Defining SLE and VLE Conditions of Hydrocarbon Fluids ... Precipitation/Defining SLE and... ·...
Transcript of Defining SLE and VLE Conditions of Hydrocarbon Fluids ... Precipitation/Defining SLE and... ·...

65f
Defining SLE and VLE Conditions ofHydrocarbon Fluids Containing Wax and Asphaltenes
Using Acoustic Resonance Technology
A. Sivaraman, D.lmer, F.B. Thomas,D.B. Bennion, A.K.M. Jamaluddin.
Hycal Energy Research Laboratories Ltd.Prepared for Presentation at the 1998 Spring National Meeting
New Orleans, LA, March 8 - 12Thennodynamic and Transport Properties 2. Asphaltenes, Waxes, Gas Hydrates
Hycal Energy Research Laboratories Ltd.Copyright ~
UNPUBLISHED
February 23, 1998
AIChE shall not be responsible for statements or opinions contained in papers or printed in its
publications.

Defining SLE and VLE Conditions of Hydrocarbon FluidsContaining Wax and Asphaltenes Using Acoustic Resonance Technology
A. Sivaraman, D.lmer, F.B. Thomas, D.B. Bennion, A.K.M. JarnaluddinHycal Energy Research Laboratories Ltd.
ABSTRACT
Acoustic resonance technology (ART) is a newly developed method for the highly accurate
non-optical detennination of phase transitions in high temperature and pressure reservoir fluids. The
acoustic resonance experiments were conducted under a depressurization mode to detect the onset
of asphaltene precipitation at a fixed experimental temperature. During the depressurization runs,
acoustic features corresponding to the onset of asphaltene precipitation or solid-liquid equilibrium
(SLE) conditions were detected as the system pressures were decreased from reservoir conditions.
Subsequently, during the same run, the acoustic features were also detected that signified the vapor-
liquid (VLE) equilibrium conditions. In addition, isobaric cooling experiments were also conducted
to identify the onset of wax precipitation. The isobaric cooling experiments detected the features
corresponding to wax precipitation (SLE) conditions.
Results defining the SLE and VLE conditions of reservoir crudes containing asphaltenes and
wax are extremely promising. Comparison of the VLE data obtained using ART with that obtained
using an optical method showed excellent agreement. In addition, comparison of SLE data obtained
using ART showed excellent agreement with that obtained using a light-scattering technique where
the light scattering technique is applicable.
Keywords: Defining, Solids-Liquid Equilibrium, Vapour Liquid Equilibrium, Acoustic ResonanceTechnology, Asphaltenes, Waxes.

INTRODUCflON
Reservoir fluids containing wax and asphaltenes are common in the petroleum industry. The
presence of these materials can cause significant operational problems during the life of the
producing wells. The identification of wax and asphaltene precipitation conditions is the first step
towards applying any remedial options. Asphaltenes are dark brown to black solid compounds with
no definite melting point. They decompose while heating and leave a carbonaceous residue. They
are non-crystalline substances or mixtures of relatively high molecular weight fractions (Briant et
a1 (1983» of bitumen with characteristics of strong aromatic polar substances (Change et a1 (1993)
and Stausz et a1 (1991». Asphaltenes are defined as the n-heptane insoluble fraction of crude oil
(Speight et al (1981, 1984». They are classified by the particular solvent used to precipitate them
(Speight et at (1985». They are generally soluble in benzene and insoluble in low molecular weight
n-alkanes (Speight (1979». Asphaltene precipitation can be determined experimentally.
Waxes are defined as normal paraffins as well as other molecules containing long chain alkyl
groups usually ranging from CI8 to C60° Solid waxes crystallize or precipitate when cooled below
their cloud point temperature. The wax precipitation process is thermodynamically reversible (Reid
et at (1977». The viscosity of crude oil is increased by the presence of the wax crystals (Ronningsen
et a1 (1991)). If the temperature is reduced sufficiently, the crude can become highly viscous and
approach its pour point temperature.
Solid (waxes and asphaltenes) precipitation from reservoir fluids causes severe operational
problems in the subsurface, surface equipment, wellhead equipment, separators and tanks (Shelton
et al (1977), Leontaritis et al (1988)). Clean-up costs can be very high in offshore oil production
(Tuttle (1983».
2

