Engineering carbonic anhydrase for highly selective ester hydrolysis
Transcript of Engineering carbonic anhydrase for highly selective ester hydrolysis

Linköping Studies in Science and Technology Dissertation No. 1085
Engineering carbonic anhydrase for
highly selective ester hydrolysis
Gunnar Höst
Molecular Biotechnology Division Department of Physics, Chemistry and Biology
Linköping University, Sweden
Linköping 2007

Cover picture:
“Pitagoras”, from Theorica Musicae By Franchino Gaffurio, 1492
© Gunnar Höst
ISBN: 978-91-85715-43-5 ISSN: 0345-7524 Printed in Sweden by LiU-tryck Linköping 2007

-Impossible things may cause a slight delay


Abstract
The main part of this thesis describes results from protein engineering experiments, in which the catalytic activity of the enzyme human carbonic anhydrase II (HCAII) is engineered by mutagenesis. This enzyme, which catalyzes the interconversion between CO2 and HCO3- in the body, also has the ability to hydrolyze ester bonds. In one project, the specificity of HCAII towards a panel of para-nitrophenyl ester substrates, with acyl chain lengths ranging from one to five carbon atoms, was changed by enlarging the substrate binding hydrophobic pocket. A variant was identified that has highly increased specificity towards substrates with long acyl chains. The mutant V121A/V143A hydrolyzes pNPV, which has four carbon atoms in the acyl chain, with an efficiency that is increased by a factor of 3000 compared to HCAII. Further, transition state analogues (TSAs) were docked to HCAII and mutant variants, and the results were correlated to the results from kinetic measurements. This indicated that automated docking could be used to some extent to construct HCAII variants with a designed specificity. Using this approach, a HCAII mutant that can hydrolyze a model benzoate ester was created. Interestingly, the resulting variant V121A/V143A/T200A was found to be highly active with other ester substrates as well. For pNPA, a kcat/KM of 1*105 M-1s-1 was achieved, which is the highest efficiency for hydrolysis of carboxylic acid esters reported for any HCAII variant.
In another project, the strong affinity between the active site zinc ion and sulfonamide was used to achieve binding of a designed substrate. Thus, the natural Zn-OH- site of HCAII was not used for catalysis, but for substrate binding. The substrate contains a benzenesulfonamide part in one end, with a para-nitrophenyl ester connected via a linker. The linker was chosen to ensure that the scissile bond is positioned close to His-64 and histidine residues introduced by mutagenesis in other positions. Using this approach, an enzyme was designed with a distinctly new two-histidine catalytic site for ester hydrolysis. The mutant, F131H/V135H, has a kcat/KM of approximately 14000 M-1s-1, which corresponds to a rate enhancement of 107 compared to a histidine mimic.
Finally, results are reported on a project aimed at cloning and producing a putative carbonic anhydrase from the malaria parasite Plasmodium falciparum. The gene was cloned by PCR and the construct was overexpressed in E. coli. However, the resulting protein was not soluble, and initial attempts to refold it are also reported.


Publications
This thesis is based on four papers, which are listed below and enclosed at the end
of the thesis. They will be referred to in the text by their roman numerals.
I Redesign of human carbonic anhydrase II for increased esterase activity and specificity towards esters with long acyl chains
Gunnar Höst, Lars-Göran Mårtensson and Bengt-Harald Jonsson Biochimica et Biophysica Acta, 2006, 1764, 1601-1606.
II Converting human carbonic anhydrase II into a benzoate ester hydrolase through rational redesign Gunnar Höst and Bengt-Harald Jonsson In manuscript
III Grafting of a cooperative two-histidine catalytic motif into a protein with an existing substrate binding capacity increases its hydrolytic efficiency Gunnar Höst, Jesus Razkin, Lars Baltzer and Bengt-Harald Jonsson Submitted
IV Cloning of a putative carbonic anhydrase from the human malaria parasite Plasmodium falciparum
Gunnar Höst, Sverker Lundman and Bengt-Harald Jonsson In manuscript
Contribution report
Paper I: I participated in the planning process, performed all experiments, analyzed the data and did a major part of the writing.
Paper II: I planned the project, performed all experiments, analyzed the data and did a major part of the writing.
Paper III: I participated in the planning process, performed a majority of the experiments, analyzed the data and did a major part of the writing.
Paper IV: I planned the project, performed a minor part of the experiments, took part in the data analysis and did a major part of the writing. I also supervised a final year student who performed most of the experiments.


Sammanfattning
I denna avhandling presenteras arbete utfört med enzymet humant karboanhydras II (HCAII). Enzymer är en typ av proteiner som accelererar (katalyserar) kemiska reaktioner, vilket är nödvändigt för allt levande. Den naturliga funktionen för HCAII är att katalysera omvandlingen av gasen koldioxid till vätekarbonat, som är löslig i vätska. Detta är viktigt bl.a. för att koldioxid som bildas i kroppen, och fraktas i blodet i form av vätekarbonat, skall hinna över till utandningsluften under den korta tid blodet är i lungorna.
Proteiner består av aminosyror som länkats samman i en lång kedja, där varje aminosyra är en av de 20 naturliga aminosyratyperna. Ett proteins struktur och egenskaper bestäms av aminosyrasekvensen, som i sin tur bestäms av genen för just det proteinet. Med genteknik kan ett proteins gen ändras (muteras), så att aminosyrasekvensen ändras, och det har här utnyttjats för att förändra HCAIIs katalytiska egenskaper. Förutom dess naturliga funktion kan HCAII även klyva (hydrolysera) vissa estrar. Mutationer gjordes så att en ’ficka’ i HCAIIs struktur, där molekylerna (substraten) som skall klyvas binder, fick en större volym. På så sätt skapades varianter med en kraftigt ökad kapacitet för att hydrolysera långa estersubstrat jämfört med icke-muterat HCAII. Som en utveckling av detta projekt skapades en mutant av HCAII, som kan hydrolysera ett än mer skrymmande substrat.
I ett annat projekt har en ny katalytisk aktivitet skapats i HCAII, som inte utnyttjar enzymets naturliga katalytiska förmåga. Ett nytt estersubstrat konstruerades, med en del som binder kraftigt till HCAII, så att en stark substratbindning erhölls. Sedan muterades vissa aminosyror till en reaktiv aminosyra som heter histidin. Valet av positioner för mutation baserades på en datormodell av enzymet med bundet substrat. Eftersom histidin kan delta i hydrolysreaktioner, får det muterade enzymet möjlighet att klyva substratet. Flera olika mutanter testades, och den effektivaste innehöll ett nära kopplat par av histidiner. Denna mutant undersöktes mer noggrannt, vilket gav viss information om den katalytiska mekanismen.
Det långsiktiga målet med detta arbete är att konstruera muterade enzymer som kan klyva giftiga ämnen, eller användas vid framställning av kemikalier. Det finns behov av nya enzymer för olika typer av substrat, och att med rationella metoder skapa nya katalytiska aktiviteter i proteiner är ett svårt vetenskapligt problem som ännu är i ett tidigt utvecklingsskede.


Acknowledgement
Min tid som doktorand har varit lärorik, ansträngande och rolig. Under tiden har jag haft förmånen att träffa en stor mängd personer, som på olika sätt bidragit till att jag nu är klar med min avhandling. Eftersom jag gjort misstaget att skriva detta kapitel sist, kvällen innan tryckningen, så har jag garanterat missat någon. Jag ber härmed om ursäkt för detta.
Först ett tack till min handledare professor Nalle Jonsson, som har lärt mig mycket om kemi, vetenskap och att hålla många bollar i luften. Det har varit väldigt givande och utvecklande.
Jag vill även tacka min biträdande handledare Lasse Mårtensson, som har lärt mig en massa om genteknik och hur man labbar.
Vår grupp har varit liten, men naggande god, och jag vill tacka mina doktorandkollegor Laila Villebeck (som stått ut med mig som rumskamrat), Martin Lundqvist (som låtit mig vinna enstaka badmintonmatcher) och Katrine Museth för deras sällskap och all hjälp. Jag vill även tacka exjobbarna som jag haft nöjet att handleda; Carin Karlsson och Sverker Lundman.
Synteskemi är ett töcken för mig, och då är det skönt att det finns kunniga människor att tillgå: Ett stort tack till Lars Baltzer, Jesus Razkin, Andreas Carlsson och Andreas Åslund för de samarbeten vi haft.
Tack till ’korridorsgänget’ Sofia, Johan R, Jonas N, Jonas W N, Helena, Patrik, Karin E, Johan V, Cissi, Gunnar D, Lotta, Klas, Kerstin, Tess, Janosch och Maria. Tack också till de nuvarande och tidigare medlemmarna i biokemigruppen för trevligt sällskap på labb och torsdagsmöten.
Tack till våra administratörer Susanne och Agneta.
Tack till IFMs innebandygäng, som sett till att jag hållit mig i form.
Jag vill också tacka min ständige lunchkompis Daniel för sällskapet, de intressanta diskussionerna och alla galna planer.
Tack till min familj, för att ni finns!
Till slut ett tack till min älskade Lina, som stöttat mig i de tunga perioderna.
Du är det bästa som hänt mig.


Contents
Introduction 1
1. Protein engineering of enzymes…………………………………………………… 3
1.1 Proteins…………………………………………………………………… 3
1.2 Methodologies for protein engineering of enzymes…………………. 4
1.2.1 Rational design………………………………………………….. 5
1.2.2 Directed evolution………………………………………………. 5
1.2.3 Semi-rational design…………………………………………….. 6
1.2.4 Computational design…………………………………………... 7
1.3 Technologies for isolation of successful enzyme variants…………... 8
1.3.1 Display methods………………………………………………… 8
1.3.2 Selection in living cells…………………………………………. 9
1.3.3 High throughput screening……………………………………. 10
2. Optimization of enzymes…………………………………………………………... 11
2.1 Enzyme catalysis……………………………………………………….. 11
2.2 Industrial applications of enzymes…………………………………… 11
2.3 Optimizing enzyme properties for industrial applications………… 12
2.4 Indicators of enzyme efficiency………… ……………………………. 13
3. Carbonic anhydrase: the model enzyme…………………………………………. 17
3.1 Carbonic anhydrase diversity…………………………………………. 17
3.2 Structure of HCAII……………………………………………………… 17
3.3 Carbonic anhydrase activity…………………………………………… 18
3.4 Binding of sulfonamide inhibitors…………………………………….. 19

Substrate structures…………………………………………………………….. 20
4. Redesigning the HCAII esterase activity………………………………………… 21
4.1 Carbonic anhydrase is an esterase…………………………………….. 21
4.2 Substrate specificity of HCAII esterase activity…………………… .. 21
4.3 Increasing activity and specificity for long chain esters…………….. 22
4.3.1 Alanine scanning of three hydrophobic pocket residues…… 23
4.3.2 Increased specificity for esters with long acyl chains……….. 23
4.3.3 Increased substrate affinity……………………………………. 25
4.3.4 Docking experiments to probe transition state stabilisation.. 26
4.4 Engineering HCAII for activity with benzoate esters..…………….. 29
4.4.1 Design of the variant V121A/V143A/T200A………………... 30
4.4.2 Activity of V121A/V143A/T200A…………………………… 30
5. Design of a His-based catalytic site in HCAII…………..………………………. 33
5.1 Histidine based catalysis of ester hydrolysis in proteins…………… 33
5.2 Introducing “two-histidine” catalytic motifs in HCAII…………… 34
5.2.1 Substrate design………………………………………………… 34
5.2.2 Creation and evaluation of “two-histidine” sites……………. 36
5.2.3 Grafting a helix “two-histidine” catalytic motif into HCAII. 38
5.2.4 Structural requirements for catalytic activity………………. 39
6. Development of a plate screening assay…………………………………………. 43
6.1 A method for screening HCAII variants for esterase activity……… 43
6.2 Screening for altered specificity………………………………………. 44
6.3 Screening for improved stability……………………………………… 44
7. Carbonic anhydrase from P. falciparum (PfCA)………………………………… 45
7.1 Malaria…………………………………………………………………… 45
7.2 P. falciparum carbonic anhydrase……………………………………… 46
7.3 Cloning and expression of PfCA……………………………………… 48
References 51

1
Introduction
During the work on which this thesis is based, my main focus of interest has
been a class of biological molecules called enzymes. Proposed by Kühne in 1878,
the word enzyme has a Greek root and means ‘in yeast’. The first recognition of an
enzyme was made by Payen and Persoz in 1833. During the second half of the 19th
century it was demonstrated, by van Manassein in 1871[1] and further by Buchner
in 1897[2], that the process of fermentation could occur in a cell-free extract, and
thus that it was dependent on chemical substances in the yeast cell rather than
dependent on the living cell as such. From its historical context it is obvious that a
defining feature of enzymes is that they are biological catalysts. Through the years
it has been discovered that the absolute majority of enzymes are protein molecules,
but catalysis can also be performed by other biological macromolecules, such as
RNA molecules. Indeed, it is now known that the massive protein synthesizing
machine known as the ribosome is dependent on one of its RNA components for
catalysis of peptide bond formation.[3] However, the topic of this dissertation is
protein enzymes.
There are many different aims that can motivate the study of enzymes.
Fundamental knowledge of how enzymes work is interesting in its own right, and
it is vital for the understanding of how living organisms function. Also, enzyme
function or loss of function is an essential part in the pathology of many diseases,
and this information can be useful for improved medical practices, or development
of new therapeutic strategies. Finally, the unique ability of enzymes to catalyze
reactions with very high specificities can be exploited industrially as a synthetic
tool in organic chemistry.

