Wegener’s Granulomatosis Kelly Mitchell July 5, 2006 Morning Report.
Wegener’s Granulomatosis: The Relationship Between Ocular and … · setting of systemic WG and...
Transcript of Wegener’s Granulomatosis: The Relationship Between Ocular and … · setting of systemic WG and...

Harper, et al: Systemic and ocular WG 1025
From the Department of Ophthalmology, Uveitis and ImmunologyService, The Massachusetts Eye and Ear Infirmary, Harvard MedicalSchool, Boston, Massachusetts, USA.
S.L. Harper, MD, MPP; E. Letko, MD; C.M. Samson, MD; P. Zafirakis,MD; V. Sangwan, MD; Q. Nguyen, MD; H. Uy, MD, Fellow in Uveitisand Immunology; C.S. Foster, MD, FACS, Professor of Ophthalmology,Department of Ophthalmology; S. Baltatzis, MD, Associate Professor ofOphthalmology, General Hospital of Athens, University Eye Clinic,Athens Medical School, Athens, Greece.
Address reprint requests to Dr. C.S. Foster, Immunology Service,Massachusetts Eye and Ear Infirmary, 243 Charles Street, Boston, MA02114. E-mail: [email protected]
Submitted February 15, 2000 revision accepted November 21, 2000.
Wegener’s granulomatosis (WG) is an etiologically obscureentity with multiple systemic manifestations. It is one ofseveral vasculitides that primarily affects small calibervessels of the arterial system. Classically, the microvas-culitis produces necrotizing granulomas in the lungs, gener-alized focal necrotizing vasculitis, and glomerulonephritis1.The focal necrotizing vasculitis can involve all organsincluding skin, joints, and eyes.
Patients with WG can be divided into 2 groups: thosewith generalized and those with limited forms of disease2.Patients with generalized WG have renal involvement.Those with limited WG do not, but the respiratory tract is
nearly always affected3-6. Very limited WG may lack respi-ratory and renal disease3,7. The diagnosis of very limitedWG can be difficult, but establishing the diagnosis can haveenormous prognostic and therapeutic importance. Closemonitoring of disease activity and lifelong surveillance todetect relapses early may result in decreases in morbidityand mortality.
A spectrum of antineutrophilic cytoplasmic antibodies(ANCA), autoantibodies against the granules of myeloidcells, may be present in patients with WG. Three majortarget antigens for ANCA were characterized as proteinase 3(PR3), myeloperoxidase (MPO), and bactericidal/perme-ability-increasing protein (BPI)8. The binding of antibody tothese antigens produces 2 immunofluorescent stainingpatterns — cytoplasmic staining (cANCA) and perinuclearstaining (pANCA). Patients with WG are typically cANCApositive; however, up to 5% are pANCA positive. Accordingto one study, a positive cANCA test yields 97% specificityfor WG9. Previous studies showed that the sensitivity ofANCA testing is greater than 88% for cANCA in patientswith active generalized WG, but only about 50% in theactive phase of limited WG, and 32% to 43% in full clinicalremission5,10,11. Classic pathologic diagnostic criteria forWG require the presence of tissue necrosis, vasculitis, and
Wegener’s Granulomatosis: The Relationship BetweenOcular and Systemic DiseaseSTEPHANIE L. HARPER, ERIK LETKO, C. MICHAEL SAMSON, PANOS ZAFIRAKIS, VIRENDER SANGWAN,QUAN NGUYEN, HARVEY UY, STEFANOS BALTATZIS, and C. STEPHEN FOSTER
ABSTRACT. Objective. Wegener’s granulomatosis (WG) is an etiologically obscure entity with multiple systemicmanifestations. Ocular involvement is present in up to 58% of patients with WG. We describe aseries of patients with ocular manifestations of WG to evaluate the presence of ocular lesions in thesetting of systemic WG and to determine the value of ocular inflammation in the diagnosis of WG.Methods. A computerized database was used to generate a list of patients cared for in the OcularImmunology Service of the Massachusetts Eye and Ear Infirmary during the 10 year period 1988–98with a diagnosis of Wegener’s granulomatosis. A detailed chart review was undertaken to determinedemographic characteristics, history, initial manifestation of WG, initial ocular presentation, biopsyresults, laboratory testing results, treatment, total followup period, and final outcome.Results. Forty-seven patients diagnosed with WG were identified. Twenty-eight were women(59.6%), 19 were men (40.4%). The average age was 53 years (range 18–90). Patients were dividedinto 4 groups. Group I included 27 patients (57.4%) who had systemic disease first and who subse-quently developed an ocular lesion. Group II included 3 patients (6.3%) who had ocular inflamma-tion first and who then subsequently developed systemic manifestations of WG. Group III included3 patients (6.3%) who presented due to ocular symptoms but, on initial evaluation by us, were foundto have occult systemic manifestations consistent with WG or biopsy evidence of WG. Group IVincluded 14 patients (30%) with ocular lesions and no history or presence of systemic disease at theirlast followup visit.Conclusion. Ocular inflammation can occur with or without obvious systemic manifestations ofWG. It may represent the first sign of WG that enables the knowledgeable physician to diagnose thispotentially lethal disease. (J Rheumatol 2001;28:1025–32)
Key Indexing Terms:WEGENER’S GRANULOMATOSIS EYE VASCULITIS
Personal non-commercial use only. The Journal of Rheumatology Copyright © 2001. All rights reserved.
