Total Synthesis New Methodology - Princeton...
Transcript of Total Synthesis New Methodology - Princeton...
What Classifies as Innovative?
Would introducing a new method in a total synthesis make it innovative? Yes but…
Some innovations are considered to be more significant than others. How do we qualify these?
In my view an innovation must dramatically change the landscape of our field or provide a method that is widely applicable.
Innovation \i-nə-ˈvā-shən\ n:
1. The act of introducing something new.
2. A new idea, method, or device.
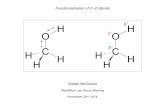
How Our Field was Born: UREA
Isolated in 1773 from human urine (hence the name) by Hilaire M. Rouelle
Intriguing to scientists who were eager to understand the mysteries of bodily functions .
H2N NH2
O
How Our Field was Born: UREA
Friedrich Wöhler: 1800-1882; student of Jöns Jakob Berzelius.
The synthesis of urea is known as the event that begot the field of synthetic organic chemistry.
“I cannot, so to say, hold my chemical water, and must tell you that I can make urea, without thereby needing to have kidneys, or anyhow, an animal, be it human or dog.”
Wöhlerʼs discovery shouldʼve refuted vitalism, the belief that organic molecules can only come from life which possesses a “vital force,” but the skeptics remained because he did not synthesize a C–C bond.
H2N NH2
O
AgNCONH4Cl
AgCl
How Our Field was Born: UREA
As if the birth of synthetic organic chemistry wasnʼt enough…
Wöhler thought he was using AgOCN (silver cyanate) instead of AgNCO (silver isocyanate), both possessing the same formula as AgCNO (silver fulminate), studied by Justus von Liebig, but having different chemical properties.
This gave rise to the notion of isomerism.
H2N NH2
O
AgNCONH4Cl
AgCl
Convincing the Skeptics
15 years later Hermann Kolbe silenced (most) skeptics by synthesizing acetic acid, literally from elements.
CFeS2
FeCS2
CS2
2Cl22SCCl4
2CCl4
pyrolysis2Cl2
Cl
ClCl
Cl
pyrolysis3HCl
Cl
ClCl
Cl Cl3C
O
OH
3H23HCl
H3C
O
OHCl3C
O
OH
Retrosynthetic Application: Tropinone
“By the imaginary hydrolysis at the points indicated by the dotted lines, the substance may be resolved into succinaldehyde, methylamine, and acetone, and this observation suggested a line of attack of the problem which has resulted in a direct synthesis.” –Sir Robert Robinson J. Chem. Soc. 1917, 762.
Sir Robertʼs analysis was one of the simplest yet most powerful suggestions of its time. The fact that it worked was a testament to his intuition.
NMe CO
Tropinone
Sir Robert Robinson
Retrosynthetic Application: Tropinone
The forward synthesis only required a slight modification of starting materials.
“In Searching for a method of synthesis of any substance it is always convenient to be able to recognize the formation of traces of the desired product…tropinone is obtained in small yield by the condensation of succinaldehyde with acetone and methylamine in aqueous solution.”
–Sir Robert Robinson J. Chem. Soc. 1917, 762-8.
CHO
CHO
CO2H
CO2H
OH2NMeCaCO3
H2O
HCl
H2O
N
O
Me
Tropinone, 42%
N
O
Me
XCaO2C
CO2CaX
Tropinone: Mechanism
“We have here a case where three rather simple molecules spontaneously unite into a complicated system, which earlier we could only build up step by step through a long series of reactions (13). We may suppose that here Sir Robert has found the key to natureʼs own way of working.”
–A. Fredga: Robinsonʼs Nobel presentation speech (1947).
CHO
CHO
CO2H
CO2H
OH2NMe
CaCO3
H2O
N
OH
Me
CO2CaX
CO2CaX
OH
N
O
Me
CO2CaX
CO2CaX
OH
N
OH
Me
CO2CaX
CO2CaX
N
O
Me
XCaO2C
CO2CaX
N
O
Me
HCl
2CO2
Reserpine
Known as one of the hallmarks of synthetic manipulation by a master of the field.