There is a strong need for a good model to predict precipitation although some theoretical
models based on the principles of colloidal suspension (Espinant et a1 (1993), Novosad et a1 (1990),
Leontaritis et al (1987)), polymer solution theory (Hirschberg et al (1984), Mansoori et al (1985),
Leontaritis (1988)), and a rigorous approach based on a wax deposition model that allows a fully
compositional representation (Thomas et al (1992), Won (1989)) are found in the literature. Full
reversibility in asphaltene precipitation is always questionable. Only a limited amount of
experimental data is available for asphaltene precipitation. Fluorescence spectrometry (Wehry et al
(1966)), conductive measurements (Nighswander (1993)), fibre optics (portland (1993)) and light-
scattering techniques (Fuhr et al (1991), J amaluddin (1996)) are being used to detect solids dropout
onsets experimentally. Most of these techniques are dependent on the oil characteristics. The most
important criterion is the color of the oil. For dark-colored oil, the popular light-scattering technique
becomes inadequate. In this case, near-infrared (NIR) light spectrum is necessary to identify any
changes in the response due to a phase change phenomenon.
An emerging technology, Acoustic Resonance Technology (ARl), has been tested at Hycal
Energy Research Laboratories Ltd. to identify the onset conditions of solids precipitation. ART is
based on the measurement of the response of a fluid or fluids, contained in a cylindrical cavity, to
acoustic variable stimulation. The most powerful use of ART is to study the state and time evolution
of the resonance response of fluids under variable and well-controlled conditions of pressure, volume
and temperature (Colgate et al (1992, 1992, 1993». In these cases, one can obtain information
related to fluid phase behaviour or phase transitions and transport properties. ART offers a sensitive
and objective method for probing and measuring bulk fluid properties and processes. The system is
applicable for any type and color of oil. The basic principle behind the technique is transmitting a
3

sound wave through the fluid and changing pressure, temperature or composition conditions to
initiate asphaltene and/or wax precipitation conditions.
The acoustic resonance experiments were conducted under a depressurization mode to detect
During thethe onset of asphaltene precipitation at a fixed experimental temperature.
depressurization runs, a sharp change in acoustic features, corresponding to the onset of asphaltene
precipitation or solid-liquid-equilibrium (SLE) conditions, was detected as the system pressures were
decreased from the reservoir conditions. Subsequently, during the same run, a significant change
in acoustic features was also detected which signified vapor-liquid (VLE) equilibrium conditions.
In addition, isobaric cooling experiments were also conducted to identify the onset of wax
precipitation. The isobaric cooling experiments detected the features corresponding to wax
precipitation (SLE) conditions. Results of the depressurization and isobaric cooling experimental
runs are presented in this paper.
EXPERIMENTAL EQUIPMENT AND PROCEDURE
The heart of the ART experimental setup is a cylindrical resonator of 0.25 inches in diameter
made of Hastelloy to resist any corrosion. Acoustic stimulation is applied by a piezo-electric
element with the transmitter forming one end of the cylindrical cavity. This element vibrates in
response to an applied voltage. Another similar element, the receiver, forms the other end of the
resonator cavity and generates a voltage principally in response to the stimulated fluid oscillations.
At certain applied stimulation frequencies, standing waves (resonances) will be set up. The pattern
of these standing waves depends on the geometry of the cavity and the nature and state of the fluids.
4

