Profiling mRNA of the Graying Human Hair Follicle Constitutes a Promising State-of-the-Art Tool to...
Transcript of Profiling mRNA of the Graying Human Hair Follicle Constitutes a Promising State-of-the-Art Tool to...

Profiling mRNA of the Graying Human HairFollicle Constitutes a Promising State-of-the-Art Toolto Assess Its Aging: An Exemplary ReportEva M.J. Peters1,2, Christiane Liezmann1,2, Katharina Spatz1, Ute Ungethum3, Ralf- Jurgen Kuban3,Maria Daniltchenko1, Johannes Kruse2, Dominik Imfeld4, Burghard F. Klapp1 and Remo Campiche4,5
Determining hitherto uninvestigated and safe targets to halt the aging process is important in our aging society.Graying is a hallmark of the aging process and may be used to identify aging tissue for comparative analysis. Herewe analyzed differential gene expressions between pigmented, gray, and white human scalp skin hair follicles(HFs) from identical donors. Forming intersections between five donors identified 194/192 downregulated and186/177 upregulated genes in gray/white HFs. These included melanogenesis (tyrosinase; tyrosinase-relatedprotein 1)- and melanosome structure (Melan-A; Pmel17)–associated genes and regulation of melanocyte relevanttyrosine kinases. Alongside these expected changes, regulated genes included nonmelanocyte-related genesassociated with aging as well as nonaging-related genes associated with melanocytes. Intriguingly, among them,genes associated with energy metabolism (i.e., glutaminase) and axon guidance (plexin C1) were altered. Theseresults were reflected by pathway analysis and exemplarily confirmed by PCR and immunohistochemical studies.Supplementing cultured HFs with glutamine or plexin C1 revealed biological relevance and pharmacointerven-tional potential of these microarray results in altering the HF aging process. Together, we present intriguing dataobtained from intra-individual sample comparison that suggest the graying HF to be a valid aging model and apromising target for testing therapeutic interventions.
Journal of Investigative Dermatology (2013) 133, 1150–1160; doi:10.1038/jid.2012.462; published online 13 December 2012
INTRODUCTIONSociodemographic data suggest that quality of life in our agingsociety depends crucially on the healthy transition from youthto old age (Crimmins, 2004). A huge industry and growingbranches in medical research—regenerative and preventivemedicine—are driven by and live off the fight against theaging process. However, safe and effective targets for inter-vention are few and we lack good markers for a healthy agingprocess or reliable read-out parameters to effectively and
economically test pharmacological intervention ex vivo(Franceschi et al., 2007).
One obvious and early hallmark of aging is hair graying.A linchpin of the graying process is the hair follicle (HF)melanocyte (Sarin and Artandi, 2007). This neural crest–derived cell population is highly sensitive for oxidative stressdamage (Commo et al., 2004; Arck et al., 2006; Papageorgiouet al., 2008), which leads to depletion of melanocytes from theHFs early on during aging (Nishimura et al., 2005; Arck et al.,2006; Wood et al., 2009). The involved mechanisms not onlyvisibly affect melanocytes (Arck et al., 2006; Wood et al.,2009) but also affect the more numerous and seemingly less-sensitive HF keratinocytes and fibroblasts. The HF melanocytethus somewhat represents a sensor for surrounding damageand changes in the HF aging process. We therefore proposethat the visible loss of pigment is a valid indicator foridentifying aged HFs and HF cell populations such askeratinocytes or fibroblasts for intra-individual comparisonwith less-aged HFs from identical donors in order to identifyaging-related processes, keeping in mind that selective deathof melanocytes may also take place under defined conditions(Wood et al., 2009; Tobin, 2011; Schallreuter et al., 2012).
To identify genes mapping the HF aging process, we choseto use a microarray approach to analyze small amounts ofRNA obtained from HFs of identical donors and in the samehair-cycle stage but with different degrees of pigmentation(Figure 1). Thereby, we took advantage of the fact that
ORIGINAL ARTICLE
1Department of Psychosomatic Medicine, ChariteCenter 12 (CC12) for InternalMedicine and Dermatology, University-Medicine Charite, Berlin, Germany;2Department of Psychosomatic Medicine and Psychotherapy, UniversityHospital Gie�en and Marburg, Gie�en, Germany; 3Charite-Laboratory ofFunctional Genomics, Berlin, Germany and 4DSM Nutritional Products,Personal Care, Basel, Switzerland
Correspondence: Eva M.J. Peters, Psychoneuroimmunology Laboratory, JLUGie�en and Charite Berlin, Ludwigstrasse 76, Gie�en D-35392, Germany.E-mail: [email protected]
5Nee Graub
Received 6 September 2011; revised 21 August 2012; accepted 16 September2012; published online 13 December 2012
Abbreviations: HF, hair follicle; HPRT, hypoxanthine phosphoribosyltransferase; KEGG, Kyoto Encyclopedia of Genes and Genomes; KIT, receptorfor stem cell factor; MET, receptor for hepatocyte growth factor; MIAME,Minimum Information About a Microarray Experiment; ROS, reactive oxygenspecies; sqPCR, semiquantitative PCR; TYR, tyrosinase; TYRP-1 and 2,tyrosinase-related protein 1 and 2
1150 Journal of Investigative Dermatology (2013), Volume 133 & 2013 The Society for Investigative Dermatology

pigmented, gray, and white HFs derived from the same donorshare an identical genetic background and environmentalinfluences. At the same time, they display differential activa-tion of a known and visible aging process—graying (Figure 1).Using this approach we show here, successfully, that grayingHFs are not only characterized by the expected decline ofknown melanocyte functions but also hint at heretoforeunexplored aging mechanisms. We exemplarily explore glu-tamate metabolism and plexin C1 signaling and show datasupporting their role in the aging process, observingchanges in the melanocyte subpopulations as an exemplaryHF cell population. In summary, our results indicate intra-individual tissue comparison as an optimal state-of-the-artmethod to identify key regulators of the HF aging process andconfirm the graying HF as a potential aging model forpharmacological studies.
