Economics, Formulation Techniques and Properties of Biodiesel: A Review
-
Upload
universal-journal-of-environmental-research-and-technology -
Category
Documents
-
view
217 -
download
0
Transcript of Economics, Formulation Techniques and Properties of Biodiesel: A Review
-
8/4/2019 Economics, Formulation Techniques and Properties of Biodiesel: A Review
1/11
-
8/4/2019 Economics, Formulation Techniques and Properties of Biodiesel: A Review
2/11
Universal Journal of Environmental Research and Technology
125
Mathur Y. B. et al.
vegetable oils used for biodiesel are mainlyRapeseed or Sunflower oil in Europe, the USA andCanada uses Soyabean, Rapeseed, other waste oilsand fats; frying oil and animal fat is the chosenoption in Ireland; Castor oil and Soyabean oil isused in Brazil; Coconut oil is preferred in Malaysia
and Philippines; Palm oil in Thailand, Malaysia,Indonesia and the Philippines; Cotton Seed oil inGreece; Linseed and Olive oil in Spain; Jatrophaand Karanja in used in India, Nicaragua and Africato produce biodiesel (Frank et al., 2009; Altin etal., 2001, Arjun et al., 2008). Several other non-edible plants such as Neem ( Azadirachta Indica),Meswak (Salvadora Species), Mahua (Madhucaindica), Rubber (Hevea Species), Castor (Ricinuscommunis), Diploknema Butracea, Garcinia Speciesand Thumba (Citrullus colocynthis) can also beused for producing biofuels in India (Barnwal and
Sharma, 2005; Mohibbe et al., 2005; Augustus etal., 2003).
1.3 Biodiesel as Fuel for Diesel Engines:Biodiesel, the renewable liquid fuel produced frombiological raw material is a good substitute forpetroleum diesel. Biodiesel contains no petroleum,but it can be blended at any level with petroleumdiesel to create a biodiesel blend. It can be used incompression ignition engines with little or nomodifications. As an alternative fuel, biodiesel canprovide almost same power output as of diesel
(Gumus and Kasifoglu, 2010; Zafer and Mevlut,2008; Christian et al., 2009, Pall et al., 2009). Theconversion process of vegetable oil into biodieselis quite efficient, in nearly one-to-one ratio.Among the many advantages of biodiesel fuelwhich includes its safe use in all conventionaldiesel engines, offers almost the sameperformance and engine durability as conventionaldiesel fuel, non-toxic and reduces tailpipeemissions, visible black smoke and noxious fumesand odours and higher cetane number whichimprove the combustion characteristics. However
NOX emission of biodiesel increases because ofrapid combustion and other fuel characteristics.Diesel fuel blends with biodiesel have superiorlubricating properties with reduced wear and tearon the diesel engine components. The mostcommon diesel fuel blends are B10 containing 10percent biodiesel and B20 containing 20 percentbiodiesel (Sirisomboon et al., 2007; Nag et al.,1995).
Lot of research work is being done to analyse theengine combustion and performance using straight
vegetable oils as well as biodiesel but fewerresearchers have analysed and compiled the
economics of biodiesel, its formulation techniquesand fuel related properties in recent past. In thispaper, a detail survey of literature is thereforeundertaken to comprehensively review thedifferent recently published research papers oneconomical viability, conversion of straight
vegetable oil into biodiesel and combustion relatedproperties of biodiesel fuel for their utilization indiesel engines.
2.0 Economics of Biodiesel:Economical feasibility of biodiesel depends on theprice of the crude petroleum and the cost oftransporting diesel through long distances. It iscertain that the cost of crude petroleum is boundto increase due to increase in its intensivedemand, limited supply, strict regulations on thearomatics and sulphur contents in diesel fuels
which will result in higher cost of production ofdiesel fuels as removal of aromatics from thedistillate fractions requires capital-intensiveprocessing equipments. The major economicfactor to consider for input costs of biodieselproduction is the feedstock (price of seed, seedcollection and oil extraction, transport of seed andoil), which is about 7580% of the total operatingcost. Other important cost related factors arelabour, methanol and catalyst for biodieselconversion for straight vegetable oil, which mustbe added to the feedstock. Cost recovery will be
through sale of oil cake and of glycerol (Mulugetta,2009). The volatile oil prices due to increaseddemand have necessitated for continuous researchand development into the biodiesel sector so as toincrease the production of biodiesel of suitablequality and at reasonable price so that it cancompete with diesel fuel. Between 2001 and 2006alone, the global annual production of biodieselgrew up by 43% (Victor et al., 2010).
