Fini, M.E. Et Al. 1997. Perspectives on Eye Development
Transcript of Fini, M.E. Et Al. 1997. Perspectives on Eye Development
-
8/13/2019 Fini, M.E. Et Al. 1997. Perspectives on Eye Development
1/11
REVIEW ARTICLE
Perspectives on Eye Development
M. ELI ZABETH FINI,* KATHERINE J . STRISSEL, AND J UDITH A. WEST-MAYS
Developmental Bi ology Un it of the Vision Research L aborat ories, Departments of Oph thal mology, and Anatomy and
Cell ul ar Bi ology, N ew Engl and M edi cal Center and Tufts U ni versity School of M edi cin e, Boston, M assachusetts
ABSTRACT The lens of the vertebrate eye wasthe classic model used to demonstrate the concepts of
inductive interactions controlling development. H ow-
ever, it is i n the D rosop hila model that the greatest
progressin understanding molecular mechanismsof eye
development have most recently been made. This
progress can be attributed to the power of molecular
genetics, an approachthatwasonce confined to simpler
systems like worms a nd flies, but is now becoming
possible in vertebrates. Thus, the use of transgenic and
knock-outgene technology, coupled with the availability
of new positional cloning methods, hasrecently initiated
a surge of progress in the mouse genetic model and has
also led to the identification of genesi nvolved i n human
inherited disorders. In addition, gene transfer techniques
have opened up opportunities for progress using chick,
Xenopus, and other classic developmental systems.
Finally, a new vertebrate genetic model, zebrafish,
appearsvery promising for molecular studies. A sa result
of the opportunitiespresented by these new approaches,
eye development has come into the limelight, hence the
timeliness of this focus issue ofD evelopm entalG enetics.
In this i ntroductory review, we discuss three areas of
current work arising through the use of these newer
genetic approaches, and pertinent to research articles
presented herein. W e also touch on related studies
reported at the first Keystone M eeting on O cular C ell
and M olecular Biology, recentlyheld in TamarronSprings,
C olorado, January712 , 1 997 . D ev. G enet. 2 0: 175
185, 1997. r 1997 W iley-Liss, Inc.
Key words:mouse; zebrafish;D rosophila;eye; devel-opment
UNITING DEVELOPMENTAL MECHANISMS I NVERTEBRATES AND FLIE S
Th e D r o sop h i l a eye is structurally and functionallyq u it e di f f e re n t f ro m t h e v e rt e bra t e e y e a n d t h e t woorgans have been historically thought to have arisensepar at ely during evolution. However, a ma jor switchin paradigm came with recent characterizat ion of the
Dr osophi la eyelessgene as the homologue of the verte-bra t e g en e , PAX6 [Quiring et a l ., 1994]. O f e q u a lsignificance, PAX6 w a s a l s o f o u n d t o f u n c t i o n a s ama ster contr ol gene, a long-sought developmenta lr eg u la t o r w i t h t h e c a p a ci t y t o s w i t ch on a n e nt i r eg en e t ic p rog ra m f or org a n f orma t io n [H a lder et a l .,1995]. This demonstrationthat ectopic expression ofeyeless could cause the appearance of fully formed andfunctional compound eyes to appear on the wings ora n t e n n a e s p a rk ed t h e ima g in a t ion of s cien t is t s a n dnonscientists alike. It is not surprising, therefore, thateyeless/ PAX6was chosen as a runner-up for molecule ofthe year by the journa l,Science, in 1995.
Th e fi rs t con n e ct ion bet we e n t h e e y e o f fl ies a n dvertebrates came with the discovery of the molecularl in k bet we e n t h e h u ma n g e ne t ic s y n dro me, a n ir idia ,t h e small eye(sey) phenotype in mice and ra ts, a nd th eeyelessphenotype in flies. It h a d been known for severa lyears tha t muta t ions in thePAX 6gene cause anir idia inhumans, a syndrome characterized by underdevelop-ment of the iris, with associated anomalies includinglens cataracts, corneal vascularizat ion, and glaucoma[Ton et al., 1991; Glaser et al., 1992; Ha nsen a nd VanHeynin gen, 1995]. Mut a tions in PAX6have since beendemonstrated to underlie other anterior segment disor-de rs s u c h a s P e t e r s a n o ma ly , wh ic h ma n if e s t s a s acentra l corneal opacifi cat ion and adh esion betw een t hecornea and lens [Ha nsen et a l ., 1994], or autosomaldominan t kera t it is , chara cterized by corneal opacifica-t ion and vascularizat ion [Mirzanya ns et al ., 1995]. Thesmall eyephenotype in mice a nd ra ts, wh ich results in asimilar spectrum of abnorma lit ies in heterozygotes,
and complete absence of eyes in homozygotes, was alsodiscovered to result from mut at ions in t he rodent PAX6gene h omologue [Wa lth er e t a l ., 1991]. Sequencing of
Received 21 Febru ar y 1997; a ccepted 4 Ma rch 1997.
*Correspondence to: M. Elizabeth Fini, Developmenta l Biology Unit ofthe Vision Research Laboratories, New England Medical Center, 750
Was hingt on St ., B ox 450, Boston, MA02111. Ema il: [email protected] s.edu
DE VELOPME NTAL GENETI CS 20:175185 (1997)
r 1997 WILEY-LISS, INC.
-
8/13/2019 Fini, M.E. Et Al. 1997. Perspectives on Eye Development
2/11
-
8/13/2019 Fini, M.E. Et Al. 1997. Perspectives on Eye Development
3/11
extended period of time [Wetts et al., 1989]. In teleostfi sh, such a s the goldfi sh or the zebrafi sh, annuli of newret ina continue to be added throughout life from thisg ermin a l zon e . As a re s ult of t h e p ers ist e n ce of t h eg ermin a l zon e , da ma g ed re t in a ca n be re g en e ra t e dthr oughout life in t hese species [Ra ymond, 1995; Sulli-
va n et al., 1997, (this issue)].Many genes controlling progress of the morphoge-
n e t ic f u rro w a n d s u bse q u en t di ff ere n t ia t io n of t h eom a t i d ia h a v e b ee n i d en t i fi e d i n Dr o so p h i l a [Ven-k a t e s h , 1993; Ca g a n , 1993]. Ce n t ra l a mo n g t h e s e isdecapentaplegic(d pp), wh ich encodes a secreted signa l-ing molecule related to vertebrate bone morphogeneticproteins (BMPs) of the TGF-b family of extracellulargrowth factors. Decapentaplegic is expressed aroundt h e r im o f t h e e y e ima g in a l dis c , be f o re t h e f u rro wbeg in s i t s p rog re ss a c ros s t h e p rog e nit or fi e ld, a n ddecapentaplegic protein is required for furrow init ia-t ion a nd progression [Cha nut an d Heberlein, 1996].The role of d ecapent apl egicis not t o contr ol cell fat e, but
to a id in synchronizat ion of the cell division cycle, bypromoting progression through the G 2 and M pha ses ofthe cell cycle [Penton et a l ., 1997]. Decapentaplegicin t era c t s w it h a s econ d g e n e, hedgehog (h g), whichencodes a signaling protein expressed just posterior tot h e f u rrow. H e dge h og p rot e in di ff u s es f orwa rd a n dtriggers expression of decapentaplegic wi t h in t h e f u r-row [Heberlein et al., 1993; Ma et al., 1993]. Decapen-taplegic is e ss en t ia l f or ma n y e a rly de ve lop men t a levents in flies including establishment of the body planin e a rly e mbry os , h owe v er , s e v era l decapentaplegica lleles a ffect only eye morphogenesis, and this h as beenuseful in ascertaining its r ole in this process. Some ofthese a lleles, such a s b l i n k (d ppbl k), cause a n a symmet-
ric loss of retinal tissue. The primary defect in b l i n k iss h o wn in t h is is s u e o f Developm ent al Genetics t o in -volve a failure to express d ecapent apl egicact ivity at thev en t ro pos t e rior ma rg in of t h e e y e dis c [Ch a n u t a n dHeber lein, 1997].
The vert ebra te homologues ofd ecapent apl egic, B M P 2and BMP-4, are both expressed in the vertebrate eye.Predictably, BMP-4 knockout mice die very early indevelopment, before formation of the eye [Winnier etal ., 1995]. Thus, the role of decapentaplegic in verte-bra t e e y e mo rp h o g e n e s is is s t i l l u n c e rt a in . M o re isknown about hedgehoghomologues; thus, in zebrafi sh,it was recently shown that two hedgehog family mem-bers, t iggy-winkle hedgehog a n d sonic hedgehog, a r e
expressed in t he fl oor of th e diencephalon portion of thede ve lop in g bra in , loca t e d bet we e n t h e s i t es of t h efuture optic stalks. Ectopic expression of either hedge-h oggene promotes proximal fate and suppresses distalfate in th e developing eye. In contra st , proximal fat eswere lost in cyclops muta nt embry os, w hich lackhedge-h og-expressing forebrain cells [Ekker et al., 1995].
