Report Biodiesel
-
Upload
prasanna-shetti -
Category
Documents
-
view
314 -
download
0
Transcript of Report Biodiesel

MINI PROJECT 6th SemesterDepartment Of Automobile Engineering, B.V.B.C.E.T. Hubli.
2009 2010
By,Prasanna.ShettiPraveen.MadiwalarSuresh.KaralattiMahammad.Wasim

Department of
AUTOMOBILE ENGINEERING
CERTIFICATE
This is to certify that the project work entitled “PREPERATION OF BIODIESEL AND
TESTING”, a bonafide work carried out by Prasanna.Shetti, of 6th semester MINI PROJECT in
Automobile Engineering, for the academic year 2009-2010. It is certified that all the
corrections/suggestions have been incorporated in the report deposited in the department
library. The project report has been approved as it satisfies the academic requirement in
respect of project work prescribed for the 6th semester Automobile Engineering curriculum.
Prof. Gireesh.N.M Prof. T. Veeramahantesh Dr. Ashok Shettar
Swamy
(PROJECT GUIDE) (HEAD OF DEPARTMENT) (PRINCIPAL)
Name of the examiner: Signature with date
1.
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 2

2. ACKNOWLEDGMENT
Behind the success of any task, there will be sincere and valuable suggestions
and co-operation of eminent people and we do take immense pleasure to
acknowledge them.
The progress would not have taken shape in uniform discipline manner, with
the guidance of our beloved, Shri T.Veeramahantesh Swamy, head of the
department of Automobile engineering, and Shri Gireesh.N.M who is constant
source of our inspiration and who rendered valuable help in channelizing our
efforts in right direction. Our special thanks to them, their thoughts and guidance
are reflected in every page of our report.
We take this opportunity to express our sincere thanks to our beloved
principal Dr. Ashok Shettar, for providing the required facilities.
We thank Mechanical Department, for providing infrastructure, without
which we would have not completed our project in the stipulated time.
We would like to thank all the staff of Automobile engineering department
for their valuable co operation in under taking this project.
Last, but not the least, we express our affectionate thanks to our parents,
friends and all those who have helped us directly and indirectly during this course
of our dissertation.
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 3

Project Title:
PREPERATION OF BIODIESEL AND TESTING
TEAM MEMBERS:
Sl No. NAME ROLL No. USN1 Prasanna.Shetti 28 2BV07AU0312 Praveen.Madiwalar 30 2BV07AU0333 Suresh.Karalatti 50 2BV07AU0564 Mahammad.Wasim 70 2BV05AU012
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 4

CONTENTSChapter-1 Introduction
1.1 Alternate fuel for internal combustion engine
1.2 Vegetable Oil resource in India
1.3 Objective of the study
Chapter-2 Review of literature
2.1 Properties of biodiesel
2.2 Advantage of biodiesel
Chapter-3 Experimental setup
3.1 Transesterification setup
3.2 Setup for Testing of oil
Chapter-Result and Discussion
4.1 Properties of fuel
4.2 Fuel testing
CONCLUSION.
REFERENCE.
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 5

CHAPTER 1.0
INTRODUCTIONEnergy is an essential requirement for economic and social
development of any country. Petroleum based fuels have become important for a country’s development in the modern and fast moving world. Currently more than 80% of the fuel consumed by internal combustion engines in the transport, agriculture and other applications is drawn from petroleum sources in the form of petrol, diesel oil, heavy oils and natural gas. It is well known that these petroleum reserves are limited and will exhaust in the long run. Due to fuel shortages, there has been a steep rise in the cost of petroleum products in recent years. India imports crude oil at a cost of approximately Rs. 2,00,000 crores in foreign exchange every year. Even 5% replacement of petroleum fuel by bio-fuel can help India save Rs.10,000 crores per year in foreign exchange.
The demand for petroleum products in 1990-91 was 58.87 Million Metric Tons (MMT). The crude oil imports by India have shown considerable increase during this decade. During 2001-2007, the crude oil imports were 69.4, 87.9, 90.4, 95.9, 99.4 and 110 MMT respectively. It is expected to reach 115 million tons in 2007-2008. By the year 2010 this import percentage is likely to increase to 82%. Diesel is mainly consumed in the transport, industrial and agricultural sectors. Furthermore, the ratio of diesel fuel to gasoline fuel consumption in India is 7:1 and with the Golden Quadrilateral project there will be further addition of the vehicles on the road. The increase in the use of
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 6

