Naming Compounds Involving Transition Metals and Polyatomic Ions.
Polyatomic compounds combine polyatomic ions with metals Poly atomic ions are groups of atoms that...
-
Upload
april-norman -
Category
Documents
-
view
228 -
download
0
description
Transcript of Polyatomic compounds combine polyatomic ions with metals Poly atomic ions are groups of atoms that...

Polyatomic compounds combine polyatomic ions with metals
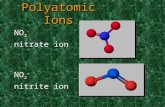
Poly atomic ions are groups of atoms that tend to stay together and carry an overall ionic charge
e.g. Nitrate - NO31-

Oxyacids: Compounds formed when hydrogen combines with polyatomic ions that contain oxygen

Molecular compounds are groups of elements that come together and “share” electrons
When elements share electrons it is called a covalent bond
This is different from ionic bonding because it is the sharing of electrons that holds the compound together and not ionic charges

In order to write formulas for molecular compounds you must know the combining capacity of the non-metals involved in forming a molecule
Combining capacity is a measure of the number of covalent bonds needed for a material to form a stable molecule (closed outer shell)
How does it work?1. Write the symbols, with their combining capacities above (always
write the symbol with the highest combining capacity first)4 2C S
2. Use the crisscross method to determine each element’s subscript4 2C S C2S4 CS2 (reduced)

Many molecular compounds have names similar to ionic compounds for example:
H2S Hydrogen Sulfide Others have common names; water (H2O), ammonia (NH3),
methane (CH4) Molecular compound names often contain prefixes that refer to
the amount of atoms of a given substance are present in each molecule:
CO2 Carbon dioxide
CO Carbon monoxide


Many nonmetallic elements exist as covalently bonded molecules.
These elements form Diatomic molecules (molecules made up of two similar atoms)