Another important gene act ing after the morphoge-netic furrow in flies isN otch, which encodes a tra nsmem-bra n e g ly c o p ro t e in s h o wn t o be imp o rt a n t in n e u ra l
development. Studies on temperature sensitive allelesin D r o sop h i l a h a v e in dica t e d t h a t Notch regulates thenumber of cells that differentiate into neurons at themorphogenetic furrow in th e eye imagina l disc [Ca ganand Ready, 1989]. N otch is also expressed in vertebrateretina. In chickens, inhibit ion of Notch expression by
delivery of a ntisense oligonucleotides resulted in asignificant overproduction of ganglion cells as opposedto other retin a l cell types [Cepkoet al., 1996]. G a nglioncells are the first of the different ret inal cell types todiff ere n t ia t e , s u g ge st in g t h a t t h e role o f Notch i s t oinhibit differentiation of retinal cell precursors so thatthey can have t ime to receive the necessary inductivesignals to become other cell types. In support of thishypothesis, infect ion of ret inas with a retrovirus thatoverexpresses a constitutively active version of Notchprotein, led to a reduction in the number of ganglioncells. Similarly, co-culture of D r o sop h i l a cells express-ing the Notch ligan d, delta, with chicken ret ina l cells,significantly inhibited differentiation of ganglion cells.
Notch is also expressed in undifferentiated precursorcells of the la te embryonic centra l retina in Xenopus(a swell as within the germinal zone), and transfect ion ofan act ivated form of the gene into isolated ret inal cellscauses them to reta in a neuroepithelial morphology[Dorsky et al., 1995]. Raymond et al . [1997] (this issue)demonstrate expression of Notch wi t h in t h e g e rmin a lzone of goldfish ret ina. These data, together with theprevious reports, suggest that Notch is responsible forma inta ining cells of the germina l zone in a undifferenti-ated state, hence the regenerative capacity of the retinain this species.
The histologically obvious pattern of organization inthe vertebrate ret ina is a strat ificat ion of the different
neural types, a layered patt ern tha t seems quite differ-ent from the repeating omatidial unit arrangement oft h e D r o sop h i l a retina. What is not obvious without theuse of specific cell type ma rkers is tha t t he vertebrateretina also contains radially arranged or columnar u n it s a l i gn ed a t cr os s -a n g l es t o t h e r et i n a l l a y er s[Reichenbach et al., 1994]. These un its a re composed ofspecies-specific numbers of the different neuronal celltypes a rra nged a round a Muller glial cell, which formsthe central frame. The arrangement of the photorecep-tor cells in teleost fi sh, which form highly regular a ndrepeatin g mosaic unit s of three or four different types ofcone cells, is even more reminiscent of th e fly omat idia[Raymond, 1995]. The omatidium in Dr o so p h i l a simi-
larly contains a regular array of eight different photore-ce pt o r t y p es (R 1 t o R 8). N e uron a l org a n iza t ion istypically chara cterized by part it ioning or clusteringin t o re p ea t in g f u n ct ion a l u n it s, a n d t h e ref ore , t h esimilar ity of ret ina l pat terning between flies and verte-brates might not necessarily indicate common origin.Nevertheless, the st riking similar ity in t he developmen-ta l processes that lead to these repeat ing units in bothcases, increases the possibility of homology. Thus, thecommitment of presumptive photoreceptor cells to a
PERSPECTIVES ON EYE DEVELOPMENT 177
-
8/13/2019 Fini, M.E. Et Al. 1997. Perspectives on Eye Development
4/11
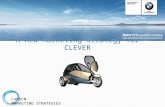
Fi g . 1 . C ompara t i ve e y e de ve lopme nt i n ve rtebrate s and fly . E y edevelopment in vertebra tes involves contr ibutions from several differ-ent embryonic tissues: the surface ectoderm, the neura l tube, and t hene ural c re st . The proc e ss be g i ns at the e nd of g astrul at i on, whe nbilatera l retinal fi elds are specified a s thickened zones in the neuroepi-
thelium of the forming neural tube at the anterior end of the embryo.The neuroepith elium in each fi eld folds to form t he optic sulcus, whichenlarges to form t he optic pit. (1) With t he closure of the n eural t ube,the optic pits are pushed outward resulting in formation of the opticvesicle (OV), connected to the brain cavity by the optic stalk (OS).
Interaction between the neuroepithelium of the optic vesicle and thesurfac e e ctoderm re sults i n i nduct i on of the l ens pl acode (L Pl ) , adisc-sha ped region of th ickened ectoderm. (2) As the optic vesiclebegins to collapse inw ar d forming t he optic cup (OC) the lens placodeinvaginates to form the lens pit (LPi). (3) The inner layer of t he opticcup ultimately forms th e neural r etina (NR); the outer la yer becomes
the re t i nal pi gme nte d e pi thel i um ( PE ) . The l e ns pi t c ont i nue s t oinvaginate and eventually separates from the surface ectoderm form-ing a hollow lens vesicle (LV) composed of a single layer of epithelialcells. The basal cells of the remaining surface ectoderm, destined tobecome the corneal epithelium, then secrete collagen, which a ccumu-lates to form the primary stroma of the cornea (C). Further develop-
ment of the cornea is initiated when a wave of neural crest cells (NC)
m i gr a t e s u n d er t h e s t r o m a t o f o r m a s in g le l a y er of ce ll s t h eendotheliumwhich secrete hyaluronic acid into the stroma causingit to swell. A second wa ve of neura l crest cells then ent ers the stroma ,re move s the ol d matri x an d re plac e s it w i th an ne w l ame l lar arra y ofco ll a g en . Th i s s eco n da r y s t r om a i s fi n a l ly d eh y d r a t e d u n d er t h e
i n fl u en ce of t h e t h y r oi d g la n d l ea d i n g t o t h e d ev el op m en t o f atran spare nt t i ssue whi ch fre e ly a l lows the passag e of l i g ht .(4) Next,the posterior cells of the vesicle elongate, forming the primary lensfi b e r s , w h i ch u l t im a t e ly ob li t er a t e t h e l u me n of t h e v es i cl e. (5)Continued differentiation gives rise to the adu lt str uctures of the eye.
The e pi the l i al c e l l s at the e q uator of the l e ns c ont i nue to unde rg odi visi on throug h to t e rm a nd throug hout l i fe formi ng the se c ondaryl e ns fibe rs. I nte rac t i on of the l e ns ( L ) wi th the ove rl y i ng surfac eectoderm leads to its differentiation into a multilayered epithelium.Development of the retin a contin ues as t he lips of the optic cup becomethe iris and ciliary body. Cells in the inner neural retina layer of the
optic cup proliferate, then differentiate to form a variety of differentne ural and g l i al c el l ty pes ar rang e d i n a l ay e red pat t e rn. I n humansan d rodents, development of the neura l retina is not yet completed atb ir t h a n d c on t i n u es p os t n a t a l l y. Ax on s f r om t h e g a n g li a of t h einnermost cell layer of neural retina grow toward a common meetingplace at t he back of the eye an d continue down the optic stalk to form
the opt i c ne rve ( O N) . C onne c t i ons are made by the se axons to the
178 FI NI E T A L .
-
8/13/2019 Fini, M.E. Et Al. 1997. Perspectives on Eye Development
5/11
s pe cifi c iden t i t y in Dr o so p h i l a in volv es a s eries ofinductive events in which the spacing and pattern oft h e o ma t idia l u n it s a re fi rs t e s t a bl is h e d by t h e e a rlycommitment of the R8 photoreceptors [Cagan, 1993].Th e re is g ro win g e v ide n ce t h a t t h is is a ls o t ru e invertebrates; for example, it has been suggested that the
Muller glial cell a cts to organize development of th eretinal columnar unit [Reichenbach et al., 1994; Snowa nd Robson, 1995] a nd th a t th e photoreceptor mosaic inteleost fish might arise from an init ial prepattern laiddown by a specifi c cone subtype [reviewed in Ra ymond,1995].