One of the greatest achievements of Woodwardʼs career especially considering only IR and elemental analysis were used as guides.
The dramatic conclusion taught chemists an invaluable lesson of thermodynamic engineering.
R. B. Woodward
Woodward, R. B.; Bader, F. E.; Bickel, H.; Frey, A. J; Kierstead, R. W. J. Am. Chem. Soc., 1956, 78, 2023.
NH
N
MeO2C
H
H
OMe
O
HMeO
O
OMe
OMe
OMe
(-)-reserpine
Reserpine: Retrosynthetic Analysis
NH
N
MeO2C
H
H
OMe
O
H
MeO
O
OMe
OMe
OMe
(+)-reserpine
NH
N
MeO2C
H
H
OMe
OAc
MeOO
Bischler-Napieralski
Reductive amination
Lactamization
MeO2C
CO2Me
H
HOMe
OAc
O
NH
NH2MeO
O
O CO2Me
H
H
Diels–AlderO
O CO2Me
p-benzoquinone
6-methoxytryptamine
O
O
MeO2CPhH, !
O
O CO2Me
H
H
endo-TS
Al(Oi-Pr)3
i-PrOH
OH
O
H
H
O
H
Br2O
O
H
H
O
H
Br
HMeONa
MeOH
O
O
H
H
O
Br
H
O
O
H
H
O
H
O
O
H
H
O
H
OMe
H
NBS
H2SO4 (aq)
O
O
H
H
O
H
OMe
HBr
HO
OMe
Reserpine
A Diels–Alder sets the first three stereocenters
Meerwein–Pondorff–Verley reduction creates the next two causing a spontaneous lactonization with the nearby ester.
O
O
MeO2CPhH, !
O
O CO2Me
H
H
endo-TS
Al(Oi-Pr)3
i-PrOH
OH
O
H
H
O
H
Br2O
O
H
H
O
H
Br
HMeONa
MeOH
O
O
H
H
O
Br
H
O
O
H
H
O
H
O
O
H
H
O
H
OMe
H
NBS
H2SO4 (aq)
O
O
H
H
O
H
OMe
HBr
HO
OMe
Reserpine: Molecular Judo
Bromoetherification sets the next two.
O
O
MeO2CPhH, !
O
O CO2Me
H
H
endo-TS
Al(Oi-Pr)3
i-PrOH
OH
O
H
H
O
H
Br2O
O
H
H
O
H
Br
HMeONa
MeOH
O
O
H
H
O
Br
H
O
O
H
H
O
H
O
O
H
H
O
H
OMe
H
NBS
H2SO4 (aq)
O
O
H
H
O
H
OMe
HBr
HO
OMe
Reserpine: Molecular Judo
Substitution of methoxide occurs with retention of configuration.
Reserpine: Molecular Judo
O
O
MeO2CPhH, !
O
O CO2Me
H
H
endo-TS
Al(Oi-Pr)3
i-PrOH
OH
O
H
H
O
H
Br2O
O
H
H
O
H
Br
HMeONa
MeOH
O
O
H
H
O
Br
H
O
O
H
H
O
H
O
O
H
H
O
H
OMe
H
NBS
H2SO4 (aq)
O
O
H
H
O
H
OMe
HBr
HO
OMe
Reserpine
O
O
H
H
O
H
OMe
HBr
O
H2Cr2O7 Zn
AcOHAcOH
Zn
H
O
CO2H
H
H
H
OMe
HBr
O
Zn
CO2H
H
H
H
OMe
OH
O
CO2Me
H
H
H
OMe
OAc
O
1. CH2N2
2. Ac2O
CO2Me
H
H
H
OMe
OAc
O
OsO4
NaClO3,H2O
OH
HO
MeO2C
CO2Me
H
H
H
OMe
OAc
1. HClO4
2. CH2N2
O
1. PhH, 1
2. NaBH4, MeOH
O
O
H
H
O
H
OMe
HBr
HO
NH
N
MeO2C
H
H
OMe
OAc
MeOO
Reserpine
NH
N
MeO2C
H
H
OMe
OAc
HMeO
(+)-methyl-O-acetylisoreserpate
N
OMeOAc
CO2Me
H
HNH
H
OMe
N
OAc
CO2MeMeO
H
H
HN
MeO
H
NH
N
MeO2C
H
H
OMe
OAc
MeOO
1. POCl3, !
2. NaBH4, MeOH
Reduction of the iminium ion happens on the convex face providing the undesired epimer.
