Cord blood stem cells Where do we stand? - MÆDICA5)_No2/2007_Vol2(5)_No2_pg... · The diagram of...
Transcript of Cord blood stem cells Where do we stand? - MÆDICA5)_No2/2007_Vol2(5)_No2_pg... · The diagram of...

Mædica A Journal of Clinical Medicine, Volume 2 No.2 2007142
SSSSSTTTTT AAAAATETETETETE -----OFOFOFOFOF----- THETHETHETHETHE -----ARARARARARTTTTT
Mædica - a Journal of Clinical Medicine
ABSTRACTUmbilical cord blood is a source of blood stem cells that can be transplanted to regenerate a patient’s
immune system. There are other parts of the human body that contain stem cells, but the only sources whichare concentrated enough to be harvested for a transplant are the bone marrow, the circulating blood, and theumbilical cord. As a source of hematopoietic stem cells for transplantation, cord blood has certain advantagesover bone marrow and peripheral blood. But there is a significant disadvantage. Because the stem cells incord blood are more primitive than those in bone marrow or peripheral blood, the engraftment process takeslonger with cord blood, leaving the patient vulnerable to fatal infections for a longer period of time. Hematopoietic stem cells are capable of evolving into all the specific cell types in the blood and immunesystem. They can be found in people of all ages. The three sources of hematopoietic stem cells which areroutinely used for medical treatments are: the bone marrow of an adult person, the peripheral blood of anadult person, the umbilical cord blood of a newborn baby. When a patient requires a Hematopoietic StemCell Transplant (HSCT), the treating physician will decide which source of stem cells to use. This willdepend on several factors, including but not limited to: the degree of match between donor and patient(sometimes the donor and patient are one and the same person)(autologous transplant), the expected speedof engraftment, and the amount of time available to search for a perfectly matching donor.
Although cord blood is so great, not everybody bank it because it costs money. Whereas a bone marrowregistry is just a data base of potential donors, a cord blood registry consists of freezers full of frozen bloodand staff to maintain them. In an ideal world, all babies would have their cord blood harvested at birth (withparental permission) and stored in public registries, much like public blood banks. In practice, only alimited number of institutions have the funding to maintain public banks which take donations for free.
Key words: cord blood, stem cells, transplant, hematopoietic stem cells
Cord blood stem cellsWhere do we stand?Ana Maria VLADAREANU, Associate Professor of Hematology, MD, PhDa,Doina MIHAILESCUb, MD, PhD, Rodica TUDOSAb, MD, PhD,Mona ZVANCAb, MD, PhD, Radu VLADAREANUb, Professor, MD, PhDaDepartament of Hematology,University Emergency Hospital, Bucharest, RomaniabDepartment of Obstetrics and Gynecology,“Elias” Emergency University Hospital, Bucharest, Romania
Address for correspondence:Radu Vladareanu, Professor, MD, PhD, Chief of Obstetrics and Gynecology Department, “Elias” University Emergency Hospital, 17Marasti Blvd, District 1, Zip Code 011461, Bucharest, Romaniaemail address: [email protected]

Mædica A Journal of Clinical Medicine, Volume 2 No.2 2007 143
CCCCCORD BBBBBLOOD SSSSSTEM CCCCCELLS – WWWWWHERE DDDDDO WWWWWE SSSSSTAND?
T he circulating blood cells are formedin bone marrow through a processcalled hematopoiesis. The bonemarrow has an enormous pro-duction capacity; it is estimated that
1010 erythrocytes and 108 to 109 leukocytes areproduced per hour in the steady state. Further-more, while cell numbers are maintained withinfairly narrow limits in normal subjects, they canbe greatly amplified on demand.
