1. Surgery of Esophagus
-
Upload
rohini-selvarajah -
Category
Documents
-
view
225 -
download
0
Transcript of 1. Surgery of Esophagus

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 1/40
Surgery Of Esophagus

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 2/40
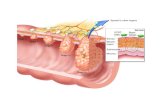
Anatomy E originates at the level of 6th cervical vertebra/posterior to the cricoid cartilage
In the thorax the E passes behing the aortic arch and the left main bronchus n enter theabdomen via the esphageal hiatus of the diaphragm
It terminates at the fundus of the stomach The length in adult :
1. from incisor teeth to cricopharngeus m. = 15-20cm
2. To aortc arch = 20-25cm
3. To inferior pulm vein = 30-35cm
4. To gastroesphageal junction = 40-45cm
Anatomic areas of narrowing :
1. At the level of cricoid cartilage/ upper esophageal sphinter (UES)
2. In the mid thorax/ due to compression of aortic ach and leftmain bronchus
3. At the level of esophageal hiatus of the diaphragam/lower esophageal sphinter (LES)
*UES = pharngoesophageal sphinter
*LES = gastroesophGEAL SPHINTER
Upper 1/3rd of the esophagus is striated m.. The lower 2/3rd smooth mm.
Arterial supplu :
1. Upper end = inferior thyroid arteries
2. Thoracic portion = bronchial aa / esophageal branches arising directly from arota/intercostal aa
3. Diaphragmatic and abdominal portion = left inferior phrenic aa/ esophagel brainches of
left gastirc aa

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 3/40
Venus drainage :
1. The majority of blood from the esophagus is drained via the esophageal veins
, which carry deoxygenatedblood from the esophagusto the azygos vein
, which in turn drains directly into the superior vena cava. (Theseveins have no part in the development of esophageal varices. )
2. The remaining blood from the esophagus is drained into the superficial veins lining the esophageal mucosa,
which drain into the coronary vein (left gastric vein), which in turn drains directly into the portal vein.3. In case the portal system gets obstructed as in cirrohsis of liver the blood is shunted upward through
coronary vein into esophagealvenous plexsus. N eventually vis axygous v to SVC
The mucosal lining of E is stratified squmaous epithelium.. It has no serosal layer = the reason why it does notheal quickly after in jury or surgery like the rest of GIT.
UES
motor innervation directly from brain ( n. Ambigus) Resting pressure 100mmHg
Esophagel body
Food moves at a speed of 3-4 cm/s
Pressure 60-140mmHg
Primary peristalisis is the wave intiated by swallowing/ secondary can be intiated by stimulating any part of e.Body aids in emptying when primary peristalisis fail/ tertiary peristaliss considered abnormal but frequesnt in elderly
LES Resting pressure 15= 24 mmHg
During thetime of swallowing the LES relaes a bit for 5-10s (mediated by vasoactive intestinal polypeptide n NO both noradrenegic and noncholinergic neurotrasmitters)

