Lecture Notes Chapter-3: Lipids · Chapter-3: Lipids Introduction The lipids are a heterogeneous...
Transcript of Lecture Notes Chapter-3: Lipids · Chapter-3: Lipids Introduction The lipids are a heterogeneous...

T.Y.B.Sc. SemIII Paper VI CH-336C Introduction to Biochemistry and Molecular Biology
Prof. Ansari Mujahid Kazafi Page 1 Department of Chemistry, G.D.A.B. Arts, Commerce and Science College, Malegaon
Lecture Notes
Chapter-3: Lipids
Introduction
The lipids are a heterogeneous group of compounds, including fats, oils, steroids, waxes, and
related compounds, which are related more by their physical than by their chemical
properties. Lipids are a class of compounds distinguished by their insolubility in water and
solubility in nonpolar solvents. Lipids are important in biological systems because they form
the cell membrane, a mechanical barrier that divides a cell from the external environment.
Lipids also provide energy for life and several essential vitamins are lipids.
Biological role of Lipids
Lipids have the common property of being relatively insoluble in water and soluble in
nonpolar solvents such as ether and chloroform. They are important dietary constituents not
only because of their high energy value but also because of the fat-soluble vitamins and the
essential fatty acids contained in the fat of natural foods. Fat is stored in adipose tissue,
where it also serves as a thermal insulator in the subcutaneous tissues and around certain
organs. Nonpolar lipids act as electrical insulators, allowing rapid propagation of
depolarization waves along myelinated nerves. Combinations of lipid and protein
(lipoproteins) are important cellular constituents, occurring both in the cell membrane and in
the mitochondria, and serving also as the means of transporting lipids in the blood.
Knowledge of lipid biochemistry is necessary in understanding many important biomedical
areas, e.g., obesity, diabetes mellitus, atherosclerosis, and the role of various polyunsaturated
fatty acids in nutrition and health.
Classification of Lipids
Lipids are classified as follows:
A. Simple lipids or Homolipids. These are esters of fatty acid with farious alcohols.
1. Fats and oils (triglycerides, triacylglycerols).
These are esters of fatty acids with a trihydroxy alcohol, glycerol. A fat is solid at ordinary
room temperature wheras an oil is liquid.
2. Waxes. These are esters of fatty acids with high molecular weight monohydroxy alcohols.
B. Compound lipids or Heterolipids. These are esters of fatty acids with alcohol and
possess additional group(s) also.
1. Phospholipids (phosphatids), These are compounds containing, in addition to fatty acids
and glycerol, a phosphoric acid, nitrogen bases and other substituents.
2. Glycolipids (cerebrosides). These are the compounds of fatty acids with carbohydrates and
contain nitrogen but no phosphoric acid. The glycolipids also include certain structurally-
related compounds comprising the groups, gangliosides, sulfolipids and sulfatids.