These huge cell numbers are immediatedescendants of maturing precursors that arisefrom a smaller pool of progenitors. The pro-genitors in turn arise from an even smaller poolof hematopoietic stem cells (HSC) that arethought to be mostly in a resting or nondividingstate and have the capacity to self-renew (andthus maintain their numbers). Hematopoieticstem cells are multipotent and have the capacityto differentiate into the cells of all ten bloodlineages – erythrocytes, platelets, neutrophils,eosinophils, basophils, monocytes, T and Blymphocytes, natural killer cells, and dendriticcells (1).
Originally, patients who received chemo-therapy which wiped out the immune systemwould receive a transplant of blood stem cells(“hematopoietic” stem cells) from the bonemarrow of a matched donor. The USA NationalMarrow Donor Program (NMDP) maintains apublic registry of adults who are willing toconsider bone marrow donation, and similar
registries exist in other countries. Despite theseregistries, about 50% of all patients needing astem cell transplant cannot find a matching bonemarrow donor.
Umbilical cord blood is also a source ofblood stem cells that can be transplanted toregenerate a patient’s immune system. Thereare other parts of the human body that containstem cells, but the only sources which areconcentrated enough to be harvested for atransplant are the bone marrow, the circulatingblood, and the umbilical cord.
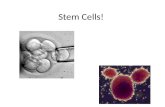
Do not confuse blood, or “hematopoetic”,stem cells with “pluripotent” stem cells that comefrom embryos (Figure 1). Medical research hasfound it is possible to use the fundamental, orpluripotent, stem cells from discarded humanembryos to grow all manner of human tissues.Obviously, there are serious ethical issues in suchresearch. Pluripotent stem cells are not the topicof discussion here. We are only discussing bloodstem cells, which can be removed from theumbilical cord without harm to the mother orthe baby, and which can generate a completeimmune system of blood cells.
As of late 2000, medical researchers arefinding that under some circumstances, stemcells from cord blood can grow into other tissuetypes, in addition to blood cells. Their growth isnot as diverse as what the “pluripotent” em-bryonic cells can do, but the potentialapplications are staggering. If cord blood couldbe a non-controversial source of cells fortreatments like nerve repair, it will revolutionizemedicine. The hope is that, eventually, therewill be established procedures to infuse cordblood into human beings, get the stem cells toturn into nerve cells, and have those nerve cellsfunction in the body to fix the patient’s problem.Hematopoietic reconstitution in a child withFanconi´s anemia by means of umbilical-cordblood from a HLA-identical sibling was the firstcord blood transplant proceded by chemo-therapy (Paris, 1988) (2,3,4).
Advantages of cord blood banking
Birth is a one time opportunity to help societyby donating child’s cord blood to a public
bank. There are three sources of stem cells fortransplant patients: bone marrow, circulatingblood, and cord blood. The first two exist in allhealthy adults, but cord blood can only beharvested and stored at birth. It is easier toFIGURE 1. The diagram of stem cells (adapted from 21)

Mædica A Journal of Clinical Medicine, Volume 2 No.2 2007144
CCCCCORD BBBBBLOOD SSSSSTEM CCCCCELLS – WWWWWHERE DDDDDO WWWWWE SSSSSTAND?
match transplant patients with cord blood thanthe two sources of adult blood. Hence,establishing public banks of stored cord bloodfrom donors with diverse tissue types can savemany lives.
Birth is also a one time opportunity for thefamily to save their own child’s cord blood.Transplant patients recover better when theyreceive stem cells from a related donor, insteadof an unrelated donor. If there are new medicaladvances developed in the future which canrepair the body with stem cells from cord blood,then families which saved cord blood will haveaccess to those treatments (2,3,4).