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 4/40
Esophageal motility disorders. Achalasia
Diffuse esophageal spasm
Nutcracker E
Hypertensive lower esophageal sphinter
Presents witha combination of dysphagia/regurgitation/ chest pain/heartburn/
Key test is = e. Manometry.
1) Achalasia.
Absence of e. Peristalisis n increased pressure in the LES which fails to relas completelty in response to swallowing
This results in incomlete propulsion of food along E. And stasis of food.
More coomin in men/ can occur at any age
Incidence 1 in 100 000 people.
Pathogenesis. / unkonwn, but 2 theories exisit
1. Degenerative disease of the neurons
2. Infections of the neurons by virus (HZV) or other organisms
* similar finding in Chagas disease (american trypanosomiasis) = orgaism destroy parasympathetic ganglion cellsthroughout the body/heart,git,urinary.respirato tracts
dgreneration of myentric plexus of Aurebach leads to loss of postganglionc inhibitory neurons which contain VIP + NO,
which is responsible for LES relaxation.but as the postganglionc cholinergic neurons are spared this leads to increase in LES resting presure + e. Contraction.
Clinical findings.
Symptoms and signs.
Dysphagia MC
Regurgitaion of undigested food 60 %
Heartburn 40% = not due to GERD but due to stasis of food n fermentaion
Chest pain 40% - due to esophageal distension usually experienced at the time of meal.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 5/40
Imaging Studies
A barium swallow - shows narrowing at the level of the gastroesophageal junction /A dilated, sigmoid esophagus may be present in patients with longstanding achalasia.
Endoscopy is performed to rule out a tumor of the gastroesophageal junction.
Special Tests
Esophageal manometry is the key test/The classic manometric findings are(1) absence of esophageal peristalsis and
(2) hypertensive LES (in about 50% of patients) that relaxes only partially in response toswallowing.
* When the esophagus is dilated and sigmoid in shape, it may be difficult to pass thecatheter through the gastroesophageal junction into the stomach: In these cases, thecatheter may be placed under fluoroscopic or endoscopic guidance.
Differential Diagnosis
Benign strictures due to gastroesophageal reflux and esophageal carcinoma
infiltrating tumor of the gastroesophageal junction /This condition is called secondary orpseudoachalasia and should be suspected in patients older than 60 years with recentonset of dysphagia (less than 6 months) and excessive weight loss. An endoscopicultrasound or a CT scan can help establishing the diagnosis.
Complications Aspiration of retained and undigested food can cause repeated episodes of pneumonia.
Achalasia is also a risk factor for esophageal cancer/Squamous cell carcinoma orAdenocarcinoma can occur in patients who develop gastroesophageal reflux after eitherpneumatic dilatation or myotomy.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 6/40
Treatment - palliative, and it is directed toward relief of symptoms
1. Medical Therapy
Calcium-channel blockers are used to decrease LES pressure. only 10% of patients benefit /it should be used primarily in elderly patients
2. Endoscopic Treatment
Intrasphincteric in jection of botulinum toxin
1. is used to block the release of acetylcholine at the level of the LES, thereby restoring the balance between excitatory and inhibitory
neurotransmitters.2. Only 60% of treated patients still have relief of dysphagia 6 months after treatment, and this number further decreases to 30% (even after multiple in jections) 2.5 years later.
3. In addition, it often causes an inflammatory reaction at the level of the gastroesophageal junction, which makes a subsequent myotomymore difficult.
4. It should be used primarily in elderly patients
Pneumatic dilatation
1. A balloon is inflated at the level of the gastroesophageal junction to rupture the muscle fibers while trying to leave the mucosa intact.
2. The initial success rate is between 70% and 80%, but it decreases to 50% at 10 years, even after multiple dilatations.
3. The perforation rate is 25%. I
4. f a perforation occurs, patients are taken emergently to the operating room, where closure of the perforation and a myotomy areperformed through a left thoracotomy.
5. The incidence of postdilatation gastroesophageal reflux is about 2535%.
6. Patients who fail pneumatic dilatation are usually treated by a Heller myotomy.
3.Surgical Treatment
A laparoscopic Heller myotomy and partial fundoplication is the procedure of choice for esophageal achalasia.
The operation consists of a controlled division of the muscle fibers (myotomy) of the lower esophagus (6 cm) and proximal stomach (2 cm),followed by an anterior or a posterior partial fundoplication to prevent reflux.
Patients remain in the hospital for 2448 hours and return to regular activities in about 2 weeks.
Esophagectomy is reserved for patients with severe dysphagia who have failed both dilatation and myotomy.
Prognosis
A laparoscopic Heller myotomy allows excellent relief of symptoms in the majority of patients and should be preferred to pneumaticdilatation
Botulinum toxin and medications should be used only in patients who are not candidates for pneumatic dilatation or laparoscopic Hellermyotomy.
Periodic follow-up by endoscopy is recommended to rule out the development of esophageal cancer.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 7/40
2) Diffuse Esophageal Spasm
cause - unknown. /Stress might play a role. /can progress to achalasia (complete loss of esophageal peristalsis).
Clinical Findings
Symptoms and Signs
intermittent chest pain - MC
dysphagia, Imaging studies
The barium swallow is abnormal in about 70% of patients.
Fluoroscopic studies show segmental spasms, areas of narrowing, and irregular uncoordinated peristalsis (corkscrew esophagus) in about30% of patients.
An epiphrenic diverticulum is sometimes present.
Manometry
Esophageal manometry is the key test for establishing the diagnosis of diffuse esophageal spasm. The classic manometric findings are
(1) alternation of esophageal peristalsis and simultaneous contractions (> 10% and < 100%)contrary to old beliefs, the contractions arenot hypertensive but of normal or even low amplitudeand
(2) normal LES function or abnormalities similar to those seen in achalasia (elevated resting pressure and decreased relaxation in responseto swallowing).
Ambulatory 24-Hour pH Monitoring to differntiate from GERD.
Differential Diagnosis
When chest pain is the predominant symptom, a complete cardiac workup is necessary to exclude a cardiac reason for the pain.
Once the heart disease has been excluded, ambulatory pH monitoring must be performed to rule out abnormal gastroesophageal reflux,which is the most common cause of noncardiac chest pain.
Esophageal manometry is the only test that distinguishes diffuse esophageal spasm from other primary esophageal motor disorders.
An endoscopy should be performed to confirm the absence of intraluminal lesions.
Complications
Regurgitation and aspiration may occur, possibly leading to repeated pneumonic infections.
An epiphrenic diverticulum may be present, secondary to the motor disorder.
Treatment
The therapeutic approach to diffuse esophageal spasm is similar to that of achalasia.
Medical therapy (long-acting nitrates, calcium-channel blocking agents) is relatively ineffective.
Pneumatic dilatation improves the dysphagia in about 25% of patients.
Intrasphincteric in jection of botulinum toxin has also given poor results.
In contrast, a laparoscopic Heller myotomy and partial fundoplication (as for patients with achalasia) improves both dysphagia and chestpain in about 80% of patients.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 8/40
3) Nutcracker Esophagus
The cause - unknown.
Clinical Findings
Symptoms and Signs = Chest pain + dysphagia.
Imaging Studies
The barium swallow is usually normal.
An epiphrenic diverticulum is sometimes present.
Manometry
Esophageal manometry is the key test The classic manometric findings are as follows:
(1) normal propagation of the peristalsis waves (there are no simultaneous contractions)theperistaltic waves in the distal esophagus, however, have very high amplitude (> 180 mm
Hg) and duration (> 6 seconds)(2) normal LES function or abnormalities similar to those seen in achalasia and diffuse
esophageal spasm.
Ambulatory 24-Hour pH Monitoring
Complications
Regurgitation and aspiration may occur, possibly leading to repeated pneumonic infections.An epiphrenic diverticulum may be present, secondary to the motor disorder.
Treatment The nutcracker esophagus is not as well defined
Initially, it was thought that the high pressure of the peristaltic contractions were the causeof the chest pain, so treatment was aimed at decreasing the high amplitude of the peristalticwaves. However, calcium-channel blockers are unable to improve the chest pain even though they decrease the strength of the contractions.
Similarly, the results of surgery have been disappointing, as chest pain persists aftermyotomy in about 50% of patients. Dysphagia is improved in 80% of patients.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 9/40
Esophageal Diverticulaabove the UES (pharyngoesophageal or Zenker diverticulum) or
the LES (epiphrenic diverticulum). General Considerations
This is the most common of the esophageal diverticulaand is three times more frequent in men than in women. Most patients are over age 60. The condition originates from the posterior wall of the esophagus, in a triangular area of weakness (Killian triangle), limited inferiorly by the cricopharyngeus muscle and superiorlyby the inferior constrictor muscles of the pharynx. As the diverticulum enlarges, it tends to deviate from the midline, mostly to the left.
Pathogenesis A Zenker diverticulum is due either to lack of coordination between the pharyngeal contraction and the opening time of the UES or to a hypertensive UES. Because of
the increased intraluminal pressure, there is progressive herniation of mucosa and submucosa through the Killian triangle. Occasionally, UES dysfunction can occur in the absence of a diverticulum (cricopharyngeal achalasia). A hereditary syndrome called oculopharyngeal muscular dystrophy, consisting of ptosis and dysphagia, hasbeen described in patients of French-Canadian ancestry. The dysphagia is the result of weak pharyngeal musculature in the face of normal UES function; it isconsiderably improved by UES myotomy. This syndrome also manifests with cervical dysphagia. A chronic cough may develop in some patients from aspiration of salivaand ingested food.
Clinical Findings
Symptoms
Dysphagia is the most common symptom. Regurgitation of undigested food from the diverticulum often occurs and can lead to aspiration into the tracheobronchialtree and pneumonia. Patients frequently have halitosis and can hear gurgling sounds in the neck. About 30% of patients have associated GERD.
Imaging Studies
A barium swallow clearly shows the position and size of the diverticulum or a prominent cricopharyngeal bar without diverticulum (Figure 206). In some patients, ahiatal hernia is present.
Special Tests
Esophageal manometry shows lack of coordination between the pharynx and the cricopharyngeus muscle and often a hypertensive UES. In addition, it can show ahypotensiveLES and abnormal esophageal peristalsis. Ambulatory pH monitoring determines if abnormal esophageal acid exposure is present.
Endoscopy may be dangerous because the instrument can enter the diverticulum rather than the esophageal lumen and cause a perforation.
Differential Diagnosis
Differential diagnosis includes esophagealstricture, achalasia, and esophageal cancer. Pulmonary infection is the most frequent serious complication, and manypatients are first seen after experiencing repeated episodes of pneumonia.
Treatment
The standard treatment consists of excision of the diverticulum and myotomy of the cricopharyngeus muscle and the upper 3 cm of the posterior esophageal wall. Forsmall diverticula (< 2 cm), the myotomy alone is sufficient. As an alternative to the conventional treatment, a transoral endoscopic approach (using an endoscopic
stapling device that ablates the septum between the diverticulum and the cervical esophagus) can be used for diverticula between 3 and 6 cm in size. If gastroesophagealreflux is present, it should be corrected before dividing the UES in order to avoid aspiration.
Prognosis
The prognosis is excellent in about 90% of cases. Complications are rare and the patients are usually able to eat the day after the procedure.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 10/40
Epiphrenic Diverticulum
Essentials of Diagnosis
Dysphagia.
Regurgitation.
Diverticulum evident on barium swallow.
Esophageal motility disorder shown by esophageal manometry.
General Considerations
Epiphrenic diverticula are located just above the diaphragm. The diverticulum is not a primary anatomic abnormality but ratherthe consequence of an underlying motility disorder of the esophagus (achalasia is the most common, followed by diffuseesophageal spasm and nutcracker esophagus). The disorder causes an outflow obstruction at the level of the gastroesophageal junction, with consequent increase in intraluminal pressure and progressive herniation of mucosa and submucosa through theesophageal muscle layers.
Clinical Findings
Symptoms
The symptoms experienced by the patient are in part due to the underlying motility disorder (dysphagia, chest pain) and in partdue to the diverticulum per se (regurgitation with the risk of aspiration). Some diverticula, however, can be asymptomatic.
Imaging Studies A chest radiograph can show an air-fluid level in the posterior mediastinum. A barium swallow clearly shows the position and
size of the diverticulum (Figure 207).
Special Tests
In the majority of cases, esophageal manometry shows the underlying motility disorder. Sometimes it is difficult to position themanometry catheter, and endoscopic or fluoroscopic guidance might be necessary.
Differential Diagnosis
A paraesophageal hernia can be confused with an epiphrenic diverticulum. The barium swallow and the endoscopy help in establishing the diagnosis.
Treatment The treatment is surgical, and the laparoscopic approach is preferred. It consists of the following:
1. Resection of the diverticulum.
2. Long myotomy. This is performed in the side of the esophagus opposite to where the diverticulum is located. It extendsproximally to the upper border of the neck of the diverticulum and distally for about 2 cm onto the gastric wall.
3. A partial fundoplication to prevent gastroesophageal reflux.
Prognosis
A laparoscopic diverticulectomy, with myotomy and fundoplication, is successful in 8090% of cases.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 11/40
EsophagealManifestations in Scleroderma & Other Systemic Diseases
Scleroderma and several other systemic diseases may involve the esophagus.