The cylindrical cavity is oriented vertically so that resonance patterns will depend on the geometry
of any multiple phases present as well as the nature of their interfaces.
The volume of the cylindrical cavity resonator used in this acoustic resonance assembly is
precision variable using a piston that allows for density and pressure sweeps of the contents. The
resonator assembly is housed in a well-insulated circulating air bath, thermally controllable in both
The maintenance and sweep control of pressure, volume andisothennal and sweep modes.
temperature is supplied by precise and very stable elements (high precision strain gauge transducers
for pressure, a Linear Velocity Displacement Transducer (L VDT) for volume and a calibrated
platinum resistance themlometer for temperature) read by precision Keithley digital multimeters each
interfaced to a control computer. Also interfaced to the control computer are the pressure, volume
and temperature control elements (a Stepper motor operating the piston for pressure and volume
control, and a liquid nitrogen servo valve and heater element for temperature control). These, in
combination with the respective sensors and multimeters, provide feedback loops and are used in
the control of the instrument. The control program uses a Proportional Integral Differential type
algorithm. The custom software allows the operator to see immediately the results of any tuning
change for each control variable in real time through a graphical interface. One can control the setup
and operation of pressure, volume and temperature sweeps very precisely through the control
computer. Sweeps of anyone or a combination of two control variables can be performed over nearly
The temperature andany desired paths within the physical operating limits of the instrument.
pressure limits of the current acoustic resonance assembly are -40°C to 150°C and atmospheric to
10,000 psia, respectively.
s

A second computer controls the acoustic excitation of the resonator and acquires acoustic
response data. An interfaced function generator supplies the signal necessary to excite the
transmitter. The acoustic response is processed through a low noise pre-amplifier and then through
a fast high precision analog to digital converter (ADC). Acoustic data, at a sampling rate of 100
kHz, acquired by ADC is synchronized by a trigger signal generated by the function generator. This
second computer displays the acoustic spectrum (frequency domain) through a graphic interface and
The acquisition computer is interfaced to the control computer in a networkalso stores the data.
configuration. Pressure, volume and temperature data, gathered during acoustic data acquisition, are
also displayed and stored. During sweeps, control of all system functions, including those of the
acquisition computer, is directed by the control computer. The raw time domain data collected are
processed to obtain the frequency domain data using a custom software. The fingerprint of the
spectrum is the representation of various excited modes in the fluid contained in the cylindrical
resonator. Tracking any of the modes with temperature, pressure or volume shows the physical and
chemical changes as well as phase transition happening in the system during the process. During
the onset of phase transition, the acoustic response shows a drastic change.
The resonance frequency in a reservoir fluid confined to a cylinder of length "1" and radius
"a" is given by:
"'21(Xn.
f=~2
-1 a
6

where c is the sonic speed in the fluid, Dz is an integer corresponding to different modes or roots of
the Bessel function (Dz = 1 first radial mode; Dz = 2; second radial mode), and "mn is a tabulated
= 0 for radial mode).eigen value «<mn
The above equation shows that the acoustic response is proportional to the sonic speed.
Hence, the onset of phase transition can be observed as a sharp change in the response behaviour
from the acoustic response track of any of the modes with temperature, pressure or volume. The
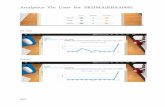
schematic illustration of the AR system is shown in Figure 1
- - " --~ ~
1
: 1 ~.O~ h1 1~~&D8 U
IP-.~II
-.:L2
~~
~
~
-_0..------...~J
to J - --
FIgI.- 1: A - ~ of DIe .. - ~ ~ ~b' 8y8I8nIn a typical acoustic resonance experiment, the system is first heated up to the experimental
temperature. Subsequently, the system is charged with the reservoir fluid at a pre set pressure and
at the experimental temperature. The system is then depressurized at an initial rate of 40 psi/minute.
The rate of depressurization decreases with time and reaches about 5 psi/minute towards the end of
The acoustic data with volume, temperature, and pressure are collected, andthe experiment.
subsequently, analyzed to identify the acoustic responses related to various phase change
phenomena.
7

Procedure to Detect the Phase Change Phenomena
j'iE<
I_I
-\.,
1~L~1-~. '.. ~. )~ ~
Figure 2: A Typical Transformed Frequency Domain Spectrum
The following is a step-by-step procedure to identify the specific signature related to a phase
change phenomenon from the acoustic resonance responses. A typical amplitudinal response curve
as a function of frequency is presented in Figure 2.
8