Many of the genes involved in D r o sop h i l a o ma t idia ldifferentiat ion ha ve been identified. B y contra st , thereha s been correspondingly less progress ma de in studieson t h e v ert e bra t e re t in a [re viewe d in Adler, 1993;Cepko et a l ., 1996]. O n e a re a t h a t is p rog re ss in g invertebrate work, however, involves cellcell and cellma trix a dhesion m olecules. The vertebrate r et ina wa st h e mode l u s e d in cla s s ic s t u dies by M os con a ; col-
leagues demonstrat ing the requirement for cell adhe-sion molecules in histogenesis of a t issue and furthershowed t heir loss dur ing development [reviewed inG ru n w a ld, 1996]. G ru n w a ld et a l . [1997] (this issue)de mon s t ra t e t h a t de ve lop men t a l down re g u la t io n ofN-cadherin is correlated with phosphorylat ion of thismolecule by tyrosine kinases. Other recent studies invertebra te ret ina have implicat ed a dhesion moleculesin ret inal pat terning and cell fate determination [e.g. ,Hunter et al., 1992; Sha h et al., 1992; Br adsha w et al.,1995], and the formation of electrical connections [e.g.,Kljavin e t a l ., 1994; B a ls a mo et a l ., 1995]. Adhesionmolecu les h a v e a ls o re ce n t ly bee n implica t e d in fl yretinal morphogenesis. Thus, a three-dimensional net-
work of cellcell conta cts m ediated by adh erens junc-tions, and cellextracellular ma trix conta cts mediatedb y f oca l a d h es ion s , d efi n e s t h e a r c hi t ect u r e of t h eomatidia. The development of these structures is dis-
ru p t ed in s ev era l mu t a n t s in ce llma t r ix ( in t e g rin )receptors [Longley a nd R ead y, 1995].
The omat idia of the D r o sop h i l a eye and the columnarunits of the vertebrate ret ina represent the functiona lu n it s f or phototransduction (i .e ., t ra n s mit t a l of t h esignals for sight) . The study of phototransduction in
Dr o so p h i l a h a s a d v a n ce d r a p i dl y a s a r es u lt of t h ea dv a n t a g es o f t h is org a n is m a s a g e ne t ic s y s t em. A d-vances in mouse knock-out technology have more re-cently offered the opportunity for genetic dissection oft h is p roce ss in v ert e bra t e s, a s we ll. Ve rt ebra t e s a n din v e rt e bra t e s s h a re a g re a t de a l o f s imila r i t y in t h eoverall molecular components used for phototransduc-tion even though the wa y they put t hese par ts t ogetherdiffers [Zuker, 1996]. Defects in components of thep h ot o t ra n s du ct ion s ign a l in g p a t h wa y a re of t e n ma n i-fested as a degeneration of photoreceptors in the Dr o - sophila re t in a in re s p o n s e t o l ig h t . I n h e ri t e d re t in a ldegenerat ive disorders a re a lso seen in a nimal models,including the retinal degenerat ion (r d) mouse, which
involves a defect in th e gene encodingb-phosphodiester-a s e . Su c h mu t a t io n s a re p a rt ic u la r ly re le v a n t t o h u -ma ns, since a large number of genetic defects involveretinal degenerat ion. One of these, ret init is pigmen-tosa, is t he most common cause of inherited blindness, ad is or d er t h a t a f fe ct s 1 i n 3, 500 A me ri ca n s a n d a nestima ted 1.5 million people w orldwide [St eele, 1994].Importantly, more than 25%of all retinitis pigmentosacases appear to be caused by domina nt mut a tions in thegene encoding t he visua l pigment, rh odopsin. The grea tma j o ri t y o f t h e s e mu t a n t s a p p e a r t o p ro du c e a mis -f olded g en e p rodu ct , wh ich ca u s e s a p op t os is of t h ephotoreceptor cells. Understanding the genetic mecha-nisms behind retina l cell degenerat ion offers t he oppor-
tunity for correction of these disorders in humans ashas been recently demonstrated to be possible in theretinal degenerat ion mouse [Bennett et al., 1996]. Per -h a p s Dr o so p h i l a genetics will help elucidate mecha-nism. Two r ecent pa pers describe rhodopsin mut a tionst h a t a c t do min a n t ly t o c a u s e re t in a l de g e n e ra t io n inDr o so p h i l a [Kura da a nd OTousa 1995; C olley et a l .,1995]; severa l of these m uta tions correspond to identi-cal substitut ions in huma n a utosoma l dominan t ret ini-t is p ig men t o sa p a t ien t s . D e g en e ra t ion in t h e D r o - sophilamuta nts w as found to result from interferenceby t h e mu t a n t p ro t e in wit h ma t u ra t io n o f wi ld- t y p erhodopsin, trigg ering photoreceptor cell a poptosis. Othert y pe s o f h u m a n r et i na l d eg en er a t i on s m a y a l s o b e
elucidat ed through D r o sop h i l a genetics. Isolat ion a ndcharacterizat ion of the human homologue of the Dr o - sophila retinal degeneration B (r d g B ) gene is describedby G u o a n d Yu [1997] (t h is is su e ). Th is g en e is amember of t he phosphatidylinositol tra nsfer proteinfam ily (P ITP ) of calcium binding proteins a nd is thoughtto be involved in regulat ing intracellular protein traf-fi cking in the photoreceptor [Liscovitch a nd Ca ntly,1995]. Human retinal degenerat ion B w a s m a p p e d t ochromosome 11q13, a region known to contain several
optic tectum of the bra in.The Drosophilacompound eye develops fromthe e y e i mag i nal di sc , an unpat te rne d e pi the l i um that prol i fe rate s
during the first and second stages of larval development. Differentia-tion begins in mid-third insta r lar vae, contr olled by a gene hierar chy:eyeless, dachshund, sine oculi s, a n d eyes absent. An indenta tion of theepithelium known as the morphogenetic furrow, forms a t t he posteriorma rgin of th e eye disc due to th e constr iction of epithelial cells a longtheir apicalbasal axis. The morphogenetic furrow, under control of
the genes,h edgehoga n d decapentapl egic(d pp), then sw eeps across thedisc from posterior to a nterior over the course of a pproximat ely 2 days.During this period, the disc continues to grow, increasing in size byapproximately eightfold. Ahead of the morphogenetic furrow, cellsdivide asynchronously. In th e furrow, cells arrest t ra nsiently in the G 1
phase of the cell cycle. They then undergo dramatic changes in genee xpre ssi on and be gi n t o a rrang e i nto e venl y spac e d c l usters, t heoma tidia l precursors. As cells exit from the furrow, they begin to differenti-at e into photoreceptor neurons. Differentiat ion is a synchronous; cells arerecruited into maturing omatidia by reproducible and stepwise process.The fina l product, the adult eye is composed of about 800 individual
omatidia or unit eyes, arra nged in a precise hexagonal a rra y.
PERSPECTIVES ON EYE DEVELOPMENT 179
-
8/13/2019 Fini, M.E. Et Al. 1997. Perspectives on Eye Development
6/11
retinopathy loci. In D r o sop h i l a , calcium cha nnel block-ers ha ve been shown t o inhibit ret ina l degenera t ion int h e retinal degeneration B mu t a n t [Sa h ly et al., 1992],suggesting a possible therapeutic approach, should theconnection to t he disea se loci hold t rue.
LE NS: A CLASSIC DEVELOPMENTAL MODEL,NOW AMENABLE TO GENETIC
INVESTIGATION
The fi rst observat ions and experiments defi ning theconcept of embryonic induction ca me from th e an a lysisof lens development in the vertebrate eye [reviewed inG ra inger, 1996]. This w ork suggested t ha t t he develop-ing optic vesicle induces forma tion of th e lens pla code,wh ich then goes on to form th e lens (see Fig. 1). Dur ingthe pa st decade, however, a r evised view of these tissueinteractions has emerged. Lens induction is now thoughtto begin during mid-gastrula phase, when embryonicectoderm becomes competent to respond to inductive
s ig n a l s. E v i de nce p r es en t e d b y G r a i n ge r et a l .[1997](this issue) suggests that competence is followedb y a p er i od of b ia s f or l en s f or m a t i on , w h e n l en sect od er m ca n f or m a l en s i n r es pon se t o a w e a kinducing environment. Final lens specificat ion occursa t t he time of neura l tube closure w hen th e optic vesicleis ju s t a p p roa c h ing t h e p res u mpt iv e le n s a re a . Th eoptic vesicle is not thought to be a ma jor lens indu cer a te a r ly s t a g es ; i n st e a d , i t s r ol e i s t o p rov id e f a ct or srequired for lens differentia t ion. In t his new view, thetissue interact ions responsible for lens induction re-ma in t o be de fi n e d, a s do t h e mo le c u la r f a c t o rs t h a tdetermine lens-forming competenceand lens-formingbia s [G ra inger, 1996].