In scleroderma or progressive systemic sclerosis, there is involvement of the gastrointestinal tract in up to 90% of patients. The most common site of gastrointestinal involvement is the smooth muscle portion of the esophagus, where atrophy and fibrosis occur. The upper esophagus (striatedmuscle) and the UES are not involved. As a consequence, the LES has a low pressure and the peristalsis is weak (low amplitude or abnormalpropagation of the peristaltic waves). These changes can be followed by an increased amount of gastroesophageal reflux with delayed clearance of the refluxed gastric contents. Esophageal symptoms usually appear in patients with the characteristic skin changes and Raynaudsyndrome. In addition to heartburn and regurgitation, patients may have respiratory symptoms due to the upward extent of the gastric refluxate and aspiration.Dysphagia may be due to the abnormal peristalsis or to the presence of a peptic stricture. The diagnostic approach is similar to that of patients withGERD:
A barium swallow may show a hiatal hernia or a stricture.
Endoscopy shows esophagitis in 5060% of patients. Barrett esophagus is present in about 10% of patients.
Esophagealmanometry usually shows a hypotensive LES. Dysmotilityis frequent and can progress to complete loss of peristalsis.
Ambulatory pH monitoring is essential to establish the diagnosis. It can also measure the presence of acid in the proximal esophagusand pharynx in patients with cough or vocal cord problems.
Gastric scintigraphy is indicated in patients who experience postprandial bloating and fullness to measure the gastric emptying of solids and liquids.
Similar esophageal changes may also occur in rheumatoid arthritis, Sjögren syndrome, Raynaud disease, and systemic lupus erythematosus. Similarmotor abnormalities are occasionally seen in alcoholism, diabetes mellitus, myxedema, multiple sclerosis, and amyloidosis.
Medical management should always be tried first. A proton pump inhibitor is the drug of choice. If gastroparesis is present, a prokinetic medication such as metoclopramide should be added. A fundoplication should be considered particularly in patients with regurgitation, cough, or vocal cordproblems.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 12/40
Gastroesophageal Reflux Disease
Essentials of Diagnosis
Heartburn.
Regurgitation.
Sliding hiatal hernia on barium swallow.
Esophagitis on endoscopy.
Abnormal esophageal motility on manometry.
Abnormal esophageal exposure on ambulatory pH monitoring.
General Considerations
GERD is the most common upper gastrointestinal disorder of the Western world and accounts for about 75% of esophageal diseases. Heartburn, usually considered synonymous with thepresence of abnormal gastroesophageal reflux, is experienced by 2040% of the adult population of Western countries. However, because many symptomatic patients treatthemselveswith over-the-counter medications without consulting a physician, the prevalence of the disease is probably higher than reported. The incidence of reflux symptoms increases with age,
and both sexes seem to be equally affected. Symptoms are more common during pregnancy, probably due to hormonal effects on the LES and the increased intra-abdominal pressuredue to the enlarging uterus. Recent studies have demonstrated a link between obesity and GERD whereby the body mass i ndex is an independent factor and has a direct effect on theseverity of reflux.
Pathogenesis
GERD is caused by the abnormal retrograde flow of gastric contents into the esophagus, resulting in symptoms and mucosal damage. A defective LES is the most common cause of GERD.Transient LES relaxations account for the majority of reflux episodes in patients without mucosal damage or with mild esophagitis, while a short and hypotensive LES is more frequentlyfound in patients with more severe esophagitis. In 4060% of patients with GERD, abnormalities of esophageal peristalsis are also present. Because esophageal peristalsis is the main determinant of esophageal clearance (the ability of the esophagus to clear gastric contents refluxed through the LES), patients with abnormal esophageal peristalsis have more severereflux and slower clearance. Therefore, these patients often have more severe mucosal in jury and more frequent atypical symptoms such as cough or hoarseness. A hiatal hernia alsocontributes to the incompetence of the gastroesophageal junction by altering the anatomic relationship between the esophageal crusa nd the LES. As the gastroesophageal junction isdisplaced above the diaphragm, the pinchcock action of the esophageal crus is lost. In patients with large hiatal hernias, the LES is usually shorter a nd weaker, and the amount of reflux isgreater.
Clinical Findings
Symptoms
Heartburn, regurgitation, and dysphagia are considered typical symptoms of GERD. However, a clinical diagnosis of GERD based on these symptoms is correct in only 70% of patients(when compared with the results of pH monitoring). A good response to therapy with proton pump inhibitors is a good predictor of the presence of abnormal reflux. GERD can also cause
atypical symptoms such as cough, wheezing, chest pain, hoarseness, and dental erosions. Two mechanisms have been postulated for GERD-induced respiratory symptoms: (1) a vagalreflex arc resulting in bronchoconstriction and (2) microaspiration into the tracheobronchial tree. Ear, nose, and throat symptoms such as hoarseness or dental erosions are insteadsecondary to the upward extent of the acid with direct damage of the vocal cords or teeth.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 13/40
Barium Swallow
A barium swallow provides information about the presence and size of a hiatal hernia, the presence and length of a stricture, and the length of theesophagus. This test, however, is not diagnostic of GERD, as a hiatal hernia or reflux of barium can be present in the absence of abnormal reflux.
Endoscopy
Fifty percent of patients with abnormal reflux do not have esophagitis on endoscopy. Therefore, endoscopy is useful for diagnosing complications of GERD such as esophagitis, Barrett esophagus, or a stricture. In addition, there is major interobserver variation among endoscopists for the low gradesof esophagitis (Table 202).
Table 202. Endoscopic Grading System for Esophagitis.
Grade 1 Reddening of the mucosa without ulceration Grade 2 Linear ulcerations lined with granulation tissue that bleeds easily when touched Grade
3 Ulcerations have coalesced to leave islands of epithelium Grade 4 Stricture EsophagealManometry
This test provides information about the LES (resting pressure, length, and relaxation) and the quality of esophageal peristalsis. In addition,manometry is essential for proper placement of the pH probe for ambulatory pH monitoring (5 cm above the upper border of the LES).
Ambulatory pH Monitoring
This test has a sensitivity and specificity of about 92% and is considered the gold standard for diagnosing GERD (Table 203). Medications that affectthe production of acid by the parietal cells must be stopped 3 days (H2-blocking agents) to 14 days (proton pump inhibitors) prior to the study. Dietand exercise are unrestricted during the test in order to mimic a typical day of the patient's life. This test should be performed (1) in patients who donot respond to medical therapy, (2) in patients who relapse after discontinuation of medical therapy, (3) before antireflux surgery, or (4) when evaluating atypical symptoms such as cough, hoarseness, and chest pain. Because fewer than 50% of these patients experience heartburn or haveesophagitis on endoscopy, a pH monitoring study becomes the only way to establish a link between reflux and symptoms. A pH probe with 2 sensors,located 5 cm and 20 cm above the LES, allows determination of the upward extent of the reflux. Tracings are analyzed for a temporal correlation between symptoms and episodes of reflux.
Table 203. Normal Values for Ambulatory 24-Hour pH Monitoring.
Percentage of total time pH < 4.0 4.5% Percentage of upright time pH < 4.0 8.4% Percentage of supine time pH < 4.0 3.5% Number of episodes of reflux < 4.0 47 Number of episodes > 5 minutes 3.5 Longest episode (minutes) 20 Composite score1
14.71The composite score indicates the extent to which the patient's values deviate from the normal means of the six variables. It allows one to expressin a single figure the degree of the patient's abnormality. Calculation of the composite score is explained in Stein HJ et al: Outpatient physiologictesting and surgical management of foregut motility disorders.Curr Probl Surg 1992;24:495.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 14/40
Differential Diagnosis
Heartburn can be the presenting symptom of irritable bowel syndrome, achalasia, cholelithiasis, coronary artery disease, or psychiatric disorders.Esophagealmanometry and pH monitoring are essential to determine with certainty if GERD is present and if reflux is the cause of the symptoms.
Complications
Esophagitis is the most common complication. Peptic strictures are uncommon, particularly in the era of proton pump inhibitors. Barrett esophagus(metaplasia of the esophageal mucosa from squamous to columnar epithelium) is found in about 1015% of patients with reflux documented by pHmonitoring. Some patients may eventually progress to high-grade dysplasia and adenocarcinoma. Respiratory complications vary from chronic coughto asthma, aspiration pneumonia, and even pulmonary fibrosis. Vocal cord and dental damage can also occur.
Treatment
Lifestyle Modifications
Patients should eat frequent small meals during the day (to avoid gastric distention), avoiding fatty foods, spicy foods, and chocolate, as they lowerLES pressure. The last meal should be no less than 2 hours before going to bed. In order to increase the effect of gravity, the head of the bed shouldbe elevated over 4-inch to 6-inch blocks.
Medical Therapy
Antacids are useful for patients with mild intermittent heartburn. Acid-suppressing medications are the mainstay of medical therapy. H2-blockingagents are usually prescribed for patients with mild symptoms or mild esophagitis. Proton pump inhibitors are superior to H2-blocking agentsbecause they determine a more profound control of the acid secretion, with healing of esophagitis in 8090% of patients. However, symptoms andesophagitis tend to recur in the majority of patients after discontinuation of therapy, so most patients need chronic maintenance therapy. In addition, about 50% of patients on maintenance proton pump inhibitors require increasing doses to maintain healing of esophagitis. Medical therapyis largely ineffective for the treatment of the extraesophageal manifestations of GERD due to the upward extension of the refluxate. In thesepatients, acid-suppressing medications only alter the pH of the gastric refluxate, but reflux and aspiration can still occur because of an incompetentLES and ineffective esophageal peristalsis. Proton pump inhibitors can interfere with calcium absorption causing fractures. In addition, they havebeen shown to cause delay in gastric emptying and abnormal cardiac contractility.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 15/40
Surgical Therapy
In the past, antireflux operation was considered only for patients who did not respond to medical treatment with antacids or H2-blocking agents.Today the ideal patient is the one whose heartburn is well controlled by proton pump inhibitors and in whom ambulatory pH monitoring showsabnormal reflux. The operation is indicated in (1) young patients who require chronic therapy with proton pump inhibitors for control of symptoms,(2) patients in whom regurgitation persists during therapy, (3) patients with respiratory symptoms (cough, asthma, aspiration pneumonia, pulmonaryfibrosis), (4) patients with vocal cord damage, and (5) patients with Barrett esophagus. Recent evidence suggests that an effective antirefluxoperation may promote regression of the columnar epithelium in up to 50% of patients who have a short segment of Barrett esophagus (< 3 cm). In addition, it may arrest the progression from metaplasia to dysplasia. However, since the response to therapy is unpredictable, endoscopicsurveillance after laparoscopic fundoplication in patients with Barrett esophagus is recommended.
The goal of surgical therapy is to restore the competence of the LES. A laparoscopic Nissen fundoplication (360°) is considered today the procedure of choice (Figure 208) because it increases the resting pressure and length of the LES and decreases the number of transient LES relaxations. The
success of the operation is based on the following technical elements: . Dissection of the esophagus in the posterior mediastinum to allow 34 cm of esophagus to lie without tension below the diaphragm. By bringing the
entire stomach and gastroesophageal junction below the diaphragm, a sliding hiatal hernia is reduced.
2. Division of the short gastric vessels in order to create a "floppy" fundoplication.
3. Approximation of the esophageal crus to decrease the size of the esophageal hiatus, thereby avoiding herniation of the wrap.
4. Construction of a 360° fundoplication over a 5660 French bougie.
The hospital stay is usually 1 day only, and the postoperative discomfort is minimal. Most patients return to work within 23 weeks.
Prognosis
Control of typical symptoms is obtained in about 90% of patients after a fundoplication. Failures are treated with either medications or a secondoperation. The success rate is in the 7090% range for patients with atypical symptoms, because it is often more difficult to establish preoperativelya strong correlation between gastroesophageal reflux and symptoms.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 16/40
Barrett Esophagus
Essentials of Diagnosis
GERD symptoms (typical and atypical).
Endoscopic evidence of "salmon pink" epithelium above gastroesophageal junction.
Specialized columnar epithelium on esophageal biopsy.
General Considerations
Barrett esophagus is caused by a change in the esophageal mucosa with replacement of the squamous epithelium by columnar epithelium. About1012% of patients undergoing endoscopy for symptoms of GERD are found to have Barrett epithelium, usually classified in short segment (less than 3 cm in length) or long segment (3 cm or longer). It occurs more frequently in white men older than 50 years. This metaplasia may progress to high-grade dysplasia and eventually adenocarcinoma. Thus, adenocarcinoma represents the final step of a sequence of events in which a benign disease
(GERD) evolves into a preneoplastic disease and eventually into cancer. Pathogenesis
Barrett esophagus is due to reflux of gastric contents (acid and duodenal juice) into the esophagus. Barrett metaplasia is considered an advancedstage of GERD, characterized by a panesophageal motor disorder. When compared with patients with GERD with no mucosal in jury or less severeesophagitis, patients with Barrett esophagus have a shorter and weaker LES and decreased amplitude of esophageal peristalsis. As a consequence,the amount of reflux is greater and esophageal clearance is slower. In addition, hiatal hernia is more common in patients with Barrett metaplasia.
Clinical Findings
Symptoms