Advantages – As a source of hematopoieticstem cells for transplantation, cord blood hascertain advantages over bone marrow andperipheral blood:
Cord blood contains a greater concentrationof highly proliferative hematopoietic cells.Although each cord blood unit contains arelatively small total number of hema-topoietic stem cells, these cells are fullycapable of reconstituting hematopoiesis inboth children and adults (5,6).Cord blood collection can be performed ina manner that entails less discomfort andmorbidity for the donor than collection ofbone marrow or peripheral blood stem cells.It is less expensive to store and, once banked,can be retrieved much more quickly thanperipheral blood or bone marrow (7).Cord blood has been shown to have lowerrates of infection with cytomegalovirus (CMV)than bone marrow, potentially resulting in areduction of post-transplant complicationscaused by this virus (8,9).Once a cord blood unit has been collectedand preserved, it is immediately available fortransplantation. In contrast, compatiblebone marrow and peripheral blood stemcells are usually available only after theidentified donor has been medically clearedand the bone marrow or peripheral bloodstem cell collection completed, resulting insignificant delays (10).Because the stem cells in cord blood are lessmature than those in bone marrow orperipheral blood, they carry much lowerincidence of graft versus host disease(GVHD). Thus, cord blood transplants donot require a “perfect match” between thedonor and the patient. In the world oftransplants, this is a very big advantage (11).
In a study where all patients received anHLA-matched transplant from a sibling, andthe results were controlled for age, therelative risk of cord blood versus bonemarrow was 0.41 for acute GVHD and 0.35for chronic GVHD.The accepted explanation is that cord bloodcarries much less GVHD than bone marrowbecause the newborn baby is “immunolo-gically immature” – ie., the immune systemhas not had time to be exposed to variousforeign bodies and develop reactions againstthem (12).
Disadvantages of cord blood transplantsversus bone marrow transplants orperipheral blood stem cells
Because the stem cells in cord blood are moreprimitive than those in bone marrow or
peripheral blood, the engraftment process takeslonger with cord blood, leaving the patientvulnerable to a fatal infection for a longer periodof time. This is a significant disadvantage (13,14).
Research is underway to speed up engraft-ment. Expanding the number of stem cells incord blood makes it possible to transplant biggerpatients, but does not speed up engraftment.In August 2004, a research team at led by HalBroxmeyer at Indiana University School ofMedicine announced that they can speed upengraftment by inhibiting or deleting CD26, anenzyme on the surface of stem cells. The initialpublication describes research on mice, buthuman trials are planned (15).
More and more diseases are now recognizedto have a genetic basis. We have always knownthat some diseases are passed by inheritence.Now we also recognize that some geneticmutations confer a greater predisposition todisease. Many of these inherited predispositionsdo not manifest until adulthood. Plus, if thedonor has one of these predispositions, someof the drugs that transplant patients take couldtrigger a malignancy in the donor cells. On onehand, older donors are better because theyhave more medical history to rule out inheritedmutations. On the other hand, older donorsare worse because they are more likely to havean acquired mutation, from exposure to diseaseor chemicals, etc. At this time, it is not clearwhether older or younger donors are better. Itis possible to test donor cells for inherited

Mædica A Journal of Clinical Medicine, Volume 2 No.2 2007 145
CCCCCORD BBBBBLOOD SSSSSTEM CCCCCELLS – WWWWWHERE DDDDDO WWWWWE SSSSSTAND?
mutations, which would be present in every cell.But with hundreds of known mutations, only alimited number can be screened with a reason-able amount of blood (16,17).
The long-term survival among recipients ofbone marrow transplants versus cord bloodtransplants
Among pediatric patients, the overall survivalrates are comparable for the two transplant
types, but the causes of death differ with each.Among cord blood transplants, the mostcommon cause of death was complicationsduring the long wait for engraftment. Amongbone marrow transplants, more patients diedof severe Graft-Versus-Host Disease. Despite thefact that cord blood seems to lack graft-versus-tumor activity, cord blood transplants were notassociated with higher rates of patient relapse(18).
