Incremental yield of dysplasia detection in Barrett's esophagus …2.pdf · nosing dysplasia over...
Transcript of Incremental yield of dysplasia detection in Barrett's esophagus …2.pdf · nosing dysplasia over...

ORIGINAL ARTICLE: Clinical Endoscopy
www
Incremental yield of dysplasia detection in Barrett’s esophagususing volumetric laser endomicroscopy with and withoutlaser marking compared with a standardized randombiopsy protocol
.giejo
Mohammad Alshelleh, MD, Sumant Inamdar, MD, Matthew McKinley, MD, Molly Stewart, BS,Jeffrey S. Novak, MD, Ronald E. Greenberg, MD, Keith Sultan, MD, Bethany Devito, MD, Mary Cheung, MD,Maurice A. Cerulli, MD, Larry S. Miller, MD, Divyesh V. Sejpal, MD, Anil K. Vegesna, MD,Arvind J. Trindade, MD
New Hyde Park, New York, USA
GRAPHICAL ABSTRACT
Background and Aims: Volumetric laser endomicroscopy (VLE) is a new wide-field advanced imaging technol-
ogy for Barrett’s esophagus (BE). No data exist on incremental yield of dysplasia detection. Our aim is to reportthe incremental yield of dysplasia detection in BE using VLE.Methods: This is a retrospective study from a prospectively maintained database from 2011 to 2017 comparingthe dysplasia yield of 4 different surveillance strategies in an academic BE tertiary care referral center. The groupswere (1) random biopsies (RB), (2) Seattle protocol random biopsies (SP), (3) VLE without laser marking (VLE),and (4) VLE with laser marking (VLEL).
Results: A total of 448 consecutive patients (79 RB, 95 SP, 168 VLE, and 106 VLEL) met the inclusion criteria. Afteradjusting for visible lesions, the total dysplasia yield was 5.7%, 19.6%, 24.8%, and 33.7%, respectively. Whencompared with just the SP group, the VLEL group had statistically higher rates of overall dysplasia yield (19.6%vs 33.7%, P Z .03; odds ratio, 2.1, P Z .03). Both the VLEL and VLE groups had statistically significant differencesin neoplasia (high-grade dysplasia and intramucosal cancer) detection compared with the SP group (14% vs 1%,P Z .001 and 11% vs 1%, P Z .003).
Conclusion: A surveillance strategy involving VLEL led to a statistically significant higher yield of dysplasia andneoplasia detection compared with a standard random biopsy protocol. These results support the use of VLELfor surveillance in BE in academic centers. (Gastrointest Endosc 2018;88:35-42.)
(footnotes appear on last page of article)
1
INTRODUCTIONPatients with the known precursor to esophageal cancer,Barrett’s esophagus (BE), are at increased risk of developing
urnal.org
esophageal adenocarcinoma. In the United States, BE isdefined as specialized intestinal metaplasia in the tubularesophagus.2 It is important to detect dysplasia in BE, as thisincreases the risk of developing esophageal cancer. Ablation
Volume 88, No. 1 : 2018 GASTROINTESTINAL ENDOSCOPY 35

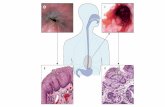
Figure 1. Examples of volumetric laser endomicroscopy images. A, Normal esophagus showing normal layering. B, Gastric cardia showing normal cryptand pit pattern. C, Barrett’s esophagus without dysplasia showing an abnormal villiform epithelium. D, Barrett’s esophagus with high-grade dysplasiashowing a high surface intensity with atypical glands.
Incremental yield of dysplasia detection with VLE with laser marking in Barrett’s Alshelleh et al
of known dysplastic BE can reduce the progression toadvanced disease and cancer.3,4 However, without properdetection, dysplasia can be missed and can lead to advancedneoplasia.5 The current standard of care for surveillance inknown BE is to perform random biopsies usually using theSeattle protocol method,2 which involves taking biopsysamples in a 4-quadrant fashion every 1 to 2 cm of the BEsegment. Unfortunately, this method can miss advancedneoplasia and samples only a minority of the BE segment.5
Thus, there is a need for red flag technologies or advancedimaging to help pinpoint or target dysplasia. In tertiary careBE referral centers, it is acceptable to survey with advancedimaging technology in lieu of 4-quadrant random biopsiesif certain diagnostic thresholds are met.6
In 2013, a new advanced imaging technology, volu-metric laser endomicroscopy (VLE), became commerciallyavailable in the United States. VLE is a second-generationoptical coherence tomography (OCT) technology. It usesinfrared light to produce real-time high-resolution cross-sectional imaging of the tubular esophagus. VLE can scana 6-cm length of the esophagus in approximately 90 sec-onds, providing surface and subsurface wide-field cross-sectional imaging with an axial resolution of 7 mm, and toa depth of 3 mm.7,8 Scoring systems for OCT and VLEhave been developed to help detect neoplasia (high-gradedysplasia and intramucosal cancer).9,10 These scoring sys-tems have been used in clinical practice to locate neoplasia
36 GASTROINTESTINAL ENDOSCOPY Volume 88, No. 1 : 2018
in BE.11–13 Figure 1 shows typical VLE images of normalesophagus and BE. Currently the latest upgrade to theimaging platform, introduced in May 2016, includes theability to perform superficial laser marking of theesophageal epithelium to suspicious areas (Fig. 2) toprovide accurate targeting for biopsies or endoscopicresection.14 Previously, physicians were targetingsuspicious areas by matching up the registration lines ofthe VLE scan to the balloon in relation to thegastroesophageal junction.
