heteroaryl direct arylation review
Transcript of heteroaryl direct arylation review

DOI: 10.1002/cctc.200900074
Palladium-Catalyzed C3 or C4 Direct Arylation ofHeteroaromatic Compounds with Aryl Halides by C�HBond ActivationJulien Roger, Aditya L. Gottumukkala, and Henri Doucet*[a]
20 � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim ChemCatChem 2010, 2, 20 – 40

1. Introduction
There exists a variety of routes for the construction of aryl–arylbonds. Some of the most common methods are through theuse of transition metal-mediated reactions. Among thesemethods, the Suzuki–Miyaura, Stille, and Negishi couplingsrepresent some of the most important procedures.[1] However,these reactions require the preliminary preparation of organo-metallic or organoboron aryl derivatives, and provide anorganometallic salt (MX) as a byproduct.
If aryl C�H bonds can be used as functional groups, theyprovide a valuable and straightforward technique for thesynthesis of biaryls. In recent years, direct C2 or C5 arylation ofheteroaromatic compounds with aryl halides by C�H bondactivation provides simple access to the corresponding aryl–heteroaryl derivatives.[2–9] The concept of using a directinggroup to control the regioselectivity of the subsequent transi-tion metal insertion into a C�H bond is often employed for theintermolecular direct arylation of aromatic carbocycles. Con-versely, this concept is rarely employed in the presence ofaromatic heterocycles. Unlike the intermolecular direct aryla-tion of carbocyclic arene systems, the inherent electronic biasof the heterocycle itself is often sufficient to control the regio-selectivity of direct arylation reactions. Using 2-substituted thi-ophenes, furans, thiazoles, oxazoles, or imidazoles, the 5-arylat-ed products were selectively obtained in most cases,[2, 10–16]
whereas 5-substituted thiazoles or oxazoles gave the 2-arylatedderivatives.[2, 17, 18] The selective 2-arylation of unsubstitutedoxazoles or imidazoles has also been reported.[2, 15, 19] With ben-zoxazoles or benzothiazoles as the substrate, 2-arylbenzoxa-zoles or 2-arylbenzothiazoles were obtained in good yields.[2, 20]
The arylation of unsubstituted triazoles preferentially gave the5-arylated compounds. The 2-arylation of pyridine or diazinederivatives also proceeded in high yields.[2] For selected hetero-aromatic compounds, the high reactivity of some of the cata-lysts employed in these reactions allowed the use of as little as0.01 mol % catalyst loadings, making such arylation reactionsindustrially attractive.[10, 12, 13, 16] The most favorable positions for
direct arylation of various heteroaromatics are represented inFigure 1.
Although palladium-catalyzed direct C2 and C5 arylations ofheteroaromatic compounds are strongly favored in general,the regioselectivity in intermolecular direct arylation reactions
is also influenced by the substituents present on the hetero-cycle and the nature of the catalyst, base, solvent, or additivesemployed. Until recently, relatively little effort had been ex-pended towards developing such direct arylation reactions forthe synthesis of 3- or 4-arylated pyrroles, thiophenes, furans,thiazoles, oxazoles, imidazoles, isoxazoles, triazoles or pyridines(Figure 2), as reactions with such substrates are generally con-
In recent years, palladium-catalyzed direct C2 or C5 arylation ofheteroaromatic compounds with aryl halides by C�H bond ac-tivation has become a popular method for generating carbon–carbon bonds. For this reaction, a wide variety of heteroaro-matics, such as furans, thiophenes, pyrroles, thiazoles, oxazoles,imidazoles, pyrazoles, indoles, triazoles, or even pyridines, canbe employed. C3 and C4 arylations of heteroaromatics by C�Hbond activation have also been described. Such reactions ini-tially attracted much less attention than the C2 or C5 arylationsdue to the lower reactivity of the C3 and C4 positions. How-ever, in more recent years, several results from using modifiedand improved catalysts and reaction conditions have beenreported, which permit C3 and C4 arylations in syntheticallyuseful yields. Several intramolecular cyclizations of 2-substitut-ed heterocycles have been described, with formation of a C�Cbond on C3 resulting in the formation of five- to nine-mem-
bered rings incorporating pyrroles, indoles, thiophenes, furans,isoxazoles, or pyridines. Intermolecular C3 or C4 direct aryla-tions are still quite rare for some heteroaromatics and are inseveral cases not highly regioselective. For such reactions, thebest results have been obtained using pyrroles, thiophenes, orfurans. For selected substrates, regioselective arylation at C3 orC4 of the heteroaromatic compounds took place under appro-priate reaction conditions. Only a few examples of intermolec-ular couplings using oxazoles, thiazoles, imidazoles, isoxazoles,pyrazoles, triazoles, or pyridines have been reported. For mostof these reactions, aryl iodides or bromides have been used ascoupling partners, although a few examples with aryl chloridesare also known. This method allows the synthesis of complexmolecules in only a few steps, and will provide access to avery wide variety of new heteroaryl derivatives in the nextyears.
Figure 1. Most favorable positions for direct arylation of heteroaromatic sub-strates.
[a] J. Roger, A. L. Gottumukkala,+ Dr. H. DoucetInstitut Sciences Chimiques de RennesUMR 6226 CNRS-Universit� de Rennes 1Campus de Beaulieu, 35042 Rennes (France)Fax: (+ 33) 0223236939E-mail : [email protected]
[+] Present address:Stratingh Institute for Chemistry, University of GroningenNijenborgh 4, 9747 AG Groningen (The Netherlands)
ChemCatChem 2010, 2, 20 – 40 � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim www.chemcatchem.org 21

sidered to be much slower and gave mixtures of isomersduring initial attempts. However, such arylations on C3 or C4are possible, especially in the presence of 2,5-disubstitutedpyrroles, furans, or thiophenes, and of 5-substituted isoxazoles,pyrazoles, or triazoles. Several catalysts, such as novel palladi-um–phosphane complexes, and new reaction conditions havebeen employed to give such coupling products in moderate tohigh yields.
This procedure is of particular interest as relatively few C3/C4-arylated heteroaromatic compounds are readily availablecommercially at an affordable cost. A chief advantage of thisprocedure is that the major byproducts are HX associated to abase, rather than metallic salts (as with classical coupling pro-cedures). Moreover, no prior preparation of an organometallicderivative is required, reducing the number of steps to preparethese compounds. For these reasons, such procedures shouldgive economically viable and environmentally attractive accessto C3 or C4 arylated heterocycles.
Although mechanistic studies have not been performed formost of the reactions discussed in this review, some mecha-nisms have been proposed (Scheme 1). The first step of the
catalytic cycle is certainly the oxidative addition of palladiuminto the aryl halide bond. Then, the reaction might proceed byseveral pathways. One proposed mechanism for of p-excessivearomatic substrates, such as indoles, is the electrophilic aro-matic substitution (SEAr).[3, 21] In SEAr reactions, the attack of theArPdX intermediate may occur at the most reactive position ofthe heteroaromatic substrate. The Heck-type process[2] and,more recently, the concerted metalation–deprotonation (CMD)pathway[22] have also been suggested. Fagnou and co-workersreported that the experimental and computational datasupport the involvement of a CMD pathway for p-excessivearomatics. They found a remarkable similarity between thecomputationally predicted and experimentally observedreaction outcomes.
The palladium-catalyzed direct arylation of heteroaromaticsubstrates has been discussed in recent years in several rele-vant review articles and accounts.[4–8] However, these articleswere mostly concerned with C2 or C5 arylation reactions offive-membered heteroaromatics or direct intramolecular or
Henri Doucet received his PhD in
chemistry working with Prof. P. H.
Dixneuf and Dr. C. Bruneau at the
University of Rennes 1 in 1994. After
post-doctoral appointments at Oxford
University (J. M. Brown) and Nagoya
University, Japan (R. Noyori), he moved
to the University of Marseille as CNRS
researcher. In 2006 he returned to the
University of Rennes 1. His research
interests include organic synthesis by
metal-catalyzed processes, ligand
synthesis and green chemistry.
Julien Roger received his graduate
education in chemistry at the Universi-
ty of Rennes 1. Currently, he is working
towards his PhD on palladium-cata-
lyzed C�H activation for the direct ary-
lation of heteroaromatic compounds
at the University of Rennes 1, under
the supervision of Dr. Henri Doucet.
Aditya L. Gottumukkala was born in
Tirupati, India. He received his under-
graduate education in chemistry at
Loyola College, Chennai. Upon receiv-
ing a grant from EGIDE, he joined the
University of Rennes 1 for his masters
program, during which he investigated
palladium-catalyzed C�H activation,
under the guidance of Dr. Henri
Doucet. Currently, he is working
towards his PhD, in the research group
of Prof. Adriaan J. Minnaard, at the
University of Groningen, the Nether-
lands.
Figure 2. Less favorable positions for direct arylation of heteroaromatic sub-strates.
Scheme 1. Three potential pathways for the arylation of aromaticheterocycles. Y = heteroatom.
22 www.chemcatchem.org � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim ChemCatChem 2010, 2, 20 – 40
H. Doucet et al.

