Basel Stent Cost-effectiveness Trial-Late Thrombotic Events (BASKET LATE) Trial Basel Stent...
-
Upload
caroline-hawkins -
Category
Documents
-
view
213 -
download
0
Transcript of Basel Stent Cost-effectiveness Trial-Late Thrombotic Events (BASKET LATE) Trial Basel Stent...

Basel Stent Cost-effectiveness Trial-Late Basel Stent Cost-effectiveness Trial-Late Thrombotic Events (BASKET LATE) TrialThrombotic Events (BASKET LATE) Trial Basel Stent Cost-effectiveness Trial-Late Basel Stent Cost-effectiveness Trial-Late Thrombotic Events (BASKET LATE) TrialThrombotic Events (BASKET LATE) Trial
Presented atPresented atThe American College of Cardiology The American College of Cardiology
Scientific Session 2006Scientific Session 2006
Presented by Dr. Matthias E. PfistererPresented by Dr. Matthias E. Pfisterer
BASKET LATE TrialBASKET LATE TrialBASKET LATE TrialBASKET LATE Trial

www. Clinical trial results.org
BASKET LATE Trial: BackgroundBASKET LATE Trial: BackgroundBASKET LATE Trial: BackgroundBASKET LATE Trial: Background
• In the original BASKET trial, a rather complex patient population was enrolled In the original BASKET trial, a rather complex patient population was enrolled and randomized to receive one of the following: a bare metal stent, paclitaxel-and randomized to receive one of the following: a bare metal stent, paclitaxel-eluting stent, or sirolimus-eluting stent and the cost effectiveness of drug-eluting eluting stent, or sirolimus-eluting stent and the cost effectiveness of drug-eluting stents vs. bare metal stents was evaluated.stents vs. bare metal stents was evaluated.
• In the BASKET LATE trial, the 743 patients from the BASKET trial who were In the BASKET LATE trial, the 743 patients from the BASKET trial who were MACE free at six months were followed for an additional 12 months after the MACE free at six months were followed for an additional 12 months after the cessation of clopidogrel treatment.cessation of clopidogrel treatment.
• The BASKET LATE trial pooled the paclitaxel- and sirolimus-eluting stent groups The BASKET LATE trial pooled the paclitaxel- and sirolimus-eluting stent groups into one drug eluting stent group for analysis. into one drug eluting stent group for analysis.
• The goal of BASKET LATE was to evaluate late thrombotic events among The goal of BASKET LATE was to evaluate late thrombotic events among patients treated with drug eluting stents vs. bare metal stents after clopidogrel patients treated with drug eluting stents vs. bare metal stents after clopidogrel discontinuation.discontinuation.
• In the original BASKET trial, a rather complex patient population was enrolled In the original BASKET trial, a rather complex patient population was enrolled and randomized to receive one of the following: a bare metal stent, paclitaxel-and randomized to receive one of the following: a bare metal stent, paclitaxel-eluting stent, or sirolimus-eluting stent and the cost effectiveness of drug-eluting eluting stent, or sirolimus-eluting stent and the cost effectiveness of drug-eluting stents vs. bare metal stents was evaluated.stents vs. bare metal stents was evaluated.
• In the BASKET LATE trial, the 743 patients from the BASKET trial who were In the BASKET LATE trial, the 743 patients from the BASKET trial who were MACE free at six months were followed for an additional 12 months after the MACE free at six months were followed for an additional 12 months after the cessation of clopidogrel treatment.cessation of clopidogrel treatment.
• The BASKET LATE trial pooled the paclitaxel- and sirolimus-eluting stent groups The BASKET LATE trial pooled the paclitaxel- and sirolimus-eluting stent groups into one drug eluting stent group for analysis. into one drug eluting stent group for analysis.
• The goal of BASKET LATE was to evaluate late thrombotic events among The goal of BASKET LATE was to evaluate late thrombotic events among patients treated with drug eluting stents vs. bare metal stents after clopidogrel patients treated with drug eluting stents vs. bare metal stents after clopidogrel discontinuation.discontinuation.
Presented at ACC 2006Presented at ACC 2006