India has rich and abundant forest resources witha wide range of plants and oilseeds. The
production of these oilseeds can be stepped upmany folds for producing diesel fuels. Economicalfeasibility of biodiesel depends on the price of thecrude petroleum and the cost of transportingdiesel to long distances to remote markets in India.Further, the strict regulations on the aromaticsand sulphur contents in diesel fuels will result inhigher cost of production of conventional dieselfuels. The reason for high biodiesel prices are thelimited availability of biodiesel feedstocks. Thebiodiesel program in any country has a time lagbetween policy planning and actual
implementation and hence the introduction couldbe gradual, gaining the maturity only after 4-5
-
8/4/2019 Economics, Formulation Techniques and Properties of Biodiesel: A Review
3/11
Universal Journal of Environmental Researc
years. This is especially applicablebiodiesel is proposed to be made foils. Presently, the availability of tlimited and the price of such oilspetro-diesel. For successful launavailability of oil on large scale has
at reasonable price and abundancbiodiesel feedstocks. Palm oil andoil are the main options thinternationally. The costs for biodifrom palm oil, soybean oil and Jestimated about Rs 45.0 /litre, Rs.39.0 /litre, respectively (Demirbas,sources in India, estimate cufinished production costs at anywh32.0 to 45.0 per litre (Joseph, 2006;The Ministry of Petroleum an
announced the biodiesel purcOctober 2005. The Policy propurchase of biodiesel at 20 specentres in 12 different states in I/litre (inclusive of taxes/duties) froThis price was subject to review eThe last price revision of biodiesDecember 2006, when it was fixed(Altenburg et al., 2008). The Indialso announced, on 23rd Deattractive incentives to encoplantation in wastelands and to ut
bio-mass feed stocks for productioaddresses the issues across the enfrom plantations, and processingbiofuels. Indias new policy onblending at least 20 percent biofupetrol by 2017. This implies that 13of biodiesel will be required. Danalysis of vegetable oil based biwas done by Dorado et al. (2006).that the price of the feedstockmost significant factors. Also, glycto be a valuable by-product that c
final manufacturing costs of the6.5% depending on the raw feedstother researchers (Prueksakorn etal., 2006; Zhou and Thomson, 20Govindasamy et al., 2009; Wu etet al., 2009; Comporn andHuanguang et al., 2010; Foidl et alal., 1999) stated the possibilitiesof energy crops and biodiesel inJapan, Thailand, China, and India.
and Technology
126
Mathur Y. B. et al.
to India whererom non-edibleese oils is veryis higher than
h of biodieselo been assured
e availability ofefined soybeant are traded
esel productionatropha oil are4.0/litre and Rs2009). Industryrent biodieselere between RsSingh, 2009).
Natural Gas
ase policy invided for thecified purchasendia at Rs 25.0
January 2006.ery six months.
el was done inat Rs 26.50/litrean governmentcember 2009,rage biofuelsilise indigenous
n of biofuels. Ittire value chaino marketing ofiofuels targetsls in diesel and.38 million tonsetail economicofuels in SpainThey identifiedas one of the
erol was founduld reduce the
process up tock used. Someal., 2010; Hill et09; Ben, 2009;l., 2009; Naoko
Shabbir, 2009;., 1996; Bona etf developmenturope, Mexico,
3. Fuel Formulation TeOne of the main problemsdiesel engine is their highbecause of heavier triglyceridue to which problems o
atomization, ring-sticking, cpiston, cylinder head, ringvegetable oils are not suitaengines; since they have totheir combustion relatedmineral diesel. This fuelaimed at reducing the viscoof flow/ atomization relatpyrolysis, dilution/ blendingand transesterification artechniques available to overelated issues associated wiin diesel engine and to mwith the hydrocarbon-base2007; Bajpai and Tyagi, 2006The petro-diesel moleculesbranched molecules withbetween 12 and 19 whereusually triglycerides generabranched chains of differemixture of organic compsimple straight chain costructure of proteins and fatypical molecular structu
molecule is shown in Figurerepresent hydrocarbon chachemical composition of difpresented in Table 1.
Figure 1: Molecular Str
Vegetable Oil
hniques:of vegetable oil use iner kinematic viscositydes and phospholipids,ccur in pumping and
arbon deposits on thegrooves, etc. Straightble as fuels for dieselbe modified to bringproperties closer toodification is mainly
sity in order to get ridd problems. Heating/, micro-emulsification,
some well knownrcome higher viscosityh use of vegetable oilake them compatiblediesel fuels (Agarwal,
; Sharma, 2009).
re saturated and non-arbon atoms ranging
as, Vegetable oils arelly with a number oft lengths and are theounds ranging from
pound to complex-soluble vitamins. The
re of vegetable oil
1, where R1, R2 and R3in of fatty acids. Theerent vegetable oils is
cture of a Typical
olecule
-
8/4/2019 Economics, Formulation Techniques and Properties of Biodiesel: A Review
4/11
Universal Journal of Environmental Research and Technology
127
Mathur Y. B. et al.
