Yanoff _ Duker, Ophthalmology, 3rd Ed- Glaucome Section
description
Transcript of Yanoff _ Duker, Ophthalmology, 3rd Ed- Glaucome Section
-
1162
groups from 4.7 (per 100 000 per year in the population aged 30 years and older) in Finland21 to 11.4 in Japan22 and 12.2 in Singapore.19
Risk Factors 1. Demographic factors:
inthepresenceofapatentperipheraliridectomy.Inplateauirisconfigurationtheciliaryprocessesareanteriorlypositioned,creatinganarrowangle,buttheangleisnotoccludablefollowingperipheraliridectomy.
n Angleclosureassociatedwithaposteriorpushingmechanism(e.g.,INTRODUCTIONAngle-closure glaucoma (ACG) was probably the first glaucoma to be recognized, when St Yves, in 1722, described its symptoms, signs, and prognosis. It was not until 1923, however, when Raeder proposed that glaucoma be classified into two main types, one with a shallow anterior
a. Age (> 60 years old) b. Female sex c. Chinese ethnic origin23 d. Family history (especially first-degree relatives, because ocular
anatomic features are inherited) 2. Anatomic factors:2428 a. Shallow anterior chamber depth, especially peripherally b. Thick/anteriorly positioned/increased anterior curvature of lens c. Short axial length d. Small diameter/increased curvature of cornea e. Plateau iris configuration/thick peripheral iris roll
vitreoushydration,suprachoroidalorsubretinalhemorrhage,etc.),isassociatedwithanaxially shallowanteriorchamber.Angle-Closure GlaucoJovina L. S. See and Paul T. K. Chew
Definition: Agroupofglaucomascharacterizedbymechanicalob-structionofthetrabecularmeshworkbyeitherpupillaryblock(primaryangle-closureglaucoma),ananteriorpullingmechanism,oraposteriorpushingmechanism.Theclosureinprimaryangle-closureglaucomamaybeduetoappositionoftheperipheraliristothetrabecularmeshworkortosynechialangleclosure,eitherofwhichmayresultinamechanicalobstructionofaqueousoutflow.
Key featuresn Angleclosuremaybeacute,subacute,orchronic.n Acuteangleclosuremayresultinsuddenpain,blurredvision,
photophobia,coloredhaloesaroundlights,ocularinjection,headache,nausea,andvomiting.
n Subacuteangleclosuremaybesymptomaticwithheadaches,oftenmistakenformigrainebybothpatientandnonophthalmicphysician,orasymptomatic.
n Chronicangleclosureisgenerallyasymptomatic.
Associated featuresn Acuteangleclosure: Peripherallyshallowanteriorchamber. Shortaxiallengthiscommon. Pupillaryblock. Hazycornea. Mid-dilatedpupil. Glaukomflecken(anteriorsubcapsularlensopacityduetolens
epithelialcellnecrosis,rarelyseen). Irisbombe(associatedwithprominentocularinflammation).n Chronicangleclosureisoftenasymptomaticandfrequently
misdiagnosedasprimaryangle-closureglaucoma.n Angleclosureassociatedwithplateauirissyndromewilloccur
PART 10 GLAUCOMASECTION 3 SpecificTypesofGlaucomachamber and the other with a normal or deep chamber, that ACG was distinguished from open-angle glaucoma (OAG).
Population-based surveys of the prevalence of eye diseases in Europe1 and the United States24 suggest a much greater rate of OAG compared to ACG. Hence, little was published about the epidemiology of ACG, until recent epidemiologic studies in Asia reported that Eskimos,56 Mongolians,7 and Chinese89 had significantly higher rates of ACG. It is now confirmed that not only is ACG more common than originally thought, but also it is associated with a much higher visual morbidity than OAG. ACG, if recognized and treated early, results in a good visual prognosis. Visual morbidity can be prevented if ACG is detected early; hence, early detection is key.
EPIDEMIOLOGY AND PATHOGENESIS
PrevalenceThe prevalence of primary angle-closure glaucoma (PACG) in Whites is reported to be 0.6% in Italy10 and 0.5% in Wales11 in the 40 plus age group, and 0.1% in the 55 plus age group in Sweden.12 The prevalence in Eskimos is some 20 to 40 times higher.5, 6, 13, 14 The prevalence in East Asia and Southeast Asia has been reported to range from 1.4% to 4.3%, depending on the age group.7, 9, 15
Based on various population-based studies of the prevalence of ACG, it is estimated that in the year 2010, there will be a total of 60.5 million people with OAG or ACG, this number increasing to 79.6 million by 2020.16 Of these, 26% will have ACG. The prevalence of ACG in 2010 among those aged 40 years and older is estimated to be 1.26% in China and 1.20% in Southeast Asia, compared to 0.25% in Europe and 0.16% in Africa. Given the high prevalence of ACG in Asia,17, 18 Asians are predicted to represent 87% of those with ACG. Women will comprise 69.5% of cases of ACG, due to the higher prevalence of the disease in women,19 as well as their greater longevity.
The World Health Organization currently ranks glaucoma as the sec-ond most common cause of blindness.20 By 2010, bilateral blindness is estimated to be present in 3.9 million people with ACG, rising to 5.3 million people in 2020. The number of people blinded by ACG is nearly equal to the number blinded by OAG because of the higher morbidity of the former disease.
IncidenceThe incidence of acute PACG varies widely among different ethnic
ma 10.12
-
10.12
1163
Angle-ClosureG
laucoma
3. Precipitating factors: a. Dim illumination (including extremes of temperature causing
people to stay indoors)19, 2931 b. Drugs i. Anticholinergic agents (topical, e.g., atropine, cyclopentolate,
and tropicamide, or systemic, e.g., antihistamine, antipsy-chotic (especially antidepressants), anti-parkinsonian, atro-pine, and gastrointestinal spasmolytic drugs)
ii. Adrenergic agents (topical, e.g., epinephrine and phenyleph-rine, or systemic, e.g., vasoconstrictors, central nervous sys-tem stimulants, bronchodilators, appetite depressants, and hallucinogenic agents)
c. Emotional stress (possibly due to mydriasis secondary to increased sympathetic tone)
ACG may be broadly subdivided into: 1. Primary ACG: no cause other than anatomic predisposition is identi-
fied. 2. Secondary ACG: angle closure is the result of a specific pathologic
condition that may arise in any part of the eye, e.g., neovascular glaucoma and anterior uveitis.