bimolecular arylation of benzene derivatives, or strongly em-phasized the author’s own work. Herein we focus on palladi-um-catalyzed direct C3 or C4 arylation of heteroaromatic com-pounds with aryl halides, outlining the developments and ad-vances in both the intramolecular and intermolecular reactions.Aryl–heteroaryl bond formations using stoichiometric amountsof metals or by oxidative coupling reactions are not discussed.Firstly, we will discuss the C3 or C4 arylation of pyrroles and C3arylation of indole derivatives, followed by C3 or C4 arylationof furans and of thiophenes. The subsequent sections are con-cerned with coupling reactions of aryl halides with thiazoles,oxazoles, imidazoles, isoxazoles, and pyrazoles and the recentlyreported C4 arylation of triazoles, before we present several ex-amples of C3 or C4 arylations of pyridines and, to conclude,describe the remaining challenges in the field.
2. 3- or 4-Arylation of Pyrrole or IndoleDerivatives
The palladium-catalyzed direct arylation of furans or thio-phenes generally gives the 2- or 5-arylated products with highregioselectivity. Conversely, the electronic properties ofpyrroles or indoles appear to be much less favorable to regio-selective 2-arylations. As a consequence, when appropriatereaction conditions are employed, the direct regioselective3-arylation of indoles or pyrroles is possible. Several examplesof direct arylations on C3 of indoles using either intra- or inter-molecular reactions have been reported. Some examples ofintramolecular cyclizations on C3 of pyrroles have also beendescribed. On the other hand, the bimolecular coupling on C3using pyrroles has attracted less attention.
Intramolecular palladium-catalyzed direct 3-arylation reac-tions of pyrroles or indoles have been utilized in organic syn-thesis as a simple route for the synthesis of complex polycyclicring systems. The first part of this section focuses on thedevelopments of this important reaction. In 1991, Ma andKozikowski reported the intramolecular cyclization of an indolesubstituted by 2-bromobenzoyl at C2 [Eq. (1)] .[23] This reactionwas performed in the presence of [Pd ACHTUNGTRENNUNG(PPh3)4] as catalyst andKOAc as base at 130 8C in N,N-dimethylacetamide (DMAc). Thetetracyclic product was obtained in 95 % yield. In the course ofthis reaction, a five-membered ring was formed. Using thesame catalyst, a six-membered ring was formed by M�rour andco-worker, in the course of the palladium-catalyzed cyclizationof an indole substituted on C2 by a 2-bromophenyl ester.[24]
Similar intramolecular cyclizations of 2-substituted indoles re-sulting in the formation of seven-membered rings have alsobeen described [Eq. (2) and (3)] . Indoles bearing a 2-iodoben-zylcarbamate substituent at C2 gave the expected cyclizationproducts in 90–96 % yield using a catalyst system of 5 mol %Pd ACHTUNGTRENNUNG(OAc)2 and 10 mol % PPh3 with Ag2CO3 as a base.[25] Using asimilar catalyst and CsOAc as base, an indole with a (2-bromo-benzyl)butylamine derivative as 2-substituent gave thedihydrobenzoazepine-fused indole in high yield.[26]
Intramolecular cyclization of an indolizine substituted by a2-bromobenzoyl group at C2 gave a tetracyclic compoundwith the formation of a five-membered ring in 43 % yield, in-
stead of the desired benzocyclazinone derivative [Eq. (4)] .[27]
Again, Pd ACHTUNGTRENNUNG(OAc)2/PPh3 was employed as the catalyst andAg2CO3 as the base.
Several intramolecular cyclizations of 2-substituted pyrroleshave also been reported [Eq. (5)–(10)] . For example, intramo-lecular cyclization of 1,5-dichloroanthracene substituted at C9and C10 by two 1-methylpyrrol-2-yl substituents allowed thesynthesis of substituted rubicenes [Eq. (5)] .[28] However, a rela-tively low yield was obtained, probably due to some polymerformation or decomposition.
1-Methylpyrroles with 2-iodobenzylcarbamate substituentsat C2 have also been cyclized using conventional or microwaveheating [Eq. (6)] .[29] With conventional heating, satisfactoryyields could only be obtained from aryl iodides. A significantamount of unreacted starting material was recovered, even
ChemCatChem 2010, 2, 20 – 40 � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim www.chemcatchem.org 23
Palladium-Catalyzed C3 or C4 Direct Arylation of Heteroaromatic Compounds

after 72 h, from a substrate with a bromopyridine substituent.However, with this more challenging substrate, an impressiveyield of 90 % was obtained after only 30 min using microwaveheating. Using a similar reactant, Cavell and co-workers did notsucceed to obtain the desired cyclized product [Eq. (7)] .[30] De-spite several attempts, they were unable to detect the productby either GC-MS or NMR spectroscopy.
The cyclization of 2-substituted pyrroles is not limited to theformation of five- or six-membered rings. Seven- and nine-membered rings have also been formed in such reactions[Eq. (8) and (9)] .[31, 32] In both cases, the catalysts systems were10 mol % Pd ACHTUNGTRENNUNG(OAc)2 with monophosphine ligands. The forma-tion of the nine-membered lactam in 47 % yield, by cyclizationof a pyrrole bearing an alkyl chain terminated by 2-iodopheny-lacetamide, was the key step for the synthesis of biologicallyactive rhazinilam. A pentacyclic compound was obtained by adouble C�H bond activation/arylation of a trisubstitutedpyrrole [Eq. (10)] .[33] During the course of this reaction, two six-membered rings were formed. However, the yield of thetarget product was quite low.
Although intramolecular palladium-catalyzed C3 direct aryla-tion of pyrroles has been described in several publications, theintermolecular version of this reaction has attracted much lessattention, probably owing to the more challenging control ofthe regioselectivity of such arylations. In 1992, Filippini and co-workers reported the direct arylation of pyrrolyl anions withbromobenzene [Eq. (11)] .[34] Pyrrol-1-ylzinc bromide or chlorideunderwent coupling with bromobenzene in the presence of[PdCl2ACHTUNGTRENNUNG(PPh3)2]/PPh3 in N-methylpyrrolidone (NMP) to give amixture of 2- and 3-phenylpyrrole. The nature of the cation onthe pyrrole ring had an important effect on the regioselectivityof the reaction. However, the highest reported yield of theC3-arylated product was only 10 %
Also in 1992, Ohta and co-workers reported the direct 3-arylation of N-phenylsulfonylpyrrole with 3,6-dialkyl-2-chloro-pyrazines [Eq. (12)] .[35] A mixture of 2- and 3-arylated pyrroleswas obtained, which could not be separated. The presence ofbulky groups on the pyrazine ring favored the production of 3-substituted pyrroles. The reaction with 2-chloro-3,6-dimethyl-pyrazine gave an equimolar mixture of 2- and 3-arylatedpyrroles, whereas that with 2-chloro-3,6-diisobutylpyrazinegave the 2- and 3-arylated pyrroles in a 2:5 ratio.
Recently, the direct C3 arylations of 1,2,5-trimethylpyrrole,2,5-dimethyl-1-phenylpyrrole, and 2,5-dimethylpyrrole usingPd ACHTUNGTRENNUNG(OAc)2 as catalyst were reported [Eq. (13) and (14)] .[36] The
24 www.chemcatchem.org � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim ChemCatChem 2010, 2, 20 – 40
H. Doucet et al.

coupling of para-substituted electron-deficient aryl bromides,such as 4-bromoacetophenone, 4-bromopropiophenone,4-bromobenzaldehyde, or 4-trifluoromethylbromobenzene, to1,2,5-trimethylpyrrole afforded the expected products in55–61 % yields. Slightly lower yields were obtained using themeta-substituted aryl bromides, 3-bromoacetophenone or 3-bromonitrobenzene. The electron-poor heteroaryl bromides, 3-bromopyridine, 3-bromoquinoline, and 4-bromoisoquinoline,gave the 3-arylated pyrroles in 53, 54, and 65 % yields, respec-tively. Quite similar results to those for the coupling with 1,2,5-trimethylpyrrole, were obtained using 2,5-dimethyl-1-phenyl-pyrrole [Eq. (14)] . With 4-bromobenzophenone or 4-bromoben-zonitrile, the C3-arylated products were obtained in 41 and50 % yields, respectively. The reactivity of free (NH) 2,5-dime-thylpyrrole was also examined [Eq. (14)] . With this substrate,the target compounds were formed in moderate yields using4-bromobenzonitrile or 4-trifluoromethylbromobenzene. Theformation of 2,5-dimethyl-1-arylpyrroles was not detected.
This group has also demonstrated that, using an unsymmet-rically 2,5-disubstituted pyrrole, control of the regioselectivityof the arylation is possible. By coupling 1,5-dimethyl-2-pyrrole-carbonitrile with 4-bromoacetophenone, they initially obtaineda mixture of C3- and C4-arylated products [Eq. (15)] .[36] Howev-er, the ratio of regioisomers and the yield strongly dependedon the reaction conditions. The use of KOAc as base, DMAc assolvent and Pd ACHTUNGTRENNUNG(OAc)2 as catalyst led to a complete conversionof the aryl bromide. Moreover, these reaction conditions gavea high selectivity in favor of the C4 arylation product (C3/C4ratio = 1:4). Other palladium sources, such as PdCl2, [Pd2ACHTUNGTRENNUNG(dba)3](dba = trans,trans-dibenzylideneacetone), and [{PdCl ACHTUNGTRENNUNG(C3H5)}2] ,gave moderate conversions and selectivities. The presence ofthe phosphine ligands PPh3 or dppe was not found to beadvantageous. Under these reaction conditions, 4-bromoben-zophenone, 4-bromonitrobenzene, and 4-bromobenzaldehyde
were coupled with 1,5-dimethyl-2-pyrrolylcarbonitrile to givethe desired 4-arylated products in 46–60 % yields. Conversely,when the electron-rich aryl bromide 4-bromoanisole was usedas a coupling partner, no arylation product was detected. Inthe presence of sterically congested bromobenzene deriva-tives, the coupling products were obtained. Ortho-substituted2-bromobenzonitrile reacted with 1,5-dimethyl-2-pyrrolylcarbo-nitrile to give the C4-arylated pyrrole in 64 % yield. The reactiv-ity of heteroaryl bromides has also been explored. A selectivereaction also took place with 4-bromoisoquinoline. With thissubstrate the C4 arylation product was obtained in 64 % yield.
A great deal of attention has been given in recent years tothe Pd-catalyzed intermolecular direct 3-arylation of indoles[Eqs. (16)–(26)] . N-Phenylsulfonylindole reacted with 3,6-dialkyl-2-chloropyrazines to give, very regioselectively, the 3-arylatedindoles [Eq. (16)] .[37, 38] Again, more bulky groups on the chloro-pyrazine ring led to higher regioselectivities in favor of 3-aryla-tion. In the presence of isopropyl or isobutyl substituents onpyrazines, 3-arylindoles were obtained highly regioselectively(C2/C3 ratio = 1:50). Substitution at the nitrogen atom of theindole also had a determining effect on the regioselectivity ofthis coupling reaction. Under the same reaction conditions, thecoupling of 1-methylindole or 1-benzylindole with chloropyra-zines led regioselectively to the 2-arylated indoles.
Sames and co-workers studied the regioselectivity of this re-action using aryl iodides. They initially reported that the aryla-tion of 1-methylindole using Pd ACHTUNGTRENNUNG(OAc)2/PPh3 as catalyst andCsOAc as base in DMAc led highly selectively to the 2-arylatedindoles when using para-substituted aryl iodides, whereas, anortho-substituted aryl iodide gave a mixture of 2- and 3-arylat-ed indoles in 29:38 ratio [Eq. (17)] .[39] The formation of mixturesof 2- and 3-arylated indoles using congested haloarenes wasexplained by a slower migration of palladium from C3 of theindole to C2 with such substrates. The deprotonation of theC3-metalated indole produces the 3-arylindole.[21, 40] Consider-ing that the regioselectivity of such arylations can be influ-enced by several factors, Sames and co-workers explored theinfluence of the nature of the magnesium salt on indoles onthe regioselectivity in the presence of iodobenzene. CongestedMeMgCl/tmeda or [MgACHTUNGTRENNUNG(HMDS)2] gave 57 and 74 % yields of3-phenylindole, respectively [Eq. (18); tmeda = N,N,N’,N’-tetra-methylethylenediamine, HMDS = hexamethyldisilazide] .
ChemCatChem 2010, 2, 20 – 40 � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim www.chemcatchem.org 25
Palladium-Catalyzed C3 or C4 Direct Arylation of Heteroaromatic Compounds