Reserpine
NH
N
MeO2C
H
H
OMe
OAc
HMeO
(+)-methyl-O-acetylisoreserpate
N
OMeOAc
CO2Me
H
HNH
H
OMe
N
OAc
CO2MeMeO
H
H
HN
MeO
H
NH
N
MeO2C
H
H
OMe
OAc
MeOO
1. POCl3, !
2. NaBH4, MeOH
Thermodynamics favors the wrong conformation for an acid catalyzed epimerization.
Reserpine
NH
N
MeO2C
H
H
OMe
OAc
HMeO
(+)-methyl-O-acetylisoreserpate
N
OMeOAc
CO2Me
H
HNH
H
OMe
N
OAc
CO2MeMeO
H
H
HN
MeO
H
NH
N
MeO2C
H
H
OMe
OAc
MeOO
1. POCl3, !
2. NaBH4, MeOH
NH
N
H
H
NH
N
H
NH
N
H H
H
NH
N
H
H
NH
N
H
Reserpine: Molecular Judo
N
OAc
CO2MeMeO
H
H
HN
MeO
H
1. KOH, MeOH
2. DCC, pyr
N
O
MeO
H
H
HN
MeO
H
O
N
OMeOAc
CO2Me
H
HNH
H
OMe
N
OAc
CO2MeMeO
H
H
HN
MeO
H
Hydrolysis and lactone formation lock the molecule in the thermodynamically unfavorable conformation.
Reserpine: Molecular Judo
N
O
MeO
H
H
HN
MeO
H
O
xylenes, !
t-BuCO2H
N
O
MeO
H
H
HN
OMe
O H
NaOMe, MeOH, !
Pyridine
OMe
OMe
OMe
Cl
O
NH
N
MeO2C
H
H
OMe
OH
HMeO
NH
N
MeO2C
H
H
OMe
OH
HMeO
O
OMe
OMe
OMe
(+)-reserpine
Epimerization is now favored, correcting the stereochemistry
Reserpine
N
O
MeO
H
H
HN
MeO
H
O
xylenes, !
t-BuCO2H
N
O
MeO
H
H
HN
OMe
O H
NaOMe, MeOH, !
Pyridine
OMe
OMe
OMe
Cl
O
NH
N
MeO2C
H
H
OMe
OH
HMeO
NH
N
MeO2C
H
H
OMe
O
HMeO
O
OMe
OMe
OMe
(+)-reserpine
Reserpine
When defeated by both kinetics and thermodynamics, Woodward manipulated the system so that it worked in his favor.
“It is sometimes said that you have demonstrated that nothing is impossible in organic synthesis. This is perhaps a slight exaggeration. You have, however, in a spectacular way expanded and enlarged the domain of the possible.” –A. Fredga: Woodwardʼs Nobel presentation speech (1965).
Woodward: Organic Ninja
NH
N
MeO2C
H
H
OMe
O
HMeO
O
OMe
OMe
OMe
(-)-reserpine
(+)-reserpine
1. (+)-CSA2. NaOH
Progesterone
O
Me
Me
H
H
H
H
O
Me
(+)-progesterone
A synthesis inspired by the polyene cyclization of squalene oxide to dammaradienol in the biosynthesis of lanosterol.
Displayed a synthetically useful domino reaction and demostrated the utility of the Johnson–Claisen rearrangement.
W. S. Johnson
Johnson, W. S.; Gravestock, M. B.; McCarry, B. E. J. Am. Chem. Soc., 1973, 95, 20, 6832.