This traditional classification evolved from clinical observations and is based on symptoms that are subjective and may be highly variable (Box 10-12-1). The lack of standardization and the frequent overlap in clinical presentation make it difficult for comparison in epidemiologic studies. Furthermore, this form of classification does not offer any in-sight as to the natural history of the disease, or the presence or absence of glaucomatous optic neuropathy and is therefore not useful for visual
BOX 10-12-1 TRADITIONAL CLASSIFICATION OF ANGLE-CLOSURE GLAUCOMA (BASED ON CLINICAL PRESENTATION AND SYMPTOMATOLOGY)ACUTESuddenonsetofIOPelevationresultingfromtotalangleclosure,accom-
paniedbysymptomsofsevere,usuallyunilateral,ocularpain,redeye,blurredvision,haloes,headache(ipsilateralfrontal),nausea,andvomiting
SUBACUTE/INTERMITTENT/CREEPINGAnepisodeofsuddenIOPelevationthatisspontaneouslyaborted,sothat
symptomsaremildorevenabsent.SuchsubacuteIOPelevationsmayberecurrentandthereforetermedintermittentangleclosure.Intermit-tentepisodescanresultinprogressivePASformation,termedcreepingangleclosure
CHRONICChronicIOPelevationduetothepresenceofperipheralanteriorsynechiae
(PAS)thatpermanentlyclosetheanteriorchamberangle.Symptomsareusuallyabsent
LATENTEvidencethatanopenbutnarrowanglecananddoescloseundercertain
circumstances.Asymptomatic,butPASisoftenfoundongonioscopy
BOX 10-12-2 CLASSIFICATION BASED ON NATURAL HISTORY PRIMARY ANGLE-CLOSURE SUSPECT (PACS)Aneyeinwhichappositionalcontactbetweentheperipheralirisand
posteriortrabecularmeshworkispresentorconsideredpossible,intheabsenceofelevatedIOP,PAS,disc,orVFchanges.Epidemiologically,thishasbeendefinedasanangleinwhich180270oftheposteriortrabecularmeshworkcannotbeseengonioscopically
PRIMARY ANGLE CLOSURE (PAC)PACSwithstatisticallyraisedIOPand/orprimaryPAS,withoutdiscorVF
changes
PRIMARY ANGLE CLOSURE GLAUCOMA (PACG)PACwithglaucomatousopticneuropathyandcorrespondingVFloss
IOP,intraocularpressure;PAS,peripheralanteriorsynechiae;VF,visualfield.FromFosterPJ,BuhrmannRR,QuigleyHA,JohnsonGJ.Thedefinitionandclassificationofglaucomainprevalencesurveys.BrJOphthalmol.2002;86:23842.prognostication. Hence, the International Society of Ophthalmic Epide-miology developed a classification that is based on the natural history of the disease (Box 10-12-2).
This classification is evidence-based and is therefore more objective. These definitions have been widely used in the classification of subjects in research, and have been adopted in the Asia Pacific Glaucoma Guide-lines. However, it does not identify the pathophysiologic mechanism that is responsible for angle closure, and hence does not facilitate the clinician in choosing an appropriate treatment. A classification devised by Ritch and colleagues33 is useful for this purpose and should be used in parallel (Box 10-12-3; Figs 10-12-1 to 10-12-7).
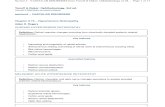
Pupillary BlockPupillary block represents the commonest mechanism underlying angle closure. In pupillary block, iridolenticular contact at the pupil limits the flow of aqueous from its site of production at the nonpigmented cili-ary epithelium to the anterior chamber, resulting in a pressure gradient between the posterior and anterior chambers that further pushes the
C
A B
Fig. 10-12-1 Pupil block.(A)Photographofeyewithpupilblock.(B)Anteriorseg-mentopticalcoherencetomographyimage.(C)Ultrasoundbiomicroscopyimage.
BOX 10-12-3 CLASSIFICATION BASED ON ANATOMIC LEvELS OF OBSTRUCTION TO AqUEOUS FLOw (PATHOPHYSIOLOGY OF ANGLE-CLOSURE GLAUCOMA)Appositionoftheiristothetrabecularmeshworkinangle-closure
glaucomamaybeduetoforcesactingat4anatomiclevels:
IRISPupillaryblock(Fig.10-12-1)*Non-pupilblock/anglecrowdingmechanisms,e.g.,thickperipheralirisroll
(Fig.10-12-2)Contraction of fibrovascular membrane in neovascular glaucomaContraction of fibrin in angle secondary to anterior uveitis or hyphemaEndothelial proliferation (iridocorneoendothelial syndromes)Epithelial downgrowth
CILIARY BODYPlateauirisconfiguration(forwardrotationoftheciliarybodyoranterior
positionofciliaryprocesses)(Fig.10-12-3)Ciliary body cysts (pseudoplateau iris) (Fig.10-12-4)
LENSPhacomorphicglaucoma(thicklens)(Fig.10-12-5)Phakotopicglaucoma(anteriorlypositionedlens)Subluxedlens(e.g.,pseudoexfoliationsyndrome,traumatic)(Fig.10-12-6)
vECTORS POSTERIOR TO LENSAqueousmisdirection(malignantglaucoma)(Fig.10-12-7)Serous or hemorrhagic choroidal detachment or effusionSpace-occupying lesion (gas bubble, vitreous substitutes, tumor)Retrolenticular tissue contracture (retinopathy of prematurity, persistent
hyperplastic primary vitreous)
-
10
1164
GLA
UCO
MAiris anteriorly. Anterior bowing of the peripheral iris narrows the angle and may then cause iridotrabecular apposition and angle closure. Laser iridotomy re-establishes aqueous flow from the posterior to the anterior chamber and relieves the pressure gradient, thereby allowing the iris to flatten and the angle to widen.
Non-Pupil Block MechanismsThe variable efficacy of laser iridotomy in many cases of angle closure as well as ultrasound biomicroscopy imaging suggests that pupillary block may not be the only mechanism responsible. The role of angle crowd-ing, for example that caused by a thick peripheral iris roll, has been increasingly recognized in many cases of angle closure. This has been added to Ritchs classification, for the sake of completeness. In many such cases, the peripheral iris stroma is thick. Upon pupil dilatation,
A
B
Fig. 10-12-2 Peripheral iris roll.Anteriorsegmentopticalcoherencetomographyimagesofthesameeyetakenin(A)lightand(B)darkconditions.