Although there have been case reports and case seriesshowing that VLE can aid in the identification and diagnosisof dysplasia,11,12,15,16 there are limited data outside of thesereports demonstrating the incremental yield of VLE in diag-nosing dysplasia over the current standard of care randombiopsies. The goal of this study was to compare the yield ofdysplasia detection in BE using 4 surveillance strategies:(1) random biopsies (RB), (2) random biopsies using theSeattle protocol (SP), (3) surveillance with VLE withoutlaser marking (VLE), and (4) surveillance with VLE withlaser marking (VLEL).
METHODS
This is a retrospective study from a prospectively main-tained endoscopy database of consecutive patients with BE
www.giejournal.org

Figure 2. An example of volumetric laser endomicroscopy (VLE) with laser marking. A, An area in a long-segment Barrett’s esophagus showing an areasuspicious for high-grade dysplasia on VLE with atypical glands and partial effacement being targeted for laser marking (each red line is where a laser markis placed). B, Laser marks placed in the corresponding area. C, Histology showing intestinal metaplasia of the esophagus with high-grade dysplasia.
Alshelleh et al Incremental yield of dysplasia detection with VLE with laser marking in Barrett’s
undergoing endoscopic surveillance at an academic tertiarycare referral center that specializes in BE. Our centerroutinely uses VLE for surveillance in BE since its inceptionin 2014. Institutional review board approval was obtainedfor this study. Consecutive patients were included if theywere age 18 years and older, had a diagnosis of BE, andwere undergoing a surveillance examination with biopsies.Surveillance examinations included patients withoutknown dysplasia, surveillance of patients with knowndysplasia (eg, a patient with low-grade dysplasia undergo-ing surveillance) who were treatment naive, and post-treatment surveillance. The post-treatment surveillancegroup included patients who achieved complete eradica-tion of intestinal metaplasia and had endoscopic evidenceof recurrence, as well as patients with persistent intestinalmetaplasia after ablation. It is our standard to perform sur-veillance endoscopy after 3 endoscopy sessions with abla-tions to rule out progression of disease. Patients wereexcluded if they were less than 18 years old, did nothave biopsy specimens taken, or if a visible lesion suspi-cious for dysplasia was present on white-light endoscopyor narrow-band imaging (NBI). Including these patientswould artificially inflate the dysplasia yield for the corre-sponding group.
Consecutive groups were abstracted from the database(Fig. 3) and divided into 4 groups. The first 2 groupsincluded patients undergoing surveillance by means ofrandom biopsies from 2011 to 2015. The first group (RB)consisted of consecutive patients with BE undergoingendoscopic surveillance with random biopsies at thediscretion of the endoscopist. The second groupconsisted of Seattle protocol (SP) biopsies. Seattleprotocol biopsies were performed as previouslydescribed in the literature with specimens taken every 1to 2 cm of the length of the BE in a 4-quadrant fashionat the discretion of the endoscopist. Pathology reportswere abstracted to ensure that the correct numbers ofbiopsy samples were taken per the BE length reported,in order to be classified as a Seattle protocol biopsy.High-definition endoscopy was used for all patients(Olympus H180, Center Valley, Pa). Full-time academicphysicians with more than 10 years of endoscopy experi-
www.giejournal.org
ence performed all of the procedures in this group. Theuse of NBI was at the discretion of the endoscopist.
