FastTrack MAG mRNA Isolation Kits -...
Transcript of FastTrack MAG mRNA Isolation Kits -...

FastTrack® MAG mRNA Isolation Kits For isolating high-quality mRNA from total RNA, cells, and tissue
Catalog Numbers K1580-01 and K1580-02
Document Part Number 25-0754 Publication Number MAN0000475 Revision 2.0
For Research Use Only. Not for use in diagnostic procedures.
USER GUIDE

2

3
Contents
Experienced Users Procedure to Isolate mRNA from Total RNA ........................................................................................................ 4
Experienced Users Procedure to Isolate mRNA from Cells ............ 6
Experienced Users Procedure to Isolate mRNA from Tissues ....... 8
Kit Contents and Storage ................................................................... 10
Introduction ............................................................................ 12
System Overview ................................................................................ 12
Handling RNA .................................................................................... 14
Methods .................................................................................. 15
Isolating mRNA from Total RNA ..................................................... 15
Isolating mRNA from Cells ............................................................... 19
Isolating mRNA from Tissues ........................................................... 24
Determining mRNA Yield and Quality ........................................... 30
Troubleshooting .................................................................................. 31
Appendix ................................................................................ 33
Accessory Products ............................................................................. 33
Technical Support ............................................................................... 34
References ............................................................................................ 35

4
Experienced Users Procedure to Isolate mRNA from Total RNA
Introduction This quick reference sheet is included for experienced users of
the FastTrack® MAG mRNA Isolation Kits. If you are a first time user, refer to the details in the manual.
Step Procedure
Total RNA Protocol
Use the listed amount of reagents with the specified amount of total RNA for the purification procedure described below.
Amount Micro Maxi
Total RNA <1 μg 1–50 μg 50–500 μg 500–2000 μg
FastTrack® MAG Beads 20 μL 50 μL 100 μL ≥200 μL
Binding Buffer B6 100 μL 200 μL 500 μL 500 μL
Wash Buffer W7 100 μL × 6 200 μL × 6 500 μL × 6 500 μL × 6
1. Isolate total RNA as described on page 15. Perform DNase I digestion, if you wish to use the mRNA for RT-PCR.
2. Preheat the specified volume of Binding Buffer B6 (see table this page) at 65°C to 70°C.
3. Add RNase-free water to your total RNA sample to a total volume equal to the volume of Binding Buffer B6 in Step 2. Place the sample tube on ice until use.
4. Gently pipet the FastTrack® MAG Beads up and down to thoroughly resuspend the beads. Transfer the specified amount of resuspended beads (see table) to an RNase-free microcentrifuge tube.
5. Insert the tube into the magnetic particle separator (MPS) for ∼0.5–2 minutes. Remove and discard the supernatant.
6. Immediately add the specified volume of Wash Buffer W7.
7. Remove the tube from the MPS and resuspend the beads by pipetting gently up and down.
8. Repeat Steps 5–6 one more time.
9. Insert the tube into the MPS for ∼0.5–2 minutes. Remove and discard the supernatant.

5
Experienced Users Procedure to Isolate mRNA from Total RNA, Continued
Step Procedure
Total RNA Protocol, continued
10. Immediately add the total RNA sample from Step 3, and the heated Binding Buffer B6 from Step 2, previous page.
11. Remove the tube from the MPS and resuspend beads by pipetting gently up and down.
12. Incubate the capped tube at 65°C to 70°C for 2–5 minutes.
13. Transfer the tube to a rotator and rotate the sample for 10 minutes at room temperature.
14. Insert the tube in the MPS for ∼0.5–2 minutes. Remove and save the supernatant.
15. Immediately add the specified volume of Wash Buffer W7.
16. Remove the tube from the MPS and resuspend the beads by pipetting gently up and down.
17. Reinsert the tube in the MPS for ∼0.5–2 minutes. Remove and discard the wash buffer from the tube.
18. Repeat Steps 15–17 three more times.
19. Immediately add 5–20 μL RNase-free water for <50 μg total RNA starting amount or add 20–50 μL RNase-free water for >50 μg total RNA starting amount.
20. Remove the tube from the MPS and resuspend beads by pipetting. Incubate at 37°C for 2–5 minutes.
21. Insert the tube into the MPS for∼0.5–2 minutes. Remove and save the supernatant.
Important: The supernatant contains the isolated mRNA.
22. Repeat Steps 19–21 using 5 μL RNase-free water for <50 μg total RNA and 10 μL RNase-free water for >50 μg total RNA. Again, save the supernatant.
23. Combine the supernatants from Steps 21 and 22 containing your isolated mRNA.
24. Store mRNA at –80ºC.

6
Experienced Users Procedure to Isolate mRNA from Cells
Introduction This quick reference sheet is included for experienced users of
the FastTrack® MAG mRNA Isolation Kits. If you are a first time user, refer to the details in the manual.
Step Procedure
Cells Protocol
Use the listed amount of reagents with the specified amount of cells for the purification procedure described below.
Amount Micro Maxi
Cells <1 × 105 105–106 106–107 107–5 × 107
FastTrack® MAG Beads 20 μL 20–50 μL 50–100 μL 100–200 μL
Binding Buffer B6 100 μL 200 μL 500 μL 500 μL
Wash Buffer W6 100 μL 200 μL 500 μL 500 μL
Wash Buffer W7 100 μL × 5 200 μL × 5 500 μL × 5 500 μL × 5
1. Harvest cells and prepare cell lysate as described on page 20.
2. Preheat the specified volume of Binding Buffer B6 (see table) at 65°C to 70°C.
3. Gently pipet the FastTrack® MAG Beads up and down to thoroughly resuspend the beads. Transfer the specified amount of resuspended beads (see table) to an RNase-free microcentrifuge tube.
4. Insert the tube into the MPS for ∼0.5–2 minutes. Remove and discard the supernatant.
5. Immediately add the specified volume of Wash Buffer W7.
6. Remove the tube from the MPS and resuspend the beads by pipetting gently up and down.
7. Repeat Steps 4–6 one more time.
8. Insert the tube into the magnetic particle separator (MPS) for ∼0.5–2 minutes. Remove and discard the supernatant.
9. Immediately add the cell lysate (page 20), and the heated Binding Buffer B6 from Step 2.