petroleum products will also proportionately increase the risks of health hazards. The harmful gases emitted by the burning of fossil fuels will pose a big threat to the environment. The leakage of crude oil during transportation spells disaster for both the flora and fauna of the area and the cleaning operations are expensive. Thus, India faces the major challenge of meeting the high demand of oil to meet the growing energy needs.
The increasing price factor coupled with increased awareness about environmental degradation has prompted governments and scientific community world over to look for suitable alternative fuels. During the last decade the use of alternative fuels for diesel engines has received renewed attention. It is important to explore the feasibility of substitution of diesel with an alternative fuel, which can be produced within the country on a massive scale for commercial utilization. As far as our country is concerned, the need to search for alternative fuels is more urgent, as India is heavily dependent upon the import of crude oil to meet its demands for automotive, agricultural and power sectors.
Moreover continuous utilization of fossil fuels have been greatly polluting and thereby harming our environment. One such alternative fuel for C I Engine application is use of vegetable oils. Vegetable oils are renewable, biodegradable and clean burning fuels.
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 7

Alternative Fuel for Internal combustion Engine:
In this context, a few fuels that are gaining prominence are as follows;
1. Biodiesels
2. Compressed Natural gas (CNG)
3. Biogas
4. Producer gas
5. Liquefied Petroleum Gas (LPG)
6. Hydrogen
7. Alcohol fuels (Methanol and Ethanol)
1.2 Vegetable Oil Resources in India:
Research in this direction with vegetable oils has yielded encouraging results. Vegetable oils present a very promising alternative to diesel oil since they are renewable and have similar properties. Many researchers have studied the use of vegetable oils in diesel engines. Vegetable oils offer almost the same power output with slightly lower thermal efficiency when used in diesel engine. Reduction of engine emissions is a major research aspect in engine development with the increasing concern on environmental protection and the stringent exhaust gas regulation (EGR).
The alternative liquid fuels must be technically and environmentally acceptable and economically competitive. The prime liquid alternative fuels are alcohols and vegetable oils. Both alcohols and vegetable oils are derived from biomass i.e. from renewable fuel compared to diesel fuel; vegetable oils have acceptable heating value
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 8

and ignition quality. Vegetable oil is a triglyceride, which are esters of long chain fatty acids and polyalcohol glycerol. Vegetable oils typically have large molecules, with carbon, hydrogen and oxygen being present. The typical molecular structures of diesel and vegetable oil are presented in Fig 1.1. Vegetable oils and their methyl esters are such alternative renewable fuels for compression ignition engines. Inherent properties of vegetable oils make them suitable for use in diesel engines solely with an acceptable loss in efficiency. Vegetable oils have comparable energy density and cetane number to mineral diesel. Vegetable oils are considered as good alternatives to diesel as their properties are close to diesel. Thus, they offer the advantage of being able to be readily used in existing diesel engines without any modifications. They have a structure similar to diesel fuel, but differ in the type of linkage of the chains and have a higher molecular mass and viscosity. The presence of oxygen in vegetable oils raises the stoichiometric fuel air ratio. The carbon residue of vegetable oil is higher than diesel fuel.
DIESEL
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 9

Vegetable oil
Fig. 1.1 Typical molecular structure of diesel and vegetable oil
1.3 Objectives of the study
To prepare biodiesel from palm oil and sunflower oil known as and determine its different properties.
To test the flash point and fire point of biodiesel To test the viscosity of the biodiesel Compare the biodiesel with diesel
CHAPTER 2 Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 10

REVIEW OF LITERATURE The internal combustion engine designed, built, and
demonstrated by Rudolf Diesel at the 1900 Paris World's Fair ran on peanut oil. This was the product of his dream-an efficient internal combustion engine, powered by crude oil or even vegetable oil. He believed that the engine that can be fed with vegetable oils would help considerably in the development of agriculture in countries, which use it. A lot of research work has been done with neat vegetable oils and biodiesel blends. Research on vegetable oils like Pongamia Pinnata and rice Bran oil is done in various laboratories all over the world.
For large scale production there are two steps that would need to be taken for producing biodiesel on a large scale — growing the feedstock, and processing them into biodiesel. The main issue that is often contested is whether or not we would be able to grow enough crops to provide the vegetable oil (feedstock) for producing the amount of biodiesel that would be required to completely replace petroleum as a transportation fuel. So, that is the main issue that will be addressed here. The point of this article is not to argue that this approach is the only one that makes sense, or that we should ignore other options. Rather, the point is merely to look at one option for producing biodiesel, and see if it would be capable of meeting our needs. For any biodiesel to succeed at replacing a large quantity of petroleum, the yield of fuel per acre needs to be as high as possible.
2.1 Properties of biodiesel
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 11