The third group (VLE) consisted of consecutive patientswith BE undergoing endoscopic surveillance from 2014 toJuly 2016 using VLE without laser marking, because lasermarking was not commercially available during the timeperiod. The VLE procedure was performed as previouslydescribed in the literature.7,8,12 The Evans criteria wereused to define areas suspicious for dysplasia.9 High-definition endoscopy and NBI were used for all patients(Olympus H180 or H190, Center Valley, Pa). Patients alsounderwent Seattle protocol biopsies in this group at thediscretion of the endoscopist. In tertiary care BE referralcenters, it is acceptable to survey with advanced imagingtechnology in lieu of 4-quadrant random biopsies if certaindiagnostic thresholds are met.6 VLE targeted biopsies wereperformed before random biopsies in all cases. Allprocedures were performed by 2 expert endoscopists inadvanced imaging in BE (A.J.T. and M.M.).
The fourth group (VLEL) consisted of consecutivepatients with BE undergoing endoscopic surveillancefrom July 2016 to April 2017 using VLE with laser marking.VLE with laser marking was performed as described in theliterature.14,17 The number of suspicious areas targetedwas at the discretion of the endoscopist. The Evans criteriawere used to define areas suspicious for dysplasia.9 High-definition endoscopy and NBI were used for all patients(Olympus H190, Center Valley, Pa). Patients also under-went Seattle protocol biopsies in this group at the discre-tion of the endoscopist. VLE targeted biopsies wereperformed before random biopsies in all cases. All VLELprocedures were performed by 2 experts in advancedimaging in BE (A.T. and M.M.).
Biopsies of raised lesions targeted by high-definitionwhite-light endoscopy were excluded in the analysis forall groups. Biopsies of areas targeted by NBI were excludedin the analysis for all groups. Dysplasia detection was thencompared among the 4 groups in order to compare theoverall yield of each strategy. Dysplasia detection of VLEversus random biopsies was not compared within eachgroup for the following reasons: (1) not every patienthad random biopsies performed if advanced imaging was
Volume 88, No. 1 : 2018 GASTROINTESTINAL ENDOSCOPY 37

Random groupassigned foreligibility (N=79)
VLE group assignedfor eligibility(N=168)
VLEL group assignedfor eligibility(N=106)
Raised lesions(N=26)
Raised lesions(N=3)
Raised lesions(N=19)
Raised lesions(N=14)
Random Group(N=53)
Seattle Group(N=92)
VLE Group(N=149)
VLEL Group(N=92)
* Yield of Indefinite forDysplasia = 1
* Yield of LGD = 0
* Yield of neoplasia(HGD/IMCA) =2
* Yield of Indefinitefor Dysplasia = 11
* Yield of LGD = 7
* Yield of neoplasia(HGD/IMCA) =13
Overall dysplasiayield = 5.7%
Overall dysplasiayield = 19.6%
Overall dysplasiayield = 24.8%
Overall dysplasiayield = 33.7%
* Yield of Indefinitefor Dysplasia = 5
* Yield of LGD = 12
* Yield of neoplasia(HGD/IMCA) =1
* Yield of Indefinitefor Dysplasia = 11
* Yield of LGD = 9
* Yield of neoplasia(HGD/IMCA) = 17
Seattle groupassigned foreligibility (N=95)
Figure 3. The flowchart of the patients included and excluded for this study for each group with corresponding yields of dysplasia.
Incremental yield of dysplasia detection with VLE with laser marking in Barrett’s Alshelleh et al
used because this is acceptable in expert centers.6 (2)Once a VLE targeted biopsy specimen was taken, arandom specimen would not be taken from the samespot. Comparing these 2 groups would bias the resultsbecause the VLE targeted biopsies alter how randombiopsy specimens are usually obtained. In addition, theVLE targeted biopsies are biasing against the yield ofrandom biopsies, because the random biopsy will nevertarget a suspicious area defined by VLE. (3)Histopathologic collection practices did not allow forcomparison in many cases. For example, in many casesall histology at one level of the esophagus was placed inone jar (except for visible lesions on white-light endoscopyor NBI, which are placed in separate jars) to minimize pa-thology costs regardless of whether it was a random biopsyor VLE targeted. This allows a certain level of the esoph-agus to be targeted in future endoscopies but does notallow for comparison of random versus VLE targeted bi-opsies for the whole group.
38 GASTROINTESTINAL ENDOSCOPY Volume 88, No. 1 : 2018
Patient characteristics were extracted from the chartsand endoscopic characteristics of the BE were extractedfrom the endoscopy report. The length of the Barrett’ssegment was based on the maximal height or extent ofthe BE. The VLE procedure was performed as previouslydescribed in the literature.7,14 An expert in GI pathologyreviewed all pathology. If a diagnosis of dysplasia or cancerwas made, then the histology was reviewed in a consensusmeeting with 5 GI pathologists experienced in readingBE histology.