PAX6 is foremost on the list of candidate genes forcontrolling formation of the lens. In the normal mouseembryo, Pax6 is expressed in a large part of the headregion during early development, but expression pro-gressively becomes restricted t o the region of the form-ing lens pla code a nd the optic vesicle (see Fig. 1). Thephenotype of sma l l eye h et e rozy g ot e mice a n d ra t s(wh ich h a v e on e n u ll a l le le f or Pax6) i s v a r i a b l e i nt e rms of e y e s ize a n d de fe ct s ; h owe v er , t h e len s iss ma ller t h a n n orma l a n d i t o f t en f a i ls t o de t a c h c om-p le t ely f rom t h e corn e a . I n h omozy g ot e s , t h e op t icvesicle is present but a bnorma l, and the lens placode isnever induced. Co-culture experiments between ecto-derm and optic vesicles from normal and small eyer a t
e mbry os s u g ge s t ed t h a t Pax6 i s n ot n e ce ss a ry f orinduction of the lens placode but is required for deter-mining the competence of surface ectoderm for lensformation [Fujiwara et al., 1994]. These conclusions aresupported by more recent st udies of small eyechimericmice [Quinn et al., 1996]. Neural crest cells accumulateabnorma lly around the eye in rat small eyemu t a n t s . I thas been suggested that this could also play a part inthe fa ilure of lens induction [Matsuo et al., 1993]. PAX6a lso has a more direct effect on lens differentia tion a s it
part icipat es in controlling a ct ivity of lens crystallingene promoters [Cvekl e t a l ., 1994; Richardson e t a l .,1995; reviewed in Cvekl and Piatigorsky, 1996]. How-ever, this is only one aspect in the complex transcrip-t ional r egulat ion of crysta llin promoters, as discussedby Li et al.[1997] (th is iss ue).
Another candidate gene for controlling lens forma-tion is AP-2a. A cell type-specific transcription factor,AP -2a i s e x p re s s e d in s u rf a c e e c t o de rm, bra in , a n dneural crest cells and their derivat ives in mice. Miceheterozygous for an AP-2a null allele are normal, buthomozygotes a re born dead, with mult iple craniofacialand other abnormalit ies [Zhang et al., 1996; Schorle etal ., 1996]. On first examination, AP-2a null mice ap-p e a r t o la c k e y e s , bu t a c t u a l ly t h e e y e s a re p re s e n tembedded deep in the head and covered by an over-growth of neura l t issue. As reported a t the Tam ar ronSprings meeting, these eyes range in phenotype fromthe complete lack of lenses to abnormal lenses that arereduced in size [West-May s et al., 1997]. There are now
k n o w n t o b e t h r e e AP-2 g e n es (AP-2a, AP-2b, a n dAP-2g) ; the details of their individual expression pat-tern during eye development is st ill under investiga-tion. However, pan-specific AP-2 antibodies reveal ex-pression of AP-2 in epithelial tissues of the developinga n d a du lt mo u s e e y e , in a p a t t e rn t h a t is s t r ik in g lys imila r t o Pax6 [Koroma e t a l ., 1997], although withsome interest ing differences [Koroma et a l ., 1997].Thus, in contrast to Pax6, AP-2 is not expressed in theoptic vesicle or optic cup during early developmentalstages, however, AP-2 expression appears in the innernuclear and ganglion cell layers a s differentiat ion pro-ceeds [St rissel, 1997], in a pat tern tha t closely mir rorst h e re st r ict e d p a t t e rn of Pax6 e xp res s ion t h a t a ls o
develops with t ime [Martin e t a l ., 1992]. Also unlikePax6, AP-2 is expressed in neural crest cells w hichcontr ibute t o th e developing eye. The possible intera c-ti on of AP -2awith P ax6 is under investigat ion.
Like Pax6, AP-2probably controls or intera cts w ithg e n e s t h a t a re bo t h u p s t re a m a n d do wn s t re a m in t h ee y e de v elop men t ca s c a de . E x a min a t ion of t h e q u a i lPax6promoter [Pla za et al., 1995] suggests a number ofpossible binding sites for AP-2 proteins. AP-2 proteinsa r e k n ow n t o r eg u la t e d ir ect l y t h e t r a n s cr i pt i on a lpromoters of a number of genes expressed in the corneaand lens, including cellcell adhesion molecules [Be-hrens et a l ., 1991], e pi t h el ia l ba s e men t me mbra n ecomponent s [Ta ma i et al., 1994], and the extracellular
ma t r ix p rot e in a s e , g e la t in a s e B [F in i et a l ., 1994].AP-2a overexpression in cultured eye epithelial cellscauses poor adhesion to the underlying substratum andcell clumping, w hich could r esult from alt erat ion infunction of these genes [West-Mays et al., 1996]. AP -2protein also has the potential to affect the cell cycle ora p o p t o s is in e y e c e l ls t h ro u g h i t s in t e ra c t io n wit h ascond t ra nscription factor, c-m yc[G a u ba t z et al., 1996].Finally,AP-2a expression a nd a ctivity ofAP-2 tra nscrip-t ion factor is responsive to ret inoids, which are also
180 FI NI E T A L .
-
8/13/2019 Fini, M.E. Et Al. 1997. Perspectives on Eye Development
7/11
known to influence lens development [Luscher e t a l .,1989].
A third gene implicated in the control of lens forma-tion encodes a member of the TG F-b family of cyto-kines, bone morph ogenetic prot ein-7 (BMP -7). Approxi-ma tely one-thir d ofB M P -7 null m ice do not form lenses,
while the rest form lenses partially to completely [Luoet a l ., 1995; Dudley et a l ., 1995]. Pax6 expressiona p p e a rs t o o c c u r in bo t h de f e c t iv e a n d n o rma l e y e s .B m p - 7 t ranscripts are found in both the neuroepithe-l iu m of t h e op t ic v e s icle a n d t h e ov erly in g s u rfa c eectoderm. Following the init iat ion of lens formation,B m p - 7 is further expressed in the mesenchymal cellsadjacent to the optic cup, but expression in the lens isn ot a p p a re n t . Th is s u g g e st s t h a t B m p - 7 m ig h t b einvolved in delivering the signal for lens specificationf rom t h e op t ic cu p . I n s u pp ort of t h is h y p ot h e s is ,a n t i-B M P -7 a n t ibodies ca n block len s f orma t ion incultured rat embryos [Luo e t a l ., 1995]. However, thev a ria ble p e n e t ra n c e o f t h e mu t a n t p h e n o t y p e in t h e
B m p - 7 null mice indicates that genes segregating in F2a nima ls ca n suppress this a spect of the B mp - 7 -deficientphenotype.
The embryonic lens vesicle of vertebra tes is a t fi rst ahollow ba ll of epith elia l cells (see Fig. 1). The beginn ingof differentiation occurs with elongation of the epithe-lial cells at the posterior of the vesicle to fill the cavity.These cells a re called t he prima ry lens fi bers. The cellsat the a nterior of the lens remain epithelial. Agermina -tive ring of mitotic epithelial cells is found around thec e n t ra l re g io n o f t h e le n s . D a u g h t e r c e l ls f ro m t h isregion move into the equatorial region or bow of thelens, elongat e, a nd differentiat e into the seconda ry lensfi bers which form concentric layers ar ound the primary
fibers. This process of differentiation continues slowlyduring post-nat al an d a dult l ife, result ing in continua lincrease in the size of th e lens. A good d eal of evidencehas implicated cytokines of the fibroblast growth factorfamily as regulators of fiber cell differentiat ion [e.g. ,Robinson et al., 1995; Chow et al., 1995]. These fa ctorsar e located in the vitreous humor w ithin t he posteriorchamber of the eye, so tha t only t he posterior portion ofthe lens w ould normally be exposed to them.
Only th e lens epithelium conta ins mit otic cells in thelens; once cells move into the equatorial region anddifferentia te, t hey become postm itotic. The cyclin depen-dent kinases have a well-established role in regulatingthe cell cycle; however, recent studies suggest tha t t hey
ma y a lso play a par t in contr olling a poptosis or differen-t ia t ion . E v iden ce s u g ge s t s t h a t a s pe cifi c me mbe r ofthis family, Cdk5, along with accessory proteins p35and p25, regulate cytoskeletal dynamics, neurite out-g rowt h , a n d a x on a l t ra n s p ort in n e rv ou s t is s u e. B a s a lcdk 5 is f ou n d in ma n y ce ll t y p es ; h o we v er , a ct iv ep35/cdk5 ha s been r eport ed previously only in postmi-totic neurons of the centra l nervous syst em [Tsa i et al .,1994]. As discussed a t the Ta ma rron S prings m eeting,a ctive p35/cdk5 kina se is demonstr a ted w ithin the lens
e p it h e l ia l c e l ls a n d fi be r c e l ls [G a o et a l ., 1997](thisissue). This is interest ing, in view of a potent ial fun da -mental link between placodally derived sensory tissuesa nd a nterior neura l tissue [reviewed in G ra inger, 1996].For exa mple, neura l retina ca n regenerat e into a lens insome cases and ret inal cells can t ra nsdifferentia te into
lens-like cells. It is suggested t ha t cdk5 in the lens ma yplay a part in controlling the cytoskeletal rearrange-ments associated with elongation and denucleat ion ofdifferentiat ing lens fi ber cells, much a s it is t hought todo in neurite outgrowth.
