1 molecular shape, architecture and size of p2x4 receptors ...
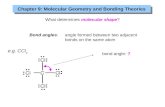
How Molecular Shape Determines Molecular Behavior
89
How Molecular Shape Determines Molecular Behavior Examples of Non-Polar Covalent Molecules H 2 O 2 Cl 2
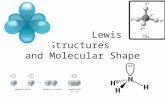
Transcript of How Molecular Shape Determines Molecular Behavior
How Molecular Shape Determines Molecular Behavior
Examples
of Non-Polar
Covalent Molecules
H2
O2
Cl2
HCl Fountain Demo
Polar Covalent Molecules
have a positive end and a negative end.
H2O
HCl
NH3
Ammonia Fountain Demo
Is NH3 Polar Covalent or Non-Polar Covalent ?
Trigonal Pyramidal Shape of NH3 from VSEPR Theory
Ammonia Fountain Demo
Ionic Solids, like salts, form extremely
strong Ionic Bonds due to strong
Electrostatic forces of attraction
Between positive and negative ions.
Because of dipole-dipole interaction,
The positive hydrogen end of one water
molecule “bonds” with the negative
oxygen end of another water molecule.
This is called Hydrogen Bonding.
Can Crush Demo
Barrel Crush Demo