7
Experienced Users Procedure to Isolate mRNA from Cells, Continued
Step Procedure
Cell Protocol, continued
10. Remove the tube from MPS and resuspend the beads by pipetting up and down.
11. Incubate the capped tube at 65°C to 70°C for 2–5 minutes.
12. Transfer the tube to a rotator and rotate the sample for 10 minutes at room temperature.
13. Insert the tube in the MPS for ∼2–5 minutes. Remove and save the supernatant.
14. Immediately add the specified volume of Wash Buffer W6.
15. Remove the tube from the MPS and resuspend the beads by pipetting.
16. For >1 × 106 cells and/or performing downstream RT-PCR: Add 1 μL (1 unit) of DNase I, Amplification Grade, per 100 μL Wash Buffer W6 to the tube. Mix by pipetting gently up and down and incubate at 25°C for 5–10 minutes.
17. Insert the tube in the MPS for ∼0.5–2 minutes. Remove and discard the wash buffer from the tube.
18. Immediately add the specified volume of Wash Buffer W7.
19. Remove the tube from the MPS and resuspend the beads by pipetting gently up and down.
20. Insert the tube in the MPS for ∼0.5–2 minutes. Remove and discard the wash buffer.
21. Repeat Steps 18–20 using Wash Buffer W7 two more times.
22. Immediately add 5–20 μL RNase-free water for a starting amount of <1 × 106 cells or add 20–50 μL RNase-free water for a starting amount of >1 × 106 cells.
23. Remove the tube from the MPS and resuspend the beads by pipetting. Incubate at 37°C for 2–5 minutes.
24. Insert the tube into the MPS for ∼0.5–2 minutes. Remove and save the supernatant.
Important: The supernatant contains the isolated mRNA.
25. Repeat Steps 22–24, using 5 μL RNase-free water for <1 × 106 cells and 10 μL RNase-free water for >1 × 106 cells.
26. Combine the supernatants from Steps 24 and 25 containing your isolated mRNA. Store mRNA at –80°C.

8
Experienced Users Procedure to Isolate mRNA from Tissues
Introduction This quick reference sheet is included for experienced users of
the FastTrack® MAG mRNA Isolation Kits. If you are a first time user, refer to the details in the manual.
Step Procedure
Tissue Protocol
Use the listed amount of reagents with the specified amount of tissue for the purification procedure described below.
Amount Micro Maxi
Tissue <10 mg 10–50 mg 50–200 mg 200–500 mg
FastTrack® MAG Beads 20 μL 20–50 μL 50–100 μL 100–200 μL
Binding Buffer B6 100 μL 200 μL 500 μL 500 μL
Wash Buffer W6 100 μL 200 μL 500 μL 500 μL
Wash Buffer W7 100 μL × 5 200 μL × 5 500 μL × 5 500 μL × 5
1. Harvest tissue and prepare tissue lysate as described on page 26.
2. Preheat the specified volume of Binding Buffer B6 (see table) at 65°C to 70°C.
3. Gently pipet the FastTrack® MAG Beads up and down to thoroughly resuspend the beads. Transfer the specified amount of resuspended beads (see table) to an RNase-free microcentrifuge tube.
4. Insert the tube into the MPS for ∼0.5–2 minutes. Remove and discard the supernatant.
5. Immediately add the specified volume of Wash Buffer W7.
6. Remove the tube from the magnetic particle separator (MPS) and resuspend the beads by pipetting gently up and down.
7. Repeat Steps 4–6 one more time with Wash Buffer W7.
8. Insert the tube into the MPS for ∼0.5–2 minutes. Remove and discard the supernatant.
9. Immediately add the tissue lysate (page 26), and the heated Binding Buffer B6 from Step 2.

9
Experienced Users Procedure to Isolate mRNA from Tissues, Continued
Step Procedure
Tissue Protocol, continued
10. Remove the tube from MPS and resuspend the beads by pipetting up and down.
11. Incubate the capped tube at 65°C to 70°C for 2–5 minutes.
12. Transfer the tube to a rotator and rotate the sample for 10 minutes at room temperature.
13. Insert the tube in the MPS for ∼2–5 minutes. Remove and save the supernatant.
14. Immediately add the specified volume of Wash Buffer W6.
15. Remove the tube from the MPS and resuspend the beads by pipetting gently up and down.
16. For >50 mg tissue and/or performing downstream RT-PCR: Add 1 μL (1 unit) DNase I, Amplification Grade, per 100 μL Wash Buffer W6 to the tube. Mix by pipetting and incubate at 25°C for 5–10 minutes.
17. Insert the tube in the MPS for∼0.5–2 minutes. Remove and discard the wash buffer from the tube.
18. Immediately add the specified volume of Wash Buffer W7.
19. Remove the tube from the MPS and resuspend the beads by pipetting gently up and down.
20. Insert the tube in the MPS for ∼0.5–2 minutes. Remove and discard the wash buffer from the tube.
21. Repeat Steps 18–20 using Wash Buffer W7 two more times. 22. Immediately add 5–20 μL RNase-free water for <50 mg
tissue starting material or add 20–50 μL RNase-free water for >50 mg of tissue starting material.
23. Remove the tube from the MPS and resuspend the beads by pipetting. Incubate at 37°C for 2–5 minutes.
24. Insert the tube into the MPS for ∼0.5–2 minutes. Remove and save the supernatant.
Important: The supernatant contains the isolated mRNA.
25. Repeat Steps 22–24, using 5 μL RNase-free water for <50 mg of tissue and 10 μL RNase-free water for >50 mg of tissue. Again, save the supernatant.
26. Combine the supernatants from Steps 24 and 25 containing your isolated mRNA. Store mRNA at –80°C.

