CTSI Research Associates Program: Building Future Leaders ... · Poster Print Size: This poster...
Transcript of CTSI Research Associates Program: Building Future Leaders ... · Poster Print Size: This poster...

Poster PrintSize:This poster template is 42” high by42” wide. Itcan be used to printanyposter with a1:1 aspectra@o.
Placeholders:ThevariouselementsincludedinthisposterareonesweoBenseeinmedical,research,[email protected],move,add,anddeleteitems,orchangethelayouttosuityourneeds.Alwayscheckwithyourconferenceorganizerforspecificrequirements.
ImageQuality:Youcanplacedigitalphotosorlogoartinyourposterfilebyselec@ngtheInsert,Picturecommand,orbyusingstandardcopy&paste.Forbestresults,allgraphicelementsshouldbeatleast150-200pixelsper inchintheir finalprintedsize.Forinstance,a1600x1200pixelphotowillusuallylookfineupto8“-10”wideonyourprintedposter.Topreviewtheprintqualityofimages,selectamagnifica@onof100%whenpreviewingyourposter.Thiswillgiveyouagoodideaofwhatitwilllooklikeinprint.Ifyouarelayingoutalargeposterandusinghalf-scaledimensions,besuretopreviewyourgraphicsat200%toseethemattheirfinalprintedsize.Pleasenotethatgraphicsfromwebsites(suchasthelogoonyourhospital'soruniversity'shomepage)willonlybe72dpiandnotsuitableforprin@ng.
[Thissidebarareadoesnotprint.]
ChangeColorTheme:Thistemplateisdesignedtousethebuilt-incolorthemesinthenewerversionsofPowerPoint.Tochangethecolortheme,selecttheDesigntab,thenselecttheColorsdrop-downlist.Thedefaultcolorthemeforthistemplateis“Office”,soyoucanalwaysreturntothataBertryingsomeofthealterna@ves.
Prin@ngYourPoster:Onceyourposterfileisready,visitwww.genigraphics.comtoorderahigh-quality,affordableposterprint.EveryorderreceivesafreedesignreviewandwecandeliverasfastasnextbusinessdaywithintheUSandCanada.Genigraphics®hasbeenproducingoutputfromPowerPoint®longerthananyoneintheindustry;da@ngbacktowhenwehelpedMicrosoB®designthePowerPoint®soBware.USandCanada:1-800-790-4001Email:[email protected]
[Thissidebarareadoesnotprint.]
CTSIResearchAssociatesProgram:BuildingFutureLeadersinClinicalResearch
KELSEYJIANG,STEVYDENNISONGGO,MICHELLEGUAN,JAR-YEELIU,NoahFederman,LaurieShaker-Irwin
UniversityofCalifornia,LosAngeles,LosAngeles,CA
The UCLA CTSI Research Associates Program (CTSI-RAP) aims toprovide UCLA undergraduate students with the opportunity togain exposure to hospital-based medicine as well as clinicalresearch. The program is designed to strengthen interest inbiomedical research ini@a@ves by allowing first-hand interac@onbetween UCLA faculty physicians and inves@gators withundergraduatestudents.Researchassociatesplayakeyroleintheimplementa@on of research protocols when they are given theopportunitytoassistprimaryinves@gatorswithvariousaspectsoftheir project including, but not limited to, consen@ng pa@ents,collec@ng secureddata, and co-authoring abstracts, posters, andpapers. In addi@on, students are given the opportunity tomakeroundswiththemedicalteam,observecommonprocedures,andexperience didac@c teaching sessions during the course of theirinvolvement in the program. Outside of direct researchexperience, the CTSI-RAP students are exposed to situa@onsmeanttoeducateandpreparethemforacareerinhealthcare.Asaresult, researchassociatesbecomecomfortablewiththe inner-workings of a hospital and gain skills in professionalism, pa@entcommunica@on,and researchmethodology.Theuniqueblendoffirst-handclinicalexperienceandscien@ficresearchwillgiveCTSI-RAP alumni an all-encompassing perspec@ve on tackling healthcarechallengesandconduc@ngclinicalresearch.
Abstract
PI:Dr.DanielDumesic
Background:Polycys@c Ovary Syndrome (PCOS) is an endocrine disorder thatleadstothedevelopmentoffluid-filledovariancysts,resul@ng inabnormallyhighquan@@esofmalesexhormones.Thisimbalanceleads to irregular menstrual cycles, pelvic pain, infer@lity, andinsulin resistance, with associated long-term complica@onsincludingTypeIIdiabetes,heartdisease,andendometrialcancer.The purpose of this study is to u@lize diagnos@c informa@on tofurthercharacterizetheeffectsofPCOSandcomparethemtotheresultsofunaffectedwomen.
CTSI-RAPInvolvement:
CTSI-RAPstudentsfunc@onasclinicalcoordinatorassistantswithinthe research study. Students conduct pa@ent screenings todeterminestudyeligibility forprospec@vepar@cipants,auditandmaintain pa@ent files, op@mize and conduct study par@cipantrecruitment strategies, andobserveand scribe forPCOSmedicalprocedures.
