Smog
-
Upload
gueste38f2d -
Category
Education
-
view
1.840 -
download
2
Transcript of Smog

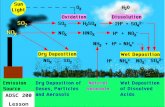
SMOGSMOG

Reactions in reducing SmogReactions in reducing Smog
The gas mainly responsible is sulfur dioxide.The gas mainly responsible is sulfur dioxide.
It is called a reducing smog because the sulfur It is called a reducing smog because the sulfur dioxide reduces other gases in the atmosphere dioxide reduces other gases in the atmosphere producing a range of other pollutants.producing a range of other pollutants.
At the same time the sulfur dioxide is oxidized At the same time the sulfur dioxide is oxidized to sulfur trioxide which dissolves in water to prto sulfur trioxide which dissolves in water to produce a mist or smog.oduce a mist or smog.

Reducing SmogReducing Smog
This can happen in 2 ways:This can happen in 2 ways:
Heterolytic catalyst. Heterolytic catalyst.
Free radical catalysts.Free radical catalysts.

Heterolytic catalyst. Heterolytic catalyst. Unburnt hydrocarbons or metal ions in the air Unburnt hydrocarbons or metal ions in the air
are called particulates are called particulates
Oxygen atoms can absorb these particulates, Oxygen atoms can absorb these particulates, weakening the bond and catalyzing their reactweakening the bond and catalyzing their reaction with SO2 into SO3ion with SO2 into SO3..

SO2(g) + O2(g)SO2(g) + O2(g) -------- SO3(g) SO3(g)

It then reacts with water in the It then reacts with water in the atmosphere to form acidic atmosphere to form acidic
smogsmogSO3(g) + H2O(l)SO3(g) + H2O(l) H2SO4(aq)H2SO4(aq)

The free radicals is produced by the fissiThe free radicals is produced by the fission of a nitrogen dioxide molecule in the on of a nitrogen dioxide molecule in the presence of UV lightpresence of UV light
Which in turns produce oxygen free radiWhich in turns produce oxygen free radicals.cals.
NO2(g) --NO(g) + O*(g)NO2(g) --NO(g) + O*(g)
Free radical catalysts. Free radical catalysts.

These highly reactive oxygen atThese highly reactive oxygen atoms can react directly with SO2. oms can react directly with SO2.
SO2(g) +O* --- SO3(g)SO2(g) +O* --- SO3(g)

This free radical will then catalyze the reThis free radical will then catalyze the reaction of either sulfur dioxide or nitrogeaction of either sulfur dioxide or nitrogen oxides n oxides
Giving off sulfuric or nitric acid mist/smoGiving off sulfuric or nitric acid mist/smog g