Antiparanodal antibodies and IgG subclasses in acute autoimmune neuropathy · ARTICLE OPEN ACCESS...
Transcript of Antiparanodal antibodies and IgG subclasses in acute autoimmune neuropathy · ARTICLE OPEN ACCESS...

ARTICLE OPEN ACCESS
Antiparanodal antibodies and IgG subclasses inacute autoimmune neuropathyLuise Appeltshauser MD Anna-Michelle Brunder Annika Heinius Peter Kortvelyessy MD
Klaus-Peter Wandinger MD Ralf Junker MD Carmen Villmann PhD Claudia Sommer MD
Frank Leypoldt MD and Kathrin Doppler MD
Neurol Neuroimmunol Neuroinflamm 20207e817 doi101212NXI0000000000000817
Correspondence
Dr Appeltshauser
Appeltshau_Lukwde
AbstractObjectiveTo determine whether IgG subclasses of antiparanodal autoantibodies are related to diseasecourse and treatment response in acute- to subacute-onset neuropathies we retrospectivelyscreened 161 baseline serumCSF samples and 66 follow-up serumCSF samples
MethodsWe used ELISA and immunofluorescence assays to detect antiparanodal IgG and their sub-classes and titers in serumCSF of patients with Guillain-Barre syndrome (GBS) recurrentGBS (R-GBS) Miller-Fisher syndrome and acute- to subacute-onset chronic inflammatorydemyelinating polyradiculoneuropathy (A-CIDP) We evaluated clinical data retrospectively
ResultsWe detected antiparanodal autoantibodies with a prevalence of 43 (7161) more often inA-CIDP (423 174) compared with GBS (3114 26) Longitudinal subclass analysis inthe patients with GBS revealed IgG23 autoantibodies against Caspr-1 and against antindashcontactin-1Caspr-1 which disappeared at remission At disease onset patients with A-CIDPhad IgG23 antindashCaspr-1 and antindashcontactin-1Caspr-1 or IgG4 antindashcontactin-1 antibodiesIgG3 being associated with good response to IV immunoglobulins (IVIg) In the chronic phaseof disease IgG subclass of one patient with A-CIDP switched from IgG3 to IgG4
ConclusionOur data (1) confirm and extend previous observations that antiparanodal IgG23 but notIgG4 antibodies can occur in acute-onset neuropathies manifesting as monophasic GBS (2)suggest association of IgG3 to a favorable response to IVIg and (3) lend support to thehypothesis that in some patients an IgG subclass switch from IgG3 to IgG4 may be thecorrelate of a secondary progressive or relapsing course following a GBS-like onset
RELATED ARTICLE
EditorialIsotyping paranodalantibodies in inflammatoryneuropathies One stepcloser to precision care
Page e843
From the Department of Neurology (LA A-MB CS KD) University Hospital of Wurzburg Neuroimmunology Section (AH K-PW RJ FL) Institute of Clinical ChemistryUniversity Hospital of Schleswig-Holstein Campus Kiel Department of Neurology (PK) University Hospital of Magdeburg and Institute for Clinical Neurobiology (CV) UniversityHospital of Wurzburg Germany
Go to NeurologyorgNN for full disclosures Funding information is provided at the end of the article
The Article Processing Charge was funded by the authors
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives License 40 (CC BY-NC-ND) which permits downloadingand sharing the work provided it is properly cited The work cannot be changed in any way or used commercially without permission from the journal
Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the American Academy of Neurology 1
Autoantibodies against the paranodal antigens contactin-1contactin-associated protein-1 (Caspr-1) and neurofascin-155(NF155) have been described as biomarkers for a new entity ofinflammatory neuropathies classified as paranodopathies1ndash3 Inthe chronic phase of disease IgG4-seropositive patients do notrespond to IV immunoglobulins (IVIg) but to rituximab4ndash7
Noninflammatory IgG4 autoantibodies are pathogenic possiblyby inhibition of the interaction between contactin-1Caspr-1and NF155 and by NF155 depletion8ndash11 Autoantibodies of theIgG3 subclass have been described (1) in monophasic disease(2) at the subacute onset (3) in patients with antindashpan-neurofascin autoantibodies and severe course of disease and(4) most recently in chronic inflammatory demyelinating poly-radiculoneuropathy (CIDP) with clinical features indistinguish-able from seronegative patients but with a good response toIVIg6711ndash13 Proinflammatory IgG3 antibodies lead to comple-ment deposition in vitro14 and in vivo resulting in reversibleconduction failure in Lewis rats intraneurally injected withantindashcontactin-1 IgG315 and may therefore play a role in theacute onset of paranodopathies However data on antiparanodalautoantibodies in Guillain-Barre syndrome (GBS) or the acuteonset of CIDP are scarce because previous studies mostly fo-cused on CIDP and patients were recruited during the chronicphase of disease IgG subclass distribution and the associatedclinical phenotype have never been investigated longitudinallyWe therefore aimed at determining the prevalence and IgGsubclass of paranodal autoantibodies in a cohort of patients withacute to subacute inflammatory neuropathies including follow-up of seropositive patients We hypothesize that IgG subclassand titer are related to the course of disease and therapeuticresponse in acute-onset paranodopathy
MethodsPatients and controlsOne hundred sixty-one patients with suspected GBS andsubacute inflammatory neuropathy (peak of symptoms le90days) who had undergone diagnostic lumbar puncture at theUniversity Hospitals of Kiel andMagdeburg between 2001 and2016 were included into the study We assessed clinical dataretrospectively by analysis of discharge letters and documentedlaboratory electrophysiologic and MRI examinations Tablee-1 linkslwwcomNXIA274 summarizes demographic dataThe diagnosis of GBS was confirmed in n = 114 patients withdiagnostic certainty according to the Brighton criteria1617 level1 in 73 patients level 2 in 29 patients level 3 in 6 patients andlevel 4 in 6 patients We classified 6 patients as recurrent GBS(R-GBS) according to the criteria adapted from Kuitwaard
et al18 and 18 patients as Miller-Fisher syndrome (MFS) In 23patients the initial diagnosis was GBS but was later reverted toCIDP because of a disease progression gt2 months (diagnosticcertainty according to the EFNS criteria19 definite CIDP in 10patients probable CIDP in 3 patients possible CIDP in 1patient and EFNS electrodiagnostic criteria not fulfilled in923 patients) Eighteen of 23 patients with CIDP fulfilled thecriteria for acute-onset CIDP (A-CIDP) proposed in previouspublications20ndash22 and 523 patients with CIDP showed a sub-acute-onset (peak le90 days) In the following all patients withacute- to subacute-onset CIDP are referred to as part of theA-CIDP cohort Follow-up sera and CSF samples were avail-able in 66 patients including 3 seropositive patients We in-cluded sera of 40 healthy controls recruited in former studies6
Sera of all patients had already been tested for anti-NF155autoantibodies in a previous study12
Binding assays on murine teased fibersWe used binding assays on murine teased fibers at a serum di-lution of 1100 and 1500 to screen all patientsrsquo and controlsrsquo serafor antiparanodal autoantibodies as previously described23 Tit-ers of seropositive samples were measured with a dilution seriesusing Cy3TM-conjugated goat anti-human IgG 1300 (JacksonWest Grove) In positive sera we performed double immuno-fluorescence with sera diluted 150 and 1100 and rabbit antindashCaspr-1 diluted 11000 (Abcam Cambridge United Kingdom)using MFP488-conjugated goat anti-human IgG diluted 1500(MoBiTec Gottingen Germany) and Cy3TM-conjugated don-key anti-rabbit IgG diluted 1300 (Jackson) as secondary anti-bodies For subclass analysis we used FITC-conjugatedsecondary antibodies diluted 1100 namely mouse anti-humanIgG1 IgG4 (Abcam) mouse anti-human IgG2 (Merck Darm-stadt Germany) and sheep anti-human IgG3 (RocklandImmunochemicals Inc Pottstown PA) A healthy control se-rum and serum of patients with antindashCaspr-1 IgG3 and IgG4antibodies from a previous study7 stained on separate slidesserved as negative and positive controls for double immunoflu-orescence and subclass analysis We tested CSF of seropositivepatients of the first assessment and follow-up on teased fibers ina dilution of 120 and 150 as described above CSF of fivepatients who were seronegative served as negative controls
ELISAWe performed ELISA for detection of antindashcontactin-1 induplets with sera of all patients and 40 healthy controls as pre-viously described6122324 For antindashCaspr-1 ELISA we coatedChinese hamster ovary cell linendashderived recombinant humanCaspr-1 protein (Research and Diagnostic Systems Inc Min-neapolis MN) at a dilution of 2 μgmL on MaxiSorb 96-well
GlossaryA-CIDP = acute-onset chronic inflammatory demyelinating polyradiculoneuropathyCaspr-1= contactin-1-associated protein-1CBA = cell-based assay CIDP = chronic inflammatory demyelinating polyradiculoneuropathy GBS = Guillain-Barresyndrome HEK = human embryonic kidney HLA = human leukocyte antigen ICU = intensive care unit IVIg = IVimmunoglobulin MFS = Miller-Fisher syndrome NF = neurofascin OD = optical density R-GBS = recurrent GBS
2 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
plates (Thermo Scientific Fisher Minneapolis MN) and testedbaselinefollow-up sera of seropositive patients and 40 healthycontrols or rabbit antindashCaspr-1 (Lifespan Biosciences SeattleWA) in duplets using corresponding horseradish peroxidasendashconjugated secondary antibodies6 and conjugated anti-rabbitIgG (DakoCytomation Glostrup Denmark) For each samplewe subtracted corresponding values of uncoated duplet wells toreduce background signals We set the threshold for both assaysat 5 SDs above the mean of healthy controls Antibody titerswere determined by serial dilutions Subclass-specific secondaryantibodies served for subclass analysis as previously described12
We tested CSF of seropositive patients in a dilution of 120 inantindashcontactin-1 ELISA and antindashCaspr-1 ELISA (exceptionpatient 4 baseline CSF in both ELISAs and patient 3 in anti-Caspr-1 ELISA due to lack of material) as described above withCSF of 5 seronegative patients as negative controls
Preabsorption experimentsTo determine the specific paranodal target of the autoanti-bodies we serially preincubated seropositive sera and serumfrom a healthy control with contactin-1 (CNTN1)ndash andCaspr-1 (CNTNAP1)ndashtransfected human embryonic kidney(HEK) 293 cells as previously described7 and afterwardperformed binding assays on murine teased fibers as de-scribed above
Cell-based assayFor specification we tested sera of patients positive in teasedfibers assay or ELISA and sera of five controls on HEK293 cellstransfected with plasmids of human Caspr-1 (CNTNAP1) andrat contactin-1 (CNTN1) as previously described723 and usedpolyclonal rabbit antindashcontactin-1 IgG (Abcam) and mono-clonal mouse antindashCaspr-1 IgG1 (Santa Cruz BiotechnologyDallas TX) for double immunofluorescence in positivepatients with corresponding Cy3TM-conjugated donkeyanti-rabbit IgG (Abcam) donkey anti-mouse IgG andMFP488-conjugated goat anti-human IgG (MoBiTec) sec-ondary antibodies Sera of patients with antindashCaspr-1 andantindashcontactin-1 antibodies from previous studies67 servedas positive controls and sera of healthy controls as negativecontrols in the double immunofluorescence assays
Statistical analysisWe performed statistical analysis (descriptive statisticscalculation of OR and 95 CI) with SPSS 250 (IBMArmonk NY)
Standard protocol approvals registrationsand patient consentsThe Ethics Committees of the University of Wurzburg andthe University of Kiel approved to conduct this study Writteninformed consent was obtained from all participants in thestudy
Data availabilityData not published within this article are available at theUniversity Hospital of Wurzburg or will be shared in an
anonymized manner on request from any qualified in-vestigator for purposes of replicating procedures and results
ResultsAntiparanodal autoantibodies of different IgGsubclasses in 43 of the patientsWe applied immunofluorescence binding assays on murineteased fibers as a screening tool for detection of anti-paranodal autoantibodies We observed serum binding tothe paranodal junctional region colocalizing with the com-mercial antindashCaspr-1 antibody (figure 1A) in seven patientsof the total cohort (7161 prevalence 43) in none of thepatients of the R-GBS (06) andMFS (018) subcohort norin the healthy controls (040) but in 3114 (26) of theGBS subcohort and 423 (174) of the A-CIDP subcohortThus paranodal autoantibodies occurred more frequentlyin A-CIDP compared with GBS (OR = 779 95 CI161ndash3795) Two of the sera with paranodal binding (1 GBSand 1 A-CIDP) had been identified as antindashNF155 sero-positive in a recent study12 therefore experimental findingsand clinical associations of these patients are not reportedhere Target antigens of the other five seropositive patientswere determined using cell-based assay (CBA figure 1B)and ELISA (see below) and confirmed by preabsorptionexperiments (figure 1C) In the GBS subcohort the twoseropositive patients showed antindashCaspr-1 (patient 1) andantindashcontactin-1Caspr-1 (patient 2) antibodies Seroposi-tive A-CIDP patients carried antibodies against either con-tactin-1 (patient 3) contactin-1Caspr-1 (patient 4) or anti-Caspr-1 (patient 5) At the onset of disease the two patientswith GBS showed IgG2 and IgG3 antibodies (table 1)During the acute phase at onset sera of the three patientslater classified as A-CIDP carried IgG24 (patient 3) andIgG23 (patients 4 and 5) as detected by binding assays andELISA (figure 2 A and B) Autoantibody titers are shown intable 1 The results of the binding assays on teased fibersELISA and CBA showed concordance regarding the specifictarget antigen of the antibodies and the predominating IgGsubclass There were only minor differences of the antibodytiter and coexisting subclasses in ELISA and teased fibersassay In case of double positivity for anti-contactin-1Caspr-1 (patients 2 and 4) preabsorption assays detectedonly the dominating antibody (table 1)
Subclass switch from IgG3 to IgG4 at follow-upin one patientIn 66161 patients follow-up serum and CSF samples wereavailable including three seropositive patients (1 GBS and 2A-CIDP) None of the follow-up sera of initially seronega-tive patients or controls showed positive results in the teasedfibers assay (data not shown) nor in the contactin-1 ELISA(figure 3A) Serum of the patient with GBS acquired inremission 55 months after the first assessment when thepatient did not show any remaining neurologic deficits wasseronegative in all experimental assays (figure 3 B and C)
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 3
Follow-up serum of one patient with A-CIDP two monthsafter onset ie still in the acute phase of disease (patient 5)showed IgG3 autoantibodies against Caspr-1 with no dif-ference in antibody titer and subclass compared with onset(figure 3 B and C) A follow-up-sample during the chronicstage (28 months after the onset) revealed a reduction of thetiter (150 in teased fibers negative in ELISA table 1) ofantindashCaspr-1 IgG autoantibodies with subclasses not mea-surable due to the low titer (data not shown) The patientnow presented with only very mild sensorimotor impair-ment and ameliorated nerve conduction studies Follow-upserum and CSF of the other patient with A-CIDP (patient 4)who was initially IgG3ndashanti-contactin-1Caspr-1 doublepositive were acquired 120 months after the acute onsetwhen the patient had developed relapsing-remitting senso-rimotor CIDP At follow-up we could no longer detectantindashcontactin-1 but detected and confirmed antindashCaspr-1autoantibodies via antindashCaspr-1 ELISA CBA and pre-absorption assay (table 1 and figure 3 B and C) Subclassanalysis now revealed reactivity against IgG24 but notIgG3 subclass anymore giving evidence of a switch of IgG
subclasses between the acute and chronic stage of the disease(figure 2C)
Intrathecal antiparanodal autoantibodies inpatients with high antibody titer and blood-brain barrier dysfunctionELISA with CSF samples of the seropositive patients and fivedisease controls showed elevated optical density (OD) ata dilution of 120 only in antindashcontactin-1 patient 3 witha very high serum titer (see appendix e-1 linkslwwcomNXIA275) and antindashCaspr-1 patient 5 with signs of se-verely disrupted blood-brain barrier (high CSF protein MRIcauda equina enhancement see appendix e-1 linkslwwcomNXIA275) but equally low OD values for the otherseronegative and seropositive patients (see figure e-1 linkslwwcomNXIA273) Binding assays on murine teasedfibers showed weak binding of CSF to the paranodes in thesetwo patients but not in the other seropositive patients orcontrols None of the seropositive patients showed signs ofintrathecal autoantibody production with CSF IgG indexand antiparanodal autoantibody specific ASI (antibody
Figure 1 Detection of antiparanodal autoantibodies by binding assays on teased fibers CBA and preabsorption assay
(A) Colocalization overlay photomicrographs of double immunofluorescence assays with commercial autoantibody displayed in red and human serum ingreen Colocalization at the paranodes (arrows) appears yellow and indicates antiparanodal autoantibodies in patients 1ndash5 (AbndashAf) but not in the negativecontrol serum (Aa) (B) The specific target of the autoantibodies is illustrated by colocalization overlay photomicrographs on transfected HEK293 cells(arrowheads) with commercial antibody in red serum in green and nucleus staining in blue Colocalization appears yellow (merge) and confirms antindashcontactin-1 in patients 2 (Bc) 3 (Bd) and 4 (Be) but not in patients 1 (Bb) 5 (Bf) and the negative control (Ba) Sera of patients 1 2 4 and 5 (Bh Bi Bk andBl) colocalize on Caspr-1 (CNTNAP1)ndashtransfected HEK293 cells as proof of antindashCaspr-1 autoantibodies whereas sera of the negative control (Bg) and patient3 (Bj) donot showantindashCaspr-1 positivity (C) Single immunofluorescence onmurine teased fibers afterHEK293 contactin-1 andCaspr-1 preabsorption showsthat antiparanodal autoantibodies disappear after contactin-1 preabsorption in patient 3 (Cd) and after Caspr-1 preabsorption in patient 4 (Cf) provingspecificity to the respective target antigen Scale bar = 10 μm CBA = cell-based assay HEK = human embryonic kidney
4 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
Table 1 Summary of the autoantibody status of seropositive patients 1ndash5 at baseline and follow-up
CSF Serum
Teased fibers and ELISA Preabsorption CBA
Paranodal bindingOD Titer IgG subclass
Patient 1 (baseline no follow-up)
Teased fibers Negative Positive 1100 IgG3
Contactin-1 0202 0465 nd nd Negative Negative
Caspr-1 0072 2961 1200 nm Positive Positive
Patient 2 (baseline)
Teased fibers Negative Positive 1200 nd
Contactin-1 0247 3538 1200 IgG2 Negative Positive
Caspr-1 0129 3965 11000 IgG2 Positive Positive
Patient 2 (follow-up)
Teased fibers nd Negative nd nd
Contactin-1 nd 0039 nd nd nd Negative
Caspr-1 nd 0025 nd nd nd Negative
Patient 3 (baseline no follow-up)
Teased fibers Negative Positive 130000 IgG4
Contactin-1 0516 4492 140000 IgG42 Positive Positive
Caspr-1 nd 0073 nd nd Negative Negative
Patient 4 (baseline)
Teased fibers Negative Positive 1500 IgG3
Contactin-1 0267 2294 1200 IgG3 Negative Positive
Caspr-1 nd 3011 1500 IgG32 Positive Positive
Patient 4 (follow-up)
Teased fibers Negative Positive 1500 IgG4
Contactin-1 0227 0206 nd nd Negative Negative
Caspr-1 0138 3618 1500 IgG24 Positive Positive
Continued
Neurolo
gyorgN
NNeurologyN
euroim
munology
ampNeuroinflam
mation
|Volum
e7N
umber
5|
Septem
ber2020
5
Table 1 Summary of the autoantibody status of seropositive patients 1ndash5 at baseline and follow-up (continued)
CSF Serum
Teased fibers and ELISA Preabsorption CBA
Paranodal bindingOD Titer IgG subclass
Patient 5 (baseline)
Teased fibers Positive Positive 1100 IgG3
Contactin-1 0219 0260 nd nd Negative Negative
Caspr-1 0136 nd nd nd Positive Positive
Patient 5 (first follow-up)
Teased fibers Positive Positive 1100 IgG3
Contactin-1 0236 0243 nd nd Negative Negative
Caspr-1 0364 3802 1200 IgG32 Positive Positive
Patient 5 (second follow-up)
Teased fibers nd Positive 150 nm
Contactin-1 nd 0168 nd nd nd Negative
Caspr-1 nd 0013 nd nd nd Negative
Abbreviations A-CIDP = acute-onset chronic inflammatory demyelinating polyradiculoneuropathy Caspr-1 = contactin-1ndashassociated protein-1 CBA = cell-based assay GBS = Guillain-Barre syndrome nd = not done nd =not done due to lack of material nm = not measurable OD = optical densityResults of teased fibers assays are displayed in the first row for each patient contactin-1 and Caspr-1-specific results are displayed in lines 2 and 3 ODs above the threshold and results considered positive are highlighted inbold letters There is a 100 concordance regarding target specificity in the teased fibers assay ELISA and CBA but minor differences in IgG titer and coexisting subclass between the teased fibers assay and ELISA In case ofdouble positivity preabsorption assays only detect the target antigen with the higher antibody titer (see ELISA titer)
6NeurologyN
euroim
muno
logyampNeuroinflam
mation
|Vo
lume7N
umber
5|
Septem
ber
2020NeurologyorgN
N
specificity index) being in a normal range (see appendix e-1linkslwwcomNXIA275)
Clinical features of seropositive patientsFigure 4 schematically summarizes the clinical course relatedto the experimental results Appendix e-1 linkslwwcomNXIA275 summarizes clinical data of seropositive patients1ndash5 and provides a detailed description of the clinical diseasecourse in individuals The two seropositive patients withGBS were both middle-aged women presenting with severemotor but only mild sensory involvement Cranial nerveinvolvement autonomic symptoms and neuropathic painwere present in 12 patients with GBS only Both patientsrequired treatment on the intensive care unit (ICU) The
three patients with A-CIDP presented with moderate to severeparesis and multimodal sensory impairment at disease onsetDuring the course of disease all three patients with A-CIDPreported neuropathic pain requiring pregabalingabapentinor even opioid treatment Two of three patients withA-CIDP presented sensory ataxia Nerve conduction studiesshowed demyelinating features in all seropositive patientswith conduction block in 35 patients Sural nerve biopsywas performed in one patient (patient 5) six months after theonset and showed axonal loss without signs of demyelinationCSF analysis revealed cytoalbuminologic dissociation andblood-brain barrier dysfunction in all seropositive patientsThe patient with subacute-onset CIDP highly elevated CSFprotein and detection of antindashCaspr-1 in both serum and
Figure 2 Serum subclass analysis at baseline and at follow-up with IgG subclass switch
(A) Results of antindashcontactin-1 and antindashCaspr-1 ELISA with baseline sera and subclass-specific IgG1-4 autoantibodies (grayscale) are shown as of the OD ofthe total IgG (using anti-human IgG autoantibody) on y-axis In patient 1 ELISA IgG subclasses were not measurable ELISA revealed mainly IgG2 subclass inpatient 2 (GBS) IgG2IgG4 in patient 3 (A-CIDP) and IgG23 in patients 4 and 5 (A-CIDP) (B) Acute phase teased fibers subclass analysis with baseline serashowed binding to the paranodal regions (arrows) only when using IgG3-specific secondary antibody in patients 1 (GBS) and 4 (A-CIDP) visualized byphotomicrographs of single immunofluorescence staining on teased fibers (C) AntindashCaspr-1 IgG subclass ELISA with follow-up serum of patient 4 nowrevealed IgG 24 subclass Photomicrographs show immunofluorescence staining of murine teased fibers with subclass-specific IgG3 and IgG4 autoanti-bodies at follow-up Paranodal binding disappeared using IgG3 antibody but occurred using IgG4 subclass-specific antibody proving subclass switch inpatient 4 Scale bar = 10 μm A-CIDP = acute-onset chronic inflammatory demyelinating polyradiculoneuropathy GBS = Guillain-Barre syndrome
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 7
CSF (patient 5) developed action tremor during the courseof disease
Antibody status is concordant with diseaseactivity and therapy responseOne of two patients with GBS (patient 2) received IVIgwhich did not lead to amelioration This patient was IgG2 gtIgG3 seropositive (patient 2 figure 2A) Both patients withGBS responded well to plasma exchange The two patientswith A-CIDP and IgG3 autoantibodies (patients 4 and 5)showed good response to IVIg treatment in the acute phase ofdisease Ongoing IVIg treatment led to further improvementof symptoms and was stopped after 25 cycles in patient 5because of a remission of symptoms In the other patient withA-CIDP and antindashcontactin-1Caspr-1 IgG3 at disease onset(patient 4) IVIg showed a good therapeutic effect in the acutephase and led to remission not requiring further therapy Inthe chronic phase IVIg was initiated again and stopped after26 cycles because of a loss of the therapeutic effect At thatstage the autoantibody subclass had already switched fromIgG3 to IgG4 (for detailed treatment response in the course ofdisease see appendix e-1 linkslwwcomNXIA275) Thepatient with A-CIDP and high-titer antindashcontactin-1 IgG4autoantibodies (patient 3) had to be treated on ICU becauseof the severity of symptoms developed renal dysfunction didnot respond to IVIg treatment and did not ameliorate duringthe course of disease
DiscussionWedetected antiparanodal autoantibodies in 43of patientswithacute to subacute immune-mediated neuropathy more frequentlyin CIDP with acute to subacute onset than in GBS We foundIgG23 in monophasic GBS which supports the notion of pre-vious studies that IgG3 is the predominant subclass inmonophasicdisease7 By analyzing sera from different time points we couldshow that the antibody status was concordant with the diseaseseverity Therefore our data support pathogenicity of autoanti-bodies and suggest that autoantibody status and titer are validindicators for disease activity as proposed in previous studies onparanodopathy and further IgG4-related neurologic diseases25ndash27
In one patient we could demonstrate a subclass switch fromIgG3 in acute disease to IgG4 in chronic disease a finding thatwe had hypothesized in a previous study6 In other autoimmunediseases eg in membranous glomerulonephritis subclassswitch of anti-PLA2R autoantibodies from IgG1 to IgG4 alsoassociates with progression to chronic disease28 According toa linear model of autoantibody response unspecific IgM re-sponse is followed by IgG isotype produced by antigen-experi-enced B cells These IgG are of the IgG3 subclass andsequentially switch to IgG1 2 and 42930 Concurrently auto-antibodies strongly gain antigen affinity with IgG4 showing thehighest affinity to its target3132 Disease progression in para-nodopathy might therefore depend on IgG subclass properties
Figure 3 Antiparanodal IgG autoantibodies at follow-up assessment
(A) Contactin-1 IgG ELISA of all patients at first assessment of 66161 patients at follow-up and of 40 healthy controls Optical density (OD) at 450 nm isdisplayed on the y-axis The threshold for positive results is set at 5 SDs above the mean of controls (0615) Patients 2ndash4 show positive results at firstassessment The other patients and controls show values below the threshold Sera of patients 2 4 and 5 are negative in antindashcontactin-1 IgG ELISA at follow-up as well as 63 other follow-up sera Patients 1 and 3were lost to follow-up (B) Overlay photomicrographs of teased fibers double immunofluorescence and(C) contactin-1ndash and Caspr-1 (CNTNAP1)ndashtransfected HEK293 cells show loss of antindashcontactin-1Caspr-1 autoantibodies in patient 2 (GBS) at follow-up (BaCa Cd) but colocalization of commercial antindashCaspr-1 antibody and sera at paranodes in patients 4 and 5 with A-CIDP (Bb Bc arrows) Both sera bind toCaspr-1 (CNTNAP1)ndashtransfected HEK293 cells (Ce Cf arrowheads) but not to contactin-1ndashtransfected cells (Cb Cc) Scale bar = 10 μm A-CIDP = acute-onsetchronic inflammatory demyelinating polyradiculoneuropathy GBS = Guillain-Barre syndrome HEK = human embryonic kidney
8 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
and affinities Gain of specificity and affinity at switch from IgG3to IgG4 might explain different binding characteristics of anti-paranodal autoantibodies in the patient whose IgG3 autoanti-bodies reacted both against contactin-1Caspr-1 epitopes butwhose IgG4 autoantibodies were specific to Caspr-1 Here wereport IgG24 (1) in patient 4 in the chronic phase after subclassswitch and (2) in another patient at disease onset showingnonameliorating disease with high disability similar to previousreports on antindashcontactin-1 IgG4-related disease111333 So farstudies have only reported antiparanodal IgG4 autoantibodies inpatients with chronic disease7113435 We therefore suggest thathigh-affinity IgG4 autoantibody binding leads to chronic diseasewith functional impairment possibly caused by disturbance of theantigen interaction paranodal architecture and paranodalcomplex formation as demonstrated previously68ndash11
Patients of our study with predominant IgG3 subclass showedgood response to IVIg whereas patients with IgG2 and IgG4initially did not respond to IVIg or lost responsiveness in thedisease course Several studies described good responses toIVIg in IgG3-associated paranodopathy but poor responses inIgG4-associated disease56111334 IgG3 can initiate comple-ment activation opsonization antigen cross-linking and in-ternalization whereas IgG2 shows little C1q binding and IgG4completely lacks these Fc-mediated effector functions31 In anin vivo study on antindashcontactin-1 pathogenicity we suggestedcomplement-mediated functional impairment at the paranodesas the pathophysiologic correlate in IgG3-mediated para-nodopathy earlier and could show in vitro that IVIg inhibitscomplement deposition and activation dose-dependently1415
Our data therefore support the hypothesis that therapy re-sponsiveness might depend on IgG subclass and subclass-related pathomechanism of antiparanodal autoantibodies If
validated in larger studies these findings might have a directimpact on the treatment regime in affected patients Testing ofautoantibody subclass during the course of disease may beuseful as an indicator of a positive response to IVIg in case ofIgG3 whereas IgG4-related paranodopathy shows very goodresponse to rituximab46711
The trigger of the autoantibody production and the patho-mechanism behind possible IgG switching are still unknownThree of five seropositive patients in this study had diabetesmellitus type 2 as a comorbidity diagnosed years before theonset of disease Previous studies report disruption of the blood-brain barrier and the nodal architecture in patients with diabetesmellitus thereby possibly exposing paranodal antigens to theimmune response3637 A recent study has identified humanleukocyte antigen (HLA)-DRB15 as a risk factor for CIDPassociated with anti-NF155 antibodies and another studyreported different HLA antibody patterns with subclass switchafter renal transplantation3839 Whether interindividual differ-ences in antibody production and switching during the course ofdisease depend on (1) HLA alleles (2) antigen exposure or (3)further immunologic mechanisms has to be investigated infurther studies
Regarding characteristic clinical features of paranodopathy wereport neuropathic pain in antindashCaspr-1ndashrelated disease aspreviously described in patients with higher autoantibody tit-ers7 Different titers might explain discrepancies between ourresults and a recent study that did not report pain in antindashCaspr-1ndashpositive patients711 In our patients further features of IgG4-related chronic paranodopathy such as tremor sensory ataxiaand nephropathy561340 were only present in the course ofdisease suggesting that they evolve due to chronic autoantibody
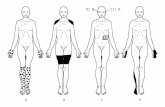
Figure 4 Scheme of the autoantibody status in the course of disease in seropositive patients
The course of disease is shown on the x-axis by pseudo-logarithmic display of the time course Time point 0 is set at baseline assessment of serumCSF Thecolor code indicates severity of symptoms Results of serum assessment are displayed in black Patients 1 and 3 were lost to follow-up
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 9
exposure The pathomechanistic correlate of action tremorwhich recently has also been described in antindashCaspr-1 IgG3-related paranodopathy11 is still under research but studiessuggest cerebellar origin5612 In this study we detected in-trathecal autoantibodies in a patient with tremor as previouslyreported in a case of antindashpan-neurofascinndashpositive para-nodopathy12 The antiparanodal autoantibodies most probablydiffuse into the intrathecal compartment due to a blood-brainbarrier dysfunction as indicated by normal IgG and ASI indicesin our patients Our CSF data suggest that detection of anti-paranodal antibodies in CSF shows little sensitivity and dependson either high titer or strong leakage of the blood-brain barrierWe therefore encourage further studies on intrathecal anti-paranodal autoantibodies targeting sensitivity pathogenicityand association to clinical symptoms
Low prevalence of autoantibodies against paranodal proteins inacute inflammatory neuropathy retrospective assessment and thelow number of seropositive patients available for follow-up limitour study Clinical characteristics at acute onset and subclass-related seropositivity as a possible risk factor for chronic pro-gression when detected at the onset should be investigated in largemulticentric studies considering lowoverall prevalence ofGBS andA-CIDP as well as further possible confounders in analysis ofparanodopathy-specific clinical symptoms eg coinciding painfuldiabetic neuropathy The question of sequential subclass switchingin paranodopathy needs to be addressed in larger prospectivestudies Nevertheless our data (1) propose autoantibody subclassscreening to be considered in patients with GBS and A-CIDP asresults might have direct implications on diagnosis and therapeuticregime during the course of disease and (2) pave the way forfurther clinical and pathomechanistic studies on antiparanodalautoantibodies in acute to subacute immune-mediated neuropathy
AcknowledgmentThe authors thank Barbara Reuter and Antonia Kohl for theirsuperb technical assistance They thankMaximilian Frommer forprovision and processing of clinical data and PD Dr JakobMatschke for provision of slides and images of supplementarymorphologic data The study was supported by a grant of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg to KD and CV and a grant of the GermanResearch Foundation to KD (DFG DO-22191-1) LA issupported by a research fellowship as a clinician scientist of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg FL receives funding from the GermanMinistry of Education and Research (BMBF 01GM1908A) andthe German Research Foundation (DFG LE30642-1) CSreceives funding from the German Research Foundation (DFG3289-1 DFG UE 1714-1 DFG SO 32810-1 DFG SFB1158 and DFG SO 328 131) from the German Ministry ofEducation and Research (BMBF CMT-Net PI) and from theEuropean Union (Horizon 2020)
Study fundingThis study was supported by the Open Access PublicationFund of the University of Wurzburg
DisclosureL Appeltshauser K-P Wandinger R Junker C Sommer FLeypoldt and K Doppler work for an academic institutionoffering commercial antibody diagnostics AM Brunder AHeinius P Kortvelyessy and C Villmann report no dis-closures relevant to the manuscript C Sommer has served onscientific advisory boards for Algiax Alnylam Air LiquideAkcea Astellas Bayer Grifols Takeda and UCB She reportsbeing a member of speakersrsquo bureau and receiving speakerhonoraria from Akcea Alnylam Novartis Pfizer Sanofi-Aventis and Teva C Sommer served as a journal editorassociate editor or editorial advisory board member for theEuropean Journal of Neurology PLoS ONE and PAIN ReportsF Leypoldt reports speaker honoraria from Bayer RocheNovartis and Fresenius travel funding from Merck Grifolsand Bayer and serving on advisory boards for Roche Biogenand Alexion Go to NeurologyorgNN for full disclosures
Publication historyReceived by Neurology Neuroimmunology amp NeuroinflammationMarch 2 2020 Accepted in final form May 19 2020
Appendix Authors
Name Location Contribution
LuiseAppeltshauserMD
University Hospital ofWurzburg
Major role in the acquisitionanalysis and interpretationof the data and drafting andrevising the manuscript forintellectual content
Anna-MichelleBrunder
University Hospital ofWurzburg
Major role in the acquisitionand analysis of the data
Annika Heinius University Hospital ofSchleswig-HolsteinCampus Kiel
Major role in the acquisitionand analysis of the data
PeterKortvelyessyMD
University Hospital ofMagdeburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Klaus-PeterWandinger MD
University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Ralf Junker MD University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
CarmenVillmann PhD
University Hospital ofWurzburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
ClaudiaSommer MD
University Hospital ofWurzburg
Conceptualization of thestudy revising themanuscript for intellectualcontent and major role ininterpretation of data
Frank LeypoldtMD
University Hospital ofSchleswig-HolsteinCampus Kiel
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy and major role ininterpretation of data
10 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
References1 Fehmi J Scherer SS Willison HJ Rinaldi S Nodes paranodes and neuropathies
J Neurol Neurosurg Psychiatry 20188961ndash712 Uncini A Vallat JM Autoimmune nodo-paranodopathies of peripheral nerve the
concept is gaining ground J Neurol Neurosurg Psychiatry 201889627ndash6353 Querol L Illa I Paranodal and other autoantibodies in chronic inflammatory neu-
ropathies Curr Opin Neurol 201528474ndash4794 Querol L Rojas-Garcia R Diaz-Manera J et al Rituximab in treatment-resistant
CIDP with antibodies against paranodal proteins Neurol Neuroimmunol Neuro-inflamm 20152e149 doi 101212NXI0000000000000149
5 Querol L Nogales-Gadea G Rojas-Garcia R et al Neurofascin IgG4 antibodies inCIDP associate with disabling tremor and poor response to IVIg Neurology 201482879ndash886