2
In the following two chapters a brief review is given, covering some important
methodological tools and concepts for developing enzymes (chapter 1) with new
and improved properties (chapter 2). The experimental system used in this study,
the enzyme carbonic anhydrase, is introduced in chapter 3. My work on
engineering the catalytic activity of this enzyme is presented in chapter 4 – 6.
Chapter 4 is based on paper I and II, and covers my attempts to change the
specificity of the ester hydrolysis activity of carbonic anhydrase. Chapter 5 is based
on paper III and deals with the creation of a completely new activity in carbonic
anhydrase. Chapter 6 describes a method that was developed for screening of
mutant libraries of carbonic anhydrase. It is based on paper I. Finally, in chapter 7
results from an ongoing project aimed at cloning and expressing carbonic
anhydrase from the human malaria parasite Plasmodium falciparum are presented.
This chapter is based on paper IV.

3
1. Protein engineering of enzymes
1.1 Proteins
Proteins are linear polymeric molecules, with peptide residues as the basic
building blocks. Peptide residues are made from amino acids, which are connected
together by peptide bonds that link the carboxyl group of one amino acid to the
amino group of the next. Amino acids differ from each other in the side chain
group, which is connected to the α-carbon atom between the amino group and the
carboxyl group. In natural proteins, 20 different amino acids are the main
constituents.[4]
Proteins differ in length and amino acid sequence, giving rise to a potentially
extreme diversity in protein structure and function. However, protein function
depends on a precisely determined, relatively stable, three dimensional form for
each protein. Information about this form is contained in the one dimensional
sequence of amino acids, and the form is achieved through the protein folding
process. The native form of the protein must be accessible by folding of the
unfolded polypeptide chain and sufficiently stable once formed. This places
restrictions on the possible sequences, so that only a small subset of the protein
sequence space is actually found in nature.
The structural organization of a protein is usually described as consisting of
four different levels. The first level, called the primary structure, is the sequence of
amino acids. Usually, some stretches of amino acids in the primary structure tend
to form local stable structures through hydrogen bonding interactions between
groups in the peptide backbone. This is called the secondary structure, and
contains elements such as α-helices and β-sheets. The tertiary structure is the

4
spatial organization of all amino acids, including those that are far apart in the
primary structure, and defines the three dimensional structure of a polypeptide
chain. Interactions between side chains participate in the forming of the tertiary
structure. In some cases, pieces of secondary structure form a distinct constellation,
so that this part of the tertiary structure forms more or less independently from
other parts of the protein. This type of protein substructure is usually called a
domain. Some proteins consist of more than one polypeptide chain. The large scale
architecture of polypeptide chains that form a multimeric protein, is known as the
quaternary structure, and the individual polypeptide chains are called subunits.[4]
1.2 Methodologies for protein engineering of enzymes
The relation between protein form and function, or structure and activity, is an
important topic in biochemistry, where a lot of effort is put into structure
determination of proteins. Several approaches for exploring the relation between
form and function exist. The absolute majority of enzyme engineering work takes
advantage of the possibility to clone a gene and incorporate mutations by
molecular biological methods. This mutagenesis approach is a versatile tool for
investigating how protein properties depend on the amino acid composition,[5]
and it is known as protein engineering. It was pioneered by Winter and coworkers
in the early 1980s, in a classic study of tyrosyl tRNA synthetase.[6] It is also the
approach taken in the present work. Using protein engineering, the effects caused
by alterations in the amino acid sequence of a protein can be studied, and changes
in the properties of e.g. enzymes can be achieved. Some alterations destabilize the
folded protein or block the correct folding pathway, and some alterations do not
have much effect. There are generally also residues that are important for the fine
tuning of protein parameters, without being critical for the structural integrity. By
introducing variation in such positions the catalytic performance of an enzyme can
be changed without destroying its ability to fold.
Prior to the advent of recombinant technology and protein engineering, the
only way to introduce changes in a protein was by introducing chemical

5
modifications.[7] It remains a valuable tool because it allows introduction of a
wide variety of cofactors, with functionalities that can not be achieved by the
naturally occurring amino acids. In addition to changing the properties of existing
proteins, it is also possible to create completely new proteins. The de novo design
of functional folded polypeptides is a critical test of the current understanding of
the factors that determine folding and stability.[8, 9]
Different methodologies can be distinguished in work on protein engineering
of enzymes. They differ in the amount of structural and functional knowledge that
is used in the design of experiments, and also in the use of randomization. Below,
four different methodologies are described, although in practice the distinctions
are not always so clear cut.
1.2.1 Rational design
In rational design, structural knowledge about the studied enzyme, together
with general knowledge about proteins, is used to generate hypotheses of what
alterations are likely to influence a certain property. Site directed mutagenesis can
then be used to perform the chosen alterations. After expressing the enzyme, the
hypothesis can be tested by assessing the property of interest.[10] A large amount
of studies have been published, in which rational design has been applied to
enzymes, ranging from early work on tRNA synthetase,[6] changes in coenzyme
specificity of a dehydrogenase [11] to improving enantioselectivity of a lipase.[12]
A drawback of rational design is that it requires a high degree of knowledge about
the protein under study. Also, the effect of a mutation is difficult to predict, and
therefore it is unlikely that potential complementary mutations in multiple
positions will be considered.[13]
1.2.2 Directed evolution
Another common methodology for enzyme engineering is based on random
alterations. Starting from one or many protein genes, a library of variants is

6
created in one of several ways, e.g. error prone PCR or gene shuffling. Successful
variants can then be isolated with respect to the desired property. In some studies,
this procedure of randomizing the gene followed by isolation of successful
variants is repeated more than once, basing later generations on the successful
variants from the prior round. This procedure is often referred to as directed
evolution or in vitro evolution, because of its resemblance to the natural process of
evolution by natural selection.[14-16] Several examples of directed evolution are
described in section 1.3. There are some drawbacks of directed evolution, e.g. for
some enzymatic activities it might be difficult to find sufficiently efficient
screening or selection systems.[13]
1.2.3 Semi-rational design
As described above, both rational design and directed evolution of enzymes
have their limitations. A way to circumvent these problems is to combine the
rational choice of positions for mutagenesis, with the combinatorial power of
directed evolution, an approach that is termed semi-rational design. In this
approach, structural and functional information is used to guide the choice of
positions to be randomized, increasing the proportion of variants in a library that
have alterations in positions that can be expected to be important for catalysis.[13]
In a study of the enantioselectivity of ester hydrolysis by Pseudomonas fluorescens
esterase, it was found that saturation mutagenesis of active site residues was a
more effective method than random mutagenesis to find variants with increased E-
values, and the best variants had higher enantioselectivities than those found by
random mutagenesis in an earlier study.[17, 18] It was suggested that mutations
close to the substrate binding site is often better, but distant sites are more likely to
be found by fully random methods.[17] Similarly, randomization of pairs of
residues in the active site was used to expand the substrate range for a lipase from
Pseudomonas aeruginosa, yielding variants with greatly increased activity for
various bulky substrates.[19] It was found that combinations of the identified
mutations yielded variants with still better enantioselectivities and higher

7
activities.[20] By creating small targeted libraries, it becomes possible to screen
enough variants to cover most of the variation. In cases when no good high-
throughput screening method is available, this is particularly valuable.
An interesting recent development is the use of multivariate statistical tools to
guide the search of sequence space in enzyme engineering studies.[21-23] By
determining the sequence and catalytic properties of a relatively small number of
library variants, the impact of individual mutations can be analyzed, and positive
mutations chosen to be propagated in a secondary library. This approach takes
advantage of the structure-activity information that is present in the screened
library diversity. Using this method, an enzyme was improved so that it fulfilled
the requirements for use in an industrial process, resulting in a variant with 4000-
fold increased productivity.[24]
1.2.4 Computational design
Structure-based computational design techniques can be used to construct
enzyme variants with new or improved activities. A computational approach has
been used successfully to introduce a histidine based active site for ester
hydrolysis into a protein scaffold. This was accomplished by scanning the protein
with a high energy intermediate for the reaction between a histidine and the
substrate. For each position that was evaluated, the surrounding residues were
allowed to change into alanine, so that the transition state of the reaction could be
accommodated.[25] In another study, an algorithm was used to screen a library in
which residues close to the active site were randomized. The initially very large
library was computationally evaluated, by energy calculations, and a subset was
selected by choosing mutations that were not disruptive to the protein structure. In
this way, a pre-screen was performed, and the results were used to create a library
of a size that could be handled experimentally. The strategy yielded a variant of β-
lactamase with improved hydrolysis activity with cefotaxime.[26] Computational
design has also been used to convert a ribose-binding protein into a triose
phosphate isomerase. First, a combinatorial search of a potential substrate binding

8
cavity was performed, to identify positions that would allow placement of
catalytic residues in a suitable geometric relation to each other and to the substrate.
Second, the surrounding residues were computationally varied so that a
complementary surface was formed. Using this method, biologically active
variants, containing 18-22 mutations, were identified.[27]
1.3 Technologies for isolation of successful enzyme variants
Generally, a screening method or a selection regime is applied to a library to
isolate successful enzyme variants, for which the property of interest has changed
in the desired direction. In selection methods (e.g. phage display and selection in
living cells), the variants are exposed simultaneously to selective conditions, such
that only variants with the desired properties are retained. In screening methods,
individual variants are tested, and successful ones are isolated. In both screening
and selection schemes, it is essential that the gene and its gene product are
somehow linked, so that a successful protein variant can be traced back to the gene
that encodes it. This link can be a direct physical link, as in display techniques, or
an indirect link, achieved by compartmentalization, as in screening of clones in
multi-well plates or selection in living cells.
Isolation of mutant enzyme variants with a desired catalytic activity from a
library of variants is in some ways more challenging, than isolating proteins based
on binding interactions, e.g. antibody fragments. The reason is that efficient
catalytic activity is not straightforwardly translated into binding interactions.
Here, some technologies for isolating enzyme variants from libraries will be
reviewed.
1.3.1 Display methods
The most common of the display methods is phage display, but other formats
such as ribosome and cell surface display of enzymes can also be used.[28, 29]
Phage display technology, in which a protein or a peptide fragment is positioned

9
on the surface of a bacteriophage, was pioneered in the 1980´s by Smith.[30] It
exploits the fact that a gene fragment that is fused to a gene encoding a phage coat
protein will be expressed, as a fusion protein which is ‘displayed’ on the surface of
the phage particle. Since the phage particle contains the gene for the displayed
fragment, there is a direct physical link between the gene and the gene product,
allowing for phage display selection experiments. Selection for catalytic activity of
phage displayed enzymes is complicated, since it requires a correlation between
catalytic efficiency and binding properties. Several approaches have been
presented to solve this problem. One such solution is to use a transition state
analogue for a reaction, and select variants that are capable of binding this
structure.[31] Other approaches address catalytic reactivity directly. Several
selection systems have been presented that use mechanism based inhibitors and
suicide substrates, in which the catalyzed reaction produces a reactive agent that
covalently traps the active phage displayed enzyme.[32-36] A more general
approach, that directly selects for turnover, is based on capture of enzyme-phage
particles that are linked to product. The substrate is attached to the phage particle,
so that the displayed enzyme can react with it. Separation of active enzyme-phage
variants can be done either by retaining inactive variants on the solid support
while eluting active variants,[37] or by retaining active variants, e.g. by a product
specific antibody.[38-41]
1.3.2 Selection in living cells
Selection of active enzyme variants can be achieved by coupling the activity of
the enzyme to survival of the cell in which it is expressed. The cell is a natural
compartment in which the reaction is allowed to take place, and selection occurs
by classic Darwinian principles. In a fascinating study, an enzyme active site was
transplanted between two enzymes that share the same fold, but have different
activities. The engineered enzyme was designed by a combination of rational
design and random mutagenesis followed by recombination. A strain of E. coli,
that is auxotrophic for tryptophan, was used for successful in vivo selection of

10
mutant variants that were able to complement the non-functional biosynthetic
pathway.[42] The strategy of selection based on cell survival has two main
limitations. First, it restricts the selected activities to those that can be coupled to
the ability of the cell to grow and divide. Second, the strong selection pressure is
focused on cell survival, and not explicitly on the engineered enzymatic activity.
Consequently, cells may find other ways to survive, e.g. by natural mutation in an
endogenous protein.
1.3.3 High throughput screening
In a library screening experiment, a large number of individual variants are
tested and successful ones are isolated, and the probability of finding successful
variants is therefore dependent on efficient screening. A common screening
method is to grow clones in multi-well plates, and screen bacterial lysates for
activity.[43, 44] Another interesting alternative is to screen clones on a solid phase.
An advantage of this is that it can be used for problematic substrates, such as
polymers. High efficiency has been reported for a solid phase screening method, in
which digital imaging spectroscopy was used, with a capacity for screening of
80000 mutants per day of a galactose oxidase enzyme.[45] Fluorescence-activated
cell sorting (FACS) has also been applied to high-throughput screening of enzyme
libraries. Active variants of a serine protease, displayed on the cell surface of E.
coli, have been isolated based on their capacity to cleave a substrate. Hydrolysis of
the scissile bond removed a fluorescence quencher from the cell surface, allowing
detection of active enzyme variants by fluorescence.[29] FACS was also used to
screen a library of phosphotriesterase mutants, which were in vitro
compartmentalized using a water-in-oil emulsion. Microbeads in aqueous
compartments were coupled to the gene and the resulting enzyme. Reaction by
active enzymes resulted in coupling of product to the bead, so that beads carrying
an active enzyme were decorated with product. Using a fluorescently labeled anti-
product antibody, beads with active enzymes could be sorted by FACS, yielding a
highly active variant.[46]

