BT5 DOC Biodiesel Report
-
Upload
shwet-kumar -
Category
Documents
-
view
10 -
download
1
description
Transcript of BT5 DOC Biodiesel Report
-
PRODUCTION OF BIODIESEL BY TRANSESTERIFICATION OF DIFFERENT PLANT OILS
PROJECT REPORT
Submitted in partial fulfillment of the Requirements for the award of the degree of
Bachelor Of Technology
In Biotechnology
By
G. SINDHU (07241A2320)
M. SWETHA (07241A2324)
DEPARTMENT OF BIOTECHNOLOGY
Gokaraju Rangaraju Institute Of Engineering And Technology
(Affiliated to Jawaharlal Nehru Technological University)
Hyderabad
2011
-
DEPARTMENT OF BIOTECHNOLOGY
Gokaraju Rangaraju Institute Of Engineering And Technology
(Affiliated to Jawaharlal Nehru Technological University)
Hyderabad
CERTIFICATE This is to certify that the project entitled
PRODUCTION OF BIODIESEL BY TRANSESTERIFICATION OF DIFFERENT PLANT OILS
has been submitted by
G. SINDHU (07241A2320) M. SWETHA (07241A2324)
In partial fulfillment of the requirements for the award of degree of Bachelor
of Technology in Biotechnology from Jawaharlal Nehru Technological University, Hyderabad.
The results embodied in this project have not been submitted to any other University or Institution for the award of any degree or diploma.
Internal Supervisor Head of Department Ramesh Dr. D. Sailaja Asst Professor Professor & HOD Dept of Biotechnology Dept of Biotechnology
-
CERTIFICATE
This is to certify that Ms G. SINDHU, Ms M. SWETHA, students of final year Biotechnology of Gokaraju Rangaraju Institute Of Engineering And Technology, affiliated to Jawaharlal Nehru technological university have completed a project work entitled PRODUCTION OF BIODIESEL BY TRANSESTERIFICATION OF DIFFERENT PLANT OILS at our biotechnology division of laboratory Gokaraju Rangaraju Institute Of Engineering And Technology, Bachupally, Hyderabad. Head of Department External Examiner Dr. D. Sailaja Professor & HOD Dept of Biotechnology
-
CONTENTS
Acknowledgements Abstract Contents
1. Introduction 1.1 What is biodiesel? 1.2 Benefits of biodiesel 1.3 Biodiesel production 1.4 Advantages and disadvantages of biodiesel
2. Biodiesel production from Jatropha Curcus
2.1 Introduction about Jatropha Curcus 2.2 Production of biodiesel by transesterification process 2.3 Materials and methods 2.4 Results and discussions
2.5 Conclusion
3. Biodiesel production from Vegetable oil 3.1 Introduction about vegetable oil 3.2 Methanol transesterification of vegetable oil 3.3 Materials and methods 3.4 Results and discussion 3.5 Conclusion
-
A C K N O W L E D G E M E N T There are many people who have helped us directly or indirectly in the successful completion of our project. We would like to take this opportunity to thank one and all. First of all we would like to express our deep sense of gratitude towards our project guide Mr Ramesh, for always being available whenever we require his guidance as well as for motivating us through out the project work. We are also grateful to the Dr. D.Sailaja, (H.O.D) for allowing us to do the project in GRIET and for her valuable guidance during our project, which helped me in the successful completion of my project. We would like to thank all our friends for their help and constructive criticism during our project period. Finally, we are very much indebted to our parents for their moral support and encouragement to achieve higher goals. We have no words to express our gratitude and still we are very thankful to our parents who have shown us this world and for every support they gave us. Signature Signature G. SINDHU (07241A2320) M.SWETHA (07241A2324)
-
WHAT IS BIODIESEL?
Biodiesel is an alternative fuel similar to conventional or fossil diesel. Biodiesel can be produced from straight vegetable oil, animal oil/fats, tallow and waste cooking oil. The process used to convert these oils to Biodiesel is called transesterification. This process is described in more detail below. The largest possible source of suitable oil comes from oil crops such as rapeseed, palm or soybean. In the UK rapeseed represents the greatest potential for biodiesel production. Most biodiesel produced at present is produced from waste vegetable oil sourced from restaurants, chip shops, industrial food producers such as Birdseye etc. Though oil straight from the agricultural industry represents the greatest potential source it is not being produced commercially simply because the raw oil is too expensive. After the cost of converting it to biodiesel has been added on it is simply too expensive to compete with fossil diesel. Waste vegetable oil can often be sourced for free or sourced already treated for a small price. (The waste oil must be treated before conversion to biodiesel to remove impurities). The result is Biodiesel produced from waste vegetable oil can compete with fossil diesel. More about the cost of biodiesel and how factors such as duty play an important role can be found here.
WHAT ARE THE BENEFITS OF BIODIESEL?