RESULTSThe study design proves to be reliable and traceable andidentifies differentially expressed probe sets in aging HFs
Gray and white HFs were compared with pigmented HFs ofthe same donor (an example of free multidimensional
condition analysis, present calls, and numbers of regulatedgenes per donor are given in Supplementary Figure S1 onlineand Supplementary S1 and S2 online). Intersections betweenall five investigated donors were then calculated to minimizethe individual background: only genes that were regulated inat least three out of five donors were added to the intersectionlists and taken into final consideration as detailed below. Thisapproach markedly reduced the number of regulated genes forfurther analysis and resulted in the selection of 194 probe setsthat were downregulated comparing gray HFs with pigmentedHFs and 192 probe sets that were downregulated comparingwhite HFs with pigmented HFs. Upregulated were 186 probesets comparing gray HFs with pigmented HFs and 177 probesets comparing white HFs with pigmented HFs(Supplementary Figure S1 online).
Gray and white HFs compared with pigmented displaydownregulation of melanocyte function–associated genes andpathways
As was to be expected, intensive literature survey identified anumber of genes on the intersection lists to have a role inmelanocyte homeostasis and pigment production (Table 1 and
irshs
orspu
dp
hm m hm
dp dp
em
rm
ors ors
irs irs
hs hspu
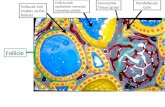
Pigmented Gray White
unpigmented hair shaftno visible melanocytes
clod-like pigment in hair shaftrounded oligo-dendriticmelanocytes in pigmentary-unitdermal papilla becomes visible
ors ors
irshs
hmrm em
hmdp
rm
em
hsirs
ors
irs
pu
hm
fully pigmenteddermal papilla hiddenno single melanocytes
• ••
•
••
••
Figure 1. The top row shows schematic representations of differentiated bulbar melanocyte distribution patterns in different stages of graying. The bottom row
shows corresponding photographs of exemplary hair follicle (HF) bulbs as viewed under the dissection microscope. Hair bulbs shown are in the growth stage of the
hair cycle, anagen VI, when differentiated melanocytes (m) in the pigmentary unit (pu) above dermal papilla fibroblasts (dp) produce pigment to be passed on to
HF keratinocytes of the proximal hair shaft (hs). Keratinocytes also constitute the inner root sheath (irs), the hair matrix (hm), and the outer root sheath (ors). (a) Fully
pigmented hair bulb. (b) Gray (partially pigmented) hair bulb with ectopically differentiating melanocyte precursor (em) and rounded melanocytes in the
pigmentary unit (rm). (c) White (unpigmented) hair bulb. Bar¼ 50mm.
EMJ Peters et al.Profiling the Aging Process
www.jidonline.org 1151

Supplementary Table S2 online). The analysis of biologicalpathways confirmed regulation of melanogenesis-associatedgenes (Kyoto Encyclopedia of Genes and Genomes (KEGG))(Supplementary Table S3 online). The respective microarrayresults investigated could be effectively confirmed by semi-quantitative PCR (sqPCR) and immunohistochemical studies(Figure 2). Markers that were strongly downregulated by arrayanalysis showed the most striking reduction, indicating thatlevels detected by microarray analysis are a good measure foractual regulation.
In detail, we found a stepwise sequential decrease oftyrosinase-related protein 1 (TYRP-1, melanin-processing),tyrosinase (TYR, rate-limiting enzyme for pigment production),
Pmel17 (structural protein central for early melanosomematuration, Hoashi et al., 2005, 2010), Melan-A (Pmel17regulator), KIT (tyrosinekinase receptor for stem cell factor),and MET (tyrosinekinase receptor for hepatocyte growthfactor) (Figure 2): in gray HFs, Pmel17 and MET showed atendency and TYRP-1 was significantly reduced; in white HFs,all markers were reduced with emphasis on TYR, Melan-A,and KIT.
Immunohistochemical analysis confirmed messenger RNA(mRNA) results and identified differential expression indefined melanocyte subpopulations: melanocytes in the hairbulb, which expressed differentiation markers TYR and NKI-beteb (immunohistochemical label for Pmel1), showed
Table 1. Regulated genes that have a role in melanocyte biology
Genesymbol Gene title
MEANFC
Genesymbol Gene title
MeanFC
LIFR Leukemia-inhibitory factor receptor alpha 0.46 APP Amyloid beta (A4) precursor protein (peptidase nexin-II,
Alzheimer’s disease)
0.62
HES1 Hairy and enhancer of split 1, (Drosophila) 0.41 NTRK3 Neurotrophic tyrosine kinase, receptor, type 3 0.51
PTGER3 Prostaglandin E receptor 3 (subtype EP3) 0.58
Gene expression in pigmented hairfollicles serves as control
Upregulated
Downregulated
White
White
Gray
Gray
Pigmented
Fold change min. 1.5 Regulated in at leastthree probands
TYR Tyrosinase (oculocutaneous albinism IA) 3.73 TYRP1 Tyrosinase-related protein 1 116.58
TYRP1 Tyrosinase-related protein 1 2.94 SILV Silver homolog (mouse) 36.21
SILV Silver homolog (mouse) 2.92 TYR Tyrosinase (oculocutaneous albinism IA) 26.36
CAPN3 Calpain 3, (p94) 2.7 MLANA Melan-A 25.02
MLANA Melan-A 2.48 TRPM1 Transient receptor potential cation channel, subfamily M,
member 1
8.58
SLC45A2 Solute carrier family 45, member 2 2.44 SLC45A2 Solute carrier family 45, member 2 5.21
GPR143 G protein-coupled receptor 143 2.42 GPR143 G protein-coupled receptor 143 3.88
MLANA Melan-A 2.34 CAPN3 Calpain 3, (p94) 3.68
TRPM1 Transient receptor potential cation channel,
subfamily M, member 1
2.29 PLXNC1 Plexin C1 3.64
SLC45A2 Solute carrier family 45, member 2 2.1 KIT v-kit Hardy–Zuckerman 4 feline sarcoma viral oncogene
homolog
2.81
PLXNC1 Plexin C1 2.09 PAX3 Paired box 3 2.23
IL6R Interleukin 6 receptor 1.87 OLFM1 Olfactomedin 1 2.06
TSHZ1 Teashirt zinc finger homeobox 1 1.87 MET Met protooncogene (hepatocyte growth factor receptor) 2.04
GPX3 Glutathione peroxidase 3 (plasma) 1.56 HPS1 Hermansky–Pudlak syndrome 1 1.86
OSTM1 Osteopetrosis-associated transmembrane protein 1 1.66
EDNRB Endothelin receptor type B 1.65
The table shows all known melanocyte biology–relevant regulated genes in gray or white hair follicles (HFs) at the time of analysis compared with pigmentedHFs of the same donor, identified by donor intersection as described in Materials and Methods. FC indicates the mean fold change downregulation comparedwith that of pigmented HFs averaged over all five samples in gray and white follicles, respectively, as assessed by microarray analysis.