Th e ma j or p rot e in s f ou n d in t h e de ve lop in g a n dm a t u r e l en s a r e t h e cr y s t a ll in s , a d i ve rs e g r o up ofsoluble proteins which make possible the refraction oflight. Cry sta llins were recruited for their refractive rolefrom pre-existing proteins, typically stress-related orhousekeeping enzymes. Ma ny crystallin genes a re ex-pressed almost exclusively in the lens. This ha s ma de itpossible t o use t he t ra nscriptional promoters of th esegenes to target specific gene expression to lens cells in
t ra n s g e n ic mic e , a n d t h u s s t u dy t h e e f f e c t s o f g e n eperturba tion in isolat ion, w ithout disrupting develop-ment in other parts of the embryo [Cvekl et al., 1994;Cvekl a nd P iat igorsky, 1996]. Thus, a unique opportu-nity is creat ed to use the lens a s a genera l in vivo modelfor studies of cell cycle dynamics and differentiation. Ane xa mp le of s u c h a s t u dy is p res en t e d by F romm a n dOverbeek [1997] (this issue), the authors expressed theoncogene, bcl-2, in tra nsgenic mice under contr ol of thelens-specific aA-crysta llin promoter t o exa mine its pa r-t icipat ion in cell cycle dynamics, apoptosis, and fibercell ma tura t ion. The results indicate t ha t the lenses oftransgenic mice represent a valuable in vivo setting forsuch studies.
A second promising genetic approach in mice forstudies on cell cycle dyna mics in the lens w as discusseda t t he Ta ma rron Sprin gs meeting [Liegeois et al ., 1996].This involves a complementa t ion system tha t ma kesu s e o f a m u t a n t m o u s e s t r a i n , a p h a k i a (ak), a cell-autonomous defect in which homozygotes fail to developan ocular lens. Microinjection of wild-type embryonicstem cells into homozygous a p h a k i a blastocysts pro-duces chimeras with normal ES -cell derived lenses.Microinjection of stem cells homozygous for a r eti noblas-to ma gene deletion, on the other hand, results in a lenswit h a l l t h e f e a t u re s o f dis ru p t e d di f f e re n t ia t io n o b-ta ined through conventiona l gene t argeting methodol-ogy. The ava ilability of such a syst em thus circumvents
the need for germline tra nsmission of null a lleles a ndwill facilitate the study of the genetics of lens differen-t ia t io n wit h re g a rd t o t h o s e g e n e s t h a t g iv e a le t h a lphenotype, when inactivated.
ZEBRAFISH AS THE NEW GENETIC MODELFOR VERTEBRATE EYE DEVELOPMENT
P rogress in understa nding mamm alia n genetics basedon h omolog y t o in s ect s s u ch a s Dr o so p h i l a cannot
PERSPECTIVES ON EYE DEVELOPMENT 181
-
8/13/2019 Fini, M.E. Et Al. 1997. Perspectives on Eye Development
8/11
address fundamental developmental questions aboutstructures and functions not represented in inverte-bra t e s .D r o sop h i l a lacks a neur a l crest, for exa mple, animporta nt contributor to development of the cornea a ndanterior chamber of the vertebrate eye (Fig. 1). Theperception of th is ina dequacy ha s lead to the explora -
t ion of vertebrate models that could enable the samet y p e o f ra p id g e n et ic s c ree n s p os s ible wit h fl ie s orworms. I t now appears that the zebrafish (D a n i o r er i o ),with its small size, ease of care, and rapid generat iont i m e, w i ll fi l l t h i s n ee d. Wi t h t h e d ev el op me nt ofappropriate methodology, large scale mutant screenshave become possible in this species.
Several recent publicat ions report the chara cteriza-t io n o f e y e mu t a n t s in ze bra fi s h . T h e fi rs t de s c ribe smutants in ret inal function isolated using an optoki-netic behavioral screen [Brockerhoff e t a l ., 1995] thatmeasured the randomness of eye movements. Eighteenoptokinetic-defective mu ta nts were identifi ed. Two ofthese showed no obvious developmental defects, but a
defect in ret inal function could be detected in one byelectroretinogram. The second report occurred in thejournal, Development, w hich devoted a special 1996issue to the r esults of major screens by la bora tories inB oston and Tubingen [G ra na to an d Nusslein-Volhard ,1996]. A paper from the Boston laboratory [Malicki etal ., 1996] reported 49 different eye development mu-tants based on a morphological screen. These could begrouped into phenotypic cat egories: loss of lam inarp a t t e rn in t h e re t in a , c y c lo p ia , lo s s o f o u t e r re t in a lla y e rs (mo st ly p h ot o rece pt o rs), g rowt h re t a rda t ion ,s ma ll e y es a s s ocia t e d wit h re t in a l de ge n era t io n , a n dretinal degenerat ion associated with general pigmentd ef ect s . Tw o m u t a n t s d id n ot fi t i n t o a n y of t h es e
ca t e g or i es , t h e fi r s t s h ow i n g a n a b s en ce of v en t r a lre t in a a n d t h e s econ d, a n e y e-s pe cifi c p ig me nt a t iondefect. A third study, from the laboratory that reportedon the optokinetic screen, describes a second group ofeye mutations isolated by microscopic examination formorphological defects [Fadool et al., 1997](thi s is sue). Atotal of 27 different muta nts were selected. S evera l ofthese did not fall rea dily into the groups described byMalickiet al .[1996].M a sk(m sk) is char acterized by thea bnorma l position of the lens with respect to the retina .Tw o m u t a t i on s , i d en t i fi e d b y a s m a l l o r r ou n d e yephenotype, r o u n d eye (r d e) a n d m a r g i n a l ey e (m r e),re su lt in s p e cifi c c el l dea t h a t t h e re t in a l ma rg in a n dapparent loss of the ret ina l prolifera t ive zone. Both ar e
embryonic letha ls. The a r ch i e (ar c) muta t ion is cha ra c-terized by the conspicuous protrusion of the lens fromthe optic cup. Retina of larvae at 6 days postfert iliza-t ion (dpf) have a considerably t hinner ga nglion cellla y e r a n d a s l ig h t ly t h in n e r in n e r n u c le a r la y e r t h a tappears to be the result of cell type-specific degenera-t ion. All displayed some degree of dorsal curvat ure oft h e b od y a x i s a n d a b n or m a l s w i m mi n g m ov em en t .Dea th occurred a t da ys 810 dpf. The retina l phenotypeis highly reminiscent of the recently described knock-
out mice for POU domain transcription factor Brn3.2(Br n-3b), w hich results in specifi c loss of ret inal gan-glion cells [Er kma n et al., 1996; Gan et al ., 1996].
Despite adva nces, la rge-scale screening of zebrafi shmu t a n t s is s t i l l a ma j o r u n de rt a k in g t h a t ma y n e v e requal the D r o sop h i l a m odel for efficiency. The Tubin gen
group screened approximat ely 3,200 zebrafi sh lines,wh ich resulted in an a llele frequency of 2.4 muta nts pergene [G ra nat o and Nusslein-Volhar d, 1996]. This isonly a bout one-half t he a llele frequency tha t ha s beenpossible wit h D r o sop h i l a and is less than that requiredf or s a t u ra t ion . Th e 49 e y e mu t a t ion s iden t ifi e d byMalicki et al. [1996] represent 36 different loci indicat-ing a n a llele frequency of 1.4 mut a nts per gene. Clear ly,ma n y g e n e s a re imp o rt a n t f o r e y e de v e lo p me n t t h a thave not yet been identifi ed. Another draw back tha t istrue of all genetic screens is tha t ma ny muta t ions withpleiotropic effects might result in death of the embryobefore the eye phenotype is manifested. Also, complexphenotypes are not usua lly easy t o follow up, since they
can result in ra ther general defects such a s retar dat ionor degeneration, yet these phenotypes might hide veryimportant genes.