Table 1: Fatty acid Composition of Some Vegetable Oils
Vegetable
Oils
Fatty Acids Composition (%)
14:0 16:0 18:0 18:1 18:2 18:3 20:0 22:0 22:1 24:0
Jatropha - 12-17 6.70 37-63 19-41 - - - - -
Karanja - 3.7-7.9 2.4-8.9 44.5-71.3 10.8-18.3 - - - - 1.1-3.5
Rapeseed - 3 1 64 22 8 - - - -
Neem 0.2-0.26 13.6-16.2 14.4-24.1 49.1-61.9 2.3-15.8 - 0.8-3.4 - - -
Sunflower - 9 2 12 78 - - - -
Soyabean - 6-10 2-5 20-30 50-60 5-11 - - - -
Corn 1-2 8-12 2-5 19-49 34-62 Traces Traces - - -
Peanut - 11 2 48 32 1 1 2 - 1
Mahua 16-28.2 20-25.1 41-51 8.9-13.7 - 0-3.3 - - -
Rice Bran 0.4-0.6 11.7-16.7 1.7-2.5 39.2-43.7 26.5-35.1 - 0.4-0.6 - - 0.4-0.9
Palm 1.5 43 5 40 10 - 0.5 - - -
Castor - - 2-3 3-5 3-5 80-90 - - - -
Olive - 9-10 2-3 73-84 10-12 Traces - - - -Tallow 3-6 24-32 24-31 37-43 2-3 - - - -
3.1 Heating/ Pyrolysis:
Heating/ Pyrolysis is the process in which highmolecular weight compound breaks in to smallercompounds by means of heat with or withoutcatalyst. Pyrolysis refers to a chemical changecaused by the application of heat energy in theabsence of air or oxygen. The liquid fractions ofthe thermally decomposed vegetable oils are likelyto get converted into liquid oils. Many
investigators have studied the Pyrolysis oftriglycerides to obtain products suitable for dieselengines (Babu, 2008; Bridgwater, 2003). ThePyrolyzate oils have almost same viscosity, flashpoint, and pour point compared to diesel fuel withequivalent calorific value. The cetane number ofthe Pyrolyzate oil has been found to be lower. ThePyrolyzate oils from vegetable oils containacceptable sulphur content, water and sedimentand give acceptable copper corrosion values butunacceptable ash and carbon residue. Mechanismsfor the thermal decomposition of triglycerides are
likely to be complex because of many structuresand multiplicity of possible reactions of mixedtriglycerides (Manurung et al., 2009).
3.2 Dilution/ Blending:
High viscosity fuels like vegetable oils can be mixedwith low viscosity fuel like diesel to overcomeoverall viscosity. These blends can then be used asdiesel engine fuels. Dilution of vegetable oils canbe accomplished with a solvent, methanol orethanol. Vegetable oil can be directly mixed withdiesel and may be used to run diesel engines.
Blending of vegetable oil with diesel have beenexperimented successfully by many researchers.
The dilution of Sunflower oil with diesel fuels inthe ratio of 1:3 by volume has been studied andengine tests were carried out by Ziejewski et al.(1983). They concluded that the blend could notbe recommended for long term use in the directinjection diesel engines due to density difference.
Pryor et al. (1983) had conducted the short term
and long term performance tests with blends ofvegetable oil with diesel. In short termperformance test, crude-degummed Soybean oiland Soybean ethyl ester were found suitablesubstitutes for diesel fuel. A longer termevaluation of the engine when using 100% crudesoybean oil was prematurely terminated severeinjector chock led to decreases in power outputand thermal efficiency. Parmanik (2003) studiedthe properties and use of Jatropha oil and dieselfuel blends in compression ignition engine. Theheating value of the vegetable oil was comparable
with ordinary diesel fuel and cetane number wasslightly lower than diesel oil. He also studied theeffect of blending Jatropha oil with diesel fuel incompression ignition engines. Significantimprovement in engine performance was observedas compared to pure vegetable oil. The exhaust gastemperature was reduced due to reduced viscosityof the vegetable oil diesel blends. It was found thatthe fuel consumption was increase with a higherproportion of the Jatropha oil in the blends.Acceptable thermal efficiencies of the engine wereobtained with blends containing up to 50% (by
volume) of Jatropha oil.
-
8/4/2019 Economics, Formulation Techniques and Properties of Biodiesel: A Review
5/11
Universal Journal of Environmental Researc
The tests were conducted by Foron a single-cylinder direct-injoperated on diesel fuel, Jatropha oidiesel and Jatropha oil in propor2.6%; 80%/ 20%; and 50%/ 50%test results showed that Jatrop
conveniently used as a diesel substengine. The test results further shobrake thermal efficiency, brakreduction of specific fuel consumptoil and it blends with diesel.
3.3 Micro-Emulsification:
A micro-emulsion is a system condispersed, with or without an eimmiscible liquid, usually in droplcolloidal size. Micro-emulsions areor translucent thermodyna
dispersions of oil, water, surfactasmall amphiphilic molecule, calleThe droplet diameters in micro-efrom 100 to 1000 . A micro-emade of vegetable oils withdispersant (co-solvent), or of vegetalcohol and a surfactant and a cwith or without diesel fuels.because of their alcohol contevolumetric heating values than diesalcohols have high latent heat of vtend to cool the combustion c
would reduce nozzle choking. A mimethanol with vegetable oils canas well as diesel fuels. Ziejewskshowed that the engine performafor a micro-emulsion of 100 % suthe 25 % blend of sunflower oil in d3.4 Transesterification Process:
The major problems associated wivegetable oil operation in diesel eto their high fuel viscosity andTransesterification of vegetable o
significant reduction in viscoenhancing the physical and chemiof vegetable oil to improveperformance (Tapanes et al., 2008ethyl esters of vegetable oils (callerevealed fuel properties similar ttransesterification process involtriglycerides, present in vegetaalcohols such as methanol or epresence of a catalyst (usually sodiat about 70C to give the este
and Technology
128
Mathur Y. B. et al.
on et al.(2004)ection enginel and blendes ofions of 97.4%/y volume. The
ha oil can be
itute in a dieseled increases in
e power andion for Jatropha
sists of a liquidulsifier, in an
ts smaller thenisotropic, clear,ically stable
t, and often aco-surfactant.
mulsions rangeulsion can be
an ester andble oils with antane improver,icro-emulsions,t have lower
el fuels, but theaporization andhamber, which
cro-emulsion ofperform nearlyi et al. (1984)nce were samenflower oil andiesel.