EMJ Peters et al.Profiling the Aging Process
1152 Journal of Investigative Dermatology (2013), Volume 133

Tyrosinase hs
hs
hs
hs
hs
hs hs hs
hs hs
hs hs
hs hs
hs hs
dp
dp
dp
dp
dp
dpdp
dp
dpdp
dpdp
dp dp
dp dp
dp dp
hs hs
Pmel17
Melan-A
KIT
MET
**n =5;
2.0
2.5
2.0
1.5
1.0
0.5
0.0
2.5
2.0
1.5
1.0
0.5
0.0
RN
A a
mou
nt c
ompa
red
with
con
tent
inpi
gmen
ted
HF
s =
1
2.0
1.5
1.0
0.5
0.0
2.0
1.5
1.0
0.5
0.0
RN
A a
mou
nt c
ompa
red
with
con
tent
inpi
gmen
ted
HF
s =
1
1.5
1.0
0.5
0.02.0
1.5
1.0
0.5
0.0
RN
A a
mou
nt c
ompa
red
with
con
tent
inpi
gmen
ted
HF
s =
1
Pigmented Gray White
P =0.01
Trp1
**
n =3;n =3;
P =0.037P =0.037
*
*
n =4,P =0.037
n =3,P =0.037
*n =6,
P =0.040
*n =4,
P =0.05
Pigmented Gray White
Pigmented Gray White
Figure 2. Microarray confirmation of melanocyte function associated genes in native hair follicles (HFs). sqRNA (donors’ N indicated in figure) and
representative immunofluorescense are shown. Dotted lines highlight hair bulb borders and basement membranes separating the dermal papilla. Arrows point at
melanocytes in the pigmentary unit. (a) Pigmentation: note label in the pigmentary unit only (differentiated) and the growing distance of immunoreactive cell
bodies from the dermal papilla (dp) and orientation toward the hair shaft (hs) (magnified inserts) through which terminally differentiated melanocytes may leave
in the graying HFs. (b) Melanosomes: note punctual staining of Melan-A and the presence Pmel17þ melanocytes in the outer root sheath even in white HFs
(melanocyte precursors, magnified inserts). (c) Tyrosine kinase receptors: note dendricity of KITþ (receptor for stem cell factor) melanocytes only in pigmented
HFs (differentiation, magnified inserts). METþ (receptor for hepatocyte growth factor) melanocytes are found only in pigmented HFs. *Po0.05, **Po0.01.
EMJ Peters et al.Profiling the Aging Process
www.jidonline.org 1153

progressive rounding and loss of dendricity as signs of terminaldifferentiation (Figure 2), whereas melanocytes in the outerroot sheath, where stem and transiently amplifying melano-cyte precursors reside throughout the hair cycle, showed signsof premature maturation (Supplementary Figure S2 online).Similar observations have been reported in mouse skin understress, in which permanent graying occurs because of differ-entiation of melanocyte precursors rather than because of theirapoptosis or induction of senescence (Inomata et al., 2009;Nishimura, 2011).
These observations manifest termination of melanin proces-sing in gray HFs and termination of pigment generation andhandling in white HFs as successively observed duringchronological HF aging. This suggests that pigmented HFscan be considered biologically younger or better equipped tohandle processes that promote aging compared with gray orwhite HFs. Accordingly, we expected other regulated genes toalso relate to individual HF age.
Leads for aging analysis taken from microarray comparison ofgray and white HFs with pigmented HFs of the same donorIn summary, we found that the pathways regulated in gray HFsare suggestive for altered energy utilization and enhancedtissue remodeling, whereas tissue degeneration takes place inwhite HFs. In detail, these pathways included (SupplementaryTable S3 online) the following: gray HFs compared withpigmented HFs (BioCarta)—vascular endothelial growth fac-tor, hypoxia, and angiogenesis, indicating remodeling ofperifollicular vasculature; glutamate metabolism, involved inenergy metabolism; the integrin signaling and cell-to-celladhesion signaling pathways, influencing cellular shape,mobility, and progression through the cell cycle; and thephospholipids as signaling intermediaries, regulating prolifera-tion and survival. KEGG—axon guidance, an indispensablefeature of the neuronal network formation; glycolysis andgluconeogenesis, key pathways of energy metabolism; regula-tion of actin skeleton, crucial for intracellular transport andchange in cell morphology; and ADP-ribosilation factor,indicating regulations of vesicular trafficking and actin remo-deling. White HFs compared with pigmented HFs (BioCarta)—FAS signaling pathway, indicating enhanced apoptosis; reg-ulation of transcriptional activity by promyelocytic leukemiaprotein, indicating regulation of apoptosis-relevant transcrip-tional activity and tumor suppression. KEGG—Alzheimer’sdisease, the most prevalent neurodegenerative disease.
Targeting selected pathways in ex vivo assays for HF aging
We and others have earlier been able to identify oxidativestress as a driving force in the graying process in analogy toaging in general (Arck et al., 2006; Wood et al., 2009).Hypoxia was among the regulated pathways in graying HFsonly. We thus decided to test for the presence of reactiveoxygen species (ROS) and found a significant upregulation ingray HFs only, indicating that these HFs suffer from the highestlevels of oxidative stress potentially promoting prematuremelanocyte death (Arck et al., 2006) (Figure 3).
Among the other regulated pathways, energy metabolismstood out with two altered pathways. We chose to further
investigate glutamate metabolism (Figure 4) and used pigmen-ted anagen HFs from aging individuals brought into organculture as an assay to test potential aging effects. As glutamine,the nondepolarizing precursor of glutamate, is a standardingredient of the HF culture medium, we analyzed culturesprocessed without glutamine as well as cultures with sub-optimal glutamine concentrations above and below thestandard concentration commonly used in cell culture experi-ments (20 and 2,000 mM). We found that HFs treated with theoptimum and standard concentration of 200 mM glutaminemaintained anagen VI—early catagen morphology (growthand early regression stages of the hair cycle) (Table 2,Supplementary Figure S3d online). Accordingly, they retainedhair growth with an elongation rate of over 80% over theirinitial length (Supplementary Figure S3b online). They alsomaintained pigmentation and contained healthy and func-tional melanocyte cell populations with appropriately shapedearly differentiating melanocytes (TYRP-2þ ) and differen-tiated pigmentary-unit melanocytes (NKI-betebþ ) but nomigrating melanocyte precursors (KITþ ) (SupplementaryFigure S3c and d online). Moreover—as important indicatorsfor regulated non–tumor-promoting growth—cell proliferationin general (Ki67þ cells in hair bulb) and of melanocytes(double label with NKI-beteb) together with the expressionof a marker for senescence (p16) was readily detec-table (Supplementary Figure S3e and f online).