Biodiesel has many environmentally beneficial properties. The main benefit of biodiesel is that it can be described as carbon neutral. This means that the fuel produces no net output of carbon in the form of carbon dioxide (CO2). This effect occurs because when the oil crop grows it absorbs the same amount of CO2 as is released when the fuel is combusted. In fact this is not completely accurate as CO2 is released during the production of the fertilizer required to fertilize the fields in which the oil crops are grown. Fertilizer production is not the only source of pollution associated with the production of biodiesel, other sources include the esterification process, the solvent extraction of the oil, refining, drying and transporting. All these processes require an energy input either in the form of electricity or from a fuel, both of which will generally result in the release of green house gases. To properly assess the impact of all these sources requires use of a technique called life cycle analysis. Our section on LCA looks closer at this analysis. Biodiesel is rapidly biodegradable and completely non-toxic, meaning spillages represent far less of a risk than fossil diesel spillages. Biodiesel has a higher flash point than fossil diesel and so is safer in the event of a crash.
-
BIODIESEL PRODUCTION
As mentioned above biodiesel can be produced from straight vegetable oil, animal oil/fats, tallow and waste oils. There are three basic routes to biodiesel production from oils and fats:
Base catalyzed transesterification of the oil. Direct acid catalyzed transesterification of the oil. Conversion of the oil to its fatty acids and then to biodiesel.
The Transesterification process is the reaction of a triglyceride (fat/oil) with an alcohol to form esters and glycerol. A triglyceride has a glycerine molecule as its base with three long chain fatty acids attached. The characteristics of the fat are determined by the nature of the fatty acids attached to the glycerine. The nature of the fatty acids can in turn affect the characteristics of the biodiesel. During the esterification process, the triglyceride is reacted with alcohol in the presence of a catalyst, usually a strong alkaline like sodium hydroxide. The alcohol reacts with the fatty acids to form the mono-alkyl ester, or biodiesel and crude glycerol. In most production methanol or ethanol is the alcohol used (methanol produces methyl esters, ethanol produces ethyl esters) and is base catalysed by either potassium or sodium hydroxide. Potassium hydroxide has been found to be more suitable for the ethyl ester biodiesel production, either base can be used for the methyl ester. A common product of the transesterification process is Rape Methyl Ester (RME) produced from raw rapeseed oil reacted with methanol.
The figure below shows the chemical process for methyl ester biodiesel. The reaction between the fat or oil and the alcohol is a reversible reaction and so the alcohol must be added in excess to drive the reaction towards the right and ensure complete conversion.
The products of the reaction are the biodiesel itself and glycerol.
-
A successful transesterification reaction is signified by the separation of the ester and glycerol layers after the reaction time. The heavier, co-product, glycerol settles out and may be sold as it is or it may be purified for use in other industries, e.g. the pharmaceutical, cosmetics etc.
Straight vegetable oil (SVO) can be used directly as a fossil diesel substitute however using this fuel can lead to some fairly serious engine problems. Due to its relatively high viscosity SVO leads to poor atomisation of the fuel, incomplete combustion, coking of the fuel injectors, ring carbonisation, and accumulation of fuel in the lubricating oil. The best method for solving these problems is the transesterification of the oil.
The engine combustion benefits of the transesterification of the oil are:
Lowered viscosity Complete removal of the glycerides Lowered boiling point Lowered flash point Lowered pour point
-
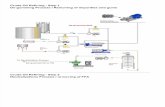
PRODUCTION PROCESS
An example of a simple production flow chart is proved below with a brief explanation of each step.
Mixing of alcohol and catalyst The catalyst is typically sodium hydroxide (caustic soda) or potassium hydroxide (potash). It is dissolved in the alcohol using a standard agitator or mixer. Reaction. The alcohol/catalyst mix is then charged into a closed reaction vessel and the oil or fat is added. The system from here on is totally closed to the atmosphere to prevent the loss of alcohol. The reaction mix is kept just above the boiling point of the alcohol (around 160 F) to speed up the reaction and the reaction takes place. Recommended reaction time varies from 1 to 8 hours, and some systems recommend the reaction take place at room temperature. Excess alcohol is normally used to ensure total conversion of the fat or oil to its esters. Care must be taken to monitor the amount of water and free fatty acids in the incoming oil or fat. If the free fatty acid level or water level is too high it may cause problems with soap formation and the separation of the glycerin by-product downstream.
Separation Once the reaction is complete, two major products exist: glycerin and
biodiesel. Each has a substantial amount of the excess methanol that was used in the reaction. The reacted mixture is sometimes neutralized at this step if needed. The glycerin phase is much more dense than biodiesel phase and the two can be gravity separated with
-
glycerin simply drawn off the bottom of the settling vessel. In some cases, a centrifuge is used to separate the two materials faster.