www.jrheum.orgDownloaded on December 25, 2020 from

granulomatous inflammation1, but finding the classic triadin biopsy samples varies depending on the tissue biopsied.When 126 biopsy specimens from the head and neck werereviewed by Devaney and associates, for example, only16% were found to have all 3 features12. Evaluation oforbital biopsies has revealed the presence of the triad in 54%of specimens13. Open lung biopsies appear to provide theclassic triad most commonly (91%)5.
Ocular manifestations occur in up to 58% of patients withWG3,5,14,15. The most common findings are conjunctivitis,scleritis, and orbital inflammation resulting in prop-tosis14,16–18. Contiguous ocular manifestations may arisefrom granulomatous inflammation of the sinuses with exten-sion to ocular structures (for example, in orbital inflamma-tion with proptosis). Focal ocular lesions result from a focalvasculitis (for example, in scleritis)15. Up to 33% of patientswith WG lose vision due to severe involvement of theeyes5,16, and patients with an ocular manifestation at thetime of WG diagnosis (up to 45%) can have progressivesystemic manifestations that may ultimately result in deathif appropriate diagnostic evaluation, followup, and treat-ment is not instituted.
We describe a series of patients with ocular manifesta-tions of WG, to evaluate the presence of ocular lesions in thesetting of systemic WG and to determine the value of ocularinflammation in the diagnosis of WG.
MATERIALS AND METHODSPatient identification. A computerized database was used to generate a listof patients cared for in the Ocular Immunology Service of theMassachusetts Eye and Ear Infirmary during the 10 year period 1988–98with a diagnosis of WG. The records were screened to determine whichpatients with systemic disease met the American College of Rheumatology(ACR) 1990 criteria for WG19. In addition, we screened the records todetermine which patients with disease exclusively located to the eye(s) andno history or presence of systemic involvement had distinctive pathologicfeatures on ocular biopsy as described20.
Inclusion criteria. The inclusion criteria for this study were the presence ofan ocular manifestation previously reported to occur as a consequence ofWG (Table 1), plus at least one of 2 features: (1) In patients with systemicdisease (groups I to III) presence of at least 2 of the following 4 ACRcriteria19: abnormal urinary sediment (red cell casts or > 5 red blood cellsper high power field), abnormal findings on chest radiograph (nodules,cavities, or fixed infiltrates), oral ulcers or nasal discharge, and granuloma-tous inflammation on biopsy. (2) In patients with disease located exclu-
sively to the eye(s) and no history or presence of systemic involvementprior to or at the last followup visit (group IV), evidence of at least 3 of thefollowing pathologic findings on ocular biopsy: granulomatous foci,collagen necrosis, plasma cells, neutrophils or their nuclear dust,eosinophils, or vasculitis20.
In patients with systemic disease who underwent biopsy of tissues unre-lated to the eye, pathologic inclusion criteria included one or more of thefollowing: the presence of necrosis or obliterative vasculitis, small vesselvasculitis, granulomatous inflammation, and tissue eosinophilia (Figure 1).