h the straightgines are dueoor volatility.ils provides a
ity, therebycal propertiesthe engine
). Methyl andd as biodiesel)
diesel. Thelves reactingle oils withhanol in the
um hydroxide)and the by
product, glycerine andreported that the methylvegetable oil can result in sthan neat vegetable oils. Bicontain oxygen, which muststorage problems (Jon, 20
2009; Vivek and Gupta, 20The transesterification reactthe general equation
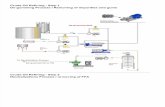
The base catalysed progenerally occur using the fo
The first step is the converdiglycerides, followed bydiglycerides to monoglycerito glycerol, yielding onefrom each glyceride
ater. It has beenand ethyl esters ofuperior performancediesel fuels naturallybe stabilized to avoid05; Patil and Deng,
4; Jha et al., 2007).ion is represented by
uction of biodiesel,llowing steps:
ion of triglycerides tothe conversion of
es and of diglyceridesethyl ester molecule
at each step.
-
8/4/2019 Economics, Formulation Techniques and Properties of Biodiesel: A Review
6/11
Universal Journal of Environmental Researc
Figure 2: Tr
Meher et al. (2006) studied the efconcentration, alcohol/oiltemperature, and rate oftransesterification of Karanja oilThey found that the optimum reafor methanolysis of Karanja oilcatalyst, MeOH/oil molar ratiotemperature 65C and rate of m
rpm for a period of three hrs. Theesters was found to be higher thaminutes and reaction was almost chours with a yield of 97 %. With 12:NaOH/oil or higher, the reactionwithin one hr. The reaction was inlow rate of stirring (180 rpm).optimization study, Meher et al. (2the yield of methyl ester from Kathe optimal condition was 97-98%.
Rathore and Madran (2008) studie
transesterification of Karanja oil intin supercritical methanol and eusing any catalyst. The effect ofreaction temperature on alkyl ewas investigated. A micro-emulsiwith vegetable oils can perform ndiesel fuels. Darnoko and Cheryanexperimental data on Palm oilobserved that the rate of alkali-transesterification in a batch rewith temperature up to 60C. Furtemperatures did not reduce the ti
maximum conversion.
and Technology
129
Mathur Y. B. et al.
ansesterification Process for Biodiesel Production
ects of catalystmolar ratio,
mixing onwith methanol.tion conditionsas 1% KOH as
6:1, reactionixing was 360
yield of methyl85% in fifteen
omplete in two1 molar ratio ofwas completedomplete with aFurther in an
006) found thatranja oil under
the kinetics of
o its alkyl estersthanol without
olar ratio andters formationn of methanol
early as well as(2000) reportedinetics. It wasatalyzed (KOH)ctor increasedher increase in
me to reach the
The free fatty acid and mstarting material are thedetermining the viabilitytransesterification process.et al. (1984) the free fatty alower than 1% to carry oreaction. In their study, theythe acid value was greater t
required to neutralize the falso caused soap formationcatalyst and reduced catresulting soaps caused anformation of gels and resultof glycerol quite difficult. Mthe effects of free fatty acidtransesterification of beef twater had more ntransesterification than frconcluded that for best resand free fatty acids content
be kept below 0.06 %respectively.
Zullaikah et al. (2005) hbiodiesel from Rice Bran oacids content. A twomethanolysis process waefficient conversion of Ricemethyl esters. Hawash ettransesterification of Jsupercritical methanol in tunder different temp
Ramadhas et al. (2004) repcatalyst followed by alkaliprocess using Rubber seed
isture content in thekey parameters for
of the vegetable oilccording to Freedman
cid quantity should bet the alkali catalysedhave observed that ifan 1, more NaOH was
ree fatty acids. Water, which consumed thealyst efficiency. Theincrease in viscosity,
ed into the separationa et al. (1999) studieds and water content inllow. The presence ofgative effect on
ee fatty acids. Theylts, the water contentin beef tallow should
/w and 0.5 % w/w
d successfully madeil with high free fatty-step acid-catalyzed
employed for theBran oil into fatty acidal. (2009) studied theatropha oil usinge absence of catalysterature conditions.
orted the use of acidcatalyst in a singleil with high free fatty
-
8/4/2019 Economics, Formulation Techniques and Properties of Biodiesel: A Review
7/11
Universal Journal of Environmental Research and Technology
130
Mathur Y. B. et al.
acids content. The objective of this study was todevelop a process for producing biodiesel from alow-cost feedstock like crude Rubber seed oil. Isoet al. (2001) have studied the transesterification byimmobilized lipase in non- aqueous conditions.Noureddini et al. (2005) have investigated the
biodiesel production by lipase catalyst. The timetaken to get the 67 % yield of biodiesel was 72hours at room temperature. However, the energyinput was zero. The reaction time and the cost oflipase were hurdles to commercialize lipaseprocesses.
