Atoms, Molecules, and Ions · Atoms, Molecules, and Ions. Chapter 2 yThe Atomic Theory ( 2.1) yThe...
Transcript of Atoms, Molecules, and Ions · Atoms, Molecules, and Ions. Chapter 2 yThe Atomic Theory ( 2.1) yThe...

Atoms, Molecules, and Ions

Chapter 2The Atomic Theory (2.1)The Structure of the Atom (2.2)Atomic Number, Mass Number, and Isotopes (2.3)The Periodic Table (2.4)Molecules and Ions (2.5)Chemical Formulas (2.6)Naming Compounds (2.7)Introduction to Organic Compounds (2.8)

2.1 The Atomic Theory
What are the four hypotheses of Dalton’s Atomic Theory?
Figure 2.1, p. 30

2.1 The Atomic Theory
Law of Multiple Proportions:
If two elements can combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element are in ratios of small whole numbers.

Example:
Nitrogen and oxygen combine to form 2 different compounds
Compound 1:10.00 g of oxygen combine to form 18.75 g of the compound
Compound 2:10.00 g of oxygen combine to form 14.38 g of the compound
What is the mass ratio of nitrogen between compound 1 and compound 2?
Figure 2.2, p. 31

Key Definitions
Law of conservation of massAtomElementCompound

Discovery of the Electron (J.J.Thomson, 1898-1903)Thomson experimented using cathode-ray tubes: ◦ high voltage was applied to the tube◦ ray was emitted by the negatively charged electrode
The ray was repelled by the negative pole of an applied electric field and also deflected by a magnetic field.
The beam consists of negatively charged particles, electrons.
Experiments Leading to the Discovery of the Subatomic Particles
2.2 The Structure of the Atom

Experiments Leading to the Discovery of the Subatomic Particles
2.2 The Structure of the Atom
Figure 2.3, p. 32

Experiments Leading to the Discovery of the Subatomic Particles
2.2 The Structure of the Atom
Figure 2.4, p. 32

Discovery of the Electron (Millikan, 1908)Measured charge of the electron:
by observing the rate of the fall of charged and uncharged oil droplets
Used the determined charge to calculate the mass of the electron
1923 Nobel Prize in Physics
Experiments Leading to the Discovery of the Subatomic Particles
2.2 The Structure of the Atom

Experiments Leading to the Discovery of the Subatomic Particles
2.2 The Structure of the Atom

Plum Pudding Model
Figure 2.6 p. 34

(Uranium compound)
α-rays (positive charge)β-rays (negative charge)γ-rays (high energy-no charge)
charged metal plates
Figure 2.5 p. 33

Discovery of the Nucleus (E. Rutherford, 1910)Rutherford used a very thin gold foil and alpha particlesThe alpha particles went through the gold foil largely undeflected.A small number were slightly deflected and some of those were deflected back at the source.
Positive charge of the atom is concentrated in the nucleus – protons have an opposite charge of electrons and a mass of approximately 1800x an electron
Experiments Leading to the Discovery of the Subatomic Particles
2.2 The Structure of the Atom

Experiments Leading to the Discovery of the Subatomic Particles
2.2 The Structure of the Atom
Figure 2.7, p. 34

atomic radius ~ 100 pm = 1 x 10-10 m
nuclear radius ~ 5 x 10-3 pm = 5 x 10-15 m
Rutherford’s Model of the Atom
“If the atom is the Houston Astrodome, then the nucleus is a marble on the 50-yard line.”
electrons
Figure 2.8, p. 35

Key Definitions
ElectronProtonNeutron
Give relative mass, charge and location of each subatomic particle
Table 2.1, p. 36

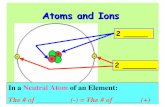
2.3 Atomic Number, Mass Number, and Isotopes
True/False: An atom is neutral
True/False: The nucleus is always positively charged
Define atomic number (Z)Define atomic mass (A)Define isotope

The Isotopes of Hydrogen
Margin figure, p. 37

2.3 Atomic Number, Mass Number, and Isotopes
True/False: An atom is neutral
True/False: The nucleus is always positively charged
Define atomic number (Z)Define atomic mass (A)Define ion, cation, anion

2.4 The Periodic Table
Figure 2.9, p. 38

Key DefinitionsGroupPeriodNonmetal◦ Halogen◦ Noble gasesMetal◦ Alkali metal◦ Alkaline earth metal◦ Transition metal◦ Inner transition metal

Period
Group
Alkali M
etal
Noble G
as
Halogen
Alkali E
arth Metal
Figure 2.9, p. 38

Cha
pter
2 –
Prac
tice

Hydrogen and water molecules are shown in the figure. What are the classifications of these substances?
A. Both are elements.B. Both are compounds.C. Hydrogen is a compound and water is an element.D. Hydrogen is an element and water is a compound.C
hapt
er 2
–Pr
actic
e

Which nucleus is shown in the figure? A. 3He B. 2HeC. 3H D. 2H
What is the nuclear charge for palladium-104?A. +46 B. +58 C. +60 D. +104
How many electrons are in Al3+?A. 3 B. 10 C. 13 D. 16Cha
pter
2 –
Prac
tice

2.5 Molecules and Ions
Key Definitions:◦ Molecule
Diatomic moleculePolyatomic molecule
◦ Molecular compounds◦ Molecular elements

Practice (Moodle)Pr
actic
e ex
erci
se –
Moo
dle

Practice (Moodle)Pr
actic
e ex
erci
se –
Moo
dle

2.5 Molecules and Ions
Key Definitions:◦ Ion
Monoatomic ionPolyatomic ion
◦ Cation◦ Anion
S
OO
O
O 2-

Monoatomic Ions
Figure 2.10, p. 40

Table 2.3, p. 46

2.6 Chemical Formulas
Key Definitions:◦ Molecular formula◦ Empirical formula

2.6 Chemical Formulas
Figure 2.11, p. 41

Practice (Moodle)Pr
actic
e ex
erci
se –
Moo
dle

2.6 Chemical Formulas
Formula of ionic compounds◦ How do ionic compounds exist?◦ How do we write a formula for an ionic
compound (is it a molecular or empirical formula)?
Figure 2.12, p. 43

Table 2.3, p. 46

Practice (Moodle)
Which are ionic compounds?
Prac
tice
exer
cise
–M
oodl
e

2.7 Naming Compounds
Ionic compounds◦ Transition metal compounds◦ Zinc, silver, cadmium, lead, tin◦ Polyatomic ions

Table 2.3, p. 46

2.7 Naming Compounds
Ionic compounds◦ Transition metal compounds◦ Zinc, silver, cadmium, lead, tin◦ Polyatomic ions
Molecular compounds◦ Compounds containing hydrogen◦ Common names

Table 2.4, p. 48

Figure 2.13, p. 49

2.7 Naming CompoundsIonic compounds◦ Transition metal compounds◦ Zinc, silver, cadmium, lead, tin◦ Polyatomic ionsMolecular compounds◦ Compounds containing hydrogen◦ Common namesAcids◦ Binary◦ OxoacidsHydrates

Figure 2.14, p. 51

Tables 2.5 and 2.6, p. 50-51

Which group on the periodic table contains the greatest number of molecular elements?
Cha
pter
2 –
Prac
tice

What is this figure attempting to show?
What makes this figure confusing?
How does that relate to scale?
Margin Figure, p. 50