10
Kit Contents and Storage
Shipping and Storage
All components of the FastTrack® MAG Micro mRNA Isolation Kit (Cat. no. K1580-01) and the FastTrack® MAG Maxi (Cat. no. K1580-02) mRNA Isolation Kit are shipped at room temperature.
Upon receipt, store all components at room temperature. Note: Do not freeze the beads.
Kit Configurations
The FastTrack® MAG mRNA Isolation Kits are designed to isolate mRNA from different size sample ranges.
The following table shows the maximum sample size for each kit (based on the amount of beads provided and the number of reactions specified).
Maximum Sample Size
Total RNA Cells Tissue
Micro Kit 50 μg 1 × 106 50 mg
Maxi Kit 2000 μg 5 × 107 500 mg
Micro Kit Components
The FastTrack® MAG Micro mRNA Isolation Kit provides reagents for 12 reactions using ≤50 μL of beads per reaction.
Component Amount
FastTrack® MAG beads 600 μL
Lysis Buffer L4 3 mL
Proteinase K, 20 mg/mL in storage buffer (proprietary) 75 μL
Binding Buffer B6 3 mL
Wash Buffer W6 3 mL
Wash Buffer W7 18 mL
RNase-Free Water 750 μL

11
Kit Contents and Storage, Continued
Maxi Kit Components
The FastTrack® MAG Maxi mRNA Isolation Kit provides reagents for 6 reactions using ≤200 μL of beads per reaction.
Component Amount
FastTrack® MAG beads 1.2 mL
Lysis Buffer L4 4.8 mL
Proteinase K, 20 mg/mL in storage buffer (proprietary) 96 μL
Binding Buffer B6 4.8 mL
Wash Buffer W6 4.8 mL
Wash Buffer W7 28.8 mL
RNase-Free Water 750 μL
DNase I, Amplification Grade (1 U/μL) 100 μL

12
Introduction
System Overview
Introduction The FastTrack® MAG mRNA Isolation Kit uses oligo(dT)-
conjugated magnetic beads to isolate poly A+ RNA directly from cell lysate, tissue lysate, or total RNA in 1–1.5 hours using conventional laboratory equipment (Morrissey et al., 1989; Stone et al., 1996). The high quality and quantity of the isolated mRNA makes it suitable for use in a variety of applications, including cDNA library construction, microinjection, in vitro translation, RT-PCR, Northern blotting. The mRNA captured on the magnetic beads may also be used in some applications without elution.
Using the kit, you can isolate mRNA from varying amounts of cells (<1 × 105 – 5 × 107 cells), tissue samples (<10 mg–>300 mg of tissue), or total RNA (<1 μg–2000 μg). Separate procedures are provided for each type of sample.
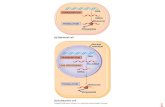
Workflow Using the FastTrack® MAG mRNA Isolation Kit, you first
isolate total RNA using a method of choice, or prepare cells/tissue samples using the reagents provided in this kit. Then you prewash the FastTrack® MAG Beads, bind the sample, and perform a series of wash steps. Finally, you elute the mRNA from the beads using RNase-free water provided in the kit.
20–30 minutes
20–30 minutes
10–20 minutes
5–10 minutes
Isolate Total RNA Lyse Cells
Prewash FastTrack® MAG Beads and bind sample
Wash FastTrack® MAG Beads
Elute sample
HomogenizeTissue

13
System Overview, Continued
Advantages of the Kit
The FastTrack® MAG mRNA Isolation Kit offers the following advantages:
• Isolates high-quality mRNA in less than 1 hour from total RNA and 1.5 hours from cells or tissue
• Isolated mRNA has minimal ribosomal RNA and genomic DNA contamination
• Higher yields of mRNA than comparable systems
• mRNA may be eluted at a high concentration for specific downstream applications
• Compatible with high-throughput applications
Typical Yields Source Recovery of mRNA
1 mg total RNA (from mammals) >30 μg (>3%)
1 mg total RNA (from plants) >15 μg (>1.5%)
1 × 106 cells 1 μg
1 g tissue 5–80 μg* *Yields of mRNA from tissue are highly dependent on the type of tissue.
Magnetic Particle Separator
This kit requires the use of a magnetic particle separator (MPS) with holes for 1.5-mL tubes to separate the FastTrack® MAG beads in solution. The Magna-Sep™ MPS is a 6-hole magnetic particle separator available from Life Technologies that can hold 1.5–2.0-mL tubes (Cat. no. K1585-01). You may also use separators from other manufacturers.

14
Handling RNA
General Handling of RNA
When working with RNA:
• Do not use plasticware or glassware without first eliminating possible ribonuclease contamination.
• Wear latex gloves while handling reagents and RNA samples to prevent RNase contamination from the surface of the skin.
• Always use proper microbiological aseptic technique when working with RNA.
RNase AWAY® Reagent
We recommend RNase AWAY® Reagent for removing RNase and DNA contamination from surfaces. This reagent is nonabrasive, noncarcinogenic, and nonbiologically corrosive. See page 33 for ordering information.
RNase-free Plasticware
• Use disposable, individually wrapped, sterile plasticware.
• Use only sterile, new pipette tips and microcentrifuge tubes.
• All plasticware provided in the FastTrack® MAG mRNA Isolation Kit is RNase-free.