Table 2: Fuel Related Properties of Biodiesel Produced From Different Vegetable Oils
Biodiesel Kinematic
viscosity
(cSt, 40C)
Density
(kg/m3)
Heating
Value
(MJ/kg)
Cloud
point
(C)
Pour
point
(C)
Flash
point
(C)
Cetane
Number
Carbon
Residue
(w/w)
Jatropha 5.65 879 38.5 13 - 175 50 -Karanja 6.87 897 37.9 - -1 187 49 0.05Rapeseed 7.2 883 37.37 - -12 - 51 -Neem 15 882 38.5 - - 180 47 -Sunflower 4.6 868 40.58 1 - 183 45-52 -Soyabean 4.5 872 39.76 1 -7 178 37-45 1.7
Coconut 3.36 866 36.1 - -4 122 56 0.03Peanut 4.9 883 - 5 - 176 54 -Palm 5.7 880 - 13 - 164 62 -Babassu 3.6 879 - 4 - 127 63 -Thumba 3.83-5.86 883 39.37 .5 - 142 53 -Tallow - - - 12 9 96 -- -
4. Properties of Biodiesel:Biodiesel is a better fuel than diesel fuel in termsof sulphur content, flash point, and aromaticcontent. The fuel characteristics of biodiesel areclose to diesel fuels, and therefore biodiesel
becomes a strong alternative to replace the dieselfuels (Demirbas, 2009). The conversion oftriglycerides into methyl or ethyl esters throughthe transesterifcation process reduces themolecular weight to one-third that of thetriglyceride reduces the viscosity by a factor ofabout eight and increases the volatility marginally.Biodiesel has viscosity close to diesel fuels. Theseesters contain 10 to 11% oxygen by weight, whichmay improve combustion as compared tohydrocarbon based diesel fuels in an engine (Ejazand Younis, 2008). The cetane number of
biodiesel is around 50 which are higher thandiesel. The use of tertiary fatty amines and amidescan be effective in enhancing the ignition qualityof the diesel fuel without having any negativeeffect on its cold flow properties. Since thevolatility increases marginally, the startingproblem persists in cold conditions. Biodiesel haslower volumetric heating values (about 12%) thandiesel fuels but has a high cetane number andflash point (Sylvain et al., 2009; Achten et al.,2008; Houfang et al., 2009) Some of the desirablefuel properties of biodiesel derived from differentvegetable oils are presented in Table 2.5.
5.0 Conclusions:Many energy fuels are being recently investigatedas potential substitutes for the current high-pollutant diesel fuel derived from diminishingcommercial sources. In the past 15 years, biodiesel
has progressed from the research stage to a full-production scale in many developed countries.Vegetable oil either from seasonal plant crops orfrom perennial forest trees origin, after beingformulated, have been found suitable forutilization in diesel engines. Many traditional seedoils like Pongamia Glabra, Jatropha (JatrophaCurcas), Mallous Philippines, Garcinia Indica,Thumba, Karanja and Madhuca Indica etc. areavailable in the country, which can be exploited forbiodiesel production purposes. Although biodieseloffers several advantages, including technical,
environmental, and socio-economic, it has somedisadvantages. Major disadvantages of biodieselare higher viscosity, lower energy content, highercloud point and pour point, marginal increase innitrogen oxides (NOX) emissions, lower enginespeed and power, engine oil degradation, injectorcoking, engine compatibility, high price ofbiodiesel and higher engine wear. Importantoperating disadvantages of biodiesel incomparison with petro-diesel are cold startproblems, the lower energy content, higher copperstrip corrosion and fuel pumping difficulty. Someof the salient conclusions are drawn on the basisof available literature for economics of bio-diesel,
-
8/4/2019 Economics, Formulation Techniques and Properties of Biodiesel: A Review
8/11
Universal Journal of Environmental Research and Technology
131
Mathur Y. B. et al.
its formulation techniques and combustion relatedproperties:1.The costs for biodiesel production from palm oil,
soybean oil and Jatropha oil are estimated aboutRs 45.0 /litre, Rs. 44.0/litre and Rs 39.0 /litre,respectively.
2.The major economic factor to consider for inputcosts of biodiesel production is the feedstock(price of seed, seed collection and oil extraction,transport of seed and oil), which is about 75-80% of the total operating cost.
3.Other important cost related factors are labor,methanol and catalyst for biodiesel conversionfor straight vegetable oil, which must be addedto the feedstock. Cost recovery will be throughsale of oil, cake and of glycerol.
4.Mechanisms for the thermal decomposition(Pyrolysis) of for biodiesel production are likely
to be complex because of many structures andmultiplicity of possible reactions of mixedtriglycerides.
5.Direct blending of vegetable oil with diesel couldnot be recommended for long term use in thedirect injection diesel engines due to densitydifference.
6.A micro-emulsion of methanol with vegetableoils can perform nearly as well as diesel fuels.
7.Transesterification of vegetable oils provides asignificant reduction in viscosity, therebyenhancing the physical and chemical properties
of vegetable oil to improve the engineperformance.
8.The fuel properties of biodiesel are close todiesel fuels, and therefore biodiesel becomes astrong alternative to replace the diesel fuel.
References:1. Achten, W. M. J., Verchot, L., Franken, Y. J.,
Mathijs, E., Singh, V. P., Aerts, R. and Muys,B.(2008): Review: Jatropha BiodieselProduction and Use. J. Biomass and Bioenergy,32: 1063-1084.
2. Agarwal, Avinash (2007): Biofuels (Alcoholsand Biodiesel): Applications as Fuels forInternal Combustion Engines. Progress inEnergy and Comb. Sci., 33: 233271.
3. Altenburg, Tilman, Hildegard, Dietz, Matthias,Hahl, Nikos, Nikolidakis, Christina, Rosendahland Kathrin, Seelige (2008): Biodiesel Policiesfor Rural Development in India: A Report.D.I.A, German Development Institute, Bonn,27th May, 2008. 15-48.