Patients with Barrett esophagus typically have a long history of GERD. While most patients experience both typical and atypical symptoms of GERD,others may become asymptomatic over time due to the decreased sensitivity of the metaplastic epithelium.
Imaging Studies
Barium swallow may show ulcerations, a hiatal hernia, or a stricture. Endoscopy shows presence of "salmon pink" epithelium above the
gastroesophageal junction, replacing the whitish squamous epithelium. The diagnosis is confirmed by pathologic examination of the esophagealmucosa and requires the identification of intestinal type epithelium, characterized by the presence of goblet cells.
Special Tests
Esophagealmanometry often shows a short and hypotensive LES and abnormal esophageal peristalsis (decreased amplitude of peristaltic waves,simultaneous waves). Ambulatory pH monitoring usually shows a severe amount of acid reflux. Esophageal exposure to duodenal juice can bequantified by a fiberoptic probe that measures intraluminal bilirubin as a marker for duodenal reflux. In GERD patients, the prevalence of esophagealbilirubin exposure parallels the degree of mucosal in jury, being higher in patients with Barrett esophagus.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 17/40
Treatment
Barrett Esophagus: Metaplasia
The treatment options are similar to those of patients with GERD without metaplasia and consist of either proton pump inhibitors or afundoplication. A surgical approach might offer an advantage over medical therapy for the following reasons:
1. Successful elimination of reflux symptoms with proton pump inhibitors does not guarantee control of acid reflux. When pH monitoring isperformed in asymptomatic Barrett patients treated with these medications, up to 80% of them still have abnormal acid reflux.
2. Proton pump inhibitors do not eliminate the reflux of bile, a major contributor to the pathogenesis of Barrett esophagus. In contrast, an antirefluxoperation prevents any form of reflux by restoring the competence of the gastroesophageal junction
3. A fundoplication may promote regression of the columnar epithelium. Many studies have shown that regression occurs in 1550% of patientswhen the length of the Barrett segment is less than 3 cm. Regardless of the effect of the fundoplication on symptoms, surveillance endoscopy should
be performed every 1224 months. Barrett Esophagus: Low-Grade Dysplasia
Patients with low-grade dysplasia should be treated for 12 months with high doses of proton pump inhibitors (34 pills per day), and subsequentlythe endoscopy should be repeated with multiple biopsies. The rationale for this approach is to decrease the mucosal inflammation by blocking acidsecretion, allowing the pathologist a more accurate reading. If the repeated biopsies show metaplasia or high-grade dysplasia, the patient will betreated accordingly. If low-grade dysplasia is confirmed, the patient can continue taking acid-reducing medications or have a laparoscopicfundoplication. There is evidence that regression to metaplasia or even disappearance of the columnar epithelium can occur after a successfulfundoplication. Surveillance endoscopy should be performed every 612 months.
Barrett Esophagus: High-Grade Dysplasia
When high-grade dysplasia is found (the diagnosis must be confirmed by two experienced pathologists), two treatment options are available:
(1) Patients can enroll in a program of strict endoscopic surveillance, with endoscopy performed every 3 months and 4-quadrant biopsies obtainedfor every centimeter of Barrett epithelium. The goal is to detect cancer as soon as it develops but before it becomes invasive and spreads to lymphnodes. Progression from high-grade dysplasia to cancer occurs in about 50% of patients 5 years after the initial diagnosis is established. This approach
is reasonable if the patient is willing to undergo endoscopy every 3 months but unwilling to have an esophagectomy or if severe comorbid conditions(cardiac or respiratory disease) are present.
(2) For young and medically fit patients who are unwilling to undergo endoscopy every 3 months, an esophagectomy should be considered. Therationale for an operation is based on the following considerations: (a) cancer is already found in about 30% of patients thought to have high-gradedysplasia; (b) cancer develops in about 50% of patients during follow-up; (c) recent studies have shown that in specialized centers the operation can be performed with minimal morbidity, no mortality, and postoperative quality of life similar to that of the general population; and (d) because theprognosis depends on the pathologic staging, waiting exposes patients to the risk of development of invasive cancer with lymph node metastases.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 18/40
Endoscopic Treatment Modalities
Because either acid-reducing medications or a fundoplication determineregression in some patients with a short segment only, and because thereis no evidence that they block progression to cancer, different modalitieshave been developed for the endoscopic ablation of the Barrett
epithelium. Photodynamic therapy is based on the administration of aphotosensitizing drug, which is retained in the Barrett epithelium. Light of proper wavelength is then delivered endoscopically, producing an oxidative reaction with destruction of the abnormal mucosa. Completedestruction of the columnar epithelium can be achieved in about 50% of patients. This technique, however, is associated with the development of esophageal strictures in about 30% of patients. In addition, islands of
columnar epithelium can still be present under the regenerated squamousepithelium. A new technique based on radio frequency ablation seems toavoid these problems and is effective in about 70% of patients. In selectedpatients with short islands of Barrett epithelium, the mucosa can beresected endoscopically.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 19/40
ParaesophagealHiatal Hernia
Essentials of Diagnosis
May be asymptomatic.
Symptoms secondary to mechanical obstruction: dysphagia, epigastric discomfort, bleeding.
Symptoms secondary to gastroesophageal reflux: heartburn, regurgitation.
General Considerations
There are two types of esophagealhiatal hernia: paraesophageal and sliding (the sliding type was discussed in the section on GastroesophagealReflux Disease); see Figures 209 and 2010. Obesity, aging, and general weakening of the musculofascial structures set the stage for enlargement of the esophageal hiatus and herniation of the stomach into the posterior mediastinum.
There are two types of paraesophageal hernia. In one type, the less common, part of the stomach herniatesinto the thorax immediately adjacent to
an undisplacedgastroesophageal junction (Figures 2011 and 2012). Since the gastroesophageal sphincteric mechanism functions normally in mostof these cases, reflux of gastric contents is uncommon. More commonly, however, the paraesophageal herniation occurs in association with thesliding type, and symptoms due to gastroesophageal reflux may occur along with symptoms secondary to the mechanical obstruction.
Clinical Findings
Symptoms usually develop late in adult life. Patients can experience epigastric discomfort, postprandial bloating, or dysphagia or have anemiasecondary to gastric erosions. In addition, they may experience symptoms due to gastroesophageal reflux.
Diagnosis
A barium swallow will delineate the anatomy and the type of hiatal hernia. Endoscopy is important to determine if gastric or esophagealinflammation is present and to rule out cancer. If reflux symptoms are present, manometry and pH monitoring should be performed.
Complications
The most frequent complications of paraesophageal hernia are hemorrhage, incarceration, obstruction, and strangulation. The herniated portion of the stomach often becomes congested, and bleeding occurs from erosions of the mucosa. Obstruction may occur, most often at the esophagogastric
junction as a result of torsion and angulation at this pointespecially if a large portion (or all) of the stomach herniates into the chest. In paraesophagealhiatal herniain contrast to the sliding typeother viscera such as the small and large intestines and spleen may also enter themediastinumalong with the stomach.
Treatment
Operative repair is indicated in symptomatic patients. The usual method is to return the herniated stomach below the diaphragm into the abdomen,repair the enlarged esophagealhiatus, and then add a fundoplication. In most cases, the operation can be performed laparoscopically.
Prognosis
The results of surgical management are excellent in about 90% of patients.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 20/40
Tumors of the Esophagus
Benign Tumors of the Esophagus
Essentials of Diagnosis
Dysphagia, epigastric discomfort.
Radiographic demonstration of a smooth filling defect within the esophageal lumen.
General Considerations
Esophageal leiomyomas are the most common benign tumors of the esophagus. They represent 10% of all gastrointestinal leiomyomas. Theyoriginate in the smooth muscle layers, mostly in the lower two thirds of the esophagus, and they narrow the esophageal lumen. These tumors consistof smooth muscle cells surrounded by a capsule of fibrous tissue. The mucosa overlying the tumor is generally intact, but occasionally it may becomeulcerated as a result of pressure necrosis by an enlarging lesion. Leiomyomas are not associated with the development of cancer. Other tumors such
as fibromas, lipomas, fibromyomas, and myxomas are rare.Congenital cysts or duplications of the esophagus (the second-most common benign lesion after leiomyomas) may occur at any level, although they are most common in the lower esophagus.
Clinical Findings
Many benign lesions are asymptomatic and are discovered incidentally during upper gastrointestinal fluoroscopic examination. Benign tumorsorcysts grow slowly and become symptomatic only after reaching a size of 5 cm or more. On barium swallow, leiomyomas appear as a smooth fillingdefect within the esophageal lumen (Figure 2013). An intraluminal mass covered by normal mucosa can be easily recognized during endoscopy, butbiopsies should not be taken because they may make subsequent enucleation of the tumor more difficult. Endoscopic ultrasound and chest CT helpin the characterization of the tumor and in the differential diagnosis.
Differential Diagnosis
Leiomyomas, cysts, and duplications can be distinguished from cancer by their c lassic radiographic appearance. Intraluminal papillomas, polyps, orgranulomas may be indistinguishable radiographically from early carcinoma, so their exact nature must be confirmed histologically.
Treatment
Small polypoid intraluminal lesions may be removed endoscopically. The treatment of choice for symptomatic leiomyomas is enucleation. While in the past a thoracotomyor a laparotomy was used to expose the esophagus and remove the tumor, today enucleation can be accomplished by eithera thoracoscopic or a laparoscopic approach.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 21/40
Carcinoma of the Esophagus
Essentials of Diagnosis
Progressive dysphagia, initially for solids and later for liquids.
Progressive weight loss.
Diagnosis established by endoscopy and biopsies.
Staging established by endoscopic ultrasound, computed tomography of chest and abdomen, and positron emission tomography. Bronchoscopyindicated for cancer of the mid thoracic esophagus.
General Considerations
In the United States, esophageal carcinoma accounts for 10,00011,000 deaths per year. The epidemiology of esophageal cancer in the United Stateshas changed considerably during the last 30 years. In the 1970s, squamous cell carcinoma was the most common type of esophageal cancer,
accounting for about 90% of the total cases. It was located in the thoracic esophagus and affected mostly black men. Over the past three decades,there has been a progressive increase in the incidence of adenocarcinoma of the distal esophagus and gastroesophageal junction, so that today itaccounts for more than 70% of all new cases of esophageal cancer. Adenocarcinoma is more frequent in white men with GERD. Squamous cell canceris still the most common type worldwide. Esophageal cancer occurs mostly during the fifth to seventh decades of life and is more common in men than in women.
Pathogenesis
The most common contributing factors for squamous cell carcinoma are cigarette smoking and chronic alcohol exposure. Chronic ingestion of hotliquids or foods, poor oral hygiene, and nutritional deficiencies may play a role. Certain medical conditions such as achalasia, caustic in juries of theesophagus, and Plummer-Vinson syndrome are associated with an increased incidence of squamous cell cancer. GERD is the most common predisposing factor for adenocarcinoma of the esophagus, where adenocarcinoma represents the last event of a sequence that starts with GERD andprogresses to metaplasia, high-grade dysplasia, and cancer. Esophageal cancer arises in the mucosa and subsequently invades the submucosa andthe muscle layers. Ultimately, structures located next to the esophagus may be infiltrated (tracheobronchial tree, aorta, recurrent laryngeal nerve).At the same time, the tumor tends to metastasize to the lymph nodes (celiac, mediastinal, cervical) and to the liver, lungs, adrenals, and bones.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 22/40
Clinical Findings
Symptoms
Dysphagia is the most common presenting symptom. Dysphagia is initially for solids but eventuallyit progresses to liquids. Weight loss occurs in more than 50% of patients. Patients can have pain when swallowing. Pain over bony structures may be due to metastases. Hoarseness is usually dueto invasion of the right or left recurrent laryngeal nerves with paralysis of the ipsilateral vocal cord.Respiratory symptoms may be due to regurgitation and aspiration of undigested food or to invasion
of the tracheobronchial tree, with development of a tracheoesophageal fistula. Imaging Studies
Barium swallow shows the location and the extent of the tumor. Esophageal cancer usuallypresents as an irregular intraluminal mass or a stricture (Figure 2014). Endoscopy allows directvisualization and biopsies of the tumor. For tumors of the upper and midesophagus, bronchoscopyis indicated to rule out invasion of the tracheobronchial tree.
Special Tests
After the diagnosis is established, it is important to determine the staging of the cancer (Table 20
4). Abdominal and chestC
T scans are useful to detect distant organ metastases (M, metastases) andinvasion of structures next to the esophagus. Alternatively, PET can be used. Endoscopic ultrasoundis the most sensitive test to determine the penetration of the tumor (T, tumor), the presence of enlarged periesophageal lymph nodes (N, nodes), and invasion of structures next to the esophagus.Fine-needle aspiration of enlarged periesophageal lymph nodes can be done under ultrasoundguidance. A bone scan is indicated in patients with new onset of bone pain.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 23/40