T.Y.B.Sc. SemIII Paper VI CH-336C Introduction to Biochemistry and Molecular Biology
Prof. Ansari Mujahid Kazafi Page 2 Department of Chemistry, G.D.A.B. Arts, Commerce and Science College, Malegaon
C. Derived lipids. These are the substances derived from simple and compound lipids by
hydrolysis. These include fatty acids, alcohols, mono- and diglycerides, steroids, terpenes
and carotenoids.
Glycerides and cholesterol esters, because of their uncharged nature, are also called neutral
lipids. However, Conn and Stumpf (1976) have traditionally classified lipids into following 6
classes :
1. Acyl glycerols
2. Waxes
3. Phospholipids
4. Sphingolipids
5. Glycolipids
6. Terpenoid lipids including carotenoids and steroids
Simple Lipids
FATS AND OILS
( = Triglycerides or Triacylglycerols)
The triglycerides are the most abundant of all lipids. They constitute about 98% of total
dietary lipids ; the remaining 2% consists of phospholipids and cholesterol and its esters.
They are the major components of storage or depot fats in plant and animal cells but are not
normally found in membranes. They are nonpolar, hydrophobic molecules since they contain
no electrically charged or highly polar functional groups. In animals, the fat cells or
adipocytes contain very large quantities of triglycerides in the form of fat droplets, which fill
almost the entire cell volume. Adipocytes are Abundantly found under the skin, in the
abdominal cavity and in the mammary glands. Triglycerides can be stored in quantities,
sufficient to supply the energy needs of the body for many months, as in the case of obese
persons. On the contrary, the body can store the carbohydrate glycogen in meagre amounts,
sufficient to supply energy need of a day only. Triglycerides are much better adapted than
glycogen to serve as storage form of energy. They are not only stored in large amounts but
also yield over twice as much energy as carbohydrates. Since fats tend to remain in the
stomach longer than carbohydrates and are digested more slowly, they also have greater
satiety value than carbohydrates. The arctic and Antarctic animals such as whales, seals,
walruses and penguins are amply padded with triglycerides
to serve both as energy storage depots and as an insulation against very low temperatures.
Most fats and oils, upon hydrolysis, yield several fatty acids as well as glycerol. However,
the milk of spiny anteater is an exception in that it comprises almost pure triolein. Human
body contains enough fat to make 7 bars of soap !
Saturated Fatty Acids
A saturated fat is a type of fat, in which the fatty acids all have single bonds.

T.Y.B.Sc. SemIII Paper VI CH-336C Introduction to Biochemistry and Molecular Biology
Prof. Ansari Mujahid Kazafi Page 3 Department of Chemistry, G.D.A.B. Arts, Commerce and Science College, Malegaon
A fat is made of two kinds of smaller molecules: monoglyceride and fatty acids. Fats are
made of long chains of carbon (C) atoms. Some carbon atoms are linked by single bonds (-C-
C-) and others are linked by double bonds (-C=C-). Double bonds can react with hydrogen to
form single bonds. They are called saturated, because the second bond is broken up and each
half of the bond is attached to (saturated with) a hydrogen atom. Most animal fats are
saturated. The fats of plants and fish are generally unsaturated. Saturated fats tend to have
higher melting points than their corresponding unsaturated fats, leading to the popular
understanding that saturated fats tend to be solids at body temperatures, while unsaturated
fats tend to be liquid oils.
Various fats contain different proportions of saturated and unsaturated fat. Examples of foods
containing a high proportion of saturated fat include animal fat products such as cream,
cheese, butter, other whole milk dairy products and fatty meats which also contain dietary
cholesterol. Certain vegetable products have high saturated fat content, such as coconut oil
and palm kernel oil. Many prepared foods are high in saturated fat content, such as pizza,
dairy desserts, and sausage.
Some common examples of fatty acids:
Butyric acid with 4 carbon atoms (contained in butter)
Lauric acid with 12 carbon atoms (contained in coconut oil, palm kernel oil, and breast milk)
Myristic acid with 14 carbon atoms (contained in cow's milk and dairy products)

T.Y.B.Sc. SemIII Paper VI CH-336C Introduction to Biochemistry and Molecular Biology
Prof. Ansari Mujahid Kazafi Page 4 Department of Chemistry, G.D.A.B. Arts, Commerce and Science College, Malegaon
Palmitic acid with 16 carbon atoms (contained in palm oil and meat)
Stearic acid with 18 carbon atoms (also contained in meat and cocoa butter)
Unsaturated Fatty Acids:
An unsaturated fat is a fat or fatty acid in which there is at least one double bond within the
fatty acid chain. A fatty acid chain is monounsaturated if it contains one double bond, and
polyunsaturated if it contains more than one double bond.
Where double bonds are formed, hydrogen atoms are subtracted from the carbon chain. Thus,
a saturated fat has no double bonds, has the maximum number of hydrogens bonded to the
carbons, and therefore is "saturated" with hydrogen atoms. In cellular metabolism,
unsaturated fat molecules contain somewhat less energy (i.e., fewer calories) than an
equivalent amount of saturated fat. The greater the degree of unsaturation in a fatty acid (i.e.,
the more double bonds in the fatty acid) the more vulnerable it is to lipid peroxidation
(rancidity). Antioxidants can protect unsaturated fat from lipid peroxidation.
Examples of unsaturated fatty acids are palmitoleic acid, oleic acid, myristoleic acid, linoleic
acid, and arachidonic acid. Foods containing unsaturated fats include avocado, nuts, and
vegetable oils such as canola and olive oils. Meat products contain both saturated and
unsaturated fats.