Chart review. A detailed chart review was undertaken to determine demo-graphic characteristics, history, initial manifestation of WG, initial ocularpresentation, biopsy results, laboratory testing results, treatment, totalfollowup period, and final outcome.
RESULTSForty-seven patients were identified who met the inclusioncriteria and who therefore form the basis of this report.Twenty-eight were women (59.6%), 19 were men (40.4%).The average age was 53 years (range 18–90). Patients weredivided into 4 groups (Table 2). Group I included thosepatients who had systemic disease first and who subse-quently developed an ocular lesion. This group represented57.4% of the total (27 patients). We separately analyzedthose patients with a history of systemic disease symptomsand an already established diagnosis of WG (group Ia) andthose with a history of symptoms from systemic disease butwithout a known diagnosis of WG at the time of initialocular presentation (group Ib). Group II included patientswho had ocular inflammation first and who then subse-quently developed systemic manifestations of WG. Thisgroup represented 6.3% of the total (3 patients). Group IIIincluded patients who presented due to ocular symptomsbut, on initial evaluation by us, were found to have occultsystemic manifestations consistent with WG or biopsy
The Journal of Rheumatology 2001; 28:51026
Table 1. Ocular manifestations of Wegener’s granulomatosis.
Conjunctivitis Retinal vasculitisGranuloma formation Retinal detachmentSubconjunctival hemorrhage Retinal artery occlusionsEpiscleritis Orbital inflammationScleritis Orbital cellulitisPeripheral ulcerating keratitis Nasal lacrimal system obstructionUveitis Optic neuropathy
Table 2. Grouping system.
Groups No. of Patients % of Total No.
I. Systemic disease first, then ocular disease 27 57.4Ia. Diagnosis of WG 15 N/AIb. No diagnosis of WG 12 N/A
II. Ocular disease first, then systemic disease 3 6.4III. Ocular symptoms first, systemic disease diagnosed 3 6.4
on initial ocular evaluationIV. Ocular disease only 14 30
Personal non-commercial use only. The Journal of Rheumatology Copyright © 2001. All rights reserved.
www.jrheum.orgDownloaded on December 25, 2020 from

evidence of WG. This group represented 6.3% of the total (3patients). Group IV included patients with ocular lesionsand no evidence of systemic disease prior to or at the lastfollowup visit. This group represented 30% of the total (14patients). The diagnosis of WG was made in 64% of patientsbased on 2 of the required criteria (clinical history +/- char-acteristic biopsy +/- ANCA positivity). All 3 features werepresent in 36% of patients. Two or more characteristic find-ings were present in 76%.
Group I Group Ia. Only 56% of patients with systemic diseaseconsistent with WG prior to ocular presentation carried anestablished diagnosis of WG (Group Ia). The average agewas 55 years (range 18–86). Thirty-four percent of thesepatients were male and 66% were female (Table 3).Systemic manifestations included lesions of the followingorgan systems: sinus (40%), kidney (13%), lung (20%),nasal mucosa (27%), skin (33%), and CNS (7%) (Table 4).Most patients in this group had scleritis (Table 5), present in11 patients (73%). Thirty-six percent of these had scleritiswith associated peripheral ulcerative keratitis (PUK). Othermanifestations included chronic conjunctivitis, proptosis,subconjunctival hemorrhage, and retinal vasculitis. ANCAresults were available for 11 of these 15 patients; 55% (6patients) were ANCA positive (83% cANCA positive, Table6). The average time to ocular presentation was 29 months(range 0.5–60).
Group Ib. Forty-four percent of patients with systemicdisease prior to ocular presentation did not carry a diagnosisof WG (Group Ib). The average age for members of thisgroup was 52 years (range 22–71). There were 6 women and
6 men (Table 3). Systemic manifestations included involve-ment of the sinuses (33%), lungs (33%), kidneys (25%),nasal mucosa (25%), skin (17%), joints (17%), and colon(8%) (Table 4). The average time to ocular presentation andthus diagnosis of WG by us was 64.5 months (range11–168). Most patients (83%) had scleritis. In 50% ofpatients the scleritis was associated with peripheral cornealdisease (Figures 2 and 3). Other ocular manifestationsincluded nodular episcleritis and central retinal artery occlu-sion (Table 5). ANCA results were available for all patientsin this group since we were pursuing a diagnosis (Table 6).ANCA positivity was noted on ocular presentation in 67%;all patients revealed the cANCA pattern. Groups Ia and Ibare compared in Table 7.