Thus, it is disappointing t hat the screens t urned upm os t ly r et i n a l m u t a t i on s w i t h s o f ew m u t a t i on s i nt h o se s t ru c t u res , s u ch a s corn e a a n d len s , t h a t a reu n iq u e t o v ert e bra t e s . I t is n ot s u rpris in g , h o we v er,since retinal defect would have been the easiest sort tospot by the stereomicroscopic screening method used.Tra nspa rency of the tissues of the an terior chamber ofthe eye would ma ke a nterior chamber defects diff icultto see. The development of specialized screening meth-od s w i ll b e i m por t a n t f or r ev ea l i ng t h es e s or t s ofdefects.
Agenetic linkage map in zebrafi sh is now a vailable sothat mutant genes can potentially be isolated by posi-tional cloning [Postlethwait et al., 1994]. Furthermore,tra nsgenic techniques a re under development in ze-bra fi s h [L in et a l ., 1994; Amsterdam et a l ., 1996].I de n t ifi ca t io n o f zebra fi s h mu t a n t s s h ou ld t h e ref orebegin to occur with in the not too dista nt fut ure, and t hispromises to bring a wealth of new information to ourunderst a nding of development of the vertebra te eye.
FUTURE DIRECTIONS
Clearly the control of eye development requires thecomplex intera ction of multiple genes, a fa ct tha t is notsurprising considering the complexity of this organ,
especially in vertebrates. We predict that analysis ofmou s e mu t a n t s a s we ll a s ch a ra ct e riza t io n of mu t a n t si n t h e n ew z eb r a fi s h g en et i c m od el w i ll l ea d t o t h ei de nt i fi ca t i on of m a n y n ew g en es w i t h in t h e n e a rfuture. This an alysis w ill be aided by t he relat ive easewit h w hich species bar riers can now be crossed throughthe use of techniques such as polymerase chain reac-tion. With th e newly emerging underst a nding of geneticconservation in eye development across evolution, theset e ch n i q ue s w i l l u n d ou b t ed l y b e a p p li ed m or e f r e-
182 FI NI E T A L .
-
8/13/2019 Fini, M.E. Et Al. 1997. Perspectives on Eye Development
9/11
quently in the future. In fact, this sort of cross-speciesa p p ro a c h wa s t a k e n t o a n a ly ze a n e w c a n dida t e f o r ama s t e r re g u la t o r in Xenopus e y e , a s re p o rt e d a t t h erecent Ta ma rron m eeting. This new gene, encoding apaired-like homeobox protein called Rx (for ret inalhomeobox), w a s isolat ed from Xenopusembryos as part
of a screen for genes induced during development of thehead [Mathers e t a l ., 1995]. Rx is the earliest knowns pe ci fi c m a r k er f or ce ll s of t h e r et i n a l a n d p in ea llineage. The homologue of R x h a s n o w be e n is o la t e df rom ze bra fi s h , fl y a n d mou s e. K n ock ou t t e ch n iq u eswere used t o show its essentia l role in eye development.I t wi l l be e x c i t in g t o le a rn wh e t h e r a n d h o w R x a n drelated genes interact and what downstream genes arecontr olled. This ty pe of a na lysis should be facilita ted bythe use of gene transfer techniques tha t t arget specifi ct i ss u es i n t h e e ye , s u ch a s t h os e d es cr i be d i n t h i sreview. Clearly, eye development will continue to stayin th e limelight for some time to come.
ACKNOWLEDGMENTS
Th is wo rk is s u pp ort e d by N a t io n a l I n s t i t u t es ofH e a l t h p roj ect g ra n t s (E Y 09828 a n d AR 42981)(t oM.E.F.), a Na tional Research Service Awa rd (EY06719)(t o K . J . S . ), a n d a n a w a rd f rom t h e M edica l R e se a rchCouncil of Ca na da (to J .A.W.-M.). M.E .F. is a J ules a ndDoris Stein Research to Prevent Blindness Professor.
REFERENCES
Adler R (1993): Plasticity and differentiation of retinal precursor cells.
Int Rev C ytol 146:145190.Amsterdam A, Lin S, Hopkins N (1996): The Aequorea victoria green
fl u o r es ce n t p r ot e in c a n b e u s e d a s a r e por t e r i n l iv e z e br a fi s hembryos. Dev Biol 171:123129.
Ba l samo J , E r nst H, Za ni n MK, Hoffman S, L i l l ie n J (1 99 5): Theinteraction of the retinal cell surface N-acetylgalactosaminylphos-
photransferase with an endogenous proteoglycan ligand results ininhibition of cadh erin-mediated adh esion. J Cell B iol 129:13911401.
Behrens J , Lowrick O, Klein-Hipass L, Birchmeier W (1991): Thee-cadh erin promoter: Fun ctiona l ana lysis of th e G /C-rich region and
an epithelial cell-specific palindromic regulatory region. Proc NatlAcad S ci 88:1149511499.
B ennet t J , Ta na be T, Sun D, Zeng Y, Kjeldye H, Gour a s P, Magu ire AM(1996): Photoreceptor cell rescue in retinal degeneration(r d) mice byin vivo gene therapy. Nature Med 2:649654.
Boni ni NM, C hoi K-W ( 19 95 ): E ar l y de ci sions i n Drosophila ey e
morphogenesis. Curr Opin Genet Dev 5:507515.Br ads ha w AG, McNagny KM, Gervin DB, Ca nn GM, Gr aff T, Clegg DO
(1995): I nt egrin a2b1 me diate s i nte rac t i ons be twe en de vel opi ngembryon ic retina l cells a nd collagen. D evelopment 121:35933602.
Broc ke rhoff S E , Hurl e y J B, J ansse n-Bi e nhol d U , Ne uhauss SC F,Driever W, Dowling J E (1995): A beha vioral screen for isolat ing
z e brafish mutants wi th vi sual sy ste m de fe c ts. Proc Nat l Ac ad Sc iUSA92:1054510549.
Cagan RL, Ready DF (1989): Notch is required for successive celldivisions in the developing Drosophilaretin a . Gen es Dev 3:393399.
Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D (1996): Cellfate determination in the vertebrate retina. Proc Natl Acad Sci USA
93:589595.Chanut F, Heberlein U (1996): Role of decapentaplegic in initiation
and prog re ssion of the morphog ene ti c furrow i n the de vel opi ngDrosophila retina. Development (in press).
Cha nut F, Heberlein U (1997): Retina l morphogenesis in Drosophila:
Hints from an eye-specific d ecapent aplegica llele. Dev Genet 20:197
207.
C how RL , Roux GD, Rog hani M, Pal me r MA, Ri fki n DB, Mosc ate l l i
DA, Lang RA(1995): FGF suppresses apoptosis and induces differen-
tia tion of fi ber cells in the mous e lens. Development 121:43834393.
Colley NJ , Cassill J A, Baker EK, Zuker CS (1995): Defective intracel-
l ular t ran sport i s the mole cul ar basi s of rhodopsi n-de pe ndent
dominant retinal degeneration. Proc Natl Acad Sci USA 92:3070
3074.
Cvekl A, P iatigorsky J (1996): Lens development an d crysta llin gene
expression: Many roles for Pax-6. Bioessays 18:621680.
Cvekl A, Sa x CM, B resnick EH, P iatigorsky J (1994): A compex arra y
o f p os i t iv e a n d n e ga t i v e e le m en t s r e gu l a t e s t h e c h ic ke n aA-
crysta llin gene: Involvement of Pa x-6, USF, CRE B, an d/or CREM,
a nd AP -1 proteins. Mol Cell B iol 14:73637376.
Cvekl A, Ka sha nchi F, Epstein J , Rauchma n M, Maa s R, Pia tigorsky J
(1996): PAX6, a master gene for eye development: Tar get genes
and mechanism of action. Invest Ophthalmol Vis Sci 37(suppl):S150.
Della NG , Senior PV, Bowt ell DD (1993): Isolation a nd cha ra cteriza-
tion of murine homologues of the Drosophila seven in absentiagene
(sina). Developm ent 117:13331334.
Dorsky RI , Rapaport DH, Ha rri s WA (1 99 5): Xotch inhibits cell
differentia tion in the Xenopusretin a. Neuron 14:487496.
Dud ley AT, Lyons K M, Robert son E J (1995): A requir ement for bonemorphogenetic protein-7 during development of the ma mma lian
kidney a nd eye. G enes Dev 9:27952819.
Ekker SC , Un gar AR, Gr eenstein P, von Kessler DP, P orter J A, MoonRT, B eachy P A (1995): P at terning activities of vertebra te h edgehog
proteins in the developing eye and brain. Curr Biol 5:944955.
E rkman L , Mc E vi l ly RJ , L ou L , Ry a n AK, H ooshmand F, O C onne l l
SM, Keithley EM, Rapaport DH, Ryan AF, Rosenfeld MG (1996):
Role of t ra nsc ript i on fac tors Br n-3 .1 an d Brn-3.2 i n a udi tory and
visual system development. Nature 381:603606.