The raw time domain amplitudinal responses collected during the AR runs are processed toobtain the amplitude versus frequency plots using the Fast Fourier Transformation (FF1)technique.
.
By plotting the changes in amplitude as a function of specific frequencies, corresponding tothe modes of the Bessel functions, the points of phase transition can be inferred.
Subsequently, the specific changes in frequencies are plotted as a function of independentvariables (i.e., pressure, temperature, or volume). The specific change in the frequencycorresponding to a phase change phenomenon is clearly identified from the plots offrequency versus pressure, for example.
.
Sample Characterization
The composition of the reservoir crude containing wax is presented in Table I.
Table I: Compositional Analvsis of Wax v Crude (Samnle 1)
HydrocarbonComponents
CompositionMole Fraction
HydrocarbonComponents
CompositionMole Fraction
c, 0.004 Cl7 0.034
i-C4 0.003 CIa 0.027
n-C4 0.010 Ct, 0.025
i-Cs 0.012 ~ 0.025
n-Cs 0.013 C:u 0.022
Cyclo Cs 0.002 ~ 0.021
c, 0.040 C2i 0.020
Mycyclo Cs 0.001 ~ 0.019
Benzene 0.014 ~ 0.017
Cyclo C, 0.018 c. 0.016
Co, 0.082 Cn 0.014
Mycyclo C6 0.005 Ca 0.013
Toluene 0.002 c. 0.012
c. 0.079 ~ 0.009
EB/MP Xylene 0.017 ~I 0.008
O-Xylene 0.005 ~2 0.006
9

HydrocarbonComponents
CompositionMole Fraction
HydrocarbonComponents
CompositionMole Fraction
0.069 Cu 0.005~TMB 0.011 ~ 0.005
0.053 c,s 0.003C1O
c.. 0.048 ~ 0.003
0.003CIa 0.047 ~Ct) 0.040 c. 0.002
0.042 c. 0.002C'4
0.037 C40 0.001CIS
C1. 0.031
The solids content of all three reservoir fluid samples are presented in Table ll.
Table II: Solids Content of Reservoir Fluid SamDles
Sample Asphaltene Content(% by Weight)
Wax Content
(% by Weight)
1 23.1
2 4.6 1.1
0.73
As seen in the table, the total wax content of the waxy fluid Sample 1 is 23.1 % (by weight).
Two different fluid samples containing asphaltenes were used in these experiments. As seen in the
table, Sample 2 contains both asphaltene and wax. The asphaltene content of Sample 2 is 1.1 % (by
weight) and the wax content is 4.6% (by weight). The asphaltene content of Sample 3 is 0.7% (by
weight).
10

DESCRIPTION OF THE EXPERIMENTS
Identification of Wax Solidification
An isobaric cooling experiment was conducted to detennine the onset of wax precipitation
using Sample 1. In this nIn, the ART data were processed to detect the onset of the first wax crystal
(cloud point), and subsequently detect the temperature condition at which agglomeration of the
individual wax crystals took place (pour point).
Identification of the Onset of Asphaltene Precipitation
In the first set of experiments, depressurization runs were conducted using live oil Sample
2 to verify if the changes in temperature and pressure could cause asphaltene precipitation during the
production phase of the well. In this case, the sample was charged into the cylindrical acoustic
resonator' at a pressure higher than the reservoir pressure and at a preset temperature. Subsequently,
the pressure was reduced from the experimental pressure to a pressure below the expected bubble
point pressure. Acoustic signature corresponding to the appearance of the asphaltene particles and
the bubble point pressure were detected. The AR depressurization runs were repeated at various
temperatures.
In the second sets of experiments, two solvents (CO2 and NGL) have been tested to evaluate
the enhanced oil recovery potential. These EOR solvents have the potential to precipitate asphaltene
in contact with oil. Therefore, the ART experiments were conducted to define the SLE and SLVE
conditions. During these testing, NGL (composition given in Table III) was mixed with Sample 3
at various proportions at a pressure much higher than the expected asphaltene precipitation pressure.
1