Statistical methodsThe mean and median of quantitative variables (eg, age
and length of BE in centimeters) were compared among 3groups (non-SP, VLE, and VLEL groups) with the referencecohort (SP). The Wilcoxon-Mann-Whitney test was used forcomparisons of median values and the paired t test usedfor comparison of mean values. The categorical variableswere compared using c2 tests or Fisher exact tests. All
www.giejournal.org

TABLE 1. Patient and procedure characteristics
Seattleprotocol
biopsy (N [ 92)
Non-Seattleprotocol
random biopsy(N [ 53) P value
Non-lasermarking VLE
directed biopsy(N [ 149) P value
Laser markingVLE directed
biopsy(N [ 92) P value
Age (years), mean 66 65 NS 66 NS 67 NS
Female (%) 32.61 (30/92) 26.4 NS 27.5 NS 28.3 NS
Length of Barrett’s (cm), median 3 1 <.0001 2 NS 3 NS
Length of Barrett’s (cm), average 3.36 1.61 <.0001 3.37 NS 3.68 NS
Treatment-naive, surveillance(no known dysplasia) (%)
58.7 (54/92) 50.9 (27/53) NS 22.8 (34/149) <.0001 18.5 (17/92) <.0001
Treatment-naive, surveillance(known dysplasia) (%)
15.2 (14/92) 9.4 (5/53) NS 20.8 (31/149) NS 21.7 (20/92) NS
Post-treatment surveillance (%) 26.1 (24/92) 39.6 (21/53) NS 56.4 (84/149) <.0001 59.8 (55/92) <.0001
Use of narrow-band imaging (%) 36 (33/92) 43 (23/53) NS 100 (149/149) <.0001 100 (92/92) <.0001
EMR (%) 0 3.8 (2/53) NS 6.0 (9/149) .0165 15.2 (14/92) <.0001
Seattle protocol biopsies performed (%) 100 0 <.0001 25.5 (38/149) <.0001 30.4 (28/92) <.0001
P values are based on and compared with the Seattle protocol biopsy group.VLE, Volumetric laser endomicroscopy without laser marking; NS, not significant (P > .05).
Alshelleh et al Incremental yield of dysplasia detection with VLE with laser marking in Barrett’s
statistical tests were 2-sided, and P< .05 was consideredsignificant. All analyses were conducted using SAS version9.4 (SAS Institute Inc, Cary, NC).
RESULTS
A total of 448 consecutive patients (79 RB, 95 SP, 168VLE, and 106 VLEL) met the inclusion criteria. However, atotal of 386 consecutive patients were analyzed. Sixty-twopatients were excluded for raised or suspicious lesionsseen on high-definition white-light endoscopy or NBI (seeFig. 3 for details). Fifty-three patients were analyzed in theRB group, 92 in the SP group, 149 in the VLE group, and92 in the VLEL group. Patient and endoscopic characteristicsfor each group can be found in Table 1. The average lengthof BE was similar in each group except the RB group. Thepercentage of treatment-naive patients in each group was60%, 74%, 44%, and 40%, respectively, for the RB, SP,VLE, and VLEL groups. A similar rate, 25.5% versus 30.4%of patients, underwent Seattle protocol biopsies in theVLE versus VLEL groups, respectively (P Z .4054).
The overall dysplasia yield for each group was 5.7%,19.6%, 24.8%, and 33.7% for the RB, SP, VLE, and VLELgroups, respectively (Table 2). The VLEL group had astatistically higher yield of dysplasia detection (P Z .03versus the SP group with an odds ratio of 2 (P Z .03).The VLE group did not have statistically significantdifferences in dysplasia detection compared with the SPgroup (P Z .35). The VLE and VLEL groups did not havestatistically significant differences in overall dysplasia yieldwhen compared with each other (P Z .14). The RBgroup had a statistically significant lower yield ofdysplasia detection versus the SP group (P Z .02). The
www.giejournal.org
odds ratios for detection of dysplasia compared with theSP group were 0.25 (95% confidence interval [CI], 0.07-0.88), 1.36 (95% CI, 0.7-2.6), and 2.1 (95% CI, 1.07-4.1)for the RB, VLE, and VLEL groups, respectively. Thespecific details for each dysplasia grade can be found inTable 2. Both VLEL and VLE groups had statisticallysignificant differences in neoplasia (high-grade dysplasiaand intramucosal cancer) detection compared with theSP group (14% vs 1%, P Z .001 and 11% vs 1%,P Z .003, respectively).
Subgroup analysis was performed to determine ifdysplasia yield was biased by the surveillance category(pre-treatment vs post-treatment surveillance) becausethe VLE groups contained more patients who underwentpost-treatment surveillance. There were 202 patients inthe treatment-naive group and 184 patients were surveyedafter treatment. Twenty-two percent of patients from thetreatment-naive group had dysplasia versus 24% of patientsfrom the post-treatment group. There was no statisticallysignificant difference between these 2 groups (22% vs24%, P Z .7).