11
2. Optimization of enzymes
2.1 Enzyme catalysis
By definition, catalysis involves the acceleration of a reaction by a species, the
catalyst, which is not consumed in the reaction. Increasing the rate of a reaction by
catalysis requires either lowering of the transition state energy, or increasing the
transmission coefficient of the reaction. In general, the former mechanism is most
important in enzyme catalysis.[47] Pauling suggested that enzymes accomplish the
catalysis of reactions by being complementary in structure to the activated
complex of the reaction, which is similar to the modern concept of the transition
state, rather than actively participating in the chemical reactions.[48] This implies
that enzymes use non covalent binding energy to achieve the differential binding
of the transition state compared to the initial state. Enzymes can also lower the
transition state energy by using covalent binding energy, and thereby participate
in the chemical reaction. It has been suggested that the majority of highly
proficient enzymes use covalent binding energy for catalysis.[49] While the
transition state energy is lowered in covalent catalysis, the actual structure of the
transition state of the reaction can be different from the reaction in solution. In this
case, the presence of an enzyme opens an alternative route for the reaction to
proceed.
2.2 Industrial applications of enzymes
The natural function of enzymes is to catalyze reactions that occur inside and
outside of cells in organisms. Through evolution, the enzymatic parameters have

12
been tuned to closely fit the environment in which the enzymes are situated.
However, enzymes can also be used for industrial biotransformations, in which
they convert substrates that may not be involved in the enzyme’s natural
processes. It has also been recognized that many enzymes, in addition to being
able to react with many different substrates, also have the capacity to catalyze
more than one type of reaction. [50, 51] These aspects of enzymes can be exploited
in the industrial production of fine chemicals. The occurrence of enzymatic steps in
industrial processes is increasing, and although the number of industrial
applications of enzymes is still limited, it is expected to grow further.[52, 53]
Hydrolases are involved in a large proportion of the industrial
biotransformations.[52] The hydrolysis reactions catalyzed, e.g. the kinetic
resolution of racemates and enzymatic removal of protecting groups, frequently
involve ester bonds, and the commonly used enzyme classes are proteases, lipases
and esterases.[54] Other areas of industrial application for hydrolases beside the
chemical industry include production of food and beverages, as well as the
pharmaceutical and agricultural industries.[55] A well-known application of
hydrolytic enzymes is to improve the performance of detergents, by hydrolyzing
e.g. proteinaceous and greasy stains.[56] Preprocessing by lipases can be used to
improve biodegradation of wastewater with high levels of oil and grease, from
industries such as dairies,[57] potentially leading to increased yield of biogas
production from such organic material.[58]
2.3 Optimizing enzyme properties for industrial applications
The search for a suitable enzyme catalyst for a particular biocatalytic operation
can be difficult and time-consuming. It might therefore be desirable to alter the
properties of a previously used and well-known enzyme. Protein engineering
allows the designer of a biocatalytic system to reverse the standard procedure,
which includes first choosing an enzyme and then trying to fit the parameters of
the overall process to the requirements of the enzyme, so that instead the enzyme
is changed to fit the process parameters.[59]

13
A variety of properties are of interest for optimization by protein engineering,
depending on the intended application of the enzyme. Improvement of protein
stability is important for enzymes which are intended for use in industrial
processes, since it will prolong the half life of the protein. Another important set of
parameters is the optimal working conditions for the enzyme, including optimum
pH, temperature, salts and solvent polarity. These parameters can all be altered
using techniques of protein engineering.[14, 59]
The most important requirement is that the catalytic performance of the
enzyme is well matched to the desired reactions in the bioprocess. This includes
both reaction and substrate specificity of the enzyme, and much work is devoted
to the problem of engineering these parameters.[60, 61] An increased enzyme
activity means that more reaction products can be produced per enzyme unit in a
given time interval, which has obvious economic implications for industrial
processes. Good enantioselectivity is essential for the production of chiral products
intended for drug use, since different stereoisomers can have widely different
biological properties.[62] Redesigning an enzyme to achieve suitable catalysis can
in principle be done in two ways, either by augmenting the existing activity of an
enzyme or by creating a new activity in a protein.[63, 64] Penning and Jez calls the
creation of new catalytic activities in enzymes the ‘Holy grail’ of enzyme
redesign.[63] These authors mention a few general strategies that can be employed
to achieve this, such as diverting a covalent intermediate in a new direction,
optimizing side reactions, modifying the active site to participate in an alternative
reaction mechanism and inserting a catalytic activity in a ligand binding site.
2.4 Indicators of enzyme efficiency
Enzymes are widely known for their spectacular catalytic powers. Their ability
to catalyze a reaction can be evaluated and compared in many different ways. In
this section, some of the relevant expressions for catalytic competence are
summarized.

14
Efficiency and specificity
For a single substrate enzyme catalyzed reaction, the standard Michaelis
Menten equation for the reaction rate can be formulated as:
v = (kcat / KM) * [E] * [S], (equation 2.1)
in which [E] and [S] are the free enzyme and substrate concentrations,
respectively.[65] At low substrate concentrations (so that [S]<<KM), [E] is equal to
the total enzyme concentration, and the term kcat/KM is formally equivalent to a
second order rate constant for the reaction. This rate constant is limited by the
diffusion rate of the participating species. The diffusion limited rate constant for
enzymes is estimated to be around 1*108 M-1s-1. At this maximal rate, every
encounter between enzyme and substrate is productive. Therefore, the value of the
kcat/KM term for an enzyme is an indication of its efficiency. From an evolutionary
perspective, enzymes that approach the diffusion limit in their efficiency are
kinetically perfect, since further gains in efficiency can only come through
increases in the diffusion rate.[65]
Specificity, understood as the discrimination between substrates that compete
for the same active site, is determined by the ratio of reaction rates for the
competing substrates. Thus, it can be seen from equation 2.1 that the
discrimination between two substrates will be determined by the ratio:
(kcat/KM)substrate 1 / (kcat/KM)substrate 2. Therefore, kcat/KM is sometimes called the
specificity constant.
Rate enhancement
Enzymes catalyze most of the reactions in living organisms, and therefore they
control the flow of materials and energy in the complex network of reactions that
constitutes life. In the absence of enzymes, some of these reactions are exceedingly
slow. An estimate of the half-time for the hydrolysis of a peptide bond in the
absence of enzymes is around 400 years at room temperature. Other reactions are
fast, e.g. the half-life for the hydration of carbon dioxide is around 5 s. However,

15
the catalyzed rate constants (kcat) for most reactions show much less divergence as
most of them fall between 102 -103 s-1.[66]
A measure of the rate enhancement performed by an enzyme is obtained by
comparing the rate constant for a catalyzed reaction to the rate constant for the
corresponding non-catalyzed reaction, giving the ratio (kcat/knon). While the rate
constants for catalyzed reactions occupy a relatively narrow range, there is a great
spread in the rate enhancement between enzymes.[66] Thus, the rate enhancement
is an interesting indicator of the extent of catalysis that an enzyme performs. As an
extreme example, the decarboxylation of orotic acid has an estimated half-time of
78 million years, and is efficiently catalyzed by orotidine 5’-phosphate
decarboxylase, with a rate enhancement of 1017.[67]
Interesting information can also come from the ratio between second order rate
constants. The impact of having catalytic groups positioned in a well-defined
protein environment can be seen, by comparing the reaction rate to that achieved
by the catalytic groups in solution.[68]
Proficiency
As dicussed, rate enhancement is achieved by lowering the transition state
energy for the reaction. Therefore, an efficient enzyme has a high affinity for the
transition state. In a dilute solution, it is expected that the formal dissociation
constant of the substrate in the transition state is lower than the dissociation
constant of the ground state structure (KM). The factor between the two
dissociation constants should be the same as the ratio between the rate constants
for the catalyzed reaction (kcat) and the non-catalyzed reaction (knon). Hence, the
formal dissociation constant for the transition state is equal to KM * (knon / kcat).
This is equal to knon divided by the specificity constant, and is called the
proficiency of the enzyme.[66]

16

17
3. Carbonic anhydrase: the model enzyme
3.1 Carbonic anhydrase diversity
Since the discovery in 1933 of carbonic anhydrase (CA; carbonate hydro-lyase,
EC 4.2.1.1) in mammalian erythrocytes,[69, 70] this very common zinc enzyme [71]
has been found in virtually all living organisms that have been tested for its
presence. There are at least three independently evolved classes of carbonic
anhydrase, known as α-, β- and γ-CA.[72] The existence of a fourth class, δ-CA,
has also been suggested.[73] All mammals, including humans, have α-type CA. In
mammals, 16 different isozymes have been identified, and there are at least ten
active human isozymes, of which four are cytoplasmic, two are mitochondrial, and
four are membrane bound.[74] The most studied isozyme is human carbonic
anhydrase II (HCA II), which is found in many different tissue types, including
erythrocytes. Erythrocytes also contain large amounts of HCA I.[75]
3.2 Structure of HCAII
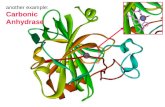
The crystal structure for HCA II, the isozyme used in the work for this thesis,
has been determined to high resolution (see figure 3.1).[76-78] It is an almost
spherical monomer of 259 amino acids, with no disulfide bonds. Its dimensions are
roughly 50 * 40 * 40 Å3. The molecule is divided into two halves by a large, 10-
stranded, twisted β-sheet. On both sides there are some short α-helices. The amino
terminal part of the polypeptide chain is loosely associated to the rest of the
molecule, which consists more or less of one domain.

18
The active site is situated in a large conical cleft, approximately 15 Å deep. At
the bottom of the cleft, a zinc ion is coordinated by three histidine residues. A
water molecule with a pKa of approximately 7 completes the tetrahedral
coordination geometry around the zinc ion.[79] Hydrogen bonds to an outer shell
of residues are involved in positioning the zinc ligand.
Figure 3.1: Structure of HCAII. The active site contains a zinc ion (yellow), coordinated to three histidine residues and a water molecule with pKa 7. A histidine (His-64) at the edge of the active site cavity functions as a proton shuttle. Pdb accession code 2cba.[78]
3.3 Carbonic anhydrase activity
Carbonic anhydrase catalyzes the reversible hydration of carbon dioxide: CO2 +
H2O ↔ HCO3- + H+. The catalytic reaction contains two steps. In the first step, the
substrate carbon dioxide is subjected to a nucleophilic attack by the zinc-
coordinated hydroxide ion. A water molecule from the bulk solvent displaces the
resulting metal-bound HCO3- ion. In the second step, a proton from the zinc-

19
bound water molecule is transferred to the surrounding buffer medium via His-64,
which acts as a proton shuttle group (see figure 3.1).[80, 81] The catalyzed reaction
between CA and CO2 is exceedingly fast. In fact, HCAII has one of the largest kcat
values known (1.4 *106 s-1), and with a kcat/KM value of 1.5 *108 M-1s-1, this enzyme
operates close to the limit set by the rate of diffusion.[65, 82]
For a long time, it was believed that carbonic anhydrase exhibited absolute
specificity, i.e. that it would only catalyze the interconversion between CO2 and
HCO3-. However, in the 1960s it was discovered that the enzyme also catalyzes
hydration of various aldehydes [83, 84] as well as hydrolysis of esters.[85-88]
Recently, the catalytic versatility of carbonic anhydrase has been further expanded,
as manganese substituted carbonic anhydrase has been shown to display
peroxidase and epoxide synthase activities.[89, 90] In chapter 4, the HCAII esterase
activity is further examined, and the methods of protein engineering are used to
change the substrate specificity and catalytic efficiency of the reaction.
3.5 Binding of sulfonamide inhibitors
It has been known since the 1940s that sulfonamides are potent inhibitors of
carbonic anhydrase.[91] This property has been exploited for medical purposes,
e.g. in the treatment of glaucoma with the sulfonamide drug acetazolamide.
Crystal structures and UV spectroscopy have revealed that the sulfonamide group
binds directly to the zinc ion with the negatively charged nitrogen atom.[76, 92] It
has been suggested that the high affinity between sulfonamides and carbonic
anhydrase might be explained by the similarity between the bound complex and
the transition state for the CO2 hydration reaction.[93] In chapter 5, the strong and
well-defined binding of sulfonamide molecules with a substituted benzene group
is used to provide binding for a designed ester substrate. By introducing catalytic
residues into HCAII, a new enzyme substrate system is created that is completely
independent of the natural catalytic activity of HCAII.