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 24/40
Differential Diagnosis
The differential diagnosis includes peptic strictures due to reflux, achalasia, and benign esophageal tumors.
Treatment
Patients with esophageal cancer are considered candidates for esophageal resection if the following criteria are met: (1) there is no evidence of spread of the tumor to structures next to the esophagus such as the tracheobronchial tree, the aorta, or the recurrent laryngeal nerve; (2) there is noevidence of distant metastases; (3) the patient is fit from a cardiac and respiratory point of view. An esophagectomy can be performed by using an abdominal and a cervical incision (with blunt dissection of the thoracic esophagus through the esophageal hiatus; transhiatal esophagectomy) or byusing an abdominal and a right chest incision (transthoracic esophagectomy). After removal of the esophagus, continuity of the gastrointestinal tractis reestablished by using either the stomach or the colon. The transhiatal esophagectomy offers the advantage of avoiding the chest incision, withdecreased compromise of lung function and decreased postoperative discomfort. The validity of the transhiatal esophagectomy as a canceroperation was initially questioned because part of the operation is not done under direct vision and because of the small number of resected lymph
nodes. However, many retrospective studies and prospective randomized trials have shown no difference in survival between the two operations,suggesting that it is not the type of operation that influences survival but rather the stage of the disease at the time the operation is performed. Themorbidity rate of the operation is around 30%, and it is mostly due to cardiac (arrhythmias), respiratory (atelectasis, pleural effusion), and septiccomplications (anastomotic leak, pneumonia). The mortality rate in specialized centers is less than 5%. As with other complex operations (cardiacoperation, liver and pancreatic resections), a lower mortality rate is obtained in "high-volume centers" and is due to the presence of an experiencedteam composed of surgeons, anesthesiologists, intensivists, cardiologists, radiologists, and nurses.
Because most patients already have lymph node metastases at the time of surgery, the 5-year survival for this disease remains poor. Neoadjuvanttherapy based on a combination of radiotherapy and chemotherapy is used in order to improve local (radiotherapy) and distant control of thedisease (chemotherapy). Overall, it seems that the combination of neoadjuvant therapy followed by surgery offers the best survival benefit. This isparticularly true in the subgroup of patients (about 20%) who have a "complete pathologic response" (no tumor found in the specimen).
Nonoperative therapy is reserved for patients who are not candidates for surgery because of local invasion of the tumor, metastases, or a poorfunctional status. The goal of therapy in these patients is palliation of the dysphagia, allowing them to eat. The following treatment modalities areavailable to achieve this goal:
1. Expandable, coated, metallic stents can be deployed by endoscopy under fluoroscopic guidance in order to keep the esophageal lumen open.They are particularly useful when a tracheoesophageal fistula is present.
2. Laser therapy (Nd:YAG laser) relieves dysphagia in up to 70% of patients. However, multiple sessions are usually required to keep the esophageallumen open.
3. Radiation therapy is successful in relieving dysphagia in about 50% of patients.
Prognosis
The stage of the disease is the most important prognostic factor. Overall 5-year survival for esophageal cancer remains around 25%.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 25/40
erforation of the Esophagus
Essentials of Diagnosis
History of recent instrumentation of the esophagus or severe vomiting.
Pain in the neck, chest, or upper abdomen.
Signs of mediastinal or thoracic sepsis within 24 hours.
Radiographic evidence of an esophageal leak.
General Considerations
Esophageal perforations can result from iatrogenic instrumentation (eg, endoscopy, balloon dilation), severevomiting, external trauma, and other rare causes. The subsequent clinical manifestations are influenced by thesite of the perforation (ie, cervical or thoracic) and, in the case of thoracic perforations, whether or not themediastinal pleura has been ruptured. Morbidity resulting from esophageal perforation is principally due toinfection. Immediately after in jury, the tissues are contaminated by esophageal contents, but infection has notbecome established; surgical closure of the defect will usually prevent the development of serious infection. If more than 24 hours have elapsed since the time of in jury, severe contamination has occurred. At this time, theesophageal defect usually breaks down if it is surgically closed, and measures to treat mediastinitis and empyemamay not be adequate to avoid a fatal outcome. Although serious infection usually occurs if surgical repair isdelayed, a few cases of minor instrumental perforations can be managed by antibiotics without operation.
Instrumental Perforations
Medical instrumentation is the most common cause of esophageal perforation (diagnostic or therapeuticendoscopy). Instrumental perforations are most likely to occur in the cervical esophagus. The endoscope maypress the posterior wall of the esophagus against osteoarthritic spurs of the cervical vertebrae, causing contusion or laceration. The cricopharyngeal area is the most common site of in jury. Perforations of the thoracic esophagusmay occur at any level but are most common at the natural sites of narrowing, at the level of the left main stembronchus and at the diaphragmatic hiatus. Perforations during pneumatic dilatation for achalasia (26%) occurproximal to the gastroesophageal junction