Among adult patients, perfectly matchedbone marrow is preferable to cord blood, butmis-matched bone marrow and mis-matchedcord blood yield comparable outcome. In2004, two studies were published in the NewEngland Journal of Medicine which announcedthat cord blood transplants are suitable foradults who lack a perfectly matched HLAdonor. The USA study found no differencebetween outcomes of cord blood transplants(150 patients) with 1 or 2 HLA mis-matchesversus bone marrow transplants (83 patients)with one HLA mis-match. A perfect bonemarrow match (367 patients) is still better thancord blood for adult patients (19). TheEuropean study compared 98 cord bloodtransplants (98 patients, 94% of them mis-matched) with bone marrow transplants (584patients, all matched). There were no significantdifferences in occurance of chronic GVHD,transplantation-related mortality, relapse rate,and leukemia-free survival (20).
Arguments in favor of private banking
One is that, as today’s children grow up andsome of them develop cancer as adults,autologous (self) cord blood transplants willbecome more common. The reason is thatpediatric cancers and adult cancers arecompletely different diseases at the cellularlevel. While pediatric cancer patients rarelyreceive autologous transplants, among adultcancer patients the autologous transplants
are more common than transplants fromdonors.Secondly, recent news reports constantlyannounce new medical advances using stemcells. Future applications will probablyinclude tissue repair to various organs of thebody.Another factor families should consider iswhether the odds given for the “averagebaby” apply to you. Some families do havea higher predisposition to cancer andimmune disorders.Finally, if a family has children of mixedethnic background, it may be impossible tofind an adult bone marrow donor who is aperfect match. In that event, cord bloodfrom even a partially matched sibling wouldbe invaluable. It takes at least a month lessto search for a cord blood donor than tofind a bone marrow donor. Median time tochose a cord blood unit is 13.5 days (range,2-387), compared to median time toapprove an unrelated bone marrow donorof 49 days (range, 32-293), in a summaryof 171 donor searches over the course of ayear at the University of Minnesota. The“additional time taken to obtain a BM donorwas predominantly due to the additional 30-day interval (range, 10-101 days) requiredto clear the donor” (21). A person can receive a cord blood transplant
until the age 70. A study at the University ofMinnesota transplanted patients who were noteligible for high-dose chemotherapy, either dueto age, co-existing medical problems, orprevious treatment. The median age was 49(range from 19 to 69). They received reducedconditioning chemotherapy, also known as a“mini transplant”. Sustained engraftment was89% overall, and 98% in patients who hadablative chemotherapy (enough to wipe outbone marrow) within three months prior totransplant (22).
Limitations to banked cord blood as life-long “medical insurance”
1. Diseases which require transplantation ofblood stem cells are still rare, although thelist of diseases amenable to such treatmentis steadily growing. They are very rare amongchildren and become more common inadults.
2. The banked cord blood only providesinsurance for so long as the frozen cells are

Mædica A Journal of Clinical Medicine, Volume 2 No.2 2007146
CCCCCORD BBBBBLOOD SSSSSTEM CCCCCELLS – WWWWWHERE DDDDDO WWWWWE SSSSSTAND?
still viable. So far, research has confirmedthe long-term viability of cord blood for upto 15 years, but the existing literature oncryogenic storage of living cells indicates thatstorage for decades is feasible (23, 24, 25).
3. The banked cord blood only providesinsurance providing there are enough stemcells in the sample for a successful transplant,even after your new baby grows up tobecome a much larger adult. Again, on-goingresearch is exploring several methods totransplant large adults with banked cordblood.
4. Even among adults, where autologoustransplants with one’s own stem cells aremore common, there are alternate sourcesof stem cells in the patient’s bone marrowor circulating blood. Over the course of alifetime up to age 70, the probability that aperson will require a transplant of her ownstem cells (“autologous” transplant) is 1 in450 or 0.23% and the probability ofrequiring any transplant, from yourself oranother person (“autologous” or “allogeneic”transplant), is 1 in 220 or 0.46% (26).