Alcohol Removal Once the glycerin and biodiesel phases have been separated, the excess alcohol in each phase is removed with a flash evaporation process or by distillation. In others systems, the alcohol is removed and the mixture neutralized before the glycerin and esters have been separated. In either case, the alcohol is recovered using distillation equipment and is re-used. Care must be taken to ensure no water accumulates in the recovered alcohol stream.
Glycerin Neutralization The glycerin by-product contains unused catalyst and soaps that are neutralized with an acid and sent to storage as crude glycerin. In some cases the salt formed during this phase is recovered for use as fertilizer. In most cases the salt is left in the glycerin. Water and alcohol are removed to produce 80-88% pure glycerin that is ready to be sold as crude glycerin. In more sophisticated operations, the glycerin is distilled to 99% or higher purity and sold into the cosmetic and pharmaceutical markets.
Methyl Ester Wash Once separated from the glycerin, the biodiesel is sometimes purified by washing gently with warm water to remove residual catalyst or soaps, dried, and sent to storage. In some processes this step is unnecessary. This is normally the end of the production process resulting in a clear amber-yellow liquid with a viscosity similar to petrodiesel. In some systems the biodiesel is distilled in an additional step to remove small amounts of color bodies to produce a colorless biodiesel.
Product Quality Prior to use as a commercial fuel, the finished biodiesel must be analyzed using sophisticated analytical equipment to ensure it meets any required specifications. The most important aspects of biodiesel production to ensure trouble free operation in diesel engines are:
Complete Reaction Removal of Glycerin Removal of Catalyst Removal of Alcohol Absence of Free Fatty Acids
-
ADVANTAGES OF BIODIESEL
It is made from renewable resources. It performs just as well as the normal diesel fuel. It causes less pollution as compared to diesel-powered engines. It is relatively less inflammable compared to the normal diesel. It can be mixed with normal diesel fuel. It is biologically degradable and reduces the danger of contamination of soil and
underground water during transport, storage and use. It contains no sulphur, the element responsible for acid rain. There are no extra costs for the conversion of engines in comparison to other
biological fuels. It is suitable for catalytic convertor. Engines last longer when using it. Its refineries are comparitively simpler and environmental-friendly in design than
typical petrochemical refineries. It produces 78% less carbon dioxide (CO2) than normal diesel fuel. It has a higher cetane and lubricity rating than pure petroleum-based diesel fuel,
which improves engine efficiency and operating life cycle.
DISADVANTAGES OF BIOFUELS
Growing crops for biofuel absorbs the carbon that biofuels emit, but it does not absorb the fossil fuel emissions created in planting, fertilizing, treating, harvesting, transporting and processing these crops before they can be converted into fuel. There are also considerable carbon emissions from the coal or gas required to heat the required raw materials in the manufacturing process. Its production can also lead to environmental destruction. Brazil, for example, produces ethanol from sugar cane but to do so is cutting down the Amazon rain forest, thus causing great damage to the environment.
Too much concentration on running vehicles on plant oil would set up a direct competition between feeding the cars and feeding the people. This would not increase our self-reliance but would increase our food and energy vulnerability.
-
BIODIESEL PRODUCTION FROM
JATROPHA CURCAS
-
JATROPHA CURCUS
INTRODUCTION Jatropha curcus is a drought-resistant perennial, growing well in marginal/poor soil. It is easy to establish, grows relatively quickly and lives, producing seeds for 50 years. Jatropha the wonder plant produces seeds with an oil content of 37%. The oil can be combusted as fuel without being refined. It burns with clear smoke-free flame, tested successfully as fuel for simple diesel engine. The by-products are press cake a good organic fertilizer, oil contains also insecticide. It is found to be growing in many parts of the country, rugged in nature and can survive with minimum inputs and easy to propagate. Medically it is used for diseases like cancer, piles, snakebite, paralysis, dropsy etc. Jatropha grows wild in many areas of India and even thrives on infertile soil. A good crop can be obtained with little effort. Depending on soil quality and rainfall, oil can be extracted from the jatropha nuts after two to five years. The annual nut yield ranges from 0.5 to 12 tons. The kernels consist of oil to about 60 percent; this can be transformed into biodiesel fuel through esterification. Family: Euphorbiaceae Synonyms: Curcas purgans Medic. Vernacular/common names: English- physic nut, purging nut; Hindi - Ratanjyot Jangli erandi; Malayalam - Katamanak; Tamil - Kattamanakku; Telugu - Pepalam; Kannada - Kadaharalu; Gujarathi - Jepal; Sanskrit - Kanana randa.
Distribution and habitat
It is still uncertain where the centre of origin is, but it is believed to be Mexico and Central America. It has been introduced to Africa and Asia and is now culti-vated world-wide. This highly drought-resistant spe-cies is adapted to arid and semi-arid conditions. The
current distribution shows that introduction has been most successful in the drier regions of the tropics with annual rainfall of 300-1000 mm. It occurs mainly at lower altitudes (0-500 m) in areas with average an-nual temperatures well above 20C but can grow at higher altitudes and tolerates slight frost. It grows on well-drained soils with good aeration and is well adapted to marginal soils with low nutrient content.