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 26/40
Spontaneous (Postemetic) Perforation (Boerhaave Syndrome)
Spontaneous perforation usually occurs in the absence of preexisting esophageal disease, but 10% of patients have reflux esophagitis, esophagealdiverticulum, or carcinoma. Most cases follow a bout of heavy eating and drinking. The rupture usually involves all layers of the esophageal wall andmost frequently occurs in the left posterolateral aspect, 35 cm above the gastroesophageal junction. The tear results from excessive intraluminalpressure, usually caused by violent retching and vomiting. Some cases have also been associated with childbirth, defecation, convulsions, heavylifting, and forceful swallowing. The overlying pleura are also torn, so both the mediastinum and the pleural cavity are contaminated with esophagealcontents. The second-most common site of perforation is at the midthoracic esophagus, on the right side at the level of the azygous vein.
Clinical Findings
Signs and Symptoms
The principal early manifestation is pain, which is felt in the neck with cervical perforations and in the chest or upper abdomen with perforations of the thoracic esophagus. The pain may radiate to the back. With cervical perforations, pain is followed by crepitus in the neck, dysphagia, and signs of
infection. Perforations of the thoracic esophagus, which communicate with the pleural cavity in about 75% of cases, are usually accompanied bytachycardia, tachypnea, dyspnea, and the early development of hypotension. With perforation into the chest, pneumothorax is produced, followedby hydrothorax and, if not promptly treated, empyema. The left chest is involved in 70% and the right chest in 20%; involvement is bilateral in 10%.Escape of air into the mediastinum may result in a "mediastinalcrunch," which is produced by the heart beating against air-filled tissues (Hammansign). If the pleura remain intact, mediastinal emphysema appears more rapidly, and pleural effusion is slow to develop.
Imaging Studies
X-ray studies are important to demonstrate that perforation has occurred and to locate the site of the in jury. In perforations of the cervicalesophagus, x-rays show air in the soft tissues, especially along the cervical spine. The trachea may be displaced anteriorly by air and fluid. Later,widening of the superior mediastinum may be seen. With thoracic perforations, mediastinal widening and pleural effusion with or withoutpneumothorax are the usual findings. An esophagogram using water-soluble contrast medium should be performed promptly in every patientsuspected of having an esophageal perforation (Figure 2015). If a leak is not seen, the examination should be repeated using barium. A CT scan of the chest is also useful to localize the perforation and eventually to drain mediastinal fluid collections.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 27/40
Special Studies
Thoracentesis will reveal cloudy or purulent fluid, depending on how much time has passed since the time of perforation. The amylase content of thefluid is elevated, and serum amylase levels may also be high as a result of absorption of amylase from the pleural cavity.
Treatment
Antibiotics should be given immediately. The infection is usually polymicrobial with Staphylococcus, Streptococcus, Pseudomonas, and Bacteroides.Early operation is appropriate for all but a few cases, and every effort should be made to operate before the perforation is 24 hours old. For lesionstreated within this time limit, the operation should consist of closure of the perforation and external drainage. External drainage alone may sufficefor small cervical perforations, which may be difficult to find. Patients with achalasia in whom perforation has resulted from balloon dilation shouldhave the tear in the esophagus repaired and a Heller myotomy performed on the opposite side of the esophagus. Definitive therapy (eg, resection)should also be performed in patients with other surgical conditions, such as esophageal carcinoma.
Primary repair has a high failure rate if the perforation is older than 24 hours. The classic recommendation in this situation has been to isolate the
perforation (ie, to minimize further contamination) by performing a temporary cervical esophagostomy, ligating the esophagus just proximal to thegastroesophageal junction, and placing a feeding jejunostomy for enteral nutrition. Alternatively, the segment of esophagus where the perforation islocated can be resected, bringing the proximal end of esophagus out through the neck and closing the distal end. The mediastinum is drained, and afeeding jejunostomy is created. Later, the esophagostomy is taken down, and stomach or colon are interposed to bridge the gap. Bluntesophagectomy may be feasible as emergency treatment of instrumental perforation in a patient with lye stricture.
Nonoperative management consisting of antibiotics alone may be all that is necessary in a few selected cases of instrumental perforation. Thisapproach should be confined to patients without thoracic involvement (eg, pneumothorax or hydrothorax) whose esophagogram demonstrates justa short extraluminalsinus tract without wide mediastinal spread (ie, the contamination is limited) and who have no systemic signs of sepsis (eg,hypotension and tachypnea). Recently, esophageal stents have been placed for the treatment of iatrogenic, intrathoracic esophageal perforations.
Prognosis
The survival rate is 90% when surgical treatment is accomplished within 24 hours. The rate drops to about 50% when treatment is delayed.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 28/40
Esophageal Atresia
Complete obliteration (stenosis = narrowing)
Embryological abnorm.
Assoc. w/ other abnorm.: heart, vertebral, renal, Downs syn.
85% w/ tracheo-esoph. fistula
Dx. = NGT arrested by obliteration
confirm w/ contrast XR
gas in stomach = tracheo-esoph. fistula
Tx. = ligation & division