5. Many regenerative therapies are beingdeveloped which use the patient’s own stemcells. One of the most common andpromising is the use of stem cells for heartrepair. Adult patients who have banked cordblood would have a ready source of stemcells for regenerative medicine. But they alsohave a rich source of stem cells in their bonemarrow.When parents bank the cord blood from a
new baby, in the near term they are most likelyproviding medical insurance for that child’ssiblings, and only in the long term when thedonor grows up will they have value for self-use.
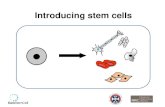
It’s not clear yet which cord blood collectionmethod is optimum, gravity drip or syringe.Studies have shown that pulling the blood outwith a syringe tends to draw a bigger samplethan simply letting the blood drip by gravity intoa bag (27). We are currently using the gravitydrip method, with large quantity samplesconfirmed (Figure 2). We have started inNovember 2006 the cord blood collection, inElias Hospital, and we have by now a numberof samples confirmed in private banking.
The optimum cord blood storagecontainer – bags or vials?
Existing scientific studies do not show asignificant difference between the cell dose
and cell viability of cord blood stored in bagsversus vials. There have been successfultransplants with cord blood that had beenstored for years using either method. Historic-ally, the use of bags and vials have existed inparallel for over a decade. On one hand, publiccord blood banks have always stored cordblood in traditional blood bags, presumablybecause they were founded by traditionalblood banks and this was a consistent practicefor them. But, on the other hand, researchinstitutions usually stored cord blood in vials,which allow multiple portions per collection andare less expensive to maintain. There havealways been pros and cons to vials versus bagsas a storage method. Vials have the advantagethat the cord blood is stored in multipleportions, which gives the family the option touse part of the collection and save the rest. Vialshave the disadvantage that because they arevery small, they are very prone to temperaturefluctuations, and should not be transfered inthe lab without insulation. Bags have the ad-vantage that they are a “closed system” wherethe blood can be processed and stored in thesame container, thus minimizing the risk ofcontamination during a transfer. Bags have thedisadvantage that they shatter if not handledcarefully.
FIGURE 2. Collection of cord blood stem cells during a cesariansection

Mædica A Journal of Clinical Medicine, Volume 2 No.2 2007 147
CCCCCORD BBBBBLOOD SSSSSTEM CCCCCELLS – WWWWWHERE DDDDDO WWWWWE SSSSSTAND?
Diseases Treated by Blood Stem Cells
Hematopoietic stem cells are capable ofevolving into all the specific cell types in
the blood and immune system. They can befound in people of all ages.
When a patient requires a HematopoieticStem Cell Transplant (HSCT), the treating
physician will decide which source of stem cellsto use. This will depend on several factors,including but not limited to: the degree of matchbetween donor and patient (sometimes thedonor and patient are one and the sameperson), the expected speed of engraftment,and the amount of time available to search fora perfectly matching donor (Table 1)
TABLE 1. The main diseases that benefits from cord blood transplant or bone marrow transplant (adaptedfrom 21)

Mædica A Journal of Clinical Medicine, Volume 2 No.2 2007148
CCCCCORD BBBBBLOOD SSSSSTEM CCCCCELLS – WWWWWHERE DDDDDO WWWWWE SSSSSTAND?

Mædica A Journal of Clinical Medicine, Volume 2 No.2 2007 149
CCCCCORD BBBBBLOOD SSSSSTEM CCCCCELLS – WWWWWHERE DDDDDO WWWWWE SSSSSTAND?

Mædica A Journal of Clinical Medicine, Volume 2 No.2 2007150
CCCCCORD BBBBBLOOD SSSSSTEM CCCCCELLS – WWWWWHERE DDDDDO WWWWWE SSSSSTAND?
Legend:Yes = By using cord blood, the transplant can be performed and we can obtain the cure
= By using bone marrow, the transplant can be tempt and the goal of the procedure is curativeYes- = The transplant is being used but we can’t obtain the healing, only the increase of life expectation(Yes) = The transplant can be used and lately it was performed experimentally but in the present the results are not well precised± = In some cases we use the transplant and the definitive healing is possible but the procedure is not a standard one because of high riskexp = Just an experimental methodNo = Transplant is not performed
The crucial thing for a successful transplantis not the volume of the blood sample, but thenumber of stem cells it contains. These aremeasured with a stain ”CD34+” that picks outall mononuclear cells, including stem cells.