In contrast, HFs cultured in the absence of glutaminemaintained only some 50% hair shaft elongation, gained asomewhat mummified appearance resembling anagen VI, anddisplayed enhanced eosinophilia and bending of the hair bulbas signs of degeneration (Supplementary Figure S3a and bonline). However, no TUNELþ apoptotic cells could bedetected in these HFs (not shown), indicating growth arrestin the absence of toxic treatment effects producing a dyingrather than an aging HF.
HFs cultured in the presence of the suboptimal glutamineconcentrations 20 and 2,000 mM showed significant
*Low150
140
130
120
110
100
90
80
High
% A
ntio
xida
tive
capa
city
if c
ontr
ol=
100
Control Pigmented HF Gray HF White HF
n =3, P =0.037
Figure 3. Antioxidative capacity of aging hair follicles (HFs). The test
produces high levels of superoxide, which then causes Pholasin to
luminesce. The lower the level compared with control (buffer), the higher the
antioxidative capacity of the tested probe. Differences between HF samples
and control and between the different grades of pigmentation were calculated.
N¼ 10 HFs per donor, three donors per pigmentation status. *Po0.05.
EMJ Peters et al.Profiling the Aging Process
1154 Journal of Investigative Dermatology (2013), Volume 133

enhancement of HF depigmentation (Supplementary FigureS3c and d online). Depigmentation was associated withenhanced dendricity of hair bulb melanocytes (not shown)
as well as enhanced expression of the migration marker KITand decreased expression of the senescence marker p16,indicating enhanced mobilization of melanocyte precursors
Table 2. Summarizing HF culture experiments with glutamine
Organgrowth Regression Pigmentation
MelC precursoractivation
MelCmigration Proliferation Apoptosis Senescense
Summary of HF biologicaleffect
No
glutamine
þ þ þ þ þ � þ þ � � Growth arrest
20 mM
Glutamineþ þ þ þ þ þ � þ þ þ þ � þ Premature HF regression
200 mM
Glutamine
þ þ þ þ þ þ þ þ þ � þ þ þ � þ þ Obtimal growth condition
keeping the HF ‘young’
2,000 mM
Glutamine
þ þ þ þ þ þ � þ þ þ � � � Stress response and premature
HF aging
Abbreviation: HF, hair follicle.Detailed photomicrographic documentation and statistical analysis are provided in Supplementary Figure S3 online. Changes observed are summarizedhere in arbitrary units. � indicates not detectable. If detectable, intensity/number can range from þ ¼barely detectable to þ þ ¼well detected toþ þ þ ¼ strongly detectable.
Guanine monophosphatesynthetase (GMPS)
Epidermis
1.5*
n =3, P =0.037
n =3,P =0.037
1.0
0.5
0.0
1.5
1.0
0.5
0.0
5.0
4.0
3.0
2.0
1.0
0.0
Pigmented
Glutaminase (GLS)
Glutamate decarboxylase-1(GAD 1)
*
n =3,P =0.037*
Gray White
Pigmented Gray White
Pigmented Gray White
RN
A a
mou
nt c
ompa
red
with
cont
ent i
n pi
gmen
ted
HF
s =
1R
NA
am
ount
com
pare
d w
ithco
nten
t in
pigm
ente
d H
Fs
= 1
RN
A a
mou
nt c
ompa
red
with
cont
ent i
n pi
gmen
ted
HF
s =
1
Pigmented Gray White
Epidermis Pigmented Gray White
Epidermis Pigmented Gray White
epidermis pigmented Gray White
Glutamine
GMPS
GLS
GAD 1
GABA(inhibitory
neurotrans-mitter)
Guaninenucleotides
GTP(DNA/RNAsynthesis,
cell divison)
Glutamine
Glutamate
Glucose(energy:
gluconeo-genesis)
Gluta-thione
(oxidativedefense) NO (iNOS)
(apoptosisand microbial
defense)Urea
(nitrogenexcretion)
�-keto-Glutarate
(citric acid cycle)
No promotionof cell growthand wealth
Blokade ofcell growth
hs hs hs
dp
hs
dp
hs hs
dp dp
cts
cts
hs
dp
hs hs
dpdp
dp dp
Figure 4. Analysis of glutamate metabolism in aging hair follicles (HFs). Semiquantitative messenger RNA (mRNA) and immunohistochemical studies (IHC)
confirm microarray results. (a) GMPS mRNA and IHC are lowest in gray HFs; IHC labels epidermal and outer root sheath (ors) keratinocytes, oligodendritic
epidermal melanocytes, and connective tissue sheath (cts) fibroblasts in gray and white HFs. (b) GLS mRNA and IHC are lowest in gray HFs; IHC labels basal
epidermal and ors keratinocytes, oligodendritic putative melanocytes in the epidermis, and hair bulb ors (arrows in inserts). (c) GAD1 mRNA and IHC are highest
in gray HFs; IHC labels differentiating epidermal and HF keratinocytes and occasional hair bulb melanocytes exclusively in gray HFs. (d) The schematic drawing
indicates upregulation and downregulation of relevant enzymes and also indicates the functional consequences. *Po0.05. cts, connective tissue sheath;
dp, dermal papilla fibroblasts; GABA, gamma-aminobutyric acid; hs, hair shaft.
EMJ Peters et al.Profiling the Aging Process
www.jidonline.org 1155

under suboptimal glutamine supply as is the case under stressconditions and in chronological HF aging (Nishimura et al.,2005; Peters et al., 2011) (Supplementary Figure S3e and fonline).
Targeting individual leads in ex vivo assays for HF aging
As plexin C1 was individually highlighted and also highlightedby the pathway analysis (axon guidance), especially in whiteHFs, we next decided to determine plexin C1 levels usingsqPCR and again confirmed our microarray results(Supplementary Figure S4a online). When cultured HFs weresubmitted to plexin C1 treatment, addition of soluble plexinC1 to the culture medium markedly decreased pigmentation
and melanocyte precursor activation in both tested concentra-tions (5 and 50 ng ml�1). Both concentrations reduced theproliferation of keratinocytes in the hair bulb, but only thehigh concentration terminated proliferation of melanocytes(Table 3, Supplementary Figure S4b–g online). Thus, solubleplexin C1 competing with endogenous plexin C1 for HF-produced ligand is a promotor of premature aging in organ-cultured pigmented HFs.