Table m: NGL Comoosition
Composition(Mole Fraction)
Components
~ 0.0161
c, 0.4395
i-C4 0.1518
n-C. 0.3623
i-C, 0.0223
n-C, 0.0078
c+6 0.0002
Subsequently, the depressurization nms were conducted at the reservoir temperature of 2 5°C
to detect the onset pressure of asphaltene precipitation. Similarly, Sample 3 was mixed with various
proportions of CO2, again at a pressure much higher than the expected pressure of the onset of
asphaltene precipitation. Depressurization nms were conducted at the reservoir temperature of25°C
In the second sets of experiments, the mixing of solvents with oil was conducted in a cell and
transferred to another cell through a filter to see any asphaltenes have precipitated out during the
mixing process. No asphaltenes were seen to appear in the filter during the sample transfer
RESULTS AND DISCUSSIONS
Cloud and Pour Points Measurements
In these experiments, the cell was first cleaned with solvents and dried with argon and
evacuated. The system was then maintained at a temperature of 65 °C, the live waxy crude Sample
, maintained at a temperature of 65 °C, was transferred to the evacuated cell through a heated line
in liquid state. Then the system valve was closed. Once the system was stabilized, the resonator was
12

cooled down to 30°C at a mte of 0.1 °C/min. Time domain data was collected at each set point along
with pressure, temperature and volume data. Once the run was over, the data was processed to
obtain the frequency spectrum.
The normalized acoustic response obtained from a particular frequency track of the FFT data
as a function of temperature is presented in Figure 3
u
lIt..u
F\c1Ire 3: Aceastic Detenaiaatioe of aolld aod Pour Poi.to of Sample 1
As seen in the figure, the acoustic response shows some variations as the temperature is
reduced. At about 40°C temperature, the acoustic response starts to increase and this point is
characterized as the cloud point temperature. This increase in the acoustic response is due to the
increase in the density of the fluid undergoing a cooling cycle. Further reduction in temperature
provided another sharp change in the acoustic response, and this corresponds to the pour point of the
system. The cloud point was determined to be 41.4OC. An optical laser method was used to detect
the cloud point of this oil sample and this method detected the cloud point at a temperature of
13

42.5°C. Therefore, the results are comparable. The pour point of the system was determined to be
20°C.
Onset of Asphaltene Precipitation Pressure
Depressurization Experiments Using Live Reservoir Fluid
Three acoustic resonance depressurization experiments were conducted at three specified
In these runs, the experimentaltemperatures of 49, 57, and 66°C using live oil Sample 2.
temperature was held constant and the system pressure was decreased from 55 MPa to 10 MPa. The
55 MPa pressure is higher than the reservoir pressure of 43 MPa. The acoustic response is basically
the sonic frequency in Hertz and the normalized values of the acoustic responses are presented in the
scale of 0 to I in the example figure. The nominal values of these acoustic responses varied between
0 a 50 kHz.
A typical normalized acoustic response curve is presented as a function of pressure in
Figure 4. The onset of solids precipitation (SLE) and bubblepoint pressures (VLE) are identified by
their respective sharp and drastic changes in acoustic response and these specific signatures are
labelled in Figure 4.
Figure 4: A.oati. Deter8li.alioa or 0- or Asphaltetle Precipitation..d Bubblepoint In Uve 011 @ 66OC
14

As seen in Figure 4, the acoustic response starts to slightly increase with a decrease in
pressure from an experimental pressure of 55 MPa. The AR response attains a sharp increase at
around a pressure of 42 MPa. This sharp increase in acoustic response is characterized as the onset
of solids precipitation. Further reduction in pressure results in decreasing the acoustic response until
a low AR response is attained. This low AR response is characterized as the bubble point pressure
of the reservoir fluid (37 MPa). The increases in the AR response after the appearance of the solids
are believed to be due to the higher sonic speed in solids than that in the liquid phase.
The onset of asphaltene precipitation (solid-liquid equilibrium, SLE) and the bubble point
pressures (vapor-liquid equilibrium, VLE) at various temperatures are summarized in Table IV and
graphically presented in Figure 5.
The bubble point pressures measured using a visual high pressure cell are also presented inFigure 5.
15

