ChemInform Abstract: A Novel Anionic Domino Process for the Synthesis of...
-
Upload
sarvesh-kumar -
Category
Documents
-
view
213 -
download
0
Transcript of ChemInform Abstract: A Novel Anionic Domino Process for the Synthesis of...
2008
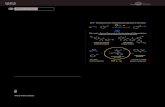
NitrilesQ 0520 A Novel Anionic Domino Process for the Synthesis of o-Cyanoaryl-Methylthio/
Alkyl/Aryl/Heteroaryl Acetylenes. — Rearrangement of 3,3-bis(methylthio) o-bro-moarylacrylonitriles (I) provides an efficient access towards o-(methylthioethynyl) benzonitriles (II). Variations at the ethynyl group are achieved by either quenching of the reaction mixture with electrophilic agents [cf. (III) or (V)] or by direct substitution of one methylthio group by (het)aryl or alkyl group [cf. (VII)]. Interestingly, cyclic dithioacetals like (VIII) undergo direct acetal ring opening—recyclization without shift of the nitrile group. — (KUMAR, S.; PERUNCHERALATHAN, S.; ILA*, H.; JUNJAPPA, H.; Org. Lett. 10 (2008) 5, 965-968; Dep. Chem., Indian Inst. Technol., Kanpur 208 016, India; Eng.) — Mischke
32- 081