The Periodic Table & Element Classes. The Periodic Table is the organization of known elements...
-
Upload
thomas-marsh -
Category
Documents
-
view
222 -
download
0
Transcript of The Periodic Table & Element Classes. The Periodic Table is the organization of known elements...

The Periodic Table & Element
Classes

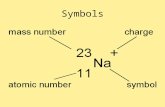
The Periodic Table is the organization of known elements arranged by increasing atomic number (# of protons).
This design was based on research by Henry Moseley (23 November 1887 – 10 August 1915)

The first periodic table was created by Dmitri Mendeleev a Russian chemist and inventor.
Using the table he predicted the properties of elements yet to be discovered!

Group or Family Period

CA StandardsCA Standards
Students know how to use the periodic table to identify alkali metals, alkaline earth metals, transition metals, metals, semimetals (metalloids), nonmetals, halogens and noble gases.

Metals

Properties of Metals
Shiny—metallic luster
Malleable—can be hammered or rolled into sheets
Ductile—can be stretched into fine wires
Tensile strength— resists breaking
Most are solid at room temperature (except mercury)
Good conductors of electricity and heat

Alkali Metals

Alkali MetalsAlkali Metals• NEVER found pure in nature NEVER found pure in nature
because they are too reactivebecause they are too reactive
• Reactivity of these elements Reactivity of these elements increases down the groupincreases down the group
• Group 1= Have 1 valence Group 1= Have 1 valence electronelectron
Potassium, K reacts with water and must be stored in kerosene

Alkaline Earth Metals

Alkaline Earth Metals• less reactive than alkali metals
• not found pure in nature; they are too reactive
• The word “alkaline” means “basic” – common bases include salts of the metals
• Ca(OH)2
• Mg(OH)2
• Group 2 = 2 valence electrons

Transition Metals

Transition Transition MetalsMetals
(still a metal just transitioning to non-metal)
Copper, Cu, is a relatively soft metal, and a very good electrical conductor.
Mercury, Hg, is the only metal that exists as a liquid at room temperature

Metaloides

Properties of Metalloids
• have properties of both metals and nonmetals.
• more brittle than metals, less brittle than most nonmetallic solids
• semiconductors of electricity
• Some metalloids possess metallic luster

Silicon, Si – A MetalloidSilicon, Si – A Metalloid
• Silicon has metallic luster• Silicon is brittle like a
nonmetal• Silicon is a semiconductor
of electricity
Other metalloids include:
Boron, B Germanium, Ge Arsenic, As Antimony, Sb Tellurium, Te

Nonmetals

Nonmetals- 20% of elements
• Brittle
• Gases at room temperature (except: bromine= liquid, iodine, sulfur, selenium, phosphorus &carbon are all solid)
• Poor conductors of electricity and heat
• HYDROGEN is a nonmetal
Carbon, the graphite in “pencil lead” is a great example of a nonmetallic element.

Examples of Nonmetals
Sulfur, S, was once known as “brimstone”
Microspheres of phosphorus, P, a reactive nonmetal
Graphite is not the only pure form of carbon, C. Diamond is also carbon; the color comes from impurities caught within the crystal structure

HalogensHalogens• never found pure in nature; they
are too reactive
• in their pure form are diatomic molecules (F2, Cl2, Br2, and I2)
• Group 7= 7 valence electrons
Chlorine is a yellow-green poisonous gas

Noble Gases

Noble Gases
• They are ONLY found pure in nature – they are chemically unreactive!!
• Colorless, odorless and unreactive; they were among the last of the natural elements to be discovered
• Group 8 = 8 valence electrons (except helium, which has only 2)

• Complete Periodic Table ActivityComplete Periodic Table Activity
• Matter and Change Review WorksheetMatter and Change Review Worksheet
• Chapter 1 Quiz Chapter 1 Quiz

1.1. Get colored pencils from the back and open Get colored pencils from the back and open your book to pg 17.your book to pg 17.
2.2. Color and label each section of the periodic Color and label each section of the periodic table that we discussed today.table that we discussed today.
3.3. Make a key at the top of your chart. Make a key at the top of your chart.