Chemical Bonding: An Introduction 1. chemical bond: -ionic bond: -covalent bond:
Chapter 2 Minerals. Vocabulary Element Atomic number Energy level mass number Compound ...
-
Upload
priscilla-simon -
Category
Documents
-
view
225 -
download
1
Transcript of Chapter 2 Minerals. Vocabulary Element Atomic number Energy level mass number Compound ...

Chapter 2Chapter 2
MineralsMinerals

VocabularyVocabulary Element Atomic number Energy level mass number Compound Chemical bond Ion Ionic bond Covalent bond Fracture Density
Element Atomic number Energy level mass number Compound Chemical bond Ion Ionic bond Covalent bond Fracture Density
Metallic bond Mineral Silicate Silicon-oxygen tetrahedron
Streak Luster Crystal form Hardness Mohs scale cleavage
Metallic bond Mineral Silicate Silicon-oxygen tetrahedron
Streak Luster Crystal form Hardness Mohs scale cleavage

I. Elements and the Periodic Table
I. Elements and the Periodic Table
A. An element is a substance that cannot be broken down into simpler substances by chemical or physical means.1. There are 112 known elements, 92 occur
naturally, while others are man made.2. Only eight elements make up the Earth’s
crust.1. Oxygen, Silicon, Aluminum, Iron, Calcium,
Sodium, Potassium, Magnesium
3. Organized for there properties.a. Rows are called periodsb. Columns are called groups.
A. An element is a substance that cannot be broken down into simpler substances by chemical or physical means.1. There are 112 known elements, 92 occur
naturally, while others are man made.2. Only eight elements make up the Earth’s
crust.1. Oxygen, Silicon, Aluminum, Iron, Calcium,
Sodium, Potassium, Magnesium
3. Organized for there properties.a. Rows are called periodsb. Columns are called groups.

II. AtomsII. AtomsA. Ions are charged B. An atom is the smallest particle of matter
that contains the characteristics of an element.1. Center is known as the nucleus.
a. Contains protons and neutrons1. Protons are positive, and has about the same mass as the
neutron.2. The atomic number is the number of protons that are
located in that element.3. Neutrons are neutral
2. Electrons are negative charged and are located around the outside of the nucleus.a. They are the smallest particles in the atom.b. They are located in energy levels, which they move up
and down when they get excited.
A. Ions are charged B. An atom is the smallest particle of matter
that contains the characteristics of an element.1. Center is known as the nucleus.
a. Contains protons and neutrons1. Protons are positive, and has about the same mass as the
neutron.2. The atomic number is the number of protons that are
located in that element.3. Neutrons are neutral
2. Electrons are negative charged and are located around the outside of the nucleus.a. They are the smallest particles in the atom.b. They are located in energy levels, which they move up
and down when they get excited.

III. Why Atoms bond III. Why Atoms bond
A. A compound is a substance that consists of two or more elements that are chemically combined in specific proportions.1. They form when atoms are more stable.2. They want to complete their outer
shells by obtaining 8 electrons on the outside shell.
A. A compound is a substance that consists of two or more elements that are chemically combined in specific proportions.1. They form when atoms are more stable.2. They want to complete their outer
shells by obtaining 8 electrons on the outside shell.

IV. Types of Chemical Bonds
IV. Types of Chemical Bonds
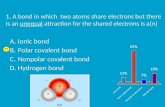
A. Ionic Bond form between positive and negative ions.1. They are rigid solids with high
melting and boiling points.2. Poor conductors of electricity.3. Usually consist of groups 1 and 2
with elements from groups 16 and 17.
A. Ionic Bond form between positive and negative ions.1. They are rigid solids with high
melting and boiling points.2. Poor conductors of electricity.3. Usually consist of groups 1 and 2
with elements from groups 16 and 17.

B. Covalent Bonds form when atoms share electrons.1. They have low melting and boiling
points. C. Metallic Bonds form when electrons
are shared by metal ions. 1. Can be easily shaped.
B. Covalent Bonds form when atoms share electrons.1. They have low melting and boiling
points. C. Metallic Bonds form when electrons
are shared by metal ions. 1. Can be easily shaped.

V. MineralsV. Minerals
A. A mineral is a naturally occurring, inorganic solid with an orderly crystalline structure and a definite chemical composition.
B. All have the following characteristics1. Naturally occurring2. Solid substance3. Orderly crystalline structure4. Definite chemical composition5. Generally considered inorganic.
A. A mineral is a naturally occurring, inorganic solid with an orderly crystalline structure and a definite chemical composition.
B. All have the following characteristics1. Naturally occurring2. Solid substance3. Orderly crystalline structure4. Definite chemical composition5. Generally considered inorganic.

