A CASE STUDY ON COVALENT BONDING By: Wong Wei Cong (31) 2A1.
-
Upload
alexina-constance-clarke -
Category
Documents
-
view
214 -
download
0
Transcript of A CASE STUDY ON COVALENT BONDING By: Wong Wei Cong (31) 2A1.
What is Covalent Bonding
Sharing of electrons so as to achieve stable electronic configuration of noble gas
Electrostatic attraction between nuclei of the atoms and the pair(s) of shared electrons
Molecules are formed Generally, covalent bonds are formed between
atoms of non-metals
Electronegativity
Describes tendency of an atom to attract electrons towards itself and thus the tendency to form anions
Affected by both the number of protons and the distance that the valence electrons reside from the nucleus
As you go across a period, electronegativity increases As you go down a group, electronegativity decreases
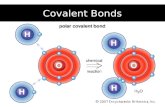
Polar Bonds
If two atoms of equal electronegativity bond together… Both have same tendency to attract bonding pair of
electrons Electron pair found on average half way between the
atoms Electron pair shared evenly between the atoms “Pure” covalent bonds
If B is a lot more electronegative than A… Electron pair dragged right over to B's end of the bond A has lost control of its electron B has complete control over both electrons Ionic bonds
Polar Bonds (II)
If B is slightly more electronegative than A… B attracts electron pair more than A does B’s end of the bond slightly negative A’s end of the bond slightly positive Polar bond
Water is a polar covalent bond!
Electronegativity & Bond Type
Electronegativity Difference
Ionic Character (%)
Covalent Character (%)
Bond Type
0.0 0 100 Covalent
0.5 5 95 Covalent
1.0 20 80 Covalent
1.5 40 60 Polar
2.0 60 40 Polar
2.5 75 25 Ionic
3.0 90 10 Ionic
So… yes! You guessed it…
Aluminium Chloride is a polar covalent bond!
Electronegativity difference = 1.4
Electronegativity difference = 1.4Beryllium Chloride is a polar
covalent bond!
Formation of Aluminium Chloride
Strongly heated aluminium foil burns in chlorine to form aluminium chloride
Aluminium Chloride
Imagine that aluminum chloride is ionic instead
Contain Al3+ and Cl- ions Aluminium ion: small & packed with
3 positive charges High charge density – polarise
chlorine ions Electron pairs dragged back towards
aluminium to such extent that the bonds become covalent
Chlorine more electronegative than aluminium – electron pairs will not be pulled half way between the atoms
Polar covalent bond formed
Factors Affecting Polarising Ability
In aluminium chloride, aluminium ions polarise chloride ions
Positive ions can polarise nearby negative ions The smaller the positive ion and the larger the number
of charges, the greater the polarising ability The bigger the negative ion, the easier it is to polarise it
Aluminium iodide is covalent as electron pair is easily dragged away from iodide ion
Aluminium fluoride is ionic as aluminium ion cannot polarise small fluoride ion sufficiently to form a covalent bond
The Mystery of Aluminium Chloride
At room temperature, each aluminium surrounded by 6 chlorines Ionic crystal structure with a lot of covalent
character At ordinary atmospheric pressure, it sublimes at
about 180°C If pressure raised to just over 2 atmospheres, it
melts at 192°C Comparatively weak attractions between
molecules Each aluminium now surrounded by 4 chlorines
rather than 6 Original lattice converted into Al2Cl6 molecules
As temperature increases further, it increasingly breaks up into simple AlCl3 molecules
Beryllium Chloride
Imagine that beryllium chloride is ionic instead
Contain Be2+ and Cl- ions Beryllium ion: small & packed with
2 positive charges High charge density – polarise
chlorine ions Electron pairs dragged back
towards beryllium to such extent that the bonds become covalent
Chlorine more electronegative than beryllium – electron pairs will not be pulled half way between the atoms
Polar covalent bond formed
As a gas, Beryllium Chloride is a linear molecule with all three atoms in a straight line
As a solid, the molecules form long chains (polymers) Coordinate bonds
The Mystery of beryllium Chloride
Gas
Solid
Arrows goes from the atom supplying the pair of electrons to the atom with the empty orbital
Why is Beryllium Chloride not Ionic?
Beryllium has quite a high electronegativity compared with the rest of Group II
Attracts bonding pair of electrons towards itself more strongly
In order for an ionic bond to form, the beryllium has to let go of its electrons, but it is too electronegative to do that
Lewis acid – accepts lone pair of electrons Boils at 520°C – low boiling point, so cannot contain ions Reacts vigorously with water, forming acidic, steamy
hydrogen chloride gas – typical of covalent chlorides
Chemistry Music Video – Enjoy!
Two atoms met on one fine dayOne asked if he could bondWith the other atom thereOf whom he was really fondThe second atom shrugged and saidWhat’s your pleasure, son?Are you up for electron transferOr electron-sharing fun!
Ionic or covalent?What kind of bonds are these?Involve valence electronsAnd form compounds with ease!
Metal atoms lose electronsAnd become a charge of plusNon-metals gain them happily
Look negative to all of us.The opposite charged ionsAttract to make ionic bondsThe E.N.D. one point seven plusThey dissolve real well in ponds!
Ionic or covalent?What kind of bonds are these?Involve valence electronsAnd form compounds with ease!
If the E.N.D. is point-five or moreAnd two non-metals hadYou have a polar covalent bondOne atoms happy, the others sad!The atom with less E.N.Gets a positive charge that’s slight
And the other, more greedy atom?Slightly negative to our sight!
Ionic or covalent?What kind of bonds are these?Involve valence electronsAnd form compounds with ease!
If the E.N.D. is point-four or lessTwo non-metal atoms bondWith equal pull on electronsEach atom is equal fondNo charges will developAnd, because of that, no polesThat’s why the bond is called non-polarOn and on and on we roll!
Ionic or covalent?Now its easy for you to tellJust look up the E.N.D.And you will do real well!
Chemistry Music Video – Enjoy!
YouTube Link: http://www.youtube.com/watch?v=oNBzyM6TcK8
Bibliography
http://www.chemguide.co.uk/atoms/bonding/electroneg.html
http://www.chemguide.co.uk/inorganic/period3/chlorides.html
http://www.chemguide.co.uk/inorganic/group2/beryllium.html
http://en.wikipedia.org/wiki/Electronegativity http://en.wikipedia.org/wiki/Covalent_bond http://en.wikipedia.org/wiki/Aluminium_chloride http://en.wikipedia.org/wiki/Beryllium_chloride