6 Doppler K Appeltshauser L Wilhelmi K et al Destruction of paranodal architecturein inflammatory neuropathy with anti-contactin-1 autoantibodies J Neurol Neuro-surg Psychiatry 201586720ndash728
7 Doppler K Appeltshauser L Villmann C et al Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathyBrain 20161392617ndash2630
8 Manso C Querol L Mekaouche M Illa I Devaux JJ Contactin-1 IgG4 antibodiescause paranode dismantling and conduction defects Brain 20161391700ndash1712
9 Manso C Querol L Lleixa C et al Anti-Neurofascin-155 IgG4 antibodies preventparanodal complex formation in vivo J Clin Invest 20191292222ndash2236
10 Labasque M Hivert B Nogales-Gadea G Querol L Illa I Faivre-Sarrailh C Specificcontactin N-glycans are implicated in neurofascin binding and autoimmune targetingin peripheral neuropathies J Biol Chem 20142897907ndash7918
11 Cortese A Lombardi R Briani C et al Antibodies to neurofascin contactin-1 andcontactin-associated protein 1 in CIDP clinical relevance of IgG isotype NeurolNeuroimmunol Neuroinflamm 20197e639 doi 101212NXI0000000000000639
12 Stengel H Vural A Brunder AM et al Anti-pan-neurofascin IgG3 as a marker offulminant autoimmune neuropathy Neurol Neuroimmunol Neuroinflamm 20196e603 doi 101212NXI0000000000000603
13 Miura Y Devaux JJ Fukami Y et al Contactin 1 IgG4 associates to chronic inflammatorydemyelinating polyneuropathy with sensory ataxia Brain 20151381484ndash1491
14 Appeltshauser L Weishaupt A Sommer C Doppler K Complement depositioninduced by binding of anti-contactin-1 auto-antibodies is modified by immunoglo-bulins Exp Neurol 201728784ndash90
15 Doppler K Schuster Y Appeltshauser L et al Anti-CNTN1 IgG3 induces acuteconduction block and motor deficits in a passive transfer rat model J Neuro-inflammation 20191673
16 Sejvar JJ Kohl KS Gidudu J et al Guillain-Barre syndrome and Fisher syndrome casedefinitions and guidelines for collection analysis and presentation of immunizationsafety data Vaccine 201129599ndash612
17 Fokke C van den Berg B Drenthen J Walgaard C van Doorn PA Jacobs BCDiagnosis of Guillain-Barre syndrome and validation of Brighton criteria Brain 201413733ndash43
18 Kuitwaard K van Koningsveld R Ruts L Jacobs BC van Doorn PA RecurrentGuillain-Barre syndrome J Neurol Neurosurg Psychiatry 20098056ndash59
19 Van den Bergh PY Hadden RD Bouche P et al European Federation of NeurologicalSocietiesPeripheral Nerve Society guideline on management of chronic in-flammatory demyelinating polyradiculoneuropathy report of a joint task force of theEuropean Federation of Neurological Societies and the Peripheral Nerve Societymdashfirst revision Eur J Neurol 201017356ndash363
20 Ruts L Drenthen J Jacobs BC van Doorn PA Distinguishing acute-onset CIDP fromfluctuating Guillain-Barre syndrome a prospective study Neurology 2010741680ndash1686
21 McCombe PA Pollard JD McLeod JG Chronic inflammatory demyelinating poly-radiculoneuropathy A clinical and electrophysiological study of 92 cases Brain 19871101617ndash1630
22 Alessandro L Pastor Rueda JM Wilken M et al Differences between acute-onsetchronic inflammatory demyelinating polyneuropathy and acute inflammatory de-myelinating polyneuropathy in adult patients J Peripher Nerv Syst 201823154ndash158
23 Doppler K Appeltshauser L Kramer HH et al Contactin-1 and neurofascin-155-186 are not targets of auto-antibodies in multifocal motor neuropathy PLoS One201510e0134274
24 Ng JK Malotka J Kawakami N et al Neurofascin as a target for autoantibodies inperipheral neuropathies Neurology 2012792241ndash2248
25 Fujita A Ogata H Yamasaki RMatsushita T Kira JI Parallel fluctuation of anti-neurofascin155 antibody levels with clinico-electrophysiological findings in patients with chronic in-flammatory demyelinating polyradiculoneuropathy J Neurol Sci 2018384107ndash112
26 Niks EH van Leeuwen Y Leite MI et al Clinical fluctuations in MuSK myastheniagravis are related to antigen-specific IgG4 instead of IgG1 J Neuroimmunol 2008195151ndash156
27 Huijbers MG Vink AF Niks EH et al Longitudinal epitope mapping in MuSKmyasthenia gravis implications for disease severity J Neuroimmunol 201629182ndash88
28 Huang CC Lehman A Albawardi A et al IgG subclass staining in renal biopsies withmembranous glomerulonephritis indicates subclass switch during disease progressionMod Pathol 201326799ndash805
29 Collins AM Jackson KJ A temporal model of human IgE and IgG antibody functionFront Immunol 20134235
30 Valenzuela NM Schaub S The Biology of IgG subclasses and their clinical relevanceto transplantation Transplantation 2018102S7ndashS13
31 Vidarsson G Dekkers G Rispens T IgG subclasses and allotypes from structure toeffector functions Front Immunol 20145520ndash532
32 Bruhns P Iannascoli B England P et al Specificity and affinity of human Fcgamma receptorsand their polymorphic variants for human IgG subclasses Blood 20091133716ndash3725
33 Querol L Nogales-Gadea G Rojas-Garcia R et al Antibodies to contactin-1 inchronic inflammatory demyelinating polyneuropathy Ann Neurol 201373370ndash380
34 Devaux JJ Miura Y Fukami Y et al Neurofascin-155 IgG4 in chronic inflammatorydemyelinating polyneuropathy Neurology 201686800ndash807
35 Ogata H Yamasaki R Hiwatashi A et al Characterization of IgG4 anti-neurofascin155 antibody-positive polyneuropathy Ann Clin Transl Neurol 20152960ndash971
36 Doppler K Frank F Koschker AC Reiners K Sommer C Nodes of Ranvier in skinbiopsies of patients with diabetes mellitus J Peripher Nerv Syst 201722182ndash190
37 Banks W The blood-brain barrier interface in diabetes mellitus dysfunctionsmechanisms and approaches to treatment Curr Pharm Des 2020261438ndash1447
38 Arnold ML Ntokou IS Doxiadis II Spriewald BM Boletis JN Iniotaki AG Donor-specific HLA antibodies evaluating the risk for graft loss in renal transplant recipientswith isotype switch from complement fixing IgG1IgG3 to noncomplement fixingIgG2IgG4 anti-HLA alloantibodies Transpl Int 201427253ndash261
39 Martinez-Martinez L Lleixa MC Boera-Carnicero G et al Anti-NF155 chronic in-flammatory demyelinating polyradiculoneuropathy strongly associates to HLA-DRB15 J Neuroinflammation 201714224
40 Hashimoto Y Ogata H Yamasaki R et al Chronic inflammatory demyelinatingpolyneuropathy with concurrent membranous nephropathy an anti-paranode andpodocyte protein antibody study and literature survey Front Neurol 20189997
Appendix (continued)
Name Location Contribution
KathrinDoppler MD
University Hospital ofWurzburg
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy major role in analysisand interpretation of dataand study supervision
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 11
DOI 101212NXI000000000000081720207 Neurol Neuroimmunol Neuroinflamm
Luise Appeltshauser Anna-Michelle Brunder Annika Heinius et al Antiparanodal antibodies and IgG subclasses in acute autoimmune neuropathy
This information is current as of July 24 2020
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm
ServicesUpdated Information amp
httpnnneurologyorgcontent75e817fullhtmlincluding high resolution figures can be found at
References httpnnneurologyorgcontent75e817fullhtmlref-list-1
This article cites 40 articles 9 of which you can access for free at
Citations httpnnneurologyorgcontent75e817fullhtmlotherarticles
This article has been cited by 1 HighWire-hosted articles
Subspecialty Collections
httpnnneurologyorgcgicollectionperipheral_neuropathyPeripheral neuropathy
httpnnneurologyorgcgicollectionguillainbarre_syndromeGuillain-Barre syndrome
nating_polyneuropathyhttpnnneurologyorgcgicollectionchronic_inflammatory_demyeliChronic inflammatory demyelinating polyneuropathy
httpnnneurologyorgcgicollectionautoimmune_diseasesAutoimmune diseases
httpnnneurologyorgcgicollectionall_neuromuscular_diseaseAll Neuromuscular Diseasefollowing collection(s) This article along with others on similar topics appears in the
Permissions amp Licensing
httpnnneurologyorgmiscaboutxhtmlpermissionsits entirety can be found online atInformation about reproducing this article in parts (figurestables) or in
Reprints
httpnnneurologyorgmiscaddirxhtmlreprintsusInformation about ordering reprints can be found online
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm

Autoantibodies against the paranodal antigens contactin-1contactin-associated protein-1 (Caspr-1) and neurofascin-155(NF155) have been described as biomarkers for a new entity ofinflammatory neuropathies classified as paranodopathies1ndash3 Inthe chronic phase of disease IgG4-seropositive patients do notrespond to IV immunoglobulins (IVIg) but to rituximab4ndash7
Noninflammatory IgG4 autoantibodies are pathogenic possiblyby inhibition of the interaction between contactin-1Caspr-1and NF155 and by NF155 depletion8ndash11 Autoantibodies of theIgG3 subclass have been described (1) in monophasic disease(2) at the subacute onset (3) in patients with antindashpan-neurofascin autoantibodies and severe course of disease and(4) most recently in chronic inflammatory demyelinating poly-radiculoneuropathy (CIDP) with clinical features indistinguish-able from seronegative patients but with a good response toIVIg6711ndash13 Proinflammatory IgG3 antibodies lead to comple-ment deposition in vitro14 and in vivo resulting in reversibleconduction failure in Lewis rats intraneurally injected withantindashcontactin-1 IgG315 and may therefore play a role in theacute onset of paranodopathies However data on antiparanodalautoantibodies in Guillain-Barre syndrome (GBS) or the acuteonset of CIDP are scarce because previous studies mostly fo-cused on CIDP and patients were recruited during the chronicphase of disease IgG subclass distribution and the associatedclinical phenotype have never been investigated longitudinallyWe therefore aimed at determining the prevalence and IgGsubclass of paranodal autoantibodies in a cohort of patients withacute to subacute inflammatory neuropathies including follow-up of seropositive patients We hypothesize that IgG subclassand titer are related to the course of disease and therapeuticresponse in acute-onset paranodopathy
MethodsPatients and controlsOne hundred sixty-one patients with suspected GBS andsubacute inflammatory neuropathy (peak of symptoms le90days) who had undergone diagnostic lumbar puncture at theUniversity Hospitals of Kiel andMagdeburg between 2001 and2016 were included into the study We assessed clinical dataretrospectively by analysis of discharge letters and documentedlaboratory electrophysiologic and MRI examinations Tablee-1 linkslwwcomNXIA274 summarizes demographic dataThe diagnosis of GBS was confirmed in n = 114 patients withdiagnostic certainty according to the Brighton criteria1617 level1 in 73 patients level 2 in 29 patients level 3 in 6 patients andlevel 4 in 6 patients We classified 6 patients as recurrent GBS(R-GBS) according to the criteria adapted from Kuitwaard
et al18 and 18 patients as Miller-Fisher syndrome (MFS) In 23patients the initial diagnosis was GBS but was later reverted toCIDP because of a disease progression gt2 months (diagnosticcertainty according to the EFNS criteria19 definite CIDP in 10patients probable CIDP in 3 patients possible CIDP in 1patient and EFNS electrodiagnostic criteria not fulfilled in923 patients) Eighteen of 23 patients with CIDP fulfilled thecriteria for acute-onset CIDP (A-CIDP) proposed in previouspublications20ndash22 and 523 patients with CIDP showed a sub-acute-onset (peak le90 days) In the following all patients withacute- to subacute-onset CIDP are referred to as part of theA-CIDP cohort Follow-up sera and CSF samples were avail-able in 66 patients including 3 seropositive patients We in-cluded sera of 40 healthy controls recruited in former studies6
Sera of all patients had already been tested for anti-NF155autoantibodies in a previous study12
Binding assays on murine teased fibersWe used binding assays on murine teased fibers at a serum di-lution of 1100 and 1500 to screen all patientsrsquo and controlsrsquo serafor antiparanodal autoantibodies as previously described23 Tit-ers of seropositive samples were measured with a dilution seriesusing Cy3TM-conjugated goat anti-human IgG 1300 (JacksonWest Grove) In positive sera we performed double immuno-fluorescence with sera diluted 150 and 1100 and rabbit antindashCaspr-1 diluted 11000 (Abcam Cambridge United Kingdom)using MFP488-conjugated goat anti-human IgG diluted 1500(MoBiTec Gottingen Germany) and Cy3TM-conjugated don-key anti-rabbit IgG diluted 1300 (Jackson) as secondary anti-bodies For subclass analysis we used FITC-conjugatedsecondary antibodies diluted 1100 namely mouse anti-humanIgG1 IgG4 (Abcam) mouse anti-human IgG2 (Merck Darm-stadt Germany) and sheep anti-human IgG3 (RocklandImmunochemicals Inc Pottstown PA) A healthy control se-rum and serum of patients with antindashCaspr-1 IgG3 and IgG4antibodies from a previous study7 stained on separate slidesserved as negative and positive controls for double immunoflu-orescence and subclass analysis We tested CSF of seropositivepatients of the first assessment and follow-up on teased fibers ina dilution of 120 and 150 as described above CSF of fivepatients who were seronegative served as negative controls
ELISAWe performed ELISA for detection of antindashcontactin-1 induplets with sera of all patients and 40 healthy controls as pre-viously described6122324 For antindashCaspr-1 ELISA we coatedChinese hamster ovary cell linendashderived recombinant humanCaspr-1 protein (Research and Diagnostic Systems Inc Min-neapolis MN) at a dilution of 2 μgmL on MaxiSorb 96-well
GlossaryA-CIDP = acute-onset chronic inflammatory demyelinating polyradiculoneuropathyCaspr-1= contactin-1-associated protein-1CBA = cell-based assay CIDP = chronic inflammatory demyelinating polyradiculoneuropathy GBS = Guillain-Barresyndrome HEK = human embryonic kidney HLA = human leukocyte antigen ICU = intensive care unit IVIg = IVimmunoglobulin MFS = Miller-Fisher syndrome NF = neurofascin OD = optical density R-GBS = recurrent GBS
2 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
plates (Thermo Scientific Fisher Minneapolis MN) and testedbaselinefollow-up sera of seropositive patients and 40 healthycontrols or rabbit antindashCaspr-1 (Lifespan Biosciences SeattleWA) in duplets using corresponding horseradish peroxidasendashconjugated secondary antibodies6 and conjugated anti-rabbitIgG (DakoCytomation Glostrup Denmark) For each samplewe subtracted corresponding values of uncoated duplet wells toreduce background signals We set the threshold for both assaysat 5 SDs above the mean of healthy controls Antibody titerswere determined by serial dilutions Subclass-specific secondaryantibodies served for subclass analysis as previously described12
We tested CSF of seropositive patients in a dilution of 120 inantindashcontactin-1 ELISA and antindashCaspr-1 ELISA (exceptionpatient 4 baseline CSF in both ELISAs and patient 3 in anti-Caspr-1 ELISA due to lack of material) as described above withCSF of 5 seronegative patients as negative controls
Preabsorption experimentsTo determine the specific paranodal target of the autoanti-bodies we serially preincubated seropositive sera and serumfrom a healthy control with contactin-1 (CNTN1)ndash andCaspr-1 (CNTNAP1)ndashtransfected human embryonic kidney(HEK) 293 cells as previously described7 and afterwardperformed binding assays on murine teased fibers as de-scribed above
Cell-based assayFor specification we tested sera of patients positive in teasedfibers assay or ELISA and sera of five controls on HEK293 cellstransfected with plasmids of human Caspr-1 (CNTNAP1) andrat contactin-1 (CNTN1) as previously described723 and usedpolyclonal rabbit antindashcontactin-1 IgG (Abcam) and mono-clonal mouse antindashCaspr-1 IgG1 (Santa Cruz BiotechnologyDallas TX) for double immunofluorescence in positivepatients with corresponding Cy3TM-conjugated donkeyanti-rabbit IgG (Abcam) donkey anti-mouse IgG andMFP488-conjugated goat anti-human IgG (MoBiTec) sec-ondary antibodies Sera of patients with antindashCaspr-1 andantindashcontactin-1 antibodies from previous studies67 servedas positive controls and sera of healthy controls as negativecontrols in the double immunofluorescence assays
Statistical analysisWe performed statistical analysis (descriptive statisticscalculation of OR and 95 CI) with SPSS 250 (IBMArmonk NY)
Standard protocol approvals registrationsand patient consentsThe Ethics Committees of the University of Wurzburg andthe University of Kiel approved to conduct this study Writteninformed consent was obtained from all participants in thestudy
Data availabilityData not published within this article are available at theUniversity Hospital of Wurzburg or will be shared in an
anonymized manner on request from any qualified in-vestigator for purposes of replicating procedures and results
ResultsAntiparanodal autoantibodies of different IgGsubclasses in 43 of the patientsWe applied immunofluorescence binding assays on murineteased fibers as a screening tool for detection of anti-paranodal autoantibodies We observed serum binding tothe paranodal junctional region colocalizing with the com-mercial antindashCaspr-1 antibody (figure 1A) in seven patientsof the total cohort (7161 prevalence 43) in none of thepatients of the R-GBS (06) andMFS (018) subcohort norin the healthy controls (040) but in 3114 (26) of theGBS subcohort and 423 (174) of the A-CIDP subcohortThus paranodal autoantibodies occurred more frequentlyin A-CIDP compared with GBS (OR = 779 95 CI161ndash3795) Two of the sera with paranodal binding (1 GBSand 1 A-CIDP) had been identified as antindashNF155 sero-positive in a recent study12 therefore experimental findingsand clinical associations of these patients are not reportedhere Target antigens of the other five seropositive patientswere determined using cell-based assay (CBA figure 1B)and ELISA (see below) and confirmed by preabsorptionexperiments (figure 1C) In the GBS subcohort the twoseropositive patients showed antindashCaspr-1 (patient 1) andantindashcontactin-1Caspr-1 (patient 2) antibodies Seroposi-tive A-CIDP patients carried antibodies against either con-tactin-1 (patient 3) contactin-1Caspr-1 (patient 4) or anti-Caspr-1 (patient 5) At the onset of disease the two patientswith GBS showed IgG2 and IgG3 antibodies (table 1)During the acute phase at onset sera of the three patientslater classified as A-CIDP carried IgG24 (patient 3) andIgG23 (patients 4 and 5) as detected by binding assays andELISA (figure 2 A and B) Autoantibody titers are shown intable 1 The results of the binding assays on teased fibersELISA and CBA showed concordance regarding the specifictarget antigen of the antibodies and the predominating IgGsubclass There were only minor differences of the antibodytiter and coexisting subclasses in ELISA and teased fibersassay In case of double positivity for anti-contactin-1Caspr-1 (patients 2 and 4) preabsorption assays detectedonly the dominating antibody (table 1)
Subclass switch from IgG3 to IgG4 at follow-upin one patientIn 66161 patients follow-up serum and CSF samples wereavailable including three seropositive patients (1 GBS and 2A-CIDP) None of the follow-up sera of initially seronega-tive patients or controls showed positive results in the teasedfibers assay (data not shown) nor in the contactin-1 ELISA(figure 3A) Serum of the patient with GBS acquired inremission 55 months after the first assessment when thepatient did not show any remaining neurologic deficits wasseronegative in all experimental assays (figure 3 B and C)
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 3
Follow-up serum of one patient with A-CIDP two monthsafter onset ie still in the acute phase of disease (patient 5)showed IgG3 autoantibodies against Caspr-1 with no dif-ference in antibody titer and subclass compared with onset(figure 3 B and C) A follow-up-sample during the chronicstage (28 months after the onset) revealed a reduction of thetiter (150 in teased fibers negative in ELISA table 1) ofantindashCaspr-1 IgG autoantibodies with subclasses not mea-surable due to the low titer (data not shown) The patientnow presented with only very mild sensorimotor impair-ment and ameliorated nerve conduction studies Follow-upserum and CSF of the other patient with A-CIDP (patient 4)who was initially IgG3ndashanti-contactin-1Caspr-1 doublepositive were acquired 120 months after the acute onsetwhen the patient had developed relapsing-remitting senso-rimotor CIDP At follow-up we could no longer detectantindashcontactin-1 but detected and confirmed antindashCaspr-1autoantibodies via antindashCaspr-1 ELISA CBA and pre-absorption assay (table 1 and figure 3 B and C) Subclassanalysis now revealed reactivity against IgG24 but notIgG3 subclass anymore giving evidence of a switch of IgG
subclasses between the acute and chronic stage of the disease(figure 2C)
Intrathecal antiparanodal autoantibodies inpatients with high antibody titer and blood-brain barrier dysfunctionELISA with CSF samples of the seropositive patients and fivedisease controls showed elevated optical density (OD) ata dilution of 120 only in antindashcontactin-1 patient 3 witha very high serum titer (see appendix e-1 linkslwwcomNXIA275) and antindashCaspr-1 patient 5 with signs of se-verely disrupted blood-brain barrier (high CSF protein MRIcauda equina enhancement see appendix e-1 linkslwwcomNXIA275) but equally low OD values for the otherseronegative and seropositive patients (see figure e-1 linkslwwcomNXIA273) Binding assays on murine teasedfibers showed weak binding of CSF to the paranodes in thesetwo patients but not in the other seropositive patients orcontrols None of the seropositive patients showed signs ofintrathecal autoantibody production with CSF IgG indexand antiparanodal autoantibody specific ASI (antibody
Figure 1 Detection of antiparanodal autoantibodies by binding assays on teased fibers CBA and preabsorption assay
(A) Colocalization overlay photomicrographs of double immunofluorescence assays with commercial autoantibody displayed in red and human serum ingreen Colocalization at the paranodes (arrows) appears yellow and indicates antiparanodal autoantibodies in patients 1ndash5 (AbndashAf) but not in the negativecontrol serum (Aa) (B) The specific target of the autoantibodies is illustrated by colocalization overlay photomicrographs on transfected HEK293 cells(arrowheads) with commercial antibody in red serum in green and nucleus staining in blue Colocalization appears yellow (merge) and confirms antindashcontactin-1 in patients 2 (Bc) 3 (Bd) and 4 (Be) but not in patients 1 (Bb) 5 (Bf) and the negative control (Ba) Sera of patients 1 2 4 and 5 (Bh Bi Bk andBl) colocalize on Caspr-1 (CNTNAP1)ndashtransfected HEK293 cells as proof of antindashCaspr-1 autoantibodies whereas sera of the negative control (Bg) and patient3 (Bj) donot showantindashCaspr-1 positivity (C) Single immunofluorescence onmurine teased fibers afterHEK293 contactin-1 andCaspr-1 preabsorption showsthat antiparanodal autoantibodies disappear after contactin-1 preabsorption in patient 3 (Cd) and after Caspr-1 preabsorption in patient 4 (Cf) provingspecificity to the respective target antigen Scale bar = 10 μm CBA = cell-based assay HEK = human embryonic kidney
4 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
Table 1 Summary of the autoantibody status of seropositive patients 1ndash5 at baseline and follow-up
CSF Serum
Teased fibers and ELISA Preabsorption CBA
Paranodal bindingOD Titer IgG subclass
Patient 1 (baseline no follow-up)
Teased fibers Negative Positive 1100 IgG3
Contactin-1 0202 0465 nd nd Negative Negative
Caspr-1 0072 2961 1200 nm Positive Positive
Patient 2 (baseline)
Teased fibers Negative Positive 1200 nd
Contactin-1 0247 3538 1200 IgG2 Negative Positive
Caspr-1 0129 3965 11000 IgG2 Positive Positive
Patient 2 (follow-up)
Teased fibers nd Negative nd nd
Contactin-1 nd 0039 nd nd nd Negative
Caspr-1 nd 0025 nd nd nd Negative
Patient 3 (baseline no follow-up)
Teased fibers Negative Positive 130000 IgG4
Contactin-1 0516 4492 140000 IgG42 Positive Positive
Caspr-1 nd 0073 nd nd Negative Negative
Patient 4 (baseline)
Teased fibers Negative Positive 1500 IgG3
Contactin-1 0267 2294 1200 IgG3 Negative Positive
Caspr-1 nd 3011 1500 IgG32 Positive Positive
Patient 4 (follow-up)
Teased fibers Negative Positive 1500 IgG4
Contactin-1 0227 0206 nd nd Negative Negative
Caspr-1 0138 3618 1500 IgG24 Positive Positive
Continued
Neurolo
gyorgN
NNeurologyN
euroim
munology
ampNeuroinflam
mation
|Volum
e7N
umber
5|
Septem
ber2020
5
Table 1 Summary of the autoantibody status of seropositive patients 1ndash5 at baseline and follow-up (continued)
CSF Serum
Teased fibers and ELISA Preabsorption CBA
Paranodal bindingOD Titer IgG subclass
Patient 5 (baseline)
Teased fibers Positive Positive 1100 IgG3
Contactin-1 0219 0260 nd nd Negative Negative
Caspr-1 0136 nd nd nd Positive Positive
Patient 5 (first follow-up)
Teased fibers Positive Positive 1100 IgG3
Contactin-1 0236 0243 nd nd Negative Negative
Caspr-1 0364 3802 1200 IgG32 Positive Positive
Patient 5 (second follow-up)
Teased fibers nd Positive 150 nm
Contactin-1 nd 0168 nd nd nd Negative
Caspr-1 nd 0013 nd nd nd Negative
Abbreviations A-CIDP = acute-onset chronic inflammatory demyelinating polyradiculoneuropathy Caspr-1 = contactin-1ndashassociated protein-1 CBA = cell-based assay GBS = Guillain-Barre syndrome nd = not done nd =not done due to lack of material nm = not measurable OD = optical densityResults of teased fibers assays are displayed in the first row for each patient contactin-1 and Caspr-1-specific results are displayed in lines 2 and 3 ODs above the threshold and results considered positive are highlighted inbold letters There is a 100 concordance regarding target specificity in the teased fibers assay ELISA and CBA but minor differences in IgG titer and coexisting subclass between the teased fibers assay and ELISA In case ofdouble positivity preabsorption assays only detect the target antigen with the higher antibody titer (see ELISA titer)
6NeurologyN
euroim
muno
logyampNeuroinflam
mation
|Vo
lume7N
umber
5|
Septem
ber
2020NeurologyorgN
N
specificity index) being in a normal range (see appendix e-1linkslwwcomNXIA275)
Clinical features of seropositive patientsFigure 4 schematically summarizes the clinical course relatedto the experimental results Appendix e-1 linkslwwcomNXIA275 summarizes clinical data of seropositive patients1ndash5 and provides a detailed description of the clinical diseasecourse in individuals The two seropositive patients withGBS were both middle-aged women presenting with severemotor but only mild sensory involvement Cranial nerveinvolvement autonomic symptoms and neuropathic painwere present in 12 patients with GBS only Both patientsrequired treatment on the intensive care unit (ICU) The
three patients with A-CIDP presented with moderate to severeparesis and multimodal sensory impairment at disease onsetDuring the course of disease all three patients with A-CIDPreported neuropathic pain requiring pregabalingabapentinor even opioid treatment Two of three patients withA-CIDP presented sensory ataxia Nerve conduction studiesshowed demyelinating features in all seropositive patientswith conduction block in 35 patients Sural nerve biopsywas performed in one patient (patient 5) six months after theonset and showed axonal loss without signs of demyelinationCSF analysis revealed cytoalbuminologic dissociation andblood-brain barrier dysfunction in all seropositive patientsThe patient with subacute-onset CIDP highly elevated CSFprotein and detection of antindashCaspr-1 in both serum and
Figure 2 Serum subclass analysis at baseline and at follow-up with IgG subclass switch
(A) Results of antindashcontactin-1 and antindashCaspr-1 ELISA with baseline sera and subclass-specific IgG1-4 autoantibodies (grayscale) are shown as of the OD ofthe total IgG (using anti-human IgG autoantibody) on y-axis In patient 1 ELISA IgG subclasses were not measurable ELISA revealed mainly IgG2 subclass inpatient 2 (GBS) IgG2IgG4 in patient 3 (A-CIDP) and IgG23 in patients 4 and 5 (A-CIDP) (B) Acute phase teased fibers subclass analysis with baseline serashowed binding to the paranodal regions (arrows) only when using IgG3-specific secondary antibody in patients 1 (GBS) and 4 (A-CIDP) visualized byphotomicrographs of single immunofluorescence staining on teased fibers (C) AntindashCaspr-1 IgG subclass ELISA with follow-up serum of patient 4 nowrevealed IgG 24 subclass Photomicrographs show immunofluorescence staining of murine teased fibers with subclass-specific IgG3 and IgG4 autoanti-bodies at follow-up Paranodal binding disappeared using IgG3 antibody but occurred using IgG4 subclass-specific antibody proving subclass switch inpatient 4 Scale bar = 10 μm A-CIDP = acute-onset chronic inflammatory demyelinating polyradiculoneuropathy GBS = Guillain-Barre syndrome
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 7
CSF (patient 5) developed action tremor during the courseof disease
Antibody status is concordant with diseaseactivity and therapy responseOne of two patients with GBS (patient 2) received IVIgwhich did not lead to amelioration This patient was IgG2 gtIgG3 seropositive (patient 2 figure 2A) Both patients withGBS responded well to plasma exchange The two patientswith A-CIDP and IgG3 autoantibodies (patients 4 and 5)showed good response to IVIg treatment in the acute phase ofdisease Ongoing IVIg treatment led to further improvementof symptoms and was stopped after 25 cycles in patient 5because of a remission of symptoms In the other patient withA-CIDP and antindashcontactin-1Caspr-1 IgG3 at disease onset(patient 4) IVIg showed a good therapeutic effect in the acutephase and led to remission not requiring further therapy Inthe chronic phase IVIg was initiated again and stopped after26 cycles because of a loss of the therapeutic effect At thatstage the autoantibody subclass had already switched fromIgG3 to IgG4 (for detailed treatment response in the course ofdisease see appendix e-1 linkslwwcomNXIA275) Thepatient with A-CIDP and high-titer antindashcontactin-1 IgG4autoantibodies (patient 3) had to be treated on ICU becauseof the severity of symptoms developed renal dysfunction didnot respond to IVIg treatment and did not ameliorate duringthe course of disease
DiscussionWedetected antiparanodal autoantibodies in 43of patientswithacute to subacute immune-mediated neuropathy more frequentlyin CIDP with acute to subacute onset than in GBS We foundIgG23 in monophasic GBS which supports the notion of pre-vious studies that IgG3 is the predominant subclass inmonophasicdisease7 By analyzing sera from different time points we couldshow that the antibody status was concordant with the diseaseseverity Therefore our data support pathogenicity of autoanti-bodies and suggest that autoantibody status and titer are validindicators for disease activity as proposed in previous studies onparanodopathy and further IgG4-related neurologic diseases25ndash27
In one patient we could demonstrate a subclass switch fromIgG3 in acute disease to IgG4 in chronic disease a finding thatwe had hypothesized in a previous study6 In other autoimmunediseases eg in membranous glomerulonephritis subclassswitch of anti-PLA2R autoantibodies from IgG1 to IgG4 alsoassociates with progression to chronic disease28 According toa linear model of autoantibody response unspecific IgM re-sponse is followed by IgG isotype produced by antigen-experi-enced B cells These IgG are of the IgG3 subclass andsequentially switch to IgG1 2 and 42930 Concurrently auto-antibodies strongly gain antigen affinity with IgG4 showing thehighest affinity to its target3132 Disease progression in para-nodopathy might therefore depend on IgG subclass properties
Figure 3 Antiparanodal IgG autoantibodies at follow-up assessment
(A) Contactin-1 IgG ELISA of all patients at first assessment of 66161 patients at follow-up and of 40 healthy controls Optical density (OD) at 450 nm isdisplayed on the y-axis The threshold for positive results is set at 5 SDs above the mean of controls (0615) Patients 2ndash4 show positive results at firstassessment The other patients and controls show values below the threshold Sera of patients 2 4 and 5 are negative in antindashcontactin-1 IgG ELISA at follow-up as well as 63 other follow-up sera Patients 1 and 3were lost to follow-up (B) Overlay photomicrographs of teased fibers double immunofluorescence and(C) contactin-1ndash and Caspr-1 (CNTNAP1)ndashtransfected HEK293 cells show loss of antindashcontactin-1Caspr-1 autoantibodies in patient 2 (GBS) at follow-up (BaCa Cd) but colocalization of commercial antindashCaspr-1 antibody and sera at paranodes in patients 4 and 5 with A-CIDP (Bb Bc arrows) Both sera bind toCaspr-1 (CNTNAP1)ndashtransfected HEK293 cells (Ce Cf arrowheads) but not to contactin-1ndashtransfected cells (Cb Cc) Scale bar = 10 μm A-CIDP = acute-onsetchronic inflammatory demyelinating polyradiculoneuropathy GBS = Guillain-Barre syndrome HEK = human embryonic kidney
8 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
and affinities Gain of specificity and affinity at switch from IgG3to IgG4 might explain different binding characteristics of anti-paranodal autoantibodies in the patient whose IgG3 autoanti-bodies reacted both against contactin-1Caspr-1 epitopes butwhose IgG4 autoantibodies were specific to Caspr-1 Here wereport IgG24 (1) in patient 4 in the chronic phase after subclassswitch and (2) in another patient at disease onset showingnonameliorating disease with high disability similar to previousreports on antindashcontactin-1 IgG4-related disease111333 So farstudies have only reported antiparanodal IgG4 autoantibodies inpatients with chronic disease7113435 We therefore suggest thathigh-affinity IgG4 autoantibody binding leads to chronic diseasewith functional impairment possibly caused by disturbance of theantigen interaction paranodal architecture and paranodalcomplex formation as demonstrated previously68ndash11
Patients of our study with predominant IgG3 subclass showedgood response to IVIg whereas patients with IgG2 and IgG4initially did not respond to IVIg or lost responsiveness in thedisease course Several studies described good responses toIVIg in IgG3-associated paranodopathy but poor responses inIgG4-associated disease56111334 IgG3 can initiate comple-ment activation opsonization antigen cross-linking and in-ternalization whereas IgG2 shows little C1q binding and IgG4completely lacks these Fc-mediated effector functions31 In anin vivo study on antindashcontactin-1 pathogenicity we suggestedcomplement-mediated functional impairment at the paranodesas the pathophysiologic correlate in IgG3-mediated para-nodopathy earlier and could show in vitro that IVIg inhibitscomplement deposition and activation dose-dependently1415
Our data therefore support the hypothesis that therapy re-sponsiveness might depend on IgG subclass and subclass-related pathomechanism of antiparanodal autoantibodies If
validated in larger studies these findings might have a directimpact on the treatment regime in affected patients Testing ofautoantibody subclass during the course of disease may beuseful as an indicator of a positive response to IVIg in case ofIgG3 whereas IgG4-related paranodopathy shows very goodresponse to rituximab46711
The trigger of the autoantibody production and the patho-mechanism behind possible IgG switching are still unknownThree of five seropositive patients in this study had diabetesmellitus type 2 as a comorbidity diagnosed years before theonset of disease Previous studies report disruption of the blood-brain barrier and the nodal architecture in patients with diabetesmellitus thereby possibly exposing paranodal antigens to theimmune response3637 A recent study has identified humanleukocyte antigen (HLA)-DRB15 as a risk factor for CIDPassociated with anti-NF155 antibodies and another studyreported different HLA antibody patterns with subclass switchafter renal transplantation3839 Whether interindividual differ-ences in antibody production and switching during the course ofdisease depend on (1) HLA alleles (2) antigen exposure or (3)further immunologic mechanisms has to be investigated infurther studies
Regarding characteristic clinical features of paranodopathy wereport neuropathic pain in antindashCaspr-1ndashrelated disease aspreviously described in patients with higher autoantibody tit-ers7 Different titers might explain discrepancies between ourresults and a recent study that did not report pain in antindashCaspr-1ndashpositive patients711 In our patients further features of IgG4-related chronic paranodopathy such as tremor sensory ataxiaand nephropathy561340 were only present in the course ofdisease suggesting that they evolve due to chronic autoantibody
Figure 4 Scheme of the autoantibody status in the course of disease in seropositive patients
The course of disease is shown on the x-axis by pseudo-logarithmic display of the time course Time point 0 is set at baseline assessment of serumCSF Thecolor code indicates severity of symptoms Results of serum assessment are displayed in black Patients 1 and 3 were lost to follow-up
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 9
exposure The pathomechanistic correlate of action tremorwhich recently has also been described in antindashCaspr-1 IgG3-related paranodopathy11 is still under research but studiessuggest cerebellar origin5612 In this study we detected in-trathecal autoantibodies in a patient with tremor as previouslyreported in a case of antindashpan-neurofascinndashpositive para-nodopathy12 The antiparanodal autoantibodies most probablydiffuse into the intrathecal compartment due to a blood-brainbarrier dysfunction as indicated by normal IgG and ASI indicesin our patients Our CSF data suggest that detection of anti-paranodal antibodies in CSF shows little sensitivity and dependson either high titer or strong leakage of the blood-brain barrierWe therefore encourage further studies on intrathecal anti-paranodal autoantibodies targeting sensitivity pathogenicityand association to clinical symptoms