C. How minerals form1. There are four major processes by which
minerals form:a. Crystallization from magma
1. Rich in iron, calcium, and magnesium start then at end,
sodium, potassium, and aluminum form.
b. Precipitation1. Water on Earth contains
dissolved substances. Evaporation leaves these
behind to condense into minerals.
C. How minerals form1. There are four major processes by which
minerals form:a. Crystallization from magma
1. Rich in iron, calcium, and magnesium start then at end,
sodium, potassium, and aluminum form.
b. Precipitation1. Water on Earth contains
dissolved substances. Evaporation leaves these
behind to condense into minerals.

c. Hydrothermal Solutions1. Very hot solution, temps.
Between 100oC and 300oC,
come in contact with other
minerals and then you get a
chemical reaction.d. Pressure and Temperature1. Minerals are subjected to
extreme temperature and/or pressure
changes.
c. Hydrothermal Solutions1. Very hot solution, temps.
Between 100oC and 300oC,
come in contact with other
minerals and then you get a
chemical reaction.d. Pressure and Temperature1. Minerals are subjected to
extreme temperature and/or pressure
changes.

D. Properties of Minerals.1. Color
a. The first thing to notice, small amounts of different elements can give the same mineral different colors.2. Streak
a. The color of a mineral in its
powdered form.3. Luster
a. Is used to describe how light is reflected from the surface of a mineral.
D. Properties of Minerals.1. Color
a. The first thing to notice, small amounts of different elements can give the same mineral different colors.2. Streak
a. The color of a mineral in its
powdered form.3. Luster
a. Is used to describe how light is reflected from the surface of a mineral.

4. Crystal Forma. This is the visible expression of a
mineral’s internal arrangement of
atoms.5. Hardness (Mohs Scale)
a. 1 (softest) - 10 (hardest)6. Cleavage
a. The tendency of a mineral to cleave, or
break along flat even surfaces.7. Fracture
a. Minerals that do not show cleavage when broken, but rather break unevenly.
4. Crystal Forma. This is the visible expression of a
mineral’s internal arrangement of
atoms.5. Hardness (Mohs Scale)
a. 1 (softest) - 10 (hardest)6. Cleavage
a. The tendency of a mineral to cleave, or
break along flat even surfaces.7. Fracture
a. Minerals that do not show cleavage when broken, but rather break unevenly.

8. Densitya. The property of all
matter that is the ratio of an
objects mass to its volume. D=M/V g/cm3
E. Distinctive properties1.Talc=Soapy 2. Graphite=write3. Magnitite=Magnetic
4.Carbonate=fizz
8. Densitya. The property of all
matter that is the ratio of an
objects mass to its volume. D=M/V g/cm3
E. Distinctive properties1.Talc=Soapy 2. Graphite=write3. Magnitite=Magnetic
4.Carbonate=fizz

VI. Mineral GroupsVI. Mineral Groups
A.Carbonate Minerals.1. The carbonate groups is made of
one carbon atom combined with three oxygen atoms, and has a negative charge of two.2. The rocks limestone and marble are
made almost entirely of carbonate
minerals.
A.Carbonate Minerals.1. The carbonate groups is made of
one carbon atom combined with three oxygen atoms, and has a negative charge of two.2. The rocks limestone and marble are
made almost entirely of carbonate
minerals.

3. Types of Carbonatesa. The most common carbonate
mineral is calcite.1. Has three prefect cleavages
that meet at oblique angels, and can
be easily identified using the acid test.b. Dolmite
1. Is a calcium magnesium carbonate, that has a hardness of
3.5-4 and cleaves into rhombs.
3. Types of Carbonatesa. The most common carbonate
mineral is calcite.1. Has three prefect cleavages
that meet at oblique angels, and can
be easily identified using the acid test.b. Dolmite
1. Is a calcium magnesium carbonate, that has a hardness of
3.5-4 and cleaves into rhombs.

Calcite
Calcite
Dolmite

B. Iron Oxide and Sulfide minerals1. An oxide is a mineral consisting of a
metal element combined with oxygen.2. Types of minerals.a. Hematite is the most common
iron oxide mineral, with a hardness of 5-6 and is red with
an earthy luster, and will have a
red-brown streak.b. Magnetite is a black magnetic iron
oxide, with a hardness of 5.5-6.5 and is highly magnetic.
B. Iron Oxide and Sulfide minerals1. An oxide is a mineral consisting of a
metal element combined with oxygen.2. Types of minerals.a. Hematite is the most common
iron oxide mineral, with a hardness of 5-6 and is red with
an earthy luster, and will have a
red-brown streak.b. Magnetite is a black magnetic iron
oxide, with a hardness of 5.5-6.5 and is highly magnetic.

