polymers The most important biological compounds are polymers many Polymers (poly = many) proteins,...
-
Upload
richard-douglas -
Category
Documents
-
view
233 -
download
3
Transcript of polymers The most important biological compounds are polymers many Polymers (poly = many) proteins,...
The most important biological compounds are polymers
Polymers (poly = many)
The polymers are: proteins, carbohydrates, lipids (fats), and nucleic acids (DNA/RNA).
A polymer is made up of a chain of many monomers linked together
MONOMERS (mono = one)
Monomers are: amino acids, sugars, fatty acids, and nucleotides.
These are made (dehydration synthesis) or broken down (hydrolysis) over and over in living cells.
Large polymers are also called _______________ macromolecules
Macromolecules are formed by _________________, usually by reactions involving the loss of water = ________________________.
joining monomers
DEHYDRATION SYNTHESIS
____________ are joined together during dehydration synthesis.
Chains of monomers are called _________
MONOMERS
POLYMERS
Note: enzymes that speed up dehydration synthesis reactions are called _____________.dehydrogenases
Note: enzymes that speed up hydrolysis reactions are called __________hydrolases
The breaking of a polymer into units is ______________ (i.e. done by adding water to polymer).
HYDROLYSIS
http://science.nhmccd.edu/biol/dehydrat/dehydrat.html
Polymersa) Carbohydratesb)c)d)
Monomersa) Simple sugarsb)c)d)
H2O & Energy
HydrolysisDehydration
Synthesis
Polymersa) Carbohydratesb)c)d)
Monomersa) Simple sugarsb)c)d)
H2O & Energy
HydrolysisDehydration
Synthesis
H2O & Energy
Polymersa) Carbohydratesb) Proteinsc)d)
Monomersa) Simple sugarsb)c)d)
H2O & Energy
HydrolysisDehydration
Synthesis
H2O & Energy
Polymersa) Carbohydratesb) Proteinsc)d)
Monomersa) Simple sugarsb) Amino Acidsc)d)
H2O & Energy
HydrolysisDehydration
Synthesis
H2O & Energy
Polymersa) Carbohydratesb) Proteinsc) Lipids (fats)d)
Monomersa) Simple sugarsb) Amino Acidsc)d)
H2O & Energy
HydrolysisDehydration
Synthesis
H2O & Energy
Polymersa) Carbohydratesb) Proteinsc) Lipids (fats)d)
Monomersa) Simple sugarsb) Amino Acidsc) Fatty Acids & Glycerold)
H2O & Energy
HydrolysisDehydration
Synthesis
H2O & Energy
Polymersa) Carbohydratesb) Proteinsc) Lipids (fats)d) DNA/RNA (nucleic acids)
Monomersa) Simple sugarsb) Amino Acidsc) Fatty Acids & Glycerold)
H2O & Energy
HydrolysisDehydration
Synthesis
H2O & Energy
Polymersa) Carbohydratesb) Proteinsc) Lipids (fats)d) DNA/RNA (nucleic acids)
Monomersa) Simple sugarsb) Amino Acidsc) Fatty Acids & Glycerold) Nucleotides
H2O & Energy
HydrolysisDehydration
Synthesis
H2O & Energy
Polymersa) Carbohydratesb) Proteinsc) Lipids (fats)d) DNA/RNA (nucleic acids)
Monomersa) Simple sugarsb) Amino Acidsc) Fatty Acids & Glycerold) Nucleotides
H2O & Energy
HydrolysisDehydration
Synthesis
H2O & Energy
These reactions require:
1.
Polymersa) Carbohydratesb) Proteinsc) Lipids (fats)d) DNA/RNA (nucleic acids)
Monomersa) Simple sugarsb) Amino Acidsc) Fatty Acids & Glycerold) Nucleotides
H2O & Energy
HydrolysisDehydration
Synthesis
H2O & Energy
These reactions require:
1. ATP energy
Polymersa) Carbohydratesb) Proteinsc) Lipids (fats)d) DNA/RNA (nucleic acids)
Monomersa) Simple sugarsb) Amino Acidsc) Fatty Acids & Glycerold) Nucleotides
H2O & Energy
HydrolysisDehydration
Synthesis
H2O & Energy
These reactions require:
1. ATP energy
2. Water
Polymersa) Carbohydratesb) Proteinsc) Lipids (fats)d) DNA/RNA (nucleic acids)
Monomersa) Simple sugarsb) Amino Acidsc) Fatty Acids & Glycerold) Nucleotides
H2O & Energy
HydrolysisDehydration
Synthesis
H2O & Energy
These reactions require:
1. ATP energy
2. Water
3. Enzymes
Where does the name come from?Hydrated Carbons: (CH20)n
Carbohydrates have the empirical formula of (CH20)n where n = the # of times the chain is repeated.