Group II Patients in group II had ocular inflammation and subse-quently developed systemic WG. There were 3 patients inthis group, representing 6.3% of the total number of
Harper, et al: Systemic and ocular WG 1027
Table 3. Background characteristics.
Group Group Group Group GroupIa Ib II III IV
n 15 12 3 3 14Women 10 6 2 1 8Men 5 6 1 2 6Average age, yrs 49 52 69 41 57
Table 4. Systemic manifestations (percentages).
Organ Group Group Group GroupIa Ib II III
Kidney 13 25 66 33.3Lung 20 33 66 0Sinus 40 33 0 33.3Nares 27 25 0 33.3Skin 33 17 0 0Arthritis 0 17 0 0CNS 7 0 0 0Colon 0 8 0 0
CNS: central nervous system.
Table 5. Ocular manifestations (percentages).
Group Group Group Group GroupIa Ib II III IV
Scleritis 73 83 100 67 71Necrotizingdisease (scleritis +/– 36 50 66 33 11corneal disease)Others 27 17 0 33 29
Table 6. ANCA results.
N/Total Positive, % cANCA, % pANCA, % Negative, %
Group IIa 11/15 55 83 17 45Ib 12/12 67 100 0 33
Group II 2/3 100 Not Not 0available available
Group III 3/3 66 50 50 34Group IV 12/14 92 64 36 8
Personal non-commercial use only. The Journal of Rheumatology Copyright © 2001. All rights reserved.
www.jrheum.orgDownloaded on December 25, 2020 from

patients. The patients were 76, 68, and 70 years old; 2 werewomen, and all presented with scleritis (necrotizing in 2)(Tables 3 and 5). Systemic manifestations of WG developedan average of 22 months after the ocular presentation andincluded involvement of the kidneys (2 patients) and lung (2patients) (Table 4). Two of these 3 patients were ANCApositive on presentation. One patient did not have an ANCAvalue available at the time of chart review (Table 6).
Group III These patients presented with ocular inflammation and werediscovered to have occult systemic manifestations of WG.There were 3 patients in this group (6.3% of total), onewoman and 2 men. They were younger than the Group IIpatients (average age 41 years) (Table 3). The ocular lesionwas scleritis in 2 patients (necrotizing in one) and a conjunc-tival granuloma in one (Table 5). Sinus, nasal, and renallesions were each present in one patient (Table 4). Twopatients were ANCA positive and one was ANCA negativeat the time of presentation (Table 6).
Group IVAt last followup, 30% of patients had only ocular disease
(14 patients). The ages ranged from 16 to 90 years (averageage 57). There were 8 (57%) women and 6 (43%) men(Table 3). Ten patients (71%) had scleritis (one necrotizing).Other ocular manifestations included PUK, episcleritis, andan inferior rectus muscle mass (Table 5). The biopsy ofocular tissue (sclera 10, conjunctiva 3, inferior rectusmuscle mass 1) was indicated after noninvasive diagnosticmethods failed to detect underlying cause of inflammationin the eye. Histopathologic findings in biopsied tissuerevealed granulomatous foci, eosinophils, and vasculitis inall 14 patients. ANCA testing results were available for 12of these patients. The test was positive in 92% (11 patients).Sixty-four percent were cANCA positive and 36% werepANCA positive (Table 6). Average followup period was 33months (range 3–115) excluding 2 patients with < 3 monthsof followup.
DISCUSSIONThis report documents the ocular and systemic manifesta-tions of WG in 47 patients followed for an average of 34months at a tertiary care institution. We believe this is thelargest series of its kind to date. We wished to understandthe relationship between ocular and systemic disease in WG,
The Journal of Rheumatology 2001; 28:51028
Table 7. Group Ia vs Group Ib.
Percentage of Average Sex Time to Ocular Scleritis, Renal RenalGroup Age, Presentation, mo % Disease, Disease,
I yrs % %
Group 56 49 10 women 29 73 13 20Ia 5 men
Group 44 52 6 women 64.5 83 25 33Ib 6 men
Figure 1. Scleral biopsy from a patient with WG. Note the eosinophilic infiltration.