Botanical Features It is a small tree or shrub with smooth gray bark, which
-
exudes a whitish colored, watery, latex when cut. Normally, it grows between three and five meters in height, but can attain a height of up to eight or ten meters under favourable conditions.
Leaves
It has large green to pale-green leaves, alternate to sub-opposite, three-to five-lobed with a spiral phyllotaxis.
Flowers The petiole length ranges between 6-23 mm. The inflorescence is formed in the leaf axil. Flowers are formed terminally, individually, with female flowers usually slightly larger and occurs in the hot seasons. In conditions where continuous growth occurs, an unbalance of pistillate or staminate flower production results in a higher number of female flowers.
Fruits
Fruits are produced in winter when the shrub is leafless, or it may produce several crops during the year if soil moisture is good and temperatures are sufficiently high. Each inflorescence yields a bunch of approximately 10 or more ovoid fruits. A three, bi-valved cocci is formed after the seeds mature and the fleshy exocarp dries.
Seeds The seeds become mature when the capsule changes from green to yellow, after two to four months. Flowering and fruiting habit
The trees are deciduous, shedding the leaves in the dry season. Flowering occurs during the wet season and two flowering peaks are often seen. In permanently hu-mid regions, flowering occurs throughout the year. The seeds mature about three months after flowering. Early growth is fast and with good rainfall conditions nursery plants may
-
bear fruits after the first rainy season, direct sown plants after the second rainy season. The flowers are pollinated by insects especially honey bees.
Ecological Requirements Jatropha curcas grows almost anywhere , even on gravelly, sandy and saline soils. It can thrive on the poorest stony soil. It can grow even in the crevices of rocks. The leaves shed during the winter months form mulch around the base of the plant. The organic matter from shed leaves enhance earth-worm activity in the soil around the root-zone of the plants, which improves the fertility of the soil. Regarding climate, Jatropha curcas is found in the tropics and subtropics and likes heat, although it does well even in lower temperatures and can withstand a light frost. Its water requirement is extremely low and it can stand long periods of drought by shedding most of its leaves to reduce transpiration loss. Jatropha is also suitable for preventing soil erosion and shifting of sand dunes.
Biophysical limits
Altitude: 0-500 m, Mean annual temperature: 20-28 deg. C, Mean annual rainfall: 300-1000 mm or more. Soil type: Grows on well-drained soils with good aeration.
-
PRODUCTION OF BIODIESEL BY TRANSESTERIFICATION OF JATROPHA OIL USING IMMOBILIZED
Pseudomonas fluorescens
ABSTRACT Transesterification of vegetable oils is an important reaction that produces fatty acid alkyl esters, methyl and ethyl esters which are excellent substitutes for diesel fuel. Biodiesel prepared by catalyzed mild tranesterification has become of much current interest for alternative fuel production. In the present study the ability of a commercial immobilized Pseudomonas fluorescens to catalyze the transesterification of Jatropha oil and methanol was investigated. The cell of P. fluorescens was easily immobilized within the sodium alginate during batch process. The important parameters like temperature, pH, reaction time and amount of beads was studied. From the study it was found that maximum yield of biodiesel was obtained at the optimum conditions of at 40 , pH of 7.0, reaction time 48hrs and amount of beads 3g. the physical properties of the products were analyzed and the results were compared with the other sources of oils like used vegetable oils.
-
INTRODUCTION For more than two centuries, the worlds energy supply has relied heavily on non-renewable crude oil derived liquid fuels. Out of which 90% is estimated as to be consumed for energy generation and transportation. It is also known that emissions from the combustion of these fuels such as CO2, CO, NOx and sulfur containing residues are the principal causes of global warming. On the other hand, known crude oil reserves could be depleted in less than 50years at the present rate of consumption. Thus, increased environmental concerns, tougher clean air act standards necessitates the search for a viable alternative fuels, which are environmentally friendly. Oil seed crops such as palm, soyabean, sunflower, peanut, olive etc are by far the largest group of exploitable renewable biomass resource for liquid fuel and energy generation. The attractive features of bio-diesel fuel are:
- It is a plant derived, not petroleum derived, and such its combustion does not increase current net atmospheric levels of CO2, a greenhouse gas.
- It can be domestically produced, offering the possibility of reducing petroleum imports.
- It is biodegradable. - Relative to conventional diesel fuel, its combustion products have reduced
level of particulates, carbon monoxide, sulfur oxides, hydrocarbons etc. - Vegetable oils can be used in diesel engines as they have high octane
number and calorific value very close to diesel.