Biodiesel fuel is reliable, renewable, biodegradable and non-toxic.
It contains practically no sulfur and substantially reduces emissions of unburned hydrocarbon (HC), carbon monoxide, sulfates, polycyclic aromatics and particulate matter.
It has fuel properties comparable to diesel and can be used in standard diesel engines with minor or no modifications at all.
It can be produced from any kind of vegetable oil.
2.2 Advantages of BIODIESEL
Biodiesel reduces carbon dioxide emissions, carbon monoxide (CO) emissions, sulfur dioxide emissions, hydrocarbons (HC) and other particulate matter (PM) emissions.
Biodiesel can be used in existing diesel engine with few or no modifications.
Non-toxic and Biodegradable
Biodiesel use helps to stabilize greenhouse gases.
When biodiesel is used in place of conventional diesel, auto ignition, fuel consumption, power output, and engine torque are relatively unaffected.
CHAPTER 3
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 12

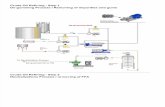
EXPERIMENTAL SET UPThe present work involves the Transesterification process to
obtain vegetable oil biodiesel and experimental investigations of properties of palm oil and sunflower oil biodiesel.
3.1 Transesterification set up
The Transesterification set up consists of a 2 liter capacity round bottom flask with three necks to it and is placed in a water container for heating the oil [Fig 3.1]. A heater with temperature regulator was used for heating the oil in the round bottom flask. A high speed motor with a magnetic stirrer in the form of rotating element was used for mixing the oil vigorously. In the Transesterification process triglycerides of vegetable oil react with methyl alcohol in the presence of a catalyst (NAOH/KOH) to produce fatty acid ester and glycerol.
In this process 1000 ml of vegetable oil, 200 ml methanol and 4 gm sodium hydroxide were taken in a round bottom flask. All the contents were heated up to 70 0C and stirred by the magnetic stirrer vigorously for one hour when the ester formation begins. The mixture was transferred to a separating funnel and allowed to settle down under gravity for overnight. The upper layer in the separating funnel forms the ester and the lower layer being glycerol was removed from the mixture [Fig 3.3]. The separated ester was mixed with 250 gm of hot water and allowed to settle under gravity for 24 hours. Water washing removes the fatty acids and catalyst dissolved in the lower layer and was separated [Fig 3.4].
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 13

Fig 3.1 Equipment for Transesterification
Fig3.2 Methanol, Sodium hydroxide Sodium Sulfate and Silica gel
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 14

Fig 3.3 Ester precipitation
Fig 3.4 Ester separation and biodiesel washing
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 15

Pensky Marten apparatus
Red wood viscometer
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 16

Procedure to carry out testing of viscosity of biodiesel:
1. Apparatus is cleaned and leveled
2. Oil is poured into the cup upto the mark.
3. Water is heated with the help of the heater.
4. When steady state is reached, a clean dry 50ml flask is placed below the jet properly.
5. The ball valve is lifted and the stop watch is started simultaneously.
6. Time taken for collecting 50cc of oil is noted down and tabulated.
Procedure to carry out testing of Flash and fire point of biodiesel:
1. Cup and accessories is well cleaned.
2. Cup is filled with oil upto the mark and thermometer is inserted.
3. Heat is increased at a rate of 3-6degree/min with continuous stirring at the rate of 1-2 rev/sec.
4. Test flame is brought near the cup surface for observing the phenomenon.
5. Flash point is taken as the temperature when the flash appears at any point to the surface of oil inside the cup.
6. The temperature at which vapor burns continuously for 5 second is noted down as the fire point.
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 17

CHAPTER 4
RESULT AND DISCUSSION
4.1 Viscosity of fuel
1. Palm oil Biodiesel
Sl No. Temperature of oil in 0C
Weight of empty jar`w1` grams
Weight of oil with jar`w2` grams
Weight of oil `w2-w1` in grams
Time taken to collect 50c.c oil in `t` seconds
1 45 73.7 116.7 43 402 48 73.7 114.2 40.5 393 51 73.7 113.9 40.2 37
Density oil`ρ` in gram/c.c
Specific gravity `S`
Kinematic viscosity in centiStokes
Absolute viscosity in centiPoise
Relative viscosity in %
0.86 0.88 6.1 5.2 7.190.81 0.88 5.7 4.6 70.80 0.88 5.2 4.16 6.65
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 18

2. Sunflower oil
Sl No. Temperature of oil in 0C
Weight of empty jar`w1` grams
Weight of oil with jar`w2` grams
Weight of oil `w2-w1` in grams
Time taken to collect 50c.c oil in `t`seconds
1 40 73.7 119.7 46 462 43 73.7 116.0 42.3 453 47 73.7 115.3 41.8 42
Density oil`ρ` in gram/c.c
Specific gravity `S`
Kinematic viscosity in centiStokes
Absolute viscosity in centiPoise
Relative viscosity in %
0.92 0.92 8.2 7.5 8.640.84 0.92 7.9 6.6 8.450.83 0.92 6.8 5.6 7.89
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 19