www. Clinical trial results.org
BASKET LATE Trial: Study DesignBASKET LATE Trial: Study DesignBASKET LATE Trial: Study DesignBASKET LATE Trial: Study Design
Primary Endpoint: Composite cardiac death or nonfatal MI.Primary Endpoint: Composite cardiac death or nonfatal MI. Other Endpoints: Other Endpoints:
- “Thrombosis-related events”:- “Thrombosis-related events”:
- angiographically documented stent thrombosis- angiographically documented stent thrombosis
- cardiac death/ target vessel MI- cardiac death/ target vessel MI
- Target vessel revascularization (TVR)- Target vessel revascularization (TVR)
Primary Endpoint: Composite cardiac death or nonfatal MI.Primary Endpoint: Composite cardiac death or nonfatal MI. Other Endpoints: Other Endpoints:
- “Thrombosis-related events”:- “Thrombosis-related events”:
- angiographically documented stent thrombosis- angiographically documented stent thrombosis
- cardiac death/ target vessel MI- cardiac death/ target vessel MI
- Target vessel revascularization (TVR)- Target vessel revascularization (TVR)
743 patients all undergoing PCI irrespective of indication for PCI and without target 743 patients all undergoing PCI irrespective of indication for PCI and without target vessel diameter vessel diameter ≥4mm, restenotic lesions, or an event during the on-clopidogrel phase.≥4mm, restenotic lesions, or an event during the on-clopidogrel phase.
Original BASKET study randomized patients in 1:1:1 strategy. Present study pooled DES patients into one group.Original BASKET study randomized patients in 1:1:1 strategy. Present study pooled DES patients into one group.21% female, 21% ST-elevation MI, 37% unstable angina, 42% stable angina, 67% with multivessel disease, 51% with 21% female, 21% ST-elevation MI, 37% unstable angina, 42% stable angina, 67% with multivessel disease, 51% with
LAD culprit lesions, average 1.9 stents per patient, mean age 63 years, mean follow-up 18 months.LAD culprit lesions, average 1.9 stents per patient, mean age 63 years, mean follow-up 18 months.Concomitant medications: aspirin indefinitely.Concomitant medications: aspirin indefinitely.
743 patients all undergoing PCI irrespective of indication for PCI and without target 743 patients all undergoing PCI irrespective of indication for PCI and without target vessel diameter vessel diameter ≥4mm, restenotic lesions, or an event during the on-clopidogrel phase.≥4mm, restenotic lesions, or an event during the on-clopidogrel phase.
Original BASKET study randomized patients in 1:1:1 strategy. Present study pooled DES patients into one group.Original BASKET study randomized patients in 1:1:1 strategy. Present study pooled DES patients into one group.21% female, 21% ST-elevation MI, 37% unstable angina, 42% stable angina, 67% with multivessel disease, 51% with 21% female, 21% ST-elevation MI, 37% unstable angina, 42% stable angina, 67% with multivessel disease, 51% with
LAD culprit lesions, average 1.9 stents per patient, mean age 63 years, mean follow-up 18 months.LAD culprit lesions, average 1.9 stents per patient, mean age 63 years, mean follow-up 18 months.Concomitant medications: aspirin indefinitely.Concomitant medications: aspirin indefinitely.
Presented at ACC 2006Presented at ACC 2006
Bare metal stents (BMS)Bare metal stents (BMS)
n=244n=244
Bare metal stents (BMS)Bare metal stents (BMS)
n=244n=244
Drug-eluting stents (DES)Drug-eluting stents (DES)(pooled paclitaxel and (pooled paclitaxel and sirolimus DES groups) sirolimus DES groups)
n=499n=499
Drug-eluting stents (DES)Drug-eluting stents (DES)(pooled paclitaxel and (pooled paclitaxel and sirolimus DES groups) sirolimus DES groups)
n=499n=499

www. Clinical trial results.org
BASKET LATE Trial: Primary Composite EndpointBASKET LATE Trial: Primary Composite EndpointBASKET LATE Trial: Primary Composite EndpointBASKET LATE Trial: Primary Composite Endpoint
4.9
1.3
0
1
2
3
4
5
6
DES BMS
4.9
1.3
0
1
2
3
4
5
6
DES BMS
• In the year following In the year following clopidogrel clopidogrel discontinuation, the discontinuation, the primary composite primary composite endpoint of cardiac endpoint of cardiac death or MI occurred death or MI occurred significantly more significantly more frequently in the DES frequently in the DES group than in the group than in the BMS group (4.9% vs. BMS group (4.9% vs. 1.3%, p=0.01).1.3%, p=0.01).
Composite of Cardiac death or nonfatal MI (%)Composite of Cardiac death or nonfatal MI (%)
p=0.01p=0.01
% p
atie
nts
% p
atie
nts
Presented at ACC 2006Presented at ACC 2006

