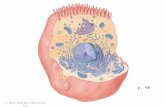
Bellringer-December 5, 2014 (Label the letters) Cytoplasm Extracellular Fluid.
-
Upload
kenneth-jenkins -
Category
Documents
-
view
215 -
download
0
Transcript of Bellringer-December 5, 2014 (Label the letters) Cytoplasm Extracellular Fluid.
December 5, 2014
1) What types of molecules passes easily?2) What types of molecules does not pass easily
and needs assistance?
Answers1) a-glycoprotein2) B-glycolipid3) C-Oligosaccharide chains (attached to glycoprotein)4) D-nonpolar tails (fatty acid tails) (hydrophobic tails)5) E-Phosopholipid bilayer6) F-Polar heads (hydrophilic heads) (phosphate head)7) G-Peripheral protein8) H-cholesterol9) I-Integral Protein10) J-Cytoskeleton
Membrane structure LEADS TO selective permeability
• A cell must exchange materials with its surroundings–a process controlled by the selectively permeable plasma membrane
Cell Transport• Means moving things INTO and OUT
of the cell• Cells need to take in –Food–Get rid of waste products (excretion) –Give out such useful substances as
hormones and enzymes (secretion).
Permeability and Cell Transport• Hydrophobic (non polar) molecules– Are lipid soluble (can dissolve) – can pass through membrane easily• Ex: Hydrocarbons, CO2, O2
• Hydrophilic (Polar) molecules– Are NOT lipid soluble (can’t dissolve)• Lipid INsoluble
– Do not cross membrane easily• Ex: Na+, Cl- , Glucose/ other sugars–NOTE: CHARGED molecules need “help” to cross
membrane–NOTE: LARGE molecules, POLAR molecules need
“help”
Types of Cellular Transport
• Passive Transport cells do NOT use energy
1. Diffusion2. Facilitated Diffusion3. Osmosis
• Active Transportcells DO use energy
1. Protein Pumps2. Endocytosis3. Exocytosis
high
low
This is going to be hard!
high
low
Weee!
1) Diffusion• Diffusion: • Movement of molecules from a high
concentration to a lower concentration– Does not use energy– Can occur in non-living systems (ex: dye)
• Concentration: – Number of molecules of a substance in a given
volume• Concentration gradient: – Difference in concentration of a substance from
one location to another10
Types of Passive Transport
Diffusion is the tendency for a population of molecules (of ANY substance) to spread out evenly into available space–A “net” movement • Ex: Perfume, a fart , tea, food coloring in
water (see demo) • http://www.indiana.edu/~phys215/lecture/lecnotes/lecgraphics/diffusion2.gif• http://www.biosci.ohiou.edu/introbioslab/Bios170/diffusion/Diffusion.html
DIFFUSION • In absence of other forces…–Molecules move (diffuse) from area of HIGH [ ]
to an area of lower [ ] –A.k.a. Molecules move DOWN its OWN
concentration gradient•No chemical work (ATP energy) is used diffusion is spontaneous!
Substances diffuse down their OWN concentration gradient
Net diffusion
Net diffusion
Net diffusion
Net diffusion Equilibrium
Equilibrium
Factors Affecting Diffusion
1. Temperature• Higher temperature more kinetic
energy molecules move faster2. Pressure• Higher pressure molecules move faster• ExampleTea: Higher temperature and
more pressure (twirling/stirring) it around) makes diffusion happen faster.
2) Facilitated Diffusion• Molecules move down (not against) a
concentration gradient with the aid of special proteins (channel or carrier proteins)– Speeds up the process– Proteins CAN change SHAPE
• Does NOT use energy (NO ATP NEEDED) = type of passive transport
• Moves things from high to low concentrations–Moves POLAR molecules can NOT
easily pass through the membrane on it’s own. • Example: ions, smaller polar molecules (ex: sugar)
17
Channel proteins-Provide “tunnels”
EXTRACELLULARFLUID
Channel proteinSolute
CYTOPLASM
A channel protein (purple) has a channel through which water molecules or a specific solute can pass.