In addition, subgroup analysis was performed looking attotal dysplasia and neoplasia (high-grade dysplasia/intra-mucosal cancer) yield for each surveillance strategy(SP vs VLE vs VLEL) for each surveillance category (pre-treatment vs post-treatment) to determine whether thepost-treatment surveillance group was confounding the re-sults (Table 3). Table 3 shows that the VLEL surveillancestrategy yielded more neoplasia detection than the SPstrategy for the treatment-naive surveillance cohort aswell as the post-treatment surveillance cohort. For thetreatment-naive with known dysplasia cohort, there wereno statistical differences in dysplasia or neoplasia yield be-tween the VLEL and SP groups.
Volume 88, No. 1 : 2018 GASTROINTESTINAL ENDOSCOPY 39

TABLE 2. Dysplasia yield of surveillance strategies
Seattle protocolbiopsy (N [ 92)
Non-Seattleprotocol randombiopsy (N [ 53) P value
Non-lasermarking VLE
directed biopsy(N [ 149) P value
Laser markingVLE directed
biopsy (N [ 92) P value
Yield of IND (%) 5.4 (5/92) 1.9 (1/53) NS 7.4 (11/149) NS 12.0 (11/92) NS
Yield of LGD (%) 13.0 (12/92) 0 NS 6.0 (9/149) NS 7.6 (7/92) NS
Yield of neoplasia (HGD/IMCA) (%) 1.1 (1/92) 3.8 (2/53) NS 11.4 (17/149) .003 14.1 (13/92) .001
Overall dysplasia/neoplasia yield (%) 19.6 (18/92) 5.7 (3/53) NS 24.8 (37/149) NS 33.7 (31/92) .03
P values are with regard to a positive yield of dysplasia seen in comparison with Seattle protocol biopsies.VLE, Volumetric laser endomicroscopy without laser marking; IND, indefinite for dysplasia; NS, not significant (P > .05); LGD, low-grade dysplasia; HGD, high-grade dysplasia;IMCA, intramucosal cancer.
Incremental yield of dysplasia detection with VLE with laser marking in Barrett’s Alshelleh et al
The percentage of patients undergoing EMR of flat areaswithin the BE segment was 3.8%, 0%, 6%, and 15.2% in theRB, SP, VLE, and VLEL groups, respectively. Whencompared with the control group (SP), there was a statisti-cally higher rate of EMR performed on flat areas in the VLEand VLEL groups (Table 2). There was a statistically higherrate of EMR of flat areas between VLE and VLEL (6% vs15%, P Z .02). In the VLE group, 6 of 9 patients whohad EMR were positive for any form of dysplasia. Of 14patients who had EMR in the VLEL group, 4 werepositive for any form of dysplasia.
In order to adjust for a learning curve of VLE, the first 25cases from each attending who performed VLE in thiscohort were excluded from the VLE without laser groupto determine if this affected the results. We found thatexcluding the first 50 cases (25 from each attending) didnot change the overall results. The overall dysplasia yieldin the VLE group was 26.3%. There was no statistically sig-nificant difference between the SP group and VLE group inoverall dysplasia detection (P Z .28). The overall neoplasiayield in the VLE group was statistically significant comparedwith the SP group overall neoplasia yield (13.1% vs 1%;P Z .0015). There was no statistically significant differencebetween both VLE groups (VLE vs VLEL) when they werecompared with each other (P Z .26).
DISCUSSION
In this study, we aimed to show the incremental yield ofsurveying BE with VLE and VLEL compared with a standardof care random biopsy cohort. We found that surveying BEwith VLEL resulted in a statistically significant higher yieldof dysplasia detection and advanced neoplasia comparedwith the standard SP group. These results support theuse of surveillance of BE with VLEL in academic centers,especially because there is a high interobserver agreementin image interpretation in VLE between academiccenters.18 These results are encouraging as previousliterature has shown that SP protocols can missadvanced neoplasia,5 and thus VLEL is a potential tool toovercome this.
40 GASTROINTESTINAL ENDOSCOPY Volume 88, No. 1 : 2018
These results also show, not surprisingly, that VLE withlaser marking can target more dysplasia than VLE withoutlaser marking because of the placement of precise lasermarks to target tissue.17 The laser marking technologyallows for marking of suspected neoplasia. Before themarking technology, trying to target a suspicious areawith VLE, based on the registration lines from the scanand the balloon, was challenging. The increased yield ofdysplasia in the VLEL group is not related to an increasedrate of EMR (increased surface area of mucosa sampledin an EMR specimen vs a pinch biopsy). Surprisingly, theyield of dysplasia of the EMR specimens for the VLELgroup was lower than for the VLE group. However, it isencouraging that the pinch biopsies from the laser marksallow for precise targeting of dysplasia.