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 29/40
Pharyngeal Pouch
Mucosal protrusion cricopharyngeus + thyropharyngeus
= Killians dehiscence
luminal P., elderly,
dysphagia, regurg. of food in pouch
palpable swelling in neck
food in pouch: fetor, aspiration pneumonia, lung abscess
Dx. = Ba2+ swallow Tx. = excision of pouch
post. myotomy of cricopharyngeus

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 30/40
Achalasia (Of Cardia)
NM failure of relaxation at lower esophagus
- progressive dil.
- tortuosity
- hypertrophy of esoph. above
- incoordination of peristalsis Indistinguishable from Chagas dis. = Trypanosoma Cruzi destroy ggl. cells
dysphagia
regurg. aspiration pneumonia
mal. in dil. esoph.
Dx. = CXR, Ba2+ swallow (birds beak/rats tail), esophagoscopy
Tx. = intra-sphincteric in j. w/ botulinum toxin
Heller cardiomytomy (lengthwise cut: above LES stomach)
+ partial fundoplication xs. reflux
forcible dil. of gastro-esoph. junct. w/ hydrostatic bag

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 31/40
Reflux Esophagitis
incompetent cardiac sphincter
reflux of peptic juice lower esophagus
inflamm., ulceration & stricture
Assoc.: rep. vomiting w/ gastric juice acidity
long-standing NG intubation
resection of cardia w/ gastro-esoph. anastomosisBarrets esoph. = ectopic acid-secr. mucosa in esoph.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 32/40
Dx.
Fiberoptic esophagoscopy
24-hr pH studies
Acid-infusion test = dil. HCl pain; relieved by saline
Ba2+ swallow outline & assoc. structures
tilting head reflux
Diff. dx. = cholecystitis
peptic ulcer
angina pectoris