The optimal (transplant) dose is about 20million nucleated cells per kilogram of bodyweight (one kilogram equals 2.2 pounds).
“...patients who received no more than 10million nucleated cells per kilogram had a 75percent probability of death, whereas recipientsof at least 30 million nucleated cells per kilogramhad a 30 percent probability of death” (28).
On average, a cord blood sample contains8.6 million nucleated cells per millileter. Theoptimal transplant dose requires harvesting 1millileter of cord blood for every pound ofpatient weight (1 ml and 1 cc are the sameamount) (28, 29).
A few private cord blood banks freeze theblood whole, while most process it. There aretwo stages of processing: Volume Reduction andSeparation.
Volume Reduction can be accomplishedeither by sedimentation or spinning in a cen-trifuge. (Note: sedimentation uses a chemicalcalled “Hespan Starch” or “Hetastarch” whichis made by Dupont Pharmaceuticals.) After-wards, the blood components are separatedso that: red cells are on the bottom, plasma (aclear white liquid) is on top, and in the middleis a pinkish layer called the “buffy coat” whichcontains the white blood cells, including stemcells. At this point, a gentle spin in a centrifugeis sufficient to isolate the white blood cells. Mostpublic banks stop processing at this point andstore the white blood cells in bags.
Separation of mononuclear cells isaccomplished by further centrifuge spinning ofthe white cells. One protocol for doing this whichis routinely used by stem cell researchers iscalled “Ficoll-Hypaque” density gradientcentrifuge. The separation step reduces thevolume of the collection to only a few milliLiters,which makes storage in vialsfeasible.
MonoNuclear Cells: Hematopoietic stemcells are identified with a characteristic surfacemolecule called “CD34+”, but only 1-2% ofthe MNC are actually stem cells. When parentsbank cord blood, and they receive a follow-uplab report saying that so many billions of cellswere stored, the lab is actually counting MNC,not stem cells. This is the standard medicalprocedure.
Common sense suggests there is no reasonto process a sample when you don’t know if itwill ever be used. Separation of MNC adds tothe laboratory cost of processing, and this costis passed on to the consumer.
Financial Pro: Lower storage costs
The smaller is the volume of the final sample,the less cryogenic nitrogen it takes to preserveit.
Medical Pro: Advantages of removing redblood cells
This minimizes any reaction of the patientto incompatibilities with the ABO/Rh bloodtype of the donor. (Strange but true: twopeople can have “matching” HLA types butdifferent blood types).Moreover, the cryo-preservation solvent thatis added before freezing tends to rupture

Mædica A Journal of Clinical Medicine, Volume 2 No.2 2007 151
CCCCCORD BBBBBLOOD SSSSSTEM CCCCCELLS – WWWWWHERE DDDDDO WWWWWE SSSSSTAND?
red cells. Thus, when the stem cells arethawed for transplant they must be washedto remove the solvent and the hemoglobinreleased from the ruptured cells (it is toxicto kidneys).Given the above, some banks will brag abouthaving a very low “final hematocrit”, whichrefers to the red cell count after processing.
Medical Counter-argument: Red cells don’thave to be removed
Cord blood transplants are less sensitive toblood type incompatibilities than transplants
of adult bone marrow. The earliest cord bloodtransplants used whole blood. Nonetheless, atthis time it has become standard medicalpractice in the USA to separate out red cellsprior to freezing (2,3,4).
