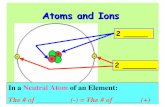
Chapter 2 Atoms, Molecules, Ions HW: 4 11 23 25 31 35 39 45 50 53 55 59 61 63 65 67 69 71 102.
-
Upload
aleesha-tyler -
Category
Documents
-
view
219 -
download
2
Transcript of Chapter 2 Atoms, Molecules, Ions HW: 4 11 23 25 31 35 39 45 50 53 55 59 61 63 65 67 69 71 102.

Chapter 2Atoms, Molecules, Ions
HW: 4 11 23 25 31 35 39 45 50 53 55 59 61 63 65 67 69 71 102

2.1 – Atomic TheoryDalton’s Atomic Theory1. Elements are made of
extremely small particles called atoms
2. Atoms of an element are identical
3. Atoms are not created or destroyed
4. Compounds are combinations of atoms
(1766-1844)

2.1 – Atomic TheoryLaw of Definite Proportions (ConstantComposition)
-Joseph Proust-A compound always has the same proportion of its elements
Law of Conservation of Mass (Matter) = Matter cannot be created or destroyed
Law of Multiple Proportions-Two or more compounds with the same elements must have different proportions of the elements-Example: Water vs. Hydrogen peroxide

2.2 – Discovery of Atomic Structure
Atom = Basic unit of an element that can enter into a chemical reaction
Subatomic Particle = Particles that make up an atom (protons, neutrons, electrons are as small as we will study)

-Cathode (Ray) Tube / Crooke’s Tube / = Glass tube w/2 metal plates. Connected to high voltage source. Emits ray.
2.2 – Discovery of Atomic Structure
Crooke – Determined that the ray was made of negative particles

2.2 – Discovery of Atomic StructureJJ Thomson = 1897 – Credited w/ finding
electrons Determined charge to mass ratio to be -1.76 108 coulombs/g.
Millikan = 1909 - Performed the Oil Drop Experiment. Found the charge of an electron (-1.60 x 10-19C). Could then calculate mass of the electron

2.2 – Discovery of Atomic Structure
a = + charge = Helium nucleus = High massb = - charge (high speed electrons) = Low massg = No charge. High energy = No mass
Radioactivity = Spontaneous emission of radiation

2.2 – Discovery of Atomic Structure
• 1900 - “Plum pudding” model, put forward byJJ Thompson.
• Positive sphere of matter with negative electrons imbedded in it.

2.2 – Discovery of Atomic Structure
Protons = + charge particles in the nucleus
Rutherford = 1919- Gold Foil Experiment-Most alpha particles when through, some deflected

2.2 – Discovery of Atomic StructureThis proved that Thomson’s model was incorrect
Results of Gold Foil Experiment:
Rutherford postulated a very small, dense nucleus with the electrons around the outside of the atom.
-Most of the volume of the atom is empty space.

2.2 – Discovery of the Structure of the Atom
Mass of electron so small it is ignored
-We will only discuss protons, neutrons and electrons because they are the only ones that affect chemical behavior-Charge on proton = +1.602 x 10-19 C. Assigned a +1 charge.-Charge on an electron = -1.602 x 10-19 C. Assigned a -1 charge.-Neutrons – Electrically neutral. Similar mass to protons. Discovered by Chadwick.

2.3 – Modern Atomic Theory
amu = atomic mass unit. 1 amu = 1.66054 x10-24 g. Based on 12 amu = mass of one atom of Carbon-12. 1 amu is approximately equal to the mass of a proton/neutron.
Angstrom (Å) = Unit used to measure atom size.1 Angstrom = 1 x 10-10 m

2.3 – Modern Atomic TheoryAtomic Numbers, Mass Numbers
and IsotopesSymbols:
2. Carbon - 12
1.
Mass Number

2.3 – Atomic Number, Etc.
All atoms of the same element have the same number of protons: The atomic number (Z)
Atomic Number:

2.3 – Atomic Number, Etc.
The total number of protons and neutrons in the atom.
Mass Number:

2.3 – Atomic Number, Etc.Isotopes = Atoms of the same element with
different masses.-Isotopes have different numbers of neutrons.-Isotopes have different mass numbers.
116 C 12
6 C 136 C
146 C
Carbon-11 Carbon-13Carbon-12 Carbon-14

2.4 – Atomic Weights
Average Atomic Masses – Based on abundance of isotopes and mass of each isotope. =Atomic Weight
Example 2.4 – Page 47

2.5 - Periodic Table
• The rows on the periodic table are periods.• Columns are groups.• Elements in the same group have similar chemical
properties.
Periodic Table – Arranged by atomic number with elements with similar properties in same column.
Mendeleev – Organized first accepted Periodic Table

Periodic TableMetals are on the left side of the chart.
-Low electronegativity-Good conductors of heat and electricity-Malleable, Ductile, Luster-Readily lose electrons

Periodic TableNonmetals are on the right side of the periodic table (with the exception of H).
-High electronegativity-Readily gain electrons

Periodic TableMetalloids border the stair-step line (with the exception of Al and Po).
-Properties are intermediate between metals and nonmetals.

Groups
These five groups are known by their names.
12161718

2.6 – Molecules and Molecular CompoundsChemical Formula = Shows the atoms present in a substance
Molecule – Two or more atoms in a definite arrangement held by covalent bonds. Can be the same or different atoms.
ex – H2, H2O, C6H12O6
Diatomic Molecule = Molecule made of only 2 atoms total
Molecular Compound – Must have DIFFERENT elements
ex- H2O is a compound, but H2 is NOT

2.6 – Molecules
REVIEW:Bonds –
-Nonpolar Covalent – Equal sharing of electrons-No partial charges-Electronegativity of two atoms is close-Difference of electronegativities is <0.3
-Polar Covalent – Unequal sharing of electrons-Partial charges exist on atoms-Electronegativity difference is 0.3-1.7

2.6 - Molecules
Molecular Formula = Gives actual number and type of atoms in a molecule
Empirical Formula = Simplest whole number ration of atoms
Example = Glucose

2.6 - MoleculesStructural Formula – Similar to a Lewis
Structure. Shows bonds, but not shape.

2.6 - Molecules

• When atoms lose or gain electrons, they become ions.– Cations are positive and are formed by elements on
the left side of the periodic chart.– Anions are negative and are formed by elements on
the right side of the periodic chart.
2.7 – Ions and Ionic Compounds

2.7 - Ions• Examples:

2.7 - IonsIonic compounds (such as NaCl) are formed by ionic bonds. Contain both + and – ions.Ionic bonds are generally formed between metals (cations) and nonmetals (anions).
Monatomic – one atom only with a chargePolyatomic – more than one atom with an overall
charge

Metallic Bond
• Metal ions with an electron sea.

2.8 – Naming Inorganic CompoundsIonic
Names and Formulas of Ionic Compounds:1. Cations
a. One charge only = name onlyZn+2 = Zinc ionCa+2 = Calcium ion
b. Multiple charges possible = use Roman NumeralCu+1 = Copper (I)Cu+2 = Copper (II)-Old system uses –ous / -ic
c. Polyatomics – hydronium, ammonium

Common Cations

2.8 – Inorganic CompoundsIonic
Names and Formulas of Ionic Compounds:2. Anions:
a. Monotomic – Change ending to –ideb. Polyatomic – MEMORIZE 8-ates and rules!
c. H+ added ions = use bi- or hydro-prefixExample – bicarbonate = hydrogen carbonate
• Carbonate CO3-2
• Nitrate NO3-1• Sulfate SO4
-2
• Chlorate ClO3-1
• Chromate CrO4-
2
• Bromate BrO3-1
• Phosphate PO4-3
• Iodate IO3-1

Common Anions

2.8 – Inorganic CompoundsIonic
Ionic Compounds – Cation + Anion-Binary – Compound of 2 “atoms”
-ex – NaCl, Al2O3
-Ternary – Compound of 3 “atoms”-ex – Mg(NO3)2

2.8 – InorganicAcids

2.8 – Inorganic CompoundsBinary Molecular
Molecular Compounds: Discrete molecular units. Contain covalent bonds. Use prefixes.

2.9 - Organic