The same group also reported that the coupling using aSEM-protected indole [SEM = 2-(trimethylsilyl)ethoxymethyl] , inthe presence of a palladium complex containing a carbeneligand and triphenylphosphine generally gave the 2-arylatedindole, except when using 2-iodotoluene [Eq. (19)] .[41] With thiscongested substrate, 11 % of the 3-arylated product was alsoformed. The reactivity of a 2-substituted free (NH) indole wasalso described by this group [Eq. (20)] .[42] The arylation of2-phenylindole with 4-trifluoromethylbromobenzene using1 mol % Pd ACHTUNGTRENNUNG(OAc)2 as catalyst gave the C3-arylated product in46 % yield. However, this unreactive substrate required thepresence of a stoichiometric amount of Bu4NCl in the reactionmixture. This additive presumably stabilizes the Pd species.
Djakovitch and co-workers also studied the 3-arylation of in-doles using Pd ACHTUNGTRENNUNG(OAc)2 as catalyst. The selectivity of the reaction(N1 vs. C3 arylation) using free (NH) 2-phenylindole was mostlydirected by electronic factors [Eq. (21)] .[43, 44] Tuning of the reac-tion conditions allowed either a selective N1 or C3 arylation. Inthe presence of 4-iodonitrobenzene, the N1-arylated productwas exclusively obtained; whereas 4-bromonitrobenzene gaveonly C3 arylation in 50 % yield. The use of AgBF4 as an additiveimproved the yield of C3 arylation to 74 %. Very different be-
havior was observed using methyl indole-2-carboxylate. Withthis substrate, the arylation using 4-iodo- or 4-bromonitroben-zene led selectively in both cases to the C3-arylated indole.This procedure is limited to activated aryl bromides. Neitherbromo- nor iodobenzene gave arylation product under thesereaction conditions.
This group also reported the 3-arylation of free (NH) indolesusing a heterogeneous palladium catalyst [Eq. (22)] .[45] Employ-ing K2CO3 as base, only 1 mol % of [Pd ACHTUNGTRENNUNG(NH3)4]2+ supported onzeolite NaY as catalyst in refluxing dioxane, unsubstituted andC2-substituted indoles gave up to 92 % conversion and up to85 % yield of the C3-arylated indoles. However, the yieldstrongly depended on the substituents on the aryl bromideand on the indole. For example, 40 % yield of coupling productwas obtained using indole and 4-bromoanisole, whereas noproduct was formed with the same aryl bromide and 2-methyl-indole or 2-phenylindole.
The direct 3-arylation of free (NH) indoles was also reportedrecently by He and co-workers [Eq. (23)] .[46] Using a palladium–phosphinous acid complex, K2CO3 or KOH, and dioxane, theyobtained selectively the 3-arylated indoles in moderate togood yields. Both electron-rich and electron-poor aryl bro-mides were employed as reaction partners. The highest yield
26 www.chemcatchem.org � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim ChemCatChem 2010, 2, 20 – 40
H. Doucet et al.

(71 %) was obtained using bromobenzene. Electron-deficientaryl bromides, such as 4-bromobenzonitrile or 4-bromonitro-benzene, led to the target compounds in lowers yields of 52and 48 % respectively. Indoles substituted at C5 with methoxyor cyano groups were also found to be useful reactants for thisreaction. However, 2-acetylindole and 5-nitroindole both failedto couple with bromobenzene.
Very recently, Rossi and co-workers examined the influenceon arylation regioselectivity of a wide variety of phosphineligands associated to Pd ACHTUNGTRENNUNG(OAc)2 for the coupling of 4-bromoani-sole with free (NH) indole (Table 1).[47] The monophosphinesPPh3 and PCy3 gave the 3-arylated indole in 60 and 57 % yields,respectively. Sterically very congested and electron-rich ligandsP ACHTUNGTRENNUNG(tBu)3 and PBu ACHTUNGTRENNUNG(1-Ad)2 gave only N1 arylation. Bidentatephosphine ligands 1,1-bis(diphenylphosphanyl)ferrocene(dppf), 1,3-bis(diphenylphosphanyl)propane (dppp), and 2,2’-bis(diphenylphosphanyl)-1,1’-binaphthyl (binap) gave selective-ly the 3-arylated indole, albeit in moderate yields. However, onstudying the scope and limitations of this procedure, they es-tablished that the reaction conditions (Pd ACHTUNGTRENNUNG(OAc)2/PCy3, toluene,K2CO3 with 2-phenyl- or 2-methylindole as a coupling partner)did not produce satisfactory results. Ligand-free reaction condi-tions with added BnBu3NCl, in several cases greatly improvedthe yields. Under these conditions, the arylation of 2-methyl-or 2-phenylindole with 4-bromoanisole gave the 3-arylated in-doles in 62 and 74 % yields, respectively. On the other hand,ethyl indole-2-carboxylate in the presence of 4-bromoanisole
was recovered unreacted. This ligand-free palladium procedurealso tolerated some functionalized aryl bromides. The authorsproposed a catalytic cycle based on an electrophilic palladationpathway at the C3 position of 1-indolylpotassium salts.
Using an air-stable heteroatom-substituted secondary phos-phine oxide (HASPO) preligand and 5 mol % Pd ACHTUNGTRENNUNG(OAc)2 as thecatalytic system, Ackermann and Barf�sser were also able toarylate free (NH) indole regioselectively at C3 [Eq. (24)] .[48]
Diversely functionalized aryl bromides were employed ascoupling partners.
Beletskaya and co-workers also studied the arylation of in-doles (Scheme 2).[49] In the presence of a magnesium salt ofindole, they obtained quite selectively the 3-arylated indole,with [Pd{P ACHTUNGTRENNUNG(tBu)3}2] as catalyst. Lower selectivities were detectedfor the coupling of other indole salts (Scheme 2 a). They alsoexplored the regioselectivity of the reaction in the presence ofa variety of ligands, using [Pd ACHTUNGTRENNUNG(dba)2] as the palladium source,and, in contrast to the results obtained by Rossi, the nature of
Table 1. Effect on regioselectivity of phosphine ligands in the couplingof 4-bromoanisole with indoles.[47]
Ligand or additive R1 R2 SC3 [%][a] Yield (C3) [%][b]
PPh3 H OMe 97 60PCy3 H OMe 95 57dppf H OMe 93 53dppp H OMe 98 56binap H OMe 96 54BnBu3NCl H OMe – 56BnBu3NCl H CF3 – 80BnBu3NCl H NO2 – 0BnBu3NCl Me OMe – 62BnBu3NCl Ph OMe – 74
[a] SC3 = selectivity towards C3 regioselectivity; [b] yield of C3-regioselec-tive product ; dppf = 1,1-bis(diphenylphosphanyl)ferrocene; dppp = 1,3-bis(diphenylphosphanyl)propane; binap = 2,2’-bis(diphenylphosphanyl)-1,1’-binaphthyl.
ChemCatChem 2010, 2, 20 – 40 � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim www.chemcatchem.org 27
Palladium-Catalyzed C3 or C4 Direct Arylation of Heteroaromatic Compounds

the ligand was found to dramatically influence the regioselec-tivity of the arylation (Scheme 2 b). The highest selectivity in3-phenylindole was obtained using PACHTUNGTRENNUNG(tBu)3 as the ligand,whereas Xantphos gave almost exclusively 2-phenylindole.
The indole direct 3-arylation methodology was appliedrecently for the synthesis of fluorescent rhodamine dyes.[50]
N-Methylindole was arylated at C3 in 61 % yield using 4-bromo-3-nitroanisole as coupling partner and Pd ACHTUNGTRENNUNG(OAc)2/PPh3 ascatalyst.
In summary, the palladium-catalyzed direct arylation of in-doles is now a powerful method for the synthesis of 3-arylin-doles. For bimolecular reactions, the regioselectivity (C2 vs. C3arylation) seems to be very sensitive to the reaction conditions.In the presence of free (NH) indoles, the addition of congestedmagnesium salts to the indoles improved the regioselectivityin favor of C3 arylation. However, simpler procedures, employ-ing either a palladium–phosphinous acid complex, K2CO3, anddioxane, or Pd ACHTUNGTRENNUNG(OAc)2, toluene, BnBu3NCl, and K2CO3, also gavethis regioisomer in high selectivity. A heterogeneous palladiumcatalyst [Pd ACHTUNGTRENNUNG(NH3)4]/NaY, with K2CO3 and dioxane was also foundto produce this regioisomer.
2-Substituted pyrrole derivatives have also been relativelywidely used for intramolecular 3-arylation, and five-, six-,seven-, and even nine-membered rings have been formed suc-cessfully. Pyrroles have been only rarely used for intermoleculararylation reactions. Moreover, the selectivities in favor of 3-ary-lation and yields of these reactions have been quite low. Goodresults have been described for the 3-arylation of 2,5-dimethyl-pyrrole derivatives. In the presence of 1,5-dimethyl-2-pyrrolyl-carbonitrile, a regioselective 4-arylation was obtained underthe appropriate reaction conditions.
Several palladium catalysts bearing mono-, or bidentateligands or even ligand-free methods have been employed forthe arylation of indoles or pyrroles. With the most efficient cat-alysts, the reaction was effective with as little as 1 mol % palla-dium. These catalysts allowed the coupling of electron-defi-cient or excessive aryl bromides and iodides. So far, the cou-pling with aryl chlorides has attracted less attention. Only afew examples using chloropyrazines have been reported.Several sets of reaction conditions have been investigated.However, in most cases, K2CO3, or acetates were used as thebases, and toluene, dioxane, DMAc, or DMF were used as thesolvents. In the presence of pyrrolyl anions, the reaction couldbe performed in the absence of additional base. In general, rel-atively elevated reaction temperatures (100–150 8C) have beenemployed.
3. 3- or 4-Arylation of Furan Derivatives
Despite the fact that C2 or C5 direct arylation of furans isstrongly favored,[2, 10] several examples of successful C3 or C4direct arylations using palladium have also been reported.Both intra- and intermolecular versions of these reactions havebeen explored. In 1995, Grigg and co-workers employed a pal-ladium catalyzed cyclization process, resulting in a C3-arylatedfuran motif, as a viable route to bicyclic b-lactams(Scheme 3).[51] However, this reaction required the use of toxic
Tl2CO3 as the base, along with 10 mol % Pd ACHTUNGTRENNUNG(OAc)2 and20 mol % PPh3. In the course of these cyclizations, seven-mem-bered rings were formed. The formation of six-memberedrings from furans, substituted at C2 by a 2-iodobenzylamine,has been described more recently. Using 33 mol % of Pd ACHTUNGTRENNUNG(OAc)2,the expected product was obtained in 57 % yield [Eq. (25)] .[52, 53]
A very similar furan derivative was successfully cyclized by Bec-calli and co-workers, using 10 mol % Pd ACHTUNGTRENNUNG(OAc)2 with PPh3 as aligand.[54] With conventional heating, the target compoundwas obtained in only 35 % yield after 24 h, whereas, undermicrowave irradiation, the cyclized product was obtained in80 % yield after only 1 h [Eq. (26)] .
Such cyclizations are not limited to aryl iodides or bromides.A furoquinolinone was prepared in high yield by Fagnou andco-workers using a furan substituted at C2 by a N-(2-chloro-phenyl)amide [Eq. (27)] .[55] As the oxidative addition of aryl
Scheme 2. Arylation of indoles by Beletskaya and co-workers.[49] dba =
trans,trans-dibenzylideneacetone; dppp = 1,2-bis(diphenylphosphanyl)-ethane.
Scheme 3. Synthesis of bicyclic b-lactams by Grigg and co-workers.[51]
28 www.chemcatchem.org � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim ChemCatChem 2010, 2, 20 – 40
H. Doucet et al.

