Lipids Mini Lecture Radjewski. Lipids PDQ 1 Lipids are hydrocarbons (composed of C and H atoms);...
-
Upload
mervin-daniel-walsh -
Category
Documents
-
view
218 -
download
3
Transcript of Lipids Mini Lecture Radjewski. Lipids PDQ 1 Lipids are hydrocarbons (composed of C and H atoms);...

Lipids
Mini LectureRadjewski

Lipids PDQ 1
• Lipids are hydrocarbons (composed of C and H atoms); they are insoluble in water because of many nonpolar covalent bonds.
• When close together, weak but additive van der Waals interactions hold them together.

Functions of Lipids PDQ 2
• Lipids• Store energy in C—C and C—H bonds• Play structural role in cell membranes • Fat in animal bodies serves as thermal
insulation

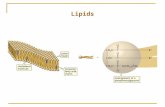
Triglycerides PDQ 3
• Three fatty acids—nonpolar hydrocarbon chain attached to a polar carboxyl group (—COOH) (carboxylic acid)
• One glycerol—an alcohol with 3 hydroxyl (—OH) groups
• Synthesis of a triglyceride involves three condensation reactions.


PDQ 4
Fatty acid chains can vary in length and structure.• In saturated fatty acids, all bonds between
carbon atoms are single; they are saturated with hydrogens.
• In unsaturated fatty acids, hydrocarbon chains contain one or more double bonds. These acids cause kinks in the chain and prevent molecules from packing together tightly.



PDQ 6
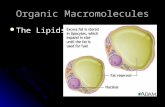
• Fatty acids are amphipathic; they have a hydrophilic end and a hydrophobic tail.
• Phospholipid—two fatty acids and a phosphate compound bound to glycerol
• The phosphate group has a negative charge, making that part of the molecule hydrophilic.

Figure 2.13 A Phospholipids

Figure 2.13 B Phospholipids

PDQ 5
• Fatty acid – component in lipids• Triglyceride – 3 fatty acids• Phospholipids – 2 fatty acids

Steroids
Examples of steroids1.Cholesterol – maintains fluidity in cell membranes2.Testosterone - hormone3.Estrogen - hormone4.Progesterone - hormone

Build a Triglyceride
• Remember triglycerides have:– 3 fatty acids– Glycerol molecule
• Do some condensation reactions.• Attach water(s). • Label one saturated fatty acid; unsaturated
fatty acid; glycerol, and the ester bonds