Fig. 10-12-3 Plateau iris configuration.Ultrasoundbiomicroscopyimage.
Fig. 10-12-4 Ciliary body cysts.Ultrasoundbiomicroscopyimage.the peripheral iris bunches up. If the angle is already narrow, this thick peripheral iris roll may become apposed to the trabecular meshwork and result in angle closure.
Plateau Iris ConfigurationOn gonioscopy, the iris assumes a steep approach at its insertion before flattening centrally. The peripheral iris is forced into the angle by ante-rior rotation of the ciliary body or anteriorly positioned ciliary processes. The development of angle closure either spontaneously or after pupil dilatation in an eye with plateau iris configuration, in the presence of a patent laser iridotomy, is termed plateau iris syndrome. Disorders of the ciliary body, such as iridociliary cysts or tumors, may result in a similar plateau iris configuration. This is termed pseudoplateau iris.
Aqueous MisdirectionThis condition, also called malignant glaucoma or ciliary block glau-coma, is characterized by shallowing or flattening of the anterior cham-ber, accompanied by a rise in intraocular pressure (IOP). It is typically seen in the postoperative period, but can arise spontaneously. It is be-lieved that aqueous passes posteriorly to the posterior segment instead of anteriorly to the posterior chamber, due to obstruction to flow caused by the anterior rotation of ciliary processes resulting in their apposition to the lens equator in the phakic eye, or against the anterior hyaloid in the aphakic eye. The accumulation of aqueous in the posterior segment causes an anterior displacement of the lensiris diaphragm. Laxity of the lens zonules allowing this forward movement has also been sug-gested to play a role in the development of this condition.
The term malignant was used originally to describe its poor re-sponse to conventional miotic treatment. Early recognition of aqueous misdirection is important in reducing its morbidity. Management
Fig. 10-12-5 Phacomorphic glaucoma.
Fig. 10-12-6 Anteriorly subluxed lens.
Fig. 10-12-7 Malignant glaucoma.Ultrasoundbiomicroscopyimage.
-
10.12
1165
Angle-ClosureG
laucoma involves prompt medical treatment with topical cycloplegic agents such as atropine, which increases zonular tension and pulls the lens posteri-orly. Atropine 1% may be given 24 times a day for weeks to months or even years. Topical beta-blockers, 2-agonists, and carbonic anhydrase inhibitors may be used to decrease aqueous production and lower the IOP. Hyperosmotic agents may also be used to decrease the vitreous vol-ume. If the condition persists beyond 5 days despite adequate medical therapy, laser or surgical intervention must be considered. Neodymium:yttriumaluminumgarnet (Nd:YAG) laser has been demonstrated to be effective in the pseudophakic and aphakic eye by disrupting the ante-rior hyaloid face. Aspiration of the anterior vitreous, anterior pars plana vitrectomy, or lens extraction with a posterior capsulotomy may be per-formed. A prophylactic laser iridotomy should also be considered for the fellow eye, as there is a significant risk that aqueous misdirection may occur following intraocular surgery in that eye.
DIAGNOSIS
External ExaminationThe majority of people with PACG do not experience any symptoms.3, 7 Characteristic findings in a patient presenting during an acute angle-closure attack include conjunctival hyperemia, a hazy cornea with cor-responding decreased visual acuity (Fig. 10-12-8), and a mid-dilated nonreactive or sluggish pupil. The pupil is mid-dilated due to ischemic paralysis of the iris sphincter muscles as a result of the greatly elevated IOP. If these muscles infarct, the pupil does not return to its normal appearance even when the IOP has been lowered, and iris whorling may become evident. Digital palpation of the eye through a closed eyelid reveals a firm (often rock-hard) eye. The patient may also experience bradycardia or arrhythmia.
Penlight ExaminationWhen a slit lamp or goniolens is unavailable, a penlight may be used to estimate the anterior chamber depth. This test is performed by shining the penlight from the temporal side of the eye. A flat iris with a deep an-terior chamber would allow the nasal iris to be illuminated, while an iris that is convex forwards with a correspondingly shallow anterior chamber would block the illumination, causing the nasal iris to be in shadow.
Slit-Lamp ExaminationIn acute angle closure, the cornea usually appears hazy due to epithelial and stromal edema secondary to the acute rise in IOP. Iris bombe is usually present due to pupillary block. Iris whorling (sectoral infarction
Fig. 10-12-8 Acute angle-closure glaucoma.
Fig. 10-12-9 Iris whorling and atrophy.of the iris sphincter leading to torsion of the iris), patchy iris stromal atrophy (Fig. 10-12-9), and lens glaucomflecken (Fig. 10-12-10) (small greywhite anterior subcapsular or capsular opacities in the pupillary zone, due to infarction of lens fibers) may also be evident if the patient has had an acute rise in IOP previously.
Both central and peripheral anterior chamber depth (ACD) may be assessed at the slit lamp. While the central ACD only weakly corre-lates with the angle width,34 peripheral ACD estimation appears to perform well in the detection of occludable angles.35 The van Herick technique36 (Fig. 10-12-11) is useful for estimating the peripheral ACD. In this technique, the illumination column is offset from the axis of the microscope by 60. The brightest, narrowest possible vertical beam of light is directed at the temporal limbus, perpendicular to the ocular surface. Viewed from the nasal aspect, the peripheral ACD is compared to the adjacent corneal thickness that is illuminated by the light beam. The angle may be occludable if the peripheral ACD is less than one fourth of the corneal thickness. The limbal chamber depth method of grading the peripheral anterior chamber is a recent modification of the van Herick test.37 Instead of the four grades used in the van Herick method, it has seven grades that are expressed as a percentage fraction of the thick-ness of the adjacent cornea: 0%, 5%, 15%, 25%, 40%, 75%, and 100%. The limbal chamber depth method has been demonstrated to perform better in the detection of established PACG and is now widely used in epidemiologic research.
Slit-lamp examination should also include a thorough check for the presence of any inflammation, hyphema, and cataract or subluxed lens. IOP is often severely elevated (often > 40 mmHg). Careful examination of the optic disc should also be performed in order to detect any evi-dence of glaucomatous optic neuropathy.