chlorides to palladium is generally quite slow with ligand-freepalladium, this reaction was carried out using Pd ACHTUNGTRENNUNG(OAc)2 withPCy3, an electron-rich and bulky phosphine ligand. Theexpected tricyclic compound was obtained in 83 % yield.
Using a 3-substituted furan, Larock and co-workers obtaineda tetracyclic compound by C�H bond activation at C4 of thefuran derivative [Eq. (28)] . For this reaction, 5 mol % of apalladacyclic catalyst was required to bring about the desiredtransformation.[56]
Palladium migration in catalyzed reactions is a fairly generalrearrangement that has been observed to occur in a wide vari-ety of systems. Such migration is synthetically useful becauseit provides an alternative way to introduce a palladium moietyat a specific position on an organic molecule, which may notbe readily accessible by conventional methods. Larock and co-workers recently detected a 1,4-migration of Pd from an arylposition to an imidoyl position.[57] Presumably, Pd0 first under-goes oxidative addition to the aryl iodide. The palladiummoiety may then undergo further oxidative addition of theimidoyl C�H bond to afford a palladacycle(IV) intermediate,which can undergo reductive elimination to form an imidoylpalladium complex. Then an intramolecular arylation followedby a reductive elimination forms the imine. Using this method,a 3-substituted furan, was converted to a cyclized compoundby C�H bond activation (Scheme 4). Interestingly, with this
substrate, the C4 position on the furan was activated, insteadof C2, which is usually more reactive for palladium-catalyzeddirect arylation reactions.
Intermolecular C3 or C4 arylation of furans by C�H bond ac-tivation has remained largely unexplored. In 2006, 2,3-diphe-nylfuran was detected as a side-product, formed in low yield inthe course of the coupling of bromobenzene with 2-furancar-boxylic acid (Scheme 5).[58] The authors postulated that the
electrophilic PdII intermediate generated from the oxidative ad-dition is first coordinated by the carboxylate moiety prior to anelectrophilic palladation at the C3 position of furan. At thisstage, two pathways are possible. A 3,2-migration of Pd andextrusion of CO2 can occur, followed by reductive eliminationto yield the 2-substituted furan. Alternatively, deprotonation toregenerate the aromatic furan can take place, after which re-ductive elimination will generate 3-phenyl-2-furancarboxylicacid. This product can in turn re-enter the catalytic cycle and
Scheme 4. Palladium-catalyzed intramolecular arylation by Larock and co-workers.[57] dppm = bis(diphenylphosphanyl)methane.
Scheme 5. Formation of 2,3-diphenylfuran as a side-product in the couplingof bromobenzene with 2-furancarboxylic acid.[58]
ChemCatChem 2010, 2, 20 – 40 � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim www.chemcatchem.org 29
Palladium-Catalyzed C3 or C4 Direct Arylation of Heteroaromatic Compounds

afford 2,3-diphenylfuran. When 2-phenylfuran was subjected tothe reaction conditions, only 2,5-diphenylfuran was formed,ruling out 2-phenylfuran as an intermediate for the formationof 2,3-diphenylfuran under these conditions.
In 2007, Daugulis and Chiong generated 2,3-diphenylbenzo-furan in 68 % yield from benzofuran and chlorobenzene[Eq. (29)] .[59] For this reaction, the catalyst system was Pd ACHTUNGTRENNUNG(OAc)2
with n-butyldi-1-adamantylphosphine.
In 2008, Doucet and Gottumukkala reported a method forthe C3 or C4 arylation of 2,5-disubstituted furans. The reactionof 2,5-dimethylfuran with electron-deficient aryl bromides gavethe 3-arylated furans in moderate yields [Eq. (30)] .[60] An elec-tron-withdrawing substituent on the furan ring favored the re-action. When using 2-acetyl-5-methylfuran, higher yields wererecorded than in the presence of 2,5-dimethylfuran. Moreover,the reaction was regioselective in favor of the 4-arylated com-pound [Eq. (31)] . This regioselectivity suggested that coordina-tion of the metal species to the carbonyl group of the furan isnot an essential step of the catalytic cycle. In the course of thisreaction, 3-arylation and 3,4-diarylation products were alsoformed. The ratio of these products was strongly dependenton the reaction conditions and catalyst. Under the optimizedcoupling conditions, [{PdCl ACHTUNGTRENNUNG(C3H5)}2] , KOAc, DMAc at 120 8C, theC4-arylated product was formed with high regioselectivity (C3/C4 ratio = 13:87). This procedure was tolerant to a variety offunctional groups on the aryl bromide, such as ester, formyl,acetyl, nitrile, nitro, fluoro, or trifluoromethyl groups.2-Formyl-5-methylfuran and 2-propionyl-5-methylfuran werealso suitable reactants.
The direct C4 arylation of 2,5-diaryl-3-fluorofurans, exploitingthe effect of the neighboring fluorine atom, was reported byZhu and co-workers (Table 2).[61] This reaction afforded 2,4,5-tri-aryl-3-fluorofurans in moderate to good yields. Several reactionconditions were tested and the best yields were obtainedwith KOAc as the base, NMP or DMF as the solvent, and[PdCl2ACHTUNGTRENNUNG(PPh3)2] as the catalyst. Several aryl bromides were suc-
cessfully coupled by this method and, interestingly, neither theelectronic nature nor the position of the substituents on the
phenyl ring exerted any considerable influence on the yields.The presence of the fluorine atom on furan seemed tofacilitate the arylation. After subjecting 2,5-diphenylfuran and4-chlorobromobenzene to similar reaction conditions, no cross-coupled product was detected.
Finally, Miura and co-workers recently reported the peraryla-tion of 3-furancarboxylic acid. Using an excess of aryl bromide,the cleavage of three C�H bonds and decarboxylation in thepresence of 10 mol % of Pd ACHTUNGTRENNUNG(OAc)2 and 40 mol % of PCy3, gavethe tetraarylated furans in 33–77 % yields [Eq. (32)] .[62] Thehighest yield was obtained using the electron-deficient arylbromide, 4-trifluoromethylbromobenzene.
In summary, the intramolecular C3 or C4 arylation of 2- or 3-substituted furans proceeds using aryl chlorides, bromides or
Table 2. Direct 4-arylation of 2,5-diaryl-3-fluorofurans.[61]
R1 R2 R2 Yield [%]
Ph Ph H 51Ph Ph 2-MeC6H4 64Ph Ph 4-FC6H4 73Ph Ph 2-ClC6H4 704-MeC6H4 Ph 2-MeC6H4 603-FC6H4 Ph 4-ClC6H4 60Et Ph 4-ClC6H4 57Ph n-pentyl 4-ClC6H4 61
30 www.chemcatchem.org � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim ChemCatChem 2010, 2, 20 – 40
H. Doucet et al.

iodides. The formation of five-, six-, and seven-mem-bered rings by such cyclization reactions has been re-ported. In general, relatively high catalyst loadingswere employed (3–33 mol %). Carbonates or acetateswere used as bases and DMAc, DMF, or even toluenewere employed assolvents.
The control of the intermolecular C3 or C4 aryla-tion of furans by C�H activation appears to be moresensitive. However, in the presence of a symmetrical-ly 2,5-disubstituted furan, the 3-arylated compoundswere obtained in moderate yields. Unsymmetrically2,5-disubstituted furans, such as 2-acetyl-5-methylfur-an, allowed selective access to the 4-arylated furans.2,5-Diaryl-3-fluorofurans were also useful reactants and provid-ed the 2,4,5-triaryl-3-fluorofurans in good yields. However,studies on the influence of the nature of the substituents onfurans need to be greatly extended to enable prediction of theyields and regioselectivities of such arylations.
4. 3- or 4-Arylation of Thiophene Derivatives
The palladium-catalyzed direct arylation of unsubstituted thio-phenes with aryl halides occurs at C2 and, in the presence of2-substituted thiophenes, generally at C5.[2, 10, 13] However,several examples of direct arylations at C3 or C4, either by in-tramolecular cyclizations, mostly using 2-substituted thio-phenes, or intermolecular reactions have also been described.In the course of intramolecular cyclization reactions, 5-, 6-, or7-membered rings were formed. For example, two thiophenessubstituted at C2 by b-lactams (Scheme 6) cyclized to give the
bicyclic b-lactams in 65 and 66 % yields, respectively, after a C�H bond activation at C3.[51] Rubicene analogues have also beenprepared by cyclization of thienyl- or dithienyl-1,5-dichloroan-thracenes (Scheme 7).[27] However, the yields of target productsare quite low (20 and 30 %), due to some polymerization anddecomposition.
Recently, Beccalli and co-workers reported the formation ofa tricyclic compound by intramolecular cyclization of a thio-phene substituted at C2 by N-(3-bromopyridin-2-yl)-N-methyl-ACHTUNGTRENNUNGamide using [Pd ACHTUNGTRENNUNG(PPh3)4] as catalyst [Eq. (33)] .[28] Under classical
heating, the desired product was obtained in only 49 % yieldafter 24 h, whereas, with microwave heating, an excellent yieldof 92 % was obtained after only 12 min. Fagnou and co-work-ers reported a very similar reaction using a thiophene bearinga N-(2-chlorophenyl)amide as substituent in position C2[Eq. (34)] .[55] As the oxidative addition of aryl chlorides is gener-ally quite slow when catalyzed by ligand-free palladium, thisreaction was performed using Pd ACHTUNGTRENNUNG(OAc)2 alongside the electron-rich and bulky phosphine ligand, PCy3. Beccalli and co-workershave also employed the microwave heating for the cyclizationof a (2-iodobenzyl)-thiophen-2-yl-carbamate [Eq. (35)] . Again,the use of microwave irradiation gave a higher yield of cyclizedproduct than conventional heating.[54] This method is not limit-ed to the formation of five- or six-membered rings. A seven-membered ring has also been formed by cyclization of a 2-sub-stituted benzothiophene [Eq. (36)] .[24, 30]
Scheme 6. Formation of bicyclic b-lactams from C2-substituted thiophenesby C�H bond activation at C3.[51]
Scheme 7. Preparation of rubicene analogues.[40]
ChemCatChem 2010, 2, 20 – 40 � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim www.chemcatchem.org 31
Palladium-Catalyzed C3 or C4 Direct Arylation of Heteroaromatic Compounds