DISCUSSIONWe here present an intriguing approach to obtain a gene profileof the aging process in human anagen HFs usingintra-individual sample comparison complemented by
Table 3. Summarizing HF culture experiments with plexin C1
Organgrowth Regression Pigmentation
MelC precursoractivation
MelCmigration Proliferation Apoptosis Senescence
Summary of putativebiological effect
No plexin
C1
þ þ þ þ þ þ þ þ þ � þ þ þ � þ þ Good growth condition
5 ngPlexin C1
þ þ þ þ þ � þ þ þ þ þ � � Stops pigmentation andprecursor turnover: aging
50 ng
Plexin C1
þ þ (þ ) þ þ þ þ � þ þ � � � þ Stops proliferation of
melanocytes: regression
Abbreviation: HF, hair follicle.Detailed photomicrographic documentation and statistical analysis are provided in Supplementary Figure S3 online. Changes observed are summarizedhere in arbitrary units. � indicates not detectable. If detectable, intensity/number can range from þ ¼barely detectable to þ þ ¼well detected toþ þ þ ¼ strongly detectable.
Table 4. Antibodies used for immunohistochemistry
Antigen Species Company Staining methodWorkingdilution
Tyrosinase (TYR) Mouse-anti-human NOVO Castra, Berlin, Germany IF (red) 1:25
Tyrosinase-related peptide
1 (TYRP-1/Mel5)
Mouse-anti-human Covance, CA IF (red) double with KIT (green) 1:500
NKI-beteb (Pmel17) Mouse-anti-human Monosan, Uden, The Netherlands IF (green) double with KIT or MET
(red)
1:20
Melan-A Mouse-anti-human Merck/Calbiochem, Darmstadt,
Germany
IF(red) double with KIT (green) 1:200
MET Rabbit-anti-human Santa Cruz Biotechnology,
Santa Cruz, CA
IF (red) double lable with NKI-
beteb (green)
1:50
KIT Rabbit-anti-human Dako, Hamburg, Germany IF (green) double with TYRP-1,
Melan-A; IF (red) double with
NKI-beteb (green)
1:100
Glutaminase (GLS) Mouse-anti-human Sigma, Munich, Germany IF (red) double with KIT (green) 1:100
Tyrosinase-related petide
2 (TYRP-2)
Goat- anti-human Santa Cruz Biotechnology,
Santa Cruz, CA
IF (red) double with NKI-beteb
(green)
1:500
p16 Rabbit-anti-human Santa Cruz Biotechnology,
Santa Cruz, CA
IF (red) double with NKI-beteb
(green)
1:500
Ki67 Rabbit-anti-human Santa Cruz Biotechnology,
Santa Cruz, CA
IF (red) double with NKI-beteb
(green)
1:50
Abbreviations: IF, immunofluorescence; KIT, receptor for stem cell factor; MET, receptor for hepatocyte growth factor.For red label tetramethyl-rhodamine isothiocyante-labeled and for green label Cy2-labeled goat anti-rabbit or goat anti-rat secondary antibodies (1:200; JacksonImmunoResearch, West Grove, PA) were used. Secondary antibodies were added for 1 hour at 37 1C in PBS with 2% normal goat serum. All sections werecounterstained with 40,6-diamidino-2-phenylindole dihydrochloride (DAPI; Boehringer Mannheim, Mannheim, Germany) for identification of cell nuclei.
EMJ Peters et al.Profiling the Aging Process
1156 Journal of Investigative Dermatology (2013), Volume 133

interindividual intersection analysis. With this approach, wedemonstrate that the graying human anagen HF offers itself asan easily accessible sample tissue to determine genes involvedin its aging process. To summarize, our observations suggestthe following: (1) altered expression of melanocyte biology–associated markers is a good measure for biological HF age,and the pathways regulated are indicative of a role in the HFaging process; (2) exemplary targeting the glutamate metabolismshows that an optimal concentration of glutamine appears to becrucial for maintaining hair growth and pigmentation, whereassuboptimal concentrations promote an aging-like process inculture; (3) Similarly, the decline in plexin C1 expression inwhite HFs seems to demarcate the completion of the HF agingprocess, as HFs in culture respond to soluble, endogenousligand-binding plexin C1 with termination of pigmentationand decreased turnover of the melanocyte precursor pool.
To date, transcriptional profiling of HF cells has beenconducted in mice and rats, effectively revealing known andnew receptors and the signaling pathways involved in themaintenance of murine HF stem cells (Morris et al., 2004;Tumbar et al., 2004; Ishimatsu-Tsuji et al., 2005; Trempuset al., 2007; Umeda-Ikawa et al., 2009). Microarray studiesthat use human HFs have so far focused on fossile mRNA(Tochio et al., 2011), compared HF fibroblast populations(Goodarzi et al., 2010; Ariza de Schellenberger et al., 2011),and plugged incomplete HFs (Kim et al., 2006) or HFsexposed to ex vivo pharmacological experiments (Gasparet al., 2010). Here we provide an intra-individual compar-ison using native microdissected HFs with melanocytes asindicators of biological age.
As gray hair is not always associated with other features of theaging process or is generally present in degenerative diseasescharacterized by premature aging, it is not necessarily represen-tative of the aging process as such. However, aging syndromessuch as progeria are always associated with graying, andclinicians frequently observe graying of hair in patients experi-encing acute and chronic inflammatory disease or other endo-genous or environmental stressors that promote aging. Thus,graying may, to some extent, reflect systemic aging processes, asindicated by the regulation of pathways such as hypoxia, energymetabolism, or axon guidance (Peters et al., 2011).
Close scrutiny of the regulated genes suggested reducedenergy production by glycolysis, whereas products of sidepaths are favored that hint at low oxygen tension andpromotion of neurodegenerative disease in the aging tissue(Supplementary Figure S5 online). Similarly, glutamate meta-bolism was diverted in the direction of inhibitory signalingmolecule production and away from cell division. In line withthe observation that altered utilization of sources for energygeneration is a possible mechanism of the aging process(Williams et al., 1993; Kealey et al., 1994), our HF organculture results suggest that an optimal supply of glutamine canhalt the aging process at least in epithelial–mesenchymalorgans, such as HFs. In contrast, activation of the melanocyteprecursor pool by suboptimal glutamine supply impliespossible negative downstream effects such as prematureexhaustion of precursor cell pools and facilitation of malignanttransformation (Qin et al., 2010).