(a)
• Channel Proteins animations
Carrier proteins-Undergo a subtle change in shape “carry” solute across the
membrane
Carrier protein Solute
3. Osmosis• Osmosis: – diffusion of water across a
membrane• Water moves across a semi-permeable membrane
from an area of high water concentration to an area of low water concentration
• If water can cross a membrane, but the solute cannot, then…
– The water moves towards the side with MORE solute to balance the concentrations
20
Effects of Osmosis on Water Balance
Osmosis
• The movement of water (water diffusion) across a semipermeable membrane– Involves the movement of FREE water
molecules down a water [ ] gradient• High solute low “free” water [ ] or….• Low solute high free water [ ]
Osmosis is affected by the concentration gradient of dissolved substances (solutes)
Osmosis animation
25
Diffusion of H2O Across A Membrane
High H2O potentialLow solute concentration
Low H2O potentialHigh solute concentration
copyright cmassengale
Osmoregulation• Osmoregulation – control of water balance
• Turgor pressure – pressure inside a cell
• Tonicity – tendency of a cell to lose or gain water based on the solution it is in
3 Types of solutions: – Isotonic– Hypotonic– Hypertonic
26
3 Different Types of SolutionsRecall: SOLUTION = a uniform mixture of 2 or more
substances** compare solutions OUTSIDE cell to inside cell
• 1. ISOTONIC-Solution has same concentration of dissolved particles as the cell (same amount of solute outside and inside the cell)
• Water moves into and out of cell at equal rates and cell size remains constant• Cell does not change shape– There will be NO net movement of
water
ISOTONIC SOLUTION
Result: Water moves equally in both directions and the cell remains same size! (Dynamic
Equilibrium)
2. If a solution is hypertonic• Solution has a higher concentration of dissolved
particles than the cell (more solute outside of the cell than it is inside the cell)– Water flows OUT of the cell – The cell shrivels up and turgor pressure decreases – “hyper” means more
» (high [solute])• Ex: when salinity increases in lake, fish can
die!
The water will move out of the fish towards the area with MORE solutes (ocean) to balance the concentrations! Poor fish…
NaCl
HYPERTONIC SOLUTION
Result: Water moves from inside the cell into the solution: Cell shrinks (Plasmolysis)!
3. If a solution is hypotonic• Solution has a lower concentration of
dissolved particles than the cell (less solute outside of the cell than it is inside the cell)– Water flows INTO the cell– The cell swells/gets big! – Ex. Happens with distilled water
• Animals:• Cytolysis (when a cell BURSTS!)
–“hypo” means “less” – (low [solute])
– Think: Hypo- sounds like hippo…hippos are big & round; cells in hypotonic solutions get big & roundAlso, think “hypo” is “low” meaning “low” solutes
SURROUNDING cell
“Hypo” is LOW!!!
HYP0TONIC SOLUTION
Result: Water moves from the solution to inside the cell): Cell Swells and bursts open
(cytolysis)!
33
Cell in ___________ Solution
CELL
10% NaCL90% H2O
10% NaCL90% H2O
What is the direction of water movement?
The cell is at ____________________.Dynamic equilibrium
ENVIRONMENT
NO NET MOVEMENT
34
Cell in ____________ Solution
CELL
10% NaCL90% H2O
20% NaCL80% H2O
What is the direction of water movement?
copyright cmassengale
ENVIRONMENT
35
Cell in ___________ Solution
CELL
15% NaCL85% H2O
5% NaCL95% H2O
What is the direction of water movement?
ENVIRONMENT
copyright cmassengale
http://www.tvdsb.on.ca/westmin/science/sbi3a1/Cells/Osmosis.htm
• Osmosis Animations for isotonic,
hypertonic, and hypotonic solutions
Water Balance of Cells with Walls
• Cell walls–Help maintain water balance
• Cell walls are in:–Plants–Prokaryotes–Fungi–Some protists
42
Plant Cells & Solutions• Isotonic (Flaccid)
• Plant cells at isotonic solutions are flaccid (can also be in hypertonic solution)• Cells are limp
• Hypotonic (Turgid)• Plant cells prefer hypotonic solutions because the cell wall provides more
support and plant cells are less likely to lyse (burst)• Turgor pressure increases (vacuole fills with water)• It is very firm• A healthy state in most plants
• Hypertonic (Plasmolysis)• Plants wilt, undergo plasmolysis• The cytoplasm and plasma membrane begin to pull
away from the cell wall• Causes cell with walls to wilt & can be lethal.
Water balance in cells with walls
Plant cell. Plant cells are turgid (firm) and generally healthiest in a hypotonic environment, where the uptake of water is eventually balanced by the elastic wall pushing back on the cell.
H2OH2OH2OH2O
Turgid (normal) Flaccid Plasmolyzed
Types of Cellular Transport
#1 • Passive Transport–DOES NOT require chemical energy (ATP)
#2 • Active Transport–DOES require chemical energy (ATP)