GonioscopyCareful gonioscopic examination of the angle is vital to make the diag-nosis of angle closure. This is best performed using first a two-mirror goniolens (e.g., Goldmann) to avoid artefactual distortion of the angle caused by inadvertent pressure on the cornea, followed by a four-mirror goniolens (e.g., Sussman, Zeiss) that allows indentation gonioscopy to reveal whether any closure is due to PAS or is merely appositional. Go-nioscopy should be performed in a dark room using a 1 mm light beam reduced to a very narrow slit, and care should be taken to avoid any light falling on the pupil, which might otherwise cause pupil constriction and angle widening. The vertical light beam should be offset horizontally for
Fig. 10-12-10 Glaucomflecken.
Fig. 10-12-11 van Herick technique.
-
10
1166
GLA
UCO
MA
yirislens interaction, and ciliary body. Thus, it can help to elucidate the
Fig. 10-12-12 Scheimpflug photography.
A
Fig. 10-12-13 (A)Ultrasoundbiomicroscopydevice;(B)ultrasoundbiomicroscopthe darkroom prone test and pharmacologic pupil dilatation. However, these tests may not be easily reversible and are associated with high false-positive and false-negative rates. They are therefore seldom prac-tised now.
DIFFERENTIAL DIAGNOSIS 1. Secondary ACG (in italics above). 2. Other causes of headache (e.g., migraine or cluster headache).
MANAGEMENT OF ACUTE ANGLE CLOSUREAcute angle closure is an ophthalmologic emergency. Measures should be taken within minutes to hours to lower the IOP and break the attack, followed by identifying the mechanism of angle closure and treating appropriately in an attempt to widen the angle (Box 10-12-4).
B
imageoftheanteriorchamberangle.the assessment of the superior and inferior angles, while the horizon-tal beam should be offset vertically for the nasal and temporal angles. Assessment of the angles should be carried out at 25 magnification. Although currently the reference standard for angle assessment, gonios-copy remains a highly subjective technique that depends on the experi-ence of the clinician as well as conditions of illumination.
Various grading systems, including Scheie, Shaffer, and Spaeth have been proposed for the recording of gonioscopic findings (see Chapter 10-28). These gonioscopic grades provide an index of the likelihood of angle closure.37 With the Scheie grading system, there is a high risk of angle closure in eyes that are graded III (only anterior TM and Schwal-bes line visible) or IV (only Schwalbes line visible). Shaffer grades I (angle width 010) and II (1020) are associated with risk of angle clo-sure, while an angle with a Spaeth grade of B20s (iris insertion behind Schwalbes line, angle width 20, steep peripheral iris contour) may be potentially occludable. Biometry gonioscopy, where a graticule in the eyepiece of the slit lamp is used to measure the distance from the iris insertion to Schwalbes line, has also been suggested as a more repro-ducible and objective method of gonioscopy.
Other Imaging TechniquesScheimpflug photographyScheimpflug photography (Fig. 10-12-12) has been used to assess angle width. However, its relatively low resolution limits its usefulness in the evaluation of angle closure.
Ultrasound biomicroscopy (UBM)UBM (Fig. 10-12-13) allows dynamic high-resolution imaging of the an-terior segment structures, including the anterior chamber angle, the iris,
underlying mechanism of angle closure in most cases, including plateau iris syndrome and iridociliary cysts, thereby allowing the appropriate treatment to be given. It is also useful in demonstrating angle occlud-ability when performed in a dark room. The major disadvantages of UBM are that it is a time-consuming procedure that requires a skilled operator and contact with the patients eye. Its high cost also limits its availability.
Anterior segment optical coherence tomography (AS-OCT)More recently, AS-OCT (Fig. 10-12-14) using light of wavelength 1310 nm has enabled high-speed imaging of the anterior segment structures. It is an easy technique to master and does not require con-tact with the patients eye. A comparison with gonioscopy has found that it may be superior in its ability to detect angle occludability.38 It suggests that gonioscopy (which uses visible light) may be underes-timating angle occludability, even when performed in ideal darkroom conditions. A comparison of AS-OCT with UBM has reported at least moderate agreement between the two techniques (J See, et al., personal communication)
Scanning peripheral anterior chamber depth analyzer (SPAC)Evaluation of the peripheral anterior chamber is also possible with the newly developed SPAC,39 for which early results are encouraging.
Provocative testsHistorically, provocative tests were used to attempt to trigger angle closure in primary angle-closure suspects (PACS), in order to identify patients for whom treatment is then recommended. These included
-
10.12
1167
Angle-ClosureG
laucomaCorneal indentation with a four-mirror goniolens or cotton-tipped applicator may be attempted at 30-second intervals in order to force open an area of appositionally closed trabecular meshwork that will al-low some aqueous to exit the eye.40 However, this technique may cause pain and momentary further increases in IOP. Hence, it may not be suitable in all cases.
Recent studies suggest that laser iridoplasty may be a useful alterna-tive to conventional systemic medication as a first-line treatment in the management of acute angle closure, especially when certain medi-cations are contraindicated, for example, in patients with pre-existing asthma, or cardiac or renal disease.4142
After 12 Hours 1. If the attack is broken and corneal edema resolves, perform laser
iridotomy. Laser iridoplasty should be performed in addition in cases of plateau iris syndrome, and can also be considered where the angle remains narrow despite a patent laser iridotomy.
2. If attack is not broken but cornea is clear, perform laser iridotomy. 3. If attack is not broken and cornea is still hazy, perform laser iri-
doplasty first, followed by laser iridotomy later when cornea edema resolves.4345
Anterior chamber paracentesis has also been suggested as an alternative to break the attack if all else fails, especially if laser is unavailable.46
LaterIf attack is still not broken, consider surgery (lens extraction if lens is the causative factor, or surgical peripheral iridectomy). The fellow eye should be evaluated by gonioscopy for risk of angle occludability and treated with prophylactic laser iridotomy if necessary. Topical ste-roids should be continued four times a day for about 57 days post-laser iridotomy, and anti-glaucoma medications discontinued when IOP returns to normal.
A
B
Fig. 10-12-14 (A)Anteriorsegmentopticalcoherencetomographydevice;(B)anteriorsegmentopticalcoherencetomographyimageofanteriorchamber.
