidaggregation a study of the GNNQQNY prion sequencerdquo PLoSComputational Biology vol 8 no 11 Article ID e1002782 2012
[94] J Nasica-Labouze M Meli P Derreumaux G Colombo andN Mousseau ldquoA multiscale approach to characterize the earlyaggregation steps of the amyloid-forming peptide gnnqqnyfrom the yeast prion sup-35rdquo PLoS Computational Biology vol7 no 5 Article ID e1002051 2011
[95] J R Lewandowski P C A van der Wel M Rigney N Grig-orieff and R G Griffin ldquoStructural complexity of a compositeamyloid fibrilrdquo Journal of the American Chemical Society vol133 no 37 pp 14686ndash14698 2011
16 BioMed Research International
[69] S N Savvides S Raghunathan K Futterer A G Kozlov T MLohman and G Waksman ldquoThe C-terminal domain of full-length E coli SSB is disordered even when bound to DNArdquoProtein Science vol 13 no 7 pp 1942ndash1947 2004
[70] C-C Chen J-K Hwang and J-M Yang ldquo(PS)2-v2 template-based protein structure prediction serverrdquo BMC Bioinformaticsvol 10 article 366 2009
[71] C Chen J Hwang and J Yang ldquo(PS)2 protein structure pre-diction serverrdquoNucleic Acids Research vol 34 pp W152ndashW1572006
[72] K Bush and J F Fisher ldquoEpidemiological expansion structuralstudies and clinical challenges of new 120573-lactamases from gram-negative bacteriardquo Annual Review of Microbiology vol 65 pp455ndash478 2011
[73] K K Kumarasamy M A Toleman T R Walsh et al ldquoEmer-gence of a new antibiotic resistance mechanism in India Pak-istan and the UK a molecular biological and epidemiologicalstudyrdquoThe Lancet Infectious Diseases vol 10 no 9 pp 597ndash6022010
[74] K Bush and M J MacIelag ldquoNew 120573-lactam antibiotics and 120573-lactamase inhibitorsrdquo Expert Opinion on Therapeutic Patentsvol 20 no 10 pp 1277ndash1293 2010
[75] K Bush ldquoAlarming 120573-lactamase-mediated resistance in multi-drug-resistant Enterobacteriaceaerdquo Current Opinion in Microbi-ology vol 13 no 5 pp 558ndash564 2010
[76] A Srivastava M Talaue S Liu et al ldquoNew target for inhibitionof bacterial RNA polymerase ldquoswitch regionrdquordquo Current Opinionin Microbiology vol 14 no 5 pp 532ndash543 2011
[77] K Chono K Katsumata T Kontani et al ldquoASP2151 a novelhelicase-primase inhibitor possesses antiviral activity againstvaricella-zoster virus and herpes simplex virus types 1 and 2rdquoJournal of Antimicrobial Chemotherapy vol 65 no 8 pp 1733ndash1741 2010
[78] M T Black and K Coleman ldquoNew inhibitors of bacterial topoi-somerase GyrAParC subunitsrdquo Current Opinion in Investiga-tional Drugs vol 10 no 8 pp 804ndash810 2009
[79] A H Marceau D A Bernstein B W Walsh W Shapiro LA Simmons and J L Keck ldquoProtein interactions in genomemaintenance as novel antibacterial targetsrdquo PLoS ONE vol 8no 3 Article ID e58765 2013
[80] D Lu D A Bernstein K A Satyshur and J L Keck ldquoSmall-molecule tools for dissecting the roles of SSBprotein inter-actions in genome maintenancerdquo Proceedings of the NationalAcademy of Sciences of the United States of America vol 107 no2 pp 633ndash638 2010
[81] I Wong and T M Lohman ldquoA double-filter method fornitrocellulose-filter binding application to protein-nucleic acidinteractionsrdquo Proceedings of the National Academy of Sciences ofthe United States of America vol 90 no 12 pp 5428ndash5432 1993
[82] M Mitas J Y Chock and M Christy ldquoThe binding-site sizesof Escherichia coli single-stranded-DNA-binding protein andmammalian replication protein A are 65 and ge 54 nucleotidesrespectivelyrdquo Biochemical Journal vol 324 no 3 pp 957ndash9611997
[83] C Urbanke G Witte and U Curth ldquoSedimentation velocitymethod in the analytical ultracentrifuge for the study of protein-protein interactionsrdquoMethods inMolecular Biology vol 305 pp101ndash114 2005
[84] S Chrysogelos and J Griffith ldquoEscherichia coli single-strandbinding protein organizes single-strandedDNA innucleosome-like unitsrdquo Proceedings of the National Academy of Sciences of theUnited States of America vol 79 no 19 pp 5803ndash5807 1982
[85] J R Casas-Finet K R Fischer and R L Karpel ldquoStructuralbasis for the nucleic acid binding cooperativity of bacteriophageT4 gene 32 protein the (LysArg)3(SerThr)2 (LAST) motifrdquoProceedings of the National Academy of Sciences of the UnitedStates of America vol 89 pp 1050ndash1054 1992
[86] G Witte R Fedorov and U Curth ldquoBiophysical analysis ofThermus aquaticus single-stranded DNA binding proteinrdquo Bio-physical Journal vol 94 no 6 pp 2269ndash2279 2008
[87] R Fedorov G Witte C Urbanke D J Manstein and UCurth ldquo3D structure of Thermus aquaticus single-strandedDNA-binding protein gives insight into the functioning of SSBproteinsrdquo Nucleic Acids Research vol 34 no 22 pp 6708ndash67172006
[88] K L Tsai C Y Huang C H Chang Y J Sun W J ChuangandC DHsiao ldquoCrystal structure of the human FOXK1a-DNAcomplex and its implications on the diverse binding specificityof winged helixforkhead proteinsrdquo Journal of Biological Chem-istry vol 281 no 25 pp 17400ndash17409 2006
[89] A H Mao N Lyle and R V Pappu ldquoDescribing sequence-ensemble relationships for intrinsically disordered proteinsrdquoBiochemical Journal vol 449 no 2 pp 307ndash318 2013
[90] R Wetzel ldquoPhysical chemistry of polyglutamine Intriguingtales of a monotonous sequencerdquo Journal of Molecular Biologyvol 421 no 4-5 pp 466ndash490 2012
[91] H T Orr ldquoPolyglutamine neurodegeneration expanded glu-tamines enhance native functionsrdquo Current Opinion in Geneticsand Development vol 22 no 3 pp 251ndash255 2012
[92] M Figiel W J Szlachcic P M Switonski A Gabka and W JKrzyzosiak ldquoMouse models of polyglutamine diseases reviewand data table Part Irdquo Molecular Neurobiology vol 46 no 2pp 393ndash429 2012
[93] J Nasica-Labouze and N Mousseau ldquoKinetics of amyloidaggregation a study of the GNNQQNY prion sequencerdquo PLoSComputational Biology vol 8 no 11 Article ID e1002782 2012
[94] J Nasica-Labouze M Meli P Derreumaux G Colombo andN Mousseau ldquoA multiscale approach to characterize the earlyaggregation steps of the amyloid-forming peptide gnnqqnyfrom the yeast prion sup-35rdquo PLoS Computational Biology vol7 no 5 Article ID e1002051 2011
[95] J R Lewandowski P C A van der Wel M Rigney N Grig-orieff and R G Griffin ldquoStructural complexity of a compositeamyloid fibrilrdquo Journal of the American Chemical Society vol133 no 37 pp 14686ndash14698 2011