15
Methods Isolating mRNA from Total RNA
Introduction This section provides procedures for isolating mRNA from total RNA.
User Supplied Materials
• Total RNA purification reagent/system
• Magnetic particle separator (the Magna-Sep™ 6-hole MPS is available from Life Technologies; see ordering information on page 33)
• Heat block or thermal cycler preheated to 65°C to 70°C
• RNase-free 1.5-mL tubes
• Pipettes
• Rotator
• Tabletop microcentrifuge
Isolating Total RNA
To isolate total RNA, we recommend TRIzol® Reagent (Cat. no. 15596-026 and 15596-018), the PureLink® RNA Mini Kit (Cat. no. 12183-018A), or the PureLink® Pro 96 total RNA Purification Kit (Cat. no. 12173-011A).
Note If the total RNA contains high-level of genomic DNA or you are performing downstream RT-PCR, perform a DNase I digestion step to minimize genomic DNA contamination prior to mRNA purification.
Amounts of Beads and Buffers
The following table lists the amounts of FastTrack® MAG Beads, Binding Buffer B6, and Wash Buffer W7 to use with the specified amount of total RNA.
Note: The isolation procedure uses six separate volumes of Wash Buffer W7, so each volume is shown × 6.
Amount Micro Maxi
Total RNA <1 μg 1–50 μg 50–500 μg 500–2000 μg
FastTrack® MAG Beads 20 μL 50 μL 100 μL ≥200 μL*
Binding Buffer B6 100 μL 200 μL 500 μL 500 μL
Wash Buffer W7 100 μL × 6 200 μL × 6 500 μL × 6 500 μL × 6
*To ensure maximum mRNA yield, the ratio of total RNA (μg) to beads (μL) should be >7.5 (i.e., at least 200 μL of beads per 1500 μg of total RNA).

16
Isolating mRNA from Total RNA, Continued
Before Proceeding
Before proceeding with the protocol, preheat the Binding Buffer B6 and add water to the total RNA sample as described. While the buffer is heating, proceed with the Prewash and Binding Procedure, page 17.
1. Add the volume of Binding Buffer B6 specified in the table on page 15 to a 1.5-mL RNase-free tube. Place the tube in a heat block or thermal cycler at 65°C to 70°C.
2. In a separate 1.5-mL RNase-free tube, add RNase-free water to your total RNA sample to a total volume equal the volume of Binding Buffer B6 in Step 1. Place the sample tube on ice.
Important When following the procedures in this section, be careful to
never let the beads dry out.
Pipetting Liquid from Tubes
When pipetting liquid from a tube in the magnetic separator, always point the pipette tip toward the opposite side of the tube bottom from the pellet to avoid touching the beads.
Bead pellet

17
Isolating mRNA from Total RNA, Continued
Prewash and Binding Procedure
While the Binding Buffer B6 is preheating (step 1 page 16), proceed with steps 1–4:
1. Thoroughly resuspend the FastTrack® MAG Beads by pipetting them gently up and down. Transfer the amount of resuspended beads specified in the table on page 15 to an RNase-free microcentrifuge tube.
2. Insert the microcentrifuge tube into the magnetic particle separator (MPS). When the beads are clearly separated from the liquid (∼0.5–2 minutes), pipette the liquid out of the tube and discard, and immediately add the volume of Wash Buffer W7 specified in the table on page 15. Important: Do not let the beads dry out.
3. Remove the tube from the magnetic particle separator and resuspend the beads by pipetting gently up and down.
4. Repeat Steps 2–3 one more time, and then proceed to Step 5.
5. Insert the tube into the magnetic separator. When the beads are clearly separated from the liquid (∼0.5–2 minutes), pipette the liquid out of the tube and discard, and immediately add the total RNA sample from Step 2, page 16, and the heated Binding Buffer B6 from Step 1, page 16.
6. Remove the tube from the magnetic separator and resuspend the beads in the solution by pipetting gently up and down. Note: Pipetting too vigorously may damage the RNA.
7. Cap the tube and place in the heat block or thermal cycler. Incubate at 65°C to 70°C for 2–5 minutes.
8. Transfer the tube to a rotator and rotate the sample for 10 minutes at room temperature. Proceed to Wash and Elution Procedure, page 18.

18
Isolating mRNA from Total RNA, Continued
Wash and Elution Procedure
1. Insert the tube from Prewash and Binding Procedure (Step 8, page 17) in the magnetic separator. When the beads are clearly separated (∼0.5–2 minutes), remove and save the supernatant.
Note: For rare samples, if your yield is low, you may want to preserve this supernatant and attempt to perform a separate isolation with it.
2. To the beads, immediately add the volume of Wash Buffer W7 specified in the table on page 15.
3. Remove the tube from the magnetic separator and resuspend the beads in the wash buffer by pipetting gently up and down.
4. Reinsert the tube in the magnetic separator. When the beads are clearly separated (∼0.5–2 minutes), remove the wash buffer from the tube and discard. Be careful to completely remove the wash buffer.
5. Repeat Steps 2–4 three more times, and proceed to Step 6.
6. If you started with <50 μg of total RNA, immediately add 5–20 μL of RNase-free water. If you started with >50 μg of total RNA, immediately add 20–50 μL of RNase-free water.
7. Remove the tube from the magnetic separator and thoroughly resuspend the beads by pipetting gently up and down. Incubate at 37°C for 2–5 minutes. Use the longer incubation time for larger samples.
8. Insert the tube into the magnetic separator. When the beads are clearly separated (∼0.5–2 minutes), remove and save the supernatant.
Important: The supernatant contains the isolated mRNA. Do not discard.
9. Repeat Steps 6–8, using 5 μL of RNase-free water for <50 μg of total RNA and 10 μL of RNase-free water for >50 μg of total RNA. Again, save the supernatant.
10. Combine the supernatants from Steps 8 and 9. This is your isolated mRNA. Store mRNA at –80°C.
The mRNA quality and yield may be determined by spectrophotometry and agarose gel electrophoresis, as described on page 30.