Personal non-commercial use only. The Journal of Rheumatology Copyright © 2001. All rights reserved.
www.jrheum.orgDownloaded on December 25, 2020 from

Harper, et al: Systemic and ocular WG 1029
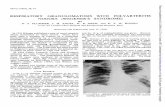
Figure 2. Scleritis in a patient with WG.
Figure 3. Severe necrotizing disease affecting the cornea and sclera in a patient with WG.
Personal non-commercial use only. The Journal of Rheumatology Copyright © 2001. All rights reserved.
www.jrheum.orgDownloaded on December 25, 2020 from

since it has been our experience that eye findings canprecede systemic findings, accompany occult or knownsystemic manifestations, or occur as the only initial mani-festation in WG.
Ocular manifestations in WG. The most common ocularcondition in our cohort of patients was scleritis, present in74% of patients. This likely reflects the nature of referrals toa tertiary ocular immunology service. Other authors havereported the most common manifestation to be orbitaldisease and conjunctivitis21. Necrotizing scleritis (Figure 3)was found in 67% of our patients, and involvement of thecornea was present in the majority. Men appeared to fareworse. Although men and women were equally likely tohave scleritis as their ocular manifestation, men were morelikely to have the necrotizing variety (71%), while womenwere more likely to have non-necrotizing disease (71%).Other ocular manifestations included peripheral ulcerativekeratitis, episcleritis, and sterile corneal ulceration, eachpresent in 2 patients, and central retinal artery occlusion,chronic conjunctivitis, retinal vasculitis, orbital disease withresulting proptosis, subconjunctival hemorrhage, inferiorrectus mass, and conjunctival granuloma, each present inone patient. Other forms of ocular involvement reported inthe literature but not seen in our patient group includeduveitis, retinal detachment, optic neuropathy, orbitalcellulitis, and nasolacrimal duct obstruction4,15,22-26.
Occurrence of ocular disease in the setting of WG. At thetime of onset of eye symptoms, most patients (Groups I, III)already had systemic manifestations of WG (30 patients,64%), but only 50% of these patients had a diagnosis ofWG. The diagnosis of WG was made in the other 15 patientsby our service after the patient was sent to us for manage-ment of the ocular inflammation. In the patients with ahistory of systemic manifestations but no established diag-nosis of WG, the ocular inflammation developed 64.5months after the onset of systemic signs and symptoms,which in retrospect were signs and symptoms of WGinflammatory foci. The systemic manifestations in ourpatients with a history of undiagnosed WG at the time ofocular inflammation presentation included sinusitis, hema-turia, hemoptosis, epistaxis, nasal mucosal ulcers, vasculitisinvolving the skin and colon, and arthritis. ANCA testing,ocular tissue biopsy, or review of previous biopsy resultsenabled us to establish the diagnosis of WG. Ten percent ofthe patients with systemic manifestations of WG in groupsIb and III had no history of systemic symptoms or signs atthe time of initial presentation in our service. However, find-ings on ocular examination resulted in investigations thatidentified systemic involvement consistent with WG inthese patients.
Forty-three percent of patients had eye symptoms prior tothe presentation of systemic signs and symptoms (groups II,III, IV). Fifteen percent of these were found to havesystemic involvement on initial evaluation (group III). In
another 15%, initial systemic investigation was negative,but systemic lesions evolved over time (average 22 mo).Two of the 3 patients in this group (Group II) developednephritis, the most rapidly progressive and severe lesion ofWG. The remaining patients with only ocular signs andsymptoms have had no evidence of systemic disease after 36months’ followup (Group IV). An association betweenhistological findings on biopsies of ocular tissues and posi-tivity of ANCA tests has been described by us as “verylimited Wegener’s granulomatosis” in patients with diseaselocated exclusively to the eye(s)20. Therefore, we make adiagnosis of “very limited WG” even in the absence ofANCA positivity if ocular findings on clinical examinationare consistent with WG and 3 or more of the followingcriteria are present on ocular biopsy: granulomatous inflam-mation, collagen necrosis, plasma cells, vasculitis,eosinophils, neutrophils or their nuclear dust; infectiousetiology is excluded by cultures, special stainings, and poly-merase chain reaction studies. Although the diagnosis of“very limited WG” may be considered controversial bysome physicians, we believe that early immunosuppressivetherapy in such cases plays an important role in haltingdisease progression and preventing potentially fatal conse-quences. Continued observation is obviously required in allpatients with limited forms of WG, since the percentage ofpatients with any given organ involved increases over thecourse of the disease2,3,5. For example, nephritis eventuallydeveloped in 85% of patients with WG, although only 11%showed evidence of it at the time of initial diagnosis in onestudy3. Lung involvement similarly can increase, fromroughly 70% at diagnosis to more than 90% over the courseof followup3. The presence of arthritis and skin lesions hasalso been shown to evolve and ultimately can be found in 67to 85% and 45 to 77% of patients, respectively3,27. Thisconcept of progressive evolution of WG with increasedorgan involvement is important, since compelling evidenceexists indicating that effective early therapeutic interventionsignificantly reduces the likelihood that the disease willprogress to the generalized and potentially lethal form5,17,28.