Low prevalence of autoantibodies against paranodal proteins inacute inflammatory neuropathy retrospective assessment and thelow number of seropositive patients available for follow-up limitour study Clinical characteristics at acute onset and subclass-related seropositivity as a possible risk factor for chronic pro-gression when detected at the onset should be investigated in largemulticentric studies considering lowoverall prevalence ofGBS andA-CIDP as well as further possible confounders in analysis ofparanodopathy-specific clinical symptoms eg coinciding painfuldiabetic neuropathy The question of sequential subclass switchingin paranodopathy needs to be addressed in larger prospectivestudies Nevertheless our data (1) propose autoantibody subclassscreening to be considered in patients with GBS and A-CIDP asresults might have direct implications on diagnosis and therapeuticregime during the course of disease and (2) pave the way forfurther clinical and pathomechanistic studies on antiparanodalautoantibodies in acute to subacute immune-mediated neuropathy
AcknowledgmentThe authors thank Barbara Reuter and Antonia Kohl for theirsuperb technical assistance They thankMaximilian Frommer forprovision and processing of clinical data and PD Dr JakobMatschke for provision of slides and images of supplementarymorphologic data The study was supported by a grant of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg to KD and CV and a grant of the GermanResearch Foundation to KD (DFG DO-22191-1) LA issupported by a research fellowship as a clinician scientist of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg FL receives funding from the GermanMinistry of Education and Research (BMBF 01GM1908A) andthe German Research Foundation (DFG LE30642-1) CSreceives funding from the German Research Foundation (DFG3289-1 DFG UE 1714-1 DFG SO 32810-1 DFG SFB1158 and DFG SO 328 131) from the German Ministry ofEducation and Research (BMBF CMT-Net PI) and from theEuropean Union (Horizon 2020)
Study fundingThis study was supported by the Open Access PublicationFund of the University of Wurzburg
DisclosureL Appeltshauser K-P Wandinger R Junker C Sommer FLeypoldt and K Doppler work for an academic institutionoffering commercial antibody diagnostics AM Brunder AHeinius P Kortvelyessy and C Villmann report no dis-closures relevant to the manuscript C Sommer has served onscientific advisory boards for Algiax Alnylam Air LiquideAkcea Astellas Bayer Grifols Takeda and UCB She reportsbeing a member of speakersrsquo bureau and receiving speakerhonoraria from Akcea Alnylam Novartis Pfizer Sanofi-Aventis and Teva C Sommer served as a journal editorassociate editor or editorial advisory board member for theEuropean Journal of Neurology PLoS ONE and PAIN ReportsF Leypoldt reports speaker honoraria from Bayer RocheNovartis and Fresenius travel funding from Merck Grifolsand Bayer and serving on advisory boards for Roche Biogenand Alexion Go to NeurologyorgNN for full disclosures
Publication historyReceived by Neurology Neuroimmunology amp NeuroinflammationMarch 2 2020 Accepted in final form May 19 2020
Appendix Authors
Name Location Contribution
LuiseAppeltshauserMD
University Hospital ofWurzburg
Major role in the acquisitionanalysis and interpretationof the data and drafting andrevising the manuscript forintellectual content
Anna-MichelleBrunder
University Hospital ofWurzburg
Major role in the acquisitionand analysis of the data
Annika Heinius University Hospital ofSchleswig-HolsteinCampus Kiel
Major role in the acquisitionand analysis of the data
PeterKortvelyessyMD
University Hospital ofMagdeburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Klaus-PeterWandinger MD
University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Ralf Junker MD University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
CarmenVillmann PhD
University Hospital ofWurzburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
ClaudiaSommer MD
University Hospital ofWurzburg
Conceptualization of thestudy revising themanuscript for intellectualcontent and major role ininterpretation of data
Frank LeypoldtMD
University Hospital ofSchleswig-HolsteinCampus Kiel
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy and major role ininterpretation of data
10 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
References1 Fehmi J Scherer SS Willison HJ Rinaldi S Nodes paranodes and neuropathies
J Neurol Neurosurg Psychiatry 20188961ndash712 Uncini A Vallat JM Autoimmune nodo-paranodopathies of peripheral nerve the
concept is gaining ground J Neurol Neurosurg Psychiatry 201889627ndash6353 Querol L Illa I Paranodal and other autoantibodies in chronic inflammatory neu-
ropathies Curr Opin Neurol 201528474ndash4794 Querol L Rojas-Garcia R Diaz-Manera J et al Rituximab in treatment-resistant
CIDP with antibodies against paranodal proteins Neurol Neuroimmunol Neuro-inflamm 20152e149 doi 101212NXI0000000000000149
5 Querol L Nogales-Gadea G Rojas-Garcia R et al Neurofascin IgG4 antibodies inCIDP associate with disabling tremor and poor response to IVIg Neurology 201482879ndash886
6 Doppler K Appeltshauser L Wilhelmi K et al Destruction of paranodal architecturein inflammatory neuropathy with anti-contactin-1 autoantibodies J Neurol Neuro-surg Psychiatry 201586720ndash728
7 Doppler K Appeltshauser L Villmann C et al Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathyBrain 20161392617ndash2630
8 Manso C Querol L Mekaouche M Illa I Devaux JJ Contactin-1 IgG4 antibodiescause paranode dismantling and conduction defects Brain 20161391700ndash1712
9 Manso C Querol L Lleixa C et al Anti-Neurofascin-155 IgG4 antibodies preventparanodal complex formation in vivo J Clin Invest 20191292222ndash2236
10 Labasque M Hivert B Nogales-Gadea G Querol L Illa I Faivre-Sarrailh C Specificcontactin N-glycans are implicated in neurofascin binding and autoimmune targetingin peripheral neuropathies J Biol Chem 20142897907ndash7918
11 Cortese A Lombardi R Briani C et al Antibodies to neurofascin contactin-1 andcontactin-associated protein 1 in CIDP clinical relevance of IgG isotype NeurolNeuroimmunol Neuroinflamm 20197e639 doi 101212NXI0000000000000639
12 Stengel H Vural A Brunder AM et al Anti-pan-neurofascin IgG3 as a marker offulminant autoimmune neuropathy Neurol Neuroimmunol Neuroinflamm 20196e603 doi 101212NXI0000000000000603
13 Miura Y Devaux JJ Fukami Y et al Contactin 1 IgG4 associates to chronic inflammatorydemyelinating polyneuropathy with sensory ataxia Brain 20151381484ndash1491
14 Appeltshauser L Weishaupt A Sommer C Doppler K Complement depositioninduced by binding of anti-contactin-1 auto-antibodies is modified by immunoglo-bulins Exp Neurol 201728784ndash90
15 Doppler K Schuster Y Appeltshauser L et al Anti-CNTN1 IgG3 induces acuteconduction block and motor deficits in a passive transfer rat model J Neuro-inflammation 20191673
16 Sejvar JJ Kohl KS Gidudu J et al Guillain-Barre syndrome and Fisher syndrome casedefinitions and guidelines for collection analysis and presentation of immunizationsafety data Vaccine 201129599ndash612
17 Fokke C van den Berg B Drenthen J Walgaard C van Doorn PA Jacobs BCDiagnosis of Guillain-Barre syndrome and validation of Brighton criteria Brain 201413733ndash43
18 Kuitwaard K van Koningsveld R Ruts L Jacobs BC van Doorn PA RecurrentGuillain-Barre syndrome J Neurol Neurosurg Psychiatry 20098056ndash59
19 Van den Bergh PY Hadden RD Bouche P et al European Federation of NeurologicalSocietiesPeripheral Nerve Society guideline on management of chronic in-flammatory demyelinating polyradiculoneuropathy report of a joint task force of theEuropean Federation of Neurological Societies and the Peripheral Nerve Societymdashfirst revision Eur J Neurol 201017356ndash363
20 Ruts L Drenthen J Jacobs BC van Doorn PA Distinguishing acute-onset CIDP fromfluctuating Guillain-Barre syndrome a prospective study Neurology 2010741680ndash1686
21 McCombe PA Pollard JD McLeod JG Chronic inflammatory demyelinating poly-radiculoneuropathy A clinical and electrophysiological study of 92 cases Brain 19871101617ndash1630
22 Alessandro L Pastor Rueda JM Wilken M et al Differences between acute-onsetchronic inflammatory demyelinating polyneuropathy and acute inflammatory de-myelinating polyneuropathy in adult patients J Peripher Nerv Syst 201823154ndash158
23 Doppler K Appeltshauser L Kramer HH et al Contactin-1 and neurofascin-155-186 are not targets of auto-antibodies in multifocal motor neuropathy PLoS One201510e0134274
24 Ng JK Malotka J Kawakami N et al Neurofascin as a target for autoantibodies inperipheral neuropathies Neurology 2012792241ndash2248
25 Fujita A Ogata H Yamasaki RMatsushita T Kira JI Parallel fluctuation of anti-neurofascin155 antibody levels with clinico-electrophysiological findings in patients with chronic in-flammatory demyelinating polyradiculoneuropathy J Neurol Sci 2018384107ndash112
26 Niks EH van Leeuwen Y Leite MI et al Clinical fluctuations in MuSK myastheniagravis are related to antigen-specific IgG4 instead of IgG1 J Neuroimmunol 2008195151ndash156
27 Huijbers MG Vink AF Niks EH et al Longitudinal epitope mapping in MuSKmyasthenia gravis implications for disease severity J Neuroimmunol 201629182ndash88
28 Huang CC Lehman A Albawardi A et al IgG subclass staining in renal biopsies withmembranous glomerulonephritis indicates subclass switch during disease progressionMod Pathol 201326799ndash805
29 Collins AM Jackson KJ A temporal model of human IgE and IgG antibody functionFront Immunol 20134235
30 Valenzuela NM Schaub S The Biology of IgG subclasses and their clinical relevanceto transplantation Transplantation 2018102S7ndashS13
31 Vidarsson G Dekkers G Rispens T IgG subclasses and allotypes from structure toeffector functions Front Immunol 20145520ndash532
32 Bruhns P Iannascoli B England P et al Specificity and affinity of human Fcgamma receptorsand their polymorphic variants for human IgG subclasses Blood 20091133716ndash3725
33 Querol L Nogales-Gadea G Rojas-Garcia R et al Antibodies to contactin-1 inchronic inflammatory demyelinating polyneuropathy Ann Neurol 201373370ndash380
34 Devaux JJ Miura Y Fukami Y et al Neurofascin-155 IgG4 in chronic inflammatorydemyelinating polyneuropathy Neurology 201686800ndash807
35 Ogata H Yamasaki R Hiwatashi A et al Characterization of IgG4 anti-neurofascin155 antibody-positive polyneuropathy Ann Clin Transl Neurol 20152960ndash971
36 Doppler K Frank F Koschker AC Reiners K Sommer C Nodes of Ranvier in skinbiopsies of patients with diabetes mellitus J Peripher Nerv Syst 201722182ndash190
37 Banks W The blood-brain barrier interface in diabetes mellitus dysfunctionsmechanisms and approaches to treatment Curr Pharm Des 2020261438ndash1447
38 Arnold ML Ntokou IS Doxiadis II Spriewald BM Boletis JN Iniotaki AG Donor-specific HLA antibodies evaluating the risk for graft loss in renal transplant recipientswith isotype switch from complement fixing IgG1IgG3 to noncomplement fixingIgG2IgG4 anti-HLA alloantibodies Transpl Int 201427253ndash261
39 Martinez-Martinez L Lleixa MC Boera-Carnicero G et al Anti-NF155 chronic in-flammatory demyelinating polyradiculoneuropathy strongly associates to HLA-DRB15 J Neuroinflammation 201714224
40 Hashimoto Y Ogata H Yamasaki R et al Chronic inflammatory demyelinatingpolyneuropathy with concurrent membranous nephropathy an anti-paranode andpodocyte protein antibody study and literature survey Front Neurol 20189997
Appendix (continued)
Name Location Contribution
KathrinDoppler MD
University Hospital ofWurzburg
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy major role in analysisand interpretation of dataand study supervision
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 11
DOI 101212NXI000000000000081720207 Neurol Neuroimmunol Neuroinflamm
Luise Appeltshauser Anna-Michelle Brunder Annika Heinius et al Antiparanodal antibodies and IgG subclasses in acute autoimmune neuropathy
This information is current as of July 24 2020
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm
ServicesUpdated Information amp
httpnnneurologyorgcontent75e817fullhtmlincluding high resolution figures can be found at
References httpnnneurologyorgcontent75e817fullhtmlref-list-1
This article cites 40 articles 9 of which you can access for free at
Citations httpnnneurologyorgcontent75e817fullhtmlotherarticles
This article has been cited by 1 HighWire-hosted articles
Subspecialty Collections
httpnnneurologyorgcgicollectionperipheral_neuropathyPeripheral neuropathy
httpnnneurologyorgcgicollectionguillainbarre_syndromeGuillain-Barre syndrome
nating_polyneuropathyhttpnnneurologyorgcgicollectionchronic_inflammatory_demyeliChronic inflammatory demyelinating polyneuropathy
httpnnneurologyorgcgicollectionautoimmune_diseasesAutoimmune diseases
httpnnneurologyorgcgicollectionall_neuromuscular_diseaseAll Neuromuscular Diseasefollowing collection(s) This article along with others on similar topics appears in the
Permissions amp Licensing
httpnnneurologyorgmiscaboutxhtmlpermissionsits entirety can be found online atInformation about reproducing this article in parts (figurestables) or in
Reprints
httpnnneurologyorgmiscaddirxhtmlreprintsusInformation about ordering reprints can be found online
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm

plates (Thermo Scientific Fisher Minneapolis MN) and testedbaselinefollow-up sera of seropositive patients and 40 healthycontrols or rabbit antindashCaspr-1 (Lifespan Biosciences SeattleWA) in duplets using corresponding horseradish peroxidasendashconjugated secondary antibodies6 and conjugated anti-rabbitIgG (DakoCytomation Glostrup Denmark) For each samplewe subtracted corresponding values of uncoated duplet wells toreduce background signals We set the threshold for both assaysat 5 SDs above the mean of healthy controls Antibody titerswere determined by serial dilutions Subclass-specific secondaryantibodies served for subclass analysis as previously described12
We tested CSF of seropositive patients in a dilution of 120 inantindashcontactin-1 ELISA and antindashCaspr-1 ELISA (exceptionpatient 4 baseline CSF in both ELISAs and patient 3 in anti-Caspr-1 ELISA due to lack of material) as described above withCSF of 5 seronegative patients as negative controls
Preabsorption experimentsTo determine the specific paranodal target of the autoanti-bodies we serially preincubated seropositive sera and serumfrom a healthy control with contactin-1 (CNTN1)ndash andCaspr-1 (CNTNAP1)ndashtransfected human embryonic kidney(HEK) 293 cells as previously described7 and afterwardperformed binding assays on murine teased fibers as de-scribed above
Cell-based assayFor specification we tested sera of patients positive in teasedfibers assay or ELISA and sera of five controls on HEK293 cellstransfected with plasmids of human Caspr-1 (CNTNAP1) andrat contactin-1 (CNTN1) as previously described723 and usedpolyclonal rabbit antindashcontactin-1 IgG (Abcam) and mono-clonal mouse antindashCaspr-1 IgG1 (Santa Cruz BiotechnologyDallas TX) for double immunofluorescence in positivepatients with corresponding Cy3TM-conjugated donkeyanti-rabbit IgG (Abcam) donkey anti-mouse IgG andMFP488-conjugated goat anti-human IgG (MoBiTec) sec-ondary antibodies Sera of patients with antindashCaspr-1 andantindashcontactin-1 antibodies from previous studies67 servedas positive controls and sera of healthy controls as negativecontrols in the double immunofluorescence assays
Statistical analysisWe performed statistical analysis (descriptive statisticscalculation of OR and 95 CI) with SPSS 250 (IBMArmonk NY)
Standard protocol approvals registrationsand patient consentsThe Ethics Committees of the University of Wurzburg andthe University of Kiel approved to conduct this study Writteninformed consent was obtained from all participants in thestudy
Data availabilityData not published within this article are available at theUniversity Hospital of Wurzburg or will be shared in an
anonymized manner on request from any qualified in-vestigator for purposes of replicating procedures and results
ResultsAntiparanodal autoantibodies of different IgGsubclasses in 43 of the patientsWe applied immunofluorescence binding assays on murineteased fibers as a screening tool for detection of anti-paranodal autoantibodies We observed serum binding tothe paranodal junctional region colocalizing with the com-mercial antindashCaspr-1 antibody (figure 1A) in seven patientsof the total cohort (7161 prevalence 43) in none of thepatients of the R-GBS (06) andMFS (018) subcohort norin the healthy controls (040) but in 3114 (26) of theGBS subcohort and 423 (174) of the A-CIDP subcohortThus paranodal autoantibodies occurred more frequentlyin A-CIDP compared with GBS (OR = 779 95 CI161ndash3795) Two of the sera with paranodal binding (1 GBSand 1 A-CIDP) had been identified as antindashNF155 sero-positive in a recent study12 therefore experimental findingsand clinical associations of these patients are not reportedhere Target antigens of the other five seropositive patientswere determined using cell-based assay (CBA figure 1B)and ELISA (see below) and confirmed by preabsorptionexperiments (figure 1C) In the GBS subcohort the twoseropositive patients showed antindashCaspr-1 (patient 1) andantindashcontactin-1Caspr-1 (patient 2) antibodies Seroposi-tive A-CIDP patients carried antibodies against either con-tactin-1 (patient 3) contactin-1Caspr-1 (patient 4) or anti-Caspr-1 (patient 5) At the onset of disease the two patientswith GBS showed IgG2 and IgG3 antibodies (table 1)During the acute phase at onset sera of the three patientslater classified as A-CIDP carried IgG24 (patient 3) andIgG23 (patients 4 and 5) as detected by binding assays andELISA (figure 2 A and B) Autoantibody titers are shown intable 1 The results of the binding assays on teased fibersELISA and CBA showed concordance regarding the specifictarget antigen of the antibodies and the predominating IgGsubclass There were only minor differences of the antibodytiter and coexisting subclasses in ELISA and teased fibersassay In case of double positivity for anti-contactin-1Caspr-1 (patients 2 and 4) preabsorption assays detectedonly the dominating antibody (table 1)
Subclass switch from IgG3 to IgG4 at follow-upin one patientIn 66161 patients follow-up serum and CSF samples wereavailable including three seropositive patients (1 GBS and 2A-CIDP) None of the follow-up sera of initially seronega-tive patients or controls showed positive results in the teasedfibers assay (data not shown) nor in the contactin-1 ELISA(figure 3A) Serum of the patient with GBS acquired inremission 55 months after the first assessment when thepatient did not show any remaining neurologic deficits wasseronegative in all experimental assays (figure 3 B and C)
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 3
Follow-up serum of one patient with A-CIDP two monthsafter onset ie still in the acute phase of disease (patient 5)showed IgG3 autoantibodies against Caspr-1 with no dif-ference in antibody titer and subclass compared with onset(figure 3 B and C) A follow-up-sample during the chronicstage (28 months after the onset) revealed a reduction of thetiter (150 in teased fibers negative in ELISA table 1) ofantindashCaspr-1 IgG autoantibodies with subclasses not mea-surable due to the low titer (data not shown) The patientnow presented with only very mild sensorimotor impair-ment and ameliorated nerve conduction studies Follow-upserum and CSF of the other patient with A-CIDP (patient 4)who was initially IgG3ndashanti-contactin-1Caspr-1 doublepositive were acquired 120 months after the acute onsetwhen the patient had developed relapsing-remitting senso-rimotor CIDP At follow-up we could no longer detectantindashcontactin-1 but detected and confirmed antindashCaspr-1autoantibodies via antindashCaspr-1 ELISA CBA and pre-absorption assay (table 1 and figure 3 B and C) Subclassanalysis now revealed reactivity against IgG24 but notIgG3 subclass anymore giving evidence of a switch of IgG
subclasses between the acute and chronic stage of the disease(figure 2C)
Intrathecal antiparanodal autoantibodies inpatients with high antibody titer and blood-brain barrier dysfunctionELISA with CSF samples of the seropositive patients and fivedisease controls showed elevated optical density (OD) ata dilution of 120 only in antindashcontactin-1 patient 3 witha very high serum titer (see appendix e-1 linkslwwcomNXIA275) and antindashCaspr-1 patient 5 with signs of se-verely disrupted blood-brain barrier (high CSF protein MRIcauda equina enhancement see appendix e-1 linkslwwcomNXIA275) but equally low OD values for the otherseronegative and seropositive patients (see figure e-1 linkslwwcomNXIA273) Binding assays on murine teasedfibers showed weak binding of CSF to the paranodes in thesetwo patients but not in the other seropositive patients orcontrols None of the seropositive patients showed signs ofintrathecal autoantibody production with CSF IgG indexand antiparanodal autoantibody specific ASI (antibody
Figure 1 Detection of antiparanodal autoantibodies by binding assays on teased fibers CBA and preabsorption assay
(A) Colocalization overlay photomicrographs of double immunofluorescence assays with commercial autoantibody displayed in red and human serum ingreen Colocalization at the paranodes (arrows) appears yellow and indicates antiparanodal autoantibodies in patients 1ndash5 (AbndashAf) but not in the negativecontrol serum (Aa) (B) The specific target of the autoantibodies is illustrated by colocalization overlay photomicrographs on transfected HEK293 cells(arrowheads) with commercial antibody in red serum in green and nucleus staining in blue Colocalization appears yellow (merge) and confirms antindashcontactin-1 in patients 2 (Bc) 3 (Bd) and 4 (Be) but not in patients 1 (Bb) 5 (Bf) and the negative control (Ba) Sera of patients 1 2 4 and 5 (Bh Bi Bk andBl) colocalize on Caspr-1 (CNTNAP1)ndashtransfected HEK293 cells as proof of antindashCaspr-1 autoantibodies whereas sera of the negative control (Bg) and patient3 (Bj) donot showantindashCaspr-1 positivity (C) Single immunofluorescence onmurine teased fibers afterHEK293 contactin-1 andCaspr-1 preabsorption showsthat antiparanodal autoantibodies disappear after contactin-1 preabsorption in patient 3 (Cd) and after Caspr-1 preabsorption in patient 4 (Cf) provingspecificity to the respective target antigen Scale bar = 10 μm CBA = cell-based assay HEK = human embryonic kidney
4 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
Table 1 Summary of the autoantibody status of seropositive patients 1ndash5 at baseline and follow-up
CSF Serum
Teased fibers and ELISA Preabsorption CBA
Paranodal bindingOD Titer IgG subclass
Patient 1 (baseline no follow-up)
Teased fibers Negative Positive 1100 IgG3
Contactin-1 0202 0465 nd nd Negative Negative
Caspr-1 0072 2961 1200 nm Positive Positive
Patient 2 (baseline)
Teased fibers Negative Positive 1200 nd
Contactin-1 0247 3538 1200 IgG2 Negative Positive
Caspr-1 0129 3965 11000 IgG2 Positive Positive
Patient 2 (follow-up)
Teased fibers nd Negative nd nd
Contactin-1 nd 0039 nd nd nd Negative
Caspr-1 nd 0025 nd nd nd Negative
Patient 3 (baseline no follow-up)
Teased fibers Negative Positive 130000 IgG4
Contactin-1 0516 4492 140000 IgG42 Positive Positive
Caspr-1 nd 0073 nd nd Negative Negative
Patient 4 (baseline)
Teased fibers Negative Positive 1500 IgG3
Contactin-1 0267 2294 1200 IgG3 Negative Positive
Caspr-1 nd 3011 1500 IgG32 Positive Positive
Patient 4 (follow-up)
Teased fibers Negative Positive 1500 IgG4
Contactin-1 0227 0206 nd nd Negative Negative
Caspr-1 0138 3618 1500 IgG24 Positive Positive
Continued
Neurolo
gyorgN
NNeurologyN
euroim
munology
ampNeuroinflam
mation
|Volum
e7N
umber
5|
Septem
ber2020
5
Table 1 Summary of the autoantibody status of seropositive patients 1ndash5 at baseline and follow-up (continued)
CSF Serum
Teased fibers and ELISA Preabsorption CBA
Paranodal bindingOD Titer IgG subclass
Patient 5 (baseline)
Teased fibers Positive Positive 1100 IgG3
Contactin-1 0219 0260 nd nd Negative Negative
Caspr-1 0136 nd nd nd Positive Positive
Patient 5 (first follow-up)
Teased fibers Positive Positive 1100 IgG3
Contactin-1 0236 0243 nd nd Negative Negative
Caspr-1 0364 3802 1200 IgG32 Positive Positive
Patient 5 (second follow-up)
Teased fibers nd Positive 150 nm
Contactin-1 nd 0168 nd nd nd Negative
Caspr-1 nd 0013 nd nd nd Negative
Abbreviations A-CIDP = acute-onset chronic inflammatory demyelinating polyradiculoneuropathy Caspr-1 = contactin-1ndashassociated protein-1 CBA = cell-based assay GBS = Guillain-Barre syndrome nd = not done nd =not done due to lack of material nm = not measurable OD = optical densityResults of teased fibers assays are displayed in the first row for each patient contactin-1 and Caspr-1-specific results are displayed in lines 2 and 3 ODs above the threshold and results considered positive are highlighted inbold letters There is a 100 concordance regarding target specificity in the teased fibers assay ELISA and CBA but minor differences in IgG titer and coexisting subclass between the teased fibers assay and ELISA In case ofdouble positivity preabsorption assays only detect the target antigen with the higher antibody titer (see ELISA titer)
6NeurologyN
euroim
muno
logyampNeuroinflam
mation
|Vo
lume7N
umber
5|
Septem
ber
2020NeurologyorgN
N
specificity index) being in a normal range (see appendix e-1linkslwwcomNXIA275)
Clinical features of seropositive patientsFigure 4 schematically summarizes the clinical course relatedto the experimental results Appendix e-1 linkslwwcomNXIA275 summarizes clinical data of seropositive patients1ndash5 and provides a detailed description of the clinical diseasecourse in individuals The two seropositive patients withGBS were both middle-aged women presenting with severemotor but only mild sensory involvement Cranial nerveinvolvement autonomic symptoms and neuropathic painwere present in 12 patients with GBS only Both patientsrequired treatment on the intensive care unit (ICU) The
three patients with A-CIDP presented with moderate to severeparesis and multimodal sensory impairment at disease onsetDuring the course of disease all three patients with A-CIDPreported neuropathic pain requiring pregabalingabapentinor even opioid treatment Two of three patients withA-CIDP presented sensory ataxia Nerve conduction studiesshowed demyelinating features in all seropositive patientswith conduction block in 35 patients Sural nerve biopsywas performed in one patient (patient 5) six months after theonset and showed axonal loss without signs of demyelinationCSF analysis revealed cytoalbuminologic dissociation andblood-brain barrier dysfunction in all seropositive patientsThe patient with subacute-onset CIDP highly elevated CSFprotein and detection of antindashCaspr-1 in both serum and
Figure 2 Serum subclass analysis at baseline and at follow-up with IgG subclass switch
(A) Results of antindashcontactin-1 and antindashCaspr-1 ELISA with baseline sera and subclass-specific IgG1-4 autoantibodies (grayscale) are shown as of the OD ofthe total IgG (using anti-human IgG autoantibody) on y-axis In patient 1 ELISA IgG subclasses were not measurable ELISA revealed mainly IgG2 subclass inpatient 2 (GBS) IgG2IgG4 in patient 3 (A-CIDP) and IgG23 in patients 4 and 5 (A-CIDP) (B) Acute phase teased fibers subclass analysis with baseline serashowed binding to the paranodal regions (arrows) only when using IgG3-specific secondary antibody in patients 1 (GBS) and 4 (A-CIDP) visualized byphotomicrographs of single immunofluorescence staining on teased fibers (C) AntindashCaspr-1 IgG subclass ELISA with follow-up serum of patient 4 nowrevealed IgG 24 subclass Photomicrographs show immunofluorescence staining of murine teased fibers with subclass-specific IgG3 and IgG4 autoanti-bodies at follow-up Paranodal binding disappeared using IgG3 antibody but occurred using IgG4 subclass-specific antibody proving subclass switch inpatient 4 Scale bar = 10 μm A-CIDP = acute-onset chronic inflammatory demyelinating polyradiculoneuropathy GBS = Guillain-Barre syndrome
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 7
CSF (patient 5) developed action tremor during the courseof disease
Antibody status is concordant with diseaseactivity and therapy responseOne of two patients with GBS (patient 2) received IVIgwhich did not lead to amelioration This patient was IgG2 gtIgG3 seropositive (patient 2 figure 2A) Both patients withGBS responded well to plasma exchange The two patientswith A-CIDP and IgG3 autoantibodies (patients 4 and 5)showed good response to IVIg treatment in the acute phase ofdisease Ongoing IVIg treatment led to further improvementof symptoms and was stopped after 25 cycles in patient 5because of a remission of symptoms In the other patient withA-CIDP and antindashcontactin-1Caspr-1 IgG3 at disease onset(patient 4) IVIg showed a good therapeutic effect in the acutephase and led to remission not requiring further therapy Inthe chronic phase IVIg was initiated again and stopped after26 cycles because of a loss of the therapeutic effect At thatstage the autoantibody subclass had already switched fromIgG3 to IgG4 (for detailed treatment response in the course ofdisease see appendix e-1 linkslwwcomNXIA275) Thepatient with A-CIDP and high-titer antindashcontactin-1 IgG4autoantibodies (patient 3) had to be treated on ICU becauseof the severity of symptoms developed renal dysfunction didnot respond to IVIg treatment and did not ameliorate duringthe course of disease
DiscussionWedetected antiparanodal autoantibodies in 43of patientswithacute to subacute immune-mediated neuropathy more frequentlyin CIDP with acute to subacute onset than in GBS We foundIgG23 in monophasic GBS which supports the notion of pre-vious studies that IgG3 is the predominant subclass inmonophasicdisease7 By analyzing sera from different time points we couldshow that the antibody status was concordant with the diseaseseverity Therefore our data support pathogenicity of autoanti-bodies and suggest that autoantibody status and titer are validindicators for disease activity as proposed in previous studies onparanodopathy and further IgG4-related neurologic diseases25ndash27
In one patient we could demonstrate a subclass switch fromIgG3 in acute disease to IgG4 in chronic disease a finding thatwe had hypothesized in a previous study6 In other autoimmunediseases eg in membranous glomerulonephritis subclassswitch of anti-PLA2R autoantibodies from IgG1 to IgG4 alsoassociates with progression to chronic disease28 According toa linear model of autoantibody response unspecific IgM re-sponse is followed by IgG isotype produced by antigen-experi-enced B cells These IgG are of the IgG3 subclass andsequentially switch to IgG1 2 and 42930 Concurrently auto-antibodies strongly gain antigen affinity with IgG4 showing thehighest affinity to its target3132 Disease progression in para-nodopathy might therefore depend on IgG subclass properties
Figure 3 Antiparanodal IgG autoantibodies at follow-up assessment
(A) Contactin-1 IgG ELISA of all patients at first assessment of 66161 patients at follow-up and of 40 healthy controls Optical density (OD) at 450 nm isdisplayed on the y-axis The threshold for positive results is set at 5 SDs above the mean of controls (0615) Patients 2ndash4 show positive results at firstassessment The other patients and controls show values below the threshold Sera of patients 2 4 and 5 are negative in antindashcontactin-1 IgG ELISA at follow-up as well as 63 other follow-up sera Patients 1 and 3were lost to follow-up (B) Overlay photomicrographs of teased fibers double immunofluorescence and(C) contactin-1ndash and Caspr-1 (CNTNAP1)ndashtransfected HEK293 cells show loss of antindashcontactin-1Caspr-1 autoantibodies in patient 2 (GBS) at follow-up (BaCa Cd) but colocalization of commercial antindashCaspr-1 antibody and sera at paranodes in patients 4 and 5 with A-CIDP (Bb Bc arrows) Both sera bind toCaspr-1 (CNTNAP1)ndashtransfected HEK293 cells (Ce Cf arrowheads) but not to contactin-1ndashtransfected cells (Cb Cc) Scale bar = 10 μm A-CIDP = acute-onsetchronic inflammatory demyelinating polyradiculoneuropathy GBS = Guillain-Barre syndrome HEK = human embryonic kidney
8 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
and affinities Gain of specificity and affinity at switch from IgG3to IgG4 might explain different binding characteristics of anti-paranodal autoantibodies in the patient whose IgG3 autoanti-bodies reacted both against contactin-1Caspr-1 epitopes butwhose IgG4 autoantibodies were specific to Caspr-1 Here wereport IgG24 (1) in patient 4 in the chronic phase after subclassswitch and (2) in another patient at disease onset showingnonameliorating disease with high disability similar to previousreports on antindashcontactin-1 IgG4-related disease111333 So farstudies have only reported antiparanodal IgG4 autoantibodies inpatients with chronic disease7113435 We therefore suggest thathigh-affinity IgG4 autoantibody binding leads to chronic diseasewith functional impairment possibly caused by disturbance of theantigen interaction paranodal architecture and paranodalcomplex formation as demonstrated previously68ndash11
Patients of our study with predominant IgG3 subclass showedgood response to IVIg whereas patients with IgG2 and IgG4initially did not respond to IVIg or lost responsiveness in thedisease course Several studies described good responses toIVIg in IgG3-associated paranodopathy but poor responses inIgG4-associated disease56111334 IgG3 can initiate comple-ment activation opsonization antigen cross-linking and in-ternalization whereas IgG2 shows little C1q binding and IgG4completely lacks these Fc-mediated effector functions31 In anin vivo study on antindashcontactin-1 pathogenicity we suggestedcomplement-mediated functional impairment at the paranodesas the pathophysiologic correlate in IgG3-mediated para-nodopathy earlier and could show in vitro that IVIg inhibitscomplement deposition and activation dose-dependently1415
Our data therefore support the hypothesis that therapy re-sponsiveness might depend on IgG subclass and subclass-related pathomechanism of antiparanodal autoantibodies If
validated in larger studies these findings might have a directimpact on the treatment regime in affected patients Testing ofautoantibody subclass during the course of disease may beuseful as an indicator of a positive response to IVIg in case ofIgG3 whereas IgG4-related paranodopathy shows very goodresponse to rituximab46711
The trigger of the autoantibody production and the patho-mechanism behind possible IgG switching are still unknownThree of five seropositive patients in this study had diabetesmellitus type 2 as a comorbidity diagnosed years before theonset of disease Previous studies report disruption of the blood-brain barrier and the nodal architecture in patients with diabetesmellitus thereby possibly exposing paranodal antigens to theimmune response3637 A recent study has identified humanleukocyte antigen (HLA)-DRB15 as a risk factor for CIDPassociated with anti-NF155 antibodies and another studyreported different HLA antibody patterns with subclass switchafter renal transplantation3839 Whether interindividual differ-ences in antibody production and switching during the course ofdisease depend on (1) HLA alleles (2) antigen exposure or (3)further immunologic mechanisms has to be investigated infurther studies
Regarding characteristic clinical features of paranodopathy wereport neuropathic pain in antindashCaspr-1ndashrelated disease aspreviously described in patients with higher autoantibody tit-ers7 Different titers might explain discrepancies between ourresults and a recent study that did not report pain in antindashCaspr-1ndashpositive patients711 In our patients further features of IgG4-related chronic paranodopathy such as tremor sensory ataxiaand nephropathy561340 were only present in the course ofdisease suggesting that they evolve due to chronic autoantibody
Figure 4 Scheme of the autoantibody status in the course of disease in seropositive patients
The course of disease is shown on the x-axis by pseudo-logarithmic display of the time course Time point 0 is set at baseline assessment of serumCSF Thecolor code indicates severity of symptoms Results of serum assessment are displayed in black Patients 1 and 3 were lost to follow-up
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 9
exposure The pathomechanistic correlate of action tremorwhich recently has also been described in antindashCaspr-1 IgG3-related paranodopathy11 is still under research but studiessuggest cerebellar origin5612 In this study we detected in-trathecal autoantibodies in a patient with tremor as previouslyreported in a case of antindashpan-neurofascinndashpositive para-nodopathy12 The antiparanodal autoantibodies most probablydiffuse into the intrathecal compartment due to a blood-brainbarrier dysfunction as indicated by normal IgG and ASI indicesin our patients Our CSF data suggest that detection of anti-paranodal antibodies in CSF shows little sensitivity and dependson either high titer or strong leakage of the blood-brain barrierWe therefore encourage further studies on intrathecal anti-paranodal autoantibodies targeting sensitivity pathogenicityand association to clinical symptoms
Low prevalence of autoantibodies against paranodal proteins inacute inflammatory neuropathy retrospective assessment and thelow number of seropositive patients available for follow-up limitour study Clinical characteristics at acute onset and subclass-related seropositivity as a possible risk factor for chronic pro-gression when detected at the onset should be investigated in largemulticentric studies considering lowoverall prevalence ofGBS andA-CIDP as well as further possible confounders in analysis ofparanodopathy-specific clinical symptoms eg coinciding painfuldiabetic neuropathy The question of sequential subclass switchingin paranodopathy needs to be addressed in larger prospectivestudies Nevertheless our data (1) propose autoantibody subclassscreening to be considered in patients with GBS and A-CIDP asresults might have direct implications on diagnosis and therapeuticregime during the course of disease and (2) pave the way forfurther clinical and pathomechanistic studies on antiparanodalautoantibodies in acute to subacute immune-mediated neuropathy