T.Y.B.Sc. SemIII Paper VI CH-336C Introduction to Biochemistry and Molecular Biology
Prof. Ansari Mujahid Kazafi Page 5 Department of Chemistry, G.D.A.B. Arts, Commerce and Science College, Malegaon
Arachidonic Acid
Compound Lipids:
PHOSPHOLIPIDS
(= Phosphatids)
Phospholipids are the most abundant membrane lipids. They serve primarily as structural
components of membranes and are never stored in large quantities. As their name implies,
phospholipids contain phosphorus in the form of phosphoric acid groups. They differ from
triglycerides in possessing usually one hydrophilic polar “head” group and usually two
hydrophobic nonpolar “tails”. For this reason, they are often called polar lipids. Thus,
phospholipids are amphipathic, whereas the storage lipids (triglycerides and waxes) are not.
In phospholipids, two of the OH groups in glycerol are linked to fatty acids while the third
OH group is linked to phosphoric acid. The phosphate is further linked to one of a variety of
small polar head groups (alcohols). Folch and Sperry (1955) have classified phospholipids
into phosphoglycerides, phosphoinositides and phosphosphingosides.
A. Phosphoglycerides
These are the major phospholipids found in membranes and contain two fatty acid molecules
or “tails” esterified to the first and second hydroxyl groups of glycerol. The third hydroxyl
group of glycerol forms an ester linkage with phosphoric acid. In addition,
phosphoglycerides contain a second alcohol, which is also esterified to the phosporic acid.
This is referred to as „head alcohol group‟ as it is present at one end („head‟) of the long
phosphoglyceride molecule. The various phosphoglycerides differ in their head alcohol
groups. However, all of them contain two nonpolar tails, each consisting of a long chain
(usually C16 or C18) fatty acid. Usually one of the fatty acids is saturated and the other

T.Y.B.Sc. SemIII Paper VI CH-336C Introduction to Biochemistry and Molecular Biology
Prof. Ansari Mujahid Kazafi Page 6 Department of Chemistry, G.D.A.B. Arts, Commerce and Science College, Malegaon
unsaturated ; the latter is always esterified to the middle or β-hydroxy group of glycerol. A
noteworthy feature of the phosphoglycerides is that they contain an asymmetric carbon atom
at position 2 in the glycerol part of their molecule. It has the L-configuration since it is
related to L-glyceraldehyde. All phosphoglycerides have a negative charge on phosphoric
group at pH 7. In addition, the head alcohol group may also have one or more electric
charges at pH 7.
1.Lecithins (= phosphatidyl cholines) − Lecithins (likithosG = yolk) are widely distributed in
nature. Various oil seeds like soybean and the yeasts are important sources from plant world.
In animals, the glandular and nervous tissues are rich in these lipids. The lecithins are
required for the normal transport and utilization of as other lipids esp., in the liver of animals.
In their absence, accumulation of lipids occurs in the liver to as much as 30% against a
normal value of 3-4%, giving rise to a condition called “fatty liver”. This fatty infiltration
may lead to fibrotic changes, characteristic of the liver disease cirrhosis. In addition to
glycerol and 2 moles of fatty acids, the lecithins also contain phosphoric acid and a nitrogen
base choline at either the end or middle carbon atom of glycerol unit. Accordingly, two forms
of lecithins, α and β are recognized.
2.Phosphatidic Acid
3. Cephalins The cephalins (kephalusG = head) are closely associated with lecithins in
animal tissues. These have also been identified from soybean oil. These are similar in
structure to the lecithins except that the choline is replaced by either ethanolamine or serine.
Serine is the biochemical precursor of ethanolamine.