Progesterone: Retrosynthetic Analysis
Me
OO
O O
PPh3
IMe
Me
CHO
Me
Me
Me
OO
O O
Me
Me
Me
O
O
Me
Me
O
Me
Me
Me
Me
OHMe
O
Me
Me
H
H
H
H
OMe
(+)-progesterone
Me
Me
H
H
H
Me
OMe
H
Me
Me
H
H
H
H
OMe
O
Me
O
Aldol/dehydration Ozonolysis
Cation-!-cyclization
Aldol/dehydration
Wittig/Schlosermodification
Cyclization of Squalene Oxide
Stork and Eschenmoser independently yet concomitantly proposed that the trans olefins in the substrate controlled stereoselectivity.
Eschenmoser states: “It seems that with polyenes of this complexity (below), acid-catalyzed cyclization ceases to be a useful reaction from the preparative point of view.”
CO2MeMe
Me
MeMe
CO2Me
Me
Me Me
OH
Me
HH
5-10%
Me
MeO
Me
Me
MeMe
MeHH
H
Me
Me
HO
Me
Me
Me Me
Me
enzyme catalyzed
Me
Me
Me
H
Me Me
HO
Me H
further enzymatic steps
dammaradienol
lanosterol
Progesterone: Right Half
Johnson–Claisen rearrangement sets the γ,δ-usaturation
Me
CHO Me
BrMgMe
Me
HO 138 °C,CH3C(OEt)3,C2H5CO2H,55% 2-steps
Me
Me
OEtO
Me
Me
OEtO
1. LiAlH4
2. CrO3,pyr, 77%
Me
Me
CHO
Progesterone: Left Half
Furan starting material installs the 1,4-diketo relationship.
nBuLi, THF–30 °C
BrBr
—20 to 23 °C, 75%
O
Me
Br
O
Me HO(CH2)2OH, p-TsOH
hydroquinone, PhH,reflux, 71%
Me
OO
O O
NaI, MgOC2H5COCH3,80 °C, 99%
Br
Me
OO
O O
IMe
OO
O O
PPh3 PPh3, PhH
80 °C, 94%
I
Progesterone: Converging Two Halves
Schlosser modification of the Wittig provides the requisite E-olefin.
Deprotection and cyclization installs the final unsaturation.
Me
OO
O O
PPh3
IMe
Me
CHO
PhLi, –70 to –30 °C
then MeOH, -30 °C
Me
Me
Me
OO
O O
0.1 N HCl (aq),MeOH (3:10),40 °C
Me
Me
Me
O
O
EtOH, reflux2% NaOH (aq)
40%
Me
Me
O
Me
Progesterone: Cation-π-Cylization
MeLi, ether99%
Me
Me
Me
OHMe O O
O
Cl(CH2)2Cl,TFA, 0°C
Me
Me
Me
Me
O
OO
Me
Me
O
Me
Me
Me
H
H
H
Me
Me
O
OO
K2CO3, H2O/MeOH,71% Me
Me
H
H
H
Me
OMe
H
Progesterone
Cation-π-cylization forges all six stereocenters in a tandem operation from the crude tertiary alcohol.
Though biomimetic cation-π-cylizations had been previously demonstrated by Stork, Eschenmoser, and van Tamlen, this is the most elegant and highest yielding example applied to a total synthesis.
O
Me
Me
H
H
H
H
OMe
(+)-progesterone
Me
Me
H
H
H
Me
OMe
H O3, MeOH/CH2Cl2–70 °C
then Zn, AcOH, H2O, –15 °C to rt, 88%
Me
Me
H
H
H
H
OMe
O
Me
O
KOH, H2O51%
Prostaglandin A2
The first example of a total synthesis using the TBS protecting group, which also directs a SN2- selective organocuprate addition.
Addition of a chiral organocuprate facilitates convergence within the synthesis.
Introduction of the TBS protecting group has been one of the most enabling innovations of the 20th century.
O
H
CO2H
OH
Me
PGA2
Elias James Corey
Corey, E. J.; Mann, J. J. Am. Chem. Soc., 1973, 95, 20, 6832.