AcknowledgmentThe authors thank Barbara Reuter and Antonia Kohl for theirsuperb technical assistance They thankMaximilian Frommer forprovision and processing of clinical data and PD Dr JakobMatschke for provision of slides and images of supplementarymorphologic data The study was supported by a grant of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg to KD and CV and a grant of the GermanResearch Foundation to KD (DFG DO-22191-1) LA issupported by a research fellowship as a clinician scientist of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg FL receives funding from the GermanMinistry of Education and Research (BMBF 01GM1908A) andthe German Research Foundation (DFG LE30642-1) CSreceives funding from the German Research Foundation (DFG3289-1 DFG UE 1714-1 DFG SO 32810-1 DFG SFB1158 and DFG SO 328 131) from the German Ministry ofEducation and Research (BMBF CMT-Net PI) and from theEuropean Union (Horizon 2020)
Study fundingThis study was supported by the Open Access PublicationFund of the University of Wurzburg
DisclosureL Appeltshauser K-P Wandinger R Junker C Sommer FLeypoldt and K Doppler work for an academic institutionoffering commercial antibody diagnostics AM Brunder AHeinius P Kortvelyessy and C Villmann report no dis-closures relevant to the manuscript C Sommer has served onscientific advisory boards for Algiax Alnylam Air LiquideAkcea Astellas Bayer Grifols Takeda and UCB She reportsbeing a member of speakersrsquo bureau and receiving speakerhonoraria from Akcea Alnylam Novartis Pfizer Sanofi-Aventis and Teva C Sommer served as a journal editorassociate editor or editorial advisory board member for theEuropean Journal of Neurology PLoS ONE and PAIN ReportsF Leypoldt reports speaker honoraria from Bayer RocheNovartis and Fresenius travel funding from Merck Grifolsand Bayer and serving on advisory boards for Roche Biogenand Alexion Go to NeurologyorgNN for full disclosures
Publication historyReceived by Neurology Neuroimmunology amp NeuroinflammationMarch 2 2020 Accepted in final form May 19 2020
Appendix Authors
Name Location Contribution
LuiseAppeltshauserMD
University Hospital ofWurzburg
Major role in the acquisitionanalysis and interpretationof the data and drafting andrevising the manuscript forintellectual content
Anna-MichelleBrunder
University Hospital ofWurzburg
Major role in the acquisitionand analysis of the data
Annika Heinius University Hospital ofSchleswig-HolsteinCampus Kiel
Major role in the acquisitionand analysis of the data
PeterKortvelyessyMD
University Hospital ofMagdeburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Klaus-PeterWandinger MD
University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Ralf Junker MD University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
CarmenVillmann PhD
University Hospital ofWurzburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
ClaudiaSommer MD
University Hospital ofWurzburg
Conceptualization of thestudy revising themanuscript for intellectualcontent and major role ininterpretation of data
Frank LeypoldtMD
University Hospital ofSchleswig-HolsteinCampus Kiel
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy and major role ininterpretation of data
10 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
References1 Fehmi J Scherer SS Willison HJ Rinaldi S Nodes paranodes and neuropathies
J Neurol Neurosurg Psychiatry 20188961ndash712 Uncini A Vallat JM Autoimmune nodo-paranodopathies of peripheral nerve the
concept is gaining ground J Neurol Neurosurg Psychiatry 201889627ndash6353 Querol L Illa I Paranodal and other autoantibodies in chronic inflammatory neu-
ropathies Curr Opin Neurol 201528474ndash4794 Querol L Rojas-Garcia R Diaz-Manera J et al Rituximab in treatment-resistant
CIDP with antibodies against paranodal proteins Neurol Neuroimmunol Neuro-inflamm 20152e149 doi 101212NXI0000000000000149
5 Querol L Nogales-Gadea G Rojas-Garcia R et al Neurofascin IgG4 antibodies inCIDP associate with disabling tremor and poor response to IVIg Neurology 201482879ndash886
6 Doppler K Appeltshauser L Wilhelmi K et al Destruction of paranodal architecturein inflammatory neuropathy with anti-contactin-1 autoantibodies J Neurol Neuro-surg Psychiatry 201586720ndash728
7 Doppler K Appeltshauser L Villmann C et al Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathyBrain 20161392617ndash2630
8 Manso C Querol L Mekaouche M Illa I Devaux JJ Contactin-1 IgG4 antibodiescause paranode dismantling and conduction defects Brain 20161391700ndash1712
9 Manso C Querol L Lleixa C et al Anti-Neurofascin-155 IgG4 antibodies preventparanodal complex formation in vivo J Clin Invest 20191292222ndash2236
10 Labasque M Hivert B Nogales-Gadea G Querol L Illa I Faivre-Sarrailh C Specificcontactin N-glycans are implicated in neurofascin binding and autoimmune targetingin peripheral neuropathies J Biol Chem 20142897907ndash7918
11 Cortese A Lombardi R Briani C et al Antibodies to neurofascin contactin-1 andcontactin-associated protein 1 in CIDP clinical relevance of IgG isotype NeurolNeuroimmunol Neuroinflamm 20197e639 doi 101212NXI0000000000000639
12 Stengel H Vural A Brunder AM et al Anti-pan-neurofascin IgG3 as a marker offulminant autoimmune neuropathy Neurol Neuroimmunol Neuroinflamm 20196e603 doi 101212NXI0000000000000603
13 Miura Y Devaux JJ Fukami Y et al Contactin 1 IgG4 associates to chronic inflammatorydemyelinating polyneuropathy with sensory ataxia Brain 20151381484ndash1491
14 Appeltshauser L Weishaupt A Sommer C Doppler K Complement depositioninduced by binding of anti-contactin-1 auto-antibodies is modified by immunoglo-bulins Exp Neurol 201728784ndash90
15 Doppler K Schuster Y Appeltshauser L et al Anti-CNTN1 IgG3 induces acuteconduction block and motor deficits in a passive transfer rat model J Neuro-inflammation 20191673
16 Sejvar JJ Kohl KS Gidudu J et al Guillain-Barre syndrome and Fisher syndrome casedefinitions and guidelines for collection analysis and presentation of immunizationsafety data Vaccine 201129599ndash612
17 Fokke C van den Berg B Drenthen J Walgaard C van Doorn PA Jacobs BCDiagnosis of Guillain-Barre syndrome and validation of Brighton criteria Brain 201413733ndash43
18 Kuitwaard K van Koningsveld R Ruts L Jacobs BC van Doorn PA RecurrentGuillain-Barre syndrome J Neurol Neurosurg Psychiatry 20098056ndash59
19 Van den Bergh PY Hadden RD Bouche P et al European Federation of NeurologicalSocietiesPeripheral Nerve Society guideline on management of chronic in-flammatory demyelinating polyradiculoneuropathy report of a joint task force of theEuropean Federation of Neurological Societies and the Peripheral Nerve Societymdashfirst revision Eur J Neurol 201017356ndash363
20 Ruts L Drenthen J Jacobs BC van Doorn PA Distinguishing acute-onset CIDP fromfluctuating Guillain-Barre syndrome a prospective study Neurology 2010741680ndash1686
21 McCombe PA Pollard JD McLeod JG Chronic inflammatory demyelinating poly-radiculoneuropathy A clinical and electrophysiological study of 92 cases Brain 19871101617ndash1630
22 Alessandro L Pastor Rueda JM Wilken M et al Differences between acute-onsetchronic inflammatory demyelinating polyneuropathy and acute inflammatory de-myelinating polyneuropathy in adult patients J Peripher Nerv Syst 201823154ndash158
23 Doppler K Appeltshauser L Kramer HH et al Contactin-1 and neurofascin-155-186 are not targets of auto-antibodies in multifocal motor neuropathy PLoS One201510e0134274
24 Ng JK Malotka J Kawakami N et al Neurofascin as a target for autoantibodies inperipheral neuropathies Neurology 2012792241ndash2248
25 Fujita A Ogata H Yamasaki RMatsushita T Kira JI Parallel fluctuation of anti-neurofascin155 antibody levels with clinico-electrophysiological findings in patients with chronic in-flammatory demyelinating polyradiculoneuropathy J Neurol Sci 2018384107ndash112
26 Niks EH van Leeuwen Y Leite MI et al Clinical fluctuations in MuSK myastheniagravis are related to antigen-specific IgG4 instead of IgG1 J Neuroimmunol 2008195151ndash156
27 Huijbers MG Vink AF Niks EH et al Longitudinal epitope mapping in MuSKmyasthenia gravis implications for disease severity J Neuroimmunol 201629182ndash88
28 Huang CC Lehman A Albawardi A et al IgG subclass staining in renal biopsies withmembranous glomerulonephritis indicates subclass switch during disease progressionMod Pathol 201326799ndash805
29 Collins AM Jackson KJ A temporal model of human IgE and IgG antibody functionFront Immunol 20134235
30 Valenzuela NM Schaub S The Biology of IgG subclasses and their clinical relevanceto transplantation Transplantation 2018102S7ndashS13
31 Vidarsson G Dekkers G Rispens T IgG subclasses and allotypes from structure toeffector functions Front Immunol 20145520ndash532
32 Bruhns P Iannascoli B England P et al Specificity and affinity of human Fcgamma receptorsand their polymorphic variants for human IgG subclasses Blood 20091133716ndash3725
33 Querol L Nogales-Gadea G Rojas-Garcia R et al Antibodies to contactin-1 inchronic inflammatory demyelinating polyneuropathy Ann Neurol 201373370ndash380
34 Devaux JJ Miura Y Fukami Y et al Neurofascin-155 IgG4 in chronic inflammatorydemyelinating polyneuropathy Neurology 201686800ndash807
35 Ogata H Yamasaki R Hiwatashi A et al Characterization of IgG4 anti-neurofascin155 antibody-positive polyneuropathy Ann Clin Transl Neurol 20152960ndash971
36 Doppler K Frank F Koschker AC Reiners K Sommer C Nodes of Ranvier in skinbiopsies of patients with diabetes mellitus J Peripher Nerv Syst 201722182ndash190
37 Banks W The blood-brain barrier interface in diabetes mellitus dysfunctionsmechanisms and approaches to treatment Curr Pharm Des 2020261438ndash1447
38 Arnold ML Ntokou IS Doxiadis II Spriewald BM Boletis JN Iniotaki AG Donor-specific HLA antibodies evaluating the risk for graft loss in renal transplant recipientswith isotype switch from complement fixing IgG1IgG3 to noncomplement fixingIgG2IgG4 anti-HLA alloantibodies Transpl Int 201427253ndash261
39 Martinez-Martinez L Lleixa MC Boera-Carnicero G et al Anti-NF155 chronic in-flammatory demyelinating polyradiculoneuropathy strongly associates to HLA-DRB15 J Neuroinflammation 201714224
40 Hashimoto Y Ogata H Yamasaki R et al Chronic inflammatory demyelinatingpolyneuropathy with concurrent membranous nephropathy an anti-paranode andpodocyte protein antibody study and literature survey Front Neurol 20189997
Appendix (continued)
Name Location Contribution
KathrinDoppler MD
University Hospital ofWurzburg
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy major role in analysisand interpretation of dataand study supervision
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 11
DOI 101212NXI000000000000081720207 Neurol Neuroimmunol Neuroinflamm
Luise Appeltshauser Anna-Michelle Brunder Annika Heinius et al Antiparanodal antibodies and IgG subclasses in acute autoimmune neuropathy
This information is current as of July 24 2020
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm
ServicesUpdated Information amp
httpnnneurologyorgcontent75e817fullhtmlincluding high resolution figures can be found at
References httpnnneurologyorgcontent75e817fullhtmlref-list-1
This article cites 40 articles 9 of which you can access for free at
Citations httpnnneurologyorgcontent75e817fullhtmlotherarticles
This article has been cited by 1 HighWire-hosted articles
Subspecialty Collections
httpnnneurologyorgcgicollectionperipheral_neuropathyPeripheral neuropathy
httpnnneurologyorgcgicollectionguillainbarre_syndromeGuillain-Barre syndrome
nating_polyneuropathyhttpnnneurologyorgcgicollectionchronic_inflammatory_demyeliChronic inflammatory demyelinating polyneuropathy
httpnnneurologyorgcgicollectionautoimmune_diseasesAutoimmune diseases
httpnnneurologyorgcgicollectionall_neuromuscular_diseaseAll Neuromuscular Diseasefollowing collection(s) This article along with others on similar topics appears in the
Permissions amp Licensing
httpnnneurologyorgmiscaboutxhtmlpermissionsits entirety can be found online atInformation about reproducing this article in parts (figurestables) or in
Reprints
httpnnneurologyorgmiscaddirxhtmlreprintsusInformation about ordering reprints can be found online
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm

Follow-up serum of one patient with A-CIDP two monthsafter onset ie still in the acute phase of disease (patient 5)showed IgG3 autoantibodies against Caspr-1 with no dif-ference in antibody titer and subclass compared with onset(figure 3 B and C) A follow-up-sample during the chronicstage (28 months after the onset) revealed a reduction of thetiter (150 in teased fibers negative in ELISA table 1) ofantindashCaspr-1 IgG autoantibodies with subclasses not mea-surable due to the low titer (data not shown) The patientnow presented with only very mild sensorimotor impair-ment and ameliorated nerve conduction studies Follow-upserum and CSF of the other patient with A-CIDP (patient 4)who was initially IgG3ndashanti-contactin-1Caspr-1 doublepositive were acquired 120 months after the acute onsetwhen the patient had developed relapsing-remitting senso-rimotor CIDP At follow-up we could no longer detectantindashcontactin-1 but detected and confirmed antindashCaspr-1autoantibodies via antindashCaspr-1 ELISA CBA and pre-absorption assay (table 1 and figure 3 B and C) Subclassanalysis now revealed reactivity against IgG24 but notIgG3 subclass anymore giving evidence of a switch of IgG
subclasses between the acute and chronic stage of the disease(figure 2C)
Intrathecal antiparanodal autoantibodies inpatients with high antibody titer and blood-brain barrier dysfunctionELISA with CSF samples of the seropositive patients and fivedisease controls showed elevated optical density (OD) ata dilution of 120 only in antindashcontactin-1 patient 3 witha very high serum titer (see appendix e-1 linkslwwcomNXIA275) and antindashCaspr-1 patient 5 with signs of se-verely disrupted blood-brain barrier (high CSF protein MRIcauda equina enhancement see appendix e-1 linkslwwcomNXIA275) but equally low OD values for the otherseronegative and seropositive patients (see figure e-1 linkslwwcomNXIA273) Binding assays on murine teasedfibers showed weak binding of CSF to the paranodes in thesetwo patients but not in the other seropositive patients orcontrols None of the seropositive patients showed signs ofintrathecal autoantibody production with CSF IgG indexand antiparanodal autoantibody specific ASI (antibody
Figure 1 Detection of antiparanodal autoantibodies by binding assays on teased fibers CBA and preabsorption assay
(A) Colocalization overlay photomicrographs of double immunofluorescence assays with commercial autoantibody displayed in red and human serum ingreen Colocalization at the paranodes (arrows) appears yellow and indicates antiparanodal autoantibodies in patients 1ndash5 (AbndashAf) but not in the negativecontrol serum (Aa) (B) The specific target of the autoantibodies is illustrated by colocalization overlay photomicrographs on transfected HEK293 cells(arrowheads) with commercial antibody in red serum in green and nucleus staining in blue Colocalization appears yellow (merge) and confirms antindashcontactin-1 in patients 2 (Bc) 3 (Bd) and 4 (Be) but not in patients 1 (Bb) 5 (Bf) and the negative control (Ba) Sera of patients 1 2 4 and 5 (Bh Bi Bk andBl) colocalize on Caspr-1 (CNTNAP1)ndashtransfected HEK293 cells as proof of antindashCaspr-1 autoantibodies whereas sera of the negative control (Bg) and patient3 (Bj) donot showantindashCaspr-1 positivity (C) Single immunofluorescence onmurine teased fibers afterHEK293 contactin-1 andCaspr-1 preabsorption showsthat antiparanodal autoantibodies disappear after contactin-1 preabsorption in patient 3 (Cd) and after Caspr-1 preabsorption in patient 4 (Cf) provingspecificity to the respective target antigen Scale bar = 10 μm CBA = cell-based assay HEK = human embryonic kidney
4 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
Table 1 Summary of the autoantibody status of seropositive patients 1ndash5 at baseline and follow-up
CSF Serum
Teased fibers and ELISA Preabsorption CBA
Paranodal bindingOD Titer IgG subclass
Patient 1 (baseline no follow-up)
Teased fibers Negative Positive 1100 IgG3
Contactin-1 0202 0465 nd nd Negative Negative
Caspr-1 0072 2961 1200 nm Positive Positive
Patient 2 (baseline)
Teased fibers Negative Positive 1200 nd
Contactin-1 0247 3538 1200 IgG2 Negative Positive
Caspr-1 0129 3965 11000 IgG2 Positive Positive
Patient 2 (follow-up)
Teased fibers nd Negative nd nd
Contactin-1 nd 0039 nd nd nd Negative
Caspr-1 nd 0025 nd nd nd Negative
Patient 3 (baseline no follow-up)
Teased fibers Negative Positive 130000 IgG4
Contactin-1 0516 4492 140000 IgG42 Positive Positive
Caspr-1 nd 0073 nd nd Negative Negative
Patient 4 (baseline)
Teased fibers Negative Positive 1500 IgG3
Contactin-1 0267 2294 1200 IgG3 Negative Positive
Caspr-1 nd 3011 1500 IgG32 Positive Positive
Patient 4 (follow-up)
Teased fibers Negative Positive 1500 IgG4
Contactin-1 0227 0206 nd nd Negative Negative
Caspr-1 0138 3618 1500 IgG24 Positive Positive
Continued
Neurolo
gyorgN
NNeurologyN
euroim
munology
ampNeuroinflam
mation
|Volum
e7N
umber
5|
Septem
ber2020
5
Table 1 Summary of the autoantibody status of seropositive patients 1ndash5 at baseline and follow-up (continued)
CSF Serum
Teased fibers and ELISA Preabsorption CBA
Paranodal bindingOD Titer IgG subclass
Patient 5 (baseline)
Teased fibers Positive Positive 1100 IgG3
Contactin-1 0219 0260 nd nd Negative Negative
Caspr-1 0136 nd nd nd Positive Positive
Patient 5 (first follow-up)
Teased fibers Positive Positive 1100 IgG3
Contactin-1 0236 0243 nd nd Negative Negative
Caspr-1 0364 3802 1200 IgG32 Positive Positive
Patient 5 (second follow-up)
Teased fibers nd Positive 150 nm
Contactin-1 nd 0168 nd nd nd Negative
Caspr-1 nd 0013 nd nd nd Negative
Abbreviations A-CIDP = acute-onset chronic inflammatory demyelinating polyradiculoneuropathy Caspr-1 = contactin-1ndashassociated protein-1 CBA = cell-based assay GBS = Guillain-Barre syndrome nd = not done nd =not done due to lack of material nm = not measurable OD = optical densityResults of teased fibers assays are displayed in the first row for each patient contactin-1 and Caspr-1-specific results are displayed in lines 2 and 3 ODs above the threshold and results considered positive are highlighted inbold letters There is a 100 concordance regarding target specificity in the teased fibers assay ELISA and CBA but minor differences in IgG titer and coexisting subclass between the teased fibers assay and ELISA In case ofdouble positivity preabsorption assays only detect the target antigen with the higher antibody titer (see ELISA titer)
6NeurologyN
euroim
muno
logyampNeuroinflam
mation
|Vo
lume7N
umber
5|
Septem
ber
2020NeurologyorgN
N
specificity index) being in a normal range (see appendix e-1linkslwwcomNXIA275)
Clinical features of seropositive patientsFigure 4 schematically summarizes the clinical course relatedto the experimental results Appendix e-1 linkslwwcomNXIA275 summarizes clinical data of seropositive patients1ndash5 and provides a detailed description of the clinical diseasecourse in individuals The two seropositive patients withGBS were both middle-aged women presenting with severemotor but only mild sensory involvement Cranial nerveinvolvement autonomic symptoms and neuropathic painwere present in 12 patients with GBS only Both patientsrequired treatment on the intensive care unit (ICU) The
three patients with A-CIDP presented with moderate to severeparesis and multimodal sensory impairment at disease onsetDuring the course of disease all three patients with A-CIDPreported neuropathic pain requiring pregabalingabapentinor even opioid treatment Two of three patients withA-CIDP presented sensory ataxia Nerve conduction studiesshowed demyelinating features in all seropositive patientswith conduction block in 35 patients Sural nerve biopsywas performed in one patient (patient 5) six months after theonset and showed axonal loss without signs of demyelinationCSF analysis revealed cytoalbuminologic dissociation andblood-brain barrier dysfunction in all seropositive patientsThe patient with subacute-onset CIDP highly elevated CSFprotein and detection of antindashCaspr-1 in both serum and
Figure 2 Serum subclass analysis at baseline and at follow-up with IgG subclass switch
(A) Results of antindashcontactin-1 and antindashCaspr-1 ELISA with baseline sera and subclass-specific IgG1-4 autoantibodies (grayscale) are shown as of the OD ofthe total IgG (using anti-human IgG autoantibody) on y-axis In patient 1 ELISA IgG subclasses were not measurable ELISA revealed mainly IgG2 subclass inpatient 2 (GBS) IgG2IgG4 in patient 3 (A-CIDP) and IgG23 in patients 4 and 5 (A-CIDP) (B) Acute phase teased fibers subclass analysis with baseline serashowed binding to the paranodal regions (arrows) only when using IgG3-specific secondary antibody in patients 1 (GBS) and 4 (A-CIDP) visualized byphotomicrographs of single immunofluorescence staining on teased fibers (C) AntindashCaspr-1 IgG subclass ELISA with follow-up serum of patient 4 nowrevealed IgG 24 subclass Photomicrographs show immunofluorescence staining of murine teased fibers with subclass-specific IgG3 and IgG4 autoanti-bodies at follow-up Paranodal binding disappeared using IgG3 antibody but occurred using IgG4 subclass-specific antibody proving subclass switch inpatient 4 Scale bar = 10 μm A-CIDP = acute-onset chronic inflammatory demyelinating polyradiculoneuropathy GBS = Guillain-Barre syndrome
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 7
CSF (patient 5) developed action tremor during the courseof disease
Antibody status is concordant with diseaseactivity and therapy responseOne of two patients with GBS (patient 2) received IVIgwhich did not lead to amelioration This patient was IgG2 gtIgG3 seropositive (patient 2 figure 2A) Both patients withGBS responded well to plasma exchange The two patientswith A-CIDP and IgG3 autoantibodies (patients 4 and 5)showed good response to IVIg treatment in the acute phase ofdisease Ongoing IVIg treatment led to further improvementof symptoms and was stopped after 25 cycles in patient 5because of a remission of symptoms In the other patient withA-CIDP and antindashcontactin-1Caspr-1 IgG3 at disease onset(patient 4) IVIg showed a good therapeutic effect in the acutephase and led to remission not requiring further therapy Inthe chronic phase IVIg was initiated again and stopped after26 cycles because of a loss of the therapeutic effect At thatstage the autoantibody subclass had already switched fromIgG3 to IgG4 (for detailed treatment response in the course ofdisease see appendix e-1 linkslwwcomNXIA275) Thepatient with A-CIDP and high-titer antindashcontactin-1 IgG4autoantibodies (patient 3) had to be treated on ICU becauseof the severity of symptoms developed renal dysfunction didnot respond to IVIg treatment and did not ameliorate duringthe course of disease
DiscussionWedetected antiparanodal autoantibodies in 43of patientswithacute to subacute immune-mediated neuropathy more frequentlyin CIDP with acute to subacute onset than in GBS We foundIgG23 in monophasic GBS which supports the notion of pre-vious studies that IgG3 is the predominant subclass inmonophasicdisease7 By analyzing sera from different time points we couldshow that the antibody status was concordant with the diseaseseverity Therefore our data support pathogenicity of autoanti-bodies and suggest that autoantibody status and titer are validindicators for disease activity as proposed in previous studies onparanodopathy and further IgG4-related neurologic diseases25ndash27
In one patient we could demonstrate a subclass switch fromIgG3 in acute disease to IgG4 in chronic disease a finding thatwe had hypothesized in a previous study6 In other autoimmunediseases eg in membranous glomerulonephritis subclassswitch of anti-PLA2R autoantibodies from IgG1 to IgG4 alsoassociates with progression to chronic disease28 According toa linear model of autoantibody response unspecific IgM re-sponse is followed by IgG isotype produced by antigen-experi-enced B cells These IgG are of the IgG3 subclass andsequentially switch to IgG1 2 and 42930 Concurrently auto-antibodies strongly gain antigen affinity with IgG4 showing thehighest affinity to its target3132 Disease progression in para-nodopathy might therefore depend on IgG subclass properties
Figure 3 Antiparanodal IgG autoantibodies at follow-up assessment
(A) Contactin-1 IgG ELISA of all patients at first assessment of 66161 patients at follow-up and of 40 healthy controls Optical density (OD) at 450 nm isdisplayed on the y-axis The threshold for positive results is set at 5 SDs above the mean of controls (0615) Patients 2ndash4 show positive results at firstassessment The other patients and controls show values below the threshold Sera of patients 2 4 and 5 are negative in antindashcontactin-1 IgG ELISA at follow-up as well as 63 other follow-up sera Patients 1 and 3were lost to follow-up (B) Overlay photomicrographs of teased fibers double immunofluorescence and(C) contactin-1ndash and Caspr-1 (CNTNAP1)ndashtransfected HEK293 cells show loss of antindashcontactin-1Caspr-1 autoantibodies in patient 2 (GBS) at follow-up (BaCa Cd) but colocalization of commercial antindashCaspr-1 antibody and sera at paranodes in patients 4 and 5 with A-CIDP (Bb Bc arrows) Both sera bind toCaspr-1 (CNTNAP1)ndashtransfected HEK293 cells (Ce Cf arrowheads) but not to contactin-1ndashtransfected cells (Cb Cc) Scale bar = 10 μm A-CIDP = acute-onsetchronic inflammatory demyelinating polyradiculoneuropathy GBS = Guillain-Barre syndrome HEK = human embryonic kidney
8 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
and affinities Gain of specificity and affinity at switch from IgG3to IgG4 might explain different binding characteristics of anti-paranodal autoantibodies in the patient whose IgG3 autoanti-bodies reacted both against contactin-1Caspr-1 epitopes butwhose IgG4 autoantibodies were specific to Caspr-1 Here wereport IgG24 (1) in patient 4 in the chronic phase after subclassswitch and (2) in another patient at disease onset showingnonameliorating disease with high disability similar to previousreports on antindashcontactin-1 IgG4-related disease111333 So farstudies have only reported antiparanodal IgG4 autoantibodies inpatients with chronic disease7113435 We therefore suggest thathigh-affinity IgG4 autoantibody binding leads to chronic diseasewith functional impairment possibly caused by disturbance of theantigen interaction paranodal architecture and paranodalcomplex formation as demonstrated previously68ndash11
Patients of our study with predominant IgG3 subclass showedgood response to IVIg whereas patients with IgG2 and IgG4initially did not respond to IVIg or lost responsiveness in thedisease course Several studies described good responses toIVIg in IgG3-associated paranodopathy but poor responses inIgG4-associated disease56111334 IgG3 can initiate comple-ment activation opsonization antigen cross-linking and in-ternalization whereas IgG2 shows little C1q binding and IgG4completely lacks these Fc-mediated effector functions31 In anin vivo study on antindashcontactin-1 pathogenicity we suggestedcomplement-mediated functional impairment at the paranodesas the pathophysiologic correlate in IgG3-mediated para-nodopathy earlier and could show in vitro that IVIg inhibitscomplement deposition and activation dose-dependently1415
Our data therefore support the hypothesis that therapy re-sponsiveness might depend on IgG subclass and subclass-related pathomechanism of antiparanodal autoantibodies If
validated in larger studies these findings might have a directimpact on the treatment regime in affected patients Testing ofautoantibody subclass during the course of disease may beuseful as an indicator of a positive response to IVIg in case ofIgG3 whereas IgG4-related paranodopathy shows very goodresponse to rituximab46711
The trigger of the autoantibody production and the patho-mechanism behind possible IgG switching are still unknownThree of five seropositive patients in this study had diabetesmellitus type 2 as a comorbidity diagnosed years before theonset of disease Previous studies report disruption of the blood-brain barrier and the nodal architecture in patients with diabetesmellitus thereby possibly exposing paranodal antigens to theimmune response3637 A recent study has identified humanleukocyte antigen (HLA)-DRB15 as a risk factor for CIDPassociated with anti-NF155 antibodies and another studyreported different HLA antibody patterns with subclass switchafter renal transplantation3839 Whether interindividual differ-ences in antibody production and switching during the course ofdisease depend on (1) HLA alleles (2) antigen exposure or (3)further immunologic mechanisms has to be investigated infurther studies
Regarding characteristic clinical features of paranodopathy wereport neuropathic pain in antindashCaspr-1ndashrelated disease aspreviously described in patients with higher autoantibody tit-ers7 Different titers might explain discrepancies between ourresults and a recent study that did not report pain in antindashCaspr-1ndashpositive patients711 In our patients further features of IgG4-related chronic paranodopathy such as tremor sensory ataxiaand nephropathy561340 were only present in the course ofdisease suggesting that they evolve due to chronic autoantibody
Figure 4 Scheme of the autoantibody status in the course of disease in seropositive patients
The course of disease is shown on the x-axis by pseudo-logarithmic display of the time course Time point 0 is set at baseline assessment of serumCSF Thecolor code indicates severity of symptoms Results of serum assessment are displayed in black Patients 1 and 3 were lost to follow-up
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 9
exposure The pathomechanistic correlate of action tremorwhich recently has also been described in antindashCaspr-1 IgG3-related paranodopathy11 is still under research but studiessuggest cerebellar origin5612 In this study we detected in-trathecal autoantibodies in a patient with tremor as previouslyreported in a case of antindashpan-neurofascinndashpositive para-nodopathy12 The antiparanodal autoantibodies most probablydiffuse into the intrathecal compartment due to a blood-brainbarrier dysfunction as indicated by normal IgG and ASI indicesin our patients Our CSF data suggest that detection of anti-paranodal antibodies in CSF shows little sensitivity and dependson either high titer or strong leakage of the blood-brain barrierWe therefore encourage further studies on intrathecal anti-paranodal autoantibodies targeting sensitivity pathogenicityand association to clinical symptoms
Low prevalence of autoantibodies against paranodal proteins inacute inflammatory neuropathy retrospective assessment and thelow number of seropositive patients available for follow-up limitour study Clinical characteristics at acute onset and subclass-related seropositivity as a possible risk factor for chronic pro-gression when detected at the onset should be investigated in largemulticentric studies considering lowoverall prevalence ofGBS andA-CIDP as well as further possible confounders in analysis ofparanodopathy-specific clinical symptoms eg coinciding painfuldiabetic neuropathy The question of sequential subclass switchingin paranodopathy needs to be addressed in larger prospectivestudies Nevertheless our data (1) propose autoantibody subclassscreening to be considered in patients with GBS and A-CIDP asresults might have direct implications on diagnosis and therapeuticregime during the course of disease and (2) pave the way forfurther clinical and pathomechanistic studies on antiparanodalautoantibodies in acute to subacute immune-mediated neuropathy
AcknowledgmentThe authors thank Barbara Reuter and Antonia Kohl for theirsuperb technical assistance They thankMaximilian Frommer forprovision and processing of clinical data and PD Dr JakobMatschke for provision of slides and images of supplementarymorphologic data The study was supported by a grant of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg to KD and CV and a grant of the GermanResearch Foundation to KD (DFG DO-22191-1) LA issupported by a research fellowship as a clinician scientist of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg FL receives funding from the GermanMinistry of Education and Research (BMBF 01GM1908A) andthe German Research Foundation (DFG LE30642-1) CSreceives funding from the German Research Foundation (DFG3289-1 DFG UE 1714-1 DFG SO 32810-1 DFG SFB1158 and DFG SO 328 131) from the German Ministry ofEducation and Research (BMBF CMT-Net PI) and from theEuropean Union (Horizon 2020)
Study fundingThis study was supported by the Open Access PublicationFund of the University of Wurzburg
DisclosureL Appeltshauser K-P Wandinger R Junker C Sommer FLeypoldt and K Doppler work for an academic institutionoffering commercial antibody diagnostics AM Brunder AHeinius P Kortvelyessy and C Villmann report no dis-closures relevant to the manuscript C Sommer has served onscientific advisory boards for Algiax Alnylam Air LiquideAkcea Astellas Bayer Grifols Takeda and UCB She reportsbeing a member of speakersrsquo bureau and receiving speakerhonoraria from Akcea Alnylam Novartis Pfizer Sanofi-Aventis and Teva C Sommer served as a journal editorassociate editor or editorial advisory board member for theEuropean Journal of Neurology PLoS ONE and PAIN ReportsF Leypoldt reports speaker honoraria from Bayer RocheNovartis and Fresenius travel funding from Merck Grifolsand Bayer and serving on advisory boards for Roche Biogenand Alexion Go to NeurologyorgNN for full disclosures
Publication historyReceived by Neurology Neuroimmunology amp NeuroinflammationMarch 2 2020 Accepted in final form May 19 2020
Appendix Authors
Name Location Contribution
LuiseAppeltshauserMD
University Hospital ofWurzburg
Major role in the acquisitionanalysis and interpretationof the data and drafting andrevising the manuscript forintellectual content
Anna-MichelleBrunder
University Hospital ofWurzburg
Major role in the acquisitionand analysis of the data
Annika Heinius University Hospital ofSchleswig-HolsteinCampus Kiel
Major role in the acquisitionand analysis of the data
PeterKortvelyessyMD
University Hospital ofMagdeburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Klaus-PeterWandinger MD
University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Ralf Junker MD University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
CarmenVillmann PhD
University Hospital ofWurzburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
ClaudiaSommer MD
University Hospital ofWurzburg
Conceptualization of thestudy revising themanuscript for intellectualcontent and major role ininterpretation of data
Frank LeypoldtMD
University Hospital ofSchleswig-HolsteinCampus Kiel
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy and major role ininterpretation of data
10 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
References1 Fehmi J Scherer SS Willison HJ Rinaldi S Nodes paranodes and neuropathies
J Neurol Neurosurg Psychiatry 20188961ndash712 Uncini A Vallat JM Autoimmune nodo-paranodopathies of peripheral nerve the
concept is gaining ground J Neurol Neurosurg Psychiatry 201889627ndash6353 Querol L Illa I Paranodal and other autoantibodies in chronic inflammatory neu-
ropathies Curr Opin Neurol 201528474ndash4794 Querol L Rojas-Garcia R Diaz-Manera J et al Rituximab in treatment-resistant
CIDP with antibodies against paranodal proteins Neurol Neuroimmunol Neuro-inflamm 20152e149 doi 101212NXI0000000000000149
5 Querol L Nogales-Gadea G Rojas-Garcia R et al Neurofascin IgG4 antibodies inCIDP associate with disabling tremor and poor response to IVIg Neurology 201482879ndash886
6 Doppler K Appeltshauser L Wilhelmi K et al Destruction of paranodal architecturein inflammatory neuropathy with anti-contactin-1 autoantibodies J Neurol Neuro-surg Psychiatry 201586720ndash728
7 Doppler K Appeltshauser L Villmann C et al Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathyBrain 20161392617ndash2630
8 Manso C Querol L Mekaouche M Illa I Devaux JJ Contactin-1 IgG4 antibodiescause paranode dismantling and conduction defects Brain 20161391700ndash1712
9 Manso C Querol L Lleixa C et al Anti-Neurofascin-155 IgG4 antibodies preventparanodal complex formation in vivo J Clin Invest 20191292222ndash2236
10 Labasque M Hivert B Nogales-Gadea G Querol L Illa I Faivre-Sarrailh C Specificcontactin N-glycans are implicated in neurofascin binding and autoimmune targetingin peripheral neuropathies J Biol Chem 20142897907ndash7918
11 Cortese A Lombardi R Briani C et al Antibodies to neurofascin contactin-1 andcontactin-associated protein 1 in CIDP clinical relevance of IgG isotype NeurolNeuroimmunol Neuroinflamm 20197e639 doi 101212NXI0000000000000639
12 Stengel H Vural A Brunder AM et al Anti-pan-neurofascin IgG3 as a marker offulminant autoimmune neuropathy Neurol Neuroimmunol Neuroinflamm 20196e603 doi 101212NXI0000000000000603
13 Miura Y Devaux JJ Fukami Y et al Contactin 1 IgG4 associates to chronic inflammatorydemyelinating polyneuropathy with sensory ataxia Brain 20151381484ndash1491
14 Appeltshauser L Weishaupt A Sommer C Doppler K Complement depositioninduced by binding of anti-contactin-1 auto-antibodies is modified by immunoglo-bulins Exp Neurol 201728784ndash90
15 Doppler K Schuster Y Appeltshauser L et al Anti-CNTN1 IgG3 induces acuteconduction block and motor deficits in a passive transfer rat model J Neuro-inflammation 20191673
16 Sejvar JJ Kohl KS Gidudu J et al Guillain-Barre syndrome and Fisher syndrome casedefinitions and guidelines for collection analysis and presentation of immunizationsafety data Vaccine 201129599ndash612
17 Fokke C van den Berg B Drenthen J Walgaard C van Doorn PA Jacobs BCDiagnosis of Guillain-Barre syndrome and validation of Brighton criteria Brain 201413733ndash43
18 Kuitwaard K van Koningsveld R Ruts L Jacobs BC van Doorn PA RecurrentGuillain-Barre syndrome J Neurol Neurosurg Psychiatry 20098056ndash59