20
Substrate structures
pNPA (para-nitrophenyl acetate) oNPA (ortho-nitrophenyl acetate)
pNPP (para-nitrophenyl propionate) pNPBenzo (para-nitrophenyl benzoate)
pNPB (para-nitrophenyl butyrate)
cocaine
pNPV (para-nitrophenyl valerate)
pNPC (para-nitrophenyl caproate) pNPSA (4-sulfamoyl(benzoylamino)
acetic acid para-nitrophenyl ester)

21
4. Redesigning the HCAII esterase activity (paper I, II)
4.1 Carbonic anhydrase is an esterase
It has been known for 45 years that carbonic anhydrase is capable of catalyzing
the hydrolysis of esters. This was first demonstrated with 1-naphtyl acetate.[85, 94]
Hydrolysis of acetate esters containing various aromatic alcohol groups have been
investigated, such as nitrophenyl [86-88] and other substituted phenols.[95]
Hydrolysis of other types of substrates has been performed as well, e.g. pyruvate
esters [96] and 2-hydroxy-5-nitro-α-toluenesulfonic acid sultone.[97]
4.2 Substrate specificity of the HCAII esterase activity
The various ester substrates are hydrolyzed with different efficiencies by
carbonic anhydrase, depending on their structure. In part, this is a consequence of
the difference in inherent stability of the ester bond in the substrates. For example,
para-nitrophenyl esters are activated compared to meta-nitrophenyl esters,
because of different pKa-values for the leaving groups. Therefore higher
efficiencies can be expected for para-nitrophenyl ester hydrolysis. More
interesting, from an enzyme engineering perspective, is the substrate selectivity
that is caused by differences in the interaction between the enzyme active site and
the substrate molecule. This specificity can be augmented by the methods of
protein engineering.
Earlier studies have shown that bovine carbonic anhydrase (BCA) catalyzes the
hydrolysis of para-nitrophenyl esters with different efficiencies depending on the
structure of the acyl part of the substrate. Ester substrates with long and bulky acyl

22
groups are hydrolyzed less efficiently than smaller substrates.[86, 98] We have
measured the activity of HCAII for hydrolysis of an isologous series of aliphatic
para-nitrophenyl esters differing in the length of the acyl chain (from one to five
carbon atoms): para-nitrophenyl acetate (pNPA), para-nitrophenyl propionate
(pNPP), para-nitrophenyl butyrate (pNPB), para-nitrophenyl valerate (pNPV) and
para-nitrophenyl caproate (pNPC). The structures for all substrates are shown on
page 20. The pattern of specificity for HCAII was similar to the bovine enzyme,
with the highest catalytic efficiency (kcat/KM) for pNPA, and steadily decreasing
efficiencies for longer substrates (see table 4.1).
The carbonic anhydrase esterase activity also displays substrate selectivity with
respect to the alcohol part of the substrate. Both BCA and HCAII hydrolyze pNPA
more efficiently than ortho-nitrophenyl acetate (oNPA), while HCAI is more
efficient with oNPA than pNPA.[86, 88] These substrates have similar pKa values
for the corresponding nitrophenol group, and it is therefore not expected that the
enzymes display activity differences based on differences in ester bond stability.
The presence of substrate recognition based on both the acyl part and the
alcohol part of the substrate suggest the possibility of engineering HCAII variants
with selectivity for a variety of different substrates. Design of enzyme variants
with the ability to discriminate between stereoisomers of a substrate is a desirable
goal.
4.3 Increasing activity and specificity for long chain esters
It is likely that the aliphatic ester substrates bind to the so-called hydrophobic
pocket, an area of the active site that is dominated by hydrophobic residues. This
area is composed of the residues Val-121, Val-143, Leu-198 and Trp-209 in
HCAII.[77] It is known that mutations in these positions affect the rate of
hydrolysis of pNPA,[99-102] and that replacement of Val-143 with alanine or
glycine results in increased efficiency of pNPP hydrolysis.[103]
Variants with altered specificity with respect to the alcohol part of the substrate
have also been found. For example, the mutant T200G is 6 times more efficient

23
with oNPA than with pNPA, while the wild type of HCAII is 23 times more
efficient with pNPA than oNPA.[103] The activity for 2-naphthyl acetate has been
increased by a factor of 40 in the mutant A65V/D110N/T200A, created by directed
evolution. In this variant, the pNPA activity was also increased, to a lesser
degree.[104]
4.3.1 Alanine scanning of three hydrophobic pocket residues
With the aim of creating HCAII variants that selectively hydrolyze ester
substrates with long acyl chains, the residues Val-121, Val-143 and Leu-198 were
mutated into alanine residues. Since alanine has a smaller side chain than the wild
type residues, this is expected to enlarge the cavity and modulate the shape of the
hydrophobic pocket. This might allow longer substrates to be accommodated in a
catalytically productive way. By combining alanine mutations, a small library of 7
mutant variants was created, comprising all single and double mutants as well as
the triple mutant. A 96-well plate screening assay was developed (see chapter 6),
and used to screen the library for variants with increased specificity for substrates
with long acyl chains. We found that the variants V143A and V121A/V143A had
an increased efficiency for long substrates compared to pNPA, and therefore these
variants were selected for detailed kinetic measurements. Wild type HCAII and
V121A were also included, to allow us to distinguish the contributions of the
individual mutations.
4.3.2 Increased specificity for esters with long acyl chains
The resulting kcat/KM values from the kinetic measurements are presented in
table 4.1. As discussed in section 2.4, specificity is defined by the relative
efficiencies (the ratio between kcat/KM values) for the reactions of an enzyme with
two different substrates. Assuming that [S] << KM (i.e. that the enzyme is far from
saturation at the substrate concentration used), kcat/KM is equal to the apparent
second order rate constant (kenz) for the reaction, calculated from the equation:

24
Table 4.1: Activities for HCAII variants a
a The measurements were carried out at 25 ˚C, pH 8.5. The apparent second order rate constants (kenz = kcat/KM), expressed in M-1s-1, are presented with a 95 % confidence interval based on triplicate measurements.
V0 = kenz * [E]0 * [S]0 ,
in which [E]0 is the total enzyme concentration, [S]0 is the total initial substrate
concentration and V0 is the initial enzyme catalyzed reaction rate. The assumption
that [S] << KM was examined for the most active variants, and found to be valid in
most cases. However, kcat/KM for V121A/V143A with pNPB and pNPV are
calculated from a Michaelis Menten analysis (see below, section 4.3.3), because the
[S] << KM assumption was not satisfied under the experimental conditions used.
It can be seen (table 4.1) that the variants V143A and V121A/V143A have
activity patterns that are very different from the wild type. V143A has a kcat/KM
maximum for pNPP, which is hydrolyzed 20 times more efficiently than pNPA.
V121A/V143A has a maximum for pNPV, which is hydrolyzed 6 times more
efficiently than pNPA. V121A has a similar pattern of efficiencies as HCAII, with a
maximum for pNPA. These results represent dramatic changes in specificity. For
example, pNPA is hydrolyzed at least 500 times more efficiently than pNPV by
HCAII. Thus, the specificity for pNPV has changed by a factor of more than 3000
between wild type HCAII and V121A/V143A.
It is interesting to compare the values of kcat/KM for the mutants with the
kcat/KM values for the wild type. In this analysis, the level of rate enhancement that
is obtained from the individual mutations becomes clear, giving a better
understanding of the observed specificity changes. In table 4.2, the relative
pNPA pNPP pNPB pNPV pNPC
HCAIIpwt 2080 ± 60 516 ± 9 47 ± 2 3.2 ± 0.6 1.7 ± 1.4
V121A 472 ± 5 88 ± 1 14.6 ± 0.4 13.6 ± 0.3 10.4 ± 1.7
V143A 650 ± 20 13900 ± 550 2710 ± 140 800 ± 30 41 ± 6
V121A/V143A 1550 ± 30 1820 ± 60 2790 ± 180 13740 ± 560 2180 ± 140

25
efficiencies are shown for the mutants. For each substrate, the value of kcat/KM for
the mutants are compared to the wild type value. It can be seen that all three
mutants are less efficient for pNPA hydrolysis than the wild type. In addition,
V121A is less efficient for pNPP and pNPB as well. This indicates that the side
chain of Val-121 contributes positive interactions with these three substrates, while
it is a steric hindrance for the interaction with pNPV and pNPC. Val-143 interact
positively with pNPA, and is a steric hindrance for all substrates longer than
pNPA, since kcat/KM for each substrate is larger for V143A than for the wild type.
Thus the side chains of both Val-121 and Val-143 have a negative effect on the
hydrolysis of pNPV and pNPC. In V121A/V143A both of these obstacles are
removed, and consequently the kcat/KM value is increased dramatically for these
substrates compared to the wild type.
Table 4.2: Relative efficiencies of mutants compared to HCAIIpwt a
V121A V143A V121A/V143A
pNPA 0.227 ± 0.004 0.310 ± 0.006 0.747 ± 0.013
pNPP 0.170 ± 0.002 27.0 ± 0.5 3.54 ± 0.06
pNPB 0.312 ± 0.009 58 ± 2 53 ± 1
pNPV 4.4 ± 0.4 258 ± 24 3150 ± 290
pNPC 13 ± 9 52 ± 35 2800 ± 1800
a For each substrate, the ratio of the apparent second order rate constant for each mutant and HCAIIpwt is shown. Mean values and 95 % confidence intervals are given, based on the range of possible ratios that can be calculated using the three individual samples measured for each substrate.
4.3.3 Increased substrate affinity
The results in table 4.1 indicate that the increased catalytic efficiencies obtained
with the mutants are due to both removal of negative interactions and addition of
positive interactions. If only removal of negative steric interactions were involved,
it would be expected that e.g. pNPB should be hydrolyzed with a similar efficiency

26
as pNPV by V121A/V143A, since the substrate binding cavity size required for
pNPV would be sufficient also for pNPB.
It is not possible to determine from the results in table 4.1 and table 4.2 if the
additional positive interactions are manifested in higher values of kcat or lower
values of KM. The values of the individual Michaelis Menten parameters can be
determined by measuring the reaction rates with many different substrate
concentrations. However, the highest substrate concentrations that can be achieved
with the ester substrates used are well below KM for carbonic anhydrase,[86]
making it difficult to accurately determine KM. As a consequence, KM can only be
determined for HCAII mutants if they have significantly increased substrate
affinity, i.e. if KM is much lower than for wild type HCAII.
Experiments were performed with V143A and V121A/V143A for the most
efficient substrates. It was only possible to determine KM for V121A/V143A using
the substrates pNPB (2.0 ± 0.9 mM) and pNPV (0.65 ± 0.09 mM). These results
support the assumption that [S]<<KM for the experimental conditions used for
kinetic measurements, except for the hydrolysis of pNPB and pNPV by
V121A/V143A, for which kcat/KM were calculated from the Micahelis Menten
analysis. Interestingly, the affinity for pNPV is stronger than for pNPB by a factor
of 3, and pNPV is hydrolyzed approximately 5 times more efficiently than pNPB.
Clearly, the high efficiencies with these substrates for V121A/V143A are at least in
part caused by stronger substrate binding. It also seems that the higher activity
with pNPV might be due to stronger affinity for this substrate compared to pNPB.
4.3.5 Docking experiments to probe transition state stabilization
The value of kcat/KM is related to the amount of stabilization of the transition
state that occurs as the reaction proceeds. Thus, it is expected that the observed
efficiencies (kcat/KM) in table 4.1 are correlated to the binding of the transition state
for the hydrolysis reactions. We have examined this by docking transition state
analogues (TSA) of pNPA, pNPP, pNPB, pNPV and pNPC to the active sites of
HCAII and the variants V121A, V143A and V121A/V143A, using the automated

27
docking software Autodock 3.0.[105-107] The ligand molecules for the docking
calculations were designed with the ester bond replaced by a phosphonate group,
a transition state analogue that is frequently used experimentally to mimic the
transition state for ester hydrolysis reaction.[108]
In most cases, the strongest binders were docked with the phosphonate oxygen
atoms close to the active site zinc ion, and the acyl chains pointing in the direction
of the hydrophobic pocket, see figure 4.1. In this figure, different binding modes of
the enzyme variants for pNPVTSA can be seen. Possibly, the low activities of HCAII
and V121A for pNPV (see table 4.1) results from the sterically restricted binding
conformations of pNPV that are observed. For these variants, the acyl chain is
bent, while pNPVTSA is allowed to dock with a more elongated acyl chain in
V143A and V121A/V143A.
Figure 4.1: Representative docked pNPVTSA molecules, resulting from automated docking to HCAIIwt, V121A, V143A and V121A/V143A, superpositioned in the active site of wild type HCAII. The active site zinc atom is shown in yellow. Black labels indicate which variant was used for docking. The green spheres indicate the free volume created when the indicated valines are mutated into alanines.
It is expected that good binding of transition state analogues are found for
substrates that are hydrolyzed efficiently, i.e. that the fraction of productively
bound TSA molecules correlates with the values of kcat/KM in table 4.1. Such
correlations can indeed be observed. For each substrate, the enzyme variant with
the highest efficiency in table 4.1 has the largest fraction of productively docked
TSA in table 4.3. For pNPB, pNPV and pNPC, ranking of the enzymes based on

28
kcat/KM values yields the same result as ranking based on fractions of docked
TSAs. Also, all enzymes except V121A have the largest fraction of productively
docked TSAs for the substrate that is hydrolyzed most efficiently. Interestingly, the
values in table 4.3 qualitatively predict the behavior of the specificity profile for
both V143A and V121A/V143A (see figure 4.2), with only minor exceptions.
Table 4.3: Fraction of Transition State Analogues (TSAs) with productive interactions a
Mutant pNPATSA pNPPTSA pNPBTSA pNPVTSA pNPCTSA
HCAIIwt 65 64 47 18 8
V121A 58 61 45 26 16
V143A 44 69 64 66 31
V121A/V143A 42 58 60 71 46
a The number of docked TSAs of 100 for which at least one of the two phosphonate oxygens is positioned close to the zinc ion and the acyl chain points in the general direction of the hydrophobic pocket.
Figure 4.2: Ratios of kcat/KM values of ester substrates for V143A and V121A/V143A. For each substrate, the ratio between the kcat/KM for that substrate and pNPA is shown.