4.2 Flash and Fire point of fuel
1. Palm oil Biodiesel
Sl no.
Temperature in 0C
Flash point Fire point
1 90 ----- -----
2 100 ----- -----
3 110 ----- -----
4 120 ----- -----
5 130 ----- -----
6 140 ----- -----
7 150 ----- -----
8 160 ----- -----
9 162 YES -----
10 164 ----- -----
11 166 ----- -----
12 168 ----- -----
13 170 ----- -----
14 172 ----- YES
15 174 ----- -----
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 20

2. Sunflower oil biodiesel
Sl no.
Temperature in 0C
Flash point Fire point
1 120 ----- -----
2 130 ----- -----
3 140 ----- -----
4 150 ----- -----
5 160 ----- -----
6 166 ----- -----
7 168 YES -----
8 170 ----- -----
9 172 ----- -----
10 174 ----- -----
11 176 ----- -----
12 178 ----- -----
13 180 ----- -----
14 182 ----- YES
15 184 ----- -----
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 21

Formulae required:
1. Density of oil, ρ=(W2-W1)/50 in grams/c.c2. Kinematic viscosity=0.0026*t-1.71/t in centistokes3. Absolute viscosity= Kinematic viscosity*Density of oil in centiPoise4. Specific gravity of oil=S5. Relative viscosity=S*t*100/535*0.915
Where,
ρ is density of oil.
W1 is weight of empty jar.
W2 is weight of jar with oil.
t is time taken to collect 50ml of oil.
S is specific gravity.
Specimen calculations:
1. Density of oil ρ=(W2-W1)/50=0.86 grams/c.c2. Kinematic viscosity=0.0026*t-1.71/t = 6.1 centistokes3. Absolute viscosity= Kinematic viscosity*Density of oil=5.2 cpoise4. Specific gravity of oil=S=0.885. Relative viscosity= S*t*100/535*0.915=7.19%
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 22

Comparison of diesel with the biodiesel
Properties Diesel Biodiesel of sunflower oil
Biodiesel of palm oil
Chemical formula C13H24 ----- -----
Density (Kg/m3) 830 920 860
Viscosity (cSt) 4.95 8.2 6.1
Flash point 56 168 162
Fire point 66 182 172
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 23

CONCLUSIONDevelopment of Experimental Hardware
Necessary hardware developed for the Transesterification of sunflower oil and palm oil was adequate to meet the project work.
Transesterification of sunflower oil and palm oil
The quantity of catalyst and methyl alcohol used is crucial for successful reaction. Washing is necessary to drain away undesired contaminants in the biodiesel. The biodiesel should be finally heated to remove excess moisture.
The optimum values of the parameter that result in highest biodiesel yield(98%) are 1% NaOH catalyst, 5:1 molar ratio of methyl alcohol, 60 minutes and 700C respectively.
Testing of Biodiesel
The properties of biodiesel prepared by sunflower oil and palm oil were comparable to that of standard diesel. The viscosity of biodiesel is reduced almost ten times from 42.24 centiStokes to 6.1 centiStokes for palm oil and from 50 centipoise to 8.2 centiPoise and also volatility is improved.
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 24

REFERENCES1. N.R. Banapurmath and P.G. Tewari- "Comparative Performance of
some esters of edible and non-edible oils in a compression ignition engine".15th ISME International Conference on Advances in Mechanical Engineering, March 18-20, 2008, Bhopal, M.P. India.
2. R K Malhotra, IOC R&D centre-Biodiesel as blending component for diesel products.
3. Celeste Peltier-Transesterification, turning used vegetable oil into clean burning biodiesel fuel.
4. Altin Recep, Cetinkaya Selim, and Yucesu Huseyin Serdar (2001)-The potential of using vegetable oil fuels as fuel for diesel engines. Energy Conversion and Management, 42, 529-538.
5. Hideki Fukuda, Akihiko Kondo and Hideo Noda (2001)-Biodiesel fuel production by Transesterification of oils. Cobe University Japan.
6. V. Lertsathapornsuk, P. Ruangying, R. Pairintra and K.Krisnangkura Continuous Transesterification of Vegetable Oils by Microwave irradiation. School of Energy and Materials. King Mongkut's University of Technology Thonburi, Bangkok, Thailand.
7. http://en.wikipedia.org
8. http://www.engineeringtoolbox.com
Automobile Department, B.V.B College Of Engineering And Technology, Hubli Page 25