19 Van den Bergh PY Hadden RD Bouche P et al European Federation of NeurologicalSocietiesPeripheral Nerve Society guideline on management of chronic in-flammatory demyelinating polyradiculoneuropathy report of a joint task force of theEuropean Federation of Neurological Societies and the Peripheral Nerve Societymdashfirst revision Eur J Neurol 201017356ndash363
20 Ruts L Drenthen J Jacobs BC van Doorn PA Distinguishing acute-onset CIDP fromfluctuating Guillain-Barre syndrome a prospective study Neurology 2010741680ndash1686
21 McCombe PA Pollard JD McLeod JG Chronic inflammatory demyelinating poly-radiculoneuropathy A clinical and electrophysiological study of 92 cases Brain 19871101617ndash1630
22 Alessandro L Pastor Rueda JM Wilken M et al Differences between acute-onsetchronic inflammatory demyelinating polyneuropathy and acute inflammatory de-myelinating polyneuropathy in adult patients J Peripher Nerv Syst 201823154ndash158
23 Doppler K Appeltshauser L Kramer HH et al Contactin-1 and neurofascin-155-186 are not targets of auto-antibodies in multifocal motor neuropathy PLoS One201510e0134274
24 Ng JK Malotka J Kawakami N et al Neurofascin as a target for autoantibodies inperipheral neuropathies Neurology 2012792241ndash2248
25 Fujita A Ogata H Yamasaki RMatsushita T Kira JI Parallel fluctuation of anti-neurofascin155 antibody levels with clinico-electrophysiological findings in patients with chronic in-flammatory demyelinating polyradiculoneuropathy J Neurol Sci 2018384107ndash112
26 Niks EH van Leeuwen Y Leite MI et al Clinical fluctuations in MuSK myastheniagravis are related to antigen-specific IgG4 instead of IgG1 J Neuroimmunol 2008195151ndash156
27 Huijbers MG Vink AF Niks EH et al Longitudinal epitope mapping in MuSKmyasthenia gravis implications for disease severity J Neuroimmunol 201629182ndash88
28 Huang CC Lehman A Albawardi A et al IgG subclass staining in renal biopsies withmembranous glomerulonephritis indicates subclass switch during disease progressionMod Pathol 201326799ndash805
29 Collins AM Jackson KJ A temporal model of human IgE and IgG antibody functionFront Immunol 20134235
30 Valenzuela NM Schaub S The Biology of IgG subclasses and their clinical relevanceto transplantation Transplantation 2018102S7ndashS13
31 Vidarsson G Dekkers G Rispens T IgG subclasses and allotypes from structure toeffector functions Front Immunol 20145520ndash532
32 Bruhns P Iannascoli B England P et al Specificity and affinity of human Fcgamma receptorsand their polymorphic variants for human IgG subclasses Blood 20091133716ndash3725
33 Querol L Nogales-Gadea G Rojas-Garcia R et al Antibodies to contactin-1 inchronic inflammatory demyelinating polyneuropathy Ann Neurol 201373370ndash380
34 Devaux JJ Miura Y Fukami Y et al Neurofascin-155 IgG4 in chronic inflammatorydemyelinating polyneuropathy Neurology 201686800ndash807
35 Ogata H Yamasaki R Hiwatashi A et al Characterization of IgG4 anti-neurofascin155 antibody-positive polyneuropathy Ann Clin Transl Neurol 20152960ndash971
36 Doppler K Frank F Koschker AC Reiners K Sommer C Nodes of Ranvier in skinbiopsies of patients with diabetes mellitus J Peripher Nerv Syst 201722182ndash190
37 Banks W The blood-brain barrier interface in diabetes mellitus dysfunctionsmechanisms and approaches to treatment Curr Pharm Des 2020261438ndash1447
38 Arnold ML Ntokou IS Doxiadis II Spriewald BM Boletis JN Iniotaki AG Donor-specific HLA antibodies evaluating the risk for graft loss in renal transplant recipientswith isotype switch from complement fixing IgG1IgG3 to noncomplement fixingIgG2IgG4 anti-HLA alloantibodies Transpl Int 201427253ndash261
39 Martinez-Martinez L Lleixa MC Boera-Carnicero G et al Anti-NF155 chronic in-flammatory demyelinating polyradiculoneuropathy strongly associates to HLA-DRB15 J Neuroinflammation 201714224
40 Hashimoto Y Ogata H Yamasaki R et al Chronic inflammatory demyelinatingpolyneuropathy with concurrent membranous nephropathy an anti-paranode andpodocyte protein antibody study and literature survey Front Neurol 20189997
Appendix (continued)
Name Location Contribution
KathrinDoppler MD
University Hospital ofWurzburg
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy major role in analysisand interpretation of dataand study supervision
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 11
DOI 101212NXI000000000000081720207 Neurol Neuroimmunol Neuroinflamm
Luise Appeltshauser Anna-Michelle Brunder Annika Heinius et al Antiparanodal antibodies and IgG subclasses in acute autoimmune neuropathy
This information is current as of July 24 2020
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm
ServicesUpdated Information amp
httpnnneurologyorgcontent75e817fullhtmlincluding high resolution figures can be found at
References httpnnneurologyorgcontent75e817fullhtmlref-list-1
This article cites 40 articles 9 of which you can access for free at
Citations httpnnneurologyorgcontent75e817fullhtmlotherarticles
This article has been cited by 1 HighWire-hosted articles
Subspecialty Collections
httpnnneurologyorgcgicollectionperipheral_neuropathyPeripheral neuropathy
httpnnneurologyorgcgicollectionguillainbarre_syndromeGuillain-Barre syndrome
nating_polyneuropathyhttpnnneurologyorgcgicollectionchronic_inflammatory_demyeliChronic inflammatory demyelinating polyneuropathy
httpnnneurologyorgcgicollectionautoimmune_diseasesAutoimmune diseases
httpnnneurologyorgcgicollectionall_neuromuscular_diseaseAll Neuromuscular Diseasefollowing collection(s) This article along with others on similar topics appears in the
Permissions amp Licensing
httpnnneurologyorgmiscaboutxhtmlpermissionsits entirety can be found online atInformation about reproducing this article in parts (figurestables) or in
Reprints
httpnnneurologyorgmiscaddirxhtmlreprintsusInformation about ordering reprints can be found online
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm

Table 1 Summary of the autoantibody status of seropositive patients 1ndash5 at baseline and follow-up
CSF Serum
Teased fibers and ELISA Preabsorption CBA
Paranodal bindingOD Titer IgG subclass
Patient 1 (baseline no follow-up)
Teased fibers Negative Positive 1100 IgG3
Contactin-1 0202 0465 nd nd Negative Negative
Caspr-1 0072 2961 1200 nm Positive Positive
Patient 2 (baseline)
Teased fibers Negative Positive 1200 nd
Contactin-1 0247 3538 1200 IgG2 Negative Positive
Caspr-1 0129 3965 11000 IgG2 Positive Positive
Patient 2 (follow-up)
Teased fibers nd Negative nd nd
Contactin-1 nd 0039 nd nd nd Negative
Caspr-1 nd 0025 nd nd nd Negative
Patient 3 (baseline no follow-up)
Teased fibers Negative Positive 130000 IgG4
Contactin-1 0516 4492 140000 IgG42 Positive Positive
Caspr-1 nd 0073 nd nd Negative Negative
Patient 4 (baseline)
Teased fibers Negative Positive 1500 IgG3
Contactin-1 0267 2294 1200 IgG3 Negative Positive
Caspr-1 nd 3011 1500 IgG32 Positive Positive
Patient 4 (follow-up)
Teased fibers Negative Positive 1500 IgG4
Contactin-1 0227 0206 nd nd Negative Negative
Caspr-1 0138 3618 1500 IgG24 Positive Positive
Continued
Neurolo
gyorgN
NNeurologyN
euroim
munology
ampNeuroinflam
mation
|Volum
e7N
umber
5|
Septem
ber2020
5
Table 1 Summary of the autoantibody status of seropositive patients 1ndash5 at baseline and follow-up (continued)
CSF Serum
Teased fibers and ELISA Preabsorption CBA
Paranodal bindingOD Titer IgG subclass
Patient 5 (baseline)
Teased fibers Positive Positive 1100 IgG3
Contactin-1 0219 0260 nd nd Negative Negative
Caspr-1 0136 nd nd nd Positive Positive
Patient 5 (first follow-up)
Teased fibers Positive Positive 1100 IgG3
Contactin-1 0236 0243 nd nd Negative Negative
Caspr-1 0364 3802 1200 IgG32 Positive Positive
Patient 5 (second follow-up)
Teased fibers nd Positive 150 nm
Contactin-1 nd 0168 nd nd nd Negative
Caspr-1 nd 0013 nd nd nd Negative
Abbreviations A-CIDP = acute-onset chronic inflammatory demyelinating polyradiculoneuropathy Caspr-1 = contactin-1ndashassociated protein-1 CBA = cell-based assay GBS = Guillain-Barre syndrome nd = not done nd =not done due to lack of material nm = not measurable OD = optical densityResults of teased fibers assays are displayed in the first row for each patient contactin-1 and Caspr-1-specific results are displayed in lines 2 and 3 ODs above the threshold and results considered positive are highlighted inbold letters There is a 100 concordance regarding target specificity in the teased fibers assay ELISA and CBA but minor differences in IgG titer and coexisting subclass between the teased fibers assay and ELISA In case ofdouble positivity preabsorption assays only detect the target antigen with the higher antibody titer (see ELISA titer)
6NeurologyN
euroim
muno
logyampNeuroinflam
mation
|Vo
lume7N
umber
5|
Septem
ber
2020NeurologyorgN
N
specificity index) being in a normal range (see appendix e-1linkslwwcomNXIA275)
Clinical features of seropositive patientsFigure 4 schematically summarizes the clinical course relatedto the experimental results Appendix e-1 linkslwwcomNXIA275 summarizes clinical data of seropositive patients1ndash5 and provides a detailed description of the clinical diseasecourse in individuals The two seropositive patients withGBS were both middle-aged women presenting with severemotor but only mild sensory involvement Cranial nerveinvolvement autonomic symptoms and neuropathic painwere present in 12 patients with GBS only Both patientsrequired treatment on the intensive care unit (ICU) The
three patients with A-CIDP presented with moderate to severeparesis and multimodal sensory impairment at disease onsetDuring the course of disease all three patients with A-CIDPreported neuropathic pain requiring pregabalingabapentinor even opioid treatment Two of three patients withA-CIDP presented sensory ataxia Nerve conduction studiesshowed demyelinating features in all seropositive patientswith conduction block in 35 patients Sural nerve biopsywas performed in one patient (patient 5) six months after theonset and showed axonal loss without signs of demyelinationCSF analysis revealed cytoalbuminologic dissociation andblood-brain barrier dysfunction in all seropositive patientsThe patient with subacute-onset CIDP highly elevated CSFprotein and detection of antindashCaspr-1 in both serum and
Figure 2 Serum subclass analysis at baseline and at follow-up with IgG subclass switch
(A) Results of antindashcontactin-1 and antindashCaspr-1 ELISA with baseline sera and subclass-specific IgG1-4 autoantibodies (grayscale) are shown as of the OD ofthe total IgG (using anti-human IgG autoantibody) on y-axis In patient 1 ELISA IgG subclasses were not measurable ELISA revealed mainly IgG2 subclass inpatient 2 (GBS) IgG2IgG4 in patient 3 (A-CIDP) and IgG23 in patients 4 and 5 (A-CIDP) (B) Acute phase teased fibers subclass analysis with baseline serashowed binding to the paranodal regions (arrows) only when using IgG3-specific secondary antibody in patients 1 (GBS) and 4 (A-CIDP) visualized byphotomicrographs of single immunofluorescence staining on teased fibers (C) AntindashCaspr-1 IgG subclass ELISA with follow-up serum of patient 4 nowrevealed IgG 24 subclass Photomicrographs show immunofluorescence staining of murine teased fibers with subclass-specific IgG3 and IgG4 autoanti-bodies at follow-up Paranodal binding disappeared using IgG3 antibody but occurred using IgG4 subclass-specific antibody proving subclass switch inpatient 4 Scale bar = 10 μm A-CIDP = acute-onset chronic inflammatory demyelinating polyradiculoneuropathy GBS = Guillain-Barre syndrome
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 7
CSF (patient 5) developed action tremor during the courseof disease
Antibody status is concordant with diseaseactivity and therapy responseOne of two patients with GBS (patient 2) received IVIgwhich did not lead to amelioration This patient was IgG2 gtIgG3 seropositive (patient 2 figure 2A) Both patients withGBS responded well to plasma exchange The two patientswith A-CIDP and IgG3 autoantibodies (patients 4 and 5)showed good response to IVIg treatment in the acute phase ofdisease Ongoing IVIg treatment led to further improvementof symptoms and was stopped after 25 cycles in patient 5because of a remission of symptoms In the other patient withA-CIDP and antindashcontactin-1Caspr-1 IgG3 at disease onset(patient 4) IVIg showed a good therapeutic effect in the acutephase and led to remission not requiring further therapy Inthe chronic phase IVIg was initiated again and stopped after26 cycles because of a loss of the therapeutic effect At thatstage the autoantibody subclass had already switched fromIgG3 to IgG4 (for detailed treatment response in the course ofdisease see appendix e-1 linkslwwcomNXIA275) Thepatient with A-CIDP and high-titer antindashcontactin-1 IgG4autoantibodies (patient 3) had to be treated on ICU becauseof the severity of symptoms developed renal dysfunction didnot respond to IVIg treatment and did not ameliorate duringthe course of disease
DiscussionWedetected antiparanodal autoantibodies in 43of patientswithacute to subacute immune-mediated neuropathy more frequentlyin CIDP with acute to subacute onset than in GBS We foundIgG23 in monophasic GBS which supports the notion of pre-vious studies that IgG3 is the predominant subclass inmonophasicdisease7 By analyzing sera from different time points we couldshow that the antibody status was concordant with the diseaseseverity Therefore our data support pathogenicity of autoanti-bodies and suggest that autoantibody status and titer are validindicators for disease activity as proposed in previous studies onparanodopathy and further IgG4-related neurologic diseases25ndash27
In one patient we could demonstrate a subclass switch fromIgG3 in acute disease to IgG4 in chronic disease a finding thatwe had hypothesized in a previous study6 In other autoimmunediseases eg in membranous glomerulonephritis subclassswitch of anti-PLA2R autoantibodies from IgG1 to IgG4 alsoassociates with progression to chronic disease28 According toa linear model of autoantibody response unspecific IgM re-sponse is followed by IgG isotype produced by antigen-experi-enced B cells These IgG are of the IgG3 subclass andsequentially switch to IgG1 2 and 42930 Concurrently auto-antibodies strongly gain antigen affinity with IgG4 showing thehighest affinity to its target3132 Disease progression in para-nodopathy might therefore depend on IgG subclass properties
Figure 3 Antiparanodal IgG autoantibodies at follow-up assessment
(A) Contactin-1 IgG ELISA of all patients at first assessment of 66161 patients at follow-up and of 40 healthy controls Optical density (OD) at 450 nm isdisplayed on the y-axis The threshold for positive results is set at 5 SDs above the mean of controls (0615) Patients 2ndash4 show positive results at firstassessment The other patients and controls show values below the threshold Sera of patients 2 4 and 5 are negative in antindashcontactin-1 IgG ELISA at follow-up as well as 63 other follow-up sera Patients 1 and 3were lost to follow-up (B) Overlay photomicrographs of teased fibers double immunofluorescence and(C) contactin-1ndash and Caspr-1 (CNTNAP1)ndashtransfected HEK293 cells show loss of antindashcontactin-1Caspr-1 autoantibodies in patient 2 (GBS) at follow-up (BaCa Cd) but colocalization of commercial antindashCaspr-1 antibody and sera at paranodes in patients 4 and 5 with A-CIDP (Bb Bc arrows) Both sera bind toCaspr-1 (CNTNAP1)ndashtransfected HEK293 cells (Ce Cf arrowheads) but not to contactin-1ndashtransfected cells (Cb Cc) Scale bar = 10 μm A-CIDP = acute-onsetchronic inflammatory demyelinating polyradiculoneuropathy GBS = Guillain-Barre syndrome HEK = human embryonic kidney
8 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
and affinities Gain of specificity and affinity at switch from IgG3to IgG4 might explain different binding characteristics of anti-paranodal autoantibodies in the patient whose IgG3 autoanti-bodies reacted both against contactin-1Caspr-1 epitopes butwhose IgG4 autoantibodies were specific to Caspr-1 Here wereport IgG24 (1) in patient 4 in the chronic phase after subclassswitch and (2) in another patient at disease onset showingnonameliorating disease with high disability similar to previousreports on antindashcontactin-1 IgG4-related disease111333 So farstudies have only reported antiparanodal IgG4 autoantibodies inpatients with chronic disease7113435 We therefore suggest thathigh-affinity IgG4 autoantibody binding leads to chronic diseasewith functional impairment possibly caused by disturbance of theantigen interaction paranodal architecture and paranodalcomplex formation as demonstrated previously68ndash11
Patients of our study with predominant IgG3 subclass showedgood response to IVIg whereas patients with IgG2 and IgG4initially did not respond to IVIg or lost responsiveness in thedisease course Several studies described good responses toIVIg in IgG3-associated paranodopathy but poor responses inIgG4-associated disease56111334 IgG3 can initiate comple-ment activation opsonization antigen cross-linking and in-ternalization whereas IgG2 shows little C1q binding and IgG4completely lacks these Fc-mediated effector functions31 In anin vivo study on antindashcontactin-1 pathogenicity we suggestedcomplement-mediated functional impairment at the paranodesas the pathophysiologic correlate in IgG3-mediated para-nodopathy earlier and could show in vitro that IVIg inhibitscomplement deposition and activation dose-dependently1415
Our data therefore support the hypothesis that therapy re-sponsiveness might depend on IgG subclass and subclass-related pathomechanism of antiparanodal autoantibodies If
validated in larger studies these findings might have a directimpact on the treatment regime in affected patients Testing ofautoantibody subclass during the course of disease may beuseful as an indicator of a positive response to IVIg in case ofIgG3 whereas IgG4-related paranodopathy shows very goodresponse to rituximab46711
The trigger of the autoantibody production and the patho-mechanism behind possible IgG switching are still unknownThree of five seropositive patients in this study had diabetesmellitus type 2 as a comorbidity diagnosed years before theonset of disease Previous studies report disruption of the blood-brain barrier and the nodal architecture in patients with diabetesmellitus thereby possibly exposing paranodal antigens to theimmune response3637 A recent study has identified humanleukocyte antigen (HLA)-DRB15 as a risk factor for CIDPassociated with anti-NF155 antibodies and another studyreported different HLA antibody patterns with subclass switchafter renal transplantation3839 Whether interindividual differ-ences in antibody production and switching during the course ofdisease depend on (1) HLA alleles (2) antigen exposure or (3)further immunologic mechanisms has to be investigated infurther studies
Regarding characteristic clinical features of paranodopathy wereport neuropathic pain in antindashCaspr-1ndashrelated disease aspreviously described in patients with higher autoantibody tit-ers7 Different titers might explain discrepancies between ourresults and a recent study that did not report pain in antindashCaspr-1ndashpositive patients711 In our patients further features of IgG4-related chronic paranodopathy such as tremor sensory ataxiaand nephropathy561340 were only present in the course ofdisease suggesting that they evolve due to chronic autoantibody
Figure 4 Scheme of the autoantibody status in the course of disease in seropositive patients
The course of disease is shown on the x-axis by pseudo-logarithmic display of the time course Time point 0 is set at baseline assessment of serumCSF Thecolor code indicates severity of symptoms Results of serum assessment are displayed in black Patients 1 and 3 were lost to follow-up
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 9
exposure The pathomechanistic correlate of action tremorwhich recently has also been described in antindashCaspr-1 IgG3-related paranodopathy11 is still under research but studiessuggest cerebellar origin5612 In this study we detected in-trathecal autoantibodies in a patient with tremor as previouslyreported in a case of antindashpan-neurofascinndashpositive para-nodopathy12 The antiparanodal autoantibodies most probablydiffuse into the intrathecal compartment due to a blood-brainbarrier dysfunction as indicated by normal IgG and ASI indicesin our patients Our CSF data suggest that detection of anti-paranodal antibodies in CSF shows little sensitivity and dependson either high titer or strong leakage of the blood-brain barrierWe therefore encourage further studies on intrathecal anti-paranodal autoantibodies targeting sensitivity pathogenicityand association to clinical symptoms
Low prevalence of autoantibodies against paranodal proteins inacute inflammatory neuropathy retrospective assessment and thelow number of seropositive patients available for follow-up limitour study Clinical characteristics at acute onset and subclass-related seropositivity as a possible risk factor for chronic pro-gression when detected at the onset should be investigated in largemulticentric studies considering lowoverall prevalence ofGBS andA-CIDP as well as further possible confounders in analysis ofparanodopathy-specific clinical symptoms eg coinciding painfuldiabetic neuropathy The question of sequential subclass switchingin paranodopathy needs to be addressed in larger prospectivestudies Nevertheless our data (1) propose autoantibody subclassscreening to be considered in patients with GBS and A-CIDP asresults might have direct implications on diagnosis and therapeuticregime during the course of disease and (2) pave the way forfurther clinical and pathomechanistic studies on antiparanodalautoantibodies in acute to subacute immune-mediated neuropathy
AcknowledgmentThe authors thank Barbara Reuter and Antonia Kohl for theirsuperb technical assistance They thankMaximilian Frommer forprovision and processing of clinical data and PD Dr JakobMatschke for provision of slides and images of supplementarymorphologic data The study was supported by a grant of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg to KD and CV and a grant of the GermanResearch Foundation to KD (DFG DO-22191-1) LA issupported by a research fellowship as a clinician scientist of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg FL receives funding from the GermanMinistry of Education and Research (BMBF 01GM1908A) andthe German Research Foundation (DFG LE30642-1) CSreceives funding from the German Research Foundation (DFG3289-1 DFG UE 1714-1 DFG SO 32810-1 DFG SFB1158 and DFG SO 328 131) from the German Ministry ofEducation and Research (BMBF CMT-Net PI) and from theEuropean Union (Horizon 2020)
Study fundingThis study was supported by the Open Access PublicationFund of the University of Wurzburg
DisclosureL Appeltshauser K-P Wandinger R Junker C Sommer FLeypoldt and K Doppler work for an academic institutionoffering commercial antibody diagnostics AM Brunder AHeinius P Kortvelyessy and C Villmann report no dis-closures relevant to the manuscript C Sommer has served onscientific advisory boards for Algiax Alnylam Air LiquideAkcea Astellas Bayer Grifols Takeda and UCB She reportsbeing a member of speakersrsquo bureau and receiving speakerhonoraria from Akcea Alnylam Novartis Pfizer Sanofi-Aventis and Teva C Sommer served as a journal editorassociate editor or editorial advisory board member for theEuropean Journal of Neurology PLoS ONE and PAIN ReportsF Leypoldt reports speaker honoraria from Bayer RocheNovartis and Fresenius travel funding from Merck Grifolsand Bayer and serving on advisory boards for Roche Biogenand Alexion Go to NeurologyorgNN for full disclosures
Publication historyReceived by Neurology Neuroimmunology amp NeuroinflammationMarch 2 2020 Accepted in final form May 19 2020
Appendix Authors
Name Location Contribution
LuiseAppeltshauserMD
University Hospital ofWurzburg
Major role in the acquisitionanalysis and interpretationof the data and drafting andrevising the manuscript forintellectual content
Anna-MichelleBrunder
University Hospital ofWurzburg
Major role in the acquisitionand analysis of the data
Annika Heinius University Hospital ofSchleswig-HolsteinCampus Kiel
Major role in the acquisitionand analysis of the data
PeterKortvelyessyMD
University Hospital ofMagdeburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Klaus-PeterWandinger MD
University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Ralf Junker MD University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
CarmenVillmann PhD
University Hospital ofWurzburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
ClaudiaSommer MD
University Hospital ofWurzburg
Conceptualization of thestudy revising themanuscript for intellectualcontent and major role ininterpretation of data
Frank LeypoldtMD
University Hospital ofSchleswig-HolsteinCampus Kiel
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy and major role ininterpretation of data
10 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
References1 Fehmi J Scherer SS Willison HJ Rinaldi S Nodes paranodes and neuropathies
J Neurol Neurosurg Psychiatry 20188961ndash712 Uncini A Vallat JM Autoimmune nodo-paranodopathies of peripheral nerve the
concept is gaining ground J Neurol Neurosurg Psychiatry 201889627ndash6353 Querol L Illa I Paranodal and other autoantibodies in chronic inflammatory neu-
ropathies Curr Opin Neurol 201528474ndash4794 Querol L Rojas-Garcia R Diaz-Manera J et al Rituximab in treatment-resistant
CIDP with antibodies against paranodal proteins Neurol Neuroimmunol Neuro-inflamm 20152e149 doi 101212NXI0000000000000149
5 Querol L Nogales-Gadea G Rojas-Garcia R et al Neurofascin IgG4 antibodies inCIDP associate with disabling tremor and poor response to IVIg Neurology 201482879ndash886
6 Doppler K Appeltshauser L Wilhelmi K et al Destruction of paranodal architecturein inflammatory neuropathy with anti-contactin-1 autoantibodies J Neurol Neuro-surg Psychiatry 201586720ndash728
7 Doppler K Appeltshauser L Villmann C et al Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathyBrain 20161392617ndash2630
8 Manso C Querol L Mekaouche M Illa I Devaux JJ Contactin-1 IgG4 antibodiescause paranode dismantling and conduction defects Brain 20161391700ndash1712
9 Manso C Querol L Lleixa C et al Anti-Neurofascin-155 IgG4 antibodies preventparanodal complex formation in vivo J Clin Invest 20191292222ndash2236
10 Labasque M Hivert B Nogales-Gadea G Querol L Illa I Faivre-Sarrailh C Specificcontactin N-glycans are implicated in neurofascin binding and autoimmune targetingin peripheral neuropathies J Biol Chem 20142897907ndash7918
11 Cortese A Lombardi R Briani C et al Antibodies to neurofascin contactin-1 andcontactin-associated protein 1 in CIDP clinical relevance of IgG isotype NeurolNeuroimmunol Neuroinflamm 20197e639 doi 101212NXI0000000000000639
12 Stengel H Vural A Brunder AM et al Anti-pan-neurofascin IgG3 as a marker offulminant autoimmune neuropathy Neurol Neuroimmunol Neuroinflamm 20196e603 doi 101212NXI0000000000000603
13 Miura Y Devaux JJ Fukami Y et al Contactin 1 IgG4 associates to chronic inflammatorydemyelinating polyneuropathy with sensory ataxia Brain 20151381484ndash1491
14 Appeltshauser L Weishaupt A Sommer C Doppler K Complement depositioninduced by binding of anti-contactin-1 auto-antibodies is modified by immunoglo-bulins Exp Neurol 201728784ndash90
15 Doppler K Schuster Y Appeltshauser L et al Anti-CNTN1 IgG3 induces acuteconduction block and motor deficits in a passive transfer rat model J Neuro-inflammation 20191673
16 Sejvar JJ Kohl KS Gidudu J et al Guillain-Barre syndrome and Fisher syndrome casedefinitions and guidelines for collection analysis and presentation of immunizationsafety data Vaccine 201129599ndash612
17 Fokke C van den Berg B Drenthen J Walgaard C van Doorn PA Jacobs BCDiagnosis of Guillain-Barre syndrome and validation of Brighton criteria Brain 201413733ndash43
18 Kuitwaard K van Koningsveld R Ruts L Jacobs BC van Doorn PA RecurrentGuillain-Barre syndrome J Neurol Neurosurg Psychiatry 20098056ndash59
19 Van den Bergh PY Hadden RD Bouche P et al European Federation of NeurologicalSocietiesPeripheral Nerve Society guideline on management of chronic in-flammatory demyelinating polyradiculoneuropathy report of a joint task force of theEuropean Federation of Neurological Societies and the Peripheral Nerve Societymdashfirst revision Eur J Neurol 201017356ndash363
20 Ruts L Drenthen J Jacobs BC van Doorn PA Distinguishing acute-onset CIDP fromfluctuating Guillain-Barre syndrome a prospective study Neurology 2010741680ndash1686
21 McCombe PA Pollard JD McLeod JG Chronic inflammatory demyelinating poly-radiculoneuropathy A clinical and electrophysiological study of 92 cases Brain 19871101617ndash1630
22 Alessandro L Pastor Rueda JM Wilken M et al Differences between acute-onsetchronic inflammatory demyelinating polyneuropathy and acute inflammatory de-myelinating polyneuropathy in adult patients J Peripher Nerv Syst 201823154ndash158
23 Doppler K Appeltshauser L Kramer HH et al Contactin-1 and neurofascin-155-186 are not targets of auto-antibodies in multifocal motor neuropathy PLoS One201510e0134274
24 Ng JK Malotka J Kawakami N et al Neurofascin as a target for autoantibodies inperipheral neuropathies Neurology 2012792241ndash2248
25 Fujita A Ogata H Yamasaki RMatsushita T Kira JI Parallel fluctuation of anti-neurofascin155 antibody levels with clinico-electrophysiological findings in patients with chronic in-flammatory demyelinating polyradiculoneuropathy J Neurol Sci 2018384107ndash112
26 Niks EH van Leeuwen Y Leite MI et al Clinical fluctuations in MuSK myastheniagravis are related to antigen-specific IgG4 instead of IgG1 J Neuroimmunol 2008195151ndash156
27 Huijbers MG Vink AF Niks EH et al Longitudinal epitope mapping in MuSKmyasthenia gravis implications for disease severity J Neuroimmunol 201629182ndash88
28 Huang CC Lehman A Albawardi A et al IgG subclass staining in renal biopsies withmembranous glomerulonephritis indicates subclass switch during disease progressionMod Pathol 201326799ndash805
29 Collins AM Jackson KJ A temporal model of human IgE and IgG antibody functionFront Immunol 20134235
30 Valenzuela NM Schaub S The Biology of IgG subclasses and their clinical relevanceto transplantation Transplantation 2018102S7ndashS13
31 Vidarsson G Dekkers G Rispens T IgG subclasses and allotypes from structure toeffector functions Front Immunol 20145520ndash532
32 Bruhns P Iannascoli B England P et al Specificity and affinity of human Fcgamma receptorsand their polymorphic variants for human IgG subclasses Blood 20091133716ndash3725
33 Querol L Nogales-Gadea G Rojas-Garcia R et al Antibodies to contactin-1 inchronic inflammatory demyelinating polyneuropathy Ann Neurol 201373370ndash380
34 Devaux JJ Miura Y Fukami Y et al Neurofascin-155 IgG4 in chronic inflammatorydemyelinating polyneuropathy Neurology 201686800ndash807
35 Ogata H Yamasaki R Hiwatashi A et al Characterization of IgG4 anti-neurofascin155 antibody-positive polyneuropathy Ann Clin Transl Neurol 20152960ndash971
36 Doppler K Frank F Koschker AC Reiners K Sommer C Nodes of Ranvier in skinbiopsies of patients with diabetes mellitus J Peripher Nerv Syst 201722182ndash190
37 Banks W The blood-brain barrier interface in diabetes mellitus dysfunctionsmechanisms and approaches to treatment Curr Pharm Des 2020261438ndash1447
38 Arnold ML Ntokou IS Doxiadis II Spriewald BM Boletis JN Iniotaki AG Donor-specific HLA antibodies evaluating the risk for graft loss in renal transplant recipientswith isotype switch from complement fixing IgG1IgG3 to noncomplement fixingIgG2IgG4 anti-HLA alloantibodies Transpl Int 201427253ndash261
39 Martinez-Martinez L Lleixa MC Boera-Carnicero G et al Anti-NF155 chronic in-flammatory demyelinating polyradiculoneuropathy strongly associates to HLA-DRB15 J Neuroinflammation 201714224
40 Hashimoto Y Ogata H Yamasaki R et al Chronic inflammatory demyelinatingpolyneuropathy with concurrent membranous nephropathy an anti-paranode andpodocyte protein antibody study and literature survey Front Neurol 20189997
Appendix (continued)
Name Location Contribution
KathrinDoppler MD
University Hospital ofWurzburg
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy major role in analysisand interpretation of dataand study supervision
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 11
DOI 101212NXI000000000000081720207 Neurol Neuroimmunol Neuroinflamm
Luise Appeltshauser Anna-Michelle Brunder Annika Heinius et al Antiparanodal antibodies and IgG subclasses in acute autoimmune neuropathy
This information is current as of July 24 2020
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm
ServicesUpdated Information amp
httpnnneurologyorgcontent75e817fullhtmlincluding high resolution figures can be found at
References httpnnneurologyorgcontent75e817fullhtmlref-list-1
This article cites 40 articles 9 of which you can access for free at
Citations httpnnneurologyorgcontent75e817fullhtmlotherarticles
This article has been cited by 1 HighWire-hosted articles
Subspecialty Collections
httpnnneurologyorgcgicollectionperipheral_neuropathyPeripheral neuropathy
httpnnneurologyorgcgicollectionguillainbarre_syndromeGuillain-Barre syndrome
nating_polyneuropathyhttpnnneurologyorgcgicollectionchronic_inflammatory_demyeliChronic inflammatory demyelinating polyneuropathy
httpnnneurologyorgcgicollectionautoimmune_diseasesAutoimmune diseases
httpnnneurologyorgcgicollectionall_neuromuscular_diseaseAll Neuromuscular Diseasefollowing collection(s) This article along with others on similar topics appears in the
Permissions amp Licensing
httpnnneurologyorgmiscaboutxhtmlpermissionsits entirety can be found online atInformation about reproducing this article in parts (figurestables) or in
Reprints
httpnnneurologyorgmiscaddirxhtmlreprintsusInformation about ordering reprints can be found online
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm

Table 1 Summary of the autoantibody status of seropositive patients 1ndash5 at baseline and follow-up (continued)
CSF Serum
Teased fibers and ELISA Preabsorption CBA
Paranodal bindingOD Titer IgG subclass
Patient 5 (baseline)
Teased fibers Positive Positive 1100 IgG3
Contactin-1 0219 0260 nd nd Negative Negative
Caspr-1 0136 nd nd nd Positive Positive
Patient 5 (first follow-up)
Teased fibers Positive Positive 1100 IgG3
Contactin-1 0236 0243 nd nd Negative Negative
Caspr-1 0364 3802 1200 IgG32 Positive Positive
Patient 5 (second follow-up)
Teased fibers nd Positive 150 nm
Contactin-1 nd 0168 nd nd nd Negative
Caspr-1 nd 0013 nd nd nd Negative
Abbreviations A-CIDP = acute-onset chronic inflammatory demyelinating polyradiculoneuropathy Caspr-1 = contactin-1ndashassociated protein-1 CBA = cell-based assay GBS = Guillain-Barre syndrome nd = not done nd =not done due to lack of material nm = not measurable OD = optical densityResults of teased fibers assays are displayed in the first row for each patient contactin-1 and Caspr-1-specific results are displayed in lines 2 and 3 ODs above the threshold and results considered positive are highlighted inbold letters There is a 100 concordance regarding target specificity in the teased fibers assay ELISA and CBA but minor differences in IgG titer and coexisting subclass between the teased fibers assay and ELISA In case ofdouble positivity preabsorption assays only detect the target antigen with the higher antibody titer (see ELISA titer)
6NeurologyN
euroim
muno
logyampNeuroinflam
mation
|Vo
lume7N
umber
5|
Septem
ber
2020NeurologyorgN
N
specificity index) being in a normal range (see appendix e-1linkslwwcomNXIA275)
Clinical features of seropositive patientsFigure 4 schematically summarizes the clinical course relatedto the experimental results Appendix e-1 linkslwwcomNXIA275 summarizes clinical data of seropositive patients1ndash5 and provides a detailed description of the clinical diseasecourse in individuals The two seropositive patients withGBS were both middle-aged women presenting with severemotor but only mild sensory involvement Cranial nerveinvolvement autonomic symptoms and neuropathic painwere present in 12 patients with GBS only Both patientsrequired treatment on the intensive care unit (ICU) The
three patients with A-CIDP presented with moderate to severeparesis and multimodal sensory impairment at disease onsetDuring the course of disease all three patients with A-CIDPreported neuropathic pain requiring pregabalingabapentinor even opioid treatment Two of three patients withA-CIDP presented sensory ataxia Nerve conduction studiesshowed demyelinating features in all seropositive patientswith conduction block in 35 patients Sural nerve biopsywas performed in one patient (patient 5) six months after theonset and showed axonal loss without signs of demyelinationCSF analysis revealed cytoalbuminologic dissociation andblood-brain barrier dysfunction in all seropositive patientsThe patient with subacute-onset CIDP highly elevated CSFprotein and detection of antindashCaspr-1 in both serum and
Figure 2 Serum subclass analysis at baseline and at follow-up with IgG subclass switch
(A) Results of antindashcontactin-1 and antindashCaspr-1 ELISA with baseline sera and subclass-specific IgG1-4 autoantibodies (grayscale) are shown as of the OD ofthe total IgG (using anti-human IgG autoantibody) on y-axis In patient 1 ELISA IgG subclasses were not measurable ELISA revealed mainly IgG2 subclass inpatient 2 (GBS) IgG2IgG4 in patient 3 (A-CIDP) and IgG23 in patients 4 and 5 (A-CIDP) (B) Acute phase teased fibers subclass analysis with baseline serashowed binding to the paranodal regions (arrows) only when using IgG3-specific secondary antibody in patients 1 (GBS) and 4 (A-CIDP) visualized byphotomicrographs of single immunofluorescence staining on teased fibers (C) AntindashCaspr-1 IgG subclass ELISA with follow-up serum of patient 4 nowrevealed IgG 24 subclass Photomicrographs show immunofluorescence staining of murine teased fibers with subclass-specific IgG3 and IgG4 autoanti-bodies at follow-up Paranodal binding disappeared using IgG3 antibody but occurred using IgG4 subclass-specific antibody proving subclass switch inpatient 4 Scale bar = 10 μm A-CIDP = acute-onset chronic inflammatory demyelinating polyradiculoneuropathy GBS = Guillain-Barre syndrome
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 7
CSF (patient 5) developed action tremor during the courseof disease