Glutamine processing results in glutamate, the most abun-dant neurotransmitter in vertebrates (Santello and Volterra,2009). Extracellular glutamate can activate receptors such asNMDA or ionotropic glutamate receptors GluR2 and 4(Hoogduijn et al., 2006), which enhance, for example,intracellular calcium levels with multiple consequences forcell growth and apoptosis. In the growing HFs, we wouldexpect premature differentiation of keratinocytes and henceregression (Fischer et al., 2004). Interestingly, mice transgenicfor the glutamate receptor subtype 1 develop melanoma(Abdel-Daim et al., 2010). Thus, excess extracellularglutamate promotes tumor development. However, we didnot observe significant changes in the relevant glutamatereceptors. Instead, we supplemented with glutamine, whichsensitizes some melanoma cell lines to tumor necrosis factor–related apoptosis-inducing ligand-induced apoptosis (Qinet al., 2010). Moreover, our microarray data suggestdecreased levels of calmodulin in white HFs. As calmodulinenhances GAD activity in the presence of calcium (Jin et al.,2005), it potentially feeds into altered gamma-aminobutyricacid production in graying HFs. Altered utilization of energysources in the aging HFs may thus maintain low glutamatelevels and be one of the reasons why malignant transformationis a very rare sight in HFs (Tobin, 2011).
With regard to plexin C1, it appears to counteract theeffects of semaphorin 7a on melanocytes and preventsdevelopment of melanoma (Scott et al., 2009). Its declinein aging HFs and its capacity to induce aging of theHF pigmentary unit by terminating pigmentation andstopping melanocyte precursors from differentiating incultured HFs suggest that its presence in pigmented and grayHFs from aging individuals has an important role in themaintenance of a functionally differentiated unit betweenmature hair bulb melanocytes and hair shaft keratinocytes,halting the HF aging process.
We conclude that whole-mount HF analysis usingpigmentation analysis maps the HF melanocyte agingprocess in a highly selective manner. In this assay, the onlydifference between HFs was the visual function or absence ofmelanocytes, which serves as an internal control to identifyhitherto uninvestigated regulators involved in the HF agingprocess. As the melanocyte subpopulation may well representthe aging process in other HF cell populations such askeratinocytes or fibroblasts, additional targets for futureresearch efforts are provided for by the many more regulatedgenes discovered.
MATERIALS AND METHODSTissue collection and donors
Following the Declaration of Helsinki principles and after obtaining
institutional approval and written informed patient consent, temporal
scalp skin was obtained by elective plastic surgery (face lifting) on
healthy postmenopausal women (51–78 years) and processed as
described before (Philpott et al., 1990; Peters et al., 2006, 2007). In
brief, HFs are plugged out of the subcutis with the help of a pair of
watchmaker forceps after surgically separating the dermis and
subcutis at the dermis–subcutis border. Isolated HFs are B2–3 mm
long and contain the bulb as well as most of the outer and inner root
EMJ Peters et al.Profiling the Aging Process
www.jidonline.org 1157

sheaths, the hair shaft, and the connective tissue sheath between the
bulge and the bulb (compare also HF photomigrographs day 1
Supplementary Figures S3a and S4b online). Approximately 30
anagen VI HFs per donor were sorted into pigmented, gray, and
white HFs under an inverted microscope (compare Figure 1) and
collected in TRIZOL Reagent (Invitrogen, Karlsruhe, Germany) for
microarray or sqPCR or further processed for immunohistochemical
studies and HF organ culture as described below.
RNA isolation
Total RNA was isolated using an RNeasy micro Kit (Qiagen,
Hilden, Germany) according to the manufacturer’s instructions
with slight modifications (Tsui et al., 2006). RNA quantity and
quality were determined using UV-spectrophotometry (NanoDrop
ND-1000, Thermo Scientific, Wilmington, DE) and a bioanalzyer
(LapChip-BioAnalyzer, Agilent Technologies, Santa Clara, CA).
Small sample target preparation
cRNA targets were generated using the small-sample protocol
(Affymetrix, vers. II, Affymetrix, Santa Clara, CA). RNAs were
amplified using T7-(dT)24 primer, DNA-dependent RNA polymerase
(TIB Molbiol, Berlin, Germany), and the Two-Cycle-cDNA-Synthesis
Kit (Affymetrix). Double-strand complementary DNA was directly
subjected to the first round of amplification using the MEGA-Script T7
Kit (Ambion, Darmstadt, Germany) and T4 DNA polymerase
(5 Uml� 1) (Invitrogen). Synthesis of biotin-labeled cRNA was per-
formed using the GeneChip IVT labeling kit (Affymetrix).
GeneChip array hybridization
A volume of 15mg of each cRNA sample was hybridized for
16 hours at 45 1C to an Affymetrix GeneChip Human Genome
U133 Plus 2.0 Array (HG U133 Plus 2.0, 54,000 probe sets
representing B38,500 genes; http://www.affymetrix.com/products_
services/arrays/specific/hgu133plus.affx) according to protocols
recommended by Affymetrix. Probe arrays were scanned at 3-mm/
1.56-mm resolution using the Affymetrix GeneChip System confocal
scanner 2,500/3,000, respectively. Raw data were analyzed
with the Affymetrix GeneChip Operating Software (GCOS 4.1). The
detection P-value of a transcript determines the detection call,
which indicates whether the transcript is reliably detected (Po0.05;
present) or not detected (absent). Data were normalized to a global
intensity of 500.
Data analysis, annotation, and pathway analysis
Data analysis was supported by using Affymetrix GeneChip
Operating Software 4.1 and Agilent Genespring software package.
Data were collected on Excel spreadsheets giving lists of regulated
genes between the white/gray and the pigmented HF transcriptome
for each individual proband (30 lists). Intersection lists were
calculated between all five individuals, and all genes that were
represented in at least three of five individuals were subjected to a
pathway analysis. Probe sets were annotated using NetAFFX analysis
center (http://www.affymetrix.com/analysis/index.affx). Pathway
enrichment and gene ontology categories were retrieved from the
database for annotation, visualization, and integrated discovery
(DAVID Bioinformatics Resources, http://david.abcc.ncifcrf.gov/
home.jsp). Pathway enrichment was calculated with the modified
Fisher’s exact test (EASE) (Dennis, 2003; Hosack et al., 2003).