BOX 10-12-4 MANAGEMENT OF ACUTE ANGLE CLOSUREImmediate(remembertocheckforanydrugallergyorcontraindications)1. Topicalbeta-blocker,2-agonist,pilocarpine2%or4%(pilocarpineis
effectiveininducingmiosisonlywhenirisischemiaisrelieved,i.e.,whenintraocularpressurefallsto
-
10
1168
GLA
UCO
MALens extractionRemoval of the lens, especially if there is any evidence of cataract, is be-lieved to be helpful in cases where either the lens thickness or anterior position is thought to be the main mechanism underlying the acute epi-sode of angle closure (Fig. 10-12-17). However, care must be taken dur-ing surgery as these eyes are usually associated with high IOP, shallow anterior chambers, cloudy cornea, decreased corneal endothelial cell counts, floppy iris due to previous ischemia, posterior synechiae, bulky lens, lax lens zonules, and a high risk of malignant glaucoma. Reports of phacoemulsification combined with goniosynechialysis, in the presence of peripheral anterior synechial closure, have been encouraging.56, 57 However, there is as yet no evidence from good-quality randomized trials or nonrandomized studies of the effectiveness of lens extraction for chronic primary ACG.58
GoniosynechialysisThis is usually performed in combination with lens extraction, and in-volves mechanical stripping of PAS away from the trabecular meshwork using viscoelastics or an irrigation cyclodialysis spatula.56, 57
TrabeculectomyTrabeculectomy is performed similarly as for OAG, with the excep-tion that a surgical peripheral iridectomy should always be performed at the time of trabeculectomy (Fig. 10-12-18). In addition, the use of antimetabolites should be considered. Trabeculectomy, alone or in combination with lens extraction, should be considered after the acute attack of angle closure, if the IOP control remains suboptimal despite
BOX 10-12-5 LASER IRIDOTOMY FOR MANAGEMENT OF CHRONIC ANGLE-CLOSURE GLAUCOMAINDICATIONSl ACl ACGl ACS,especiallyif:
ACinfelloweyeFamilyhistoryofACGNeedforrepeateddilatedexaminationsPooraccesstoregularophthalmiccare
PROCEDURE1. Pre-laser:
Instilltopical2%or4%pilocarpineand2-agonistand/ororalacetazol-amide3060minutespriortotheprocedure.Thishelpstoreducetheoccurrenceofpost-laserIOPspike
Topicalanesthesia2. Laser:
Abraham/WiseiridotomylenswithcouplingfluidChooseiriscryptoranareaofthiniris.Avoidleveloftearmeniscus
formedbylidandglobe.Aimatperipheraliris,avoidingcorneaarcus.Nd:YAG25mJ(useminimumenergy),13pulses/burstArgonlaser7001100mW,50mspotsize,100ms,1020burnscanbe
usedpriortoNd:YAGlaserinathickiristophotocoagulateandthintheirisstroma,therebyalsoreducingtheriskofirisbleeding54
Endpoint:irispigmentplume,lensvisiblethroughiridotomy,LIsizeofabout150200m.55BrownAsianiridesarethickerthanblueonesandprobablyrequirealargeriridotomy
3. Post-laser:CheckIOP1hourafterlasertoexcludeanyIOPspikeandagainat
2448hours
COMPLICATIONSCornealendothelialburns(withargon)IrishemorrhagefromsiteofLI(withNd:YAG)applyingpressureonthe
globewiththelaserlensisusuallysufficienttostopthehemorrhageIOPspikeACinflammationwithclosureofiridotomy,formationofposterior
synechiae,orraisedIOPCataractformationCornealendothelialdecompensation,malignantglaucoma,retinal
damage,cystoidmacularedema(allrare)
AC,angleclosure;ACG,angle-closureglaucoma;ACS,angle-closuresuspect;IOP,intraocularpressure;LI,laseriridotomy.medical and laser treatment, especially in more advanced cases of ACG that are associated with PAS, optic nerve or visual field damage. In acutely inflamed eyes, trabeculectomy has been reported to have low success rates.59
Glaucoma drainage implantThis may be considered in chronic ACG where trabeculectomy has failed to control the IOP, or in eyes that are deemed to be at high risk of failure with trabeculectomy (Fig. 10-12-19).
deep anterior chamber shallow anterior chamber
open angle narrow angle opening to angle
peripheraliridectomy
pupilblock
C
A B
Fig. 10-12-15 Laser iridotomy.
BOX 10-12-6 LASER IRIDOPLASTY FOR MANAGEMENT OF CHRONIC ANGLE-CLOSURE GLAUCOMAINDICATIONSAnglestilloccludableafterlaseriridotomy(e.g.,plateauiris)Inacuteangleclosure,tohelpbreaktheattackwheremedicaltherapyhas
failedoriscontraindicatedTofacilitateaccesstotrabecularmeshworkforlasertrabeculoplasty
PROCEDURE1. Pre-laser:
Asforlaseriridotomy2. Laser:
Abraham/Wise/Goldmann3-mirrorlens Aimatirisasperipheralaspossible,outsideofanycorneaarcus Argongreenorblue-green,ordiode200500mW,100500mspot
size,0.20.5seconds,singlerowof2550burnsover180360atone-spotdiameterintervals
Endpoint:irisstromalcontractionaccompaniedbyprogressiveperipheralanteriorchamberdeepeningwithincreasingnumberofburns
3. Post-laser:asforlaseriridotomy.Topicalsteroids4times/dayfor7days.
COMPLICATIONSCornealendothelialburnsIritisIOPspikePeripheralanterior&/orposteriorsynechiae
-
10.12
1169
Angle-ClosureG
laucomaCyclodestructive proceduresCyclodestructive procedures like trans-scleral cyclophotocoagulation are used for ACG eyes with end-stage disease that do not have any visual potential, especially if they are symptomatic due to high IOP (Fig. 10-12-20).
A
B
Fig. 10-12-17 Pre- versus post-phacoemulsification with intraocular lens implant.Anteriorsegmentopticalcoherencetomographyimages.
BOX 10-12-7 THE MAIN AIMS AND INDICATIONS FOR SURGICAL TREATMENT IN ANGLE CLOSUREMAIN AIMSTodecreaseIOPandreduceriskofopticnervedamageTopreventprogressiveangleclosureToreduceriskofacuteangleclosure
INDICATIONSInadequatecontrolofIOP,withprogressionofopticnerveorvisualfielddamage,despitemedicalandlasertreatmentPoorcomplianceorintolerancetomedicaltreatmentInabilitytocooperatewithlasertreatmentWorseningangleclosureand/orPASPresenceofsignificantcataractimpairingvision
IOP,intraocularpressure;PAS,peripheralanteriorsynechiae.