Antibody status is concordant with diseaseactivity and therapy responseOne of two patients with GBS (patient 2) received IVIgwhich did not lead to amelioration This patient was IgG2 gtIgG3 seropositive (patient 2 figure 2A) Both patients withGBS responded well to plasma exchange The two patientswith A-CIDP and IgG3 autoantibodies (patients 4 and 5)showed good response to IVIg treatment in the acute phase ofdisease Ongoing IVIg treatment led to further improvementof symptoms and was stopped after 25 cycles in patient 5because of a remission of symptoms In the other patient withA-CIDP and antindashcontactin-1Caspr-1 IgG3 at disease onset(patient 4) IVIg showed a good therapeutic effect in the acutephase and led to remission not requiring further therapy Inthe chronic phase IVIg was initiated again and stopped after26 cycles because of a loss of the therapeutic effect At thatstage the autoantibody subclass had already switched fromIgG3 to IgG4 (for detailed treatment response in the course ofdisease see appendix e-1 linkslwwcomNXIA275) Thepatient with A-CIDP and high-titer antindashcontactin-1 IgG4autoantibodies (patient 3) had to be treated on ICU becauseof the severity of symptoms developed renal dysfunction didnot respond to IVIg treatment and did not ameliorate duringthe course of disease
DiscussionWedetected antiparanodal autoantibodies in 43of patientswithacute to subacute immune-mediated neuropathy more frequentlyin CIDP with acute to subacute onset than in GBS We foundIgG23 in monophasic GBS which supports the notion of pre-vious studies that IgG3 is the predominant subclass inmonophasicdisease7 By analyzing sera from different time points we couldshow that the antibody status was concordant with the diseaseseverity Therefore our data support pathogenicity of autoanti-bodies and suggest that autoantibody status and titer are validindicators for disease activity as proposed in previous studies onparanodopathy and further IgG4-related neurologic diseases25ndash27
In one patient we could demonstrate a subclass switch fromIgG3 in acute disease to IgG4 in chronic disease a finding thatwe had hypothesized in a previous study6 In other autoimmunediseases eg in membranous glomerulonephritis subclassswitch of anti-PLA2R autoantibodies from IgG1 to IgG4 alsoassociates with progression to chronic disease28 According toa linear model of autoantibody response unspecific IgM re-sponse is followed by IgG isotype produced by antigen-experi-enced B cells These IgG are of the IgG3 subclass andsequentially switch to IgG1 2 and 42930 Concurrently auto-antibodies strongly gain antigen affinity with IgG4 showing thehighest affinity to its target3132 Disease progression in para-nodopathy might therefore depend on IgG subclass properties
Figure 3 Antiparanodal IgG autoantibodies at follow-up assessment
(A) Contactin-1 IgG ELISA of all patients at first assessment of 66161 patients at follow-up and of 40 healthy controls Optical density (OD) at 450 nm isdisplayed on the y-axis The threshold for positive results is set at 5 SDs above the mean of controls (0615) Patients 2ndash4 show positive results at firstassessment The other patients and controls show values below the threshold Sera of patients 2 4 and 5 are negative in antindashcontactin-1 IgG ELISA at follow-up as well as 63 other follow-up sera Patients 1 and 3were lost to follow-up (B) Overlay photomicrographs of teased fibers double immunofluorescence and(C) contactin-1ndash and Caspr-1 (CNTNAP1)ndashtransfected HEK293 cells show loss of antindashcontactin-1Caspr-1 autoantibodies in patient 2 (GBS) at follow-up (BaCa Cd) but colocalization of commercial antindashCaspr-1 antibody and sera at paranodes in patients 4 and 5 with A-CIDP (Bb Bc arrows) Both sera bind toCaspr-1 (CNTNAP1)ndashtransfected HEK293 cells (Ce Cf arrowheads) but not to contactin-1ndashtransfected cells (Cb Cc) Scale bar = 10 μm A-CIDP = acute-onsetchronic inflammatory demyelinating polyradiculoneuropathy GBS = Guillain-Barre syndrome HEK = human embryonic kidney
8 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
and affinities Gain of specificity and affinity at switch from IgG3to IgG4 might explain different binding characteristics of anti-paranodal autoantibodies in the patient whose IgG3 autoanti-bodies reacted both against contactin-1Caspr-1 epitopes butwhose IgG4 autoantibodies were specific to Caspr-1 Here wereport IgG24 (1) in patient 4 in the chronic phase after subclassswitch and (2) in another patient at disease onset showingnonameliorating disease with high disability similar to previousreports on antindashcontactin-1 IgG4-related disease111333 So farstudies have only reported antiparanodal IgG4 autoantibodies inpatients with chronic disease7113435 We therefore suggest thathigh-affinity IgG4 autoantibody binding leads to chronic diseasewith functional impairment possibly caused by disturbance of theantigen interaction paranodal architecture and paranodalcomplex formation as demonstrated previously68ndash11
Patients of our study with predominant IgG3 subclass showedgood response to IVIg whereas patients with IgG2 and IgG4initially did not respond to IVIg or lost responsiveness in thedisease course Several studies described good responses toIVIg in IgG3-associated paranodopathy but poor responses inIgG4-associated disease56111334 IgG3 can initiate comple-ment activation opsonization antigen cross-linking and in-ternalization whereas IgG2 shows little C1q binding and IgG4completely lacks these Fc-mediated effector functions31 In anin vivo study on antindashcontactin-1 pathogenicity we suggestedcomplement-mediated functional impairment at the paranodesas the pathophysiologic correlate in IgG3-mediated para-nodopathy earlier and could show in vitro that IVIg inhibitscomplement deposition and activation dose-dependently1415
Our data therefore support the hypothesis that therapy re-sponsiveness might depend on IgG subclass and subclass-related pathomechanism of antiparanodal autoantibodies If
validated in larger studies these findings might have a directimpact on the treatment regime in affected patients Testing ofautoantibody subclass during the course of disease may beuseful as an indicator of a positive response to IVIg in case ofIgG3 whereas IgG4-related paranodopathy shows very goodresponse to rituximab46711
The trigger of the autoantibody production and the patho-mechanism behind possible IgG switching are still unknownThree of five seropositive patients in this study had diabetesmellitus type 2 as a comorbidity diagnosed years before theonset of disease Previous studies report disruption of the blood-brain barrier and the nodal architecture in patients with diabetesmellitus thereby possibly exposing paranodal antigens to theimmune response3637 A recent study has identified humanleukocyte antigen (HLA)-DRB15 as a risk factor for CIDPassociated with anti-NF155 antibodies and another studyreported different HLA antibody patterns with subclass switchafter renal transplantation3839 Whether interindividual differ-ences in antibody production and switching during the course ofdisease depend on (1) HLA alleles (2) antigen exposure or (3)further immunologic mechanisms has to be investigated infurther studies
Regarding characteristic clinical features of paranodopathy wereport neuropathic pain in antindashCaspr-1ndashrelated disease aspreviously described in patients with higher autoantibody tit-ers7 Different titers might explain discrepancies between ourresults and a recent study that did not report pain in antindashCaspr-1ndashpositive patients711 In our patients further features of IgG4-related chronic paranodopathy such as tremor sensory ataxiaand nephropathy561340 were only present in the course ofdisease suggesting that they evolve due to chronic autoantibody
Figure 4 Scheme of the autoantibody status in the course of disease in seropositive patients
The course of disease is shown on the x-axis by pseudo-logarithmic display of the time course Time point 0 is set at baseline assessment of serumCSF Thecolor code indicates severity of symptoms Results of serum assessment are displayed in black Patients 1 and 3 were lost to follow-up
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 9
exposure The pathomechanistic correlate of action tremorwhich recently has also been described in antindashCaspr-1 IgG3-related paranodopathy11 is still under research but studiessuggest cerebellar origin5612 In this study we detected in-trathecal autoantibodies in a patient with tremor as previouslyreported in a case of antindashpan-neurofascinndashpositive para-nodopathy12 The antiparanodal autoantibodies most probablydiffuse into the intrathecal compartment due to a blood-brainbarrier dysfunction as indicated by normal IgG and ASI indicesin our patients Our CSF data suggest that detection of anti-paranodal antibodies in CSF shows little sensitivity and dependson either high titer or strong leakage of the blood-brain barrierWe therefore encourage further studies on intrathecal anti-paranodal autoantibodies targeting sensitivity pathogenicityand association to clinical symptoms
Low prevalence of autoantibodies against paranodal proteins inacute inflammatory neuropathy retrospective assessment and thelow number of seropositive patients available for follow-up limitour study Clinical characteristics at acute onset and subclass-related seropositivity as a possible risk factor for chronic pro-gression when detected at the onset should be investigated in largemulticentric studies considering lowoverall prevalence ofGBS andA-CIDP as well as further possible confounders in analysis ofparanodopathy-specific clinical symptoms eg coinciding painfuldiabetic neuropathy The question of sequential subclass switchingin paranodopathy needs to be addressed in larger prospectivestudies Nevertheless our data (1) propose autoantibody subclassscreening to be considered in patients with GBS and A-CIDP asresults might have direct implications on diagnosis and therapeuticregime during the course of disease and (2) pave the way forfurther clinical and pathomechanistic studies on antiparanodalautoantibodies in acute to subacute immune-mediated neuropathy
AcknowledgmentThe authors thank Barbara Reuter and Antonia Kohl for theirsuperb technical assistance They thankMaximilian Frommer forprovision and processing of clinical data and PD Dr JakobMatschke for provision of slides and images of supplementarymorphologic data The study was supported by a grant of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg to KD and CV and a grant of the GermanResearch Foundation to KD (DFG DO-22191-1) LA issupported by a research fellowship as a clinician scientist of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg FL receives funding from the GermanMinistry of Education and Research (BMBF 01GM1908A) andthe German Research Foundation (DFG LE30642-1) CSreceives funding from the German Research Foundation (DFG3289-1 DFG UE 1714-1 DFG SO 32810-1 DFG SFB1158 and DFG SO 328 131) from the German Ministry ofEducation and Research (BMBF CMT-Net PI) and from theEuropean Union (Horizon 2020)
Study fundingThis study was supported by the Open Access PublicationFund of the University of Wurzburg
DisclosureL Appeltshauser K-P Wandinger R Junker C Sommer FLeypoldt and K Doppler work for an academic institutionoffering commercial antibody diagnostics AM Brunder AHeinius P Kortvelyessy and C Villmann report no dis-closures relevant to the manuscript C Sommer has served onscientific advisory boards for Algiax Alnylam Air LiquideAkcea Astellas Bayer Grifols Takeda and UCB She reportsbeing a member of speakersrsquo bureau and receiving speakerhonoraria from Akcea Alnylam Novartis Pfizer Sanofi-Aventis and Teva C Sommer served as a journal editorassociate editor or editorial advisory board member for theEuropean Journal of Neurology PLoS ONE and PAIN ReportsF Leypoldt reports speaker honoraria from Bayer RocheNovartis and Fresenius travel funding from Merck Grifolsand Bayer and serving on advisory boards for Roche Biogenand Alexion Go to NeurologyorgNN for full disclosures
Publication historyReceived by Neurology Neuroimmunology amp NeuroinflammationMarch 2 2020 Accepted in final form May 19 2020
Appendix Authors
Name Location Contribution
LuiseAppeltshauserMD
University Hospital ofWurzburg
Major role in the acquisitionanalysis and interpretationof the data and drafting andrevising the manuscript forintellectual content
Anna-MichelleBrunder
University Hospital ofWurzburg
Major role in the acquisitionand analysis of the data
Annika Heinius University Hospital ofSchleswig-HolsteinCampus Kiel
Major role in the acquisitionand analysis of the data
PeterKortvelyessyMD
University Hospital ofMagdeburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Klaus-PeterWandinger MD
University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Ralf Junker MD University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
CarmenVillmann PhD
University Hospital ofWurzburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
ClaudiaSommer MD
University Hospital ofWurzburg
Conceptualization of thestudy revising themanuscript for intellectualcontent and major role ininterpretation of data
Frank LeypoldtMD
University Hospital ofSchleswig-HolsteinCampus Kiel
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy and major role ininterpretation of data
10 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
References1 Fehmi J Scherer SS Willison HJ Rinaldi S Nodes paranodes and neuropathies
J Neurol Neurosurg Psychiatry 20188961ndash712 Uncini A Vallat JM Autoimmune nodo-paranodopathies of peripheral nerve the
concept is gaining ground J Neurol Neurosurg Psychiatry 201889627ndash6353 Querol L Illa I Paranodal and other autoantibodies in chronic inflammatory neu-
ropathies Curr Opin Neurol 201528474ndash4794 Querol L Rojas-Garcia R Diaz-Manera J et al Rituximab in treatment-resistant
CIDP with antibodies against paranodal proteins Neurol Neuroimmunol Neuro-inflamm 20152e149 doi 101212NXI0000000000000149
5 Querol L Nogales-Gadea G Rojas-Garcia R et al Neurofascin IgG4 antibodies inCIDP associate with disabling tremor and poor response to IVIg Neurology 201482879ndash886
6 Doppler K Appeltshauser L Wilhelmi K et al Destruction of paranodal architecturein inflammatory neuropathy with anti-contactin-1 autoantibodies J Neurol Neuro-surg Psychiatry 201586720ndash728
7 Doppler K Appeltshauser L Villmann C et al Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathyBrain 20161392617ndash2630
8 Manso C Querol L Mekaouche M Illa I Devaux JJ Contactin-1 IgG4 antibodiescause paranode dismantling and conduction defects Brain 20161391700ndash1712
9 Manso C Querol L Lleixa C et al Anti-Neurofascin-155 IgG4 antibodies preventparanodal complex formation in vivo J Clin Invest 20191292222ndash2236
10 Labasque M Hivert B Nogales-Gadea G Querol L Illa I Faivre-Sarrailh C Specificcontactin N-glycans are implicated in neurofascin binding and autoimmune targetingin peripheral neuropathies J Biol Chem 20142897907ndash7918
11 Cortese A Lombardi R Briani C et al Antibodies to neurofascin contactin-1 andcontactin-associated protein 1 in CIDP clinical relevance of IgG isotype NeurolNeuroimmunol Neuroinflamm 20197e639 doi 101212NXI0000000000000639
12 Stengel H Vural A Brunder AM et al Anti-pan-neurofascin IgG3 as a marker offulminant autoimmune neuropathy Neurol Neuroimmunol Neuroinflamm 20196e603 doi 101212NXI0000000000000603
13 Miura Y Devaux JJ Fukami Y et al Contactin 1 IgG4 associates to chronic inflammatorydemyelinating polyneuropathy with sensory ataxia Brain 20151381484ndash1491
14 Appeltshauser L Weishaupt A Sommer C Doppler K Complement depositioninduced by binding of anti-contactin-1 auto-antibodies is modified by immunoglo-bulins Exp Neurol 201728784ndash90
15 Doppler K Schuster Y Appeltshauser L et al Anti-CNTN1 IgG3 induces acuteconduction block and motor deficits in a passive transfer rat model J Neuro-inflammation 20191673
16 Sejvar JJ Kohl KS Gidudu J et al Guillain-Barre syndrome and Fisher syndrome casedefinitions and guidelines for collection analysis and presentation of immunizationsafety data Vaccine 201129599ndash612
17 Fokke C van den Berg B Drenthen J Walgaard C van Doorn PA Jacobs BCDiagnosis of Guillain-Barre syndrome and validation of Brighton criteria Brain 201413733ndash43
18 Kuitwaard K van Koningsveld R Ruts L Jacobs BC van Doorn PA RecurrentGuillain-Barre syndrome J Neurol Neurosurg Psychiatry 20098056ndash59
19 Van den Bergh PY Hadden RD Bouche P et al European Federation of NeurologicalSocietiesPeripheral Nerve Society guideline on management of chronic in-flammatory demyelinating polyradiculoneuropathy report of a joint task force of theEuropean Federation of Neurological Societies and the Peripheral Nerve Societymdashfirst revision Eur J Neurol 201017356ndash363
20 Ruts L Drenthen J Jacobs BC van Doorn PA Distinguishing acute-onset CIDP fromfluctuating Guillain-Barre syndrome a prospective study Neurology 2010741680ndash1686
21 McCombe PA Pollard JD McLeod JG Chronic inflammatory demyelinating poly-radiculoneuropathy A clinical and electrophysiological study of 92 cases Brain 19871101617ndash1630
22 Alessandro L Pastor Rueda JM Wilken M et al Differences between acute-onsetchronic inflammatory demyelinating polyneuropathy and acute inflammatory de-myelinating polyneuropathy in adult patients J Peripher Nerv Syst 201823154ndash158
23 Doppler K Appeltshauser L Kramer HH et al Contactin-1 and neurofascin-155-186 are not targets of auto-antibodies in multifocal motor neuropathy PLoS One201510e0134274
24 Ng JK Malotka J Kawakami N et al Neurofascin as a target for autoantibodies inperipheral neuropathies Neurology 2012792241ndash2248
25 Fujita A Ogata H Yamasaki RMatsushita T Kira JI Parallel fluctuation of anti-neurofascin155 antibody levels with clinico-electrophysiological findings in patients with chronic in-flammatory demyelinating polyradiculoneuropathy J Neurol Sci 2018384107ndash112
26 Niks EH van Leeuwen Y Leite MI et al Clinical fluctuations in MuSK myastheniagravis are related to antigen-specific IgG4 instead of IgG1 J Neuroimmunol 2008195151ndash156
27 Huijbers MG Vink AF Niks EH et al Longitudinal epitope mapping in MuSKmyasthenia gravis implications for disease severity J Neuroimmunol 201629182ndash88
28 Huang CC Lehman A Albawardi A et al IgG subclass staining in renal biopsies withmembranous glomerulonephritis indicates subclass switch during disease progressionMod Pathol 201326799ndash805
29 Collins AM Jackson KJ A temporal model of human IgE and IgG antibody functionFront Immunol 20134235
30 Valenzuela NM Schaub S The Biology of IgG subclasses and their clinical relevanceto transplantation Transplantation 2018102S7ndashS13
31 Vidarsson G Dekkers G Rispens T IgG subclasses and allotypes from structure toeffector functions Front Immunol 20145520ndash532
32 Bruhns P Iannascoli B England P et al Specificity and affinity of human Fcgamma receptorsand their polymorphic variants for human IgG subclasses Blood 20091133716ndash3725
33 Querol L Nogales-Gadea G Rojas-Garcia R et al Antibodies to contactin-1 inchronic inflammatory demyelinating polyneuropathy Ann Neurol 201373370ndash380
34 Devaux JJ Miura Y Fukami Y et al Neurofascin-155 IgG4 in chronic inflammatorydemyelinating polyneuropathy Neurology 201686800ndash807
35 Ogata H Yamasaki R Hiwatashi A et al Characterization of IgG4 anti-neurofascin155 antibody-positive polyneuropathy Ann Clin Transl Neurol 20152960ndash971
36 Doppler K Frank F Koschker AC Reiners K Sommer C Nodes of Ranvier in skinbiopsies of patients with diabetes mellitus J Peripher Nerv Syst 201722182ndash190
37 Banks W The blood-brain barrier interface in diabetes mellitus dysfunctionsmechanisms and approaches to treatment Curr Pharm Des 2020261438ndash1447
38 Arnold ML Ntokou IS Doxiadis II Spriewald BM Boletis JN Iniotaki AG Donor-specific HLA antibodies evaluating the risk for graft loss in renal transplant recipientswith isotype switch from complement fixing IgG1IgG3 to noncomplement fixingIgG2IgG4 anti-HLA alloantibodies Transpl Int 201427253ndash261
39 Martinez-Martinez L Lleixa MC Boera-Carnicero G et al Anti-NF155 chronic in-flammatory demyelinating polyradiculoneuropathy strongly associates to HLA-DRB15 J Neuroinflammation 201714224
40 Hashimoto Y Ogata H Yamasaki R et al Chronic inflammatory demyelinatingpolyneuropathy with concurrent membranous nephropathy an anti-paranode andpodocyte protein antibody study and literature survey Front Neurol 20189997
Appendix (continued)
Name Location Contribution
KathrinDoppler MD
University Hospital ofWurzburg
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy major role in analysisand interpretation of dataand study supervision
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 11
DOI 101212NXI000000000000081720207 Neurol Neuroimmunol Neuroinflamm
Luise Appeltshauser Anna-Michelle Brunder Annika Heinius et al Antiparanodal antibodies and IgG subclasses in acute autoimmune neuropathy
This information is current as of July 24 2020
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm
ServicesUpdated Information amp
httpnnneurologyorgcontent75e817fullhtmlincluding high resolution figures can be found at
References httpnnneurologyorgcontent75e817fullhtmlref-list-1
This article cites 40 articles 9 of which you can access for free at
Citations httpnnneurologyorgcontent75e817fullhtmlotherarticles
This article has been cited by 1 HighWire-hosted articles
Subspecialty Collections
httpnnneurologyorgcgicollectionperipheral_neuropathyPeripheral neuropathy
httpnnneurologyorgcgicollectionguillainbarre_syndromeGuillain-Barre syndrome
nating_polyneuropathyhttpnnneurologyorgcgicollectionchronic_inflammatory_demyeliChronic inflammatory demyelinating polyneuropathy
httpnnneurologyorgcgicollectionautoimmune_diseasesAutoimmune diseases
httpnnneurologyorgcgicollectionall_neuromuscular_diseaseAll Neuromuscular Diseasefollowing collection(s) This article along with others on similar topics appears in the
Permissions amp Licensing
httpnnneurologyorgmiscaboutxhtmlpermissionsits entirety can be found online atInformation about reproducing this article in parts (figurestables) or in
Reprints
httpnnneurologyorgmiscaddirxhtmlreprintsusInformation about ordering reprints can be found online
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm

specificity index) being in a normal range (see appendix e-1linkslwwcomNXIA275)
Clinical features of seropositive patientsFigure 4 schematically summarizes the clinical course relatedto the experimental results Appendix e-1 linkslwwcomNXIA275 summarizes clinical data of seropositive patients1ndash5 and provides a detailed description of the clinical diseasecourse in individuals The two seropositive patients withGBS were both middle-aged women presenting with severemotor but only mild sensory involvement Cranial nerveinvolvement autonomic symptoms and neuropathic painwere present in 12 patients with GBS only Both patientsrequired treatment on the intensive care unit (ICU) The
three patients with A-CIDP presented with moderate to severeparesis and multimodal sensory impairment at disease onsetDuring the course of disease all three patients with A-CIDPreported neuropathic pain requiring pregabalingabapentinor even opioid treatment Two of three patients withA-CIDP presented sensory ataxia Nerve conduction studiesshowed demyelinating features in all seropositive patientswith conduction block in 35 patients Sural nerve biopsywas performed in one patient (patient 5) six months after theonset and showed axonal loss without signs of demyelinationCSF analysis revealed cytoalbuminologic dissociation andblood-brain barrier dysfunction in all seropositive patientsThe patient with subacute-onset CIDP highly elevated CSFprotein and detection of antindashCaspr-1 in both serum and
Figure 2 Serum subclass analysis at baseline and at follow-up with IgG subclass switch
(A) Results of antindashcontactin-1 and antindashCaspr-1 ELISA with baseline sera and subclass-specific IgG1-4 autoantibodies (grayscale) are shown as of the OD ofthe total IgG (using anti-human IgG autoantibody) on y-axis In patient 1 ELISA IgG subclasses were not measurable ELISA revealed mainly IgG2 subclass inpatient 2 (GBS) IgG2IgG4 in patient 3 (A-CIDP) and IgG23 in patients 4 and 5 (A-CIDP) (B) Acute phase teased fibers subclass analysis with baseline serashowed binding to the paranodal regions (arrows) only when using IgG3-specific secondary antibody in patients 1 (GBS) and 4 (A-CIDP) visualized byphotomicrographs of single immunofluorescence staining on teased fibers (C) AntindashCaspr-1 IgG subclass ELISA with follow-up serum of patient 4 nowrevealed IgG 24 subclass Photomicrographs show immunofluorescence staining of murine teased fibers with subclass-specific IgG3 and IgG4 autoanti-bodies at follow-up Paranodal binding disappeared using IgG3 antibody but occurred using IgG4 subclass-specific antibody proving subclass switch inpatient 4 Scale bar = 10 μm A-CIDP = acute-onset chronic inflammatory demyelinating polyradiculoneuropathy GBS = Guillain-Barre syndrome
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 7
CSF (patient 5) developed action tremor during the courseof disease
Antibody status is concordant with diseaseactivity and therapy responseOne of two patients with GBS (patient 2) received IVIgwhich did not lead to amelioration This patient was IgG2 gtIgG3 seropositive (patient 2 figure 2A) Both patients withGBS responded well to plasma exchange The two patientswith A-CIDP and IgG3 autoantibodies (patients 4 and 5)showed good response to IVIg treatment in the acute phase ofdisease Ongoing IVIg treatment led to further improvementof symptoms and was stopped after 25 cycles in patient 5because of a remission of symptoms In the other patient withA-CIDP and antindashcontactin-1Caspr-1 IgG3 at disease onset(patient 4) IVIg showed a good therapeutic effect in the acutephase and led to remission not requiring further therapy Inthe chronic phase IVIg was initiated again and stopped after26 cycles because of a loss of the therapeutic effect At thatstage the autoantibody subclass had already switched fromIgG3 to IgG4 (for detailed treatment response in the course ofdisease see appendix e-1 linkslwwcomNXIA275) Thepatient with A-CIDP and high-titer antindashcontactin-1 IgG4autoantibodies (patient 3) had to be treated on ICU becauseof the severity of symptoms developed renal dysfunction didnot respond to IVIg treatment and did not ameliorate duringthe course of disease
DiscussionWedetected antiparanodal autoantibodies in 43of patientswithacute to subacute immune-mediated neuropathy more frequentlyin CIDP with acute to subacute onset than in GBS We foundIgG23 in monophasic GBS which supports the notion of pre-vious studies that IgG3 is the predominant subclass inmonophasicdisease7 By analyzing sera from different time points we couldshow that the antibody status was concordant with the diseaseseverity Therefore our data support pathogenicity of autoanti-bodies and suggest that autoantibody status and titer are validindicators for disease activity as proposed in previous studies onparanodopathy and further IgG4-related neurologic diseases25ndash27
In one patient we could demonstrate a subclass switch fromIgG3 in acute disease to IgG4 in chronic disease a finding thatwe had hypothesized in a previous study6 In other autoimmunediseases eg in membranous glomerulonephritis subclassswitch of anti-PLA2R autoantibodies from IgG1 to IgG4 alsoassociates with progression to chronic disease28 According toa linear model of autoantibody response unspecific IgM re-sponse is followed by IgG isotype produced by antigen-experi-enced B cells These IgG are of the IgG3 subclass andsequentially switch to IgG1 2 and 42930 Concurrently auto-antibodies strongly gain antigen affinity with IgG4 showing thehighest affinity to its target3132 Disease progression in para-nodopathy might therefore depend on IgG subclass properties
Figure 3 Antiparanodal IgG autoantibodies at follow-up assessment
(A) Contactin-1 IgG ELISA of all patients at first assessment of 66161 patients at follow-up and of 40 healthy controls Optical density (OD) at 450 nm isdisplayed on the y-axis The threshold for positive results is set at 5 SDs above the mean of controls (0615) Patients 2ndash4 show positive results at firstassessment The other patients and controls show values below the threshold Sera of patients 2 4 and 5 are negative in antindashcontactin-1 IgG ELISA at follow-up as well as 63 other follow-up sera Patients 1 and 3were lost to follow-up (B) Overlay photomicrographs of teased fibers double immunofluorescence and(C) contactin-1ndash and Caspr-1 (CNTNAP1)ndashtransfected HEK293 cells show loss of antindashcontactin-1Caspr-1 autoantibodies in patient 2 (GBS) at follow-up (BaCa Cd) but colocalization of commercial antindashCaspr-1 antibody and sera at paranodes in patients 4 and 5 with A-CIDP (Bb Bc arrows) Both sera bind toCaspr-1 (CNTNAP1)ndashtransfected HEK293 cells (Ce Cf arrowheads) but not to contactin-1ndashtransfected cells (Cb Cc) Scale bar = 10 μm A-CIDP = acute-onsetchronic inflammatory demyelinating polyradiculoneuropathy GBS = Guillain-Barre syndrome HEK = human embryonic kidney
8 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
and affinities Gain of specificity and affinity at switch from IgG3to IgG4 might explain different binding characteristics of anti-paranodal autoantibodies in the patient whose IgG3 autoanti-bodies reacted both against contactin-1Caspr-1 epitopes butwhose IgG4 autoantibodies were specific to Caspr-1 Here wereport IgG24 (1) in patient 4 in the chronic phase after subclassswitch and (2) in another patient at disease onset showingnonameliorating disease with high disability similar to previousreports on antindashcontactin-1 IgG4-related disease111333 So farstudies have only reported antiparanodal IgG4 autoantibodies inpatients with chronic disease7113435 We therefore suggest thathigh-affinity IgG4 autoantibody binding leads to chronic diseasewith functional impairment possibly caused by disturbance of theantigen interaction paranodal architecture and paranodalcomplex formation as demonstrated previously68ndash11
Patients of our study with predominant IgG3 subclass showedgood response to IVIg whereas patients with IgG2 and IgG4initially did not respond to IVIg or lost responsiveness in thedisease course Several studies described good responses toIVIg in IgG3-associated paranodopathy but poor responses inIgG4-associated disease56111334 IgG3 can initiate comple-ment activation opsonization antigen cross-linking and in-ternalization whereas IgG2 shows little C1q binding and IgG4completely lacks these Fc-mediated effector functions31 In anin vivo study on antindashcontactin-1 pathogenicity we suggestedcomplement-mediated functional impairment at the paranodesas the pathophysiologic correlate in IgG3-mediated para-nodopathy earlier and could show in vitro that IVIg inhibitscomplement deposition and activation dose-dependently1415
Our data therefore support the hypothesis that therapy re-sponsiveness might depend on IgG subclass and subclass-related pathomechanism of antiparanodal autoantibodies If
validated in larger studies these findings might have a directimpact on the treatment regime in affected patients Testing ofautoantibody subclass during the course of disease may beuseful as an indicator of a positive response to IVIg in case ofIgG3 whereas IgG4-related paranodopathy shows very goodresponse to rituximab46711
The trigger of the autoantibody production and the patho-mechanism behind possible IgG switching are still unknownThree of five seropositive patients in this study had diabetesmellitus type 2 as a comorbidity diagnosed years before theonset of disease Previous studies report disruption of the blood-brain barrier and the nodal architecture in patients with diabetesmellitus thereby possibly exposing paranodal antigens to theimmune response3637 A recent study has identified humanleukocyte antigen (HLA)-DRB15 as a risk factor for CIDPassociated with anti-NF155 antibodies and another studyreported different HLA antibody patterns with subclass switchafter renal transplantation3839 Whether interindividual differ-ences in antibody production and switching during the course ofdisease depend on (1) HLA alleles (2) antigen exposure or (3)further immunologic mechanisms has to be investigated infurther studies
Regarding characteristic clinical features of paranodopathy wereport neuropathic pain in antindashCaspr-1ndashrelated disease aspreviously described in patients with higher autoantibody tit-ers7 Different titers might explain discrepancies between ourresults and a recent study that did not report pain in antindashCaspr-1ndashpositive patients711 In our patients further features of IgG4-related chronic paranodopathy such as tremor sensory ataxiaand nephropathy561340 were only present in the course ofdisease suggesting that they evolve due to chronic autoantibody
Figure 4 Scheme of the autoantibody status in the course of disease in seropositive patients
The course of disease is shown on the x-axis by pseudo-logarithmic display of the time course Time point 0 is set at baseline assessment of serumCSF Thecolor code indicates severity of symptoms Results of serum assessment are displayed in black Patients 1 and 3 were lost to follow-up
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 9
exposure The pathomechanistic correlate of action tremorwhich recently has also been described in antindashCaspr-1 IgG3-related paranodopathy11 is still under research but studiessuggest cerebellar origin5612 In this study we detected in-trathecal autoantibodies in a patient with tremor as previouslyreported in a case of antindashpan-neurofascinndashpositive para-nodopathy12 The antiparanodal autoantibodies most probablydiffuse into the intrathecal compartment due to a blood-brainbarrier dysfunction as indicated by normal IgG and ASI indicesin our patients Our CSF data suggest that detection of anti-paranodal antibodies in CSF shows little sensitivity and dependson either high titer or strong leakage of the blood-brain barrierWe therefore encourage further studies on intrathecal anti-paranodal autoantibodies targeting sensitivity pathogenicityand association to clinical symptoms
Low prevalence of autoantibodies against paranodal proteins inacute inflammatory neuropathy retrospective assessment and thelow number of seropositive patients available for follow-up limitour study Clinical characteristics at acute onset and subclass-related seropositivity as a possible risk factor for chronic pro-gression when detected at the onset should be investigated in largemulticentric studies considering lowoverall prevalence ofGBS andA-CIDP as well as further possible confounders in analysis ofparanodopathy-specific clinical symptoms eg coinciding painfuldiabetic neuropathy The question of sequential subclass switchingin paranodopathy needs to be addressed in larger prospectivestudies Nevertheless our data (1) propose autoantibody subclassscreening to be considered in patients with GBS and A-CIDP asresults might have direct implications on diagnosis and therapeuticregime during the course of disease and (2) pave the way forfurther clinical and pathomechanistic studies on antiparanodalautoantibodies in acute to subacute immune-mediated neuropathy
AcknowledgmentThe authors thank Barbara Reuter and Antonia Kohl for theirsuperb technical assistance They thankMaximilian Frommer forprovision and processing of clinical data and PD Dr JakobMatschke for provision of slides and images of supplementarymorphologic data The study was supported by a grant of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg to KD and CV and a grant of the GermanResearch Foundation to KD (DFG DO-22191-1) LA issupported by a research fellowship as a clinician scientist of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg FL receives funding from the GermanMinistry of Education and Research (BMBF 01GM1908A) andthe German Research Foundation (DFG LE30642-1) CSreceives funding from the German Research Foundation (DFG3289-1 DFG UE 1714-1 DFG SO 32810-1 DFG SFB1158 and DFG SO 328 131) from the German Ministry ofEducation and Research (BMBF CMT-Net PI) and from theEuropean Union (Horizon 2020)
Study fundingThis study was supported by the Open Access PublicationFund of the University of Wurzburg
DisclosureL Appeltshauser K-P Wandinger R Junker C Sommer FLeypoldt and K Doppler work for an academic institutionoffering commercial antibody diagnostics AM Brunder AHeinius P Kortvelyessy and C Villmann report no dis-closures relevant to the manuscript C Sommer has served onscientific advisory boards for Algiax Alnylam Air LiquideAkcea Astellas Bayer Grifols Takeda and UCB She reportsbeing a member of speakersrsquo bureau and receiving speakerhonoraria from Akcea Alnylam Novartis Pfizer Sanofi-Aventis and Teva C Sommer served as a journal editorassociate editor or editorial advisory board member for theEuropean Journal of Neurology PLoS ONE and PAIN ReportsF Leypoldt reports speaker honoraria from Bayer RocheNovartis and Fresenius travel funding from Merck Grifolsand Bayer and serving on advisory boards for Roche Biogenand Alexion Go to NeurologyorgNN for full disclosures
Publication historyReceived by Neurology Neuroimmunology amp NeuroinflammationMarch 2 2020 Accepted in final form May 19 2020
Appendix Authors
Name Location Contribution
LuiseAppeltshauserMD
University Hospital ofWurzburg
Major role in the acquisitionanalysis and interpretationof the data and drafting andrevising the manuscript forintellectual content
Anna-MichelleBrunder
University Hospital ofWurzburg
Major role in the acquisitionand analysis of the data
Annika Heinius University Hospital ofSchleswig-HolsteinCampus Kiel
Major role in the acquisitionand analysis of the data
PeterKortvelyessyMD
University Hospital ofMagdeburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Klaus-PeterWandinger MD
University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Ralf Junker MD University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
CarmenVillmann PhD