29
4.4 Engineering HCAII for activity with benzoate esters
The results from engineering of the hydrophobic pocket, described above,
indicate that docking of TSAs can be used to evaluate suitable mutations to allow
hydrolysis of a chosen substrate. Further, an expanded hydrophobic pocket was
designed by mutation of Val-121 and Val-143 into alanine. In an extension of these
results, the TSA-docking strategy was used to construct a HCAII variant capable of
hydrolyzing a benzoate ester substrate.
Benzoate esters are demanding substrates, because they have a bulky group
close to the ester bond. Therefore, many commonly used hydrolases do not have
appreciable activity with model benzoate substrates, e.g. para-nitrophenyl
benzoate (pNPBenzo). Identification and development of lipase variants that can
hydrolyze such bulky substrates as benzoate esters is performed by many
groups.[20, 109, 110] Several esterases also have activity with benzoate esters, e.g.
liver carboxylesterase from various animal sources.[111, 112] Other esterases are
evaluated for applications such as mild and selective removal of protective groups
in organic synthesis [113] and synthesis of benzoylated compounds.[114] Another
possible application for benzoate esterases is for the treatment of cocaine
overdoses. Cocaine inhibits the reuptake of dopamine in nerve terminals, which
affects the brain reward systems and thereby causes the drug’s reinforcing
effects.[115] Enzymatic removal of the active biological form of cocaine is
attractive, because of the difficulties of using small molecule therapy to inhibit an
inhibitor, and the large amounts of cocaine-specific antibodies required for
immune based therapy.[116] An efficient enzyme could lower the level of cocaine
rapidly, by hydrolyzing a benzoate ester bond, which inactivates the drug. Several
groups investigate this possibility, using various cocaine hydrolyzing enzymes
such as human butyrylcholinesterase,[117] catalytic antibodies [118] and a
bacterial esterasase from a microorganism living in the soil under the coca
shrub.[119]

30
4.4.1 Design of the variant V121A/V143A/T200A
As a model benzoate ester substrate, para-nitrophenyl benzoate (pNPBenzo)
was chosen. Cocaine was also included, to test the possibility of designing a HCAII
variant for hydrolysis of a large ester substrate. (The structures of the substrates
are shown on page 20.) Docking of TSAs for hydrolysis of these substrates, to
HCAII and the variant V121A/V143A, indicated that the double mutant could
accommodate both pNPBenzo and cocaine better than the wild type (see table 4.4).
From the docked structures, it was seen that the side chain of Thr-200 might be a
steric hinder for correct docking, by restricting the position of the alcohol moieties
of the substrates. Therefore, a triple mutant was designed, in which the T200A
mutation was incorporated. To evaluate if further enlargement of the hydrophobic
pocket would allow better positioning of pNPBenzo or cocaine, the variant
V121A/V143A/L198A/T200A was also selected after identification of a putative
steric clash between the substrate and the leucine side chain at position 198.
Table 4.4: Automated docking of TSAs for pNPBenzo and cocaine to HCAII variants. The number of successfully docked molecules of 100 are shown.
HCAII V121A/V143A V121A/V143A/T200A
V121A/V143A/
L198A/T200A
pNPBenzo 1 20 46 39
(-) cocaine 0 5 59 71
4.4.2 Activity of V121A/V143A/T200A
Kinetic measurements with the mutant V121A/V143A/T200A confirmed that it
is capable of accommodating the bulky acyl part of benzoate esters, and
hydrolyzed pNPBenzo with a modest activity (see table 4.5). By contrast, HCAII
and the variant V121A/V143A did not display any activity.
Table 4.5: Kinetic measurements with pNPBenzo. Values of kenz (M-1s-1) are shown.

31
HCAII V121A/V143A V121A/V143A/T200A V121A/V143A/
L198A/T200A
pNPBenzo n.d. n.d. 625 ± 38 ~ 60
The V121A/V143A/ L198A/T200A mutant was obtained in low yield, and
although a reaction rate above the background level could be observed, it was only
possible to roughly estimate the catalytic rate constant. Interestingly, it appears
that the docking results (table 4.4) correctly predict that V121A/V143A/T200A is
the most active variant with pNPBenzo (table 4.5), indicating that the docking
based design principle was successful. The kenz-value determined for
V121A/V143A/T200A compares favorably with values reported for some natural
esterases. Notably, the carbonic anhydrase variant is actually more efficient than
the rabbit and human liver carboxylesterases (kcat/KM = 157 and 185 M-1s-1,
respectively), while chicken liver carboxylesterase is much more efficient (1.5*108
M-1s-1).
V121A/V143A/T200A is also highly active with other substrates. From table
4.6 it can be seen that for all substrates this variant is at least 3 times more efficient
than the most efficient parent mutant. In the case of pNPA, it is 50 times more
efficient than HCAII, with a kcat/KM value that is the largest so far reported for
ester hydrolysis by any carbonic anhydrase variant.
Table 4.6: Values of kcat/KM for V121A/V143A/T200A and the most efficient parent variant.
V121A/V143A/T200A Most efficient parent
pNPA 101670 (± 4830) 2080 (± 60) HCAII
pNPP 43710 (± 1860) 13890 (± 530) V143A
pNPB 29240 (± 1630) 2790 (± 180) V121A/V143A
pNPV 46310 (± 370) 13740 (± 560) V121A/V143A
pNPC 55450 (± 1100) 2370 (± 270) V121A/V143A/L198A/T200A

32
Attempts were made to determine KM values for the reaction of
V121A/V143A/T200A with pNPB, pNPV and pNPC, but due to the low solubility
of these substrates no values could be determined. As mentioned in chapter 4.3.3,
the high activities of V121A/V143A with pNPB and pNPV were in part caused by
increased substrate binding, and KM values could be determined. It thus appears
that the T200A mutation resulted in lowered affinity and increased efficiency
simultaneously, indicating that a proportion of the available binding energy is
used to stabilize the transition state, rather than the enzyme-substrate complex.
Interestingly, the variant V121A/V143A/L198A/T200A is less active with pNPC
than V121A/V143A/T200A, and it has a measurable affinity (KM = 0.18 mM (±
0.12)) for this substrate. Apparently, the side chain of Leu-198 is involved in the
transition state binding in V121A/V143A/T200A, and removal of it allows strong
substrate binding but less strong transition state binding. The role of Leu-198 in
selective transition state stabilization should be further examined.
In addition, cocaine was tested as a substrate for V121A/V143A/T200A and
V121A/V143A/L198A/T200A. As a control, HCAII was also included in the
experiment. No enzymatic hydrolysis could be observed for any of the variants.
Possibly, this is because the benzoate ester bond in the cocaine molecule is
expected to be more stable than the activated esters at physiological pH. The pKa
value of the ecgonine alcohol group of cocaine is around 14.[120] As the pKa of the
nitrophenyl alcohol group is 7.2, the expulsion of this leaving group can not be
acid catalyzed at higher pH,[65, 121] while it might be necessary to have a
component capable of general acid catalysis for expulsion of the cocaine alcohol
group at the pH values used. Alternatively, it is possible that dynamic
reorganization in the active site is restricted for cocaine, because of its large size,
and it might therefore be unable to find an optimal position for catalysis.

33
5. Design of a His-based catalytic site in HCAII (paper III)
5.1 Histidine based catalysis of ester hydrolysis in proteins
As described in chapter 4 of this thesis, the natural active site of HCAII can
catalyze the hydrolysis of esters, based on a nucleophilic hydroxide ion bound to a
zinc ion. For industrial purposes, the most commonly used enzymes for ester
hydrolysis belong to the protease, lipase and carboxylesterase families of
enzymes.[54] For many of these enzymes, the active site contains a so-called
catalytic triad. In the reaction, a nucleophilic residue attacks the ester bond, while
the other two residues contribute general acid/base catalysis.
In recent years, histidine residues have been used to achieve catalytic activity.
Baltzer and coworkers have constructed a catalytic site on a helix-loop-helix
polypeptide scaffold, which is composed of two histidine residues.[68] Ester
hydrolysis is achieved by cooperative catalysis, in which one of the histidine
residues functions as a nucleophile, while the other residue contributes general
acid-catalysis. The histidine residues are positioned in a (i, i+4) configuration on
the surface of one of the α-helices in the helix-loop-helix construct, placing them
spatially close to each other with the side chains directed in the same orientation.
In another study, an active site with a histidine and a lysine residue was
constructed on the surface of a polypeptide. The polypeptide becomes ordered
into a helical conformation when it adsorbs to a silica nanoparticle. Thus, the His-
Lys pair is arranged in positions suitable for catalysis when the particles are added
to the solution, allowing the catalytic activity to be controlled.[122]

34
Other groups have constructed active sites in which histidine residues are
catalytically active exclusively as nucleophiles. An active site for nucleophilic
catalysis by a histidine residue in an E. coli protein scaffold has been constructed
using a computational method.[25] In a study of de novo designed proteins, based
on binary patterning of polar and non-polar amino acids, it was found that some
proteins that had not been selected or designed for catalysis, could nevertheless
hydrolyze esters.[123]
The side chain of histidine is a catalytically versatile group, capable of general
acid/base catalysis as well as nucleophilic catalysis. The reason for this is the range
of pKa values that it can have, which tend to be between 5 and 8 in proteins.[65]
Thus, at physiological pH there is usually a significant proportion of both
protonated and deprotonated species, allowing for a variety of catalytic
mechanisms.
5.2 Introducing “two-histidine” catalytic motifs into HCAII
In chapter 4, the esterase activity of the natural active site of HCAII is used as a
starting point, and the specificity of the reaction is changed dramatically by
mutagenesis. Another design principle is to take a known ligand binding
interaction as a starting point, and create an enzyme substrate system by
introducing an activity into the system. We have done this, by designing a
substrate, which binds with high affinity to HCAII, and using histidine residues
positioned appropriately in the protein scaffold to allow hydrolysis of an ester
bond in that substrate.
5.2.1 Substrate design
As mentioned in chapter 3, many molecules that contain a terminal
sulfonamide group bind to HCAII with very low dissociation constants. For
example, a KD of 14 nM is found for HCAII in complex with the inhibitor
acetazolamide.[124] The binding mode of sulfonamide inhibitors have been

35
studied extensively. Crystal structures of HCAII in complex with various
sulfonamide inhibitors with substituted benzene groups were used to guide the
design of a new substrate.[125, 126] A comparison of the bound inhibitors revealed
that the benzenesulfonamide part of the inhibitors was bound in a similar way for
most of the inhibitor molecules. The similar binding modes indicate that a
substrate molecule based on a benzenesulfonamide is likely to be bound in a
relatively rigid way. A rigid binding mode increases the likelihood of achieving
high substrate specificity in future applications, which is a desirable goal in
enzyme engineering. However, if a lower affinity is desired (e.g. if kcat is increased
so that substrate/product dissociation rates become rate limiting), KM can be
increased by choosing a sulfonamide moiety with a lower affinity or engineering
the enzyme. This should also increase the local mobility of the bound substrate,
which might be advantageous in some applications.
A substrate was constructed (see figure 5.1), containing a benzenesulfonamide
moiety in one end. A linker consisting of a glycine amino acid residue was added,
coupled via an ester bond to a para-nitrophenyl group. The substrate is called
pNPSA, based on the para-nitrophenyl alcohol moiety, and the sulfonamide
moiety. Using this linker, a close interaction between the scissile bond and the
natural His-64 residue was allowed.
Figure 5.1: Structure of the ester substrate pNPSA. Substrate binding is ensured by the benzenesulfonamide moiety, which is known to bind strongly to HCAII.

36
5.2.2 Creation and evaluation of “two-histidine” sites
Suitable sites for introducing histidine residues in HCAII were identified, based
on the crystal structure information for various benzenesulfonamide inhibitors in
complex with HCAII[125, 126]. Three sites were chosen to allow formation of three
pairs of histidine residues, in which the histidines were positioned close to each
other. The positions chosen were Asn-62/His-64, Phe-131/Val-135 and Leu-
198/Pro-202. In the first pair, the natural histidine residue, His-64, is recruited to
form a pair together with a histidine residue inserted in position 62. Because the
substrate was designed to allow interaction with His-64, the double mutants
F131H/V135H and L198H/P202H each contain three histidines at suitable
positions. To indicate this, (H64) will in some cases be added to the name of
variants in the discussion. Values of kcat for the hydrolysis of the substrate
molecule pNPSA were measured at pH 5.3 and 6.7 for the various histidine
mutants, and the results are shown in table 5.1.
Table 5.1: Values of kcat for pNPSA hydrolysis by HCAII variants a
kcat (104*min-1) at pH 5.3 kcat (104*min-1) at pH 6.7
HCAII (H64) 89 ± 7 39 ± 9
N62H (H64) < 20 72 ± 7
F131H (H64) 53 ± 5 52 ± 4
V135H (H64) 37 ± 6 < 20
F131H/V135H (H64) 66 ± 6 119 ± 18
L198H (H64) 9 ± 2 30 ± 3
P202H (H64) < 10 39 ± 4
L198H/P202H (H64) 13 ± 4 32 ± 5
a Values of kcat were determined from the initial reaction rates, based on v0 = kcat*[E]. The values are given with a 95 % confidence interval. For low activity variants, the activity is reported as less than the kcat that would give a reaction rate five times higher than background.

37
The wild type is active at both pH values, and a control measurement with the
variant H64A at pH 6.7 did not show activity, confirming that His-64 is capable of
catalyzing the reaction. The two-histidine site N62H/(H64) is less active than
HCAII (H64) at pH 5.3, but more active at pH 6.7, indicating that they constitute a
functional two-histidine pair. The site composed of histidine residues in positions
131 and 135 is the most active, and the kcat for this variant at pH 6.7 is the highest
rate in table 5.1. The site composed of positions 198 and 202, on the other hand,
apparently does not support catalysis. At pH 5.3, the activity is lower than HCAII
(H64), and at pH 6.7 it is similar to HCAII (H64). This activity might well be
caused by the histidine residue in position 64. The results indicate that there are no
beneficial interactions between histidine residues in different histidine pairs,
because single mutants (which also contain H64) are generally less active than
HCAII (H64).
In figure 5.2, the pH dependences of kcat are shown for HCAII (H64) and the
mutant F131H/V135H. A pKa value of 7.1 for deprotonation of the His-64 side
chain has been determined by NMR.[127] At conditions with low salt
concentrations, similar to the conditions used for the pNPSA hydrolysis
measurements, a pKa value of 6.9 has been determined (B.H. Jonsson, personal
communication).
Figure 5.2: pH dependence of log kcat for hydrolysis of pNPSA. The data for HCAII (H64) was fitted to a pKa of 6.9. For F131H/V135H, pKa values of 5.6 and 7.0 were obtained.