Magnetite

3. A sulfide is a metal element combined
with sulfur.4. Type of sulfide minerals.
a. Pyrite is the most common sulfide mineral and has a color from pale
brass to golden-yellow with a hardness of 6. C. Silicate Minerals1. More than 90 percent of the minerals in
Earth’s crust are made of silicates.2. They consist of four oxygen atoms
bounded to a central silicon atom.
3. A sulfide is a metal element combined
with sulfur.4. Type of sulfide minerals.
a. Pyrite is the most common sulfide mineral and has a color from pale
brass to golden-yellow with a hardness of 6. C. Silicate Minerals1. More than 90 percent of the minerals in
Earth’s crust are made of silicates.2. They consist of four oxygen atoms
bounded to a central silicon atom.

Pyrite

3. Examples of silicate minerals.a. Quartz is made entirely of silica and has
a glassy or greasy luster. It fractures are
irregular, with a hardness of 7. It is
colorless, but may have some pink, purple, brown or gray.b. Feldspar makes up 60 percent of the
crust, and had two directions of cleavage with a hardness of 6.
3. Examples of silicate minerals.a. Quartz is made entirely of silica and has
a glassy or greasy luster. It fractures are
irregular, with a hardness of 7. It is
colorless, but may have some pink, purple, brown or gray.b. Feldspar makes up 60 percent of the
crust, and had two directions of cleavage with a hardness of 6.

1. There are two types of feldspar. A. Potassium feldspar.
1. Most common and known as orthoclase. Has a white or
cream color with two cleavage
surfaces that meet at a right
angle. B. Sodium-calcite feldspar
1. Known as plagioclase and has a white to gray color. It also
has two cleavage surfaces
that meet at slightly less than
a right angle.
1. There are two types of feldspar. A. Potassium feldspar.
1. Most common and known as orthoclase. Has a white or
cream color with two cleavage
surfaces that meet at a right
angle. B. Sodium-calcite feldspar
1. Known as plagioclase and has a white to gray color. It also
has two cleavage surfaces
that meet at slightly less than
a right angle.

Potassium Feldspar
Sodium Feldspar
Quartz

C. Mica 1. A soft silicate that is found in many
rocks that is flat, shiny and flakes are easily picked out of rocks. 2. Muscovite is silvery white, and
Biotite is dark brown or black, both are soft and have a hardness of about 2.5 with perfect cleavages.D. Talc 1. The softest mineral with a white to
grayish color and had one good cleavage. This mineral also has a soapy feel.
C. Mica 1. A soft silicate that is found in many
rocks that is flat, shiny and flakes are easily picked out of rocks. 2. Muscovite is silvery white, and
Biotite is dark brown or black, both are soft and have a hardness of about 2.5 with perfect cleavages.D. Talc 1. The softest mineral with a white to
grayish color and had one good cleavage. This mineral also has a soapy feel.

Muscovite
Talc

E. Amphiboles 1. Forms long, needlelike crystals, and
is the most common form of hornblende. 2.Usually dark green had has two
good cleavages that meet at oblique angles. Has a hardness of 5-6.F. Pyroxenes 1. Augite is the most common member and
is dark green brown, and has two good
cleavages with a hardness between 5-6.
E. Amphiboles 1. Forms long, needlelike crystals, and
is the most common form of hornblende. 2.Usually dark green had has two
good cleavages that meet at oblique angles. Has a hardness of 5-6.F. Pyroxenes 1. Augite is the most common member and
is dark green brown, and has two good
cleavages with a hardness between 5-6.

Hornblende
Augite

G. Olivine 1. Is green and has a hardness
around 6.5.H. Garnet 1. Is dark red to brown had has a
hardness from 6.5-7.5.I. Kaolinite1.Usually is yellow with a hardness of 1-
2 and also has a greasy feel.
G. Olivine 1. Is green and has a hardness
around 6.5.H. Garnet 1. Is dark red to brown had has a
hardness from 6.5-7.5.I. Kaolinite1.Usually is yellow with a hardness of 1-
2 and also has a greasy feel.

Olivine
Garnet
Kaolinite