Transesterification of vegetable oils is an important reaction that produces fatty acid alkyl esters that are valuable intermediates in oleo chemistry, and methyl and ethyl esters which are excellent substitutes for diesel fuel. Transesterification as an industrial process is usually carried out by heating an excess of the alcohol with vegetable oils under different reaction conditions in the presence of an inorganic catalyst. The most commonly used catalysts are alkali hydroxides and alcoholates. Transesterification is also possible under acidic conditions, but this process requires higher reaction temperatures. Chemical methanolysis using an alkali catalysis process gives high conversion levels of triglycerides to their corresponding methyl esters in short reaction times, the reaction has several drawbacks: it is energy intensive, recovery of glycerol id difficult, the alkaline catalyst has to be removed from the product, alkaline wastewater requires treatment, and free fatty acids and water interee with the reaction. Pseudomonas species immobilized with sodium alginate gel can be used directly as a whole cell biocatalyst. In the present study, Pseudomonas fluroscence cells immobilized within sodium alginate gel as a whole cell biocatalyst was utilized for biodiesel fuel production from Jatropha oil.
-
MATERIALS The non-edible crude Jatropha oil was purchased commercially and was stored at 4 to avoid rancidity of the vegetable oil. Its quality characteristics were determined according to the standard methods of fats and oils published by the association of oil chemists, which has the density of 0.92g/ , acid value of 19.635mg KOH, saponification value of 187gm KOH and free fatty acid of 17.25mg KOH/g and it was used throughout the experimentation.
P. fluorescens, was obtained from Microbial Type Culture Collection and Gene Bank. The culture was maintained on nutrient agar medium. After three days incubation at 25 the agar slants were stored at 4 .The liquid medium for the growth of inoculums for bacteria was nutrient agar medium composed of 1.0 g/l of beef extract, 2.0 g/l of yeast extract, 5.0 g/l of NaCl.
-
INOCULA PREPARATION Inocula were grown aerobically in 250ml Erlenmeyer flasks containing the above mentioned medium at 25 in an environmental shaker at 200rpm for 24h.
Active cells were centrifuged in a clinical centrifuge (1200rpm), washed with sterile water, and were used as inoculum. IMMOBILIZATION OF Pseudomonas fluorescence CELLS BY ENTRAPMENT The sodium alginate entrapment of cells was performed according to the standard method. Alginate solution with a concentration range of 0.5 10% was used or the cell immobilization and was prepared by dissolving sodium alginate in boiling water and autoclaved at 121 for 15min. Both alginate slurry and cell suspension was mixed and stirred for 10min to get uniform mixture. The alginate solution was extruded drop by drop into a cold sterile 0.2m CaCl2 solution through a sterile 5ml pipette from 5cm height and kept for curing at 4 for 1h. the beads were hardened by resuspending into a fresh CaCl2 solution for 24h at 4 with gentle agitation. Finally these beads were washed with distilled water to remove excess calcium ions and unentraped cells. When the beads are not being used, they are preserved in 0.9% sodium chloride solution in the refrigerator.
-
METHANOLYSIS OF JATROPHA OIL Methanolysis reactions were conducted at stoichiometric molar ratio of oil/methanol; oil and methano were poured into the reaction flask and heated to the reaction temperature with constant shaking using magnetic stirrer for 48h.
In subsequent experiments, in which the effect of molar ratio of oil/methanol was investigated, the volume of the oil is kept constant and the volume of methanol is varied. Around 3ml of hexane is added to the reaction mixture to increase the solubility of the reactants. The appropriate amount of immobilized whole cells based on oil weight was added to the flask. After 48h of reaction time, the reaction was stopped and the cells were removed from the reaction mixture by filtration.
The produced ester and byproduct glycerol were separated using separate funnel. The biodiesel and glycerol were collected and stored.
-
EFFECT OF TEMPERATURE Temperature is one of the importance parameter for the production of biodiesel because the rate of reaction is strongly influenced by the reaction temperature. The effect of temperature on biodiesel production from Jatropha oil using immobilized cells of P. fluorescens was studied by conducting experiments at different temperatures 25, 30, 35, 40, 45 keeping initial cell concentration of 3g of beads, substrate concentraton of 50ml of oil (1:3 molar ratio oil/methanol) with n-hexane (3ml), reaction time of 48h and pH of 7.0 were fixed constant. Temperature 25 30 35 40 45 Yield 40% 55% 65% 70% 60%
Maximum yield of 70% biodiesel was obtained at temperature 40 and was found decreasing after 40 due to denaturation of enzyme. The optimum temperature of the maximum yield of biodiesel was fixed as 40 .
0
10
20
30
40
50
60
70
80
0 10 20 30 40 50 60
Percentageyeild
Percentageyeild
-
EFFECT OF PH pH of the reaction media is the another important parameter affecting the yield of biodiesel. In this experiment, the pH of the reaction media was varied from 5.5 to 7.5. the effect of pH on biodiesel production of Jatropha oil using immobilized cells of P. fluorescens was studied by conducting experiments at different pH 5.5, 6.0, 6.5, 7.0 and 7.5. pH 5.5 6.0 6.5 7.0 7.5 Yield 40% 50% 60% 70% 55%
The yield of biodiesel was found to be increasing and a maximum 70% was obtained at pH 7.0 and the yield of biodiesel was found to be decreasing when the pH was increased beyond 7.0.