19
Isolating mRNA from Cells
Introduction This section provides procedures for isolating mRNA from
cells.
User Supplied Materials
• Magnetic particle separator (the Magna-Sep™ 6-hole MPS is available from Life Technologies; see ordering information on page 33)
• Heat block or thermal cycler preheated to 65°C to 70°C
• Water bath or incubator at 45°C
• RNase-free 1.5-mL tubes
• Pipettes
• Rotator
• 21-gauge needle and 1-mL syringe
• Tabletop microcentrifuge
• Optional for Micro kit: DNase I, Amplification Grade (Cat. no. 18068-015)
Amounts of Beads and Buffers
The following table lists the amounts of FastTrack® MAG Beads, Lysis Buffer, Proteinase K, Binding Buffer B6, and Wash Buffer W6, and Wash Buffer W7 to use with the specified amount of cells.
Note: The isolation procedure uses five separate volumes of Wash Buffer W7, so each volume is shown × 5.
Amount Micro Maxi
Cells <1 × 105 1 × 105–106 1 × 106–107 1 × 107–5 × 107
FastTrack® MAG Beads 20 μL 20–50 μL 50–100 μL 100–200 μL
Lysis Buffer L4 100 μL 200 μL 500 μL 500 μL
Proteinase K 2.5 μL 5 μL 10 μL 10 μL
Binding Buffer B6 100 μL 200 μL 500 μL 500 μL
Wash Buffer W6 100 μL 200 μL 500 μL 500 μL
Wash Buffer W7 100 μL × 5 200 μL × 5 500 μL × 5 500 μL × 5
*For cells amounts >5 × 107, you can use more Lysis Buffer L4/Proteinase K and Binding Buffer B6 in multiple tubes or proportionally scale up to a larger tube.

20
Isolating mRNA from Cells, Continued
Preparing Binding Buffer and Lysis Buffer
Preheat the Binding Buffer B6 and prepare the Lysis Buffer L4 as described: 1. Add the volume of Binding Buffer B6 specified in the
table above to a 1.5-mL RNase-free tube. Place the tube in a heat block or thermal cycler at 65°C to 70°C.
2. In a separate 1.5-mL RNase-free tube, add the volume of Proteinase K specified in the table (page 19) to the specified volume of Lysis Buffer L4. Proceed with Cell Lysis.
Preparing Cells
Collect, wash, and pellet cells according to your standard laboratory protocol.
Cell Lysis Lyse cells as described.
1. To the cell pellet in a microcentrifuge tube, add the Lysis Buffer solution from Step 2, Preparing Binding Buffer and Lysis Buffer.
2. For <1 × 105 cells, pipette the lysate up and down at least 10 times. For >1 × 105 cells, shear the DNA by passing the lysate through a 21-gauge needle fitted to a 1-mL syringe. Pass the lysate through the needle ≥20 times for Micro kit volumes and ≥30 times for Maxi kit volumes.
3. Transfer the tube to a microcentrifuge and spin at maximum speed for 5 minutes at room temperature.
4. Carefully transfer the supernatant containing your sample to a fresh microcentrifuge tube.
5. Incubate the sample at 45°C in a water bath or incubator for 10 minutes. While the sample is incubating, proceed to Prewash and Binding Procedure, page 21.
Pipetting Liquid from Tubes
When pipetting liquid from a tube in the magnetic separator, always point the pipette tip toward the opposite side of the tube bottom from the pellet to avoid touching the beads.
Bead pellet

21
Isolating mRNA from Cells, Continued
Important When following the procedures in this section, be careful to
never let the beads dry out.
Prewash and Binding Procedure
While the lysate is incubating (Step 5 page 20), proceed with the steps 1–4:
1. Thoroughly resuspend the FastTrack® MAG Beads by pipetting them gently up and down. Transfer the amount of resuspended beads specified in the table on page 19 to an RNase-free microcentrifuge tube.
2. Insert the microcentrifuge tube into the magnetic separator. When the beads are clearly separated from the liquid (∼0.5–2 minutes), pipette the liquid out of the tube and discard, and immediately add the volume of Wash Buffer W7 specified in the table on page 19.
Important: Do not let the beads dry out.
3. Remove the tube from the magnetic separator and resuspend the beads in the wash buffer by pipetting gently up and down.
4. When the incubation time of the lysate has ∼4 minutes left (Step 5, page 20), repeat the wash procedure in Steps 2 and 3 one more time, and then proceed to Step 5.
5. Insert the tube into the magnetic separator. When the beads are clearly separated from the liquid (∼0.5–2 minutes), pipette and discard the solution, and immediately add the lysate from Cell Lysis, Step 5, page 20, and the heated Binding Buffer B6 from Preparing the Binding Buffer and Lysis Buffer, Step 1, page 20.
6. Remove the tube from the magnetic separator and resuspend the beads completely in the solution by pipetting up and down.
7. Cap the tube and place in the heat block or thermal cycler. Incubate at 65°C to 70°C for 2–5 minutes.
8. Transfer the tube to a rotator and rotate the sample for 10 minutes at room temperature. Proceed to Wash and Elution Procedure, page 22.

22
Isolating mRNA from Cells, Continued
Wash and Elution Procedure
1. Insert the tube from Prewash and Binding Procedure, Step 8, page 21, in the magnetic separator. Visually inspect the beads until they are clearly separated (∼2–5 minutes).
Note: Beads may take longer to separate depending on the viscosity of the lysate and the abundance of non-sheared genomic DNA.
2. When the beads are separated, remove and save the supernatant.
Note: For rare samples, if your yield is low, you may want to preserve this supernatant and attempt to perform a separate isolation with it.
3. To the beads, immediately add the volume of Wash Buffer W6 specified in the table on page 19.
4. Remove the tube from the magnetic separator and resuspend the beads by pipetting gently up and down.
5. If you are using >1 × 106 cells and/or performing downstream RT-PCR: Add 1 μL (1 unit) of DNase I, Amplification Grade, per 100 μL of Wash Buffer W6 to the tube. Mix by pipetting gently up and down and incubate at 25°C for 5–10 minutes.
6. Reinsert the tube in the magnetic separator. When the beads are clearly separated from the liquid (∼0.5–2 minutes), remove the wash buffer from the tube and discard. Carefully remove the wash buffer completely.
7. To the beads, immediately add the volume of Wash Buffer W7 specified in the table on page 19.
8. Remove the tube from the magnetic separator and resuspend the beads by pipetting gently up and down.
9. Reinsert the tube in the magnetic separator. When the beads are clearly separated from the liquid (∼0.5–2 minutes), remove the wash buffer from the tube and discard. Be careful to completely remove the wash buffer.
10. Repeat Steps 7–9 using Wash Buffer W7 two more times, then proceed to Step 11.
11. If you started with <1 × 106 cells, immediately add 5–20 μL of RNase-free water. If you started with >1 × 106 cells, immediately add 20–50 μL of RNase-free water.