Several examples of direct C3 or C4 arylations ofthiophenes by intermolecular couplings have alsobeen described. Bi-, tri-, and even tetraarylations ofthiophenes have been reported. In 1998, Lemaireand co-workers described the direct arylations of3-formylthiophene and 3-cyanothiophene, whichresulted in a mixtures of 2-arylthiophenes and 2,4-diarylthiophenes (Scheme 8).[63] In most cases, the 2-arylated thiophenes were obtained with high selec-tivities and 2,4-diarylthiophenes were only obtainedin low yields. The coupling of 3-cyanothiophene and1-iodonaphthalene led to a drastic change in selectiv-ity and 2,4-dinaphthyl-3-cyanothiophene was formedin 47 % yield (Scheme 8 b). The selectivity seems tobe largely related to the steric hindrance of theiodoaryl moiety.
The di- or triarylation of 2-thiophenecarboxamideshas been reported by Miura and co-workers(Scheme 9).[64] Several 2-thiophenecarboxamides havebeen arylated using an excess of aryl bromide (up tosix equivalents). In the course of these reactions, de-carbamoylation took place and the 2,3,5-triarylatedthiophenes were obtained in 52–83 % yields using a
catalyst system of 10 mol % Pd ACHTUNGTRENNUNG(OAc)2 and 20 mol % P(o-biphenyl) ACHTUNGTRENNUNG(tBu)2 (Scheme 9 a). The 2-thiophenecarboxamide ap-peared to be phenylated first at the C3 and C5 positions(Scheme 9 b). Then, the mono- and diarylated compounds un-derwent decarbamoylation to give the triarylated thiophenes.To obtain someinsight into the mechanism of decarbamoylation, Miura andco-workers heated the 3,5-diphenyl-2-thiophenecarboxamidein the presence of Pd ACHTUNGTRENNUNG(OAc)2, P(o-biphenyl) (tBu)2, and Cs2CO3
(Scheme 9 c). 2,4-Diphenylthiophene was produced quantita-tively, together with aniline. By using N-phenyl-2-thiophenecar-boxamide, the 3,5-diphenylated thiophene was obtained in62 % yield. However, when 2-phenylthiophene was used, 2,5-diphenylthiophene was obtained in 90 % yield, indicating that
the major route for the formation of 2,3,5-triphenylthiophenesdoes not proceed via the formation of 2-phenylthiophene or2,5-diphenylthiophene (Scheme 10 a). The 3-arylation of N-phenyl-2-thiophenecarboxamide probably proceeds by a coor-dination-assisted mechanism, whereas 5-arylation would pro-ceed electrophilically. The arylation of 3-substituted thiophenes
Scheme 8. Direct arylation of (a) 3-formylthiophene and (b) 3-cyanothio-phene by Lemaire and co-workers.[63]
Scheme 9. Di- and triarylation of 2-thiophenecarboxamides by Miura and co-workers.[64]
Scheme 10. Arylation of (a) 2-substituted and (b) 3-substituted thiophenesby Miura and co-workers.[64]
32 www.chemcatchem.org � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim ChemCatChem 2010, 2, 20 – 40
H. Doucet et al.

was also examined by this group. In the presence of 3-cyano-thiophene, they obtained mixtures of 2,5-diarylated thiophenesand 2,4,5-triarylated thiophenes (Scheme 10 b). The triarylatedthiophenes were obtained in relatively high yields. With theelectron-deficient 3-trifluoromethylbromobenzene, the triary-lated thiophene was the only product. Phenylation of 3-ben-zoylthiophene gave 3-benzoyl-2,5-diphenylthiophene in 44 %yield and 3-benzoyl-2,4,5-triphenylthiophene in 33 % yield.Conversely, 3-phenylthiophene was only diarylated, at C2 andC5. These results seem to confirm a coordination-assistedmechanism for the 4-arylation of 3-substituted thiophenes.
Recently, Miura and co-workers have extended these perary-lation reactions to 3-thiophenecarboxylic acid (Scheme 11 a).[62]
Using this reactant and Pd ACHTUNGTRENNUNG(OAc)2/PCy3 as catalyst, the tetraary-
lated thiophenes were obtained in 30–90 % yields. In thecourse of this reaction, three C�H bonds were cleaved and adecarboxylation occurred. The reaction using electron-neutraland electron-deficient aryl bromides afforded the perarylatedthiophenes in 60–90 % yields. However, the use of 4-bromoani-sole gave the expected compound in a lower yield of 30 %.They also examined the preparation of tetraarylthiopheneswith two pairs of different aryl groups at the 2- and 5-posi-tions, and at the 3- and 4-positions (Scheme 11 b). 2,5-Di-phenyl- and 2,5-bis(4-methoxyphenyl)-3-thiophenecarboxylateswere coupled with bromobenzene, 4-bromoanisole, or 4-tri-fluoromethylbromobenzene to give the perarylated thiophenesin 47–69 % yields. In each case, the formation of a minoramount of triarylthiophene was detected. The success of thetetraarylation appears to be due to the fact that the carbonylfunction acts as a directing group before it is substituted.
Doucet and co-workers demonstrated that, by usingPd ACHTUNGTRENNUNG(OAc)2 as the catalyst precursor, direct 3- or 4-arylation byC�H bond activation of 2,5-disubstituted thiophenes with arylbromides proceeds in moderate to good yields.[65] 3-arylatedthiophenes were obtained by arylation of 2,5-dimethylthio-phene. In the presence of 2-acetyl-5-methylthiophene, regiose-lective arylation took place at C4 [Eq. (37)] . This procedure waslimited to activated aryl bromides. However, a wide range offunctions, such as propionyl, benzoyl, formyl, nitro, nitrile,fluoro, and trifluoromethyl, are tolerated on the aryl bromide.
Several examples of 3-arylation of 2-substituted benzothio-phenes have been reported by Pellet-Rostaing and co-workers[Eq. (38)] .[66] Arylation at C3 of 2-substituted benzothiophenesafforded the corresponding coupling products in lower yieldsthan arylation at C2, despite longer reaction times. However,the coupling of 4-(trifluoromethyl)bromobenzene or 2-bromo-benzonitrile with benzothiophene-2-carbonitrile or 2-(2,2,2-tri-fluoroethoxy)benzothiophene gave the 3-arylated compoundsin 67 and 69 % yields, respectively.
Lautens and co-workers have developed a route to a varietyof polycyclic sulfur-containing heterocycles, based on a palladi-um-catalyzed alkylation/direct arylation reaction sequence.[67]
The method works well with 3-substituted thiophenesubstrates (Scheme 12 a). The authors suggest that, for theseintramolecular direct arylations of thiophenes, an electrophilicmetalation mechanism may occur. However, for 2-substitutedthiophene substrates, the expected cyclized product was notdetected (Scheme 12 b). With this procedure, there was no acti-vation of the C�H bond in position 3.
In summary, several interesting results have been reportedfor the palladium-catalyzed direct arylation of thiophenes atC3 or C4. Both intra- and intermolecular reactions have beenexplored and developed into synthetically viable protocols.Concerning intramolecular reaction, the formation of five-, six-,or seven-membered rings is possible. Aryl iodides, bromides,or chlorides were employed successfully as coupling partners.
Scheme 11. Perarylation of (a) 3-thiophenecarboxylic acid and (b) 2,5-diaryl-3-thiophenecarboxylic acid by Miura and co-workers.[62]
ChemCatChem 2010, 2, 20 – 40 � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim www.chemcatchem.org 33
Palladium-Catalyzed C3 or C4 Direct Arylation of Heteroaromatic Compounds

In general, the intramolecular cyclizations proceeded with highyields. Bimolecular couplings of thiophene or benzothiophenederivatives have also been investigated. For these reactions,some regioselective arylations have been reported. In the pres-ence of amide substituents on thiophene, a regioselective C3arylation was possible. The C3 arylation of benzothiopheneproceeded in high yields with some electron-deficient arylbromides. The synthesis of triarylthiophenes has also beendescribed. However, in several cases, only the perarylations ofthiophenes were possible.
5. 4-Arylation of Thiazole, Oxazole, or Imida-zole Derivatives
The palladium-catalyzed direct arylations of unsubstituted thia-zoles, oxazoles, and imidazoles occurs preferentially at C2 orC5.[2, 15–18] When these positions are blocked, C4 arylation at ispossible. However, only a few examples of such arylationshave been reported to date.[68–71] The C4 arylation of a 2-phenyl-5-oxazolecarboxanilide using bromobenzene has beendescribed by Miura and co-workers (Scheme 13 a).[68] In thecourse of this reaction the decarbamoylation of the oxazole oc-curred, and the diarylation took place at C4 and C5 to give2,4,5-triphenyloxazole in 33 % yield. For the coupling of 2-phenyl-5-thiazolecarboxanilide with various aryl bromides, asimilar reactivity was detected. The 2,4,5-triarylthiazoles wereformed in 52–85 % yields (Scheme 13 b). The highest yield wasobtained in the presence of 4-chlorobromobenzene. The C4 ar-ylation of 2,5-diphenyloxazole was reported very recently byFagnou and co-workers, using Pd ACHTUNGTRENNUNG(OAc)2/PCy3 as the catalyst[Eq. (39)] . The desired compound was obtained in 83 %yield.[69]
Fagnou and co-workers have also reported the 4-arylation ofthiazole derivatives (Table 3).[70] Conversion of thiazole to thia-zole N-oxide considerably increases the reactivity of the thia-zole ring system, and also alters the reactivity profile in terms
of the order of arylation. Arylation of thiazole N-oxides ob-serves a strict order, occurring at C2, then at C5, and finally atC4. Moreover, the use of thiazole N-oxide substrates led to adramatic increase in reactivity, in comparison to thiazoles, fordirect arylation at all positions of the azole ring, and the reac-tion conditions were milder than those usually required fordirect arylation of thiazoles. This permitted high-yielding aryla-tions at C4, providing a unique opportunity for exhaustivefunctionalization of the thiazole core. 2,5-diarylthiazole N-oxides or 2-tolyl-5-methylthiazole N-oxide and a variety of arylbromides were used as reactants. With a catalyst system com-prising 5 mol % Pd ACHTUNGTRENNUNG(OAc)2 and 15 mol % PPh3, the 4-arylated
Scheme 12. Synthesis of polycyclic sulfur-containing heterocycles from (a) 3-substituted and (b) 2-substituted thiophenes by Lautens and co-workers.[67]
Scheme 13. Arylation of (a) 2-phenyl-5-oxazolecarboxanilide and (b) 2-phenyl-5-thiazolecarboxanilide by Miura and co-workers.[67]
Table 3. 4-arylation of thiazole derivatives.[70]
R1 R2 Ar Yield [%]
Ph 4-CF3C6H4 4-MeC6H4 79Ph 4-MeOC6H4 3,5-(CF3)2C6H3 994-MeC6H4 Ph 1-naphthyl 65Ph 4-OMeC6H4 3,5-Me2C6H3 594-MeOC6H4 Ph 3,5-Me2C6H3 643,5-Me2C6H3 Ph 4-MeOC6H4 84
34 www.chemcatchem.org � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim ChemCatChem 2010, 2, 20 – 40
H. Doucet et al.