Prostaglandin A2: Retrosynthetic Analysis
OH
H Ketene 2 + 2
HO
CO2
!-NH3MePhO
O
H
H
Baeyer–Villiger
PGA2
O
H
(CH2)3CO2H
C5H11
OH
C5H11
OH
HO
CHO
WittigTBSO
O
OH
SN2 cuprate addition
Iodoetherificationelimination
ClCl
Cl
O
Prostaglandin A2
Ketene 2 + 2 installs requisite lactone carbons.
ClCl
Cl
O
NEt3, 0 °C, 75%Cl
Cl
OH
H
Zn, AcOH
OH
H
O
O
H
H
H2O2, H2OAcOH, 90%
NaOH, H2O0 °C, then
!-NH2MePh
HO
CO2
!-NH3MePh
1. I2, 85%
2. TBS-Cl, DMF imidazole, 72%
TBSO
I
O
ODBN, THF70 °C, 94%
TBSO
O
O
Prostaglandin A2
Baeyer-Villiger oxidation followed by hydrolysis and resolution furnishes the enantioenriched hydroxy acid.
ClCl
Cl
O
NEt3, 0 °C, 75%Cl
Cl
OH
H
Zn, AcOH
OH
H
O
O
H
H
H2O2, H2OAcOH, 90%
NaOH, H2O0 °C, then
!-NH2MePh
HO
CO2
!-NH3MePh
1. I2, 85%
2. TBS-Cl, DMF imidazole, 72%
TBSO
I
O
ODBN, THF70 °C, 94%
TBSO
O
O
Prostaglandin A2
Iodolactonization, TBS protection, and elimination provide the necessary precursor for cuprate addition.
ClCl
Cl
O
NEt3, 0 °C, 75%Cl
Cl
OH
H
Zn, AcOH
OH
H
O
O
H
H
H2O2, H2OAcOH, 90%
NaOH, H2O0 °C, then
!-NH2MePh
HO
CO2
!-NH3MePh
1. I2, 85%
2. TBS-Cl, DMF imidazole, 72%
TBSO
I
O
ODBN, THF70 °C, 94%
TBSO
O
O
Prostaglandin A2
Organicuprate addition happens in an SN2 fashion over SN2´ due to blocking from the –OTBS group.
PGA2
TBSO
O
O C5H11
OTBS 2
LiCuether/pentane
–70 ! –20 °C
H
C5H11
OTBS
TBSO
CO2H
HCl (aq),acetone, 80%
H
C5H11
OH
O
O
O
DCM, PPTS
99%H
C5H11
OTHP
O
O
1. DIBAlH, – 78 °C, toluene2. Ph3P=CH(CH2)3CO2, DMSO
HO
H
(CH2)3CO2H
C5H11
OTHP
1. Collins [O]
2. AcOH/H2O
O
H
(CH2)3CO2H
C5H11
OH
Prostaglandin A2
Olefination, deprotection, and oxidation complete the synthesis of PGA2
PGA2
TBSO
O
O C5H11
OTBS 2
LiCuether/pentane
–70 ! –20 °C
H
C5H11
OTBS
TBSO
CO2H
HCl (aq),acetone, 80%
H
C5H11
OH
O
O
O
DCM, PPTS
99%H
C5H11
OTHP
O
O
1. DIBAlH, – 78 °C, toluene2. Ph3P=CH(CH2)3CO2, DMSO
HO
H
(CH2)3CO2H
C5H11
OTHP
1. Collins [O]
2. AcOH/H2O
O
H
(CH2)3CO2H
C5H11
OH
Prostaglandin A2
TBS protecting group is one of the most widely used protecting groups in synthesis (43,200 web hits, original paper cited 2,256 times).
“Corey's most important syntheses are concerned with prostaglandins and related compounds…No other chemist has developed such a comprehensive and varied assortment of methods, often showing the simplicity of genius, which have become commonplace in organic synthesis laboratories. –S. Gronowitz: Coreyʼs Nobel presentation speech (1990).