23
Isolating mRNA from Cells, Continued
Wash and Elution Procedure, continued
12. Remove the tube from the magnetic separator and thoroughly resuspend the beads by pipetting gently up and down. Incubate at 37°C for 2–5 minutes. Use the longer incubation time for larger samples.
13. Insert the tube into the magnetic separator. When the beads are clearly separated from the liquid (∼0.5–2 minutes), remove and save the supernatant.
Important: The supernatant contains the isolated mRNA. Do not discard.
14. Repeat Steps 11–13, using 5 μL of RNase-free water for <1 × 106 cells and 10 μL of RNase-free water for >1 × 106 cells. Again, save the supernatant.
15. Combine the supernatants from Steps 13 and 14. This is your isolated mRNA. Store mRNA at –80°C.
The mRNA quality and yield may be determined and spectrophotometry and agarose gel electrophoresis, as described on page 30.

24
Isolating mRNA from Tissues
Introduction This section provides procedures for isolating mRNA from
tissue samples.
User Supplied Materials
• Magnetic particle separator (the Magna-Sep™ 6-hole MPS is available from Life Technologies; see ordering information on page 33)
• Heat block or thermal cycler, preheated to 65°C to 70°C • Water bath or incubator at 45°C
• RNase-free tubes
• Homogenizer, motor-driven, compatible with microcentrifuge tube used to prepare tissue sample (various manufacturers)
• Pipettes
• Rotator • Tabletop microcentrifuge
• Optional for Micro kit: DNase I, Amplification Grade (Cat no. 18068-015)
Amounts of Beads and Buffers
The following table lists the amounts of FastTrack® MAG Beads, Lysis Buffer, Proteinase K, Binding Buffer B6, Wash Buffer W6, and Wash Buffer W7 to use with the specified amount of tissue.
Note: The isolation procedure has five separate washes using Wash Buffer W7, so each volume is shown × 5.
Amount Micro Maxi
Tissue <10 mg 10–50 mg 50–200 mg 200–500 mg*
FastTrack® MAG Beads 20 μL 20–50 μL 50–100 μL 100–200 μL
Lysis Buffer L4 100 μL 200 μL 500 μL 500 μL
Proteinase K 2.5 μL 5 μL 10 μL 10 μL
Binding Buffer B6 100 μL 200 μL 500 μL 500 μL
Wash Buffer W6 100 μL 200 μL 500 μL 500 μL
Wash Buffer W7 100 μL × 5 200 μL × 5 500 μL × 5 500 μL × 5
*For tissue amounts >200 mg, you can use more Lysis Buffer L4/Proteinase K and Binding Buffer B6 in multiple tubes or proportionally scale up to a larger tube.

25
Isolating mRNA from Tissues, Continued
Note • The abundance of mRNA varies greatly in different
tissues. Adjust the volumes accordingly.
• To maximize the yield of mRNA from tissues, we suggest first isolating total RNA from the tissue using TRIzol® Reagent or another method and then isolating mRNA from the total RNA using this kit, as described starting on page 15.
Preparing the Binding Buffer and Lysis Buffer
Preheat the Binding Buffer B6 and prepare the Lysis Buffer L4 as described:
1. Add the volume of Binding Buffer B6 specified in the table on the previous page to an RNase-free tube. Place the tube in a heat block or thermal cycler at 65°C to 70°C.
2. In a separate RNase-free tube, add the volume of Proteinase K specified in the table on the previous page to the specified volume of Lysis Buffer L4. Proceed with Preparing the Tissue Sample, page 26.
Important Homogenize tissue in the presence of lysis buffer to ensure
immediate inactivation of any RNases that are released as the cells lyse. Complete homogenization is critical for complete cell lysis and inactivation of RNases.
Before use, clean and wash the homogenizer tip and then autoclave and bake for 3 hours or overnight at 210°C.
Pipetting Liquid from Tubes
When pipetting liquid from a tube in the magnetic separator, always point the pipette tip toward the opposite side of the tube bottom from the pellet to avoid touching the beads.
Bead pellet

26
Isolating mRNA from Tissues, Continued
Preparing the Tissue Sample
Prepare the tissue sample as described:
1. Add the Lysis Buffer L4 plus Proteinase K from Step 2, previous page, to your frozen or fresh tissue sample in a sterile microcentrifuge tube. We recommend using a 1.5-mL tube for <50 mg of tissue and a 12-mL 2059 tube for >50 mg of tissue.
2. Immediately homogenize the tissue using a motor-driven homogenizer that is compatible with your microcentrifuge tube. Start at a low speed and gradually increase speed until the homogenate appears smooth with no visible particulate matter (~15–30 seconds). Keep foaming to a minimum by adjusting the speed.
Note: Thorough homogenization is required for maximum mRNA yield.
3. Transfer the tube to a microcentrifuge and spin at maximum speed for 5 minutes at room temperature.
4. Carefully transfer the supernatant containing your sample to a fresh microcentrifuge tube.
5. Incubate the sample at 45°C in a water bath or incubator for 10 minutes. While the sample is incubating, proceed to Prewash and Binding Procedure, page 27.