It is inevitable that some stem cells will belost in the process of skimming off the buffycoat or separating the MNC. Just what fractionof the stem cells are lost is hotly debated.Everybody likes to claim that their method ofprocessing is better because they lose fewer cells– BUT – studies in the medical literature (seebelow) do not even find a significant differencebetween the viability of processed versus un-processed cord blood. There is a large uncer-tainty, about 20%, in measuring the recovery
of viable stem cells, and this uncertainty exceedsthe difference between one processing methodversus another.
Rubinstein P. reports “almost total recovery”of viable hematopoietic progenitor cells aftervolume reduction, freezing, and thawing (30).After Ficoll gradient separation of cord blood,the recovery rates of MNC and CD34+ cellswere, respectively, 55% and 107% (a recoveryover 100% indicates this experiment is not veryaccurate – editor) (31). Kletzel M. found nostatistically significant difference betweenseparated UCB versus unmanipulated UCBregarding cell viability or recovery of MNC (32).
Although cord blood is so great, not every-body bank it because it costs money. Whereasa bone marrow registry is just a data base ofpotential donors, a cord blood registry consistsof freezers full of frozen blood and staff tomaintain them. In an ideal world, all babieswould have their cord blood harvested at birth(with parental permission) and stored in publicregistries, much like public blood banks. Inpractice, only a limited number of institutionshave the funding to maintain public banks whichtake donations for free. For most parents, cordblood donation is not an option because thenumber of locations served by public banks isvery limited.
REFERENCES
1. Wilson A, Trumpp A – Bone-marrowhaematopoietic stem-cell niches. NatRev Immunol Feb 2006; 6(2):93-106
2 . Gluckman E et al – Hematopoieticreconstitution in a patient withFanconi´s anemia by means ofumbilical-cord blood from an HLA-identical sibling. N Engl J Med 1989;321(17):1174-1178
3 . Wagner JE et al – Allogeneic siblingumbilical-cord-blood transplantationin children with malignant and non-malignant disease. Lancet 1995;346(8969):214-219
4 . Hahn T et al – Use of nonvolume-reduced (unmanipulated afterthawing) umbilical cord blood stemcells for allogeneic transplantationresults in safe engraftment. BoneMarrow Transplant 2003; 32(2):145-150
5 . Wang JC, Doedens M, Dick JE –Primitive human hematopoietic cellsare enriched in cord blood comparedwith adult bone marrow or mobilizedperipheral blood as measured by thequantitative in vivo SCID-repopulating cell assay. Blood 1997Jun 1; 89(11):3919-3124
6 . Hogan CJ, Shpall EJ, McNulty O etal – Engraftment and development ofhuman CD34(+)-enriched cells fromumbilical cord blood in NOD/LtSz-scid/scid mice. Blood 1997 Jul 1;90(1):85-96
7 . Barker JN, Krepski TP, DeFor TE etal – Searching for unrelated donorhematopoietic stem cells: availabilityand speed of umbilical cord bloodversus bone marrow. Blood MarrowTransplant 2002; 8(5):257-260
8 . Wiley JM, Kuller JA – Storage ofnewborn stem cells for future use.Obstet Gynecol 1997 Feb; 89(2):300-303
9 . Smith FO, Thomson BG – Umbilicalcord blood collection, banking, andtransplantation: current status andissues relevant to perinatal caregivers.Birth 2000 Jun; 27(2):127-135
10. Takahashi S, Iseki T, Ooi J et al –Single-institute comparative analysisof unrelated bone marrowtransplantation and cord bloodtransplantation for adult patientswith hematologic malignancies. Blood2004 Dec 1; 104(12):3813-3820. Epub2004 Jul 27
11. Rubinstein P, Carrier C, ScaradavouA et al – Outcomes among 562recipients of placental-blood

Mædica A Journal of Clinical Medicine, Volume 2 No.2 2007152
CCCCCORD BBBBBLOOD SSSSSTEM CCCCCELLS – WWWWWHERE DDDDDO WWWWWE SSSSSTAND?