0
10
20
30
40
50
60
70
80
0 2 4 6 8
PercentageYeild
PercentageYeild
-
EFFECT OF REACTION TIME Effect of time on biodiesel production from Jatropha oil using immobilized cells of P. fluorescens was studied by conducting experiments with different periods of 12, 24, 36, 48 and 60h. experiments were carried out at th optimum temperature 40 , pH 7.0, immobilized cell concentration of 3g beads and substrate concentration of 50ml of oil. Reaction time
12hrs 24hrs 36hrs 48hrs 60hrs
Yield 40% 50% 60% 70% 65%
Yield of biodiesel increases till 48h and thereafter decreases. Further increase in the reactiontime does not have effect on the production of biodiesel.
01020304050607080
0 10 20 30 40 50 60 70
Percentageyeild
Percentageyeild
-
EFFECT OF AMOUNT OF BEADS The effect of amount of immobilized beads on production of biodiesel from jatropha oil using immobilized cells of P. fluorescens was studied by conducting experiments at different amounts 1, 2, 3, 4 and 5g at constant levels of substrate concentration of 50ml of oil (1:3 molar ratio of oil/methanol) and n-hexane (3ml) at optimum temperature 40 , pH 7.0 and reaction time 48h. Amount of beads
1gms 2gms 3gms 4gms 5gms
Yield 32% 45% 70% 65% 60%
The percentage of yield of biodiesel increases till 4g and thereafter decreases, so 4g was chosen as the optimum amount of beads. The maximum yield of biodiesel is 70%.
01020304050607080
0 1 2 3 4 5 6 7
PercentageYeild
PercentageYeild
-
ANALYSIS
OPTIMUM
MAXIMUM PERCENTAGE YIELD
Temperature
40
70%
pH
7
70%
Reaction time
48hrs
70%
Amount of beads
4gms
70%
Transesterification reaction was carried out using jatropha oil and short chain alcohol (methanol on hexane) using immobilized cells of P. fluorescens. The various parameters affecting biodiesel yield namely temperature, Ph, reaction time and amount of beads. The maximum yield of 70% was obtained at optimum values of temperature 40 , pH 7, reaction time 48h and amount of beads 4g and molar ratio of oil to alcohol 1:4. The biodiesel produced was analysed for its physical properties and compared with the values of petroleum based diesel. The specific gravity, flash and fire point, cloud and pour point, kinematic viscosity are slightly lower than that of diesel, whereas the diesel index is much higher and the smoke point is slightly lower.
-
BIODIESEL FROM USED VEGETABLE OIL
-
VEGETABLE OIL
Vegetable oil is an alternative fuel for diesel engines and for heating oil burners. For engines designed to burn diesel fuel, the viscosity of vegetable oil must be lowered to allow for proper atomization of the fuel, otherwise incomplete combustion and carbon build up will ultimately damage the engine. Many enthusiasts refer to vegetable oil used as fuel as waste vegetable oil (WVO) if it is oil that was discarded from a restaurant or straight vegetable oil (SVO) or pure plant oil (PPO) to distinguish it from biodiesel.
PROPERTIES
The main form of SVO/PPO used in the UK is rapeseed oil (also known as canola oil, primarily in the United States and Canada) which has a freezing point of -10C. However the use of sunflower oil, which gels at around -12C,[7] is currently being investigated as a means of improving cold weather starting. Unfortunately oils with lower gelling points tend to be less saturated (leading to a higher iodine number) and polymerize more easily in the presence of atmospheric oxygen.
Material compatibility
Free fatty acids in WVO can have a detrimental effect on metals. Copper and its alloys, such as brass, are affected. Zinc and zinc-plating (galvanization) are stripped by FFA's and tin, lead, iron, and steel are affected too. Stainless steel and aluminum are generally unaffected.
Temperature effects Some Pacific island nations are using coconut oil as fuel to reduce their expenses and their dependence on imported fuels while helping stabilize the coconut oil market. Coconut oil is only usable where temperatures do not drop below 17 degrees Celsius (62 degrees Fahrenheit), unless two-tank SVO/PPO kits or other tank-heating accessories, etc. are used.
-
HOME HEATING
When liquid fuels made from biomass as used for energy purposes other than transport they are called bioliquids.
With often minimal modification, most residential furnaces and boilers that are designed to burn No. 2 heating oil can be made to burn either biodiesel or filtered, preheated waste vegetable oil. These are generally not as clean-burning as petroleum fuel oil, but if processed at home, by the consumer, can result in considerable savings. Many restaurants will give away their used cooking oil either free or at minimal cost, and processing to biodiesel is fairly simple and inexpensive. Burning filtered Waste Vegetable Oil (WVO) directly is somewhat more problematic, since it is much more viscous, but it can be accomplished with suitable preheating. WVO can thus be an economical heating option for those with the necessary mechanical and experimental aptitude, where fire regulations and insurance policy permit it.
