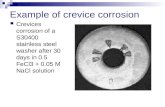
2 Corrosion Final
-
Upload
marcelo-torres -
Category
Documents
-
view
221 -
download
0
Transcript of 2 Corrosion Final

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 1/26
CorrosionTommy Lundin

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 2/26
© Metso
•Process• Corrosion

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 3/26
© Metso
Introduction
• Oxidation (and thus corrosion) is the aim of the nature to reachequilibrium
• Therefore, all boilers will corrode
• The only thing we can influence is the rate
•This summary deals only with gas side corrosion processes
• Steam side corrosion is not considered in this summary, but it shouldnot be totally neglected
• The flue gas moisture content may have a significant effect on thecorrosion process and corrosion rate of alloys

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 4/26
© Metso
• High temperature oxidation
• Hot corrosion
• Active oxidation
• Smelt induced corrosion
• Sulfidation
• Acidic sulfates
Corrosion types in recovery boilers

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 5/26
© Metso
Lower furnace:• Bottom tubes
• Wall tubes
• Air ports
Location of corrosion in recovery boilers
Upper furnace:• Superheaters
• Superheater ties
Second pass:• Boiler bank
• Economizers

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 6/26

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 7/26
© Metso
High temperature oxidation
• One of the main reasons for long-term tube wastage in boilers• Limits the use of low-alloyed steels at high steam temperatures
• Present in most corrosion processes
• Increases corrosion rate as a secondary process in connection with
the primary mechanism• Can not be totally avoided, but can be controlled by using high
chromium (Cr) alloys

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 8/26
© Metso
0.0
5.0
10.0
15.0
20.0
25.0
30.0
100 280 460 640 820 1 000
Temperature (C)
P e n e t r a t i o n ( m m )
Carbon steel AISI 304 AISI 316
AISI 310 AISI 347
High temperature oxidation
• Oxidation typically occurs whenmetals are exposed totemperatures above 300 C
• Wüstite (FeO) formation above570 C increases the oxidation
rate of low-alloyed steels• Chromium (Cr) alloying
increases oxidation resistanceof the alloys
10 000 h, 5% O2

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 9/26

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 10/26
© Metso
Hot corrosion
• Main contributors to the FMT (T0) are chlorine (Cl) and potassium (K)• High amount of carbonates (CO3) i.e. carryover lowers the FMT of
the deposits
• Even small amount of sulfides (0.1 wt.-%) in the deposits lowers theFMT about 50 C
41 Na2SO4 - 59 K2SO4 831
26 KCl - 74 K2SO4 689
50 NaCl - 50 KCl 656
48 NaCl - 52 Na2SO4 628
28 KCl - 38 NaCl - 14 K2SO4 - 20 Na2SO4 518
Deposit mixture (mol-%) FMT (°C)

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 11/26
© Metso
Hot corrosion
• Main contributors to hot corrosion are chlorine (Cl) and alkalis (Na,K)
• Pure compounds rarely cause problems, but a mixture of compoundsmay produce an extremely low FMT
• Two non-corrosive compounds may form a low melting mixture andmay become highly corrosive
• The main corrosion mechanism in hot corrosion is acidic or basic fluxing
• In practice this mean that the metal or alloy dissolves into the melt

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 12/26
© Metso
KVAERNER POWER
Chlorine influence on ash melting behaviour
• Chlorine (Cl) does not affectthe FMT of the deposits
• Increase in Cl content,increases the amount of meltin deposits
0
20
40
60
80
00
500 600 700 800
0,2%
1%
5%
Temperature, °C
M e l t , %
T70
T15
Chlorine, wt-% in ash:

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 13/26
© Metso
KVAERNER POWER
0
20
40
60
80
100
500 600 700 800
Potassium influence on ash melting behaviour
• Potassium (K) does notaffect the amount of melt inthe deposits
• Increase in K content,decreases the FMT
0,2%
2%
5%
Temperature, °C
M e l t , %
T70
T15
Potassium, wt-% in ash:

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 14/26
© Metso
KVAERNER POWER
0
20
40
60
80
00
500 600 700 800
Sulfide influence on ash melting behaviour
• Sulfide (S2-) does not affectthe amount of melt in thedeposits
• Increase in S2- content,decreases the FMT
0%
0,1%
1%
Temperature, °C
M e l t , %
T70
T15
Sulfide, wt-% in ash:

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 15/26
© Metso
Hot corrosion
• Gaseous potassium chloride (KCl) can react with chromium oxidescale, forming potassium chromates:
Cr 2O3(s) + 4 KCl(g) + 2 H2O(g) + 1.5 O2(g) 2 K2CrO4(s) + 4 HCl(g)
Cr 2O3(s) + 4 KCl(g) + 2.5 O2(g) 2 K2CrO4(s) + 2 Cl2(g)
•
Even low concentrations (~5 ppm) of gaseous KCl can be verycorrosive
• The protective properties of the oxide scale are lost
• Gaseous KCl is blamed to be one of the main contributors to thebreakdown of the protecting oxide scale of alloys

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 16/26
© Metso
Active oxidation
• Active oxidation is a chlorine-induced corrosion process- Initiated by the release of volatile chlorine from alkali chlorides or by the reaction
of alkali chlorides with the oxide scale
- Chlorine reacts selectively with Fe in the alloy, forming volatile iron chlorides
- Iron chlorides are oxidized, releasing chlorine back to the process
•Threshold temperature 450 °C
- Active oxidation is not present when metal surfaces are below this temperature
• Activated oxidation is present only when potassium chloride (KCl) isavailable

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 17/26
© Metso
Active oxidation
• Chlorine acts as a catalyst and may initiate achlorine circulation process in the material
- Released chlorine will react again with the metal- Part of the chlorine will escape from the metal and
the process will eventually cease without anexternal Cl source
• Corrosion by activated oxidation processproceeds along the grain boundaries
- Intergranular oxidation- Present only in high chromium alloys

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 18/26

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 19/26
© Metso
KVAERNER POWER
Smelt induced corrosion
• Molten alkali hydroxides are blamed to
be responsible for the primary air port corrosion
• Molten alkali hydroxide (MeOH) mayreact with chromium oxide scale, formingalkali chromates:
Cr 2O3(s) + 4 MeOH(l) + 1.5 O2(g)
2 Me2CrO4(dissolved) + 2 H2O(g)
• Formed alkali chromate dissolves into
the melt and is nonprotective

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 20/26
© Metso
Sulfidation
• Occurs when metals are exposed to temperatures above 200 °C ingases containing more than 1 ppm hydrogen sulfide gas (H2S)
• Reducing conditions may be due to low air ratio or high unburntcarbon content
• Gaseous methyl mercaptan (CH3SH) increases corrosion rate attemperatures higher than 320 °C
• The presence of gaseous hydrogen chloride (HCl) increases thecorrosion rate in reducing conditions
• The corrosion rate is related to the formation of metal sulfides, which
contain large amount of defects

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 21/26
© Metso
Sulfidation
• Sulfidation rate increases withtemperature
• High steam temperatures requirethe use of AISI 304 composite furnace wall tubing
• Alloy AISI 304 is applicable attemperatures below 350 C
• High nickel (Ni) alloys susceptible for sulfidation
0,00
0,10
0,20
0,30
0,40
0,50
0,60
0,70
0,80
0,90
1,00
250 270 290 310 330 350
Temperature (C)
P e n e t r a t i o n ( m m )
Carbon Steel AISI 304 AISI 316
AISI 310 AISI 347
1 000 ppm H2S, 20 000 h

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 22/26

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 23/26
© Metso
Acidic sulfates
by Dr. R. Backman, Åbo Akademi University

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 24/26
© Metso
Prevention of corrosion
•Flue gas inlet temperature into
- Superheater area
- Boiler bank
• Proper mixing in the furnace- Air delivery and staging
- Black liquor spraying and distribution
• Location of the burners
- CNCG burners preferentially below liquor guns
• Lower furnace materials
- High Cr alloy composite tubing
• Arrangement of heat transfer surfaces

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 25/26
© Metso
Prevention of corrosion
• High Cr content of alloys increases oxidation resistance- Wüstite formation above 570 °C
- Cr content preferably higher than 20%
- Intergranular oxidation of austenitic steels
• High Ni content increases resistance to chlorine-induced corrosion
- Ni susceptible to sulfidation
• High Cr alloys vulnerable to alkali chromate formation- Favored in high potassium atmospheres

8/13/2019 2 Corrosion Final
http://slidepdf.com/reader/full/2-corrosion-final 26/26
© Metso
Summary
• Gaseous HCl is not causing problems in oxidizing conditions, whensteam temperature is below 550 °C
• Alkali chlorides are the main cause for corrosion- Low FMT molten phase corrosion
- Sulfation of chlorides active oxidation
- Cracking of oxide scales enhanced oxidation
• Threshold material temperatures- 450 °C for chlorine-induced corrosion
- 480 °C for hot corrosion