Our study has some notable strengths. This study con-tains a standard of care group where VLE was not per-formed. This is a true control and is more sound thancomparing histology from random biopsies versus VLE tar-geted samples taken during the same procedure; asdescribed in the Methods there are flaws to this approach.This study is from a center that specializes in BE and thusthe numbers in each group are robust enough to allowcomparisons. The VLE procedures were performed by 2high-volume endoscopists for VLE, making the resultsmore generalizable than if they were from 1 endoscopist.Both endoscopists performed a similar number of VLEexaminations in both the VLE and VLEL groups. Our centerused VLE with and without laser marking; we are able tomake comparisons between these groups. Moreover, thisstudy excluded cases with visible abnormalities seen onhigh-definition white-light endoscopy and NBI, and thusevaluated only a population where advanced imagingwould be needed. Finally, histology with dysplasia was re-viewed in a consensus meeting of 5 GI pathologists expe-rienced in reading BE histology, making these results morereproducible.
This study has limitations. This study is retrospective innature and thus has the limitations inherent to retrospec-tive studies. Ideally, VLE targeted and random biopsiesfrom each level would have been placed in separate pathol-ogy jars for analysis to determine if the random biopsies in
www.giejournal.org

TABLE 3. Dysplasia and neoplasia yield analyzed by surveillance category
Seattleprotocolbiopsy
(N [ 92)
Non-Seattleprotocol randombiopsy (N [ 53) P value
Non-lasermarking VLE
directed biopsy(N [ 149) P value
Laser markingVLE directed
biopsy (N [ 92) P value
Treatment-naive, surveillance (no known dysplasia; N Z 132)
Total dysplasia (%) 16.7 (9/54) 0 (0/27) NS 8.8 (3/34) NS 35.3 (6/17) NS
Neoplasia (HGD/IMCA) (%) 0 (0/54) 0 (0/27) NS 2.9 (1/34) NS 11.8 (2/17) .01
Treatment-naive, surveillance (known dysplasia; N Z 70)
Total dysplasia (%) 50 (7/14) 60 (3/5) NS 48.4 (15/31) NS 35 (7/20) NS
Neoplasia (HGD/IMCA) (%) 7.1 (1/14) 40 (2/5) NS 19.4 (6/31) NS 5 (1/20) NS
Post-treatment surveillance (N Z 184)
Total dysplasia (%) 8.3 (2/24) 0 (0/21) NS 22.6 (19/84) NS 32.7 (18/55) .02
Neoplasia (HGD/IMCA) (%) 0 (0/24) 0 (0/21) NS 11.9 (10/84) NS 18.2 (10/55) .03
P values are with regard to a positive yield of dysplasia seen in comparison with Seattle protocol biopsies. Total dysplasia includes IND, LGD, HGD, and IMCA. Neoplasia includesHGD and IMCA.VLE, Volumetric laser endomicroscopy without laser marking; NS, not significant (P > .05); HGD, high-grade dysplasia; IMCA, intramucosal cancer; IND, indefinite for dysplasia;LGD, low-grade dysplasia.
Alshelleh et al Incremental yield of dysplasia detection with VLE with laser marking in Barrett’s
the VLE cohorts added additional yield. This would helpdetermine whether random biopsies are needed if VLE tar-geted biopsies are performed. However, in clinical practicethis is not done because it adds enormously to the pathol-ogy costs of the procedure. In practice, it is standard ofcare to separate pathology based on suspicious or raisedlesions by white-light endoscopy and NBI only and notnecessarily VLE.19
As stated in the results, the VLE groups contained agreater number of patients with post-treatment surveil-lance, whereas the SP group contained a higher numberof treatment-naive surveillance without known dysplasia.For the subgroup analysis, the total number of patientswith dysplasia did not differ between the treatment-naiveand post-treatment surveillance groups. When analyzedby surveillance strategy (Table 3), VLEL still detectedmore neoplasia in the treatment-naive group withoutknown dysplasia than in the SP group. Thus, the incremen-tal yield of dysplasia detection in the VLEL was not justseen in the post-treatment surveillance group. The sub-group analysis in our study shows that VLE with lasermarking may have its greatest benefit in treatment-naivepatients with no known dysplasia undergoing surveillanceand in post-treatment surveillance. Expectedly, VLEL didnot show an incremental yield in patients withknown dysplasia undergoing surveillance, becausedysplasia was already detected using standard biopsy pro-tocols in the past.
