neuroconscience.files.wordpress.com€¦ · Web viewInterestingly they find that correlations...
Transcript of neuroconscience.files.wordpress.com€¦ · Web viewInterestingly they find that correlations...

Is the resting BOLD signal physiological noise?
Over the past 5 years, resting-state fMRI (rsfMRI) has exploded in popularity. Literally dozens of papers are published each day examining slow (< .1 hz) or “low frequency” fluctuations in the BOLD signal. When I first moved to Europe I was caught up in the somewhat North American frenzy of resting state. I couldn’t understand why my Danish colleagues, who specialize in modelling physiological noise in fMRI simply did not take the literature seriously. The problem is essentially that the low frequencies examined in these studies are the same as those that dominate physiological rhythms. Respiration and cardiac pulsation can make up a massive amount of variability in the BOLD signal. Before resting state fMRI came along, nearly every fMRI study discarded any data frequencies lower than one oscillation every 120 seconds (e.g. 120 Hz high pass filtering). Simple things like breath holding and pulsatile motion in vasculature can cause huge effects in BOLD data, and it just so happens that these artifacts (which are non-neural in origin) tend to pool around some of our favorite “default” areas: medial prefrontal cortex, insula, and other large gyri near draining veins.
Naturally this leads us to ask if the “resting state networks” (RSNs) observed in such studies are actually neural in origin, or if they are simply the result of variations in breath pattern or the like. Naturally we can’t answer this question with fMRI alone. We can apply something like independent component analysis (ICA) and hope that it removes most of the noise- but we’ll never really be 100% sure we’ve gotten it all that way. We can measure the noise directly (e.g. “nuisance covariance regression”) and include it in our GLM- but a lot of the noise may be highly correlated with the signal we want to observe. What we need are cross-modality validations that low-frequency oscillations do exist, that they drive observed BOLD fluctuations, and that these relationships hold even when controlling for non-neural signals. Some of this is already established- for example direct intracranial recordings do find slow oscillations in animal models. In MEG and EEG, it is well established that slow fluctuations exist and have a functional role.
So far so good. But what about in fMRI? Can we measure meaningful signal while controlling for these factors? This is currently a topic of intense research interest. Marcus Raichle, the ‘father’ of the default mode network, highlights fascinating multi-modal work from a Finnish group showing that slow fluctuations in behavior and EEG signal coincide (Raichle and Snyder 2007; Monto, Palva et al. 2008). However, we should still be cautious- I recently spoke to a post-doc from the Helsinki group about the original paper, and he stressed that slow EEG is just as contaminated by physiological artifacts as fMRI. Except that the problem is much worse, because in EEG the artifacts may be several orders of magnitude larger than the signal of interest i.
Understandably I was interested to see a paper entitled “Correlated slow fluctuations in respiration, EEG, and BOLD fMRI” appear in Neuroimage today (Yuan, Zotev et al. 2013). The authors simultaneously collected EEG, respiration, pulse, and resting fMRI data in 9 subjects, and then perform cross-correlation and GLM analyses on the relationship of these variables, during both eyes closed and eyes open rest. They calculate Respiratory Volume per Time (RVT), a measure developed by Rasmus Birn, to assign a respiratory phase to each TR (Birn, Diamond et al. 2006). One key finding is that the global variations in EEG power are strongly predicted by RVT during eyes closed rest, with a maximum peak correlation coefficient of .40. Here are the two time series:

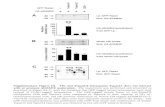
You can clearly see that there is a strong relationship between global alpha (GFP) and respiration (RVT). The authors state that “GFP appears to lead RVT” though I am not so sure. Regardless, there is a clear relationship between eyes closed ‘alpha’ and respiration. Interestingly they find that correlations between RVT and GFP with eyes open were not significantly different from chance, and that pulse did not correlate with GFP. They then conduct GLM analysis with RVT and GFP as BOLD regressors. Here is what their example subject looked like during eyes-closed rest:
Notice any familiar “RSNs” in the RVT map? I see anti-correlated executive activations and default mode deactivations! Very canonical. Too bad they are breath related. We shouldn’t be too surprised either that the GFP-BOLD and RVT-BOLD maps look similar, considering that GFP and RVT are highly correlated. After looking at these correlations separately, Yuan et al

perform RETROICOR physiological noise correction and then reexamine the contrasts. Here are the group maps:
Things look a bit less default-mode-like in the group RVT map, but the RVT and GFP maps are still clearly quite similar. In panel D you can see that physiological noise correction has a large global impact on GFP-BOLD correlations, suggesting that quite a bit of this covariance is driven by physiological noise. Yuan et al go on to examine eyes-open rest and show that, similar to their RVT-GFP cross-correlation analysis, not nearly as much seems to be happening in eyes open compared to closed:

The authors go on to conclude that “In particular, this correlation between alpha EEG and respiration is much stronger in eyes-closed resting than in eyes-open resting” and that “[the] results also suggest that eyes-open resting may be a more favorable condition to conduct brain resting state fMRI and for functional connectivity analysis because of the suppressed correlation between low-frequency respiratory fluctuation and global alpha EEG power, therefore the low-frequency physiological noise predominantly of non-neuronal origin can be more safely removed.” Fair enough- one conclusion is certainly that eyes closed rest seems much more correlated with respiration than eyes open. But then they go on to make this really strange statement, which appears through the abstract, introduction, and discussion:
“In addition, similar spatial patterns were observed between the correlation maps of BOLD with global alpha EEG power and respiration. Removal of respiration related physiological noise in the BOLD signal reduces the correlation between alpha EEG power and spontaneous BOLD signals measured at eyes-closed resting. These results

suggest a mutual link of neuronal origin between the alpha EEG power, respiration, and BOLD signals”’ (emphasis added)
That’s one way to put it! The logic here is that since alpha = neural activity, and respiration correlates with alpha, then alpha must be the neural correlate of respiration. I’m sorry guys, you did a decent experiment, but I’m afraid you’ve gotten this one wrong. There is absolutely nothing that implies alpha power cannot also be contaminated by respiration-related physiological noise. In fact it is exactly the opposite- in the low frequencies observed by Yuan et al EEG data is particularly likely to be contaminated by physiological artifacts! And that is precisely what the paper shows - in the author’s own words: “impressively strong correlations between global alpha and respiration”. This is further corroborated by the strong similarity between the RVT-BOLD and alpha-BOLD maps, and the fact that removing respiratory and pulse variance drastically alters the alpha-BOLD correlations!
So what should we take away from this study? It is of course inconclusive- there are several aspects of the methodology that are puzzling to me, and sadly the study is rather underpowered at n = 9. I found it quite curious that in all of the BOLD-alpha maps there seemed to be a significant artifact in the lateral and posterior ventricles (check out figure 2b, an almost perfect ventricle map). If their global alpha signal is specific to a neural origin, why does this artifact remain even after physiological noise correction? I can’t quite put my finger on it but it seems to me more likely that some source of noise remained even after correction- perhaps a reader with more experience in these methods can comment. Regardless, we should all take one clear message away from this study – low frequency signals are not easily untangled from physiological noise, even in the electrophysiological modality. This isn’t a damnation of all resting state research- rather it should be a clear sign that we need to measuring these signals to retain a sense of control over our data.
References:
Birn, R. M., J. B. Diamond, et al. (2006). "Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI." Neuroimage 31(4): 1536-1548 %@
1053-8119.
Monto, S., S. Palva, et al. (2008). "Very slow EEG fluctuations predict the dynamics of stimulus detection and oscillation amplitudes in humans." The Journal of Neuroscience 28(33):
8268-8272 %@ 0270-6474.
Raichle, M. E. and A. Z. Snyder (2007). "A default mode of brain function: a brief history of an evolving idea." Neuroimage 37(4): 1083-1090 %@ 1053-8119.
Yuan, H., V. Zotev, et al. (2013). "Correlated Slow Fluctuations in Respiration, EEG, and BOLD fMRI." NeuroImage %@ 1053-8119.

i Note that this is not meant to be in anyway a comprehensive review. A quick literature search suggests that there are quite a few recent papers on resting BOLD EEG. I recall a well done paper by a group at the Max Planck Institute that did include noise regressors, and found unique slow BOLD-EEG relations. I cannot seem to find it at the moment however!