AVAILABILITY Waste vegetable oil
As of 2000, the United States was producing in excess of 11 billion liters of waste vegetable oil annually, mainly from industrial deep fryers in potato processing plants, snack food factories and fast food restaurants. If all those 11 billion liters could be collected and used to replace the energetically equivalent amount of petroleum, almost 1% of US oil consumption could be offset.]Use of waste vegetable oil as a fuel competes with some other uses of the commodity, which has effects on its price as a fuel and increases its cost as an input to the other uses as well.
Pure plant oil (Straight vegetable oil)
Pure plant oil (PPO) (or Straight Vegetable Oil (SVO)), in contrast to waste vegetable oil, is not a byproduct of other industries, and thus its prospects for use as fuel are not limited by the capacities of other industries. Production of vegetable oils for use as fuels is theoretically limited only by the agricultural capacity of a given economy. However, doing so detracts from the supply of other uses of pure vegetable oil.
-
METHANOL TRANSESTERIFICATON OF VEGETABLE OIL
ABSTRACT The result of the investigation on methyl esters obtained on the basis of used vegetable oil are given in this paper. Transesterification reaction conditions that effect yield and purity of the product esters including oil quality, type and concentration of catalyst, temperature and reaction time were examined. Methanol transesterification of different oils at 60 with 1-10 % (v/v) sodium met-oxide was studied, with appropriate percent of sodium met-oxide, temperature 60 and 1 hour. Vegetable oil was sufficiently transesterified and could be used as fuel in diesel engines.
-
INTRODUCTION
Over 45 million tonnes of greenhouse gases are produced every year from the burning of diesel in trucks. Of course we are trying to find new creative ways to save our environment and one way to do this is to use vegetable oil as a renewable source of fuel for transportation and also the use of heating.
There are so many benefits of using this source to replace fossil fuels and some of these include reduced air pollution, reduced greenhouse gas emissions, and conservation of limited fossil fuels. There are two different ways that you can use vegetable oil as a fuel in engines.
The first way is that you can use straight vegetable oil either waste frying oil or fresh- pressed oil, however you will need an extra fuel tank and a system for heating and filtering the oil before it reaches the engine. The reason why you will need this is because pure vegetable oil is too thick to work in the engine unless the oil is heated up.
The other way is to simply convert the vegetable oil into biodiesel which can be used in a diesel engine without any modifications. Biodiesel is a fuel source made from vegetable oil when a chemical reaction occurs between methanol and lye. It can be created either from using waste vegetable oil from the food industry, or you have the other option of using it from fresh-pressed vegetable oil.
This is something that is now being made to sell commercially in thousands of countries all around the world, however with the right equipment and enough time it can also be made right at home. Some of the toxic air pollutants that are reduced include soot, particulates, carbon monoxide, and sulphur oxides, however nitrous oxide emissions may increase slightly.
-
MATERIALS AND METHODS Warm up oil: Pour the oil into the flask and turn the electric heater to low. We need to warm up the oil for a couple of reasons. First, the heating will make the oil less viscous so it will stir more easily. Second, the heat helps the conversion to biodiesel go faster. Temperature:
Using a thermometer bring the temperature to between 120 to 130 which is about 50 .
This will feel hot but not too hot to touch. Instead of electric heater, the flask could be placed in
hot tap water. Weigh out lye: Open the lye bottle, add sodium hydroxide until the balance reads 5.0g. Be sure to close the bottle of lye as you get right amount weighed. Lye will absorb water out of the air making it heavier and less effective. Pour methanol into NaOH: Take the lye in a bottle. Open the methanol bottle and pour some methanol into the bottle of lye. Continue adding methanol until the bottle is about full. Dissolve NaOH in the methanol: Close the lid. Tip the bottle back and forth until the NaOH dissolves. The NaOH reacts with the methanol to form a powerful substance called methoxide (C ONa).
-
The bottle will get warm as the NaOH dissolves. Place flask on magnetic stirrer:
The flask can now be placed on the magnetic stirrer. Be sure to put the magnetic bar in the flask. To keep the
temperature between 120 to 130 , adjust the temperature. Pour methoxide into oil: Open the methoxide bottle and pour it into the flask containing oil. Pour the remaining methanol into the flask containing the oil and methoxide. This extra methanol helps to convert more of the oil to biodiesel. Stir mixture:
Turn on the magnetic stirrer to mix the methoxide, oil and methanol. The stirrer should not be too fast, the meagnetic
bar may get unstable. Stirring time is normally listed as 2hrs. To get maximum yield of biodiesel, 2hrs is recommended.