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 33/40
Tx.
weight, smoking, diet, sleep propped up
H2-antag. (cimetidine)
PPI (omeprazole)
Surgery = fundoplication = fundus of stomach sutured
anti-reflux valve

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 34/40
Esophageal Ca.
MC , mid. > lower > upper esoph. xs. alcohol, cigarettes
achalasia of cardia
Barrets esoph.
caustic stricture
Benign = leiomyoma
Mal.: 1o = leiomyosarcoma, ca.
2o = from lung/stomach
Barrets esophagus = stratified sq. ep.
- long-standing reflux metaplasia
gastric type dysplasia mal.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 35/40
Spread
Local trachea, aorta, lungs
Ly. esoph.
tracheo-esoph.supraclavicular
sub-diaphragmatic
Bld liverlungs

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 36/40
Clinical
weight, anorexia, anemia
Dysphagia: solids liq.
Regurg. & aspiration Enlarged neck ly. nodes
Jaundice; hepatomegaly
L. recurrent laryngeal n. hoarsenessbovine cough

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 37/40
Dx.
Ba2+ swallow narrowing at lesion + prox. dil. shelf
CXR pneumonitis, pleural effusion, lung abscess
CT liver met.
Esophagoscopy + bronchoscopy (invade tracheobronchial tree)
Liver US

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 38/40
Tx.
Vaporize growth & restore lumen endoscopic laser tx.
Esophagectomy = via laparotomy + R. thoracotomy
- 10 cm of grossly UNinvolv. esoph. prox.
- may NOT req. thoracotomy
- NOT involv.: trachea, bronchi or aorta
- ab. + cervical incisions
Radiotx. Chemotx. platinum-based regimen + radiotx.

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 39/40
Esophageal Varices
Portal hy. collaterals
L. gastric v. + esoph. vv. gastroesoph. varices

8/8/2019 1. Surgery of Esophagus
http://slidepdf.com/reader/full/1-surgery-of-esophagus 40/40
Tx.
hemorrhage bld loss HYPOtension
Prophylaxis: -blockers, band ligation, sclerotx.
Immed. tx. = bld replacement
enema empty colon for bld
neomycin bacterial decomposition of bldglucose IV/PO
Stop hemorrhage = band ligation/sclerotx.
IV vasopressin
balloon tamponade
TIPSS
esoph. transection
porto-caval shunt