www. Clinical trial results.org
BASKET LATE Trial: Primary EndpointBASKET LATE Trial: Primary EndpointBASKET LATE Trial: Primary EndpointBASKET LATE Trial: Primary Endpoint
• Non-fatal MI was Non-fatal MI was higher in the DES higher in the DES group compared with group compared with the BMS group (4.1% the BMS group (4.1% vs. 1.3%, p=0.04).vs. 1.3%, p=0.04).
• Also, cardiac death Also, cardiac death trended higher in the trended higher in the DES group than in the DES group than in the BMS group (1.2% vs. BMS group (1.2% vs. 0%, p=0.09).0%, p=0.09).
% p
atie
nts
% p
atie
nts
Components of primary composite endpoint: nonfatal Components of primary composite endpoint: nonfatal MI/cardiac death (%)MI/cardiac death (%)
4.1
1.21.3
0.00.0
1.0
2.0
3.0
4.0
5.0
MI Cardiac Death
DES BMS
4.1
1.21.3
0.00.0
1.0
2.0
3.0
4.0
5.0
MI Cardiac Death
DES BMS
p=0.04p=0.04p=0.04p=0.04
p=0.09p=0.09p=0.09p=0.09
Presented at ACC 2006Presented at ACC 2006

www. Clinical trial results.org
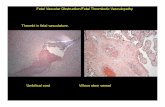
BASKET LATE Trial: “Thrombosis-Related Events”BASKET LATE Trial: “Thrombosis-Related Events”BASKET LATE Trial: “Thrombosis-Related Events”BASKET LATE Trial: “Thrombosis-Related Events”
• There was no significant There was no significant difference in the occurrence difference in the occurrence of late stent thrombosis of late stent thrombosis (combination of (combination of angiographic documented angiographic documented thrombosis and thrombotic thrombosis and thrombotic clinical events) between the clinical events) between the DES and BMS groups (2.6% DES and BMS groups (2.6% vs. 1.3%, p=0.23).vs. 1.3%, p=0.23).
• The median time of the late The median time of the late thrombotic event was 116 thrombotic event was 116 days following clopidogrel days following clopidogrel discontinuation, but events discontinuation, but events occurred throughout the occurred throughout the one year follow-up (range one year follow-up (range 362 days).362 days).
2.6
1.3
0
1
2
3
DES BMS
2.6
1.3
0
1
2
3
DES BMS
Additional endpoint of Additional endpoint of ““thrombosis-related events” (%)thrombosis-related events” (%)
p=0.23 p=0.23
Presented at ACC 2006Presented at ACC 2006
% p
atie
nts
% p
atie
nts

www. Clinical trial results.org Presented at ACC 2006Presented at ACC 2006
BASKET LATE Trial: TVRBASKET LATE Trial: TVRBASKET LATE Trial: TVRBASKET LATE Trial: TVR
• Also, there was no Also, there was no difference in difference in restenosis driven restenosis driven TVR among the two TVR among the two groups (4.5% vs. groups (4.5% vs. 6.7%, p=0.21).6.7%, p=0.21).
4.5
6.7
0
2
4
6
8
DES BMS
4.5
6.7
0
2
4
6
8
DES BMS
Restenosis driven TVR (%) Restenosis driven TVR (%) p=0.21p=0.21
Presented at ACC 2006Presented at ACC 2006
% p
atie
nts
% p
atie
nts

www. Clinical trial results.org
BASKET LATE: SafetyBASKET LATE: SafetyBASKET LATE: SafetyBASKET LATE: Safety
Presented at ACC 2006Presented at ACC 2006
• MACE rates were no MACE rates were no different between different between DES and BMS (9.3% DES and BMS (9.3% vs. 7.9%, p=0.53).vs. 7.9%, p=0.53).
9.3
7.9
0
2
4
6
8
10
DES BMS
9.3
7.9
0
2
4
6
8
10
DES BMS
MACE (%) MACE (%) p=0.53p=0.53
Presented at ACC 2006Presented at ACC 2006
% p
atie
nts
% p
atie
nts

www. Clinical trial results.org
BASKET LATE Trial: LimitationsBASKET LATE Trial: LimitationsBASKET LATE Trial: LimitationsBASKET LATE Trial: Limitations
• The present study could not identify major differences The present study could not identify major differences in thrombosis-related events because it was in thrombosis-related events because it was underpowered. underpowered.
• The present study could not identify major differences The present study could not identify major differences in thrombosis-related events because it was in thrombosis-related events because it was underpowered. underpowered.
Presented at ACC 2006Presented at ACC 2006