The carbons, hydrogens and oxygens are found in the ratio of
1:2:1 and are made up of a repeating chain of sugars.
Sugars are also known as saccarides.Carbohydrates usually end in ‘ose’. Can you think of any examples?
(CH20)3 = (CH20)6 =C3H603 C6H1206
The basic sugar molecule is GLUCOSE: C6 H12 O6.
Glucose has a ring structure.
Other monosaccharides include fructose, ribose, deoxyribose
glucose + glucose forms the sugar maltose
glucose + fructose forms the sugar sucrose
galactose + glucose forms the sugar lactose
When many sugars bind together via dehydration synthesis four types of polysaccharides may be formed:
• Starch
• Glycogen
• Cellulose
• Chitin
• The cell walls of plants are made of cellulose
• They are long chains of glucose molecules with no side chains.
• The linkage between the Carbon atoms of the sugars is different than starch and glycogen
• No mammal can break this bond
• 5. This is why we cannot digest cellulose = FIBRE.
• Plants store their energy as starch • Starch is made up of many glucose molecules linked
together
• Starch has few side chains
• Animals store their energy (extra glucose) as glycogen
• We store glycogen in our liver and muscles
• Glycogen is made up of many glucose molecules linked together
• Glycogen has many side chains
• Made by animals and fungi
• Long glucose chains linked with covalent bonds.
• Very strong
• Makes structures like exo-skeletons, fingernails, claws, and beaks
1. Energy: when the bonds between Carbon atoms are broken, the energy released can be used by cells.
Carbohydrates are the primary energy molecules for all life.
2. Structural: Cellulose is the major structural compound in plants (is used in the cell wall).
Lipids are made up of the elements C,H,O but in no set ratio.
Lipids are large molecules that are insoluble in water.
1. Composed of 3 fatty acids bonded to 1 glycerol.
2. Fatty acids contain a long chain of 16-18 Carbons with an acid end.
3. Glycerol is a small 3 Carbon chain with 3 alcohol (OH) groups
4. These two molecules bind together via dehydration synthesis
1. Saturated fats: There are no double bonds in the carbon chains of the fatty acids.
The carbons are filled with hydrogens.
Unhealthy.
They mostly come from animals. Become solid at room temperature.
Examples: lard, butter, animal fats…
2. Unsaturated fats: There are one (monounsaturated) or more
double bonds (polyunsaturated).
Mostly come from plants.
They are liquid at room temperature.
Healthy
Examples: olive oil, corn oil, palm oil…
Are used to make up the two layered cell membrane of all cells.
In phospholipids, the third fatty acid group of a triglyceride is replaced by an inorganic phosphate group (PO4
3-).
This creates a polar end:The phosphate end is water soluble (hydrophilic)The fatty acid is not water soluble (hydrophobic)
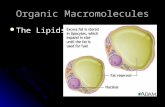
A liposome is an artificially-prepared vesicle composed of a lipid bilayer. The liposome can be used as a vehicle for administration of nutrients and pharmaceutical drugs. Liposomes can be prepared by disrupting biological membranes
Steroids structurally look very different from lipids, but are also water insoluble.
They are made up of 4 Carbon ring molecules fused together.
Examples: testosterone, estrogen, cholesterol, and vitamin D.
Used as sex hormones
1. Long term storage for energy (more efficient spacewise than glycogen or starch).
2. Insulation and protection in animals
3. Making some hormones (steroids)
4. Structure of cell membranes. Without lipids, we would have no cells.
• Found in fish and leafy vegetables • Other foods are now offering omega-3’s (eggs,
cereals, margarine…)• Help to reduce cancer• Helps with vision• Helps us think better