4. Altin, R., Cetinkaya, S. and Yucesu, H. S.(2001): The Potential of Using Vegetable Oil
Fuels as Fuel for Diesel Engines. J. Energy Con.Manag., 42: 529538.
5. Arjun, B., Chhetri, Martin S., Tango, SuzanneM., Budge, K, Chris, Watts and Rafiqul, IslamM. (2008): Non-Edible Plant Oils as NewSources for Biodiesel Production. Int. J.Molecular Sci., 9:169-180.
6. Augustus, G. D. P. S., Jayabalan, M. and Seiler,G. J. (2003): Alternative Energy Sources fromPlants of Western Ghats (Tamil Nadu, India). J.Biomass and Bioenergy, 24: 437-444.
7. Babu, B. V. (2008): Biomass Pyrolysis: a State-of-the-Art Review. J. Biofuels and Bioproducts,2:393414.
8. Baiju, B., Naik, M. K. and Das, L. M. (2009): AComparative Evaluation of CompressionIgnition Engine Characteristics Using Methyland Ethyl Esters of Karanja Oil. J. Ren. Energy,34; 1616-1621.
9. Bajpai, D. and Tyagi, V. K. (2006): Bio diesel:Source, Production, Composition, Propertiesand its Benefits. J. Oleo Sci., 55: 437-502.
10. Banapurmath, N. R., Tiwari, P. G. andHosmath, R. S. (2008): Performance andEmission Characteristics of a DI CompressionIgnition Engine Operated on Honge, Jatrophaand Sesame Oil Methyl Esters. J. Ren. Energy,33:1982-1988.
11. Barnwal, B. K. and Sharma, M. P. (2005):Prospects of Biodiesel Production fromVegetable Oils in India. J. Ren. and Sust.Energy Rev., 9: 363378.
12. Beepanraj, B. and Lawrence, P. (2011):Performance and Emission Characteristics of aDiesel Engine Fuelled With Palm Oil MethyelEster and Its Blends. J. Tech. Word, III:197-203
13. Ben, Phalan (2009): The Social andEnvironmental Impacts of Biofuels in Asia: AnOverview. J. App. Energy, 86: S21S29.
14. Bona, S., Mosca, G. and Vamerali, T. (1999):Oil Crops for Biodiesel Production in Italy. J.Ren. Energy, 16:1053-1056.
15. Bridgwater, A.V. (2003): Renewable Fuels andChemicals by Thermal Processing of Biomass.
J. Chemical Engg., 91: 87102.16. Christian, Rodriguez Coronado, Joao, Andradede Carvalho Jr., Juliana, Tiyoko Yoshioka andJose, Luz Silveira (2009): Review,Determination of Ecological Efficiency inInternal Combustion Engines: The Use ofBiodiesel. J. App. Thermal Engg., 29: 18871892.
17. Comporn, Pleanjai and Shabbir, Gheewala H.(2009): Full Chain Energy Analysis of BiodieselProduction from Palm Oil in Thailand. J. App.Energy, 86: S209S214.
18. Darnoko, D. and Cheryan, M. (2000): Kineticsof Palm Oil Transesterification in a Batch
-
8/4/2019 Economics, Formulation Techniques and Properties of Biodiesel: A Review
9/11
Universal Journal of Environmental Research and Technology
132
Mathur Y. B. et al.
Reactor. J. American Oil and Chemist Soci., 77:1263-1267.
19. Demirbas, Ayhan (2009): Progress and RecentTrends in Biodiesel Fuels. J. Energy Con. andManag., 50, 2009:1434.
20. Demirbas, Ayhan (2009): Political, Economicand Environmental Impacts of Bio fuels: AReview. J. App. Energy, 86: S108S117.
21. Dileep, K., Birur, K., Thomas, Hertel W. andWallace, Tyner E. (2007): Impact of BiofuelProduction on World Agricultural Markets: AComputable General Equilibrium Analysis.Tenth Annual Conference on Global EconomicAnalysis, Purdue University, West Lafayette,USA, 7th -9th June, 2007.
22. Dorado, M. P., Cruz, F., Palomar, J. M. andLopez, F. J. (2006): Data bank: An Approach tothe Economics of Two Vegetable Oil-Based
Biofuels in Spain. J. Ren. Energy, 31,2006:12311237.
23. Ejaz, Shahid M. and Younis, Jamal (2008): Areview of Biodiesel as Vehicular Fuel. J. Ren.and Sust. Energy Rev., 12: 2484249.
24. Foidl, N., Foidl, G., Sanchez, M., Mittelbach,M. and Hackel, S. (1996): Jatropha Curcas L. asa Source for the Production of Biofuel inNicaragua. J. Bioresource Tech., 58: 77-82.
25. Frank, Rosillo-Calle, Luc, Pelkmans andArnaldo, Walter (2009): A Global Overview ofVegetable Oils, with Reference to Biodiesel. A
Report for the IEA Bioenergy, U.S.A, 2009. 12-22.
26. Forson, F. K., Oduro, E. K. and Donkoh,Hammond E. (2004): Performance of JatrophaOil Blends in a Diesel Engine. J. Ren. Energy,29:1135-1145.
27. Freedman, B., Pryde, E. H. and Mounts, T. L.(1984): Variables Affecting the Yield of FattyEsters from Transesterified Vegetable Oils. J.American Oil and Chemist Soci., 61:1638-1643.