www. Clinical trial results.org
BASKET LATE Trial: SummaryBASKET LATE Trial: SummaryBASKET LATE Trial: SummaryBASKET LATE Trial: Summary
• Among patients with coronary artery disease treated with PCI, use of Among patients with coronary artery disease treated with PCI, use of a drug-eluting stent was associated with significantly higher rates of a drug-eluting stent was associated with significantly higher rates of cardiac death or MI compared with a bare metal stent in the year cardiac death or MI compared with a bare metal stent in the year following clopidogrel discontinuation.following clopidogrel discontinuation.
• Many trials have demonstrated a reduction in target lesion Many trials have demonstrated a reduction in target lesion revascularization with DES compared with BMS in recent years, but revascularization with DES compared with BMS in recent years, but none has ever demonstrated an effect on the hard endpoints of death none has ever demonstrated an effect on the hard endpoints of death or MI.or MI.
• Restenosis, while not desirable, is not an independent correlate of Restenosis, while not desirable, is not an independent correlate of subsequent mortality. The present study showed a more than three-subsequent mortality. The present study showed a more than three-fold increase in death or MI with DES in the year after clopidogrel fold increase in death or MI with DES in the year after clopidogrel discontinuation. Late stent thrombosis is a serious and often fatal discontinuation. Late stent thrombosis is a serious and often fatal complication.complication.
• Among patients with coronary artery disease treated with PCI, use of Among patients with coronary artery disease treated with PCI, use of a drug-eluting stent was associated with significantly higher rates of a drug-eluting stent was associated with significantly higher rates of cardiac death or MI compared with a bare metal stent in the year cardiac death or MI compared with a bare metal stent in the year following clopidogrel discontinuation.following clopidogrel discontinuation.
• Many trials have demonstrated a reduction in target lesion Many trials have demonstrated a reduction in target lesion revascularization with DES compared with BMS in recent years, but revascularization with DES compared with BMS in recent years, but none has ever demonstrated an effect on the hard endpoints of death none has ever demonstrated an effect on the hard endpoints of death or MI.or MI.
• Restenosis, while not desirable, is not an independent correlate of Restenosis, while not desirable, is not an independent correlate of subsequent mortality. The present study showed a more than three-subsequent mortality. The present study showed a more than three-fold increase in death or MI with DES in the year after clopidogrel fold increase in death or MI with DES in the year after clopidogrel discontinuation. Late stent thrombosis is a serious and often fatal discontinuation. Late stent thrombosis is a serious and often fatal complication.complication.
Presented at ACC 2006Presented at ACC 2006

www. Clinical trial results.org
BASKET LATE Trial: Summary cont.BASKET LATE Trial: Summary cont.BASKET LATE Trial: Summary cont.BASKET LATE Trial: Summary cont.
• Clopidogrel is generally prescribed for the first 6 months following Clopidogrel is generally prescribed for the first 6 months following stent placement, along with aspirin indefinitely. These data suggest stent placement, along with aspirin indefinitely. These data suggest the 6 month anti-platelet regimen of clopidogrel may not be long the 6 month anti-platelet regimen of clopidogrel may not be long enough to provide adequate protection from late thrombosis with enough to provide adequate protection from late thrombosis with DES.DES.
• It is unknown if a better strategy would be a longer duration of It is unknown if a better strategy would be a longer duration of clopidogrel, a more potent anti-platelet drug, or a different drug-clopidogrel, a more potent anti-platelet drug, or a different drug-elution kinetic pattern.elution kinetic pattern.
• The authors noted that for every 100 patients treated with a DES, 3.3 The authors noted that for every 100 patients treated with a DES, 3.3 cases of cardiac death or MI are induced for a reduction of 5 cases of cases of cardiac death or MI are induced for a reduction of 5 cases of target lesion revascularization. Further study on late thrombotic target lesion revascularization. Further study on late thrombotic events with DES is strongly warranted given these findings.events with DES is strongly warranted given these findings.
• Clopidogrel is generally prescribed for the first 6 months following Clopidogrel is generally prescribed for the first 6 months following stent placement, along with aspirin indefinitely. These data suggest stent placement, along with aspirin indefinitely. These data suggest the 6 month anti-platelet regimen of clopidogrel may not be long the 6 month anti-platelet regimen of clopidogrel may not be long enough to provide adequate protection from late thrombosis with enough to provide adequate protection from late thrombosis with DES.DES.
• It is unknown if a better strategy would be a longer duration of It is unknown if a better strategy would be a longer duration of clopidogrel, a more potent anti-platelet drug, or a different drug-clopidogrel, a more potent anti-platelet drug, or a different drug-elution kinetic pattern.elution kinetic pattern.
• The authors noted that for every 100 patients treated with a DES, 3.3 The authors noted that for every 100 patients treated with a DES, 3.3 cases of cardiac death or MI are induced for a reduction of 5 cases of cases of cardiac death or MI are induced for a reduction of 5 cases of target lesion revascularization. Further study on late thrombotic target lesion revascularization. Further study on late thrombotic events with DES is strongly warranted given these findings.events with DES is strongly warranted given these findings.
Presented at ACC 2006Presented at ACC 2006