Separating funnel: The pear-shaped glassware is called a separating funnel because it is good for separating liquids that split into two layers. In the biodiesel process, the methoxide reacts with the oil and makes two products. One is glycerin and the other is biodiesel.The glycerin will sink to the bottom. A separating funnel makes it easier to drain off the glycerin. Pour product into separating funnel:
After 2hrs of stirring, the obtained product is poured into the separating funnel. In a few minutes, the glycerin will settle down at the bottom of the separating funnel. It will
not be pure glycerin, because some of the sodium hydroxide will be with it.
-
Drain off glycerin: Turn the stopcock so the glycerin drains out. Glycerin can be saved to be used for other purposes. This glycerin has some contamination with NaOH and methanol, but the NaOH is neutralized and the methanol will evaporate. The NaOH can be neutralized with a little lemon juice to get it around a pH of 6 or 7. Add water for washing:
After draining out glycerin, add tap water to the separating funnel. To get rid of any residues of glycerin and NaOH. Mix the water and biodiesel using an air pump. After few
minutes the water and biodiesel gets separated. Drain off biodiesel: The biodiesel is collected in a beaker. The biodiesel is obtained in a Liquid gold colour. The biodiesel is dried, to remove the traces of water. After drying, the biodiesel is ready to be used in a diesel engine.
-
CONFORMATION TEST FOR GLYCEROL
1. Check to make sure the absent-minded professor has set up a pan of boiling water for you. This should be in an "on" fume hood as some chlorine gas will be produced.
2. Set up three test tubes: blank, glycerol std, and your experimental. 3. Add one ml of water to the blank; one ml of 10% glycerol to the standard; and
one ml of your fermentation mixture to the experimental tube. 4. To each, add one ml of Clorox; wait three minutes. 5. Add 4 drops of concentrated (12M) HCl to each tube. 6. Place the three tubes in the boiling water for a minute to drive off
any chlorine gas. 7. Add 200 L (0.2 mL) 5% -naphthol* 8. Add 4 mL of concentrated H2SO4 (take care to not get any of this on
your fingers or clothes!) 9. Shake the tubes carefully to mix; prevent splashing! 10. An emerald green color indicates the presence of glycerol.
-
CONFORMATION TEST FOR BIODIESEL
Clarity Testing Finished Biodiesel for Water
This is a visual inspection of the finished biodiesel. Water free biodiesel will be clear. The common method of testing is to put a sample in a jar and if you can read a newspaper through the biodiesel then it passes the test. For larger batches, the ability to see the bottom of a drum of biodiesel clearly is often used. This is a good test, however it does not detect water that is dissolved in the biodiesel. Water dissolved in biodiesel will be evenly distributed on a molecular level throughout the biodiesel. There is some debate as to the effect of dissolved water on certain types of diesel engines. One important fact is that hot biodiesel will hold more dissolved water than cold biodiesel. This results in hot or warm biodiesel passing the test and cold biodiesel failing the test. It is important to pass the test when the biodiesel is slightly colder than the expected operating temperature.
Cloud Point Testing
This test is performed by placing a sample of finished biodiesel in the freezer and watching it for cloudiness. The temperature at which the biodiesel first turns cloudy is the cloud point. This test is important for winter fuel. It is related to the "Gel Point Test". The cloud point is near the temperature at which your filter will clog from frozen biodiesel.
Gel Point Testing
In this test you place a sample of finished biodiesel in a freezer and record the temperature at which the biodiesel starts to gel. This is an important test for winter fuel. Simply put, you don't drive on biodiesel at temperatures below the gel point.
The Methanol Test from JTF or the 27/3 Test
Neither Triglycerides or Diglycerides are soluable in dry methanol, but Monoglycerides and Biodiesel will dissolve in dry methanol. If significant Triglycerides or Diglycerides present will fall to the bottom of the test. To perform the test add 1 part biodiesel to 9 parts methanol, by volume. On infopop many use 3 mL of biodiesel and 27 mL of methanol and call the test either the Jan
-
Warnqvist test or the 27/3 test. Testing has shown that for the test to appromimate ASTM level conversions the original 25ml of biodiesel and 225ml of methanol need to be used. Triglycerides and Diglycerides will form a precipitant on the bottom. Only a liquid precipitant that collects together to form a bead or bubble that rolls arond on the bottom of the test is significant. If you have a liquid precipitant after about 5 minuites, then your biodiesel is under converted. Your methanol should be dry and you perform this test at room temperature, The test has been found to be temperature sensitive. Hot biodiesel straight off the processor can give false readings as can using cold methanol stored outside in winter.
The pHLip Test
The pHLip test is a commercial test that was designed as a "Firewall" for bad fuel in the biodiesel distribution chain. It is a simple test consisting of a vial of red liquid, to which you add your sample of biodiesel, flip end over end 10 times and wait 10 minutes. It will tell you if your biodiesel is high quality or if it fails for total glycerine, soap content, free glycerine, oxidation and catalyst contamination.