O
H
CO2H
OH
Me
PGA2
Vernolepin/Vernomenin
First synthesis employing the synergistic diene: Danishefskyʼs diene (32,400 web hits, original paper cited 431 times).
Brought this concept to the scientific community, inspiring additional contributions e.g. Rawal diene.
Samuel Danishefsky
O
O
OH
HO
O
H
O
HO
O
O
HOH
vernolepin vernomenin
Danishefsky, S.; Schuda, P. F.; Kitahara, T.; Etheredge, S. J. J. Am. Chem. Soc., 1977, 99, 18, 6066.
Vernolepin: Retrosynthetic Analysis
CO2MeCO2Me
HO TMSO
OMe
HO
O
O
O
O
OH
HO
O
H
vernolepin
O
H
OH
OH CO2Me
O
O
H
OH
O
O
O
HO
O
O
O
OHC
MeO2C
H
O
O
O
O
HO
O
O
O
LactonizationEpoxide opening
Wittig
Lactonization
Directedepoxidation
Iodolactonization
Elimination
Diels–Alder
Vernolepin/Vernomenin
Sequential Diels–Alders, the second utilizing Danishefskyʼs Diene, forge the quaternary decalone.
CO2MeMeO2C
TMSO
OMe
mesitlene,reflux, 60%
CO2Me
HO
NaOH (aq),THF, quant
CO2H
HO
NaHCO3,I2, KI, 88%
HO I
O
O
PhH, DBU, 87%
HO
O
O
HO
NaOH (aq),THF, quant
CO2H
OH
Vernolepin/Vernomenin
Soponification followed by iodolactonization afford the tricyclic lactone.
CO2MeMeO2C
TMSO
OMe
mesitlene,reflux, 60%
CO2Me
HO
NaOH (aq),THF, quant
CO2H
HO
NaHCO3,I2, KI, 88%
HO I
O
O
PhH, DBU, 87%
HO
O
O
HO
NaOH (aq),THF, quant
CO2H
OH
Vernolepin/Vernomenin
E2 Elimination and a second sponification provide the allylic alcohol necessary for epoxidation.
CO2MeMeO2C
TMSO
OMe
mesitlene,reflux, 60%
CO2Me
HO
NaOH (aq),THF, quant
CO2H
HO
NaHCO3,I2, KI, 88%
HO I
O
O
PhH, DBU, 87%
HO
O
O
HO
NaOH (aq),THF, quant
CO2H
OH
Vernolepin/Vernomenin
Directed epoxidation installs the correct stereochemistry of the latent alcohol and esterification regenerates the lactone.
HO
CO2HOH
dioxane, PhH,m-CPBA
HO
CO2HOH
O
Ac2O, NaOAc
76%, 2-stepsH
O
O
O
O
OsO4, THF,B(ClO3)2, H2O,47 °C, 84%
HO
O
O
O
HO
HOPb(OAc)4, PhHMeOH, 86%OHC
MeO2C
H
O
O
O
1. LiAl(Ot-Bu)3H THF, –10 °C2. Amberlite 105 PhH, !", 93% O
HO
O
O
O
p-TsOH, PhH,MgSO4, 66%
HOOH
O
H
O
O
O
O
O
Vernolepin/Vernomenin
Dihydroxylation followed by oxidative diol cleavage prepares the molecule for γ-lactone formation.
HO
CO2HOH
dioxane, PhH,m-CPBA
HO
CO2HOH
O
Ac2O, NaOAc
76%, 2-stepsH
O
O
O
O
OsO4, THF,B(ClO3)2, H2O,47 °C, 84%
HO
O
O
O
HO
HOPb(OAc)4, PhHMeOH, 86%OHC
MeO2C
H
O
O
O
1. LiAl(Ot-Bu)3H THF, –10 °C2. Amberlite 105 PhH, !", 93% O
HO
O
O
O
p-TsOH, PhH,MgSO4, 66%
HOOH
O
H
O
O
O
O
O
Vernolepin/Vernomenin
Reduction and cyclization forge the γ-lactone functionality and surprising protection of this moiety via the orthoester happens under acetal forming conditions.