38
In the figure, a titration curve with pKa 6.9 has been fitted to the data for HCAII
(H64). The trend in the data is in agreement with the theoretical curve, with values
of kcat that declines as pH is increased. It seems reasonable to suggest that His-64 is
capable of general acid-catalysis, although the limited pH-range does not allow
accurate pKa determinations from the data. Further support for this statement
comes from measurements with the isoenzyme HCAI at pH 5.3 and 6.5. For this
enzyme, pKa of His-64 has been determined to 4.7.[128] No activity with pNPSA
could be detected for HCAI, which is expected if His-64 acts as a general acid,
because the low pKa means that a very small proportion of the enzyme molecules
are in the reactive protonated state.
5.2.3 Grafting a helix “two-histidine” catalytic motif into HCAII
It is interesting to note that the F131H/V135H variant is the most active for
pNPSA hydrolysis. These positions are situated in a helical portion of the HCAII
active site cleft, in a (i, i+4) configuration. Thus, the creation of a two-histidine
catalytic site in positions 131 and 135 represents a true grafting of the original two-
histidine catalytic motif from the helix-loop-helix construct described above into
the HCAII scaffold.[68]
From the pH-dependence of kcat for F131H/V135H (see figure 5.2), it can be
seen that the activity has a maximum around pH 6, with lower values of kcat for
higher and lower pH values. Fitting the data to the theoretical titration behavior of
a doubly ionizing system, which is active in its monoprotonated form, yields pKa
values of 5.6 and 7.0. Thus, the data support a mechanism based on cooperative
catalysis with one histidine acting as a nucleophile, and the other acting as a
general acid. However, expulsion of the para-nitrophenolate leaving group from
the tetrahedral intermediate cannot be acid-catalyzed at pH values above its pKa,
which is 7.2.[65, 121] Therefore, the pKa value for the general acid determined
from our data should be seen as a lower limit for the true pKa.
Benzenesulfonamide has been reported to bind with a KD of 0.2 µM to HCA II,
and this value decreases when 4-substituents are added. For example, a molecule

39
with similar structure as pNPSA, but without the nitrophenyl group, was found to
bind with a KD of 8 nM.[129] Because of the strong affinity between the substrate
and HCAII, the enzyme is saturated with substrate at the conditions used for the
measurements. The KM value can not be determined by varying the substrate
concentration, since the sensitivity is too low to measure reaction rates at the very
low substrate concentrations required. Instead, the KM was determined from
measurements in the presence of the strong inhibitor acetazolamide, and found to
be approximately 20 nM. Using this value, a maximum value for kcat/KM of
around 14000 M-1s-1 at pH 5.9 can be calculated. This is equal to a rate
enhancement of 104-105 over the reaction with the helix-loop-helix catalyst.
Further, it means a rate enhancement of approximately 107 over the reaction
catalyzed by the histidine mimic 4-methyl imidazole.[68, 121]
5.2.4 Structural requirements for catalytic activity
Appropriate pKa values for deprotonation of the histidine side chains are
important for the catalytic activity, and the chemical environment can have large
effects on this parameter. For example, a hydrophobic environment makes it
energetically unfavorable to have an unpaired charge because of the lower
dielectricity constant, and therefore the pKa value is depressed. The pKa values
determined for F131H/V135H from the data in figure 5.2 indicate a difference of
approximately 1.3 pH units. Interestingly, Val-135 is positioned in a hydrophobic
part of the HCAII active site cavity, and is partly buried, while Phe-131 has a less
hydrophobic environment and is accessible to solvent.[77] Thus, it seems
reasonable to suggest that V135H has the lower pKa value, and act as nucleophile,
while F131H has the higher pKa value and act as a general acid in the catalytic
reaction.
While the chemical surroundings of the histidine residues are important in both
the helix-loop-helix catalyst [68] and in two-histidine mutants of HCAII, additional
restraints on the position of the catalytic motif arise in HCAII because of the well
defined substrate binding mode. As mentioned above, the benzenesulfonamide

40
part of the substrate tends to bind in a rigid manner, and the histidines must be
appropriately positioned in relation to the substrate to allow productive catalytic
interactions. To probe this issue, automated docking experiments were performed,
in which the substrate molecule was docked to the three two-histidine variants
N62H (H64), F131H/V135H and L198H/P202H (see figure 5.3).
The results indicate that the scissile ester bond of pNPSA can come into close
proximity (< 4 Å) of histidines in all positions except L198H (>5.5 Å). This might
partly explain the low activity of the mutant L198H/P202H. Further, it was found
that pNPSA is positioned so that the histidines in positions 198 and 202 are aligned
along the length of the substrate. In the other two sites (62/64 and 131/135), the
ester bond is positioned between the histidine side chains, resulting in more
favorable positions for catalysis.
We also note that a close distance between the histidine side chains is a
requirement for cooperative catalysis. Only N62H (H64) and F131H/V135H
display a distinctly increased activity compared to HCAII (H64). The introduced
histidine residues in the single mutants apparently do not form functional two-
histidine catalytic units together with His-64, supporting the assumption that the
side chains must be positioned close together to support cooperative catalysis.

41
Figure 5.3: The substrate pNPSA, docked to the HCAII mutants N62H (H64), F131H/V135H and L198H/P202H. The left panel shows the position of the indicated histidine residues and pNPSA in the active site cavity. The zinc ion is shown as a sphere at the bottom of the substate binding cleft. To the right, the relative positions of the histidine side chains and the pNPSA ester bond are shown, with the shortest distance between the histidine side chains and the carbonyl carbon indicated. (Red atoms = oxygen, grey = aliphatic carbon, purple = aromatic carbon, blue = nitrogen and yellow = phosphorous.)

42

43
6. Development of a plate screening assay (paper I, suppl)
6.1 A method for screening HCAII variants for esterase activity
Directed evolution and other random redesign methods require that a large
amount of clones can be efficiently screened, so that variants with improved
characteristics can be isolated. To allow selection of HCAII variants with altered
properties, a plate screening method was developed, based on the catalytic
hydrolysis of para-nitrophenyl esters. Similar assay methods have been described
previously for other systems.[43, 44]
Briefly, clones of the expression strain BL21(DE3) of E. coli, containing mutated
HCAII variants, are grown in 96-well culture plates. Following induction of
protein synthesis, the cells are gently lysed by repeated freeze-thawing and the cell
debris is removed by centrifugation. The resulting enzyme-containing supernatant
is transferred to a plate suitable for spectrophotometric measurements in a plate
reader.
The enzyme concentration might vary between different clones, thus making it
difficult to compare rates. Therefore, multiple replicates of the measurement plate
are prepared from each culture plate, so that for each clone the same enzyme
concentration is used for all measurements. By normalizing measurements for
each clone with a measurement on the same clone under some standard conditions
(see section 6.2), different clones can be compared to each other.
The detection limit for the assay was determined, and it was found that it was
possible to detect enzyme activity corresponding to around 0.05 µM of wild type
HCAII using pNPA as substrate. Results from measurements on wild type HCAII,

44
produced as described above, indicated that enzyme concentrations of
approximately 0.1 µM could be obtained in the wells in the plate. Thus, the assay is
useful for finding variants with increased catalytic activities.
6.2 Screening for altered specificity
As described in chapter 4.3.1, a panel of HCAII mutants with various residues
mutated into alanine was screened using the plate assay. Measurements with
pNPA were used as a standard, and for each clone the rates of hydrolysis for other
substrates were normalized against the pNPA rate. In this way altered specificities
could be separated from the effects of varying enzyme concentrations and levels of
esterase activities.
6.3 Screening for improved stability
The plate screening assay can also be used to probe the effect of a mutation on
stability. Destabilized proteins are generally more sensitive to temperature.
Measurements of the esterase activity for a clone with and without heat treatment
yields information about the stability of the enzyme variant. The principle was
tested on purified enzyme solutions of HCAII and the variants F131H, L198H,
P202H and L198H/P202H. Enzyme solutions were subjected to 50 °C for 15
minutes, and measurements of pNPA hydrolysis were performed on heat treated
and untreated samples. It was found that P202H and L198H/P202H were 40 %
inactivated, indicating a slight destabilization. The effect of the heat treatment was
negligible on the other enzyme variants.
Pro-202 is a conserved residue,[75] and several HCAII variants with mutation
in this residue are destabilized compared to wild type HCAII.[102, 130]
Apparently, the plate screening method can be used for detecting altered stability.

45
7. Carbonic anhydrase from P. falciparum (PfCA) (paper IV)
7.1 Malaria
Malaria is a disease that affects millions of people each year. Because it has its
largest impact on poor countries where the health sector infrastructure is weak (90
% of the deadly cases are believed to occur in Africa south of Sahara [131]), it is
difficult to measure the incidence and impact of malaria. However, it has been
estimated that in 2002 as many as 500 million people were affected globally,[132]
and it is believed that approximately 1 million individuals die each year, most of
which are children.[133]
The disease is caused by infection with protozoan parasites of the Plasmodium
genus. Four species affect humans, with P. falciparum and P. vivax as the most
common. P. falciparum is the most virulent, and causes most of the severe cases of
malaria in human. The parasite’s life cycle is divided in two main parts, a sexual
phase in the gut of a mosquito of the Anopheles genus, which is the main vector for
malaria transmission, and an asexual phase in the human body. A malaria
infection cycle begins when an infected mosquito takes a blood meal, and parasites
are injected into the blood stream. Parasites then invade cells in the liver and start
to divide. As parasites are liberated from the liver, they invade red blood cells
(RBCs), in which they grow and divide until the RBC bursts. This stage is the cause
of the clinical symptoms of malaria.[134]
Development of new drugs for malaria treatment is important, because
widespread resistance has decreased the usefulness of some of the most common
and affordable antimalarial drugs, such as chloroquine.[135] An important step

46
towards development of new therapies is the identification of potential drug
targets. The P. falciparum genome sequence is now available, which should
facilitate these efforts.[136]
7.2 P. falciparum carbonic anhydrase
A gene for a protein homologous to α-CAs from various organisms has been
identified in the P. falciparum genome, and also in the species P. yoleii.[137] More
sequences have been elucidated, and highly homologous sequences can be found
in other Plasmodium species, e.g. P. berghei and P. chabaudi. The malaria parasite is
dependent on a supply of HCO3-, as this molecule is required for biosynthesis of
pyrimidines. It is believed that carbonic anhydrase meet this need, and therefore
this enzyme is a potential target for new antimalarial drugs. The presence of active
carbonic anhydrase in P. falciparum has been demonstrated, and the parasite is
sensitive to the specific carbonic anhydrase inhibitor acetazolamide.[138] A
putative carbonic anhydrase has reportedly been purified from P. falciparum and
partly characterized.[139] Other accounts report that the gene for P. falciparum
carbonic anhydrase (PfCA) has been cloned and subsequently expressed in E. coli,
yielding an enzyme with similar characteristics as the purified enzyme.[137, 140]
However, data on the characterization is very limited in these reports. For
example, it has not been established that the enzyme has CO2 hydration activity,
which is necessary to unambiguously identify it as a functional carbonic
anhydrase.
As the identified gene encodes a protein that is homologous to CAs, it can be
anticipated that it will have similar structure as confirmed CAs. Therefore, a
threading was performed, in which the amino acid sequence of PfCA is
superpositioned on a crystal structure template. The structure of Neisseria
gonorrhoeae CA (NgCA) was used, since this enzyme has a similar length (226
residues) as PfCA (235 residues).[141]

47
PfCA --MKDLKERELKNISDVYLNLFDDDNYAWNNYNKPWMKGDFFYYYEYFIKKIVINRQNNI 58 NgCA HGNHTHWGYTGHDSPESWGNLSEEFRLCSTGKNQSPVN--ITETVSGKLPAIKVNYKPSM 58 : :: .: : ** :: . . .. *:. :: : . : * :* : .:
PfCA FQIKAARDGIIP---FGVLFTTEQPAMFYADQIHFHAPSEHTFQGSGNRREIEMQIFHST 115 NgCA VDVENNGHTIQVNYPEGGNTLTVNGRTYTLKQFHFHVPSENQIKG---------RTFPME 109 .::: . * * * : : .*:***.***: ::* : * -----RTFPMEAHFV *
PfCA NYFYDIQDDKSKYKKKYGLHIYNNLKKNSKETSKKDSSRYHSYLMSFLMNSLSNEQLQNK 175 NgCA AHFVHLDENK---QPLVLAVLYEAGKTNGRLSSIWNVMPMTAGKVKLNQPFDASTLLP-- 164 :* .::::* : :*: *.*.: :* : : :.: :. * HLDENK------- . .
PfCA YNKKKRIKKMKNQYEVISITFTSAEINASTINAFKKLPSEKFLRTIINVSSAVHVGSGNK 235 NgCA --KRLKYYRFAGSLTTPPCTEGVSWLVLKTYDHIDQAQAEKFTRAVGSENNRPVQPLNAR 222 *: : :: .. . . * : : .* : :.: :*** *:: . .. . :
PfCA ---- NgCA VVIE 226 Figure 7.1: Sequence alignment between PfCA and NgCA. Zinc binding histidine residues are marked. The shaded sequence was manually aligned to allow a third histidine zinc ligand in PfCA. The alignment was performed using CLUSTAL W,[142] and the sequences were retrieved from NCBI. Symbols denote identical residues for all sequences (*), conservative substitutions (:) and semi-conservative substitutions (.), respectively.
In the threading, residues of the two enzymes were superpositioned, based on a
sequence alignment between the enzymes (see figure 7.1). A part of the PfCA
sequence, situated between two gaps in the alignment, was manually realigned
with NgCA, to allow a histidine residue in PfCA to participate in the formation of
a three-histidine zinc binding site which is present in most α-CAs. From figure 7.2,
it can be seen that PfCA is compatible with an α-CA fold, and the gaps in the
alignment are mainly positioned on turns, giving rise to loop structures in PfCA.
Further, the central β-sheet structure is largely composed of alternating
hydrophobic and hydrophilic residues, indicating that formation of a large
hydrophobic cluster below the β-sheet (similar to other carbonic anhydrases) is
possible. These observations support the idea that PfCA is an α-CA enzyme, but a
conclusive statement must await structural studies and functional characterization.