28. Gerhard, Knothe, Jon, Gerpen Van and Jurgen,Krahl (2005): The Biodiesel Handbook. AOCSPress, Champaign, Illinois, United States ofAmerica. 1-218.
29. Govindasamy, Agoramoorthy, Minna, Hsu J.,Chaudhary, Sunita and Po-Chuen Shieh (2009):Can Biofuel Crops Alleviate Tribal Poverty inIndias Dry lands?. J. App. Energy, 86:S118S124.
30. Gumus, M. and Kasifoglu, S. (2010):Performance and Emission Evaluation of aCompression Ignition Engine Using a Biodiesel(Apricot Seed Kernel Oil Methyl Ester) and itsBlends with Diesel Fuel. J. Biomass andBioenergy, 34: 134139.
31. Hawash, S., Kamal, N., Zaher, F., Kenawi, O.and Diwani G. El. (2009): Biodiesel Fuel fromJatropha Oil Via Non-Catalytic SupercriticalMethanol Transesterification. Fuel, 88: 579-582.
32. Hill, J., Nelson, E., Tilman, D., Polasky, S. andTiffany, D. (2006): Environmental, Economicand Energetic Costs and Benefits of Biodieseland Ethanol Blends. Proce. Natural andAcademic Sci., 103:11206-11210.
33. Hossain, A. K. and Davies, P. A. (2010): PlantOils as Fuels for Compression Ignition Engines:A Technical Review and Life-Cycle Analysis. J.Ren. Energy, 35: 113.
34. Houfang, Lu, Yingying, Liu, Hui, Zhou, Ying,Yang, Mingyan Chen and Bin, Liang (2009):Production of Biodiesel from Jatropha CurcasOil. J. Computers and Chemical Engineering,
33:10911096.35. Huanguang, Qiu, Jikun, Huang, Jun, Yang,
Scott, Rozelle, Yuhua, Zhang, Yahui, Zhang andYanli, Zhang (2010): Bio ethanol Developmentin China and the Potential Impacts on itsAgricultural Economy. J. App. Energy, Volume87:7683.
36. Iso, M., Chen, B., Eguchi, M., Kudo, T. andShrestha (2001): Production of Biodiesel Fuelby Triglycerides and Alcohol UsingImmobilized Lipase. J. Molecular Catalysis andEnzymatic, 53:53-58.
37. Jha, M. K., Gupta, A. K. and Kumar, Vipin(2007): Kinetics of Transesterification onJatropha Curcas Oil to Biodiesel Fuel.Proceedings of the Word Congress onEngineering and Computer Science, SenFrancisco, U.S.A., 24th -26th October, 2007.
38. Jon, Van Gerpen (2005): Biodiesel Processingand Production. Fuel Processing Tech., 86:1097 1107.
39. Joseph, Gonsalves B. (2006): An Assessment ofthe Biofuels Industry in India. United NationsConference on Trade and Development
Geneva, 18 October 2006.40. Ma, F. and Hanna, M. A. (1999): BiodiesdPrediction: A Review. J. Bioresourse Tech., 70:1-15.
41. Ma, F., Clements, L. D. and Hanna, M. A.(1998): The Effect of Catalyst, Free Fatty Acid,and Water on Transesterification of BeefTallow.Tran. ASAE, 41:1261-1264.
42. Manurung, R., Wever, D. A. Z., Wildschut, J.,Venderbosch, R. H., Hidayat, H., Van Dam, E.G., Leijenhorst, E. J., Broekhuis, A. A. andHeeres, H. J. (2009): Vaporization of JatrophaCurcas L. Plant Parts: Nut Shell Conversion toFast Pyrolysis Oil. J. Food and BioproductsProcessing, 87:187196.
-
8/4/2019 Economics, Formulation Techniques and Properties of Biodiesel: A Review
10/11
Universal Journal of Environmental Research and Technology
133
Mathur Y. B. et al.
43. Meher, L. C., Sagar, D. V. and Naik, S. N.(2006): Technical Aspect of BiodieselProduction by Transestrification: A Review. J.Ren. and Sust. Energy Rev., 10: 248-268.
44. Meher, L. C., Dharmagadda, V. S. S. and Naik,S. N. (2006): Optimization of Alkali-Catalyzed
Transesterification of Pongamta Pinnata Oilfor Production of Biodiesel. J. BioresourceTech., 7: 1392-1397.
45. Mohibbe, Azam M., Waris, Amtul and Nahar,M. N. (2005): Prospects and Potential of FattyAcid Methyl Esters of Some Non-TraditionalSeed Oils for Use as Biodiesel in India. J.Biomass and Bioenergy, 29: 293-302.
46. Mulugetta, Yacob (2009): Evaluating theEconomics of Biodiesel in Africa. J. Ren. andSust. Energy Rev., 13:15921598.
47. Nag A.N. S., Bhattacharya, M. S. and De, K. B.(1995): Utilization of Vegetable Oils. J.American Oil and Chemist Soci., 72:1591-1593.
48. Naoko, Matsumoto, Daisuke, Sano and Mark,Elder (2009): Bio fuel Initiatives in Japan:Strategies, Policies and Future Potential. J.App. Energy, 86: S69S76.
49. Noureddini, H., Gao, X. and Philkana, R. S.(2005): Immobilized Pseudomonas CepaciaLipase for Biodiesel Fuel Production fromSoybean Oil. J. Bioresource Tech., 96: 769-777.