T.Y.B.Sc. SemIII Paper VI CH-336C Introduction to Biochemistry and Molecular Biology
Prof. Ansari Mujahid Kazafi Page 7 Department of Chemistry, G.D.A.B. Arts, Commerce and Science College, Malegaon
Accordingly, two types of cephalins are recognized, phosphatidyl ethanolamine and
phosphatidyl serine. Like lecithins, the cephalins also exist in 2 forms, α and β, depending
upon the relative positions of the two substituent fatty acids.
Since the primary amino group of ethanolamine is a weaker base than the quaternary
ammonium group of choline, the cephalins are more acidic than lecithins. Moreover, the
cephalins are comparatively less souble in alcohol than lecithins
4.Phosphoinositides (=Phosphatidyl inositols)(Lipositol)
Phosphoinositides have been found to occur in phospholipids of brain tissue and of soybeans
and are of considerable importance because of their role in transport processes in cells. These
are phospholipids where a cyclic hexahydroxy alcohol called inositol replaces base. The
inositol is present as the stereoisomer, myo-inositol. On hydrolysis, the phosphoinositides
yield 1 mole of glycerol, two moles of fatty acid, 1 mole of inositol and 1, 2, or 3 moles of
phosphoric acid. Accordingly, mono-, di- or triphosphoinositides are found.
5.Sphingomyelins
These compounds are commonly found in nerve tissue esp., in the myelin sheath of the nerve
(hence their name,sphingomyelins) and apparently lack in plants and the microorganisms. In
a syndrome called Niemann−Pick disease, the sphingomyelins are stored in the brain in large
quantities. These differ from other phospholipids in their lack of glycerol and the presence of
another nitrogenous base sphingosine or a closely related dihydrosphingosine, besides
choline, in place of glycerol. Sphingomyelins are electrically charged molecules and contain
phosphocholine as their polar head groups.

T.Y.B.Sc. SemIII Paper VI CH-336C Introduction to Biochemistry and Molecular Biology
Prof. Ansari Mujahid Kazafi Page 8 Department of Chemistry, G.D.A.B. Arts, Commerce and Science College, Malegaon
1, hydroxyl, 8 sphengenine Sphingosine
6.Cholesterol
Cholesterol (choleG = bile). Cholesterol is undoubtedly the most publicized lipid in nature,
because of the strong correlation between high levels of cholesterol in the blood and the
incidence of diseases of the cardiovascular system in humans. It is not only an important
component of some cell membranes and of plasma lipoproteins but also the precursor of
many other biologically important steroids, such as bile acids and various steroid hormones.
It is the principal sterol of higher animals and is especially abundant in nerve tissues and in
gallstones. In occurs either free or as fatty esters in all animal cells. It was first isolated in
1784, from human gallstones which consist almost entirely of cholesterol and hence so
named (cholesterol literally means „solid alcohol from bile‟). Its main sources are fish liver
oils and the brain and spinal cord of cattle. White matter contains as much as 14%, gray
matter 5%, spinal cord 12% and liver about 1% cholesterol. Cholesterol is , however, not
found in plant fats. Its parent hydrocarbon is cholestane, C27H48. The structure of
cholesterol was determined by the German chemist, Adolph Windaus (LT, 1879 − 1959),
who received 1928 Nobel Prize in Chemistry. Cholesterol has a molecular formula,
C27H45OH. In addition to an OH group at C3, there is a double bond at C5. The hydroxyl
group constitutes its polar head, the rest of the molecule is hydrophobic. It is a white
crystalline solid and is optically active, [α]D 39°