Fa dool J M, Brockerhoff SE , Hy at t G A, Dowling J E (1997): Muta tions
affecting eye morphology in th e developing zebrafi sh (D a n i o r e r i o ).
Dev Genet 20:288295.Fi ni ME , Bart l e t t J D, Matsubara M, Ri ne hart WB, Mody M, Gi rard
MT, a nd Rainville M (1994): The rabbit gene for 92 kDa ma trix
meta lloproteina se: Role of AP1 and AP2 in cell type-specific tra nscrip-
tion. J Biol Chem 269:2862028628.
Fromm L , Overbeek PA (1997): Inhibit ion of cell deat h by lens-specifi coverexpression of bcl-2 in tr an sgenic mice. Dev G enet 20:276287.
Fujiwar a M, Uchida T, Osumi-Yam ash ita N, Et o K (1994): Uchida ra t
(rSey) : A ne w mutant rat wi th c rani al fac i al abnormal i t i e s re se m-
bling those of the mouse small eyemuta nt. Differentiation 57:3138.G a n L , X i a n g M , Wa g n e r D S , K l ei n W H , N a t h a n s J (1 99 6): P O U
domain fa ctor Brn -3b is required for th e development of a large set
of retinal ganglion cells. Proc Natl Acad Sci USA93:39203925.
G ao CY, Zakeri Z, Zhu Y, He H, Za lenka P S (1997): Express ion of Cdk 5,
p35, an d Cdk5-associated kina se activity in t he developing ra t lens.
Dev Genet 20:267275.
Gaubatz S, Imhof A, Dosch R, Werner O, Mitchell P, Buettner R, and
Eilers M (1996): Tra nscript ional a ctiva tion by Myc is und er nega tive
control by the transcription factor AP-2. EMBO J 14:15081519.Gehring WJ (1996): E ye evolution. [Letter to t he editor.] Science
272:467468.
Gla ser T, Walt on DS , Ma as RL (1992): Genomic structur e, evolution-
ary c onservat i on, and a ni ri dia mut at i ons i n the human PAX6gene.Nature Genet 2:232239.
Gr ainger RM (1996): New perspectives on embryonic lens induction.
Semin Cell D ev B iol 7:149155.
Gra i ng er RM, Manni on J E , C ook TL , Zy g ar C A (1 99 7): De fini ng
i nte rmedi ate stag e s i n c el l de te rmi nat i on: Ac q uisi t i on of a l ens-formi ng bi as i n he ad e ctoderm duri ng l e ns de te rmi nat i on. De v
G enet 20:246257.
Gr an at o M, Nusslein-Volhard C (1996): F ishing for genes controlling
development. Curr Opin Genet Dev 6:461468.
Grunw al d GB (1 99 6): C adhe ri n c el l a dhe sion mol ec ule s i n re t i nal
development and pathology. Prog Retinal Eye Res 15:363392.
PERSPECTIVES ON EYE DEVELOPMENT 183
-
8/13/2019 Fini, M.E. Et Al. 1997. Perspectives on Eye Development
10/11
Guo J , Yu FX (1997): Cloning and characterization of human homo-
logue of Drosophila re t i nal de ge nerat i on B: A candi dat e g e ne for
degenerative retinal diseases. Dev Genet 20:235245.
Ha lder G , Ca llaerts P, G ehring W (1995): In duction of ectopic eyes by
tar g e ted e xpre ssion of the eyeless g e ne of Drosophi la. Sc ie nce
267:17881792.
Ha nsen I, Van Heyningen V (1995): Pax6: More than me ets t he e y e.
Tren ds G enet 11:268272.
Ha nsen IM, F letcher J M, J ordan T, B rown A, Taylor D, Adams RJ ,
Punn e tt HH , a nd va n He y ni ng e n V (1 99 4): Mutat i ons a t t he PAX6
locus are found in heterogeneous anterior segment malformations,
including P eters an omaly. Natu re G enet 6:168173.
Heb erlein U , Wolff T, Ru bin G (1993): The TGF -bhomologue d ppa n d
the se g me nt pol ari ty g e ne hedgehog is required for progression of
the morphoge neti c furrow i n the Drosophila retina. Cell 75:913
926.
Hunt er DD , Murphy MD, Olsson CV, Brunken WJ (1992): S-Laminin
e xpre ssi on i n adul t and de vel opi ng re t i nas: A pote nt i al c ue for
photoreceptor m orphogenesis. Neuron 8:399413.
Kl javi n I J , L ag e naur C , Bi xby J L , Re h TA ( 19 94 ): C e l l a dhe sion
mol e c ul e s re g ul at i ng ne uri te outg rowth from amac ri ne and rod
photoreceptor cells. J Neurosci 14:50355049.
Koroma BM, Yang J -M, Sundin OH (1997): The PAX6homeobox gene
i s e xpre ssed throug hout the c orne al and c onjunct i val e pi the li a.
Inv est Oph th a lmol Vis S ci 38:108120.Kra mer H, Ca gan RL (1994): Determina tion of photoreceptor cell fat e
i n theDrosophilaretina. Curr Opin Neurobiol 4: 1420.
Kura da P, OTousa J E (1995): Retina l degenerat ion caused by domi-
nant rhodopsin mutations in Drosophila. Neuron 14:571579.
L ee M M , F i n k B D , G r u n w a l d G B (19 97): E v id e nc e t h a t t y r os i ne
phosphorylat ion regulat es N-cadherin tu rnover during retina l devel-
opment. D ev G enet 20:224234.
Li X, CveklA, Ba ssnett S, P iatigorsky J (1997): Lens-preferred a ctivity
of chicken d1 - and d2-crystallin enhancers in transgenic mice and
evidence for retinoic acid-responsive r egulation of th e d1-crystallin
gene. D ev G enet 20:258266.
Liegeois NJ , Horner J W, D ePin ho RA (1996): Lens complement a tion
s y s t em f or t h e g en e t ic a n a l y s i s o f g r o w t h , d if fe r en t i a t i on , a n d
apoptosis in vivo. Proc Natl Acad Sci USA93:13031307.
Lin S , G aia no N, Culp P, B urns J C, Fr iedman T, Yee J -K, H opkins N
(1994): In tegra tion a nd germ-line tr an smission of a pseudotyped
retroviral vector in zebrafish. Science 265:666669.
Linser PJ , Schlosshauer B, Galileo DS, Buzzi WR, Lewis RC (1997):
L ate prol i fe rat i on of re t i nal Mul l e r c el l prog e nitors fac il i tate s
pre fe rent i al tar g e t ing wi th re trovi ral ve c tors i n vi tro. De v Ge ne t
20:186196.
Liscovitch M, Cantly LC (1995): Signal transduction and membrane
tr affic: The P ITP/phosphoinositid e connection. Cell 81:659662.
Longley RL J r, Ready DF (1995): Integrins and the development of
three-dimensional structure in the Drosophila compound eye. Dev
B iol 171:415433.
Luo G, H ofmann C, B ronckers ALJ J , Sohocki M, Bra dley A, Ka rsenty
G (1995):Bm p - 7 is an inducer of nephrogenesis, an d is also required
for eye development and skeletal patterning. Genes Dev 9:2808
2820.
Luscher B, Mitchell P J , Williams T, Tjian R (1989): Regulation of
tra nsc ript i on fac tor AP-2 by the morphog en re t i noi c ac i d a nd by
second messeng ers. G enes D ev 3:15071517.Ma C, Zhou Y, Beachy PA, Moses K (1993): The segment polarity gene
hedgehog is required for progression of the morphogenetic furrow in
the developing Drosophilaeye. Cell 75:927938.
Malicki J , Neuhauss SC, Schier AF, Solnica-Krezel L, Stemple DL,
Sta inier DY, Abdelilah S, Zwa rtkru is F, Rangini Z, Driever W(1996):
Mutations affecting development of the zebrafish retina. Develop-
ment 123:263273.
Martin P, Carriere C, Dozier C, Quatannens B, Mirabel M-A, Vanden-
bunder B, Stehelin S, Saule S (1992): Characterization of a paired
box- an d homeobox-conta ining q ua il gene (Pax-QNR) expressed in
th e neuroret ina . Oncogene 7:17211727.
Mat hers P H, Miller A, Doniach T, D irksen ML, J am rich M (1995):
Initia tion of an terior head -specific gene expression in uncommitted
ectoderm of Xenopuslaevisby ammonium chloride. Dev Biol 171:641
654.
Mat hers P H, J amr ich M (1995): In duction of a novel homeobox gene
Rx in the anterior neural plate and its role in retinal proliferation.
Inv est Opht ha lmol Vis Sci 36(suppl):S213.