A
B
Fig. 10-12-16 Laser iridoplasty (performed in addition to laser iridotomy).Caution must be exercised when performing surgery in eyes with ACG because of the increased risk of malignant glaucoma.60 In addi-tion, the use of topical corticosteroids after laser or surgery in these pa-tients may be associated with steroid-induced IOP elevation following an attack of ACG.61, 62
PROGNOSISAngle closure is associated with good visual prognosis, provided it is detected early and the appropriate treatment given. Forty-two to seventy-two per cent of cases presenting with acute PAC can be satisfactorily treated with laser iridotomy alone,63, 64 and 6075% of such patients re-cover without optic disc or visual field damage, if the IOP is promptly and adequately controlled.65 However, a longer duration of the angle-closure attack or a history of intermittent angle-closure episodes is often associ-ated with the need for additional medical or even surgical therapy.6668 The presence of a significant amount of PAS, a higher presenting IOP, and a larger cup-to-disc ratio on presentation are other predictors of inadequate IOP control despite a patent laser iridotomy.6971 Most pa-tients who develop a rise in IOP after laser iridotomy do so within the first 6 months.68 Once GON and visual field damage have developed, 94100% may require further surgical treatment to control IOP.72
Several studies have shown that up to 5075% of patients with angle closure in one eye will have an attack in the fellow eye often within
Fig. 10-12-18 Trabeculectomy.
Fig. 10-12-19 Glaucoma drainage implant.
Fig. 10-12-20 Cyclodestructive procedure.
-
10
1170
GLA
UCO
MA
ExpOphthalmol.1987;225:35760. ofacuteprimaryangleclosure:mid-termresults.Eye. 64. PlayfairTJ,WatsonPG.Managementofacuteprimary
22. ShioseY,KitazawaY,TsukuharaS,et al. Epidemiologyof
glaucomainJapananationwideglaucomasurvey.JpnJOphthalmol.1991;35:13355.
23. WongTY,FosterPJ,SeahSKL,et al. RatesofhospitaladmissionsforprimaryangleclosureglaucomaamongChinese,MalaysandIndiansinSingapore.BrJOphthal-mol.2000;84:9902.
24. LoweRF.Ahistoryofprimaryangleclosureglaucoma.SurvOphthalmol.1995;40:16370.
2006;20:30914.43. LamDS,LaiJS,et al. Argon
conventionalsystemicmeofacuteprimaryangleclotive,randomized,controlle2002;109:15916.
44. LaiJS,ThamCC,et al. Lasertialtreatmentofacuteattalong-termfollow-upstudylaseriridoplastyversusdicaltherapyintreatmentsureglaucoma:aprospec-dtrial.Ophthalmology.
peripheraliridoplastyasini-ckofprimaryangleclosure:a.JGlaucoma.2002;11:4847.
angleclosureglaucoma:along-termfollow-upoftheresultsofperipheraliridectomyusedasaninitialproce-dure.BrJOphthalmol.1979;63:1722.
65. DhillonB,ChewPT,LimASM.Fieldlossinprimaryangleclosureglaucoma.Asia-PacJOphthalmol.1990;2:857.
66. BuckleySA,ReevesB,BurdonM,et al. Acuteangleclosureglaucoma:relativefailureofYAGiridotomyinaffectedeyesandfactorsinfluencingoutcome.BrJOphthalmol.1994;78:529.1 year (up to 10 years) despite miotic treatment.73, 74 The risk of this occurring is much reduced with a surgical iridectomy.75, 76
Prophylactic laser iridotomy in fellow eyes of patients presenting with unilateral acute PAC also appears to be safe and effective in pre-venting acute PAC in 100%, and in preventing long-term rise in IOP in 89%.77 However, the fact that a small proportion of fellow eyes did develop a pressure rise despite the presence of a patent laser iridotomy emphasizes the importance of long-term monitoring.
There has been a paucity of longitudinal studies looking at the rate of progression from PACS to PAC and PACG. One population-based study in Greenland78 reported an incidence of PAC (who progressed from PACS) of 16% over 10 years, while Thomas et al.79 reported that 22% of PACS developed PAC after 5 years, and 28% of PAC went on to develop PACG80 over a similar period. However, more research needs to be done in order to understand the natural history of this disease better.
REFERENCES 1. HollowsFC,GrahamPA.Intraocularpressure,glaucoma,
andglaucomasuspectsinadefinedpopulation.BrJOphthalmol.1966;50:57086.
2. TielschJM,SommerA,KatzJ,et al. Racialvariationsintheprevalenceofprimaryopenangleglaucoma:TheBaltimoreEyeSurvey.JAmMedAssoc.1991;266:36974.
3. BonomiL,MarchiniG,MarraffaM,et al. Epidemiologyofangleclosureglaucoma:prevalence,clinicaltypes,andassociationwithperipheralanteriorchamberdepthintheEgna-NeumarketGlaucomaStudy.Ophthalmology.2000;107:9981003.
4. BuhrmannRR,QuigleyHA,BarronY,et al. Thepreva-lenceofglaucomainaruraleastAfricanpopulation.InvestOphthalmolVisSci.2000;41:408.
5. AlsbirkPH.Earlydetectionofprimaryangleclosureglaucoma:limbalandaxialchamberdepthscreeninginahighriskpopulation(GreenlandEskimos).ActaOphthalmol.1988;66:55664.
6. AlsbirkPH.Primaryangleclosureglaucoma:oculometry,epidemiologyandgeneticsinahigh-riskpopulation.ActaOphthalmol.1976;54:531.
7. FosterPJ,BaasanhuJ,AlsbirkPH,et al. GlaucomainMongolia.Apopulation-basedsurveyinHovsgolprovince,northernMongolia.ArchOphthalmol.1996;114:123541.
8. HuCN.AnepidemiologicstudyofglaucomainShunyiCounty.Beijing.ChungHuaYenKoTsaChih.1989;25:1159.
9. FosterPJ,OenFTS,MachinD,et al. TheprevalenceofglaucomainChineseresidentsofSingapore.ArchOphthalmol.2000;118:110511.
10. BonomiL,MarchiniG,MarraffaM,et al. Epidemiologyofangleclosureglaucoma:prevalence,clinicaltypes,andassociationwithperipheralanteriorchamberdepthintheEgna-NeumarketGlaucomaStudy.Ophthalmology.2000;107:9981003.