Relationship between ocular inflammation and diagnosis ofWG. Ocular inflammation resulted in the diagnosis of WGin 32 of our 47 patients (68%). This includes 12 patientswith a history of undiagnosed systemic manifestations, 3patients with only ocular signs and symptoms but evidenceof systemic disease on initial evaluation, 3 patients withocular manifestations first and then evolution to systemicinvolvement, and 14 patients with ocular lesions only at thelast followup visit. This latter group forms the basis of areport by our group alerting readers to the diagnostichistopathologic characteristics of very limited WG localizedto the eye20,29. Our experience on this matter is extendedhere, with 2 patients in Group II, who sought medical atten-tion because of ocular inflammation and were ultimatelyfound (on ocular tissue biopsy) to have histopathologic
The Journal of Rheumatology 2001; 28:51030
Personal non-commercial use only. The Journal of Rheumatology Copyright © 2001. All rights reserved.
www.jrheum.orgDownloaded on December 25, 2020 from

features we believe are diagnostic of WG limited to the eye.The patients were not treated systemically (for a variety ofreasons) and subsequently evolved to have generalizeddisease. Thus, continued followup with intermittentsystemic evaluation is warranted for patients with onlyocular manifestations.
Comparison of Groups Ia and Ib suggests that the delayof 64.5 months in the diagnosis of WG in group Ib may haveresulted in more severe lesions (Table 7). Necrotizing scle-ritis with ulcerative corneal disease was present in 50% ofpatients in group Ib compared to 36% of patients in groupIa. Nephritis was present in 25% of group Ib patients but inonly 13% of Group Ia patients. Earlier diagnosis of systemicinvolvement may have resulted in less severe manifestationsas a result of earlier treatment. Cytotoxic therapy has signif-icantly decreased the morbidity and mortality of WG3,5,27.Prior to the use of cytotoxic therapy in combination withcorticosteroids, the one-year survival was less than 20%,and at 2 years 93% of patients had died18. We now know thatcyclophosphamide therapy can induce disease remission in93% of patients, and as a result, one-year survival rates havebeen reported to be as high as 100% in some series, and at51 months after treatment, 88% of patients are stillalive3,30,31. Clearly, it is imperative that the diagnosis of WGbe made early if one is to initiate treatment at a time when itmay be expected to have such a profound effect on apatient’s life.
Group comparisons. In the setting of systemic disease,women were more likely to have a diagnosis of WG at thetime of ocular presentation. They made up 66% of patientsin Group Ia. Men and women were equally likely to haveundiagnosed systemic disease (Group Ib).
The positive result rate of ANCA tests in our study waslower than that reported in the literature for patients withWG. We believe that this is a consequence of immunosup-pressive therapy at the time we requested the ANCA studies.In the group with known WG (group Ia), 67% of patientswere undergoing cyclophosphamide and prednisonetherapy. One patient was taking cyclosporine, mycopheno-late mofetil, and prednisone, and 2 were taking prednisonealone. Although most patients were on “standard therapy,”the continued development of disease manifestations (ocularinvolvement) suggests that the treatment was inadequate ingroup Ia patients. In the group without known WG at thetime of ocular presentation (group Ib), 33% were on notreatment, 50% were taking prednisone alone, and 2 patientswere being treated with cyclophosphamide and prednisonefor treatment of their sinus disease. This suggests that WGmay have been suspected in these latter 2 patients, althoughthey did not carry it as a diagnosis at the time of ocularpresentation. There were more ANCA positive results in theunknown WG group (Group Ib), consistent with the fact thatmost of these patients were on incomplete or no systemictherapy. Additionally, the results of ANCA testing depend
on the activity and extent of WG5,17. ANCA was positive in77% of patients with generalized disease in our study and in67% of patients with limited disease.