48
Figure 7.2: The threaded structure of PfCA (dark line) superpositioned on the N. gonorrhoeae CA crystal structure (filled ribbon, pdb code 1kop[141]). Arrows indicate the two main gaps in the alignment (see figure 7.1). Three histidine residues in PfCA are shown, composing a putative zinc binding site. Threading calculations were performed using the software DeepView/Swiss pdb-viewer together with Swissmodel on Expasy (http://www.expasy.org/spdbv/).[143]
7.3 Cloning and expression of PfCA
The gene for the PfCA enzyme was cloned from genomic P. falciparum DNA by
PCR, and introduced into a plasmid vector. The resulting construct has an N-
terminal His6-tag. Initial attempts to produce the enzyme failed, and therefore an
expression strain with increased rare codon tRNA transcription was used.
Production of the enzyme in this strain resulted in a high yield of protein of the
appropriate size. However, the product was not soluble. The inclusion bodies were
dissolved in urea, and the protein was purified by affinity chromatography using
an IMAC column loaded with Ni. Initial attempts to refold the protein were not
successful.

49
Problems with production of P. falciparum proteins in E. coli are common, and
several factors are involved. The malaria parasite has a very different codon usage
profile compared to E. coli, and therefore cloned P. falciparum genes contain high
levels of codons for which tRNA is scarce in E. coli.[144] Other factors that
correlate with low yield and insoluble product are highly basic pI values for the
protein and high percentage of AT in the gene with long continuous stretches of
these bases.[145] The gene for PfCA is problematic in all of these senses, i.e. it has a
high AT% (76.5) with long stretches of A or T (the longest stretch is 10 consecutive
bases), and the gene product has a high pI (calculated to be 9.55). While the codon
usage problem is solved by using an expression strain of E. coli that is designed to
allow expression of rare codons, the physical properties of the protein are more
challenging. Further study is required to evaluate the potential for refolding of
PfCA.

50

51
References
1. Tipton K, Boyce S. History of the enzyme nomenclature system. Bioinformatics 2000,16:34-40.
2. Bornscheuer UT, Buchholz K. Highlights in biocatalysis- Historical landmarks and current trends. Engineering in life science 2005,5:309-323.
3. Nissen P, Hansen J, Ban N, Moore PB, Steitz TA. The structural basis of ribosome activity in peptide bond synthesis. Science 2000,289:920-930.
4. Berg JM, Tymoczko JL, Stryer L. Biochemistry. 5 ed. New York: W H Freeman and company; 2002.
5. Brannigan JA, Wilkinson AJ. Protein engineering 20 years on. Nature 2002,3.
6. Winter G, Fersht AR, Wilkinson AJ, Zoller M, Smith M. Redesigning enzyme structure by site-directed mutagenesis: tyrosyl tRNA synthetase and ATP binding. Nature 1982,299:756-758.
7. Qi D, Tann CM, Haring D, Distefano MD. Generation of new enzymes via covalent modification of existing proteins. Chemical reviews 2001,101:3081-3111.
8. Hill RB, Raleigh DP, Lombardi A, DeGrado WF. De novo design of helical bundles as models for understanding protein folding and function. Accounts of chemical research 2000,33:745-754.
9. Venkatraman J, Shankaramma SC, Balaram P. Design of folded peptides. Chemical reviews 2001,101:3131-3152.
10. Cedrone F, Ménez A, Quéméneur E. Tailoring new enzyme functions by rational redesign. 2000,10:405-410.
11. Chen R, Greer A, Dean AM. A highly active decarboxylating dehydrogenase with rationally inverted coenzyme specificity. Proceedings of the national academy of science 1995,92:11666-11670.
12. Rotticci D, Rotticci-Mulder JC, Denman S, Norin T, Hult K. Improved enantioselectivity of a lipase by rational protein engineering. ChemBioChem 2001,2:766-770.

52
13. Chica RA, Doucet N, Pelletier JN. Semi-rational approaches to engineering enzyme activity: Combining the benefits of directed evolution and rational design. 2005,16:378-384.
14. Kuchner O, Arnold FH. Directed evolution of enzyme catalysis. Trends in biotechnology 1997,15:523-530.
15. Dalby PA. Optimising enzyme function by directed evolution. 2003,13:500-505.
16. Kaur J, Sharma R. Directed evolution: An approach to engineer enzymes. Critical reviews in biotechnology 2006,26:165-199.
17. Park S, Morley KL, Horsman GP, Holmquist M, Hult K, Kazlauskas RJ. Focusing mutations into the P. fluorescens esterase binding site increases enantioselectivity more effectively than distant mutations. Chemistry and biology 2005,12:45-54.
18. Horsman GP, Liu AMF, Henke E, Bornscheuer UT, Kazlauskas RJ. Mutations in distant residues moderately increase the enantioselectivity of Pseudomonas fluorescens esterase toward methyl 3-bromo-2-methylpropanoate and ethyl 3-phenylbutyrate. Chemistry a european journal 2003,9:1933-1939.
19. Reetz MT, Bocola M, Carballeira JD, Zha D, Vogel A. Expanding the range of substrate acceptance of enzymes: Combinatorial active-site saturation test. Angewandte chemie international edition in english 2005,44:4192-4196.
20. Reetz MT, Carballeira JD, Peyralans J, Höbenreich H, Maichele A, Vogel A. Expanding the substrate scope of enzymes: Combining mutations obtained by CASTing. Chemistry a european journal 2006,12:6031-6038.
21. Gustafsson C, Govindarajan S, Minshull J. Putting engineering back into protein engineering: Bioinformatic approaches to catalyst design. 2003,14:366-370.
22. Fox R, Roy A, Govindarajan S, et al. Optimizing the search algorithm for protein engineering by directed evolution. Protein engineering 2003,16:589-597.
23. Larsson AK, Emrén LO, Bardsley WG, Mannervik B. Directed enzyme evolution guided by multidimensional analysis of substrate-activity space. Protein engineering, design and selection 2004,17:49-55.
24. Fox RJ, Davis SC, Mundorff EC, et al. Improving catalytic function by ProSAR-driven enzyme evolution. Nature biotechnology 2007,doi:10.1038/nbt1286.
25. Bolon DN, Mayo SL. Enzyme-like proteins by computational design. Proceedings of the national academy of science 2001,98:14274-14279.
26. Hayes RJ, Bentzien J, Ary ML, et al. Combining computational and experimental screening for rapid optimization of protein properties. Proceedings of the national academy of science 2002,99:15926-15931.

53
27. Dwyer MA, Looger LL, Hellinga HW. Computational design of a biologically active enzyme. Science 2004,304:1967-1971.
28. Amstutz P, Pelletier JN, Guggisberg A, et al. In vitro selection for catalytic activity with ribosome display. Journal of the American Chemical Society 2002,124:9396-9403.
29. Olsen MJ, Stephens D, Griffiths D, Daugherty P, Georgiou G, Iverson BL. Function-based isolation of novel enzymes from a large library. Nature biotechnology 2000,18:1071-1074.
30. Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science 1985,228:1315-1317.
31. Widersten M, Mannervik B. Glutathione transferases with novel active sites isolated by phage display from a library of random mutants. Journal of molecular biology 1995,250:115-122.
32. Janda KD, Lo LC, Lo CHL, et al. Chemical selection for catalysis in combinatorial antibody libraries. Science 1997,275:945-948.
33. Legendre D, Laraki N, Gräslund T, et al. Display of active subtilisin 309 on phage: Analysis of parameters influencing the selection of subtilisin variants with changed substrate specificity from libraries using phosphonylating inhibitors. Journal of molecular biology 2000,296:87-102.
34. Dröge MJ, Rüggeberg CJ, van der Sloot AM, et al. Binding of phage displayed Bacillus subtilis lipase A to a phosphonate suicide inhibitor. Journal of biotechnology 2003,101:19-28.
35. Cesaro-Tadic S, Lagos D, Honegger A, et al. Turnover-based in vitro selection and evolution of biocatalysts from a fully synthetic antibody library. Nature biotechnology 2003,21:679-685.
36. Gao C, Lin CH, Lo CHL, et al. Making chemistry selectable by linking it to infectivity. Proceedings of the national academy of science 1997,94:11777-11782.
37. Pedersen H, Hölder S, Sutherlin DP, Schwitter U, King DS, Schultz PG. A method for directed evolution and functional cloning of enzymes. Proceedings of the national academy of science 1998,95:10523-10528.
38. Demartis S, Huber A, Viti F, et al. A strategy for the isolation of catalytic activities from repertoires of enzymes displayed on phage. Journal of molecular biology 1999,286:617-633.
39. Atwell S, Wells JA. Selection for improved subtiligases by phage display. Proceedings of the national academy of science 1999,96:9497-9502.
40. Heinis C, Huber A, Demartis S, et al. Selection of catalytically active biotin ligase and trypsin mutants by phage display. Protein engineering 2001,14:1043-1052.
41. Strobel H, Ladant D, Jestin JL. In vitro selection for enzymatic activity: A model study using adenylate cyclase. Journal of molecular biology 2003,332:1-7.

54
42. Altamirano MM, Blackburn JM, Aguayo C, Fersht AR. Directed evolution of new catalytic activity using the α/β−barrel scaffold. Nature 2000,403:617-622.
43. Reetz MT, Zonta A, Schimossek K, Jaeger KE, Liebeton K. Creation of enantioselective biocatalysts for organic chemistry by in vitro evolution. Angewandte chemie international edition in english 1997,36:2830-2832.
44. Bornscheuer UT, Ordoñez GR, Hidalgo A, et al. Selectivity of lipases and esterases towards phenol esters. Journal of molecular catalysis B: enzymatic 2005,36:8-13.
45. Delagrave S, Murphy DJ, Rittenhouse Pruss JL, et al. Application of a very high-throughput digital imaging screen to evolve the enzyme galactose oxidase. Protein engineering 2001,14:261-267.
46. Griffiths AD, Tawfik DS. Directed evolution of an extremely fast phosphotriesterase by in vitro compartmentalization. The EMBO journal 2003,22:24-35.
47. Garcia-Viloca M, Gao J, Karplus M, Truhlar DG. How enzymes work: Analysis by modern rate theory and computer simulations. Science 2004,303:186-195.
48. Pauling L. Molecular architecture and biological reactions. Chemical and engineering news 1946,24:1375-1377.
49. Zhang X, Houk KN. Why enzymes are proficient catalysts: Beyond the Pauling paradigm. Accounts of chemical research 2005,38:379-385.
50. Bornscheuer UT, Kazlauskas RJ. Catalytic promiscuity in biocatalysis: Using old enzymes to form new bonds and follow new pathways. Angewandte chemie international edition in english 2004,43:6032-6040.
51. Khersonsky O, Roodveldt C, Tawfik DS. Enzyme promiscuity: Evolutionary and mechanistic aspects. 2006,10:498-508.
52. Straathof AJJ, Panke S, Schmid A. The production of fine chemicals by biotransformations. 2002,13:548-556.
53. Schoemaker HE, Mink D, Wubbolts MG. Dispelling the myths- biocatalysis in industrial synthesis. Science 2003,299:1694-1697.
54. Faber K. Biotransformations in organic chemistry. 3 ed. Berlin: Springer; 1997.
55. Panda T, Gowrishankar BS. Production and applications of esterases. Applied microbiology and biotechnology 2005,67:160-169.
56. Galante YM, Formantici C. Enzyme applications in detergency and in manufacturing industries. Current organic chemistry 2003,7:1399-1422.
57. Cammarota MC. A review on hydrolytic enzymes in the treatment of wastewater with high oil and grease content. Bioresource technology 2006,97:2195-2210.

55
58. Mendes AA, Pereira EB, de Castro HF. Effect of the enzymatic hydrolysis pretreatment of lipids-rich wastewater on the anaerobic biodigestion. Biochemical engineering journal 2006,32:185-190.
59. Burton SG, Cowan DA, Woodley JM. The search for the ideal biocatalyst. Nature biotechnology 2002,20:37-45.
60. Bornscheuer UT. Methods to improve enantioselectivity of lipases and esterases. 2002,13:543-547.
61. Berglund P, Park S. Strategies for altering enzyme reaction specificity for applied biocatalysis. Current organic chemistry 2005,9:325-336.
62. Scott AK. Stereoisomers and drug toxicity. The value of single stereoisomer therapy. Drug safety 1993,8:149-159.
63. Penning TM, Jez JM. Enzyme redesign. Chemical reviews 2001,101:3027-3046.
64. Hult K, Berglund P. Engineered enzymes for improved organic synthesis. 2003,14:395-400.
65. Fersht A. Structure and mechanism in protein science: a guide to enzyme catalysis and protein folding. New York: W. H. Freeman and Company; 1999.
66. Wolfenden R, Snider MJ. The depth of chemical time and the power of enzymes as catalysts. Accounts of chemical research 2001,34:938-945.
67. Radzicka A, Wolfenden R. A proficient enzyme. Science 1995,267:90-93.
68. Broo KS, Nilsson H, Nilsson J, Flodberg A, Baltzer L. Cooperative nucleophilic and general-acid catalysis by the HisH+-His pair and arginine transition state binding in catalysis of ester hydrolysis reactions by designed helix-loop-helix motifs. Journal of the American Chemical Society 1998,120:4063-4068.
69. Meldrum NU, Roughton FJW. Carbonic anhydrase: Its preparation and properties. Journal of physiology (London) 1933,80:113-142.
70. Stadie WC, O´Brien H. The catalysis of the hydration of carbon dioxide and dehydration of carbonic acid by an enzyme isolated from red blood cells. The journal of biological chemistry 1933,103:521-529.
71. Keilin D, Mann T. Carbonic anhydrase. Purification and nature of the enzyme. 1940,34:1163-1176.
72. Hewett-Emmett D, Tashian RE. Functional diversity, conservation, and convergence in the evolution of the α−, β−, and γ-carbonic anhydrase gene families. Molecular phylogenetics and evolution 1996,5:50-77.
73. Tripp BC, Smith K, Ferry JG. Carbonic anhydrase: New insights for an ancient enzyme. The journal of biological chemistry 2001,276:48615-48618.
74. Esbaugh AJ, Tufts BL. The structure and function of carbonic anhydrase isozymes in the respiratory system of vertebrates. Respiratory physiology and neurobiology 2006,154:185-198.