11. GrahamP,HollowsF.Intraocularpressure,glaucomaandglaucomasuspectsinadefinedpopulation.BrJOphthalmol.1966;50:57086.
12. BengtssonB.Theprevalenceofglaucoma.BrJOphthal-mol.1981;65:469.
13. CoxJE.AngleclosureglaucomaamongAlaskanEskimos.Glaucoma.1984;6:1357.
14. DranceSM.AngleclosureglaucomaamongCanadianEskimos.ArcticOphthalmolSymposium.1973;8:2525.
15. JacobA,ThomasR,KoshiSP,et al. PrevalenceofprimaryglaucomainanurbansouthIndianpopulation.IndianJOphthalmol.1998;46:816.
16. QuigleyHA,BromanT.Thenumberofpeoplewithglaucomaworldwidein2010and2020.BrJOphthalmol.2006;90:2627.
17. CongdonN,WangF,TielschJM.Issuesintheepidemiol-ogyandpopulation-basedscreeningofprimaryangleclosureglaucoma.SurvOphthalmol.1992;36:41123.
18. FosterPJ,JohnsonGJ.GlaucomainChina:howbigistheproblem?BrJOphthalmol.2001;85:127782.
19. SeahSKL,FosterPJ,ChewPT,et al. IncidenceofacuteprimaryangleclosureglaucomainSingapore.Anisland-widesurvey.ArchOphthalmol.1997;115:143640.
20. ResnikoffS,PascoliniD,EtyaaleD,et al. Globaldataonvisualimpairmentintheyear2002.BullWorldHealthOrg.2004;82:84451.
21. TeikariJ,RaivioI,NurminenM.Incidenceofacuteglau-comainFinlandfrom1973to1982.GraefesArchClin
25. AlsbirkPH.CornealdiameterinGreenlandEskimos.Anthropometricandgeneticstudieswithspecialreferencetoprimaryangleclosureglaucoma.ActaOphthalmol.1975;53:63546.
26. AlsbirkPH.Limbalandaxialchamberdepthvaria-tions.ApopulationstudyinEskimos.ActaOphthalmol.1986;64:593600.
27. LoweRF.Etiologyoftheanatomicalbasisforprimaryangleclosureglaucoma.Biometricalcomparisonsbetweennormaleyesandeyeswithprimaryangleclosureglaucoma.BrJOphthalmol.1970;54:1619.
28. FriedmanDS,GazzardG,FosterPJ,et al. Ultrasono-graphicbiomicroscopy,Scheimpflugphotography,andnovelprovocativetestsincontralateraleyesofChinesepatientsinitiallyseenwithacuteangleclosure.ArchOphthalmol.2003;121:63342.
29. TuplingMR,JunetEJ.Meteorologicaltriggeringofacuteglaucomaattacks.TransOphthalmolSocUK.1977;97:1858.
30. HillmanJS,TurnerJDC.Associationbetweenacuteglaucomaandtheweatherandsunspotactivity.BrJOphthalmol.1977;61:5126.
31. DavidR,TesslerZ,YassurY.Epidemiologyofacuteangleclosureglaucoma:incidenceandseasonalvariations.Ophthalmologica.1985;191:47.
32. FosterPJ,BuhrmannRR,QuigleyHA,JohnsonGJ.Thedefinitionandclassificationofglaucomainprevalencesurveys.BrJOphthalmol.2002;86:23842.
33. RitchR,LoweRF.Angleclosureglaucoma.In:RitchR,ShieldsMB,KrupinT,eds.Theglaucomas.St.Louis:CVMosby;1996:801.
34. MakabeR.Comparativestudiesoftheanteriorchamberanglewidthbyultrasonographyandgonioscopy.KlinMonatsblAugenheilkd.1989;1194:6.
35. NolanWP,AungT,MachinD,et al. Detectionofnar-rowanglesandestablishedangleclosureinChineseresidentsofSingapore:potentialscreeningtests.AmJOphthalmol.2006;141:896901.
36. vanHerickW,ShafferRN,SchwartzA.Estimationofwidthofangleofanteriorchamber:incidenceandsignificanceofthenarrowangle.AmJOphthalmol.1969;68:626.
37. FosterPJ,DevereuxJG,AlsbirkPH,et al. DetectionofgonioscopicallyoccludableanglesandprimaryangleclosureglaucomabyestimationoflimbalchamberdepthinAsians:modifiedgradingscheme.BrJOphthalmol.2000;84:18692.
38. KashiwagiK,AbeK,TsukaharaS.Quantitativeevaluationofchangesinanteriorsegmentbiometrybyperipherallaseriridotomyusingnewlydevelopedscanningperiph-eralanteriorchamberdepthanalyzer.BrJOphthalmol.2004;88:103641.
39. FosterPJ,NolanWP,AungT,et al. Definingoccludableanglesinpopulationsurveys:drainageanglewidth,peripheralanteriorsynechiaeandglaucomatousopticneuropathyinEastAsianpeople.BrJOphthalmol.2004;88:48690.
40. NolanW,SeeJ,ChewP,et al.Detectionofprimaryangle-closureusinganteriorsegmentopticalcoher-encetomographyinAsianeyes.Ophthalmology(inpress).
41. AndersonDR.Cornealindentationtorelieveacuteangleclosureglaucoma.AmJOphthalmol.1979;88:1091.
42. LaiJS,ThamCC,et al. Tocompareargonlaserperipheraliridoplastyagainstsystemicmedicationsintreatment
45. RitchR.Argonlasertreatmentformedicallyunrespon-siveattacksofangleclosureglaucoma.AmJOphthal-mol.1982;94:197.
46. ShinDH.Argonlasertreatmentforreliefofmedicallyunresponsiveangleclosureglaucomaattacks.AmJOphthalmol.1982;94:821.
47. LamDS,ChuaJK,ThamCC,et al. Efficacyandsafetyofimmediateanteriorchamberparacentesisinthetreat-mentofacuteprimaryangleclosureglaucoma.Apilotstudy.Ophthalmology.2002;109:6470.
48. ChewPT,AungT.EXACTStudyGroup.Intraocularpres-sure-reducingeffectsandsafetyoflatanoprostversustimololinpatientswithchronicangleclosureglaucoma.Ophthalmology.2004;111:42734.
49. SihotaR,SaxenaR,AgarwalHC,et al. Crossovercompari-sonoftimololandlatanoprostinchronicprimaryangleclosureglaucoma.ArchOphthalmol.2004;122:1859.
50. AgarwalHC,GuptaV,SihotaR.EffectofchangingfromconcomitanttimololpilocarpinetobimatoprostmonotherapyonocularbloodflowandIOPinprimarychronicangleclosureglaucoma.JOculPharmacolTher.2003;19:10512.