HO
CO2HOH
dioxane, PhH,m-CPBA
HO
CO2HOH
O
Ac2O, NaOAc
76%, 2-stepsH
O
O
O
O
OsO4, THF,B(ClO3)2, H2O,47 °C, 84%
HO
O
O
O
HO
HOPb(OAc)4, PhHMeOH, 86%OHC
MeO2C
H
O
O
O
1. LiAl(Ot-Bu)3H THF, –10 °C2. Amberlite 105 PhH, !", 93% O
HO
O
O
O
p-TsOH, PhH,MgSO4, 66%
HOOH
O
H
O
O
O
O
O
Vernolepin/Vernomenin
O
H
O
O
O
O
O
1. DIBAlH, – 76 °C, toluene, DME, 94% O
H
OH
O
O
O
2. Ph3P=CH2, DME, 87%
O
H
OH
OH CO2Me
1. LDA, AcOH, DME then Dowex
2. EtOAc, acetone, CH2N2, 56%
p-TsOH, PhH, !"O
O
OH
HO
O
HO
HO
O
O
HOH
O
O
O
OH
HO
O
HO
HO
O
O
HOH
vernolepin vernomenin
61% 31%
LDA, THFMe2NCH2 I,then MeI, NaHCO3
Ambigiune H
Reintroduced the notion of protecting group free (PGF) synthesis with the completion of (+)- hapalindole Q in 2004.
Synthesized (+)-ambiguine H in 13 steps in a PGF manner. Provoked the chemical community to rethink its approach toward synthesis. Do we rely on protecting groups as a crutch?
Have protecting groups ultimately held us back regarding step efficiency and more importantly development of new methodology?
Phil S. Baran
H
Me
NH
Me
Me
N
C
MeMe
(+)-ambiguine H
Baran, P. S.; Maimone, T. J.; Richter, J. M. Nature, 2007, 446, 404.
Ambigiune H
O
Me
MeOH
Me
1. ClCOCCl3, Zn ether, sonication
2. NaOMe, MeOH 61%
H
MeOH
Me
H
CO2Me
OMe
1. DIBALH, DCM
2. MsCl, pyr, then AcOH/H2O, 73%
MeOMs
H
1. NaI, acetone2. DBU, THF, !, 87%
O
Me
Me
H
LiHMDS, THF,Cu(II), –78 °C, 50%
NH
Br
H
Me
NH
BrO
Me
Pd(II), NaOCHO,65%
H
Me
NH
OMe
MeH
Me
NH
Me
MeNaBH3CN,NH4OAc
HCO2H, CDMT,COCl2, 60%
N
C
Ambigiune H
H
Me
NH
Me
Me
N
C
t-BuOClprenyl-9-BBN
H
Me
N
Me
Me
N
60%
ClMe
Me
B
H
Me
NH
Me
Me
N
C
Cl
H
Me
N
Me
Me
N
Cl
BMe
Me
H
Me
N
Me
Me
N
Cl
R
B
H
H
Me
N
Me
Me
N
Cl
R
B
H
H
Me
NH
Me
Me
N
C
MeMe
h!, TEA,PhH, 41%
(+)-ambiguine H
Recap
Urea: Gave birth to our field
Tropinone: Demonstrated retrosynthetic analysis and was one of the first examples of a biomimetic synthesis.
Reserpine: Illustrated the power of diasteroselective transformations and taught us a lesson about thermodynamic engineering.
Progesterone: Exemplified how a bioinspired transformation can be applied in the lab efficiently and aslo the utility of the Johnson-Claisen.
PGA2: Gave us the TBS protecting group and demonstrated how it can be used to control reactivity.
Vernolepin: Demonstrated the concept of the synergistic diene in the Diels–Alder reaction. Danishefskyʼs Diene has inspired other useful variations.
Ambigiune H: Reminded us why we should strive to develop protecting group free syntheses. Presented an innovative example of said concept.