Patient age may be a risk factor for the development ofsystemic WG. In group II (patients who had ocular lesionsfirst and subsequently developed systemic manifestations),all patients were in their sixth or seventh decade (averageage 69 years). This was in contrast to the average age ofpatients in the total study population (53 years), patients ingroups Ia and Ib (average age 55 and 52, respectively),patients in group III (average age 41), and patients in groupIV (57 years). Two of the three patients who subsequentlydeveloped systemic manifestations had kidney involvement.This limited sample size suggests that patients in their sixthor seventh decade with only ocular lesions on initial presen-tation may be at especially high risk for the development ofgeneralized WG; however, a larger cohort of patients has tobe studied to confirm this hypothesis.
Ocular inflammation can occur with or without obvioussystemic manifestations of WG. Ocular involvement may bethe first sign of WG, and it may be the manifestation thatenables the knowledgeable physician to diagnose this poten-tially lethal disease. A delay in diagnosis may contribute to ahigher rate of severe WG manifestations, such as necrotizingscleritis with resultant loss of the eye, and nephritis with itsobvious consequences. We believe that every patient withscleritis or other ocular inflammatory lesions (such as periph-eral ulcerative keratitis) that may occur in WG should bequestioned especially carefully for evidence of extraocularabnormalities worthy of additional study; and that biopsy ofany and all inflammatory foci, including the sclera, is prudent.Radiographic studies of lungs and sinuses as well as serologicstudies, including a search for autoantibodies directed againstneutrophil proteins, are well justified in such patients. Further,reinvestigation is appropriate periodically for patients inwhom no extraocular abnormalities were discovered, but whocontinue to experience chronic or progressive ocular inflam-mation, since WG clearly may begin with ocular inflamma-tion as its sole manifestation, evolving slowly to unmistakablegeneralized (and lethal) WG.
REFERENCES1. Lie JT. Classification of pulmonary angiitis and granulomatosis:
histopathologic perspectives. Semin Respir Med 1989;10:111-21.2. Carrington CB, Liebow AA. Limited forms of angiitis and
granulomatosis or Wegener’s type. Am J Med 1966;41:497-527.3. Fauci AS, Haynes BJ, Katz P, et al. Wegener’s granulomatosis:
prospective clinical and therapeutic experience with 85 patients for21 years. Ann Intern Med 1983;98:76-85.
4. Contu RE, Klein M, Lessell S, Friedman E, Snider GL. Limitedforms of Wegener’s granulomatosis. JAMA 1975;233:868-71.
5. Hoffman GS, Kerr GS, Leavit RY, et al. Wegener’s granulomatosis:an analysis of 158 patients. Ann Intern Med 1992;116:488-98.
6. Liebow AA. The J. Burns Amberson lecture — pulmonary angiitisand granulomatosis. Annu Rev Respir Dis 1973;108:1-18.
7. Cassan SM, Coles DT, Harrison EG. The concept of limited formsof Wegener’s granulomatosis. Am J Med 1970;49:366-79.
Harper, et al: Systemic and ocular WG 1031
Personal non-commercial use only. The Journal of Rheumatology Copyright © 2001. All rights reserved.
www.jrheum.orgDownloaded on December 25, 2020 from

8. Zhao MH, Lockwood CM. A comprehensive method to purify threemajor ANCA antigens: proteinase 3, myeloperoxidase andbactericidal/permeability-increasing protein from human neutrophilgranule extract. J Immunol Methods 1996;197:121-30.
9. Hauschild S, Schmitt WH, Csernok E, et al. ANCA in systemicvasculitides, collagen vascular diseases, rheumatic disorders andinflammatory bowel disease. Adv Exp Med Biol 1993;336:245-51.