27
Isolating mRNA from Tissues, Continued
Important When following the procedures in this section, be careful to
never let the beads dry out.
Prewash and Binding Procedure
While the sample is incubating (Step 5, page 26), begin the following procedure to wash the beads and bind the sample:
1. Thoroughly resuspend the FastTrack® MAG Beads by pipetting them gently up and down. Transfer the amount of resuspended beads specified in the table on page 24 to an RNase-free microcentrifuge tube.
2. Insert the microcentrifuge tube into the magnetic separator. When the beads are clearly separated from the liquid (∼0.5–2 minutes), pipette the liquid out of the tube and discard, and immediately add the volume of Wash Buffer W7 specified in the table on page 24. Important: Do not let the beads dry out.
3. Remove the tube from the magnetic separator and resuspend the beads in the wash buffer by pipetting gently up and down.
4. When the incubation time of the sample has ∼2 minutes left (Step 5, page 26), repeat the wash procedure in Steps 2 and 3 one time, and then proceed to Step 5.
5. Insert the tube into the magnetic separator. When the beads are clearly separated from the liquid (∼0.5–2 minutes), pipette the liquid out of the tube and discard, and immediately add the sample from Step 5, previous page, and the heated Binding Buffer B6 from Step 1, page 25.
6. Remove the tube from the magnetic separator and resuspend the beads completely in the solution by pipetting up and down.
7. Cap the tube and place in the heat block or thermal cycler. Incubate at 65°C to 70°C for 2–5 minutes.
8. Transfer the tube to a rotator and rotate the sample for 10 minutes at room temperature. Proceed to Wash and Elution Procedure, page 28.

28
Isolating mRNA from Tissues, Continued
Wash and Elution Procedure
1. Insert the tube from Prewash and Binding Procedure, Step 8, page 27, in the magnetic separator. Visually inspect the beads until they are clearly separated (∼2–5 minutes).
Note: Beads may take longer to separate depending on the viscosity of the sample and the abundance of non-sheared genomic DNA.
2. When the beads are separated, remove and save the supernatant.
Note: For rare samples, if your yield is low, you may want to preserve this supernatant and attempt to perform a separate isolation with it.
3. To the beads, immediately add the volume of Wash Buffer W6 specified in the table on page 24.
4. Remove the tube from the magnetic separator and resuspend the beads in the wash buffer by pipetting gently up and down.
5. If you are using >50 mg tissue and/or performing downstream RT-PCR: Add 1 μL (1 unit) of DNase I, Amplification Grade, per 100 μL of Wash Buffer W6 to the tube. Mix by pipetting gently up and down and incubate at 25°C for 5–10 minutes.
6. Reinsert the tube in the magnetic separator. When the beads are clearly separated from the liquid (∼0.5–2 minutes), remove the wash buffer from the tube and discard. Be careful to completely remove the wash buffer.
7. To the beads, immediately add the volume of Wash Buffer W7 specified in the table on page 24.
8. Remove the tube from the magnetic separator and resuspend the beads by pipetting gently up and down.
9. Reinsert the tube in the magnetic separator. When the beads are clearly separated from the liquid (∼0.5–2 minutes), remove the wash buffer from the tube and discard. Be careful to completely remove the wash buffer.
10. Repeat Steps 7–9 using Wash Buffer W7 two more times, then proceed immediately to Step 11.

29
Isolating mRNA from Tissues, Continued
Wash and Elution Procedure, continued
11. If you started with <50 mg of tissue, immediately add 5–20 μL of RNase-free water. If you started with >50 mg of tissue, immediately add 20–50 μL of RNase-free water.
12. Remove the tube from the magnetic separator and thoroughly resuspend the beads by pipetting gently up and down. Incubate at 37°C for 2–5 minutes. Use the longer incubation time for larger samples.
13. Insert the tube into the magnetic separator. When the beads are clearly separated from the liquid (∼0.5–2 minutes), remove and save the supernatant.
Important: The supernatant contains the isolated mRNA. Do not discard.
14. Repeat Steps 11–13, using 5 μL of RNase-free water for <50 mg of tissue and 10 μL of RNase-free water for >50 mg of tissue. Again, save the supernatant.
15. Combine the supernatants from Steps 13 and 14. This is your isolated mRNA. Store mRNA at –80°C.
The mRNA quality and yield may be determined and spectrophotometry and agarose gel electrophoresis, as described on page 30.

30
1Kb+ 1 2 3
4k3k2k
1k18S
28S
5S
Determining mRNA Yield and Quality
Determining of mRNA Yield
The following general protocol may be used to calculate the yield of mRNA using A260 absorbance:
1. Aliquot 2 μL of the isolated mRNA into a clean UV cuvette and add 198 μL of TE Buffer for a 1:100 dilution.
2. Blank a UV/visible spectrophotometer using TE Buffer, and then measure the absorbance at 260 nm.
3. The A260 reading should fall within the standard specification for the spectrophotometer (typically 0.01–1.0 OD). If it falls outside this range, adjust the dilution and rescan. If the A260 reading is too low, use a lower dilution; if it’s too high, use a higher dilution.
4. Calculate the yield of mRNA using the formula below:
mRNA yield (μg/μL) = A260 × 0.04 μg/μL RNA × Dilution factor
The dilution factor is 100 for the dilution described above. For example, if you diluted 2 μL of mRNA at 1:100, and the A260 is 0.5, then 0.5 × 0.04 μg/μL RNA × 100 = 2 μg/μL.
Analyzing mRNA Quality
The quality of the isolated mRNA may be determined by agarose gel electrophoresis. The mRNA appears as a smear from 500 bp to 8 kb, with the highest intensity between ∼1–3 kb. Any contaminating ribosomal RNA will appear as bands within the mRNA smear. These bands should be faint (<20% higher intensity than the rest of the smear).
In the 1.2% E-Gel® agarose gel on the right, lane 1 and lane 2 each contain 0.825 μg of mRNA isolated from rat heart using the procedure described in this manual. Lane 3 was loaded with 2 μg of total RNA.

31
Troubleshooting
Trouble-shooting
The following table describes solutions to possible problems you may have with the kit. For additional assistance, contact Life Technologies Technical Support (see page 34).
Problem Cause Solution
Low yields of mRNA
Cells or tissues are not sufficiently homogenized
Homogenize cells/tissues until no or minimal cell clumps or tissue chucks are visible. We recommend using motor-driven homogenizers.
Cells or tissues are not completely lysed
Reduce the amount of starting material, or increase the amount of lysis buffer.
mRNA is not completely eluted from the magnetic beads
Wash the beads one more time using RNase-free water.
mRNA is degraded
Make sure that pipette tips, tubes, and other materials are RNase-free. Use RNase AWAY® Reagent to clean materials used for RNA isolation.
Add an RNase inhibitor (e.g., RNaseOUT™ Recombinant Ribonuclease Inhibitor) to the starting material (e.g., the cell medium). Dead cells/tissues can release RNases that cause RNA degradation. Minimize dead cells/tissues and immediately freeze samples to prevent RNA degradation.
Check the quality of the purified total RNA on a gel. The 28S band should have a higher intensity than the 18S band, and the 18S band should have a higher intensity than the 5S band. If you see an obvious smear at low molecular weight (between 5S to 18S rRNA), it indicates that the total RNA is partially degraded.
Samples have a low abundance of RNA
Use more starting material.