56
75. Lindskog S. Structure and mechanism of carbonic anhydrase. Pharmacology and therapeutics 1997,74:1-20.
76. Liljas A, Kannan KK, Bergstén PC, et al. Crystal structure of human carbonic anhydrase C. Nature New Biology 1972,235:131-137.
77. Eriksson AE, Jones TA, Liljas A. Refined structure of human carbonic anhydrase II at 2.0 Å resolution. Proteins: structure, function and genetics 1988,4:274-282.
78. Håkansson K, Carlsson M, Svensson LA, Liljas A. Structure of native and apo carbonic anhydrase II and structure of some of its anion-ligand complexes. Journal of molecular biology 1992,227:1192-1204.
79. Lindskog S, Ibrahim SA, Jonsson BH, Simonsson I. Carbonic anhydrase: structure, kinetics, and mechanism. In: The coordination chemistry of metalloenzymes. Edited by Bertini I, Drago RS, Luchinat C. Dordrecht: D. Reidel publishing company; 1983:49-64.
80. Liljas A, Håkansson K, Jonsson BH, Xue Y. Inhibition and catalysis of carbonic anhydrase. European journal of biochemistry 1994,219:1-10.
81. Steiner H, Jonsson BH, Lindskog S. The catalytic mechanism of carbonic anhydrase. Hydrogen-isotope effects on the kinetic parameters of human C isoenzyme. European journal of biochemistry 1975,59:253-259.
82. Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase I. Stop-flow kinetic studies on the native human isoenzymes B and C. The journal of biological chemistry 1971,246:2561-2573.
83. Pocker Y, Meany JE. The catalytic versatility of erythrocyte carbonic anhydrase. I. Kinetic studies of the enzyme-catalyzed hydration of acetaldehyde. Biochemistry 1965,4:2535-2541.
84. Pocker Y, Meany JE. The catalytic versatility of erythrocyte carbonic anhydrase. II. Kinetic studies of the enzyme-catalyzed hydration of pyridine aldehydes. Biochemistry 1967,6:239-246.
85. Tashian RE, Plato CC, Shows TB. Inherited variant of erythrocyte carbonic anhydrase in micronesians from Guam and Saipan. Science 1963,140:53-54.
86. Thorslund A, Lindskog S. Studies of the esterase activity and the anion inhibition of bovine zinc and cobalt carbonic anhydrases. European journal of biochemistry 1967,3:117-123.
87. Pocker Y, Stone JT. The catalytic versatility of erythrocyte carbonic anhydrase. III. Kinetic studies of the enzyme-catalyzed hydrolysis of p-nitrophenyl acetate. Biochemistry 1967,6:668-678.
88. Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrase B and C. The journal of biological chemistry 1967,242:4221-4229.
89. Okrasa K, Kazlauskas RJ. Manganese-substituted carbonic anhydrase as a new peroxidase. Chemistry a european journal 2006,12:1587-1596.

57
90. Fernández-Gacio A, Codina A, Fastrez J, Riant O, Soumillon P. Transforming carbonic anhydrase into epoxide synthase by metal exchange. ChemBioChem 2006,7:1013-1016.
91. Mann T, Keilin D. Sulfanilamide as a specific inhibitor of carbonic anhydrase. Nature 1940,146:164-165.
92. King RW, Burgen ASV. Sulphonamide complexes of human carbonic anhydrases ultraviolet difference spectroscopy. Biochimica et biophysica acta 1970,207:278-285.
93. Kumar K, King RW, Carey PR. Resonance Raman studies on some carbonic anhydrase-aromatic sulfonamide complexes. Biochemistry 1976,15:2195-2202.
94. Shaw CR, Syner FN, Tashian RE. New genetically determined molecular form of erythrocyte esterase in man. Science 1962,138:31-32.
95. Pocker Y, Beug MW. Kinetic studies of bovine carbonic anhydrase catalyzed hydrolyses of para-substituted phenyl esters. Biochemistry 1972,11:698-707.
96. Pocker Y, Meany JE, Davis BC, Arrigoni J, Stein JE. Bifunctional substrates of erythrocyte carbonic anhydrase. Enzyme-catalyzed hydration and hydrolysis of pyruvate esters. Journal of the American Chemical Society 1978,100:2883-2885.
97. Kaiser ET, Lo KW. The carbonic anhydrase catalyzed hydrolysis of 2-hydroxy-5-nitro-α-toluenesulfonic acid sultone. Journal of the American Chemical Society 1969,91:4912-4918.
98. Pocker Y, Storm DR. The catalytic versatility of erythrocyte carbonic anhydrase. IV. Kinetic studies of enzyme-catalysed hydrolyses of p-nitrophenyl esters. Biochemistry 1968,7:1202-1214.
99. Nair SK, Calderone TL, Christianson DW, Fierke CA. Altering the mouth of a hydrophobic pocket. Structure and kinetics of human carbonic anhydrase II mutants at residue Val-121. Journal of biological chemistry 1991,266:17320-17325.
100. Fierke CA, Calderone TL, Krebs JF. Functional consequences of engineering the hydrophobic pocket of carbonic anhydrase II. Biochemistry 1991,30:11054-11063.
101. Krebs JF, Rana F, Dluhy RA, Fierke CA. Kinetic and spectroscopic studies of hydrophilic amino acid substitutions in the hydrophobic pocket of human carbonic anhydrase II. Biochemistry 1993,32:4496-4505.
102. Krebs JF, Fierke CA. Determinants of the catalytic activity and stability of carbonic anhydrase II as revealed by random mutagenesis. Journal of biological chemistry 1993,268:948-954.

58
103. Elleby B, Sjöblom B, Lindskog S. Changing the efficiency and specificity of the esterase activity of human carbonic anhydrase II by site-specific mutagenesis. European journal of biochemistry 1999,262:516-521.
104. Gould SM, Tawfik DS. Directed evolution of the promiscuous esterase activity of carbonic anhydrase II. Biochemistry 2005,44:5444-5452.
105. Goodsell DS, Olson AJ. Automated docking of substrates to proteins by simulated annealing. Proteins: structure, function and genetics 1990,8:195-202.
106. Morris GM, Goodsell DS, Huey R, Olson AJ. Distributed automated docking of flexible ligands to proteins: parallel applications of Autodock 2.4. Journal of computer-aided molecular design 1996,10:293-304.
107. Morris GM, Goodsell DS, Halliday RS, et al. Automated docking using lamarckian genetic algorithm and an empirical binding free energy function. Journal of computational chemistry 1998,19:1639-1662.
108. Tramontano A, Janda KD, Lerner RA. Catalytic antibodies. Science 1986,234:1566-1570.
109. Mitsuhashi K, Yamashita M, Hwan YS, Ihara F, Nihira T, Yamada Y. Purification and characterization of a novel extracellular lipase catalyzing hydrolysis of oleyl benzoate from Acinetobacter nov. sp. strain KM109. Bioscience biotechnology and biochemistry 1999,63:1959-1964.
110. Goto M, Kawasaki M, Kometani T, Nonaka T, Mitsui Y. Binding site of acyl moiety in ester hydrolysis by Candida rugosa lipase. Journal of molecular catalysis B: enzymatic 2001,11:1029-1033.
111. Berndt MC, Bowles MR, King GJ, Zerner B. Inhibition of chicken liver carboxylesterase by activated carbonyls and carbonyl hydrates. Biochimica et biophysica acta 1996,1298:159-166.
112. Yoon KJP, Morton CL, Potter PM, Danks MK, Lee RE. Synthesis and evaluation of esters and carbamates to identify critical functional groups for esterase-specific metabolism. Bioorganic and medicinal chemistry 2003,11:3237-3244.
113. Barbayianni E, Fotakopoulou I, Schmidt M, Constantinou-Kokotou V, Bornscheuer UT, Kokotos G. Enzymatic removal of carboxyl protecting groups. 2. Cleavage of the benzyl and methyl moieties. Journal of organic chemistry 2005,70:8730-8733.
114. Zimmer C, Platz T, Cadez N, Giffhorn F, Kohring GW. A cold active (2R,3R)-(-)-di-O-benzoyl-tartrate hydrolyzing esterase from Rhodotorula mucilaginosa. Applied microbiology and biotechnology 2006,73:132-140.
115. Carrera MRA, Meijler MM, Janda KD. Cocaine pharmacology and current pharmacotherapies for its abuse. Bioorganic and medicinal chemistry 2004,12:5019-5030.

59
116. Ascenzi P, Clementi E, Polticelli F. The Rhodococcus sp. cocaine esterase: a bacterial candidate for novel pharmacokinetic-based therapies for cocaine abuse. Life 2003,55:397-402.
117. Stewart DJ, Inaba T, Tang BK, Kalow W. Hydrolysis of cocaine in human plasma by cholinesterase. Life sciences 1977,20:1557-1564.
118. Landry DW, Zhao K, Yang GXQ, Glickman M, Georgiadis TM. Antibody-catalyzed degradation of cocaine. Science 1993,259:1899-1901.
119. Bresler MW, Rosser SJ, Basran A, Bruce NC. Gene cloning and nucleotide sequencing and properties of a cocaine esterase from Rhodococcus sp. strain MB1. Applied and environmental microbiology 2000,66:904-908.
120. Jeanville PM, Estapé ES, Jeanville T-Nd. The effect of liquid chromatography eluents and additives on the positive ion responses of cocaine, benzoylecgonine, and ecgonine methyl ester using electrospray ionization. International journal of mass spectrometry 2003,227:247-258.
121. Nilsson J, Baltzer L. Reactive-site design in folded-polypeptide catalysts- the leaving group pKa of reactive esters sets the stage for cooperativity in nucleophilic and general-acid catalysis. Chemistry a european journal 2000,6:2214-2220.
122. Lundqvist M, Nygren P, Jonsson BH, Broo K. Induction of structure and function in a designed peptide upon adsorption on a silica nanoparticle. Angewandte chemie (international ed. in English) 2006,45:8169-8173.
123. Wei Y, Hecht MH. Enzyme-like proteins from an unselected library of designed amino acid sequences. Protein engineering, design and selection 2004,17:67-75.
124. Taylor PW, King RW, Burgen ASV. Kinetics of complex formation between human carbonic anhydrases and aromatic sulfonamides. Biochemistry 1970,9:2638-2645.
125. Boriack PA, Christianson DW, Kingery-Wood J, Whitesides GM. Secondary interactions significantly removed from the sulfonamide binding pocket of carbonic anhydrase II influence inhibitor binding constants. Journal of medical chemistry 1995,38:2286-2291.
126. Jude KM, Banerjee AL, Haldar MK, et al. Ultrahigh resolution crystal structures of human carbonic anhydrases I and II complexed with "two-prong" inhibitors reveal the molecular basis of high affinity. Journal of the American Chemical Society 2006,128:3011-3018.
127. Campbell ID, Lindskog S, White AI. A study of the histidine residues of human carbonic anhydrase C using 270 MHz proton magnetic resonance. Journal of molecular biology 1975,98:597-614.
128. Campbell ID, Lindskog S, White AI. A study of the histidine residues of human carbonic anhydrase B using 270 MHz proton magnetic resonance. Journal of molecular biology 1974,90:469-489.

60
129. King RW, Burgen ASV. Kinetic aspects of structure-activity relations: the binding of sulphonamides by carbonic anhydrase. Proceedings of the royal society of London B 1976,193:107-125.
130. Tweedy NB, Nair SK, Paterno SA, Fierke CA, Christianson DW. Structure and energetics of a non-proline cis-peptidyl linkage in a proline-202 −> alanine carbonic anhydrase II variant. Biochemistry 1993,32:10944-10949.
131. Greenwood B, Mutabingwa T. Malaria in 2002. Nature 2002,415:670-672.
132. Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 2005,434:214-217.
133. Breman JG. The ears of the hippopotamus: Manifestations, determinants, and estimates of the malaria burden. American journal of tropical medicine and hygiene 2001,64:1-11.
134. Madigan MT, Martinko JM. Brock biology of microorganisms. eleventh ed. Upper Saddle River: Pearson Prentice Hall; 2006.
135. Biagini GA, O´Neill PM, Nzila A, Ward SA, Bray PG. Antimalarial chemotherapy: Young guns or back to the future? TRENDS in parasitology 2003,19:479-487.
136. Gardner MJ, Hall N, Fung E, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 2002,419:498-511.
137. Reungprapavut S, Krungkrai SR, Krungkrai J. Plasmodium falciparum carbonic anhydrase is a possible target for malaria chemotherapy. Journal of enzyme inhibition and medicinal chemistry 2004,19:249-256.
138. Sein KK, Aikawa M. The pivotal role of carbonic anhydrase in malaria infection. Medical hypotheses 1998,50:19-23.
139. Krungkrai SR, Suraveratum N, Rochanakij S, Krungkrai J. Characterisation of carbonic anhydrase in Plasmodium falciparum. International journal for parasitology 2001,31:661-668.
140. Krungkrai J, Scozzafava A, Reungprapavut S, et al. Carbonic anhydrase inhibitors. Inhibition of Plasmodium falciparum carbonic anhydrase with aromatic sulfonamides: Towards antimalarials with a novel mechanism of action? Bioorganic and medicinal chemistry 2005,13:483-489.
141. Huang S, Xue Y, Sauer-Eriksson E, Chirica L, Lindskog S, Jonsson BH. Crystal structure of carbonic anhydrase from Neisseria gonorrhoeae and its complex with the inhibitor acetazolamide. Journal of molecular biology 1998,283:301-310.
142. Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic acids research 1994,22:4673-4680.

61
143. Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis 1997,18:2714-2723.
144. Sayers JR, Price HP, Fallon PG, Doenhoff MJ. AGA/AGG codon usage in parasites: Implications for gene expression in Escherichia coli. Parasitology today 1995,11:345-346.
145. Mehlin C, Boni E, Buckner FS, et al. Heterologous expression of proteins from Plasmodium falciparum: Results from 1000 genes. Molecular & biochemical parasitology 2006,148:144-160.