Mat suo T, Osumi-Yam ash ita N, Noji S, Ohuchi H, K oyama E, Myokai
F, Mats uo N, Tan iguchi S, D oy H, Iseki S (1993): A muta tion in the
Pax6g e ne in ra t small eye is associated with impaired migration of
midbrain crest cells. Nature Genet 3:299304.
Mirzany an s F, P earce WG, MacDona ld IM, Walter MA (1995): Muta -
tion of the PAX6g e ne in pat i ents w i th a utosomal domi nant ke rat i -
tis. Am J Hum G enet 57:539548.
O l ive r G , Mai l hos A, We hr R, C ope land NG, J e nkins NA, G russ P
(1995):Six3, a mur ine homologue of the sineoculisgene, demar cates
the most ante ri or border of the de vel opi ng ne ural pl ate a nd i s
expressed du ring eye development. Development 121:40454055.
Penton A, Selleck SB, Hoffman FM (1997): Regulation of cell cycle
synchronization by d ecapentap legicduri ng Drosophilaeye d evelop-
ment. Science 275:203206.
Pla za S , Grevin D , MacLeod K, Stehelin D, Sa ule S (1994): Pa x-QNR/
Pax6, a paired- and homeobox-containing protein, recognizes Ets
bi ndi ng si te s and c an al te r the transac t i vat i ng prope rt i e s of E ts
tra nscription factors. G ene Expr 4:4352.Pla za S, Turque N, D ozier C, B ailly M, Sa ule S (1995): c-m yb a c t s a s
transc ri pt i onal ac t i vator of the q uai l Pax6 (Pax-QNR) promoter
through two different mechanisms. Oncogene 10:329340.
P o s t le t h w a i t J H , J oh n s on S L , M id s on C N , Ta l b ot WS , G a t e s M ,
Ba l l ing e r E W, Afri ca D, Andre ws R, C arl T, E i sen J S, Horne S,
Kimmel CB, Hutchinson M, J ohnson M, Rodriguez A(1994): Science
264:699703.
Quinn J C, West J D, Hill RE (1996): Multiple functions for Pax6 in
mouse eye and nasal development. Genes Dev 10:435446.
Quiring R, Waldorf U, Kloter U, Gehring WJ (1994): Homology of the
eyelessgene of Drosophilato the small eyegene in mice and a niridia
in h uma ns. Science 265:785789.
Ray mond P A (1995): D evelopment an d morphological organiza tion of
photorec eptors. I n Djamg oz MB A, Arc her SN, Val l e rga S (e ds):
Neurobiology and Clinical Aspects of the Outer Retina. London:
C h a p m a n & H a l l.
Reichenbach A, Ziegert M, Schnitzer J , Pritz-Hohmeier S, Schaaf P,
Schober W, S chneider H (1994): D evelopment of the ra bbit r etina:
The question of columnar units. Dev Brain Res 79:7284.
Ri chardson J , C ve kl A, Wi stow G (1 99 5): Pa x-6 i s e ssent i al for
lens-specific expression of z-c ry stal l in. P roc Nat l Acad Sc i U S A
92:46764680.
Robi nson ML , O verbe ek PA, Ve rran DJ , Gri z z l e WE , Stockard C R,
Friesel R, Ma ciag T, Thompson J A(1995): Ext ra cellular F G F-1 act s
as a l ens di ffe rent i at i on fac tor i n t ran sg e nic mi c e. De vel opment
121:505514.
Sa hly I , B ar Nachum S, S uss-Toby E, Rom A, P eretz A, Kleiman J , B yk
T, Selinger Z, Minke B (1992): Ca lcium chann el blockers inhibit
re t i nal de g ene rat i on i n the retinal degenerati on B m u t a n t of D r o -
sophila. Proc Natl Acad Sci USA89:435439.
Sa na MS, S ervetnick M, Gr ainger RM (1992): Vertebrat e eye develop-
ment. Curr Opin Genet Dev 2:582588.
Schedl A, Ross A, Lee M, Engelkam p D, R ash bass P, van Heyningen V,Ha stie ND (1996): In fluence of Pax6gene dosa ge on development:
Overexpression causes severe eye abnormalities. Cell 86:7182.
Sc horl e H, Me ie r P, B uc hert M, J ae ni sche R, Mi tche l l PJ (1 99 6):
Tra nscription factor AP -2 essential for cra nial closure a nd cra niofa-
cial development . Na tur e 381:235238.
Shah BH, Rao AS, Hausman RE (1992): Role of the cell recognition
mole cul e, c og ni n, i n GABAerg i c di f fere nt i at i on i n c hic k re t i na.
Br a in Res 589:268274.
Shen W, Mar don G (1997): Ectopic eye development in Drosophila
i nduce d by di re cte d dachshund expression. Development 124:
4552.
184 FI NI E T A L .
-
8/13/2019 Fini, M.E. Et Al. 1997. Perspectives on Eye Development
11/11
Snow RL, Robson J A (1995): Migra tion a nd differentia tion of neuronsin th e retina an d optic tectum of the chick. Exp Neurol 134:1324.
Steele FR (1994): Shedding light on inherited blindness: The geneticsof retinitis pigmentosa. J Nat l Inst H ealth Res 6:5862.
Strissel KJ (1997): Transcription factor AP-2 expressed in differentiat-
ing mouse r etina l cells. In vest Oph th a lmol Vis Sci (suppl) 38:5299.Sullivan SA, Ba rthel LK , La rgent B L, Ra ymond PA (1997): A goldfi sh
notch-3homologue is expressed in neurogenic regions of embryonic,
adu lt, and r egenera ting bra in an d retina. D ev Genet 20:208223.Tam ai K , Kehua L, U itto J (1994): Identifi cation of a DNA-binding
protein recognizing a kera tinocyt e-specific regulat ory element in the230-kDa bullous pemphigoid a nt igen. J B iol Chem 269:493502.
Tomare v S, C al l ae rts P, Kos L , Zinovie va R, Hal de r G , G e hri ng W,P iatigorsky J (1997): Sq uid pax6 an d e y e de ve lopme nt . P roc Nat lAcad Sci US A(in press).
Ton, CC H irvonen H, Miw a H, Weil MM, Monagha n P, J orda n T, van
Heyningen V, Hastie ND, Meijers-Heijboer H, Drechsler M (1991):Positional cloning and characterization of a paired box and homeobox-conta ining gen e from th e an iridia regon. Cell 67:10591074.
Tsai LH, Delalle I , Ca viness VSJ , Cha e T, H ar low E (1994): p35 is aneura l-specific r egulatory subunit of cyclin-dependent kinase 5.Na tur e 371:419423.
Venkatesh T(1993): Neuronal development in the Drosophilare t i na. JNeur obiol 24:740756.
Walth er C, G uenet J L, Simon D, D eutsch U, J ostes B , Goulding MD,Pl ac hov D, Bal l i ng R, Gruss P ( 1 9 9 1 ) : Pa x: A muri ne mul t i g e nefa mily of pair ed box-conta ining gen es. G enomics 11:424434.
West-Mays J A, Sadow PM, Daryanani HA, Zieske J D, Fini ME (1996):Role of AP-2 tra nsc ript i on fac tor i n morphog ene sis of stra t i fie d
epithelium. J Invest Dermatol 106(suppl):834.West-Mays J A, William s T, S adow P M, Libby D, Zieske J D, Fini ME
(1997): AP-2 and ocular epithelial morphogenesis. In vest Ophtha l-
mol Vis S ci (suppl) 38:2280.We tts R, S e rbedz i ja GN, Frase r SE (1 98 9): C e l l l ine ag e a nal y si s
re ve al s mul i tpote nt pre c ursors i n the c i l i ary marg i n of the frogretin a . D ev B iol 136:254263.
Wi n n ie r G , B l e ss i ng M , L a b os k y P A, H o ga n B L M (19 95 ): B o n emorphogenetic protein-4 (BMP -4) is required for mesoderm forma -tion and patterning in the mouse. Genes Dev 9:21052117.
X u P -X , Woo I , H e r H , B e i er D R , M a a s R L (1 99 7): M ou s e E ya
homologues of the Dr osophil a eyes absent gene require Pax6 forexpression in lens and nasal placode. Development 124:219231.
Zhang J , Ha g opi an-Donal dson S, Se rbedz i ja G, E l se more J , P l ehn-Dujowich D , McMahon AP, F lavell RA, Williams T (1996): Neuraltube, skeletal, and body wall defects in mice lacking transcriptionfa ctor AP-2. Na tur e 381:238241.
Zuker C S (1996): The biology of vision in Drosophila. P roc Nat l Ac adSci U SA 93:571576.
PERSPECTIVES ON EYE DEVELOPMENT 185