Data presentation and study design are according to the
Minimum Information About a Microarray Experiment (MIAME) 2.0
(reviewed on http://www.mged.org/Workgroups/MIAME/miame_2.0.
html) guidelines.
Semiquantitative reverse transcriptase PCR
sqPCR exploited the 50-nuclease activity of AmpliTaq Platin (Invitro-
gen) DNA polymerase to cleave a fluorogenic probe designed for the
above transcripts (TipMolBiol, Berlin, Germany), and a fluorogenic
probe for the housekeeping gene hypoxanthine phosphoribosyl
transferase (HPRT) was used to normalize our samples in sqPCR
by calculating the difference between the CT for HPRT and the CT
for the respective transcript as DCT¼CTHPRT�CTtranscript, pooled
as mean DCT per group. The amount of mRNA was calculated as
2�DDCT (DDCT¼DCTpigmentation status�DCTpigmented) and expressed as
the difference from pigmented HF mRNA expression when pigmented
HF mRNA expression equals 1 (Livak and Schmittgen, 2001;
Schmittgen and Livak, 2008). Sequences, reaction conditions, and
primer sizes are available on request.
ROS assay
For measuring the capacity of isolated native HFs to scavenge free
radicals and other oxidants, we used an ABEL antioxidant test kit with
PHOLASIN (ABEL-21M2, Knight Scientific Limited, Plymouth, UK).
The chemiluminescent test kit was used as recommended by the
manufacturer. In brief, Pholasin emits light in the presence of free
radicals such as ROS, oxidants, and certain oxidases. Whole-isolated
HFs were immersed in solution A and PHOLASIN, and solution B was
automatically added by the multimode microplate reader (Mithras LB
940, Berthold Technologies, Bad Wildbad, Germany). Buffer with
only reagents served as the internal control.
Human HF organ cultureAnagen VI HFs were cultured for 7 days in supplemented Williams’ E
medium (Biochrom AG, Berlin, Germany) as published before (Peters
et al., 2005; Arck et al., 2006). HFs were cultured without glutamine
(L-glutamine; Biochrom AG), which is normally a standard in the HF
culture medium at a concentration of 2 mmol l� 1, with glutamine at
three concentrations of 20, 200, and 2,000 mmol l� 1 or with plexin
C1 (recombinant human Plexin C1, R&D Systems, Minneapolis, MN)
at two concentrations of 5 and 50 ng ml� 1 in the presence of
200 mmol l� 1 glutamine. On days 1, 4, and 7, the medium and
supplements were replaced and the pigmentation status and total
length of each HF were recorded. HFs were harvested on day 7 on
histogel-embedding medium (Vector Laboratories, Peterborough, UK)
for immunohistochemical analysis or by rapid snap-freezing for RNA
extraction. Culture experiments were performed on at least three
donor samples per group if not otherwise indicated in the figure
legends.
Routine and immunohistochemistry
Longitudinal cryosections of 8-mm thickness taken from full-thickness
human scalp skin and cultured HFs were processed and analyzed
using a digital image analysis system (AxioVision, Zeiss, Gottingen,
Germany). For TYR, TYRP-1 (detects Mel5 clone T99), TYRP-2,
Melan-A, NKI-beteb (detects Pmel17; Adema et al., 1996), p16,
KIT, MET, and glutaminase expressions were immunohistochemically
EMJ Peters et al.Profiling the Aging Process
1158 Journal of Investigative Dermatology (2013), Volume 133

detected following adapted, established protocols (Tables 1 and 4)
(Muller-Rover et al., 2001; Peters et al., 2005; Arck et al., 2006).
Similarly, for hair-cycle staging and dystrophy screening,
hematoxylin–eosin (Merck, Darmstadt, Germany) staining and detec-
tion of Ki67 (Tables 1 and 4) or TUNEL staining (ApopTag Plus
fluorescein in situ apoptosis detection kit; Chemicon International,
Hampshire, UK) were performed.
Statistical evaluation
Means were calculated and significant differences were determined
by performing a Mann–Whitney U-test for unpaired samples. Sig-
nificance was assumed if P*o0.05 or P**o0.01 and is indicated
accordingly in the figures.
CONFLICT OF INTERESTThe authors state no conflict of interest.
ACKNOWLEDGMENTSWe thank the Clinica Vita, the Meoclinic, and the Schlosspark Clinic, Berlin,Germany, for the highly engaged supply of human material from electiveplastic surgery.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
REFERENCES
Abdel-Daim M, Funasaka Y, Komoto M et al. (2010) Pharmacogenomics ofmetabotropic glutamate receptor subtype 1 and in vivo malignantmelanoma formation. J Dermatol 37:635–46
Adema GJ, Bakker AB, de Boer AJ et al. (1996) pMel17 is recognised bymonoclonal antibodies NKI-beteb, HMB-45 and HMB-50 and by anti-melanoma CTL. Br J Cancer 73:1044–8
Arck PC, Overall R, Spatz K et al. (2006) Towards a ‘‘free radicaltheory of graying’’: melanocyte apoptosis in the aging human hair follicleis an indicator of oxidative stress induced tissue damage. FASEB J20:1567–9
Ariza de Schellenberger A, Horland R, Rosowski M et al. (2011) Cartilageoligomeric matrix protein (COMP) forms part of the connective tissue ofnormal human hair follicles. Exp Dermatol 20:361–6
Commo S, Gaillard O, Bernard BA (2004) Human hair greying is linked to aspecific depletion of hair follicle melanocytes affecting both the bulb andthe outer root sheath. Br J Dermatol 150:435–43
Crimmins EM (2004) Trends in the health of the elderly. Annu Rev PublicHealth 25:79–98
Dennis C (2003) Draft guidelines ease restrictions on use of genome sequencedata. Nature 421:877–8
Fischer M, William T, Helmbold P et al. (2004) Expression of epidermalN-methyl-D-aspartate receptors (NMDAR1) depends on formation of thegranular layer—analysis in diseases with parakeratotic cornification. ArchDermatol Res 296:157–62
Franceschi C, Bezrukov V, Blanche H et al. (2007) Genetics of healthy aging inEurope: the EU-integrated project GEHA (GEnetics of Healthy Aging). AnnN Y Acad Sci 1100:21–45