The SP biopsy group involved experienced endoscopistswho do not perform advanced imaging, whereas the VLEgroups were performed by 2 endoscopists who specializein advanced imaging and ablation in BE. We do not feelthat the quality of SP biopsies differs between the endo-scopists because pathology reports were abstracted toensure the correct number of biopsy specimens were
www.giejournal.org
taken per BE length. Finally, adjustment for the initiallearning curve of VLE for the VLE group was not per-formed. However, the results did not change when the first50 cases were excluded from the VLE group, as describedin the results.
All procedures in each group were performed at thesame academic BE tertiary care referral center. Althoughthis controls for heterogeneity among the groups (differ-ences in medical center practices, pathology readings,etc), these results are not applicable for widespread useof VLE in the community, just to academic centers. More-over, our surveillance cohorts had a high rate of dysplasia(19%-30%) among the SP, VLE, and VLEL groups. This maybe explained by the study being performed in an academiccenter where there is referral bias (eg, low-grade dysplasiacases being referred for surveillance or patients being sur-veyed after treatment who may be at higher risk for recur-rence of dysplasia).
The use of NBI was at the discretion of the endoscop-ists. The current guidelines recommend the use of high-definition and high-resolution white-light endoscopy forsurveillance in BE.2 The routine use of NBI is notmandated in the guidelines. Not surprisingly, the use ofNBI was 100% in this study in the VLE and VLEL groupsbecause these groups were from 2014 onward when datashowed that the use of NBI can reduce the number ofbiopsies performed, although it is not superior to high-definition white-light endoscopy from a randomized con-trol trial.20 The routine use of NBI in the VLEL and VLEgroups actually bias against these groups; if a lesion wasfound on NBI, it was excluded from this study, and thuswould lower the dysplasia yield for that group. On theother hand, it is possible that the SP or RB groupsincluded a lesion that may have been detected with NBIand would have otherwise been excluded from the
Volume 88, No. 1 : 2018 GASTROINTESTINAL ENDOSCOPY 41

Use your mobile device to scan thisQR code and watch the author in-terview. Download a free QR codescanner by searching “QR Scanner”in your mobile device’s app store.
Incremental yield of dysplasia detection with VLE with laser marking in Barrett’s Alshelleh et al
study; this may artificially increase the dysplasia yields ofthese groups. This is a moot point given the VLEL grouphad a statistically higher yield of dysplasia detectionversus the SP group.
In conclusion, we show that a surveillance strategy thatinvolves VLEL may detect statistically significantly moredysplasia and neoplasia compared with the current stan-dard of care SP biopsies. In addition, it may allow formore targeted endoscopic resection of dysplasia, whichhas advantages for pathologists for diagnosing dysplasia.21
Further prospective studies, preferably randomizedcontrolled studies, need to be performed to confirm ourretrospective results on the increased yield of dysplasiadetection in BE screening with the use of VLE with lasermarking.
REFERENCES
1. Spechler SJ, Souza RF. Barrett’s esophagus. N Engl J Med 2014;371:836-45.
2. Shaheen NJ, Falk GW, Iyer PG, et al. ACG clinical guideline: diagnosisand management of Barrett’s esophagus. Am J Gastroenterol2016;111:30-50; quiz 51.
3. Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablationin Barrett’s esophagus with dysplasia. N Engl J Med 2009;360:2277-88.
4. Phoa KN, van Vilsteren FGI, Weusten BLAM, et al. Radiofrequency abla-tion vs endoscopic surveillance for patients with Barrett esophagusand low-grade dysplasia: a randomized clinical trial. JAMA 2014;311:1209-17.
5. Sharma P, Falk GW, Weston AP, et al. Dysplasia and cancer in a largemulticenter cohort of patients with Barrett’s esophagus. Clin Gastroen-terol Hepatol 2006;4:566-72.
6. Sharma P, Brill J, Canto M, et al. Advanced imaging in Barrett’s esoph-agus. Clin Gastroenterol Hepatol 2015;13:2209-18.
7. Trindade AJ, Smith MS, Pleskow DK. The new kid on the block foradvanced imaging in Barrett’s esophagus: a review of volumetric laserendomicroscopy. Therap Adv Gastroenterol 2016;9:408-16.
8. Wolfsen HC, Sharma P, Wallace MB, et al. Safety and feasibility of volu-metric laser endomicroscopy in patients with Barrett’s esophagus (withvideos). Gastrointest Endosc 2015;82:631-40.