transplants from unrelated donors. NEngl J Med 1998 Nov 26;339(22):1565-1577
12. Rocha V et al – Graft-versus-HostDisease in Children Who HaveReceived a Cord-Blood or BoneMarrow Transplant from an HLA-Identical Sibling. NEJM 2000;342:1846-1854
13. Grewal SS, Barker JN, Davies SM etal – Unrelated donor hematopoieticcell transplantation: marrow orumbilical cord blood? Blood 2003 Jun1; 101(11):4233-4244. Epub 2003 Jan9
14. Wadlow RC, Porter DL – Umbilicalcord blood transplantation: where dowe stand? Biol Blood MarrowTransplant 2002; 8(12):637-647
15. Christopherson KW II et al –Modulation of Hematopoietic StemCell Homing and Engraftment byCD26. Science 13 Aug 2004;305(5686):1000-1003
16. McMilin K – Transfusion April 12002; vol. 42(issue 4):495
17. Fraser CJ et al – First report of donorcell derived acute leukemia as acomplication of umbilical cord bloodtransplantation. Blood 23 Aug 2005
18. Wadlow & Porter – Umbilical cordblood transplantation: where do westand? Biology of Blood & MarrowTransplantation vol.8 2004; (issue12):637-647
19. Laughlin MJ et al – Outcomes aftertransplantation of cord blood or bonemarrow from unrelated donors inadults with leukemia. NEJM Nov2004; 351(22):2265-2275
20. Rocha V et al – Transplants ofumbilical-cord blood or bone marrowfrom unrelated donors in adults withacute leukemia. NEJM Nov 2004;351:2276-2285
21. Barker JN et al – Biology of Blood &Marrow Transplantation 2002;vol.8(issue 5):257-260
22. Wagner J, Barker J – Umbilical cordblood transplantation after a non-myeloablative therapy in high riskadults. Biol Blood Marrow TransplantOct 2004; 10(10):733-734
23. Kobylka P et al – Preservation ofimmunological and colony-formingcapacities of long-term (15 years)cryopreserved cord blood cells.Transplantation 1998; 65(9):1275-1278
24. Mugishima H et al – Effects of long-term cryopreservation onhematopoietic progenitor cells inumbilical cord blood. Bone MarrowTransplant 1999; 23(4):395-396
25. Broxmeyer HE et al – High-efficiencyrecovery of functional hematopoieticprogenitor and stem cells from humancord blood cryopreserved for 15 years.pnas. 0237086100, 2003
26. Pasquini MC, Logan BR, Verter F –The likelihood of hematopoietic stem
cell transplantation (HCT) in theUnited States: implications forumbilical cord blood storage. 2005;106:1330
27. Bertolini F et al – Comparativestudy of different procedures for thecollection and banking of umbilicalcord blood. J Hematother Feb 1995;4(1):29-36
28. Gluckman E – Editorial. NEJM 2001;344:1860
29. W Reed et al – Blood 2003;101(1):351
30. Rubinstein P et al – Processing andcryopreservation of placental/umbilical cord blood for unrelatedbone marrow reconstitution. Proc NatlAcad Sci USA 1995; 92(22):10119-10122
31. Sato J et al – Quantitative andqualitative comparative analysis ofgradient-separated hematopoieticcells from cord blood andchemotherapy-mobilized peripheralblood. Stem Cells 1995; 13(5):548-555
32. Kletzel M et al – Red cell depletionof umbilical cord blood (UCB):comparison between unmanipulatedand red cell-depleted UCB by Ficoll-Paque density gradient separation. JHematotherapy 1997; 6(3):269-272

![STEM CELLS EMBRYONIC STEM CELLS/INDUCED PLURIPOTENT STEM CELLS Stem Cells.pdf · germ cell production [2]. Human embryonic stem cells (hESCs) offer the means to further understand](https://static.fdocuments.in/doc/165x107/6014b11f8ab8967916363675/stem-cells-embryonic-stem-cellsinduced-pluripotent-stem-cells-stem-cellspdf.jpg)