32
Troubleshooting, Continued
Problem Cause Solution
mRNA is contaminated with rRNA
Poor removal of rRNA during wash steps
Wash the beads 1 or 2 more times.
Perform the full procedure again, using the isolated mRNA from the first procedure.
mRNA is contaminated with genomic DNA
Poor removal of genomic DNA during the wash steps
Use the optional DNase I digestion step to remove genomic DNA contamination. Be sure to use Amplification Grade DNase I.
Viscous cell lysate
Sample is too large (mRNA will bind to oligo(dT) beads if the viscosity is reduced)
Reduce the sample size, or split the sample.
Sample contains large amounts of DNA
Split the sample as above and shear the DNA thoroughly using a 21-guage needle on a 1-ml syringe.

33
Appendix
Accessory Products
Additional Products
The following additional products are available from Life Technologies.
To order, visit www.lifetechnologies.com or contact Technical Support (see page 34).
Product Quantity Catalog No.
FastTrack® MAG 96 mRNA Isolation Kit 2 × 96 preps K1580-96
Magna-Sep™ Magnetic Particle Separator for use with 1.5-mL Tubes
Each K1585-01
RNase AWAY® Reagent 250 mL 10328-011
RNaseOUT™ Recombinant Ribonuclease Inhibitor (40 units/μL)
5000 units 10777-019
TRIzol® Reagent 100 mL 200 mL
15596-026 15596-018
DNase I, Amplification Grade 100 units 18068-015
PureLink® RNA Mini Kit 50 preps 12183-018A
PureLink® Pro 96 total RNA Purification Kit 4 plates 12173-011A

34
Technical Support
Obtaining Support
For the latest services and support information for all locations, go to www.lifetechnologies.com.
At the website, you can:
• Access worldwide telephone and fax numbers to contact Technical Support and Sales facilities
• Search through frequently asked questions (FAQs)
• Submit a question directly to Technical Support ([email protected])
• Search for user documents, SDSs, vector maps and sequences, application notes, formulations, handbooks, certificates of analysis, citations, and other product support documents
• Obtain information about customer training
• Download software updates and patches
Safety Data Sheets (SDS)
Safety Data Sheets (SDSs) are available at www.lifetechnologies.com/support.
Certificate of Analysis
The Certificate of Analysis provides detailed quality control and product qualification information for each product. Certificates of Analysis are available on our website. Go to www.lifetechnologies.com/support and search for the Certificate of Analysis by product lot number, which is printed on the box.
Limited Product Warranty
Life Technologies and/or its affiliate(s) warrant their products as set forth in the Life Technologies General Terms and Conditions of Sale found on the Life Technologies web site at www.lifetechnologies.com/termsandconditions. If you have any questions, please contact Life Technologies at www.lifetechnologies.com/support.

35
References
Morrissey, D. V., Lombardo, M., Eldredge, J. K., Kearney, K. R., Groody, E. P.,
and Collins, M. L. (1989) Nucleic Acid Hybridization Assays Employing dA-tail Capture Probes. Anal. Biochem. 181, 345-359
Stone, B. B., Cohen, S. P., Breton, G. L., Nietupski, R. M., Pelletier, D. A.,
Fiandaca, M. J., Moe, J. G., Smith, J. H., Shah, J. S., and Weisburg, W. G. (1996) Detection of rRNA from Four Respiratory Pathogens Using an Automated Qβ Replicase Assay. Molecular and Cellular Probes 10, 359-370
©2012 Life Technologies Corporation. All rights reserved. The trademarks mentioned herein are the property of Life Technologies Corporation or their respective owners. RNase AWAY is a registered trademark of Molecular Bio-Products, Inc. Trizol is a registered trademark of Molecular research Center, Inc. DISCLAIMER: LIFE TECHNOLOGIES CORPORATION AND/OR ITS AFFILIATE(S) DISCLAIM ALL WARRANTIES WITH RESPECT TO THIS DOCUMENT, EXPRESSED OR IMPLIED, INCLUDING BUT NOT LIMITED TO THOSE OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, OR NON-INFRINGEMENT. TO THE EXTENT ALLOWED BY LAW, IN NO EVENT SHALL LIFE TECHNOLOGIES AND/OR ITS AFFILIATE(S) BE LIABLE, WHETHER IN CONTRACT, TORT, WARRANTY, OR UNDER ANY STATUTE OR ON ANY OTHER BASIS FOR SPECIAL, INCIDENTAL, INDIRECT, PUNITIVE, MULTIPLE OR CONSEQUENTIAL DAMAGES IN CONNECTION WITH OR ARISING FROM THIS DOCUMENT, INCLUDING BUT NOT LIMITED TO THE USE THEREOF.

36
Notes

37
Notes

38
Notes


19 September 2012
Headquarters 5791 Van Allen Way | Carlsbad, CA 92008 USA Phone +1 760 603 7200 | Toll Free in USA 800 955 6288 For support visit lifetechnologies.com/support or email [email protected] lifetechnologies.com
![Paraquat Modulates Alternative Pre-mRNA Splicing by ...muehlemann.dcb.unibe.ch/publications/Vivarelli.pdf · alternative pre-mRNA splicing (AS) [2]. Pre-mRNA splicing is a crucial](https://static.fdocuments.in/doc/165x107/606212ed67e7345b4269ee34/paraquat-modulates-alternative-pre-mrna-splicing-by-alternative-pre-mrna-splicing.jpg)