PI:Dr.KaraCalkins
Background:The CUB research study looks for biomarkers in cord blood thatmay be indicators for hepa@c issues such as Nonalcoholic FaqyLiverDisease(NAFLD).33%ofchildrenintheU.S.areclassifiedasobese, and 38% of those children develop NAFLD, the leadingcauseforlivertransplantsintheUS.Classifyingbabiesatdifferentgesta@onalagesmayhelpevaluaterisksofdevelopingNAFLD.Byanalyzing the micro-RNA in the cord blood and studying themetabolomics of the individual, this study aims to see if theseitemsarelinkedtohighersuscep@bilitytoliverdisease.
CTSI-RAPInvolvement:
CTSI-RAPstudentscontributebyscreeningpregnantmothersforaLGA (Large Gesta@onal Age), SGA (Small Gesta@onal Age), IUGR(Intrauterine Growth Restric@on), Obese, Overweight and/orGesta@onal Diabetes label. When the pa@ent qualifies for thestudy,theRAPstudentsproceedtotheLaborandDeliveryUnitofRonaldReaganHospitaltoconsentthemothersbeforethebabyisborn. RAP students also par@cipate in cord blood collec@on,immediatebloodprocessing,andmaintainingpa@entdatabases.
CordUmbilicalBlood(CUB)StudyPI:Dr.IgorBarjaktarevic
Background:Shock is a common life-threatening condi@on that occurs in theICU and early detec@on of shock is essen@al for decreasingmortality. The inadequate blood perfusion to vital organsassociated with shock can lead to organ dysfunc@on andeventuallycardiacarrestifnotproperlymanaged,whichisusuallywith intravenous fluid (IV) administra@on or vasopressormedica@on. The purpose of this study is to determine whethersystema@cinclusionofabedsideultrasoundprotocoltoevaluatefluid-responsiveness and iden@fy the e@ology of shock earlier inthe hospital course significantly impacts clinical outcomes forpa@entsstayingintheIntensiveCareUnit(ICU).
CTSI-RAPInvolvement:On the medical and transplant ICU floors of Ronald ReaganMedical Center, CTSI-RAP students are trained to iden@fy andscreen for prospec@vepa@entswho are eligible to par@cipate inthestudy.Studentscommunicatewitheligiblepa@ents toobtaintheir consent for par@cipa@ng in the study and answer anypoten@al ques@ons the pa@ent might have. ABer enrolling apa@ent in the study, students no@fy theprincipal inves@gator ofthestudy.
ShockStudy
PI:Dr.JessicaWang
Background:The ICDR aims to understand different factors influencing heart-related disease progressionwithin affected families. Segrega@onanalysis and the search for biomarkers within blood and @ssuesamplesprovideadetailedpictureofpar@cipants'lifestylesbeforeand during par@cipa@on in the ICDR. Resul@ng data will besta@s@cally analyzed to beqer understand how gene@c andenvironmental factors influence diseasemanifesta@on. Collectedsamples may also be used to advance the understanding ofdisease mechanisms within families or disease condi@ons touncovertreatmentop@onsinthefuture.
CTSI-RAPInvolvement:RAPstudentsscreenmedicalrecordsandrecruitpa@entssuitableforpar@[email protected],theygatherconsentforpar@cipa@on from pa@ents of the UCLA Adult CongenitalCardiologyClinicaswellastheCardiacCareUnit(CCU)atRonaldReagan Medical Center. Students also assist pa@ents to thepathology lab for blood collec@onand transport the samples forimmediate processing. Furthermore, they work alongside Dr.Wangandateamofresearchspecialistsandgene@cscounselorstodesignandconstructacomprehensivevirtualdatabasefortheICDR.
InheritedCardiovascularDiseaseRegistry(ICDR)
PolycysTcOvarySyndrome(PCOS)Study
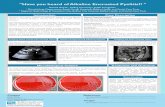
Figure1:Assetscoatedwithcopper/shamstainlesssteel
Figure3:Dr.MeeryoChoeperformingabaselineconcussionassesment
CTSI-RAPAdvisor:Dr.LaurieAnnShaker-Irwin,Ph.D.,M.S.PhysicianAdvisor:NoahCarvajalFederman,M.D.
Advisors
Buckanavage, Jack⧫ Cao, Quang ⧫ Dey, Ipsita ⧫ Dickson, Crystal ⧫ Dionson,Andrew⧫Ford,Victoria⧫Guan,Michelle⧫Habib,Omar⧫ Ho, David ⧫ Jiang, Kelsey ⧫ Jones, Adrian ⧫ Lam, Harrison ⧫ Liu, Franklin⧫Jar-Yee,Liu⧫Lu,Mimi⧫McLaughlin,Ryan⧫Ng,Cassia⧫ Nguyen,Kevin⧫Ong,Stephanie⧫Onggo,Stevyndnnis⧫Ringgenberg, ,Sienna⧫Sara,Afrida⧫So,Josh⧫Tran,Elizabeth⧫Wong,Shirley⧫ Yao,Douglas⧫Yusuf,Hamzah
CTSI-RAPStudents2016-2017
BrainSPORT,CopperTouchRounds,CTRCRounds,DepressionStudy,MethamphetamineStudy,Brain-GutInterac@onsinObesityStudy,andSystemicLupusErythematosus/AtherosclerosisStudy
AddiTonalOngoingProjects
Figure2:StudentfillingoutFentonGrowthCharts
Figure4:4ICUUnitwheretheShockandCopper
TouchStudyisconducted
Acknowledgments:ThisresearchposterwasfundedbytheCTSIthroughNCATSGrantNo:UL1TR001881.