T.Y.B.Sc. SemIII Paper VI CH-336C Introduction to Biochemistry and Molecular Biology
Prof. Ansari Mujahid Kazafi Page 9 Department of Chemistry, G.D.A.B. Arts, Commerce and Science College, Malegaon
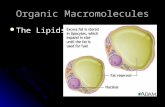
Amphipathic Lipids Aggregate
We have noted that glycerophospholipids, sphingolipids, and sterols are virtually insoluble in
water. When mixed with water, these amphipathic compounds form microscopic lipid
aggregates in a phase separate from their aqueous surroundings. Lipid molecules cluster
together with their hydrophobic moieties in contact with each other and their hydrophilic
groups interacting with the surrounding water. Recall that lipid clustering reduces the amount
of hydrophobic surface exposed to water and thus minimizes the number of molecules in the
shell of ordered water at the lipid-water interface (see Fig. 4-7), resulting in an increase in
entropy. Hydrophobic interactions among lipid molecules provide the thermodynamic
driving force for the formation and maintenance of these structures.
Depending on the precise conditions and the nature of the lipids used, three types of lipid
aggregates can form when amphipathic lipids are mixed with water (Fig. 9-14). Micelles are
relatively small, spherical structures involving a few dozen to a few thousand molecules
arranged so that their hydrophobic regions aggregate in the interior, excluding water, and
their hydrophilic head groups are at the surface, in contact with water. Micelle formation is
favored when the crosssectional area of the head group is greater than that of the acyl side
chain(s) (Fig. 9-14a), as it is in free fatty acids, lysophospholipids (which lack one fatty
acid), and the detergent SDS.
A second type of lipid aggregate in water is the bilayer, in which two lipid monolayers
combine to form a two-dimensional sheet. Bilayer formation occurs most readily when the
cross-sectional areas of the head group and side chain(s) are similar (Fig. 9-14b), as in
glycerophospholipids and sphingolipids. The hydrophobic portions in each monolayer
interact, excluding water. The hydrophilic head groups interact with water at the two surfaces
of the bilayer.

T.Y.B.Sc. SemIII Paper VI CH-336C Introduction to Biochemistry and Molecular Biology
Prof. Ansari Mujahid Kazafi Page 10 Department of Chemistry, G.D.A.B. Arts, Commerce and Science College, Malegaon
The third type of lipid aggregate is formed when a lipid bilayer folds back on itself to form a
hollow sphere called a liposome or vesicle (Fig. 9-14c). By forming vesicles, bilayer sheets
lose their hydrophobic edge regions, achieving maximal stability in their aqueous
environment. These bilayer vesicles enclose water, creating a separate aqueous compartment.
It is likely that the first living cells resembled liposomes, their aqueous contents segregated
from the rest of the world by a hydrophobic shell. We shall see in the next chapter that lipid
bilayers are fundamental to the structure of all biological membranes.
Saponification Number:
Saponification is the hydrolysis of fats or oils under basic conditions to afford glycerol and
the salt of the corresponding fatty acid. Saponification literally means "soap making". It is
important to the industrial user to know the amount of free fatty acid present, since this
determines in large measure the refining loss. The amount of free fatty acid is estimated by
determining the quantity of alkali that must be added to the fat to render it neutral. This is
done by warming a known amount of the fat with strong aqueous caustic soda solution,
which converts the free fatty acid into soap. This soap is then removed and the amount of fat
remaining is then determined. The loss is estimated by subtracting this amount from the
amount of fat originally taken for the test.
The saponification number is the number of milligrams of potassium hydroxide required to
neutralize the fatty acids resulting from the complete hydrolysis of 1g of fat . It gives
information concerning the character of the fatty acids of the fat- the longer the carbon chain,
the less acid is liberated per gram of fat hydrolysed. It is also considered as a measure of the
average molecular weight (or chain length) of all the fatty acids present. The long chain fatty
acids found in fats have low saponification value because they have a relatively fewer
number of carboxylic functional groups per unit mass of the fat and therefore high molecular
weight .
Acid Number:
It is defined as the weight of KOH in mg needed to neutralize the organic acids present in 1g
of fat and it is a measure of the free fatty acids (FFA) present in the fat or oil. An increment
in the amount of FFA in a sample of oil or fat indicates hydrolysis of triglycerides. Such
reaction occurs by the action of lipase enzyme and it is and indicator of inadequate
processing and storage conditions (i.e., high temperature and relative humidity, tissue
damage). The source of the enzyme can be the tissue from which the oil or fat was extracted
or it can be a contaminant from other cells including microorganisms. Besides FFA,
hydrolysis of triglycerides produces glycerol. The table below shows the acid value of some
common oils and bee's wax.
Iodine Number:

T.Y.B.Sc. SemIII Paper VI CH-336C Introduction to Biochemistry and Molecular Biology
Prof. Ansari Mujahid Kazafi Page 11 Department of Chemistry, G.D.A.B. Arts, Commerce and Science College, Malegaon
The iodine value (or "iodine adsorption value" or "iodine number" or "iodine index") in
chemistry is the mass of iodine in grams that is consumed by 100 grams of a chemical
substance. Iodine numbers are often used to determine the amount of unsaturation in fatty
acids. This unsaturation is in the form of double bonds, which react with iodine compounds.
The higher the iodine number, the more C=C bonds are present in the fat. It can be seen from
the table that coconut oil is very saturated, which means it is good for making soap. On the
other hand, linseed oil is highly unsaturated, which makes it a drying oil, well suited for
making oil paints.
Rancidity of Lipids:
It is the process which causes a substance to become rancid, that is, having a rank, unpleasant
smell or taste. Specifically, it is the hydrolysis and/or autoxidation of fats into short-chain
aldehydes and ketones which are objectionable in taste and odor. When these processes occur
in food, undesirable odors and flavors can result. In some cases, however, the flavors can be
desirable (as in aged cheeses). In processed meats, these flavors are collectively known as
warmed-over flavor. Rancidification can also detract from the nutritional value of food, and
some vitamins are highly sensitive to degradation. Akin to rancidification, oxidative
degradation also occurs in other hydrocarbons, e.g. lubricating oils, fuels, and mechanical
cutting fluids.
Lipoproteins:
Lipoproteins are special particles made up of droplets of fats surrounded by a single layer of
phospholipid molecules. Phospholipids are molecules of fats which are attached to a
phosphorus-containing group. They are distinctive in being amphipathic, which means they
have both polar and non-polar ends.
The types of lipoproteins with their function are as follows:
Chylomicrons – these are the largest and least dense of the lipoproteins, with the
highest triglyceride content. They consist of a protein component synthesized in the
liver, which wraps around diet-derived cholesterol and fats. It travels from the
intestinal lymphatics to the large veins, and sticks to the inner surface of the tiny
capillary blood vessels inside the muscles and the fat storage cells in various parts of
the body. There the fat is digested, while the cholesterol remains. This is now called
the chylomicron remnant. It travels to the liver, where the cholesterol is metabolized.
Thus chylomicrons deliver fats and cholesterol from the intestines to the muscles, fat
cells and the liver.
VLDL, very low density lipoprotein – this is composed of protein, fats and cholesterol
synthesized in the liver. It is associated with 5 different apoproteins, namely , B-100,
C-I, C-II, C-III and E. It is converted to IDL and LDL by removal of the apoproteins,

T.Y.B.Sc. SemIII Paper VI CH-336C Introduction to Biochemistry and Molecular Biology
Prof. Ansari Mujahid Kazafi Page 12 Department of Chemistry, G.D.A.B. Arts, Commerce and Science College, Malegaon
except for one called apoprotein B100, along with esterification of the cholesterol.
They are second only to chylomicrons in the percentage triglyceride content.
IDL – intermediate density lipoprotein, is created by the metabolism of VLDL.
LDL, low density lipoprotein – this is the last VLDL remnant, and contains chiefly
cholesterol. The only apoprotein associated with it is apoB-100. Thus all these forms
carry fats and cholesterol produced in the liver to the tissues.
HDL, high density lipoprotein – this has the highest protein: lipid ratio, and so is the
densest. It has the apoprotein A-1. This is also called „good cholesterol‟, because it
carries cholesterol away from the tissues to the liver, lowering blood cholesterol
levels. High HDL levels are associated with lowered risk of cardiovascular disease.
HDL levels are higher with exercise, higher estrogen levels, with alcohol
consumption, and weight loss.