51. AungT,ChanYH,ChewPT.EXACTStudyGroup.Degreeofangleclosureandtheintraocularpressure-loweringeffectoflatanoprostinsubjectswithchronicangleclosureglaucoma.Ophthalmology.2005;112:26771.
52. KookMS,ChoHS,YangSJ,et al. Efficacyoflatanoprostinpatientswithchronicangleclosureglaucomaandnovisibleciliarybodyface:apreliminarystudy.JOculPharmacolTher.2005;21:7584.
53. ChewPT,RojanaPongpunP,TravatanCACGStudyGroup.Intraocularpressure-loweringeffectandsafetyofTravoprost0.004%andLatanoprost0.005%forthetreatmentofchronicangleclosureglaucoma.AsianJOphthalmol.2006;8:139.
54. HoT,FanR.Sequentialargon-YAGlaseriridotomiesindarkirides.BrJOphthalmol.1992;76:32931.
55. FleckBW.Howlargemustaniridotomybe?BrJOph-thalmol.1990;74:5838.
56. HarasymowyczPJ,PapamatheakisDG,AhmedI,et al. Phacoemulsificationandgoniosynechialysisinthemanagementofunresponsiveprimaryangleclosure.JGlaucoma.2005;14:1869.
57. TeekhasaeneeC,RitchR.Combinedphacoemulsificationandgoniosynechialysisforuncontrolledchronicangleclosureglaucomaafteracuteangleclosureglaucoma.Ophthalmology.1999;106:66974.
58. FriedmanDS,VedulaSS.Lensextractionforchronicangle-closureglaucoma[Review].CochraneDatabaseSystRev.2006;3:CD005555.
59. AungT,TowSL,YapEY,et al. Trabeculectomyforacuteprimaryangleclosure.Ophthalmology.2000;107:1298302.
60. EltzH,GloorB.Trabeculectomyincasesofangleclosureglaucomasuccessesandfailures.KlinMonatsblAugenheilkd.1980;177:556.
61. AkingbehinAO.Corticosteroid-inducedocularhyper-tension.I.Prevalenceinclosedangleglaucoma.BrJOphthalmol.1982;66:536.
62. AkingbehinAO.Corticosteroid-inducedocularhypertension.II.Anacquiredform.BrJOphthalmol.1982;66:541.
63. AungT,AngLP,ChanSP,et al. Acuteprimaryangleclosure:long-termintraocularpressureoutcomeinAsianeyes.AmJOphthalmol.2001;131:712.
-
10.12
1171
Angle-ClosureG
laucoma
67. SaundersDC.AcuteclosedangleglaucomaandNd:YAGlaseriridotomy.BrJOphthalmol.1990;74:523.
68. AlsagoffZ,AungT,AngLP,et al. Long-termclinicalcourseofprimaryangleclosureglaucomainanAsianpopulation.Ophthalmology.2000;107:23004.
69. AungT,LimMC.EXACTStudyGroup.Configurationofthedrainageangle,intraocularpressureandopticdisccuppinginsubjectswithchronicangleclosureglau-coma.Ophthalmology.2005;112:2832.
70. SalmonJF.Long-termintraocularpressurecontrolafterNd:YAGlaseriridotomyinchronicangleclosureglau-coma.JGlaucoma.1993;2:2916.
71. NolanWP,FosterPJ,DevereuxJG,et al. YAGlaseriridotomytreatmentforprimaryangleclosureineastAsianeyes.BrJOphthalmol.2000;84:12559.
72. RosmanM,AungT,AngLP,et al. Chronicangleclosurewithglaucomatousdamage:long-termclinicalcourseinaNorthAmericanpopulationandcomparisonwithanAsianpopulation.Ophthalmology.2002;109:222731.
73. LoweRF.Acuteangleclosureglaucoma:thesecondeyeananalysisof200cases.BrJOphthalmol.1962;46:641.
74. RitzingerI,BenediktO,DirisamerF.Surgicalorconservativeprophylaxisofthepartnereyeafterprimaryacuteangleblocglaucoma.KlinMonatsblAugenheilkd.1974;164:645.
75. WollensakJ,EhrhornJ.Angleblockglaucomaandprophylacticiridectomyintheeyewithoutsymptoms.KlinMonatsblAugenheilkd.1975;167:791.
76. LoweRF.Primaryangleclosureglaucoma:areview5yearsafterbilateralsurgery.BrJOphthalmol.1973;57:457.
77. AngLP,AungT,ChewPT.AcuteprimaryangleclosureinanAsianpopulation:long-termoutcomeofthefel-loweyeafterprophylacticlaserperipheraliridotomy.Ophthalmology.2003;107:20926.
78. AlsbirkPH.Anatomicalriskfactorsinprimaryangleclosureglaucoma.Aten-yearfollow-upsurveybasedonlimbalandaxialanteriorchamberdepthsinahighriskpopulation.IntOphthalmol.1992;16:26572.
79. ThomasR,GeorgeR,ParikhR,et al. Five-yearriskofpro-gressionofprimaryangleclosuresuspectstoprimaryangleclosure:apopulation-basedstudy.BrJOphthal-mol.2003;87:4504.
80. ThomasR,ParikhR,MuliyilJ,et al. Five-yearriskofprogressionofprimaryangleclosuretoprimaryangleclosureglaucoma.Apopulation-basedstudy.IndianJOphthalmol.2003;51:32933.
10.12 - Angle-Closure GlaucomaINTRODUCTIONEPIDEMIOLOGY AND PATHOGENESISPrevalenceIncidenceRisk FactorsPupillary BlockNon-Pupil Block MechanismsPlateau Iris ConfigurationAqueous Misdirection
DIAGNOSISExternal ExaminationPenlight ExaminationSlit-Lamp ExaminationGonioscopyOther Imaging TechniquesScheimpflug photographyUltrasound biomicroscopy (UBM)Anterior segment optical coherence tomography (AS-OCT)Scanning peripheral anterior chamber depth analyzer (SPAC)Provocative tests
DIFFERENTIAL DIAGNOSISMANAGEMENT OF ACUTE ANGLE CLOSUREAfter 12 HoursLaterEven Later
MANAGEMENT OF CHRONIC ACGMedical TreatmentLaser TreatmentSurgical TreatmentIridectomyLens extractionGoniosynechialysisTrabeculectomyGlaucoma drainage implantCyclodestructive procedures
PROGNOSISREFERENCES