9. Evans JA, Poneros JM, Bouma BE, et al. Optical coherence tomographyto identify intramucosal carcinoma and high-grade dysplasia in Bar-rett’s esophagus. Clin Gastroenterol Hepatol 2006;4:38-43.
10. Leggett CL, Gorospe EC, Chan DK, et al. Comparative diagnostic perfor-mance of volumetric laser endomicroscopy and confocal laser endomi-croscopy in the detection of dysplasia associated with Barrett’sesophagus. Gastrointest Endosc 2016;83:880-8.e2.
11. Trindade AJ, Vamadevan AS, Sejpal DV. Finding a needle in a haystack:use of volumetric laser endomicroscopy in targeting focal dysplasia inlong-segment Barrett’s esophagus. Gastrointest Endosc 2015;82:756-7.
12. Trindade AJ, George BJ, Berkowitz J, et al. Volumetric laser endomicro-scopy can target neoplasia not detected by conventional endoscopicmeasures in long segment Barrett’s esophagus. Endosc Int Open2016;4:E318-22.
13. Leggett CL, Gorospe E, Owens VL, et al. Volumetric laser endomicro-scopy detects subsquamous Barrett’s adenocarcinoma. Am J Gastroen-terol 2014;109:298-9.
14. Trindade AJ, Leggett CL, Chang KJ. Volumetric laser endomicroscopy inthe management of Barrett’s esophagus. Curr Opin Gastroenterol2017;33:254-60.
42 GASTROINTESTINAL ENDOSCOPY Volume 88, No. 1 : 2018
15. Trindade AJ, Inamdar S, Sejpal DV, et al. Targeting neoplasia usingvolumetric laser endomicroscopy with laser marking. Endoscopy2017;49(Suppl 1):E54-5.
16. Trindade AJ, Inamdar S, Sejpal DV. Dysplasia detection in Barrett’sesophagus by use of volumetric laser endomicroscopy with lasermarking. VideoGIE 2017;2:217-8.
17. Swager A, de Groof AJ, Meijer SL, et al. Feasibility of laser marking inBarrett’s esophagus with volumetric laser endomicroscopy: first-in-man pilot study. Gastrointest Endosc 2017;86:464-72.
18. Trindade AJ, Inamdar S, Smith MS, et al. Volumetric laser endomicro-scopy in Barrett’s esophagus: interobserver agreement for interpreta-tion of Barrett’s esophagus and associated neoplasia amonghigh-frequency users. Gastrointest Endosc 2017;86:133-9.
19. Sharma P, Katzka DA, Gupta N, et al. Quality indicators for the manage-ment of Barrett’s esophagus, dysplasia, and esophageal adenocarci-noma: international consensus recommendations from the AmericanGastroenterological Association Symposium. Gastroenterology2015;149:1599-606.
20. Sharma P, Hawes RH, Bansal A, et al. Standard endoscopy with randombiopsies versus narrow band imaging targeted biopsies in Barrett’soesophagus: a prospective, international, randomised controlled trial.Gut 2013;62:15-21.
21. Wani S, Mathur SC, Curvers WL, et al. Greater interobserver agreementby endoscopic mucosal resection than biopsy samples in Barrett’sdysplasia. Clin Gastroenterol Hepatol 2010;8:783-8.
Abbreviations: BE, Barrett’s esophagus; CI, confidence interval;NBI, narrow-band imaging; OCT, optical coherence tomography;RB, random biopsies; SP, Seattle protocol; VLE, volumetric laser endo-microscopy without laser marking; VLEL, volumetric laser endomi-croscopy with laser marking.
DISCLOSURE: Dr Sejpal has served on an Advisory Board for Ninepoint.All other authors disclosed no financial relationships relevant to thispublication.
Copyright ª 2018 by the American Society for Gastrointestinal Endoscopy0016-5107/$36.00https://doi.org/10.1016/j.gie.2018.01.032
Received September 11, 2017. Accepted January 23, 2018.
Current affiliations: Zucker School of Medicine at Hofstra/Northwell,Northwell Health System, Division of Gastroenterology, Department ofMedicine, Long Island Jewish Medical Center, New Hyde Park, New York,USA.
Presented at the Esophagus Plenary Session, American College ofGastroenterology Meeting, October 18, 2017, Orlando, Florida, USA (AmJ Gastroenterol 2017;112:S168-S225).
Reprint requests: Arvind J. Trindade, Director of Endoscopy, Zucker Schoolof Medicine at Hofstra/Northwell, Long Island Jewish Medical Center,Northwell Health System, Division of Gastroenterology, 270-05, 76thAvenue, New Hyde Park, NY 11040.
If you would like to chat with an author of this article, you may contactDr Trindade at [email protected].
www.giejournal.org