3-1 Boundary of Cell
Transcript of 3-1 Boundary of Cell

3-1 At the Boundary of the Cell
• Your body is composed of trillions of cells. We must look closely at cells to understand how they function.
OBJECTIVES• Define the function of a cell membrane.• Explain why smaller cells function more
efficiently than larger cells.• Distinguish between polar and non-polar
molecules.• Compare the interaction of water molecules with
each other and with lipid molecules.

Cells: The Smallest Vessels of Life • All living things are made
of tiny compartments called cells.
• A cell is the smallest unit that can carry out all the activities necessary for life.
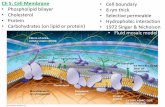
• The outer surface of a cell, called the cell membrane shields the delicate internal machinery of a cell.
• The cell membrane separates what is inside from what is outside.

Cells: The Smallest Vessels of Life • A cell membrane has
"gates" that allow the raw materials needed by the cell to enter and that allow harmful waste products to exit promptly.
• Without this flow of materials, a cell would not be able to survive.
• The cell membrane helps to maintain the internal environment of the cell by regulating what enters and leaves the cell,.
gate

What Limits the Size of a Cell?• Cell sizes vary, but most
cells are so small that you can see them only through a microscope.
• Cell parts cannot be too far from the cell membrane
• Every bit of food and information needed by the cell must enter through the cell membrane. When cells are small, no part of their complex machinery lies too far from the area outside the cell.
• Small cells work more efficiently.

A Cell's Volume Increases Faster Than Its Surface Area
• As a cell grows, it takes in more food and creates more wastes.
• These substances must pass into and out of the "gates" in the cell membrane, which must be large enough to service the cell's needs.
• As the cell grows, so does its membrane, but cells cannot grow indefinitely.
gate

A Cell's Volume Increases Faster Than Its Surface Area
• As a cell grows, its volume increases at a greater rate than its surface area.
• The ratio of a cell's surface area to its volume limits how large a cell can become.
• Cells cannot grow so large that their surface area becomes too small to take in enough food and remove enough wastes.
Surface area = 96mmVolume = 64 mm
2
3

Water and the Cell• All cells are surrounded by
water and contain water. • Single-celled creatures
swim in ponds and oceans. • Even blood or skin cells,
are surrounded by a thin film of water.
• All of the complex machinery inside the cell perform their functions in water.
• The water inside and outside the cell shapes the cell membrane.
water
lipids

Water Is a Polar Molecule
• The chemical formula for water is H2O.
• A water molecule is made of two hydrogen atoms and one oxygen atom bonded together.
• These bonds form when hydrogen and oxygen atoms share pairs of electrons.

Water Is a Polar Molecule• An important thing about a
water molecule is that the oxygen atom attracts electrons more strongly than the hydrogen atoms do.
• The electrons in the bonds between the oxygen and each hydrogen atoms are not shared equally - they are more likely to be near the oxygen atom.
• Because electrons have a negative charge, the oxygen part of the water molecule is slightly negative.

Water Is a Polar Molecule• The hydrogen atoms in the
water molecule have slightly positive charges.
• The oxygen side of the molecule has a partial negative charge and the hydrogen side of the molecule has a partial positive charge.
• A molecule with a partial negative charge on one side and a partial positive charge on the other side is called a polar molecule.
• Most of the properties of water are the result of its polarity.

• In this model of a water molecule, the area near the oxygen atom has a partial negative charge; the areas near the hydrogen atoms have partial positive charges.Oxygen H
H +’ve
–’ve
Water Is a Polar Molecule

Water Molecules Cluster Together• There is an attractive force
between particles of opposite charge.
• When together, the positive charge of one water molecule attracts the negative charge of another water molecule.
• The attraction of hydrogen atoms of one water molecule to the oxygen atom of another water molecule forms an attractive force called a hydrogen bond.
Hydrogen bonds

Water Molecules Cluster Together• Water molecules are at a
lower energy state when bonded with each other.
• All things tend toward lower energy so there is a natural tendency for water molecules to form hydrogen bonds. Water molecules are at a lower energy state when bonded with each other.
• All things tend toward lower energy so there is a natural tendency for water molecules to form hydrogen bonds. Water bonded by Hydrogen bonds

Water and The Cell Membrane• Water shapes the cell
membrane. • The cell membrane is basically
a sheet of lipids. • The interaction between water
and lipids shapes the cell membrane.
• Oil and water will not mix and soon after mixing, small beads of oil form.
• The water and oil separate into two distinct layers.

Water and The Cell Membrane
• Water molecules start to cluster together because they form hydrogen bonds with each other.
• Lipids are non-polar and are not attracted to the water.
• Because the water molecules attract one another, the oil is pushed away.

SECTION REVIEW
• What is the function of a cell membrane?

SECTION REVIEW
• Why is it advantageous for a cell to grow only to a certain size and then divide into two smaller cells?

SECTION REVIEW
• How does a polar molecule differ from a non-polar molecule? Give an example of each molecule.

SECTION REVIEW
• How do the properties of water help shape a cell membrane?

References & Acknowledgements
• Johnson, George B.: Biology, Visualizing Life, HOLT, RINEHART & WINSTON; Austin,1998.
• Miller, Kenneth R. & Levine, Joseph: Biology, Prentice Hall; Upper Saddle River, New Jersey, 2004.
• Campbell, Neil A., Williamson, Brad & Heyden, Robin J. BIOLOGY, Exploring Life, Prentice Hall; Needham, Massachusetts, 2004.
• Biggs, Alton, Hagins; Whitney Crispen; Kapica, Chris; Lundgren, Linda; Rillero, Peter; Tallman, Kathleen G.; Zike, Dinah; National Geographic Society; Biology, The Dynamics of Life, McGraw Hill, Glencoe, New York, New York, 2004.