University Hospital ofWurzburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
ClaudiaSommer MD
University Hospital ofWurzburg
Conceptualization of thestudy revising themanuscript for intellectualcontent and major role ininterpretation of data
Frank LeypoldtMD
University Hospital ofSchleswig-HolsteinCampus Kiel
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy and major role ininterpretation of data
10 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
References1 Fehmi J Scherer SS Willison HJ Rinaldi S Nodes paranodes and neuropathies
J Neurol Neurosurg Psychiatry 20188961ndash712 Uncini A Vallat JM Autoimmune nodo-paranodopathies of peripheral nerve the
concept is gaining ground J Neurol Neurosurg Psychiatry 201889627ndash6353 Querol L Illa I Paranodal and other autoantibodies in chronic inflammatory neu-
ropathies Curr Opin Neurol 201528474ndash4794 Querol L Rojas-Garcia R Diaz-Manera J et al Rituximab in treatment-resistant
CIDP with antibodies against paranodal proteins Neurol Neuroimmunol Neuro-inflamm 20152e149 doi 101212NXI0000000000000149
5 Querol L Nogales-Gadea G Rojas-Garcia R et al Neurofascin IgG4 antibodies inCIDP associate with disabling tremor and poor response to IVIg Neurology 201482879ndash886
6 Doppler K Appeltshauser L Wilhelmi K et al Destruction of paranodal architecturein inflammatory neuropathy with anti-contactin-1 autoantibodies J Neurol Neuro-surg Psychiatry 201586720ndash728
7 Doppler K Appeltshauser L Villmann C et al Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathyBrain 20161392617ndash2630
8 Manso C Querol L Mekaouche M Illa I Devaux JJ Contactin-1 IgG4 antibodiescause paranode dismantling and conduction defects Brain 20161391700ndash1712
9 Manso C Querol L Lleixa C et al Anti-Neurofascin-155 IgG4 antibodies preventparanodal complex formation in vivo J Clin Invest 20191292222ndash2236
10 Labasque M Hivert B Nogales-Gadea G Querol L Illa I Faivre-Sarrailh C Specificcontactin N-glycans are implicated in neurofascin binding and autoimmune targetingin peripheral neuropathies J Biol Chem 20142897907ndash7918
11 Cortese A Lombardi R Briani C et al Antibodies to neurofascin contactin-1 andcontactin-associated protein 1 in CIDP clinical relevance of IgG isotype NeurolNeuroimmunol Neuroinflamm 20197e639 doi 101212NXI0000000000000639
12 Stengel H Vural A Brunder AM et al Anti-pan-neurofascin IgG3 as a marker offulminant autoimmune neuropathy Neurol Neuroimmunol Neuroinflamm 20196e603 doi 101212NXI0000000000000603
13 Miura Y Devaux JJ Fukami Y et al Contactin 1 IgG4 associates to chronic inflammatorydemyelinating polyneuropathy with sensory ataxia Brain 20151381484ndash1491
14 Appeltshauser L Weishaupt A Sommer C Doppler K Complement depositioninduced by binding of anti-contactin-1 auto-antibodies is modified by immunoglo-bulins Exp Neurol 201728784ndash90
15 Doppler K Schuster Y Appeltshauser L et al Anti-CNTN1 IgG3 induces acuteconduction block and motor deficits in a passive transfer rat model J Neuro-inflammation 20191673
16 Sejvar JJ Kohl KS Gidudu J et al Guillain-Barre syndrome and Fisher syndrome casedefinitions and guidelines for collection analysis and presentation of immunizationsafety data Vaccine 201129599ndash612
17 Fokke C van den Berg B Drenthen J Walgaard C van Doorn PA Jacobs BCDiagnosis of Guillain-Barre syndrome and validation of Brighton criteria Brain 201413733ndash43
18 Kuitwaard K van Koningsveld R Ruts L Jacobs BC van Doorn PA RecurrentGuillain-Barre syndrome J Neurol Neurosurg Psychiatry 20098056ndash59
19 Van den Bergh PY Hadden RD Bouche P et al European Federation of NeurologicalSocietiesPeripheral Nerve Society guideline on management of chronic in-flammatory demyelinating polyradiculoneuropathy report of a joint task force of theEuropean Federation of Neurological Societies and the Peripheral Nerve Societymdashfirst revision Eur J Neurol 201017356ndash363
20 Ruts L Drenthen J Jacobs BC van Doorn PA Distinguishing acute-onset CIDP fromfluctuating Guillain-Barre syndrome a prospective study Neurology 2010741680ndash1686
21 McCombe PA Pollard JD McLeod JG Chronic inflammatory demyelinating poly-radiculoneuropathy A clinical and electrophysiological study of 92 cases Brain 19871101617ndash1630
22 Alessandro L Pastor Rueda JM Wilken M et al Differences between acute-onsetchronic inflammatory demyelinating polyneuropathy and acute inflammatory de-myelinating polyneuropathy in adult patients J Peripher Nerv Syst 201823154ndash158
23 Doppler K Appeltshauser L Kramer HH et al Contactin-1 and neurofascin-155-186 are not targets of auto-antibodies in multifocal motor neuropathy PLoS One201510e0134274
24 Ng JK Malotka J Kawakami N et al Neurofascin as a target for autoantibodies inperipheral neuropathies Neurology 2012792241ndash2248
25 Fujita A Ogata H Yamasaki RMatsushita T Kira JI Parallel fluctuation of anti-neurofascin155 antibody levels with clinico-electrophysiological findings in patients with chronic in-flammatory demyelinating polyradiculoneuropathy J Neurol Sci 2018384107ndash112
26 Niks EH van Leeuwen Y Leite MI et al Clinical fluctuations in MuSK myastheniagravis are related to antigen-specific IgG4 instead of IgG1 J Neuroimmunol 2008195151ndash156
27 Huijbers MG Vink AF Niks EH et al Longitudinal epitope mapping in MuSKmyasthenia gravis implications for disease severity J Neuroimmunol 201629182ndash88
28 Huang CC Lehman A Albawardi A et al IgG subclass staining in renal biopsies withmembranous glomerulonephritis indicates subclass switch during disease progressionMod Pathol 201326799ndash805
29 Collins AM Jackson KJ A temporal model of human IgE and IgG antibody functionFront Immunol 20134235
30 Valenzuela NM Schaub S The Biology of IgG subclasses and their clinical relevanceto transplantation Transplantation 2018102S7ndashS13
31 Vidarsson G Dekkers G Rispens T IgG subclasses and allotypes from structure toeffector functions Front Immunol 20145520ndash532
32 Bruhns P Iannascoli B England P et al Specificity and affinity of human Fcgamma receptorsand their polymorphic variants for human IgG subclasses Blood 20091133716ndash3725
33 Querol L Nogales-Gadea G Rojas-Garcia R et al Antibodies to contactin-1 inchronic inflammatory demyelinating polyneuropathy Ann Neurol 201373370ndash380
34 Devaux JJ Miura Y Fukami Y et al Neurofascin-155 IgG4 in chronic inflammatorydemyelinating polyneuropathy Neurology 201686800ndash807
35 Ogata H Yamasaki R Hiwatashi A et al Characterization of IgG4 anti-neurofascin155 antibody-positive polyneuropathy Ann Clin Transl Neurol 20152960ndash971
36 Doppler K Frank F Koschker AC Reiners K Sommer C Nodes of Ranvier in skinbiopsies of patients with diabetes mellitus J Peripher Nerv Syst 201722182ndash190
37 Banks W The blood-brain barrier interface in diabetes mellitus dysfunctionsmechanisms and approaches to treatment Curr Pharm Des 2020261438ndash1447
38 Arnold ML Ntokou IS Doxiadis II Spriewald BM Boletis JN Iniotaki AG Donor-specific HLA antibodies evaluating the risk for graft loss in renal transplant recipientswith isotype switch from complement fixing IgG1IgG3 to noncomplement fixingIgG2IgG4 anti-HLA alloantibodies Transpl Int 201427253ndash261
39 Martinez-Martinez L Lleixa MC Boera-Carnicero G et al Anti-NF155 chronic in-flammatory demyelinating polyradiculoneuropathy strongly associates to HLA-DRB15 J Neuroinflammation 201714224
40 Hashimoto Y Ogata H Yamasaki R et al Chronic inflammatory demyelinatingpolyneuropathy with concurrent membranous nephropathy an anti-paranode andpodocyte protein antibody study and literature survey Front Neurol 20189997
Appendix (continued)
Name Location Contribution
KathrinDoppler MD
University Hospital ofWurzburg
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy major role in analysisand interpretation of dataand study supervision
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 11
DOI 101212NXI000000000000081720207 Neurol Neuroimmunol Neuroinflamm
Luise Appeltshauser Anna-Michelle Brunder Annika Heinius et al Antiparanodal antibodies and IgG subclasses in acute autoimmune neuropathy
This information is current as of July 24 2020
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm
ServicesUpdated Information amp
httpnnneurologyorgcontent75e817fullhtmlincluding high resolution figures can be found at
References httpnnneurologyorgcontent75e817fullhtmlref-list-1
This article cites 40 articles 9 of which you can access for free at
Citations httpnnneurologyorgcontent75e817fullhtmlotherarticles
This article has been cited by 1 HighWire-hosted articles
Subspecialty Collections
httpnnneurologyorgcgicollectionperipheral_neuropathyPeripheral neuropathy
httpnnneurologyorgcgicollectionguillainbarre_syndromeGuillain-Barre syndrome
nating_polyneuropathyhttpnnneurologyorgcgicollectionchronic_inflammatory_demyeliChronic inflammatory demyelinating polyneuropathy
httpnnneurologyorgcgicollectionautoimmune_diseasesAutoimmune diseases
httpnnneurologyorgcgicollectionall_neuromuscular_diseaseAll Neuromuscular Diseasefollowing collection(s) This article along with others on similar topics appears in the
Permissions amp Licensing
httpnnneurologyorgmiscaboutxhtmlpermissionsits entirety can be found online atInformation about reproducing this article in parts (figurestables) or in
Reprints
httpnnneurologyorgmiscaddirxhtmlreprintsusInformation about ordering reprints can be found online
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm

CSF (patient 5) developed action tremor during the courseof disease
Antibody status is concordant with diseaseactivity and therapy responseOne of two patients with GBS (patient 2) received IVIgwhich did not lead to amelioration This patient was IgG2 gtIgG3 seropositive (patient 2 figure 2A) Both patients withGBS responded well to plasma exchange The two patientswith A-CIDP and IgG3 autoantibodies (patients 4 and 5)showed good response to IVIg treatment in the acute phase ofdisease Ongoing IVIg treatment led to further improvementof symptoms and was stopped after 25 cycles in patient 5because of a remission of symptoms In the other patient withA-CIDP and antindashcontactin-1Caspr-1 IgG3 at disease onset(patient 4) IVIg showed a good therapeutic effect in the acutephase and led to remission not requiring further therapy Inthe chronic phase IVIg was initiated again and stopped after26 cycles because of a loss of the therapeutic effect At thatstage the autoantibody subclass had already switched fromIgG3 to IgG4 (for detailed treatment response in the course ofdisease see appendix e-1 linkslwwcomNXIA275) Thepatient with A-CIDP and high-titer antindashcontactin-1 IgG4autoantibodies (patient 3) had to be treated on ICU becauseof the severity of symptoms developed renal dysfunction didnot respond to IVIg treatment and did not ameliorate duringthe course of disease
DiscussionWedetected antiparanodal autoantibodies in 43of patientswithacute to subacute immune-mediated neuropathy more frequentlyin CIDP with acute to subacute onset than in GBS We foundIgG23 in monophasic GBS which supports the notion of pre-vious studies that IgG3 is the predominant subclass inmonophasicdisease7 By analyzing sera from different time points we couldshow that the antibody status was concordant with the diseaseseverity Therefore our data support pathogenicity of autoanti-bodies and suggest that autoantibody status and titer are validindicators for disease activity as proposed in previous studies onparanodopathy and further IgG4-related neurologic diseases25ndash27
In one patient we could demonstrate a subclass switch fromIgG3 in acute disease to IgG4 in chronic disease a finding thatwe had hypothesized in a previous study6 In other autoimmunediseases eg in membranous glomerulonephritis subclassswitch of anti-PLA2R autoantibodies from IgG1 to IgG4 alsoassociates with progression to chronic disease28 According toa linear model of autoantibody response unspecific IgM re-sponse is followed by IgG isotype produced by antigen-experi-enced B cells These IgG are of the IgG3 subclass andsequentially switch to IgG1 2 and 42930 Concurrently auto-antibodies strongly gain antigen affinity with IgG4 showing thehighest affinity to its target3132 Disease progression in para-nodopathy might therefore depend on IgG subclass properties
Figure 3 Antiparanodal IgG autoantibodies at follow-up assessment
(A) Contactin-1 IgG ELISA of all patients at first assessment of 66161 patients at follow-up and of 40 healthy controls Optical density (OD) at 450 nm isdisplayed on the y-axis The threshold for positive results is set at 5 SDs above the mean of controls (0615) Patients 2ndash4 show positive results at firstassessment The other patients and controls show values below the threshold Sera of patients 2 4 and 5 are negative in antindashcontactin-1 IgG ELISA at follow-up as well as 63 other follow-up sera Patients 1 and 3were lost to follow-up (B) Overlay photomicrographs of teased fibers double immunofluorescence and(C) contactin-1ndash and Caspr-1 (CNTNAP1)ndashtransfected HEK293 cells show loss of antindashcontactin-1Caspr-1 autoantibodies in patient 2 (GBS) at follow-up (BaCa Cd) but colocalization of commercial antindashCaspr-1 antibody and sera at paranodes in patients 4 and 5 with A-CIDP (Bb Bc arrows) Both sera bind toCaspr-1 (CNTNAP1)ndashtransfected HEK293 cells (Ce Cf arrowheads) but not to contactin-1ndashtransfected cells (Cb Cc) Scale bar = 10 μm A-CIDP = acute-onsetchronic inflammatory demyelinating polyradiculoneuropathy GBS = Guillain-Barre syndrome HEK = human embryonic kidney
8 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
and affinities Gain of specificity and affinity at switch from IgG3to IgG4 might explain different binding characteristics of anti-paranodal autoantibodies in the patient whose IgG3 autoanti-bodies reacted both against contactin-1Caspr-1 epitopes butwhose IgG4 autoantibodies were specific to Caspr-1 Here wereport IgG24 (1) in patient 4 in the chronic phase after subclassswitch and (2) in another patient at disease onset showingnonameliorating disease with high disability similar to previousreports on antindashcontactin-1 IgG4-related disease111333 So farstudies have only reported antiparanodal IgG4 autoantibodies inpatients with chronic disease7113435 We therefore suggest thathigh-affinity IgG4 autoantibody binding leads to chronic diseasewith functional impairment possibly caused by disturbance of theantigen interaction paranodal architecture and paranodalcomplex formation as demonstrated previously68ndash11
Patients of our study with predominant IgG3 subclass showedgood response to IVIg whereas patients with IgG2 and IgG4initially did not respond to IVIg or lost responsiveness in thedisease course Several studies described good responses toIVIg in IgG3-associated paranodopathy but poor responses inIgG4-associated disease56111334 IgG3 can initiate comple-ment activation opsonization antigen cross-linking and in-ternalization whereas IgG2 shows little C1q binding and IgG4completely lacks these Fc-mediated effector functions31 In anin vivo study on antindashcontactin-1 pathogenicity we suggestedcomplement-mediated functional impairment at the paranodesas the pathophysiologic correlate in IgG3-mediated para-nodopathy earlier and could show in vitro that IVIg inhibitscomplement deposition and activation dose-dependently1415
Our data therefore support the hypothesis that therapy re-sponsiveness might depend on IgG subclass and subclass-related pathomechanism of antiparanodal autoantibodies If
validated in larger studies these findings might have a directimpact on the treatment regime in affected patients Testing ofautoantibody subclass during the course of disease may beuseful as an indicator of a positive response to IVIg in case ofIgG3 whereas IgG4-related paranodopathy shows very goodresponse to rituximab46711
The trigger of the autoantibody production and the patho-mechanism behind possible IgG switching are still unknownThree of five seropositive patients in this study had diabetesmellitus type 2 as a comorbidity diagnosed years before theonset of disease Previous studies report disruption of the blood-brain barrier and the nodal architecture in patients with diabetesmellitus thereby possibly exposing paranodal antigens to theimmune response3637 A recent study has identified humanleukocyte antigen (HLA)-DRB15 as a risk factor for CIDPassociated with anti-NF155 antibodies and another studyreported different HLA antibody patterns with subclass switchafter renal transplantation3839 Whether interindividual differ-ences in antibody production and switching during the course ofdisease depend on (1) HLA alleles (2) antigen exposure or (3)further immunologic mechanisms has to be investigated infurther studies
Regarding characteristic clinical features of paranodopathy wereport neuropathic pain in antindashCaspr-1ndashrelated disease aspreviously described in patients with higher autoantibody tit-ers7 Different titers might explain discrepancies between ourresults and a recent study that did not report pain in antindashCaspr-1ndashpositive patients711 In our patients further features of IgG4-related chronic paranodopathy such as tremor sensory ataxiaand nephropathy561340 were only present in the course ofdisease suggesting that they evolve due to chronic autoantibody
Figure 4 Scheme of the autoantibody status in the course of disease in seropositive patients
The course of disease is shown on the x-axis by pseudo-logarithmic display of the time course Time point 0 is set at baseline assessment of serumCSF Thecolor code indicates severity of symptoms Results of serum assessment are displayed in black Patients 1 and 3 were lost to follow-up
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 9
exposure The pathomechanistic correlate of action tremorwhich recently has also been described in antindashCaspr-1 IgG3-related paranodopathy11 is still under research but studiessuggest cerebellar origin5612 In this study we detected in-trathecal autoantibodies in a patient with tremor as previouslyreported in a case of antindashpan-neurofascinndashpositive para-nodopathy12 The antiparanodal autoantibodies most probablydiffuse into the intrathecal compartment due to a blood-brainbarrier dysfunction as indicated by normal IgG and ASI indicesin our patients Our CSF data suggest that detection of anti-paranodal antibodies in CSF shows little sensitivity and dependson either high titer or strong leakage of the blood-brain barrierWe therefore encourage further studies on intrathecal anti-paranodal autoantibodies targeting sensitivity pathogenicityand association to clinical symptoms
Low prevalence of autoantibodies against paranodal proteins inacute inflammatory neuropathy retrospective assessment and thelow number of seropositive patients available for follow-up limitour study Clinical characteristics at acute onset and subclass-related seropositivity as a possible risk factor for chronic pro-gression when detected at the onset should be investigated in largemulticentric studies considering lowoverall prevalence ofGBS andA-CIDP as well as further possible confounders in analysis ofparanodopathy-specific clinical symptoms eg coinciding painfuldiabetic neuropathy The question of sequential subclass switchingin paranodopathy needs to be addressed in larger prospectivestudies Nevertheless our data (1) propose autoantibody subclassscreening to be considered in patients with GBS and A-CIDP asresults might have direct implications on diagnosis and therapeuticregime during the course of disease and (2) pave the way forfurther clinical and pathomechanistic studies on antiparanodalautoantibodies in acute to subacute immune-mediated neuropathy
AcknowledgmentThe authors thank Barbara Reuter and Antonia Kohl for theirsuperb technical assistance They thankMaximilian Frommer forprovision and processing of clinical data and PD Dr JakobMatschke for provision of slides and images of supplementarymorphologic data The study was supported by a grant of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg to KD and CV and a grant of the GermanResearch Foundation to KD (DFG DO-22191-1) LA issupported by a research fellowship as a clinician scientist of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg FL receives funding from the GermanMinistry of Education and Research (BMBF 01GM1908A) andthe German Research Foundation (DFG LE30642-1) CSreceives funding from the German Research Foundation (DFG3289-1 DFG UE 1714-1 DFG SO 32810-1 DFG SFB1158 and DFG SO 328 131) from the German Ministry ofEducation and Research (BMBF CMT-Net PI) and from theEuropean Union (Horizon 2020)
Study fundingThis study was supported by the Open Access PublicationFund of the University of Wurzburg
DisclosureL Appeltshauser K-P Wandinger R Junker C Sommer FLeypoldt and K Doppler work for an academic institutionoffering commercial antibody diagnostics AM Brunder AHeinius P Kortvelyessy and C Villmann report no dis-closures relevant to the manuscript C Sommer has served onscientific advisory boards for Algiax Alnylam Air LiquideAkcea Astellas Bayer Grifols Takeda and UCB She reportsbeing a member of speakersrsquo bureau and receiving speakerhonoraria from Akcea Alnylam Novartis Pfizer Sanofi-Aventis and Teva C Sommer served as a journal editorassociate editor or editorial advisory board member for theEuropean Journal of Neurology PLoS ONE and PAIN ReportsF Leypoldt reports speaker honoraria from Bayer RocheNovartis and Fresenius travel funding from Merck Grifolsand Bayer and serving on advisory boards for Roche Biogenand Alexion Go to NeurologyorgNN for full disclosures
Publication historyReceived by Neurology Neuroimmunology amp NeuroinflammationMarch 2 2020 Accepted in final form May 19 2020
Appendix Authors
Name Location Contribution
LuiseAppeltshauserMD
University Hospital ofWurzburg
Major role in the acquisitionanalysis and interpretationof the data and drafting andrevising the manuscript forintellectual content
Anna-MichelleBrunder
University Hospital ofWurzburg
Major role in the acquisitionand analysis of the data
Annika Heinius University Hospital ofSchleswig-HolsteinCampus Kiel
Major role in the acquisitionand analysis of the data
PeterKortvelyessyMD
University Hospital ofMagdeburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Klaus-PeterWandinger MD
University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Ralf Junker MD University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
CarmenVillmann PhD
University Hospital ofWurzburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
ClaudiaSommer MD
University Hospital ofWurzburg
Conceptualization of thestudy revising themanuscript for intellectualcontent and major role ininterpretation of data
Frank LeypoldtMD
University Hospital ofSchleswig-HolsteinCampus Kiel
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy and major role ininterpretation of data
10 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
References1 Fehmi J Scherer SS Willison HJ Rinaldi S Nodes paranodes and neuropathies
J Neurol Neurosurg Psychiatry 20188961ndash712 Uncini A Vallat JM Autoimmune nodo-paranodopathies of peripheral nerve the
concept is gaining ground J Neurol Neurosurg Psychiatry 201889627ndash6353 Querol L Illa I Paranodal and other autoantibodies in chronic inflammatory neu-
ropathies Curr Opin Neurol 201528474ndash4794 Querol L Rojas-Garcia R Diaz-Manera J et al Rituximab in treatment-resistant
CIDP with antibodies against paranodal proteins Neurol Neuroimmunol Neuro-inflamm 20152e149 doi 101212NXI0000000000000149
5 Querol L Nogales-Gadea G Rojas-Garcia R et al Neurofascin IgG4 antibodies inCIDP associate with disabling tremor and poor response to IVIg Neurology 201482879ndash886
6 Doppler K Appeltshauser L Wilhelmi K et al Destruction of paranodal architecturein inflammatory neuropathy with anti-contactin-1 autoantibodies J Neurol Neuro-surg Psychiatry 201586720ndash728
7 Doppler K Appeltshauser L Villmann C et al Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathyBrain 20161392617ndash2630
8 Manso C Querol L Mekaouche M Illa I Devaux JJ Contactin-1 IgG4 antibodiescause paranode dismantling and conduction defects Brain 20161391700ndash1712
9 Manso C Querol L Lleixa C et al Anti-Neurofascin-155 IgG4 antibodies preventparanodal complex formation in vivo J Clin Invest 20191292222ndash2236
10 Labasque M Hivert B Nogales-Gadea G Querol L Illa I Faivre-Sarrailh C Specificcontactin N-glycans are implicated in neurofascin binding and autoimmune targetingin peripheral neuropathies J Biol Chem 20142897907ndash7918
11 Cortese A Lombardi R Briani C et al Antibodies to neurofascin contactin-1 andcontactin-associated protein 1 in CIDP clinical relevance of IgG isotype NeurolNeuroimmunol Neuroinflamm 20197e639 doi 101212NXI0000000000000639
12 Stengel H Vural A Brunder AM et al Anti-pan-neurofascin IgG3 as a marker offulminant autoimmune neuropathy Neurol Neuroimmunol Neuroinflamm 20196e603 doi 101212NXI0000000000000603
13 Miura Y Devaux JJ Fukami Y et al Contactin 1 IgG4 associates to chronic inflammatorydemyelinating polyneuropathy with sensory ataxia Brain 20151381484ndash1491
14 Appeltshauser L Weishaupt A Sommer C Doppler K Complement depositioninduced by binding of anti-contactin-1 auto-antibodies is modified by immunoglo-bulins Exp Neurol 201728784ndash90
15 Doppler K Schuster Y Appeltshauser L et al Anti-CNTN1 IgG3 induces acuteconduction block and motor deficits in a passive transfer rat model J Neuro-inflammation 20191673
16 Sejvar JJ Kohl KS Gidudu J et al Guillain-Barre syndrome and Fisher syndrome casedefinitions and guidelines for collection analysis and presentation of immunizationsafety data Vaccine 201129599ndash612
17 Fokke C van den Berg B Drenthen J Walgaard C van Doorn PA Jacobs BCDiagnosis of Guillain-Barre syndrome and validation of Brighton criteria Brain 201413733ndash43
18 Kuitwaard K van Koningsveld R Ruts L Jacobs BC van Doorn PA RecurrentGuillain-Barre syndrome J Neurol Neurosurg Psychiatry 20098056ndash59
19 Van den Bergh PY Hadden RD Bouche P et al European Federation of NeurologicalSocietiesPeripheral Nerve Society guideline on management of chronic in-flammatory demyelinating polyradiculoneuropathy report of a joint task force of theEuropean Federation of Neurological Societies and the Peripheral Nerve Societymdashfirst revision Eur J Neurol 201017356ndash363
20 Ruts L Drenthen J Jacobs BC van Doorn PA Distinguishing acute-onset CIDP fromfluctuating Guillain-Barre syndrome a prospective study Neurology 2010741680ndash1686
21 McCombe PA Pollard JD McLeod JG Chronic inflammatory demyelinating poly-radiculoneuropathy A clinical and electrophysiological study of 92 cases Brain 19871101617ndash1630
22 Alessandro L Pastor Rueda JM Wilken M et al Differences between acute-onsetchronic inflammatory demyelinating polyneuropathy and acute inflammatory de-myelinating polyneuropathy in adult patients J Peripher Nerv Syst 201823154ndash158
23 Doppler K Appeltshauser L Kramer HH et al Contactin-1 and neurofascin-155-186 are not targets of auto-antibodies in multifocal motor neuropathy PLoS One201510e0134274
24 Ng JK Malotka J Kawakami N et al Neurofascin as a target for autoantibodies inperipheral neuropathies Neurology 2012792241ndash2248
25 Fujita A Ogata H Yamasaki RMatsushita T Kira JI Parallel fluctuation of anti-neurofascin155 antibody levels with clinico-electrophysiological findings in patients with chronic in-flammatory demyelinating polyradiculoneuropathy J Neurol Sci 2018384107ndash112
26 Niks EH van Leeuwen Y Leite MI et al Clinical fluctuations in MuSK myastheniagravis are related to antigen-specific IgG4 instead of IgG1 J Neuroimmunol 2008195151ndash156
27 Huijbers MG Vink AF Niks EH et al Longitudinal epitope mapping in MuSKmyasthenia gravis implications for disease severity J Neuroimmunol 201629182ndash88
28 Huang CC Lehman A Albawardi A et al IgG subclass staining in renal biopsies withmembranous glomerulonephritis indicates subclass switch during disease progressionMod Pathol 201326799ndash805
29 Collins AM Jackson KJ A temporal model of human IgE and IgG antibody functionFront Immunol 20134235
30 Valenzuela NM Schaub S The Biology of IgG subclasses and their clinical relevanceto transplantation Transplantation 2018102S7ndashS13
31 Vidarsson G Dekkers G Rispens T IgG subclasses and allotypes from structure toeffector functions Front Immunol 20145520ndash532
32 Bruhns P Iannascoli B England P et al Specificity and affinity of human Fcgamma receptorsand their polymorphic variants for human IgG subclasses Blood 20091133716ndash3725
33 Querol L Nogales-Gadea G Rojas-Garcia R et al Antibodies to contactin-1 inchronic inflammatory demyelinating polyneuropathy Ann Neurol 201373370ndash380
34 Devaux JJ Miura Y Fukami Y et al Neurofascin-155 IgG4 in chronic inflammatorydemyelinating polyneuropathy Neurology 201686800ndash807
35 Ogata H Yamasaki R Hiwatashi A et al Characterization of IgG4 anti-neurofascin155 antibody-positive polyneuropathy Ann Clin Transl Neurol 20152960ndash971
36 Doppler K Frank F Koschker AC Reiners K Sommer C Nodes of Ranvier in skinbiopsies of patients with diabetes mellitus J Peripher Nerv Syst 201722182ndash190
37 Banks W The blood-brain barrier interface in diabetes mellitus dysfunctionsmechanisms and approaches to treatment Curr Pharm Des 2020261438ndash1447
38 Arnold ML Ntokou IS Doxiadis II Spriewald BM Boletis JN Iniotaki AG Donor-specific HLA antibodies evaluating the risk for graft loss in renal transplant recipientswith isotype switch from complement fixing IgG1IgG3 to noncomplement fixingIgG2IgG4 anti-HLA alloantibodies Transpl Int 201427253ndash261
39 Martinez-Martinez L Lleixa MC Boera-Carnicero G et al Anti-NF155 chronic in-flammatory demyelinating polyradiculoneuropathy strongly associates to HLA-DRB15 J Neuroinflammation 201714224
40 Hashimoto Y Ogata H Yamasaki R et al Chronic inflammatory demyelinatingpolyneuropathy with concurrent membranous nephropathy an anti-paranode andpodocyte protein antibody study and literature survey Front Neurol 20189997
Appendix (continued)
Name Location Contribution
KathrinDoppler MD
University Hospital ofWurzburg
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy major role in analysisand interpretation of dataand study supervision
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 11
DOI 101212NXI000000000000081720207 Neurol Neuroimmunol Neuroinflamm
Luise Appeltshauser Anna-Michelle Brunder Annika Heinius et al Antiparanodal antibodies and IgG subclasses in acute autoimmune neuropathy
This information is current as of July 24 2020
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm
ServicesUpdated Information amp
httpnnneurologyorgcontent75e817fullhtmlincluding high resolution figures can be found at
References httpnnneurologyorgcontent75e817fullhtmlref-list-1
This article cites 40 articles 9 of which you can access for free at
Citations httpnnneurologyorgcontent75e817fullhtmlotherarticles
This article has been cited by 1 HighWire-hosted articles
Subspecialty Collections
httpnnneurologyorgcgicollectionperipheral_neuropathyPeripheral neuropathy
httpnnneurologyorgcgicollectionguillainbarre_syndromeGuillain-Barre syndrome
nating_polyneuropathyhttpnnneurologyorgcgicollectionchronic_inflammatory_demyeliChronic inflammatory demyelinating polyneuropathy
httpnnneurologyorgcgicollectionautoimmune_diseasesAutoimmune diseases
httpnnneurologyorgcgicollectionall_neuromuscular_diseaseAll Neuromuscular Diseasefollowing collection(s) This article along with others on similar topics appears in the
Permissions amp Licensing
httpnnneurologyorgmiscaboutxhtmlpermissionsits entirety can be found online atInformation about reproducing this article in parts (figurestables) or in
Reprints
httpnnneurologyorgmiscaddirxhtmlreprintsusInformation about ordering reprints can be found online
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm

and affinities Gain of specificity and affinity at switch from IgG3to IgG4 might explain different binding characteristics of anti-paranodal autoantibodies in the patient whose IgG3 autoanti-bodies reacted both against contactin-1Caspr-1 epitopes butwhose IgG4 autoantibodies were specific to Caspr-1 Here wereport IgG24 (1) in patient 4 in the chronic phase after subclassswitch and (2) in another patient at disease onset showingnonameliorating disease with high disability similar to previousreports on antindashcontactin-1 IgG4-related disease111333 So farstudies have only reported antiparanodal IgG4 autoantibodies inpatients with chronic disease7113435 We therefore suggest thathigh-affinity IgG4 autoantibody binding leads to chronic diseasewith functional impairment possibly caused by disturbance of theantigen interaction paranodal architecture and paranodalcomplex formation as demonstrated previously68ndash11
Patients of our study with predominant IgG3 subclass showedgood response to IVIg whereas patients with IgG2 and IgG4initially did not respond to IVIg or lost responsiveness in thedisease course Several studies described good responses toIVIg in IgG3-associated paranodopathy but poor responses inIgG4-associated disease56111334 IgG3 can initiate comple-ment activation opsonization antigen cross-linking and in-ternalization whereas IgG2 shows little C1q binding and IgG4completely lacks these Fc-mediated effector functions31 In anin vivo study on antindashcontactin-1 pathogenicity we suggestedcomplement-mediated functional impairment at the paranodesas the pathophysiologic correlate in IgG3-mediated para-nodopathy earlier and could show in vitro that IVIg inhibitscomplement deposition and activation dose-dependently1415
Our data therefore support the hypothesis that therapy re-sponsiveness might depend on IgG subclass and subclass-related pathomechanism of antiparanodal autoantibodies If
validated in larger studies these findings might have a directimpact on the treatment regime in affected patients Testing ofautoantibody subclass during the course of disease may beuseful as an indicator of a positive response to IVIg in case ofIgG3 whereas IgG4-related paranodopathy shows very goodresponse to rituximab46711
The trigger of the autoantibody production and the patho-mechanism behind possible IgG switching are still unknownThree of five seropositive patients in this study had diabetesmellitus type 2 as a comorbidity diagnosed years before theonset of disease Previous studies report disruption of the blood-brain barrier and the nodal architecture in patients with diabetesmellitus thereby possibly exposing paranodal antigens to theimmune response3637 A recent study has identified humanleukocyte antigen (HLA)-DRB15 as a risk factor for CIDPassociated with anti-NF155 antibodies and another studyreported different HLA antibody patterns with subclass switchafter renal transplantation3839 Whether interindividual differ-ences in antibody production and switching during the course ofdisease depend on (1) HLA alleles (2) antigen exposure or (3)further immunologic mechanisms has to be investigated infurther studies
Regarding characteristic clinical features of paranodopathy wereport neuropathic pain in antindashCaspr-1ndashrelated disease aspreviously described in patients with higher autoantibody tit-ers7 Different titers might explain discrepancies between ourresults and a recent study that did not report pain in antindashCaspr-1ndashpositive patients711 In our patients further features of IgG4-related chronic paranodopathy such as tremor sensory ataxiaand nephropathy561340 were only present in the course ofdisease suggesting that they evolve due to chronic autoantibody
Figure 4 Scheme of the autoantibody status in the course of disease in seropositive patients
The course of disease is shown on the x-axis by pseudo-logarithmic display of the time course Time point 0 is set at baseline assessment of serumCSF Thecolor code indicates severity of symptoms Results of serum assessment are displayed in black Patients 1 and 3 were lost to follow-up
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 9
exposure The pathomechanistic correlate of action tremorwhich recently has also been described in antindashCaspr-1 IgG3-related paranodopathy11 is still under research but studiessuggest cerebellar origin5612 In this study we detected in-trathecal autoantibodies in a patient with tremor as previouslyreported in a case of antindashpan-neurofascinndashpositive para-nodopathy12 The antiparanodal autoantibodies most probablydiffuse into the intrathecal compartment due to a blood-brainbarrier dysfunction as indicated by normal IgG and ASI indicesin our patients Our CSF data suggest that detection of anti-paranodal antibodies in CSF shows little sensitivity and dependson either high titer or strong leakage of the blood-brain barrierWe therefore encourage further studies on intrathecal anti-paranodal autoantibodies targeting sensitivity pathogenicityand association to clinical symptoms
Low prevalence of autoantibodies against paranodal proteins inacute inflammatory neuropathy retrospective assessment and thelow number of seropositive patients available for follow-up limitour study Clinical characteristics at acute onset and subclass-related seropositivity as a possible risk factor for chronic pro-gression when detected at the onset should be investigated in largemulticentric studies considering lowoverall prevalence ofGBS andA-CIDP as well as further possible confounders in analysis ofparanodopathy-specific clinical symptoms eg coinciding painfuldiabetic neuropathy The question of sequential subclass switchingin paranodopathy needs to be addressed in larger prospectivestudies Nevertheless our data (1) propose autoantibody subclassscreening to be considered in patients with GBS and A-CIDP asresults might have direct implications on diagnosis and therapeuticregime during the course of disease and (2) pave the way forfurther clinical and pathomechanistic studies on antiparanodalautoantibodies in acute to subacute immune-mediated neuropathy
