Learning Objective Classify matter as a pure substance or mixture by investigating its properties...
-
Upload
cleopatra-emerald-griffin -
Category
Documents
-
view
215 -
download
0
Transcript of Learning Objective Classify matter as a pure substance or mixture by investigating its properties...

Learning Objective• Classify matter as a pure substance or
mixture by investigating its properties
Classification of Matter

• Pure substances have a defined chemical composition
Ex)
• Mixtures are formed from physically mixing two or more pure substances
Ex)
Classifications of Matter
Pure Substanc
e
Defined Composit
ion
Aluminum
Al
Oxygen O2
Water H2O
Sugar C6H12O6
Mixture Composition
Trail mix varies
Sugar water
varies
Air varies

• Element – pure substance consisting of only one type of atom
• All known elements are listed in the periodic table
• Some elements always appear as groups of two– Diatomic elements –– H2, N2, O2, F2, Cl2, Br2, I2
• Some elements exist in multiple forms– Carbon exists as graphite or diamond
Types of Pure Substances

• Compound – two or more atoms of different elements chemically combined in a fixed ratio
• Molecule – group of covalently bonded atoms with a fixed composition
• Compositions of pure substances are described by formulas– Subscripts in formulas represent how many
atoms of that element are present in the compound or molecule• Subscripts are written after the element they apply to
– C6H12O6 has six carbon atoms, 12 hydrogen atoms, and six oxygen atoms
• Subscripts of 1 are implied rather than written– H2O has two hydrogen atoms and one oxygen atom
Types of Pure Substances

• Compounds have different properties from the elements that make them upEx)
Properties of Compounds
Pure Substan
ce
Formula
Properties Image Image
Credit
Sodium Na highly reactive metal
By Dnn87 at en.wikipedia (Transferred
from en.wikipedia) [CC–BY–SA–
3.0]
Chlorine
Cl2 highly toxic
green gas
By W. Oelen (http://woelen.homescience.net/science/in
dex.html) [CC–BY–SA–
3.0]
Sodium chloride
NaCl stable white
crystal eaten
everyday
By Dubravko Sorić SoraZG on Flickr [CC–
BY–2.0]

• Law of definite proportions – a chemical compound will always contain the same ratio of elements
• Law of multiple proportions – if multiple compounds can form from the combination of elements, the elements will always combine in small, defined whole number ratios– Ratio of the elements determines the chemical
identity of the compoundEx)
Laws Governing Compounds
Common Name
Elements Present
Formula of Compound
Ratio of Elements
Water Hydrogen & Oxygen
H2O 2:1
Hydrogen Peroxide
Hydrogen & Oxygen
H2O2 2:2

• Heterogeneous mixture – a mixture that is not uniform throughout– Different properties in
different parts of the specimen
• Homogeneous mixture – a mixture that is uniform throughout– Same properties in
different parts of the specimen
– Also called a solution
Types of Mixtures
Heterogeneous MixtureBy Victor Blacus (Victor Blacus) [GFDL]
Solution

• Mixtures do not have defined ratios
• Properties of mixtures are generally a blend of the properties of the substances they are made from
Properties of Mixtures
Chocolate chip cookies
By Rdsmith4 (Own work) [CC–BY–SA–2.5]

• Separation of mixtures is a physical process– Based on
differences in the properties of the substances
• Separation of compounds is a chemical process– A chemical
reaction must occur to break a compound into elements• Elements cannot be
separated any further
Separating Mixtures and Pure Substances
Types of Physical
Separations
Reason for Separation
Filtration Particle Size
Distillation Difference in boiling points
Chromatography
Differences in attraction to various liquids or solids

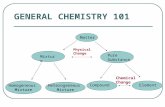
Classification of Matter
Matter
Uniform Throughout
Physically Combined
Homogeneous MixtureEx) salt water
Chemically Combined
CompoundEx) H2O
Uncombined
ElementEx) Al
Not Uniform Throughout
Heterogeneous Mixture
Ex) oil & water