-M,.-M~
45 50 55 eo 85 70T-- ('C)
r...re 5: At ~.rizatioa Sa Z at Varleu T_peo-atar..
As seen in this figure and, as expected, the bubble point pressure increases slightly with an
The bubble point pressure measured by two methods compare well.increase in temperature.
As seen in Figure 5, the onset pressure of solids precipitation decreases with an increase in
This indicates that, at a higher temperature, the solids precipitation will occur at atemperature.
lower pressure, and hence, increase the range of operating pressure conditions (from sand face
pressure to the solids onset pressure).
Depressurization Experiments Using a Mixture of Live Reservoir Oil and Solvents
In these experimental runs, the live crude oil, Sample 3, was pre-mixed with various
proportions of solvents at a pressure of 40 MPa and a reservoir temperature of25°C. Subsequently,
the AR depressurization runs were conducted to identify the SLE and VLE conditions of these live
oil and solvent mixtures. Two different solvents (NGL and COJ were tested in these experiments.
The objective of the study was to evaluate whether the addition of solvent to the live oil would
precipitate any asphaltenes from the live oil solvent mixture. This study was conducted prior to
16

injecting solvents in the reservoir to evaluate the potential for enhanced oil recovery using two types
of solvents injection.
A. SLE and VLE Usina NGL Solvent
The composition ofNOL is given in Table ill. As seen in the table, NOL primarily consists
of propane and nomlal butane and these two components are very effective in altering the
thennodynamic equilibrium of asphaltenes, and hence, precipitate asphaltenes.The acoustic
depressurization results are presented in Table V and graphically presented in Figure 6.
In these tests, three experiments were conducted using three solvent concentrations (10, 40
and 800/0 by volume). The onset of solids precipitation pressures from these three nms were obtained
from the respective acoustic response data and summarized in Figure 6. As seen in this figure, the
Thisonset of solid precipitation pressure increases with an increase in solvent concentration.
phenomenon indicates that the more solvent will enhance the onset of asphaltene precipitation and
minimi7.e the operating pressure limit above the SLE curve.
17

As expected, the bubble point pressures decreases with an increase in the solvent
concentration. The bubble point pressures at these three solvent concentrations identified using the
acoustic resonance technology were compared with that measured using conventional PVT analysis.
As seen in Figure 6, the bubble point pressures measured by two methods compare very well.
B. SLE and VLE USin2 CO2 Solvent
Similar to the NGL experiments, live oil Sample 3 was premixed with CO2 at three different
concentrations (10, 40 and 80% by volume). Subsequently, the AR depressurization runs were
The solid onset pressures (SLE) and saturation pressure (VLE) conditions areconducted.
summarized in Table V and graphically presented in Figure 7. As seen in the figure, the solids onset
pressure shows an upward trend with an increase in the CO2 concentration. If the CO2 injection
pressure is above the SLE curve, there will not be any problem with solids precipitation. Once the
injection pressure drops below the SLE curve, the asphaltene particles will appear and possibly cause
plugging problems in the porous medium.
18

The saturation pressures were also identified from the acoustic response and are presented
in Figure 7.
As seen in the figure, none of the saturation pressures identified using the ART system were
The saturation pressure measurements conducted using thehigher than the solids onset pressure.
conventional PVT method indicated two bubblepoint pressures and one dewpoint pressure. The
dewpoint pressure is found to be higher than the onset of solids precipitation pressure. This makes
logical sense because solids cannot remain in solution in the dense phase above the dewpoint. The
solids precipitation will occur in the liquid phase. The measured data point shows that the solids
appears in the liquid phase below the dewpoint. In these nms, saturation pressures identified using
the acoustic technology were also compared with that measured using the conventional PVT method.
As seen in Figure 7, the values compare very well.
The appearance of the solids precipitation pressure below the dew point pressure is logical
because of the fact that solids can only exist in the liquid phase. The crossover point of the SLE and
19