thiazoles were obtained in 59–99 % yields. This reactionmethod was tested on imidazole N-oxide [Eq. (40)] . The 4-ary-lated imidazole was obtained in 66 % yield with methyl 4-bro-mobenzoate as a coupling partner. The reactivity of the thia-zole arylation may be explained by considering the distributionof the molecular orbitals on the thiazole ring. In thiazole, thenearly equal distribution of the HOMO on all of the carbonatoms (C2/C4/C5 ratio = 25.2:30.5:29.9) led to little bias. Con-versely, on thiazole N-oxide, the HOMO is localized on C2, withvery little density on C4 or C5. Following C2 arylation, theHOMO density on C5 is much larger than that on C4, leadingto regioselective arylations.
The direct arylation of 1-methylimidazole was also reportedrecently by Rossi and co-workers [Eq. (41)] .[71] As expected, themajor product was 5-aryl-1-methylimidazole. However, byusing P ACHTUNGTRENNUNG(2-furyl)3, instead of PPh3, as a ligand, 4-aryl-1-methyl-imidazole was also formed in 11 % yield.
Although successful C4 arylation of some thiazoles, oxazoles,and imidazoles has been reported, the substrate scope inthese reactions remains largely unexplored and limited. Todate, no examples of such couplings to aryl chlorides or of in-tramolecular cyclizations have been reported. Moreover, thesereactions were all performed with 5 mol % catalyst. Therefore,more efficient procedures, permitting a broader scope of sub-strates and lower catalyst loadings, need to be developed toprovide synthetically useful access to such compounds.
6. 3- or 4-Arylation of Isoxazole or PyrazoleDerivatives
To date, very few examples of 4-arylation of isoxazoles orpyrazoles have been reported. In 1982, the intramolecularcyclization of a 3-substituted isoxazole was described by theNakamura and co-workers (Scheme 14 a).[72] Employing
Pd ACHTUNGTRENNUNG(OAc)2/PPh3 as catalyst and the carcinogenic solvent hexam-ethylphosphoramide (HMPA), the desired tricyclic compoundwas formed in 45 % yield. 5-subtituted isoxazoles have alsobeen cyclized to give tricyclic compounds (Scheme 14 b).[73]
The cyclization process led to the fused heterocycles in 9–60 %yield, with ligand-free Pd ACHTUNGTRENNUNG(OAc)2 as catalyst and microwaveheating.
The bimolecular 4-arylation of isoxazoles was also found tobe possible, although it required higher temperatures andlonger reaction times. The coupling of iodobenzene or 4-iodo-toluene with 3,5-disubstituted isoxazoles, afforded the 4-arylat-ed isoxazoles in 30-48 % yields (Scheme 15).[72] These reactions
were performed using 10 mol % of either 5 % Pd/C orPd ACHTUNGTRENNUNG(OAc)2 as catalyst and hexamethylphosphoric tria-mide (HMPT) as a solvent. Recently, the scope of thisreaction was extended to include aryl chlorides,when 1-chloronaphthalene was used as a couplingpartner [Eq. (42)] .[59] T To facilitate the oxidative addi-tion of 1-chloronaphthalene to palladium, Pd ACHTUNGTRENNUNG(OAc)2
was associated to the bulky and electron-rich ligand,butyldi-1-adamantylphosphine to act as catalyst. Theexpected product, 3,5-dimethyl-4-naphthalen-1-yli-soxazole, was obtained in 76 % yield.
Only a few examples of palladium-catalyzed direct C3 aryla-tion of a pyrazole using aryl halides have been described.Brnardic et al. utilized this reaction as the key step for ringclosure in the synthesis of pyrazoloquinolinone Chk1 kinase in-hibitors.[74] Using a 4-substituted pyrazole, and ligand-free
Scheme 14. Intramolecular cyclization of (a) 3-substituted[72] and (b) 5-substi-tuted[73] isoxazoles to give tricyclic products.
ChemCatChem 2010, 2, 20 – 40 � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim www.chemcatchem.org 35
Palladium-Catalyzed C3 or C4 Direct Arylation of Heteroaromatic Compounds

Pd ACHTUNGTRENNUNG(OAc)2 as catalyst, the intramolecular cyclization producedthe desired tetracyclic compound in 75 % yield [Eq. (43) ; CBz =
benzyloxycarbonyl THP = tetrahydropyranyl] .
Sames and co-workers recently established the regioselectiv-ity of the catalytic C�H arylation of pyrazoles (Scheme 16).[75]
SEM-pyrazole (SEM = 2-(trimethylsilyl)ethoxymethyl) was cou-pled with bromobenzene to give a mixture of products, whichindicated the higher reactivity of the 5-position relative to the4-position, and very low reactivity of the 3-position(Scheme 16 a). This trend was confirmed by examining thereaction of 1-SEM-3-phenylpyrazole, which gave a 5:2 ratio of1-SEM-3,5-diphenylpyrazole and 1-SEM-3,4,5-triphenylpyrazole(Scheme 16 b). In contrast, 1-SEM-4-phenylpyrazole was arylat-ed at C5 with high selectivity to provide 1-SEM-4,5-diphenyl-pyrazole in 80 % yield (Scheme 16 c). The regioisomer resultingfrom arylation at the C3 was not detected, and only a smallamount of the bisarylation product, 1-SEM-3,4,5-triphenylpyra-zole, was formed. Arylation of 1-SEM-5-phenylpyrazole wasalso selective, taking place at the C4 to afford 1-SEM-4,5-diphe-nylpyrazole as the major product (Scheme 16 d). These resultsdemonstrate that arylation at the C3 is inefficient, which isconsistent with the low reactivity of this site, whereas arylationoccurs at both C4 and C5, with preference for the latter. Ac-cording to Sames and co-workers, it is well established that C4of pyrazole is the most nucleophilic position and readily under-goes electrophilic substitution, whereas the 5-position carries
the most acidic C�H bond which can be selectively deproton-ated by strong bases.
The potential of Pd-catalyzed C3/C4 arylation of isoxazolesor pyrazoles remains largely unexplored. To our knowledge,the C3 arylation of isoxazoles has not, to date, been reportedand the regioselectivity of the arylation (C3 vs. C4) of 5-substi-tuted isoxazoles has not been examined. The scope and limita-tions of various aryl halide coupling partners also need to beexplored.
7. 4-Arylation of Triazole Derivatives
The earliest report of Pd-catalyzed direct arylation of 1,2,3-tria-zoles was from the Gevorgyan and co-workers,[76] who detect-ed a strong preference for C5 arylation, regardless of variationsin the electron density on the aryl halide (Scheme 17 a). Whenthe C5 position was blocked, arylation took place at C4, al-
Scheme 15. Bimolecular 4-arylation of 3,5-disubstituted isoxazoles.[72]
Scheme 16. Effect on regioselectivity of substituents on the catalyticarylation of pyrazoles.[67] SEM = CH2OCH2CH2SiMe3 ; PivOH = pivalic acid.
Scheme 17. Palladium-catalyzed arylation of 1,2,3-triazoles by Gevorgyanand co-workers.[76]
36 www.chemcatchem.org � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim ChemCatChem 2010, 2, 20 – 40
H. Doucet et al.

though the reaction was much more sluggish, requiring longerreaction time and higher catalyst loading (Scheme 17 b). A ra-tionalization for this trend was provided by density functional(DFT) calculations of the electrostatic potential charge at C4and C5 for a model triazole. Based on these calculations, anelectrophilic substitution mechanism was proposed.
Similar results were obtained by Oshima and Yorimitsu,[77]
when they attempted the arylation of 1,2,3-triazoles with arylchlorides under microwave irradiation [Eq. (44)] . This approachdrastically reduced the reaction time (15 min), although higherreaction temperatures (250 8C) were employed during thecourse of the reaction. For 1-substituted triazoles, selective 5-arylation took place. Arylation of a 1,5-disubstituted triazole re-sulted in the formation of the 4-arylated triazole in low yield(19 %). Arylation at C4 would be unfavorable because of themore localized stabilization of the cationic charge formed afterthe addition of the triazole to [ArPdCl] .
Recently, Ackermann and co-workers reported the 4-aryla-tion of 1,5-disubstituted 1,2,3-triazoles with aryl chlorides, atmuch lower temperatures and employing conventional heat-ing techniques (Scheme 18 a).[78] Again, the transformation wasdifficult to perform, whereas corresponding 5-arylation tookplace with much greater ease (Scheme 18 b).
In summary, the 4-arylation of triazoles is possible when the5-position is blocked. To date, the reported procedures haveused catalyst loadings of 4–10 mol %, but, in this case, arylchlorides have been employed as coupling partners. Thereported yields (13–60 %) have been relatively low and thesubstrate scope has been quite limited.
8. 3- or 4-Arylation of Pyridine Derivatives
The direct palladium-catalyzed arylation of pyridines generallyprovides 2-arylpyridines.[2] However, several examples of direct3- or 4-arylations of substituted pyridines have also beendescribed. Again, intramolecular cyclization reactions havebeen employed for the preparation of a variety of pyridinederivatives. In 1984, Ames and Opalko employed 2-(2-bromo-phenoxy)pyridine, as a tether for directing arylation to C3, re-sulting in the synthesis of a benzofuropyridine [Eq. (45)] .[79] De-spite a high reaction temperature and a relatively high catalystloading, the target compound was only obtained in 10 % yield,and 25 % of the starting material was recovered unreacted.
Alternatively, several C4-substituted tethers have been usedfor directing arylation at C3 position. Both five- and six-mem-bered rings have been formed using this reaction. LaVoie andco-workers used this method to prepare biologically activecompounds.[80–85] The cyclized products were generallyobtained in moderate yields (22–55 %; Scheme 19).
Maes and co-workers prepared cryptolepine derivativesusing (2-chlorophenyl)quinolin-4-ylamine or (3-chloropyridin-2-yl)pyridin-4-ylamine as reactants (Scheme 20).[86, 87] For thesecyclizations, the electron-rich phosphine ligand PACHTUNGTRENNUNG(tBu)3 wasused alongside [Pd2 ACHTUNGTRENNUNG(dba)3] as catalyst. The quinoline derivativewas more reactive than the pyridine. The reaction of (2-chloro-phenyl)quinolin-4-yl-amine gave the expected product withformation of a five-membered ring in 95 % yield, with only2.5 mol % catalyst. However, cyclization of (3-chloropyridin-2-yl)pyridin-4-ylamine gave the desired compound in only 52 %yield, with 10 mol % catalyst. In the presence of a pyridine sub-stituted at C4 by a 2-bromoaniline, the cyclization proceededin 72 % yield, with 5 mol % ligand-free Pd ACHTUNGTRENNUNG(OAc)2 as catalyst[Eq. (46)] . This improvement in yield is due to the easier oxida-tive addition of aryl bromides to palladium.[88] Intramolecularcyclization of a pyridine substituted at C4 by a 2-bromophenolgave the desired cyclized product in only 21 % yield[Eq. (47)] .[89] This low yield is probably due to the formation ofside products. Notably, the higher yields were obtained only ifair was allowed into the reaction mixture.
Scheme 18. Palladium-catalyzed arylation of 1,2,3-triazoles by Ackermannand co-workers.[78]
ChemCatChem 2010, 2, 20 – 40 � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim www.chemcatchem.org 37
Palladium-Catalyzed C3 or C4 Direct Arylation of Heteroaromatic Compounds