Gaspar E, Hardenbicker C, Bodo E et al. (2010) Thyrotropin releasinghormone (TRH): a new player in human hair-growth control. FASEB J24:393–403
Goodarzi HR, Abbasi A, Saffari M et al. (2010) MicroRNAs take part inpathophysiology and pathogenesis of male pattern baldness. Mol Biol Rep37:2959–65
Hoashi T, Sato S, Yamaguchi Y et al. (2010) Glycoprotein nonmetastaticmelanoma protein b, a melanocytic cell marker, is a melanosome-specificand proteolytically released protein. FASEB J 24:1616–29
Hoashi T, Watabe H, Muller J et al. (2005) MART-1 is required for the functionof the melanosomal matrix protein PMEL17/GP100 and the maturation ofmelanosomes. J Biol Chem 280:14006–16
Hoogduijn MJ, Hitchcock IS, Smit NP et al. (2006) Glutamate receptors onhuman melanocytes regulate the expression of MiTF. Pigment Cell Res19:58–67
Hosack DA, Dennis G Jr., Sherman BT et al. (2003) Identifying biologicalthemes within lists of genes with EASE. Genome Biol 4:R70
Inomata K, Aoto T, Binh NT et al. (2009) Genotoxic stress abrogates renewalof melanocyte stem cells by triggering their differentiation. Cell 137:1088–99
Ishimatsu-Tsuji Y, Moro O, Kishimoto J (2005) Expression profiling and cellularlocalization of genes associated with the hair cycle induced by waxdepilation. J Invest Dermatol 125:410–20
Jin H, Sha D, Wei J et al. (2005) Effect of apocalmodulin on recombinanthuman brain glutamic acid decarboxylase. J Neurochem 92:739–48
Kealey T, Williams R, Philpott MP (1994) The human hair follicle engages inglutaminolysis and aerobic glycolysis: implications for skin, splanchnicand neoplastic metabolism. Skin Pharmacol 7:41–6
Kim SJ, Dix DJ, Thompson KE et al. (2006) Gene expression in head hairfollicles plucked from men and women. Ann Clin Lab Sci 36:115–26
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data usingreal-time quantitative PCR and the 2(�Delta Delta C(T)) method.Methods 25:402–8
Morris RJ, Liu Y, Marles L et al. (2004) Capturing and profiling adult hairfollicle stem cells. Nat Biotechnol 22:411–7
Muller-Rover S, Handjiski B, van der Veen C et al. (2001) A comprehensiveguide for the accurate classification of murine hair follicles in distinct haircycle stages. J Invest Dermatol 117:3–15
Nishimura EK (2011) Melanocyte stem cells: a melanocyte reservoir in hairfollicles for hair and skin pigmentation. Pigment Cell Melanoma Res24:401–10
Nishimura EK, Granter SR, Fisher DE (2005) Mechanisms of hair graying:incomplete melanocyte stem cell maintenance in the niche. Science(New York, NY) 307:720–4
Papageorgiou N, Carpenter E, Scally AJ et al. (2008) Adult humanepidermal melanocytes for neurodegeneration research. Neuroreport19:1787–91
Peters EM, Hansen MG, Overall RW et al. (2005) Control ofhuman hair growth by neurotrophins: brain-derived neurotrophic factorinhibits hair shaft elongation, induces catagen, and stimulates folli-cular transforming growth factor beta2 expression. J Invest Dermatol 124:675–85
Peters EM, Imfeld D, Graub R (2011) Graying of the human hair follicle.J Cosmet Sci 62:121–5
Peters EM, Liotiri S, Bodo E et al. (2007) Probing the effects of stress mediatorson the human hair follicle: substance P holds central position. Am J Pathol171:1872–86
Peters EM, Stieglitz MG, Liezman C et al. (2006) p75 neurotrophin receptor-mediated signaling promotes human hair follicle regression (catagen).Am J Pathol 168:221–34
Philpott MP, Green MR, Kealey T (1990) Human hair growth in vitro. J Cell Sci97:463–71
Qin JZ, Xin H, Nickoloff BJ (2010) Targeting glutamine metabolism sensitizesmelanoma cells to TRAIL-induced death. Biochem Biophys Res Commun398:146–52
Santello M, Volterra A (2009) Synaptic modulation by astrocytes via Ca2þ -dependent glutamate release. Neuroscience 158:253–9
Sarin KY, Artandi SE (2007) Aging, graying and loss of melanocyte stem cells.Stem Cell Rev 3:212–7
Schallreuter KU, Salem MA, Gibbons NC et al. (2012) Blunted epidermalL-tryptophan metabolism in vitiligo affects immune response and ROSscavenging by Fenton chemistry, part 1: epidermal H2O2/ONOO—mediated stress abrogates tryptophan hydroxylase and dopa decarbox-ylase activities, leading to low serotonin and melatonin levels. FASEB J26:2471–85
EMJ Peters et al.Profiling the Aging Process
www.jidonline.org 1159

Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by thecomparative C(T) method. Nat Protoc 3:1101–8
Scott GA, McClelland LA, Fricke AF et al. (2009) Plexin C1, a receptor forsemaphorin 7a, inactivates cofilin and is a potential tumor suppressor formelanoma progression. J Invest Dermatol 129:954–63
Tobin DJ (2011) The cell biology of human hair follicle pigmentation. PigmentCell Melanoma Res 24:75–88
Tochio T, Tanaka H, Nakata S et al. (2011) Presence of amplifiable mRNA inacellular hair shafts: utilization to analyze gene expression profiles ofblack and white hairs. Int J Dermatol 50:530–4
Trempus CS, Dang H, Humble MM et al. (2007) Comprehensive microarraytranscriptome profiling of CD34-enriched mouse keratinocyte stem cells.J Invest Dermatol 127:2904–7
Tsui NB, Ng EK, Lo YM (2006) Molecular analysis of circulating RNA inplasma. Methods Mol Biol (Clifton, NJ) 336:123–34
Tumbar T, Guasch G, Greco V et al. (2004) Defining the epithelial stem cellniche in skin. Science (New York, NY) 303:359–63
Umeda-Ikawa A, Shimokawa I, Doi K (2009) Time-course expression profilesof hair cycle-associated genes in male mini rats after depilation of telogen-phase hairs. Int J Mol Sci 10:1967–77
Williams R, Philpott MP, Kealey T (1993) Metabolism of freshly isolated humanhair follicles capable of hair elongation: a glutaminolytic, aerobicglycolytic tissue. J Invest Dermatol 100:834–40
Wood JM, Decker H, Hartmann H et al. (2009) Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methioninesulfoxide repair. FASEB J 23:2065–75
EMJ Peters et al.Profiling the Aging Process
1160 Journal of Investigative Dermatology (2013), Volume 133