VLE curves is speculated to be the critical point. However, no analysis at hand indicates that the
crossover point could be the critical or near critical point to validate the hypothesis.
DISCUSSION
Because of dark color of the oil, the asphaltenes were not visible during the bubblepoint
experiments using the visual method. However, at the end of the AR experiments, the equipment
was reported to be plugged during the flushing and cleaning of the cell.
At the end of each experimental run (10 MPa), the system was bled off using the bottom
valve at the experimental temperature. During the bleeding process, the pressure did not drop at the
beginning. This is due to the plugging of exit lines with solids. Subsequently, the cell was charged
with toluene from the top valve at a pressure of 10 MPa and allowed to soak for one hour. After the
soak period when the bottom valve was opened, the cell content was bled off with intermittent flow.
The solids were extremely small, and therefore, were not visible under naked eyes. Following this
bleeding step the cell was rinsed with methanol and dried before the next run.
During the production phase of the live reservoir fluid or during processing of the produced
reservoir fluid, it is critical to understand the pressure-temperature conditions at which the solids will
precipitate out. Especially during the production phase of the live reservoir fluid, if the pressure falls
below the SLE condition, the solids will start to precipitate out, and hence, cause operational
problems. In addition if the pressure falls below the SLE condition in the near wellbore region, the
problem would be severe due to the solids plugging in the porous media. Therefore to take proper
preventive measure, it is recommended that the SLE conditions be determined when the operating
company is aware of the fact that the producing fluid contains solids.
20

CONCLUSION
The acoustic resonance technology is a state-of-the-art technique to precisely identify the
pressure and temperature conditions at which the solids precipitation will occur. The solid-liquid
equilibrium (SLE) condition can be defined in a live reservoir fluid using the ART system where
other techniques become inadequate due to dark color of the oil. The vapor-liquid equilibrium
Comparison of results with the(VLE) condition can also be identified using the ART system.
conventional method shows excellent agreement in the VLE data.
21

LITERATURE CITED
Briant, J. and Hotier, G.: "Etude de retat Des Asphaltenes Dans Les Melanges D'hydrocarbures" ,Revue Inst. Fran~ais Pet. 38, 83 (1983).
Change, C.L. and Fogler, H.S.: "Asphaltene Stabilization in Alkyl Solvents Using Oil-solubleAmphiphiles", paper SPE 25185 presented at the 1993 SPE International Symposium on Oil FieldChemistry, New Orleans, Louisiana, USA, March 2-5 (1993).
Colgate, S.O., McGill, K.C. Sivaraman, A. and Tatro, D.: "Acoustic Resonance Determination ofSonic Speed and the Critical Point for A Typical Retrograde Gas Condensate", Fluid PhaseEquilibria, 79, 231 (1992).
Colgate, S.O. and Sivaraman, A.: "Thermophysical Properties of Natural Gas Mixtures DerivedFrom Acoustic Cavity Measurements", Chapter 1 0 #514. Supercritical Fluid Engineering Science,Fundamentals and Applications, 121 (1993).
Colgate, S.D., Sivaraman, A. and Dejsupa, C.: "Sonic Speed and Critical Point Measurements inEthane by the Acoustic Resonance Method", Fluid Phase Equilibria, 76, 176 (1992).
Espinant, D. and Ravey, l.C.: "Colloidal Structure of Asphaltene Solutions and Heavy Oil FractionsStudied by Small Angle Neutron and X-ray Scattering", paper SPE 25187 presented at the 1993 SPEInternational Symposium on Oil Field Chemistry, New Orleans, Louisiana, USA, March 2-5 (1993).
Fortland, P ., Anfindlsen, H. and Fadness, F .H.: "Detection of Asphaltene Precipitation and AmountsPrecipitated by Measurement of Electrical Conductivity", Fluid Phase Equilibria, 82, 157 (1993).
"Properties of Asphaltene From a Waxy Crude", Fuel, 70, 1293 (1991)Fuhr, B.l. et al:
Hirschberg, A. et al: "Influence of Temperature and Pressure on Asphaltene Flocculation", SPEJ283, Trans., AIME, 277 (1984).
"De-Asphalted Oil: A NaturalJamaluddin, A.K.M., Nazarko, T. W., Sills, S. and Fuhr, B.H.Asphaltene Solvent", SPEPF, 161 (1996).
Leontaritis, K.J. and Mansoori, G.A.: "Asphaltene Deposition: A Survey ofField Experiences andResearch Approaches", J. of Petrol. Sci. & Eng., 1,229 (1988).
Leontaritis, K.J. and Mansoori, G .A.: "Asphaltene Flocculation During Oil Production andProcessing: A Thermodynamic Colloidal Model", paper SPE 16258, presented at 1987 SPEInternational Symposium on Oil Field Chemistry, San Antonio, Texas, USA, Feb. 4-6 (1987).
22