AcknowledgmentThe authors thank Barbara Reuter and Antonia Kohl for theirsuperb technical assistance They thankMaximilian Frommer forprovision and processing of clinical data and PD Dr JakobMatschke for provision of slides and images of supplementarymorphologic data The study was supported by a grant of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg to KD and CV and a grant of the GermanResearch Foundation to KD (DFG DO-22191-1) LA issupported by a research fellowship as a clinician scientist of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg FL receives funding from the GermanMinistry of Education and Research (BMBF 01GM1908A) andthe German Research Foundation (DFG LE30642-1) CSreceives funding from the German Research Foundation (DFG3289-1 DFG UE 1714-1 DFG SO 32810-1 DFG SFB1158 and DFG SO 328 131) from the German Ministry ofEducation and Research (BMBF CMT-Net PI) and from theEuropean Union (Horizon 2020)
Study fundingThis study was supported by the Open Access PublicationFund of the University of Wurzburg
DisclosureL Appeltshauser K-P Wandinger R Junker C Sommer FLeypoldt and K Doppler work for an academic institutionoffering commercial antibody diagnostics AM Brunder AHeinius P Kortvelyessy and C Villmann report no dis-closures relevant to the manuscript C Sommer has served onscientific advisory boards for Algiax Alnylam Air LiquideAkcea Astellas Bayer Grifols Takeda and UCB She reportsbeing a member of speakersrsquo bureau and receiving speakerhonoraria from Akcea Alnylam Novartis Pfizer Sanofi-Aventis and Teva C Sommer served as a journal editorassociate editor or editorial advisory board member for theEuropean Journal of Neurology PLoS ONE and PAIN ReportsF Leypoldt reports speaker honoraria from Bayer RocheNovartis and Fresenius travel funding from Merck Grifolsand Bayer and serving on advisory boards for Roche Biogenand Alexion Go to NeurologyorgNN for full disclosures
Publication historyReceived by Neurology Neuroimmunology amp NeuroinflammationMarch 2 2020 Accepted in final form May 19 2020
Appendix Authors
Name Location Contribution
LuiseAppeltshauserMD
University Hospital ofWurzburg
Major role in the acquisitionanalysis and interpretationof the data and drafting andrevising the manuscript forintellectual content
Anna-MichelleBrunder
University Hospital ofWurzburg
Major role in the acquisitionand analysis of the data
Annika Heinius University Hospital ofSchleswig-HolsteinCampus Kiel
Major role in the acquisitionand analysis of the data
PeterKortvelyessyMD
University Hospital ofMagdeburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Klaus-PeterWandinger MD
University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Ralf Junker MD University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
CarmenVillmann PhD
University Hospital ofWurzburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
ClaudiaSommer MD
University Hospital ofWurzburg
Conceptualization of thestudy revising themanuscript for intellectualcontent and major role ininterpretation of data
Frank LeypoldtMD
University Hospital ofSchleswig-HolsteinCampus Kiel
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy and major role ininterpretation of data
10 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
References1 Fehmi J Scherer SS Willison HJ Rinaldi S Nodes paranodes and neuropathies
J Neurol Neurosurg Psychiatry 20188961ndash712 Uncini A Vallat JM Autoimmune nodo-paranodopathies of peripheral nerve the
concept is gaining ground J Neurol Neurosurg Psychiatry 201889627ndash6353 Querol L Illa I Paranodal and other autoantibodies in chronic inflammatory neu-
ropathies Curr Opin Neurol 201528474ndash4794 Querol L Rojas-Garcia R Diaz-Manera J et al Rituximab in treatment-resistant
CIDP with antibodies against paranodal proteins Neurol Neuroimmunol Neuro-inflamm 20152e149 doi 101212NXI0000000000000149
5 Querol L Nogales-Gadea G Rojas-Garcia R et al Neurofascin IgG4 antibodies inCIDP associate with disabling tremor and poor response to IVIg Neurology 201482879ndash886
6 Doppler K Appeltshauser L Wilhelmi K et al Destruction of paranodal architecturein inflammatory neuropathy with anti-contactin-1 autoantibodies J Neurol Neuro-surg Psychiatry 201586720ndash728
7 Doppler K Appeltshauser L Villmann C et al Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathyBrain 20161392617ndash2630
8 Manso C Querol L Mekaouche M Illa I Devaux JJ Contactin-1 IgG4 antibodiescause paranode dismantling and conduction defects Brain 20161391700ndash1712
9 Manso C Querol L Lleixa C et al Anti-Neurofascin-155 IgG4 antibodies preventparanodal complex formation in vivo J Clin Invest 20191292222ndash2236
10 Labasque M Hivert B Nogales-Gadea G Querol L Illa I Faivre-Sarrailh C Specificcontactin N-glycans are implicated in neurofascin binding and autoimmune targetingin peripheral neuropathies J Biol Chem 20142897907ndash7918
11 Cortese A Lombardi R Briani C et al Antibodies to neurofascin contactin-1 andcontactin-associated protein 1 in CIDP clinical relevance of IgG isotype NeurolNeuroimmunol Neuroinflamm 20197e639 doi 101212NXI0000000000000639
12 Stengel H Vural A Brunder AM et al Anti-pan-neurofascin IgG3 as a marker offulminant autoimmune neuropathy Neurol Neuroimmunol Neuroinflamm 20196e603 doi 101212NXI0000000000000603
13 Miura Y Devaux JJ Fukami Y et al Contactin 1 IgG4 associates to chronic inflammatorydemyelinating polyneuropathy with sensory ataxia Brain 20151381484ndash1491
14 Appeltshauser L Weishaupt A Sommer C Doppler K Complement depositioninduced by binding of anti-contactin-1 auto-antibodies is modified by immunoglo-bulins Exp Neurol 201728784ndash90
15 Doppler K Schuster Y Appeltshauser L et al Anti-CNTN1 IgG3 induces acuteconduction block and motor deficits in a passive transfer rat model J Neuro-inflammation 20191673
16 Sejvar JJ Kohl KS Gidudu J et al Guillain-Barre syndrome and Fisher syndrome casedefinitions and guidelines for collection analysis and presentation of immunizationsafety data Vaccine 201129599ndash612
17 Fokke C van den Berg B Drenthen J Walgaard C van Doorn PA Jacobs BCDiagnosis of Guillain-Barre syndrome and validation of Brighton criteria Brain 201413733ndash43
18 Kuitwaard K van Koningsveld R Ruts L Jacobs BC van Doorn PA RecurrentGuillain-Barre syndrome J Neurol Neurosurg Psychiatry 20098056ndash59
19 Van den Bergh PY Hadden RD Bouche P et al European Federation of NeurologicalSocietiesPeripheral Nerve Society guideline on management of chronic in-flammatory demyelinating polyradiculoneuropathy report of a joint task force of theEuropean Federation of Neurological Societies and the Peripheral Nerve Societymdashfirst revision Eur J Neurol 201017356ndash363
20 Ruts L Drenthen J Jacobs BC van Doorn PA Distinguishing acute-onset CIDP fromfluctuating Guillain-Barre syndrome a prospective study Neurology 2010741680ndash1686
21 McCombe PA Pollard JD McLeod JG Chronic inflammatory demyelinating poly-radiculoneuropathy A clinical and electrophysiological study of 92 cases Brain 19871101617ndash1630
22 Alessandro L Pastor Rueda JM Wilken M et al Differences between acute-onsetchronic inflammatory demyelinating polyneuropathy and acute inflammatory de-myelinating polyneuropathy in adult patients J Peripher Nerv Syst 201823154ndash158
23 Doppler K Appeltshauser L Kramer HH et al Contactin-1 and neurofascin-155-186 are not targets of auto-antibodies in multifocal motor neuropathy PLoS One201510e0134274
24 Ng JK Malotka J Kawakami N et al Neurofascin as a target for autoantibodies inperipheral neuropathies Neurology 2012792241ndash2248
25 Fujita A Ogata H Yamasaki RMatsushita T Kira JI Parallel fluctuation of anti-neurofascin155 antibody levels with clinico-electrophysiological findings in patients with chronic in-flammatory demyelinating polyradiculoneuropathy J Neurol Sci 2018384107ndash112
26 Niks EH van Leeuwen Y Leite MI et al Clinical fluctuations in MuSK myastheniagravis are related to antigen-specific IgG4 instead of IgG1 J Neuroimmunol 2008195151ndash156
27 Huijbers MG Vink AF Niks EH et al Longitudinal epitope mapping in MuSKmyasthenia gravis implications for disease severity J Neuroimmunol 201629182ndash88
28 Huang CC Lehman A Albawardi A et al IgG subclass staining in renal biopsies withmembranous glomerulonephritis indicates subclass switch during disease progressionMod Pathol 201326799ndash805
29 Collins AM Jackson KJ A temporal model of human IgE and IgG antibody functionFront Immunol 20134235
30 Valenzuela NM Schaub S The Biology of IgG subclasses and their clinical relevanceto transplantation Transplantation 2018102S7ndashS13
31 Vidarsson G Dekkers G Rispens T IgG subclasses and allotypes from structure toeffector functions Front Immunol 20145520ndash532
32 Bruhns P Iannascoli B England P et al Specificity and affinity of human Fcgamma receptorsand their polymorphic variants for human IgG subclasses Blood 20091133716ndash3725
33 Querol L Nogales-Gadea G Rojas-Garcia R et al Antibodies to contactin-1 inchronic inflammatory demyelinating polyneuropathy Ann Neurol 201373370ndash380
34 Devaux JJ Miura Y Fukami Y et al Neurofascin-155 IgG4 in chronic inflammatorydemyelinating polyneuropathy Neurology 201686800ndash807
35 Ogata H Yamasaki R Hiwatashi A et al Characterization of IgG4 anti-neurofascin155 antibody-positive polyneuropathy Ann Clin Transl Neurol 20152960ndash971
36 Doppler K Frank F Koschker AC Reiners K Sommer C Nodes of Ranvier in skinbiopsies of patients with diabetes mellitus J Peripher Nerv Syst 201722182ndash190
37 Banks W The blood-brain barrier interface in diabetes mellitus dysfunctionsmechanisms and approaches to treatment Curr Pharm Des 2020261438ndash1447
38 Arnold ML Ntokou IS Doxiadis II Spriewald BM Boletis JN Iniotaki AG Donor-specific HLA antibodies evaluating the risk for graft loss in renal transplant recipientswith isotype switch from complement fixing IgG1IgG3 to noncomplement fixingIgG2IgG4 anti-HLA alloantibodies Transpl Int 201427253ndash261
39 Martinez-Martinez L Lleixa MC Boera-Carnicero G et al Anti-NF155 chronic in-flammatory demyelinating polyradiculoneuropathy strongly associates to HLA-DRB15 J Neuroinflammation 201714224
40 Hashimoto Y Ogata H Yamasaki R et al Chronic inflammatory demyelinatingpolyneuropathy with concurrent membranous nephropathy an anti-paranode andpodocyte protein antibody study and literature survey Front Neurol 20189997
Appendix (continued)
Name Location Contribution
KathrinDoppler MD
University Hospital ofWurzburg
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy major role in analysisand interpretation of dataand study supervision
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 11
DOI 101212NXI000000000000081720207 Neurol Neuroimmunol Neuroinflamm
Luise Appeltshauser Anna-Michelle Brunder Annika Heinius et al Antiparanodal antibodies and IgG subclasses in acute autoimmune neuropathy
This information is current as of July 24 2020
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm
ServicesUpdated Information amp
httpnnneurologyorgcontent75e817fullhtmlincluding high resolution figures can be found at
References httpnnneurologyorgcontent75e817fullhtmlref-list-1
This article cites 40 articles 9 of which you can access for free at
Citations httpnnneurologyorgcontent75e817fullhtmlotherarticles
This article has been cited by 1 HighWire-hosted articles
Subspecialty Collections
httpnnneurologyorgcgicollectionperipheral_neuropathyPeripheral neuropathy
httpnnneurologyorgcgicollectionguillainbarre_syndromeGuillain-Barre syndrome
nating_polyneuropathyhttpnnneurologyorgcgicollectionchronic_inflammatory_demyeliChronic inflammatory demyelinating polyneuropathy
httpnnneurologyorgcgicollectionautoimmune_diseasesAutoimmune diseases
httpnnneurologyorgcgicollectionall_neuromuscular_diseaseAll Neuromuscular Diseasefollowing collection(s) This article along with others on similar topics appears in the
Permissions amp Licensing
httpnnneurologyorgmiscaboutxhtmlpermissionsits entirety can be found online atInformation about reproducing this article in parts (figurestables) or in
Reprints
httpnnneurologyorgmiscaddirxhtmlreprintsusInformation about ordering reprints can be found online
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm

exposure The pathomechanistic correlate of action tremorwhich recently has also been described in antindashCaspr-1 IgG3-related paranodopathy11 is still under research but studiessuggest cerebellar origin5612 In this study we detected in-trathecal autoantibodies in a patient with tremor as previouslyreported in a case of antindashpan-neurofascinndashpositive para-nodopathy12 The antiparanodal autoantibodies most probablydiffuse into the intrathecal compartment due to a blood-brainbarrier dysfunction as indicated by normal IgG and ASI indicesin our patients Our CSF data suggest that detection of anti-paranodal antibodies in CSF shows little sensitivity and dependson either high titer or strong leakage of the blood-brain barrierWe therefore encourage further studies on intrathecal anti-paranodal autoantibodies targeting sensitivity pathogenicityand association to clinical symptoms
Low prevalence of autoantibodies against paranodal proteins inacute inflammatory neuropathy retrospective assessment and thelow number of seropositive patients available for follow-up limitour study Clinical characteristics at acute onset and subclass-related seropositivity as a possible risk factor for chronic pro-gression when detected at the onset should be investigated in largemulticentric studies considering lowoverall prevalence ofGBS andA-CIDP as well as further possible confounders in analysis ofparanodopathy-specific clinical symptoms eg coinciding painfuldiabetic neuropathy The question of sequential subclass switchingin paranodopathy needs to be addressed in larger prospectivestudies Nevertheless our data (1) propose autoantibody subclassscreening to be considered in patients with GBS and A-CIDP asresults might have direct implications on diagnosis and therapeuticregime during the course of disease and (2) pave the way forfurther clinical and pathomechanistic studies on antiparanodalautoantibodies in acute to subacute immune-mediated neuropathy
AcknowledgmentThe authors thank Barbara Reuter and Antonia Kohl for theirsuperb technical assistance They thankMaximilian Frommer forprovision and processing of clinical data and PD Dr JakobMatschke for provision of slides and images of supplementarymorphologic data The study was supported by a grant of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg to KD and CV and a grant of the GermanResearch Foundation to KD (DFG DO-22191-1) LA issupported by a research fellowship as a clinician scientist of theInterdisciplinary Center of Clinical Research of the MedicalFaculty of Wurzburg FL receives funding from the GermanMinistry of Education and Research (BMBF 01GM1908A) andthe German Research Foundation (DFG LE30642-1) CSreceives funding from the German Research Foundation (DFG3289-1 DFG UE 1714-1 DFG SO 32810-1 DFG SFB1158 and DFG SO 328 131) from the German Ministry ofEducation and Research (BMBF CMT-Net PI) and from theEuropean Union (Horizon 2020)
Study fundingThis study was supported by the Open Access PublicationFund of the University of Wurzburg
DisclosureL Appeltshauser K-P Wandinger R Junker C Sommer FLeypoldt and K Doppler work for an academic institutionoffering commercial antibody diagnostics AM Brunder AHeinius P Kortvelyessy and C Villmann report no dis-closures relevant to the manuscript C Sommer has served onscientific advisory boards for Algiax Alnylam Air LiquideAkcea Astellas Bayer Grifols Takeda and UCB She reportsbeing a member of speakersrsquo bureau and receiving speakerhonoraria from Akcea Alnylam Novartis Pfizer Sanofi-Aventis and Teva C Sommer served as a journal editorassociate editor or editorial advisory board member for theEuropean Journal of Neurology PLoS ONE and PAIN ReportsF Leypoldt reports speaker honoraria from Bayer RocheNovartis and Fresenius travel funding from Merck Grifolsand Bayer and serving on advisory boards for Roche Biogenand Alexion Go to NeurologyorgNN for full disclosures
Publication historyReceived by Neurology Neuroimmunology amp NeuroinflammationMarch 2 2020 Accepted in final form May 19 2020
Appendix Authors
Name Location Contribution
LuiseAppeltshauserMD
University Hospital ofWurzburg
Major role in the acquisitionanalysis and interpretationof the data and drafting andrevising the manuscript forintellectual content
Anna-MichelleBrunder
University Hospital ofWurzburg
Major role in the acquisitionand analysis of the data
Annika Heinius University Hospital ofSchleswig-HolsteinCampus Kiel
Major role in the acquisitionand analysis of the data
PeterKortvelyessyMD
University Hospital ofMagdeburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Klaus-PeterWandinger MD
University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
Ralf Junker MD University Hospital ofSchleswig-HolsteinCampus Kiel
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
CarmenVillmann PhD
University Hospital ofWurzburg
Revising the manuscript forintellectual content andcontribution of vitalreagentstoolspatients
ClaudiaSommer MD
University Hospital ofWurzburg
Conceptualization of thestudy revising themanuscript for intellectualcontent and major role ininterpretation of data
Frank LeypoldtMD
University Hospital ofSchleswig-HolsteinCampus Kiel
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy and major role ininterpretation of data
10 Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 NeurologyorgNN
References1 Fehmi J Scherer SS Willison HJ Rinaldi S Nodes paranodes and neuropathies
J Neurol Neurosurg Psychiatry 20188961ndash712 Uncini A Vallat JM Autoimmune nodo-paranodopathies of peripheral nerve the
concept is gaining ground J Neurol Neurosurg Psychiatry 201889627ndash6353 Querol L Illa I Paranodal and other autoantibodies in chronic inflammatory neu-
ropathies Curr Opin Neurol 201528474ndash4794 Querol L Rojas-Garcia R Diaz-Manera J et al Rituximab in treatment-resistant
CIDP with antibodies against paranodal proteins Neurol Neuroimmunol Neuro-inflamm 20152e149 doi 101212NXI0000000000000149
5 Querol L Nogales-Gadea G Rojas-Garcia R et al Neurofascin IgG4 antibodies inCIDP associate with disabling tremor and poor response to IVIg Neurology 201482879ndash886
6 Doppler K Appeltshauser L Wilhelmi K et al Destruction of paranodal architecturein inflammatory neuropathy with anti-contactin-1 autoantibodies J Neurol Neuro-surg Psychiatry 201586720ndash728
7 Doppler K Appeltshauser L Villmann C et al Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathyBrain 20161392617ndash2630
8 Manso C Querol L Mekaouche M Illa I Devaux JJ Contactin-1 IgG4 antibodiescause paranode dismantling and conduction defects Brain 20161391700ndash1712
9 Manso C Querol L Lleixa C et al Anti-Neurofascin-155 IgG4 antibodies preventparanodal complex formation in vivo J Clin Invest 20191292222ndash2236
10 Labasque M Hivert B Nogales-Gadea G Querol L Illa I Faivre-Sarrailh C Specificcontactin N-glycans are implicated in neurofascin binding and autoimmune targetingin peripheral neuropathies J Biol Chem 20142897907ndash7918
11 Cortese A Lombardi R Briani C et al Antibodies to neurofascin contactin-1 andcontactin-associated protein 1 in CIDP clinical relevance of IgG isotype NeurolNeuroimmunol Neuroinflamm 20197e639 doi 101212NXI0000000000000639
12 Stengel H Vural A Brunder AM et al Anti-pan-neurofascin IgG3 as a marker offulminant autoimmune neuropathy Neurol Neuroimmunol Neuroinflamm 20196e603 doi 101212NXI0000000000000603
13 Miura Y Devaux JJ Fukami Y et al Contactin 1 IgG4 associates to chronic inflammatorydemyelinating polyneuropathy with sensory ataxia Brain 20151381484ndash1491
14 Appeltshauser L Weishaupt A Sommer C Doppler K Complement depositioninduced by binding of anti-contactin-1 auto-antibodies is modified by immunoglo-bulins Exp Neurol 201728784ndash90
15 Doppler K Schuster Y Appeltshauser L et al Anti-CNTN1 IgG3 induces acuteconduction block and motor deficits in a passive transfer rat model J Neuro-inflammation 20191673
16 Sejvar JJ Kohl KS Gidudu J et al Guillain-Barre syndrome and Fisher syndrome casedefinitions and guidelines for collection analysis and presentation of immunizationsafety data Vaccine 201129599ndash612
17 Fokke C van den Berg B Drenthen J Walgaard C van Doorn PA Jacobs BCDiagnosis of Guillain-Barre syndrome and validation of Brighton criteria Brain 201413733ndash43
18 Kuitwaard K van Koningsveld R Ruts L Jacobs BC van Doorn PA RecurrentGuillain-Barre syndrome J Neurol Neurosurg Psychiatry 20098056ndash59
19 Van den Bergh PY Hadden RD Bouche P et al European Federation of NeurologicalSocietiesPeripheral Nerve Society guideline on management of chronic in-flammatory demyelinating polyradiculoneuropathy report of a joint task force of theEuropean Federation of Neurological Societies and the Peripheral Nerve Societymdashfirst revision Eur J Neurol 201017356ndash363
20 Ruts L Drenthen J Jacobs BC van Doorn PA Distinguishing acute-onset CIDP fromfluctuating Guillain-Barre syndrome a prospective study Neurology 2010741680ndash1686
21 McCombe PA Pollard JD McLeod JG Chronic inflammatory demyelinating poly-radiculoneuropathy A clinical and electrophysiological study of 92 cases Brain 19871101617ndash1630
22 Alessandro L Pastor Rueda JM Wilken M et al Differences between acute-onsetchronic inflammatory demyelinating polyneuropathy and acute inflammatory de-myelinating polyneuropathy in adult patients J Peripher Nerv Syst 201823154ndash158
23 Doppler K Appeltshauser L Kramer HH et al Contactin-1 and neurofascin-155-186 are not targets of auto-antibodies in multifocal motor neuropathy PLoS One201510e0134274
24 Ng JK Malotka J Kawakami N et al Neurofascin as a target for autoantibodies inperipheral neuropathies Neurology 2012792241ndash2248
25 Fujita A Ogata H Yamasaki RMatsushita T Kira JI Parallel fluctuation of anti-neurofascin155 antibody levels with clinico-electrophysiological findings in patients with chronic in-flammatory demyelinating polyradiculoneuropathy J Neurol Sci 2018384107ndash112
26 Niks EH van Leeuwen Y Leite MI et al Clinical fluctuations in MuSK myastheniagravis are related to antigen-specific IgG4 instead of IgG1 J Neuroimmunol 2008195151ndash156
27 Huijbers MG Vink AF Niks EH et al Longitudinal epitope mapping in MuSKmyasthenia gravis implications for disease severity J Neuroimmunol 201629182ndash88
28 Huang CC Lehman A Albawardi A et al IgG subclass staining in renal biopsies withmembranous glomerulonephritis indicates subclass switch during disease progressionMod Pathol 201326799ndash805
29 Collins AM Jackson KJ A temporal model of human IgE and IgG antibody functionFront Immunol 20134235
30 Valenzuela NM Schaub S The Biology of IgG subclasses and their clinical relevanceto transplantation Transplantation 2018102S7ndashS13
31 Vidarsson G Dekkers G Rispens T IgG subclasses and allotypes from structure toeffector functions Front Immunol 20145520ndash532
32 Bruhns P Iannascoli B England P et al Specificity and affinity of human Fcgamma receptorsand their polymorphic variants for human IgG subclasses Blood 20091133716ndash3725
33 Querol L Nogales-Gadea G Rojas-Garcia R et al Antibodies to contactin-1 inchronic inflammatory demyelinating polyneuropathy Ann Neurol 201373370ndash380
34 Devaux JJ Miura Y Fukami Y et al Neurofascin-155 IgG4 in chronic inflammatorydemyelinating polyneuropathy Neurology 201686800ndash807
35 Ogata H Yamasaki R Hiwatashi A et al Characterization of IgG4 anti-neurofascin155 antibody-positive polyneuropathy Ann Clin Transl Neurol 20152960ndash971
36 Doppler K Frank F Koschker AC Reiners K Sommer C Nodes of Ranvier in skinbiopsies of patients with diabetes mellitus J Peripher Nerv Syst 201722182ndash190
37 Banks W The blood-brain barrier interface in diabetes mellitus dysfunctionsmechanisms and approaches to treatment Curr Pharm Des 2020261438ndash1447
38 Arnold ML Ntokou IS Doxiadis II Spriewald BM Boletis JN Iniotaki AG Donor-specific HLA antibodies evaluating the risk for graft loss in renal transplant recipientswith isotype switch from complement fixing IgG1IgG3 to noncomplement fixingIgG2IgG4 anti-HLA alloantibodies Transpl Int 201427253ndash261
39 Martinez-Martinez L Lleixa MC Boera-Carnicero G et al Anti-NF155 chronic in-flammatory demyelinating polyradiculoneuropathy strongly associates to HLA-DRB15 J Neuroinflammation 201714224
40 Hashimoto Y Ogata H Yamasaki R et al Chronic inflammatory demyelinatingpolyneuropathy with concurrent membranous nephropathy an anti-paranode andpodocyte protein antibody study and literature survey Front Neurol 20189997
Appendix (continued)
Name Location Contribution
KathrinDoppler MD
University Hospital ofWurzburg
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy major role in analysisand interpretation of dataand study supervision
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 11
DOI 101212NXI000000000000081720207 Neurol Neuroimmunol Neuroinflamm
Luise Appeltshauser Anna-Michelle Brunder Annika Heinius et al Antiparanodal antibodies and IgG subclasses in acute autoimmune neuropathy
This information is current as of July 24 2020
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm
ServicesUpdated Information amp
httpnnneurologyorgcontent75e817fullhtmlincluding high resolution figures can be found at
References httpnnneurologyorgcontent75e817fullhtmlref-list-1
This article cites 40 articles 9 of which you can access for free at
Citations httpnnneurologyorgcontent75e817fullhtmlotherarticles
This article has been cited by 1 HighWire-hosted articles
Subspecialty Collections
httpnnneurologyorgcgicollectionperipheral_neuropathyPeripheral neuropathy
httpnnneurologyorgcgicollectionguillainbarre_syndromeGuillain-Barre syndrome
nating_polyneuropathyhttpnnneurologyorgcgicollectionchronic_inflammatory_demyeliChronic inflammatory demyelinating polyneuropathy
httpnnneurologyorgcgicollectionautoimmune_diseasesAutoimmune diseases
httpnnneurologyorgcgicollectionall_neuromuscular_diseaseAll Neuromuscular Diseasefollowing collection(s) This article along with others on similar topics appears in the
Permissions amp Licensing
httpnnneurologyorgmiscaboutxhtmlpermissionsits entirety can be found online atInformation about reproducing this article in parts (figurestables) or in
Reprints
httpnnneurologyorgmiscaddirxhtmlreprintsusInformation about ordering reprints can be found online
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm

References1 Fehmi J Scherer SS Willison HJ Rinaldi S Nodes paranodes and neuropathies
J Neurol Neurosurg Psychiatry 20188961ndash712 Uncini A Vallat JM Autoimmune nodo-paranodopathies of peripheral nerve the
concept is gaining ground J Neurol Neurosurg Psychiatry 201889627ndash6353 Querol L Illa I Paranodal and other autoantibodies in chronic inflammatory neu-
ropathies Curr Opin Neurol 201528474ndash4794 Querol L Rojas-Garcia R Diaz-Manera J et al Rituximab in treatment-resistant
CIDP with antibodies against paranodal proteins Neurol Neuroimmunol Neuro-inflamm 20152e149 doi 101212NXI0000000000000149
5 Querol L Nogales-Gadea G Rojas-Garcia R et al Neurofascin IgG4 antibodies inCIDP associate with disabling tremor and poor response to IVIg Neurology 201482879ndash886
6 Doppler K Appeltshauser L Wilhelmi K et al Destruction of paranodal architecturein inflammatory neuropathy with anti-contactin-1 autoantibodies J Neurol Neuro-surg Psychiatry 201586720ndash728
7 Doppler K Appeltshauser L Villmann C et al Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathyBrain 20161392617ndash2630
8 Manso C Querol L Mekaouche M Illa I Devaux JJ Contactin-1 IgG4 antibodiescause paranode dismantling and conduction defects Brain 20161391700ndash1712
9 Manso C Querol L Lleixa C et al Anti-Neurofascin-155 IgG4 antibodies preventparanodal complex formation in vivo J Clin Invest 20191292222ndash2236
10 Labasque M Hivert B Nogales-Gadea G Querol L Illa I Faivre-Sarrailh C Specificcontactin N-glycans are implicated in neurofascin binding and autoimmune targetingin peripheral neuropathies J Biol Chem 20142897907ndash7918
11 Cortese A Lombardi R Briani C et al Antibodies to neurofascin contactin-1 andcontactin-associated protein 1 in CIDP clinical relevance of IgG isotype NeurolNeuroimmunol Neuroinflamm 20197e639 doi 101212NXI0000000000000639
12 Stengel H Vural A Brunder AM et al Anti-pan-neurofascin IgG3 as a marker offulminant autoimmune neuropathy Neurol Neuroimmunol Neuroinflamm 20196e603 doi 101212NXI0000000000000603
13 Miura Y Devaux JJ Fukami Y et al Contactin 1 IgG4 associates to chronic inflammatorydemyelinating polyneuropathy with sensory ataxia Brain 20151381484ndash1491
14 Appeltshauser L Weishaupt A Sommer C Doppler K Complement depositioninduced by binding of anti-contactin-1 auto-antibodies is modified by immunoglo-bulins Exp Neurol 201728784ndash90
15 Doppler K Schuster Y Appeltshauser L et al Anti-CNTN1 IgG3 induces acuteconduction block and motor deficits in a passive transfer rat model J Neuro-inflammation 20191673
16 Sejvar JJ Kohl KS Gidudu J et al Guillain-Barre syndrome and Fisher syndrome casedefinitions and guidelines for collection analysis and presentation of immunizationsafety data Vaccine 201129599ndash612
17 Fokke C van den Berg B Drenthen J Walgaard C van Doorn PA Jacobs BCDiagnosis of Guillain-Barre syndrome and validation of Brighton criteria Brain 201413733ndash43
18 Kuitwaard K van Koningsveld R Ruts L Jacobs BC van Doorn PA RecurrentGuillain-Barre syndrome J Neurol Neurosurg Psychiatry 20098056ndash59
19 Van den Bergh PY Hadden RD Bouche P et al European Federation of NeurologicalSocietiesPeripheral Nerve Society guideline on management of chronic in-flammatory demyelinating polyradiculoneuropathy report of a joint task force of theEuropean Federation of Neurological Societies and the Peripheral Nerve Societymdashfirst revision Eur J Neurol 201017356ndash363
20 Ruts L Drenthen J Jacobs BC van Doorn PA Distinguishing acute-onset CIDP fromfluctuating Guillain-Barre syndrome a prospective study Neurology 2010741680ndash1686
21 McCombe PA Pollard JD McLeod JG Chronic inflammatory demyelinating poly-radiculoneuropathy A clinical and electrophysiological study of 92 cases Brain 19871101617ndash1630
22 Alessandro L Pastor Rueda JM Wilken M et al Differences between acute-onsetchronic inflammatory demyelinating polyneuropathy and acute inflammatory de-myelinating polyneuropathy in adult patients J Peripher Nerv Syst 201823154ndash158
23 Doppler K Appeltshauser L Kramer HH et al Contactin-1 and neurofascin-155-186 are not targets of auto-antibodies in multifocal motor neuropathy PLoS One201510e0134274
24 Ng JK Malotka J Kawakami N et al Neurofascin as a target for autoantibodies inperipheral neuropathies Neurology 2012792241ndash2248
25 Fujita A Ogata H Yamasaki RMatsushita T Kira JI Parallel fluctuation of anti-neurofascin155 antibody levels with clinico-electrophysiological findings in patients with chronic in-flammatory demyelinating polyradiculoneuropathy J Neurol Sci 2018384107ndash112
26 Niks EH van Leeuwen Y Leite MI et al Clinical fluctuations in MuSK myastheniagravis are related to antigen-specific IgG4 instead of IgG1 J Neuroimmunol 2008195151ndash156
27 Huijbers MG Vink AF Niks EH et al Longitudinal epitope mapping in MuSKmyasthenia gravis implications for disease severity J Neuroimmunol 201629182ndash88
28 Huang CC Lehman A Albawardi A et al IgG subclass staining in renal biopsies withmembranous glomerulonephritis indicates subclass switch during disease progressionMod Pathol 201326799ndash805
29 Collins AM Jackson KJ A temporal model of human IgE and IgG antibody functionFront Immunol 20134235
30 Valenzuela NM Schaub S The Biology of IgG subclasses and their clinical relevanceto transplantation Transplantation 2018102S7ndashS13
31 Vidarsson G Dekkers G Rispens T IgG subclasses and allotypes from structure toeffector functions Front Immunol 20145520ndash532
32 Bruhns P Iannascoli B England P et al Specificity and affinity of human Fcgamma receptorsand their polymorphic variants for human IgG subclasses Blood 20091133716ndash3725
33 Querol L Nogales-Gadea G Rojas-Garcia R et al Antibodies to contactin-1 inchronic inflammatory demyelinating polyneuropathy Ann Neurol 201373370ndash380
34 Devaux JJ Miura Y Fukami Y et al Neurofascin-155 IgG4 in chronic inflammatorydemyelinating polyneuropathy Neurology 201686800ndash807
35 Ogata H Yamasaki R Hiwatashi A et al Characterization of IgG4 anti-neurofascin155 antibody-positive polyneuropathy Ann Clin Transl Neurol 20152960ndash971
36 Doppler K Frank F Koschker AC Reiners K Sommer C Nodes of Ranvier in skinbiopsies of patients with diabetes mellitus J Peripher Nerv Syst 201722182ndash190
37 Banks W The blood-brain barrier interface in diabetes mellitus dysfunctionsmechanisms and approaches to treatment Curr Pharm Des 2020261438ndash1447
38 Arnold ML Ntokou IS Doxiadis II Spriewald BM Boletis JN Iniotaki AG Donor-specific HLA antibodies evaluating the risk for graft loss in renal transplant recipientswith isotype switch from complement fixing IgG1IgG3 to noncomplement fixingIgG2IgG4 anti-HLA alloantibodies Transpl Int 201427253ndash261
39 Martinez-Martinez L Lleixa MC Boera-Carnicero G et al Anti-NF155 chronic in-flammatory demyelinating polyradiculoneuropathy strongly associates to HLA-DRB15 J Neuroinflammation 201714224
40 Hashimoto Y Ogata H Yamasaki R et al Chronic inflammatory demyelinatingpolyneuropathy with concurrent membranous nephropathy an anti-paranode andpodocyte protein antibody study and literature survey Front Neurol 20189997
Appendix (continued)
Name Location Contribution
KathrinDoppler MD
University Hospital ofWurzburg
Drafting and revising themanuscript for intellectualcontent design andconceptualization of thestudy major role in analysisand interpretation of dataand study supervision
NeurologyorgNN Neurology Neuroimmunology amp Neuroinflammation | Volume 7 Number 5 | September 2020 11
DOI 101212NXI000000000000081720207 Neurol Neuroimmunol Neuroinflamm
Luise Appeltshauser Anna-Michelle Brunder Annika Heinius et al Antiparanodal antibodies and IgG subclasses in acute autoimmune neuropathy
This information is current as of July 24 2020
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm
ServicesUpdated Information amp
httpnnneurologyorgcontent75e817fullhtmlincluding high resolution figures can be found at
References httpnnneurologyorgcontent75e817fullhtmlref-list-1
This article cites 40 articles 9 of which you can access for free at
Citations httpnnneurologyorgcontent75e817fullhtmlotherarticles
This article has been cited by 1 HighWire-hosted articles
Subspecialty Collections
httpnnneurologyorgcgicollectionperipheral_neuropathyPeripheral neuropathy
httpnnneurologyorgcgicollectionguillainbarre_syndromeGuillain-Barre syndrome
nating_polyneuropathyhttpnnneurologyorgcgicollectionchronic_inflammatory_demyeliChronic inflammatory demyelinating polyneuropathy
httpnnneurologyorgcgicollectionautoimmune_diseasesAutoimmune diseases
httpnnneurologyorgcgicollectionall_neuromuscular_diseaseAll Neuromuscular Diseasefollowing collection(s) This article along with others on similar topics appears in the
Permissions amp Licensing
httpnnneurologyorgmiscaboutxhtmlpermissionsits entirety can be found online atInformation about reproducing this article in parts (figurestables) or in
Reprints
httpnnneurologyorgmiscaddirxhtmlreprintsusInformation about ordering reprints can be found online
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm

DOI 101212NXI000000000000081720207 Neurol Neuroimmunol Neuroinflamm
Luise Appeltshauser Anna-Michelle Brunder Annika Heinius et al Antiparanodal antibodies and IgG subclasses in acute autoimmune neuropathy
This information is current as of July 24 2020
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm
ServicesUpdated Information amp
httpnnneurologyorgcontent75e817fullhtmlincluding high resolution figures can be found at
References httpnnneurologyorgcontent75e817fullhtmlref-list-1
This article cites 40 articles 9 of which you can access for free at
Citations httpnnneurologyorgcontent75e817fullhtmlotherarticles
This article has been cited by 1 HighWire-hosted articles
Subspecialty Collections
httpnnneurologyorgcgicollectionperipheral_neuropathyPeripheral neuropathy
httpnnneurologyorgcgicollectionguillainbarre_syndromeGuillain-Barre syndrome
nating_polyneuropathyhttpnnneurologyorgcgicollectionchronic_inflammatory_demyeliChronic inflammatory demyelinating polyneuropathy
httpnnneurologyorgcgicollectionautoimmune_diseasesAutoimmune diseases
httpnnneurologyorgcgicollectionall_neuromuscular_diseaseAll Neuromuscular Diseasefollowing collection(s) This article along with others on similar topics appears in the
Permissions amp Licensing
httpnnneurologyorgmiscaboutxhtmlpermissionsits entirety can be found online atInformation about reproducing this article in parts (figurestables) or in
Reprints
httpnnneurologyorgmiscaddirxhtmlreprintsusInformation about ordering reprints can be found online
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm

ServicesUpdated Information amp
httpnnneurologyorgcontent75e817fullhtmlincluding high resolution figures can be found at
References httpnnneurologyorgcontent75e817fullhtmlref-list-1
This article cites 40 articles 9 of which you can access for free at
Citations httpnnneurologyorgcontent75e817fullhtmlotherarticles
This article has been cited by 1 HighWire-hosted articles
Subspecialty Collections
httpnnneurologyorgcgicollectionperipheral_neuropathyPeripheral neuropathy
httpnnneurologyorgcgicollectionguillainbarre_syndromeGuillain-Barre syndrome
nating_polyneuropathyhttpnnneurologyorgcgicollectionchronic_inflammatory_demyeliChronic inflammatory demyelinating polyneuropathy
httpnnneurologyorgcgicollectionautoimmune_diseasesAutoimmune diseases
httpnnneurologyorgcgicollectionall_neuromuscular_diseaseAll Neuromuscular Diseasefollowing collection(s) This article along with others on similar topics appears in the
Permissions amp Licensing
httpnnneurologyorgmiscaboutxhtmlpermissionsits entirety can be found online atInformation about reproducing this article in parts (figurestables) or in
Reprints
httpnnneurologyorgmiscaddirxhtmlreprintsusInformation about ordering reprints can be found online
Academy of Neurology All rights reserved Online ISSN 2332-7812Copyright copy 2020 The Author(s) Published by Wolters Kluwer Health Inc on behalf of the AmericanPublished since April 2014 it is an open-access online-only continuous publication journal Copyright
is an official journal of the American Academy of NeurologyNeurol Neuroimmunol Neuroinflamm