Maes and co-workers also studied the cyclization of 3-substi-tuted quinolines [Eq. (48)] .[90, 91] During the course of this reac-tion, the formation of two products was possible, resultingfrom C�H activation of the pyridine moiety either at C2 or C4.However, the C4 arylation occurred selectively. Several reac-tions conditions were tested and microwave heating wasfound to improve the yield. The use of various catalyst load-ings for this cyclization was also studied and a higher yieldwas obtained with 0.2 mol % catalyst, rather than 23 mol %,probably due to a faster aggregation of the palladium into in-active “palladium black”, at higher palladium concentration. Tohave a more general protocol for the synthesis of indoloquino-lines, they also studied the reactivity of a few other substrates.
Echavarren and co-workers studied the cyclizationof another interesting substrate [Eq. (49)] .[92, 93] Thecyclization of a 5-methyl-5-indenopyridine derivative,substituted by a 2-bromobenzyl group at C5, in thepresence of Pd ACHTUNGTRENNUNG(OAc)2 associated to a phosphineligand, proceeded with relative selectivity to the paraposition of the pyridine ring. However, the other re-gioisomer, that cyclized on the phenyl ring, was alsoobtained in 30 % yield. They also studied the reactivi-ty of substituted bromobenzyldiarylmethanes and es-tablished that electron-withdrawing substituents fa-vored reaction on the substituted ring. These resultsare incompatible with an electrophilic aromatic sub-stitution mechanism. The key step would be the ab-straction of a proton from the pyridyl ring by carbon-ate.
There have, to date, been very few examples of palladium-catalyzed intermolecular C3 or C4 arylation of pyridines witharyl halides. One of the rare cases, described by Cetinkaya andco-workers in 2005, involved the coupling of pyridine-2-carbal-dehyde and 4-chloroacetophenone in the presence of Pd ACHTUNGTRENNUNG(OAc)2
with a N-heterocyclic carbene ligand. The 3-arylated pyridineproduct was obtained in 88 % yield [Eq. (50)] .[94] Recently, the4-arylation of 2,3,5,6-tetrafluoropyridine with challenging 2-chlorotoluene or 4-bromotoluene reactants was reported byFagnou and co-workers (Scheme 21).[95, 96] The couplingproducts were obtained in 97 % and 86 % yields, respectively.
In summary, the direct arylation of pyridines at C3 or C4 pro-vides simple access to 3- or 4-arylpyridines. However, to date,the reaction has been limited to specific substrates. Intramo-lecular cyclization of 4-substituted pyridines generally gave thecyclized products with the formation of five- or six-memberedrings in good yields. Some 3-substituted pyridines cyclized atC4 of pyridine, with the formation of five- or six-memberedrings. Again, intermolecular couplings have attracted lessattention, as only pyridine-2-carbaldehyde and 2,3,5,6-tetra-fluoropyridine have been employed as reactants.
9. Conclusion and Perspectives
During the last five years, the scope and limitations of the pal-ladium-catalyzed direct C3 and C4 arylations of heteroaromaticcompounds have been greatly extended. Recent investigations
Scheme 19. Preparation of biologically active compounds by 3-arylation by LaVoie andco-workers.[80–85]
Scheme 20. Synthesis of cryptolepine derivatives from (a) (2-chlorophenyl)-quinolin-4-ylamine or (b) (3-chloropyridin-2-yl)-pyridin-4-ylamine by Maesand co-workers.[86, 87]
38 www.chemcatchem.org � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim ChemCatChem 2010, 2, 20 – 40
H. Doucet et al.

into coupling partners for this simple and elegant reactionhave demonstrated that it is actually one of the most straight-forward and powerful catalytic processes to prepare 3- or 4-arylated aromatic heterocycles. Although the highest yieldswere generally obtained using intramolecular cyclizations, in-termolecular couplings have also provided, in some cases, avery simple access to useful heteroaromatic derivatives. Severalcatalysts are available for the direct arylation of heteroaromat-ics. In most cases, aryl iodides or bromides were employed ascoupling partners. However, aryl chlorides have also been usedalongside the most efficient catalysts. Undoubtedly, this meth-odology will help both synthetic and materials chemists agreat deal, making direct C3 or C4 arylation a valuable tool fordiverse academic and industrial applications.
However, a number of challenges remain. If satisfactory re-sults were generally obtained for intramolecular cyclizations,the bimolecular reactions led in several cases to mixtures of re-gioisomers and to low yields. Moreover, the substrate scopeneeds to be greatly extended, and the influence of functionalgroups on heteroaromatic substrates and of the reaction con-ditions on the yields and selectivities need to be explored ingreater details. Although several catalytic systems are activefor the direct 3- or 4-arylation of readily available and low-costaryl chlorides, more efficient catalysts, permitting lower catalystloadings, need to be prepared in order to provide moreeconomically attractive procedures.
Regarding the mechanism involved in these reactions, con-cerning both catalytic cycles and elementary steps, many ave-nues of investigation have not been addressed. The exactmechanism for any given example seems to largely depend onthe substrates and reaction conditions employed. Determining
the reasons for the various regioselectivities for these reactionscould allow further improvement in terms of selectivity,efficiency, mildness, and reaction scope.
Keywords: arylation · C�H bond activation · heterocycles ·homogeneous catalysis · palladium
[1] E.-I. Negishi, Handbook of Organopalladium Chemistry for OrganicSynthesis, Wiley, New York, 2002.
[2] D. Alberico, M. E. Scott, M. Lautens, Chem. Rev. 2007, 107, 174–238.[3] T. Satoh, M. Miura, Chem. Lett. 2007, 36, 200–205.[4] L.-C. Campeau, D. R. Stuart, K. Fagnou, Aldrichimica Acta 2007, 40, 35–
41.[5] H. Doucet, J. C. Hierso, Curr. Opin. Drug Discovery Dev. 2007, 10, 672–
690.[6] F. Bellina, S. Cauteruccio, R. Rossi, Curr. Org. Chem. 2008, 12, 774–790.[7] B.-J. Li, S.-D. Yang, Z.-J. Shi, Synlett 2008, 949–957.[8] I. V. Seregin, V. Gevorgyan, Chem. Soc. Rev. 2007, 36, 1173–1193.[9] J. J. Li, G. W. Gribble, Palladium in Heterocyclic Chemistry, Pergamon,
Amsterdam, 2000.[10] F. Pozgan, J. Roger, H. Doucet, ChemSusChem 2008, 1, 404–407.[11] M. Parisien, D. Valette, K. Fagnou, J. Org. Chem. 2005, 70, 7578–7584.[12] A. Battace, M. Lemhadri, T. Zair, H. Doucet, M. Santelli, Organometallics
2007, 26, 472–474.[13] A. Battace, M. Lemhadri, T. Zair, H. Doucet, M. Santelli, Adv. Synth. Catal.
2007, 349, 2507–2516.[14] A. L. Gottumukkala, H. Doucet, Eur. J. Inorg. Chem. 2007, 3629–3632.[15] C. Verrier, T. Martin, C. Hoarau, F. Marsais, J. Org. Chem. 2008, 73, 7383–
7386.[16] J. Roger, F. Pozgan, H. Doucet, J. Org. Chem. 2009, 74, 1179–1186.[17] F. Besseli�vre, F. Mahuteau-Betzer, D. S. Grierson, S. Pigel, J. Org. Chem.
2008, 73, 3278–3280.[18] L. Ackermann, A. Althammer, S. Fenner, Angew. Chem. 2009, 121, 207–
210; Angew. Chem. Int. Ed. 2009, 48, 201–204.[19] F. Bellina, S. Cauteruccio, R. Rossi, J. Org. Chem. 2007, 72, 8543–8546.[20] F. Derridj, S. Djebbar, O. Benali-Baitich, H. Doucet, J. Organomet. Chem.
2008, 693, 135–144.[21] B. S. Lane, M. A. Brown, D. Sames, J. Am. Chem. Soc. 2005, 127, 8050–
8057.[22] S. I. Gorelsky, D. Lapointe, K. Fagnou, J. Am. Chem. Soc. 2008, 130,
10848–10849.[23] A. P. Kozikowski, D. Ma, Tetrahedron Lett. 1991, 32, 3317–3320.[24] B. Malapel-Andrieu, J.-Y. M�rour, Tetrahedron 1998, 54, 11079–11094.[25] A. Putey, L. Joucla, L. Picot, T. Besson, B. Joseph, Tetrahedron 2007, 63,
867–879.[26] H. Ohno, Y. Ohta, S. Oishi, N. Fujii, Angew. Chem. 2007, 119, 2345–2348;
Angew. Chem. Int. Ed. 2007, 46, 2295–2298.[27] Y. Matsuda, S. Kohra, K. Katou, T. Uemura, K. Yamashita, Heterocycles
2003, 60, 405–411.[28] M. Smet, J. Van Dijk, W. Dehaen, Synlett 1999, 495–497.[29] E. M. Beccalli, G. Broggini, M. Martinelli, G. Paladino, C. Zoni, Eur. J. Org.
Chem. 2005, 2091–2096.[30] A. M. Magill, D. S. McGuinness, K. J. Cavell, G. J. P. Britovsek, V. C. Gibson,
A. J. P. White, D. J. William, A. H. White, B. W. Skelton, J. Organomet.Chem. 2001, 617–618, 546–560.
[31] L. Joucla, A. Putey, B. Joseph, Tetrahedron Lett. 2005, 46, 8177–8179.[32] A. L. Bowie Jr. , C. C. Hughes, D. Trauner, Org. Lett. 2005, 7, 5207–5209.[33] M. G. Banwell, B. L. Flynn, D. C. R. Hockless, R. W. Longmore, A. D. Rae,
Aust. J. Chem. 1999, 52, 755–765.[34] L. Filippini, M. Gusmeroli, P. Riva, Tetrahedron Lett. 1992, 33, 1755–1758.[35] Y. Aoyagi, A. Inoue, I. Koizumi, R. Hashimoto, K. Tokunaga, K. Gohma, J.
Komatsu, K. Sekine, A. Miyafuji, J. Kunoh, R. Honma, Y. Akita, A. Ohta,Heterocycles 1992, 33, 257–272.
[36] Y. Fall, H. Doucet, M. Santelli, ChemSusChem. 2009, 2, 153–157.[37] Y. Akita, Y. Itagaki, S. Takizawa, A. Ohta, Chem. Pharm. Bull. 1989, 37,
1477–1480.[38] Y. Akita, A. Inoue, K. Yamamoto, A. Ohta, T. Kurihara, M. Shimizu, Hetero-
cycles 1985, 23, 2327–2333.[39] B. S. Lane, D. Sames, Org. Lett. 2004, 6, 2897–2900.
Scheme 21. 4-arylation of 2,3,5,6-tetrafluoropyridine with (a) 2-chlorotolueneor (b) 4-bromotoluene by Fagnou and co-workers.[95, 96]
ChemCatChem 2010, 2, 20 – 40 � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim www.chemcatchem.org 39
Palladium-Catalyzed C3 or C4 Direct Arylation of Heteroaromatic Compounds

