revison on redox-2
-
Upload
mrsmpc -
Category
Technology
-
view
1.192 -
download
3
description
Transcript of revison on redox-2

Camp science part 2
chemistry july 2011

Q6.RUSTING= SM 2007 no.6
(a)(i) State the conditions for the rusting of iron.
P
PRESENCE OF WATER AND OXYGEN

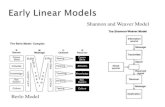
(ii) labelled diagram the rusting of iron.
Anode:
Fe Fe2+ + 2e
Cathode :
O2 + 2H2O + 4e 4OH-
2
O2 drop of water
A piece of iron
O2O2
rust
1
2
3
4 e
e
Fe 2+

(b) (i) Describe the reactions that occur after the Fe 2+ and OH- ions are
formed.
•Fe 2+ combine with OH- to form iron(II)hydroxide //
Fe 2+ + 2 OH- Fe(OH)2
•Fe(OH)2 is oxidised by air to form hydrated iron (III) oxide
•Fe2O3.xH2O is called rust

(ii) State the change in the oxidation number of iron in
6(b)(i)
Fe(OH)2 to iron(III)oxide
+ 2 to +3

c(i) Explain how the zinc plates protect the iron ship from rusting.
• Zinc is more electropositive than iron
• Zinc atom release electron to form ion zinc
• So zinc protects iron from rusting
• So iron does not rust

(ii) Write the half equation for the reaction in 6(c)(i).
Zn Zn +2 + 2e


SPM 2006
SECTION B ( NO 7)

(a) The following are the formulae of two compounds.
Al2O3 Cu2O
(i) Based on the two formulae, state the oxidation number for aluminium and copper. [2 marks]

Al2O3 = 02Al + 3O =02Al + 3(-2) =02Al = +6Al =+3
Cu2O = 02Cu + O =02Cu -2 =02Cu = -2Cu =+1
oxidation number of Al is +3oxidation number of copper is +1

(ii) Name both the compounds based on the IUPAC nomenclature system. [2 marks]
Compound IUPAC nomenclatre system
Al2O3 Aluminium Oxide
Cu2O Copper(I) Oxide

(iii) Explain the difference between the names of the two compounds based on the IUPAC nomenclature system.
[2 marks]

• Copper is a transition element , so has more than one oxidation number
• Copper(I) oxide uses a Roman number
• Aluminium has only one oxidation number ,so Aluminium does not use a Roman number
Answer:

(b) Diagram 7 shows the set up of the apparatus for an experiment to investigate electron transfer through a solution.
Diagram 7

(i) Name the oxidation agent in the
experiment. [1 mark]
Acidified Potassium manganate(VII) solution
Answer:

(ii) Write the half equations for the reactions that occur at the negative andpositive terminals. [5 marks]

Half equation at positive terminalMnO4
- + 8H+ + 5e- Mn2+ + 4H2O
Half equation at negative terminalFe2+ Fe3+
+ e-

(iii) Based on your answer in 7(b)(ii), describe the oxidation and reduction processes in terms of the electron transfer that occurs at the negative and positive terminals.
State also the changes that can be observed after 10 minutes. [8 marks]

At the negative terminal :
• Each Fe2+ donates one electron to become Fe3+
• Oxidation process takes place
• The donated electron are taken up by MnO4
- which is reduced to Mn2+ and water
Answer :

At the positive terminal :• MnO4
- ion accept electron and is reduced to Mn2+ and water
• In this process, reduction has take place

At the positive terminal• The purple colour of KMnO4 becomes lighter
or colourless • purple MnO4
- decrease in quantity because all of ions are changed to colourless Mn2+ ion
Changes after 10 minutes

At the negative terminal :• The green colour of iron(II) sulphate turns
brown.• This is due to the formation of iron(III)
sulphate which is brown in colour

End of part 2 camp science















![Unit 7: Redox Reactions and Electrochemistry Chemistry/Chapter 19 Redox Reactions … · Chapter 19: Redox Reactions and Electrochemistry ... (+6) + 4(−2) = −2] Note: Same Atom](https://static.fdocuments.in/doc/165x107/5a72fc927f8b9ab6538e143d/unit-7-redox-reactions-and-electrochemistry-chemistrychapter-19-redox-reactions.jpg)