Leontaritis, K.J., Kawanaka, G.A. and Mansoori, G.A.: "Descriptive Accounts of Thermodynamicand Colloidal Models of Asphaltene Flocculation", paper presented at the 1988 ACS meeting, ThirdCongress of North America, Toronto, Ontario, Canada, June 5-10 (1988).
Mansoori, G.A. and Jiang, T .S.: "Asphaltene Deposition and its Role in EOR Miscible Flooding",FlOC. Third AGIP SPA Improved Oil Recovery European Meeting, Rome, Italy 75 (1985).
Nighswander, J.A., Kalra, H. and Majeed, A.: "Effect of Low Molecular Weight Hydrocarbons onthe Onset Concentration and Bulk Properties of Asphaltene at In-situ Temperature and PressureConditions", paper SSE presented at the AICHE, March 28-Apr. 1 (1993).
Novosad, Z. and Costain, T .G.: "Experimental and Modelling Studies of Asphaltene Equilibria forA Reservoir Under CO2 Injection", paper SPE 20530 presented at the 1990 SPE Annual TechnicalConference, New Orleans, Louisiana, USA, Sept. 23 (1990).
"The Properties of Gases and Liquids", 3M EditionReid, RC., Prausnitz, J.M. and Sherwood, T .K.:McGraw-Hill Book Co. (1977).
Ronningsen, H.D., Bjomdal, B., Hansen, A.B. and Pedersen, W.B.: "Wax Precipitation From NorthSea Crude Oils. 1. Crystallization and Dissolution Temperatures and Newtonian and Non-
Newtonian Flow Properties", Energy & Fuels, 5, 895 (1991).
Shelton, J .L. and Yarborough, L: "Multiple Phase Behaviour in Porous Media During CO2 or Rich
Gas Flooding", J. Pet. Tech. 1171 (1977).
Speight, J.G., Wernick, D.L., Gould, K.A., Overfield, R.E., Rao, B.M.L. and Savage, D.W.:"Molecular Weight and Association of Asphaltenes: A Critical Review", Revue De L'lnstitute
Fran~ais Due Petrole, 40 (1985).
Speight, J.G. and Moschopedis, S.E.: "On the Molecular Nature of Petroleum Asphaltene",Chemistry of Asphaltene, Bunger J .S. and Li, N.C. Edited, American Chemical Society, Washington,D.C., USA, Advances in Chemistry Series No. 195 (1981).
Speight, J.G.: "Studies on Bitumen Fractionation (A) by a Cryoscopic Method, (B) Effect of Solvent
Type on Asphaltene Solubility", Information Series, ARC 84 (1979).
Speight, J.O., Long, R.B. and Trowbridge, T .D.: "Factors Influencing the Separation of Asphaltenes
From Heavy Petroleum Feed Stocks", Fuel, 63 (1984).
Stausz, D.P., Mojelsky, T.W. and La~ E.U.: "The Molecular Structure of Asphaltene: AnUnfolding Story", Proc., (1991) Eastern Oil Shale Symposium, Lexington, Kentucky, USA,November 13-15 (1991).
2)

Thomas~ F .B. et al: "Experimental and Theoretical Studies of Solids Precipitation From ReservoirFluid'1~ JCPT 31 ~ 22 (1992).
Tuttle, R.N.: "High Pour Point and Asphaltene Crude Oils and Condensates", JPT , 1192 (1983).
Wehry, E.L. and Rogers, L.B.: "Fluorescence and Phosphorescence of Organic Molecules"Fluorescence and Phosphorescence Analysis, D.M. Hercules (ed.), Interscience Publications, New
York, 81(1966).
Won, K.W.: "Thennodynamic Calculation of Cloud Point Temperatures and Wax PhaseCompositions of Refined Hydrocarbon Mixtures", Fluid Phase Equilibria 53, 377 (1989).
24