[40] B. S. Lane, M. A. Brown, D. Sames, J. Am. Chem. Soc. 2007, 129, 241.[41] B. B. Tour�, B. S. Lane, D. Sames, Org. Lett. 2006, 8, 1979–1982.[42] X. Wang, D. V. Gribkov, D. Sames, J. Org. Chem. 2007, 72, 1476–1479.[43] L. Djakovitch, P. Rouge, R. Zaidi, Catal. Commun. 2007, 8, 1561–1566.[44] L. Djakovitch, V. Dufaud, R. Zaidi, Adv. Synth. Catal. 2006, 348, 715–724.[45] G. Cusati, L. Djakovitch, Tetrahedron Lett. 2008, 49, 2499–2502.[46] Z. Zhang, Z. Hu, Z. Yu, P. Lei, H. Chi, Y. Wang, R. He, Tetrahedron Lett.
2007, 48, 2415–2419.[47] F. Bellina, F. Benelli, R. Rossi, J. Org. Chem. 2008, 73, 5529–5535.[48] L. Ackermann, S. Barf�sser, Synlett 2009, 808–812.[49] M. V. Nikulin, A. Y. Lebedev, A. Z. Voskoboinikov, I. P. Beletskaya, Dokl.
Chem. 2008, 423, 326–329.[50] E. David, J. Lejeune, S. Pellet-Rostaing, J. Schulz, M. Lemaire, J. Chauvin,
A. Deronzier, Tetrahedron Lett. 2008, 49, 1860–1864.[51] M. Burwood, B. Davies, I. Diaz, R. Grigg, P. Molina, V. Sridharan, M.
Hughes, Tetrahedron Lett. 1995, 36, 9053–9056.[52] A. Padwa, M. A. Brodney, S. M. Lynch, J. Org. Chem. 2001, 66, 1716–
1724.[53] B. Liu, A. Padwa, Tetrahedron Lett. 1999, 40, 1645–1648.[54] E. M. Beccalli, G. Broggini, M. Martinelli, S. Sottocornola, Synthesis 2008,
136–140.[55] L.-C. Campeau, M. Parisien, A. Jean, K. Fagnou, J. Am. Chem. Soc. 2006,
128, 581–590.[56] T. Yao, X. Zhang, R. C. Larock, J. Org. Chem. 2005, 70, 7679–7685.[57] J. Zhao, D. Yue, M. A. Campo, R. C. Larock, J. Am. Chem. Soc. 2007, 129,
5288–5295.[58] P. Forgione, M.-C. Brochu, M. St-Onge, K. H. Thesen, M. D. Bailey, F. Bilo-
deau, J. Am. Chem. Soc. 2006, 128, 11350–11351.[59] H. A. Chiong, O. Daugulis, Org. Lett. 2007, 9, 1449–1451.[60] A. L. Gottumukkala, H. Doucet, Adv. Synth. Catal. 2008, 350, 2183–2188.[61] P. Li, Z. Chai, G. Zhao, S.-Z. Zhu, Tetrahedron 2009, 65, 1673–1678.[62] M. Nakano, H. Tsurugi, T. Satoh, M. Miura, Org. Lett. 2008, 10, 1851–
1854.[63] L. Lavenot, C. Gozzi, K. Ilg, I. Orlova, V. Penalva, M. Lemaire, J. Organo-
met. Chem. 1998, 567, 49–55.[64] T. Okazawa, T. Satoh, M. Miura, M. Nomura, J. Am. Chem. Soc. 2002, 124,
5286–5287.[65] J. J. Dong, J. Roger, H. Doucet, Tetrahedron Lett. 2009, 50, 2778–2781.[66] J. Fournier dit Chabert, B. Marquez, L. Neville, L. Joucla, S. Broussous, P.
Bouhours, E. David, S. Pellet-Rostaing, B. Marquet, N. Moreau, M. Lem-aire, Bioorg. Med. Chem. 2007, 15, 4482–4497.
[67] A. Martins, M. Lautens, J. Org. Chem. 2008, 73, 8705–8710.[68] A. Yokooji, T. Okazawa, T. Satoh, M. Miura, M. Nomura, Tetrahedron
2003, 59, 5685–5689.[69] B. Li�gault, D. Lapointe, L. Caron, A. Vlassova, K. Fagnou, J. Org. Chem.
2009, 74, 1826–1834.[70] L.-C. Campeau, M. Bertrand-Laperle, J.-P. Leclerc, E. Villemure, S. Gorel-
sky, K. Fagnou, J. Am. Chem. Soc. 2008, 130, 3276–3277.[71] F. Bellina, S. Cauteruccio, A. Di Fiore, R. Rossi, Eur. J. Org. Chem. 2008,
5436–5445.[72] N. Nakamura, Y. Tajima, K. Sakai, Heterocycles 1982, 17, 235–245.[73] L. Basolo, E. M. Beccalli, E. Borsini, G. Broggini, S. Pellegrino, Tetrahedron
2008, 64, 8182–8187.
[74] E. J. Brnardic, R. M. Garbaccio, M. E. Fraley, E. S. Tasber, J. T. Steen, K. L.Arrington, V. Y. Dudkin, G. D. Hartman, S. M. Stirdivant, B. A. Drakas, K.Rickert, E. S. Walsh, K. Hamilton, C. A. Buser, J. Hardwick, W. Tao, S. C.Beck, X. Mao, R. B. Lobell, L. Sepp-Lorenzino, Y. Yan, M. Ikuta, S. K.Munshi, L. C. Kuo, C. Kreatsoulas, Bioorg. Med. Chem. Lett. 2007, 17,5989–5994.
[75] R. Goikhman, T. L. Jacques, D. Sames, J. Am. Chem. Soc. 2009, 131,3042–3048.
[76] S. Chuprakov, N. Chernyak, A. S. Dudnik, V. Gevorgyan, Org. Lett. 2007,9, 2333–2336.
[77] M. Iwasaki, H. Yorimitsu, K. Oshima, Chem. Asian J. 2007, 2, 1430–1435.[78] L. Ackermann, R. Vicente, R. Born, Adv. Synth. Catal. 2008, 350, 741–748.[79] D. E. Ames, A. Opalko, Tetrahedron 1984, 40, 1919–1925.[80] A. L. Ruchelman, S. K. Singh, X. Wu, A. Ray, J.-M. Yang, T.-K. Li, A. Liu, L. F.
Liu, E. J. LaVoie, Bioorg. Med. Chem. Lett. 2002, 12, 3333–3336.[81] S. K. Singh, A. L. Ruchelman, T.-K. Li, A. Liu, L. F. Liu, E. J. LaVoie, J. Med.
Chem. 2003, 46, 2254–2257.[82] A. L. Ruchelman, J. E. Kerrigan, T.-K. Li, N. Zhou, A. Liu, L. F. Liu, E. J.
LaVoie, Bioorg. Med. Chem. 2004, 12, 3731–3742.[83] A. L. Ruchelman, S. K. Singh, A. Ray, X. H. Wu, J.-M. Yang, T.-K. Li, A. Liu,
L. F. Liu, E. J. LaVoie, Bioorg. Med. Chem. 2003, 11, 2061–2073.[84] A. L. Ruchelman, P. J. Houghton, N. Zhou, A. Liu, L. F. Liu, E. J. LaVoie, J.
Med. Chem. 2005, 48, 792–804.[85] S. Zhu, A. L. Ruchelman, N. Zhou, A. Liu, L. F. Liu, E. J. LaVoie, Bioorg.
Med. Chem. 2006, 14, 3131–3143.[86] T. H. M. Jonckers, B. U. W. Maes, G. L. F. Lemi�re, G. Rombouts, L. Pieters,
A. Haemers, R. A. Dommisse, Synlett 2003, 615–618.[87] C. Meyers, G. Rombouts, K. T. J. Loones, A. Coelho, B. U. W. Maes, Adv.
Synth. Catal. 2008, 350, 465–470.[88] K. Sako, H. Aoyama, S. Sato, Y. Hashimoto, M. Baba, Bioorg. Med. Chem.
2008, 16, 3780–3790.[89] S. Prado, V. Toum, B. Saint-Joanis, S. Michel, M. Koch, S. T. Cole, F. Tille-
quin, Y. L. Janin, Synthesis 2007, 1566–1570.[90] S. Hostyn, B. U. W. Maes, L. Pieters, G. L. F. Lemi�re, P. Matyus, G. Hajos,
R. A. Dommisse, Tetrahedron 2005, 61, 1571–1577.[91] S. Hostyn, B. U. W. Maes, G. Van Baelen, A. Gulevskaya, C. Meyers, K.
Smits, Tetrahedron 2006, 62, 4676–4684.[92] D. Garcia-Cuadrado, A. A. C. Braga, F. Maseras, A. M. Echavarren, J. Am.
Chem. Soc. 2006, 128, 1066–1067.[93] D. Garcia-Cuadrado, P. de Mendoza, A. A. C. Braga, F. Maseras, A. M.
Echavarren, J. Am. Chem. Soc. 2007, 129, 6880–6886.[94] N. G�rb�z, I. Ozdemir, B. Cetinkaya, Tetrahedron Lett. 2005, 46, 2273–
2277.[95] M. Lafrance, D. Shore, K. Fagnou, Org. Lett. 2006, 8, 5097–5100.[96] M. Lafrance, C. N. Rowley, T. K. Woo, K. Fagnou, J. Am. Chem. Soc. 2006,
128, 8754–8756.
Received: April 9, 2009
Revised: May 26, 2009
Published online on October 6, 2009
40 www.chemcatchem.org � 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim ChemCatChem 2010, 2, 20 – 40
H. Doucet et al.






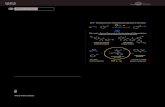



![Fluorescent hexaaryl- and hexa-heteroaryl[3]radialenes ... · Fluorescent hexaaryl- and hexa-heteroaryl[3]-radialenes: Synthesis, structures, and properties ... compounds display](https://static.fdocuments.in/doc/165x107/5b2c3c5b7f8b9abe2a8bdb3c/fluorescent-hexaaryl-and-hexa-heteroaryl3radialenes-fluorescent-hexaaryl-.jpg)