10. Cohen JW, van der Woude FJ, Fauci AS, et al. Association betweenactive Wegener’s granulomatosis and anticytoplasmic antibodies.Arch Intern Med 1989;49:2461-5.
11. Nolle B, Specks U, Ludemann J, et al. Anticytoplasmicautoantibodies: their immunodiagnostic value in Wegener’sgranulomatosis. Ann Intern Med 1989;111:28-40.
12. Devaney KO, Travis WD, Hoffman G, et al. Interpretation of headand neck biopsies in Wegener’s granulomatosis. Am J Surg Pathol1990;14:555-64.
13. Kalina PH, Lie JT, Campbell MB, Garrity JA. Diagnostic value andlimitations of orbital biopsy in Wegener’s granulomatosis.Ophthalmology 1992;99:120-4.
14. Haynes BF, Fishman ML, Fauci AS, Wolff SM. The ocularmanifestations of Wegener’s granulomatosis. Am J Med1977;63:131-41.
15. Straatsma BR. Ocular manifestations of Wegener’s granulomatosis.Am J Med 1957;63:789-99.
16. Spalton DJ, Graham EM, Page NGR, Sanders MD. Ocular changesin limited forms of Wegener’s granulomatosis. Br J Ophthalmol1981;65:553-63.
17. Novack SN, Pearson CM. Cyclophosphamide therapy in Wegener’sgranulomatosis. New Engl J Med 1971;284:938-42.
18. Walton EW. Giant-cell granuloma of the respiratory tract(Wegener’s granulomatosis). BMJ 1958;5091:265-70.
19. Leavitt RY, Fauci AS, Bloch DA, et al. The American College ofRheumatology 1990 criteria for classification of Wegener’sgranulomatosis. Arthritis Rheum 1990;33:1101-7.
20. Foster CS, Jakobiec FA, Niffenegger JH, Gion N. Diagnosis of verylimited Wegener’s granulomatosis: distinctive pathologic featureswith ANCA test confirmation. Invest Ophthalmol Vis Sci1999;40:S557.
21. Duncker G, Beigel A, Lehmann H. Wegener’s granulomatosis:ocular manifestations, diagnosis and therapy. Klin MonatsblAugenheilkd 1982;181:184-7.
22. Bullen CL, Liesegang TJ, McDonald TJ, DeRemee RA. Ocularcomplications of Wegener’s granulomatosis. Ophthalmology1983;90:279-90.
23. Cogan DG. Corneoscleral lesions in periarteritis nodosa andWegener’s granulomatosis. Trans Am Ophthalmol Soc1955;53:321-44.
24. Hardwig PW, Bartley GB, Garrity JA. Surgical management ofnasolacrimal duct obstruction in patients with Wegener’sgranulomatosis. Ophthalmology 1992;99:133-9.
25. Janknecht P, Mittelviefhaus H, Loffler KU. Sclerochoroidalgranuloma in Wegener’s granulomatosis simulating a uvealmelanoma. Retina 1995;15:150-3.
26. Rao NA, Marak GE, Hidayat AA. Necrotizing scleritis.Ophthalmology 1985;92:1542-9.
27. Brandwein S, Esdaile J, Danoff D, et al. Wegener’s granulomatosis.Arch Intern Med 1983;143:476-9.
28. Hollander D, Manning RT. The use of alkylating agents in thetreatment of Wegener’s granulomatosis. Ann Intern Med1967;67:393-8.
29. Soukiasian SH, Foster CS, Niles JL, et al. Diagnostic value ofantineutrophil cytoplasmic antibodies in scleritis associated withWegener’s granulomatosis. Ophthalmology 1992;99:125-32.
30. Reza MJ, Dornfeld L, Goldberg LS, et al. Wegener’sgranulomatosis: Long-term follow-up of patients treated withcyclophosphamide. Arthritis Rheum 1975;18:501-6.
31. Israel HL, Patchefsky AS, Saldana MJ. Wegener’s granulomatosis,lymphomatoid granulomatosis, and benign lymphocytic angiitis andgranulomatosis of lung. Ann Intern Med 1977;87:691-9.
The Journal of Rheumatology 2001; 28:51032
Personal non-commercial use only. The Journal of Rheumatology Copyright © 2001. All rights reserved.
www.jrheum.orgDownloaded on December 25, 2020 from