50. Pall, Amit, Verma, Ashish, Kachhwaha, S. S.and Mali, S. (2010): Biodiesel ProductionThrough Hydrodynamic Cavitation andPerformance Testing. J. Ren. Energy, 35:619-624.
51. Parikh, J. (2005): Growing Our Own Oils.Biofuels India, 3:164-169.
52. Parmanik, K. (2003): Properties and Use ofJatropha Curcas Oil and Diesel Fuel Blends inCompression Ignition Engine. J. Ren. Energy,28: 239-248.
53. Patil, Prafulla D. and Deng, Shuguang (2009) :Optimization of Biodiesel Production from
Edible and Non-Edible Vegetable Oils. J. Fuel,88:13021306.54. Prueksakorn, Kritana, Shabbir, Gheewala H.,
Pomthong, Malakul and Sebastien, Bonnet(2010): Energy analysis of Jatropha plantationsystems for biodiesel production in Thailand.J. Energy for Sust. Develop. 2010.
55. Pryor, R. W., Manna, M. A., Schnistock, J. L.and Bashford, L. (1983): Soybean Oil Fuel in aSmall Diesel Engine. Tran. SAE, 1983: 333-342.
56. Ramadhas, A. S., Jayaraj, S. andMuraleedharan, C. (2004): BiodieselProduction from High FFA Rubber Seed Oil.Fuel, 2004.
57. Rathore, V. and Madran, G. (2008): Synthesisof Biodiesel from Edible and Non-Edible Oils inSupercritical Alcohols and Enzymatic Synthesisin Supercritical Carbon Dioxide. J. Fuel, 2008.
58. Sahoo, P.K. and Das, L. M. (2009): CombustionAnalysis of Jatropha, Karanja and Polanga
Based Biodiesel as Fuel in a Diesel Engine.Fuel, 88; 994-999.
59. Sharma, Y. C. and Singh, B. (2009):Development of Biodiesel: Current Scenario.Ren. and Sust. Energy Rev., 13:16461651.
60. Singh, Santosh (2009): Indian Bio fuels AnnualReport 2009. Global Agriculture InformationNetwork Report IN 9080, New Delhi, 15th June2009.
61. Sirisomboon, P., Kitchaiya, P., Pholpho, T. andMahuttanyavanitch, W. (2007): Physical andMechanical Properties of Jatropha Curcas L.
Fruits, Nuts and Kernels. J. Biosystems Engg,97: 201 207.
62. Srivastava, A. and Prasad, R. (2000):Triglycerides-Based Diesel Fuels. J. Ren. andSust. Energy Rev., 4:111144.
63. Sylvain, Leduc, Karthikeyan, Natarajan, Erik,Dotzauer, Ian, McCallum and Michael,Obersteiner (2009): Optimizing biodieselproduction in India. J. App. Energy, 86: S125S131.
64. Tapanes, N., Aranda, D., Carneiro, J. andAntunes, O. (2008): Transesterification of
Jatropha Curcas Oil Glycerides: Theoreticaland Experimental Studies of BiodieselReaction. Fuel, 87: 22862295.
65. Victor, Kraemer, Wermelinger Sancho Araujo,Silvio Hamacher and Luiz Felipe Scavarda(2010): Economic Assessment of BiodieselProduction from Waste Frying Oils, J. Bio-resource Tech., 2010.
66. Vivek and Gupta, A. K. (2004): BiodieselProduction from Karanja Oil. J. Scien. and Ind.Res., 63:39 47.
67. Wu, C. Z., Yin, X. L., Yuan, Z. H., Zhou, Z .Q. andZhuang, X. S. (2009): The Development of Bioenergy Technology in China. J. Energy, 4:16.
68. Xuezheng, Liang, Shan, Gao, Jianguo, Yang andMingyuan, He (2009): Highly EfficientProcedure for the Transesterification ofVegetable Oil. J. Ren. Energy, 34: 22152217.
69. Zafer, Utlu and Mevlut, Sureyya Kocak (2008):The Effect of Biodiesel Fuel Obtained fromWaste Frying Oil on Direct Injection DieselEngine Performance and Exhaust Emissions. J.Ren. Energy, 33: 19361941.
70. Zhou, Adrian and Thomson, Elspeth (2009):The Development of Biofuels in Asia. J. App.Energy, 86: S11S20.
-
8/4/2019 Economics, Formulation Techniques and Properties of Biodiesel: A Review
11/11
Universal Journal of Environmental Research and Technology
134
Mathur Y. B. et al.
71. Ziejewski, M. Z., Kaufman, K. R. and Pratt, G. L.(1983): Vegetable Oil as Diesel Fuel. USDAAgri. Rev., 28:106 -111.
72. Ziejewski, M. Z., Kaufman, K. R., Schwab, A. W.and Pryde, E. H. (1984): Diesel EngineEvaluation of a Nonionic Sunflower Oil-
Aqueous Ethanol Micro-emulsion. J. AmericanOil and Chemist Soci., 61: 1620-1626.
73. Zullaikah, S., Lai, C. C., Ramjan, S. and Ju, Y. H.(2005): A Two Step Acid Catalyzed Processfrom Rice bran Oil. J. Bioresource Tech.,96:1889-1896.
74. 4th R&D Report on Tree Born Oilseeds 2008 -2009, National Oilseed and Vegetable OilDevelopment Board, Ministry of Agriculture,Govt. of India, Gurgaon, Feburary 2009.