· Web viewA galvanic cell is constructed of two half cells – the oxidation half-cell and the...
Transcript of · Web viewA galvanic cell is constructed of two half cells – the oxidation half-cell and the...

PRODUCTION OF MATERIALS
1. Fossil fuels provide both energy and raw materials such as ethylene, for the production of other substances
- Identify the industrial source of ethylene from the cracking of some of the fractions from the refining of petroleum
Cracking is the process in which heavier fractions of crude oil are converted into lighter fractions with more market demand. Catalytic cracking uses a zeolite catalyst, an aluminium silicate, to crack long-chained hydrocarbons into shorter chains. As a result of this, a lower temperature is required (about 500°C) compared to thermal cracking, the other main cracking process (about 750°C-900°C). Catalytic cracking yields a shorter-chain hydrocarbon and ethylene or propene.
Catalytic cracking is an industrial source of ethylene.
C10H22 (g) C8H18 (g) + C2H4 (g)
Zeolites are suitable catalysts due to their high thermal stability, large surface area since they are porous and are non-toxic.
- Identify that ethylene, because of the high reactivity of its double bond, is readily transformed into many useful products
Alkanes are unreactive. They can only undergo substitution reactions in the presence of UV light. Alkenes are reactive due to their double bond, consisting of a strong sigma bond and weak pi bond. In addition reactions, the double bond opens up to form two single bonds, and a compound (usually diatomic) is added across the bond.
An addition reaction between an alkene and hydrogen gas is called hydrogenation. (Ni, Pd, Pt catalyst required)An addition reaction between an alkene and a halogen is called halogenation.An addition reaction between an alkene and a hydrogen halide is called hydrohalogenation.
- Identify that ethylene serves as a monomer from which polymers are made
Ethylene is a starting material for other monomers such as vinyl chloride, styrene and tetrafluoroethene, itself being a monomer in the production of polyethylene.
- Identify polyethylene as an addition polymer and explain the meaning of this term
An addition polymer is a polymer that is formed when many identical monomers join without a by-product. Polyethylene, polystyrene , polyvinylchloride and Teflon are all addition polymers.
- Outline the steps in the production of polyethylene as an example of a commercially and industrially important polymer

Addition polymerisation
Low density polyethylene is polymerised using an initiator, while recently the Ziegler-Natta process has been used in the production of high density polyethylene.
Steps in polymerisation using an initiator:
Initiation
An initiator molecule is added to start the polymerisation, usually peroxide such as benzoyl peroxide. The initiator is decomposed by laser light or heat to form an initiator radical, which reacts with a monomer unit to form a monomer radical.
R−O + CH∙ 2=CH2 R−O−CH2−CH2∙Propagation
The ethylene monomer radical reacts with another ethene monomer, forming a dimer (two monomers that have been bonded together) radical. More and more alkene monomers are added and the polymer chain grows. Back biting may occur leading to chain branching.
R−O−CH2−CH2 + n[CH∙ 2=CH2] R−O−[CH2−CH2]n−CH2−CH2 ∙Termination
Chain growth terminates when two polymer radicals of variable chain length react with each other, forming a longer
chain. The initiator molecule is part of the chain, so it is not a “catalyst”.
R−O−[CH2−CH2]n−CH2−CH2 + R−O−[CH∙ 2−CH2]n−CH2−CH2 R−O−[CH∙ 2−CH2]n−O−R
- Identify the following as commercially significant monomers by both their systematic and common names
Vinyl chloride
Systematic name: chloroethene. Used as a monomer in the production of polyvinyl chloride.
Styrene
Systematic name: phenyl ethene or ethenylbenzene. Used as a monomer in the production of polystyrene.
- Describe the uses of the polymers made from the above monomers in terms of their properties

PROPERTIES
Average chain length
The longer the average polymer chain length, the higher the molecular mass leading to higher dispersion forces. This means higher melting and boiling points and increased hardness due to stronger dispersion forces.
Chain branching
Long, unbranched chains of polymers allow the chains to align closely and pack together. This leads to an orderly crystalline arrangement with high density. As a result, little chain branching leads to high density, higher melting point and a harder, less flexible material due to the stronger dispersion forces between polymer molecules. However, a high degree of chain branching prevents an orderly alignment of the chains, forming a non-crystalline polymer with many amorphous regions. A polymer with a high degree of chain branching exhibits lower density, lower melting point and greater flexibility.
Side chain
Bulky side chains like the benzene ring of polystyrene restrict movement of the polymer molecules and make it much stiffer and more rigid.
Cross-linking
Polymer chains can be linked by cross-linking covalent or ionic bonds to link individual linear chains to each other, forming an extended 2D lattice. Cross-linking gives rise to higher melting point, rigidity, strength and elasticity. Vulcanising rubber involves adding -S-S- links between chains.
USES
Low density polyethylene (LDPE)
LDPE is produced at high pressure (1000-3000 atm) and high temperatures (300°C) to induce a high degree of chain branching, leading to its low density and flexibility. It is largely transparent since it is amorphous, and is chemically inert. It is used in the production of plastic bags, food wrap, squeeze bottles, electrical insulation etc.
High density polyethylene (HDPE)
HDPE can be produced at lower temperatures of less than 100°C and pressures of less than 50 atm by the use of the Ziegler-Natta catalyst, leading to long unbranched chains and high density and melting point. It is rigid and crystalline, and is translucent as a result of the crystalline areas scattering and refracting light, and is also chemically resistant. HDPE is used for harder items including buckets, crates, rubbish bins and pipes.
PVC
Pure PVC is a hard, brittle polymer due to the stiffness from its Cl side chain. However, the C-Cl bond makes it susceptible to attack from UV light. The properties of PVC are modified by additives; UV absorbers help prevent UV decomposition, and plasticisers soften PVC.
Rigid PVC is used for credit cards, plumbing, guttering etc, while flexible PVC is used in garden hoses, flexible tubing and very commonly as household insulation.
Polystyrene
Polystyrene is commonly manufactured in two forms: crystal and expanded polystyrene, trademarked as Styrofoam. Crystal polystyrene is clear and stiff due to the bulky benzene ring side chain. It can be transparent or translucent and can be coloured to suit the application. It is used for clear CD cases, clear drinking glasses, tool handles etc.

Expanded polystyrene, commonly known as Styrofoam, is produced by blowing gas through liquid polystyrene and allowing it to cool and solidify. It is lightweight, an excellent insulator and is spongy. The sponginess and the insulating properties arise from the gas trapped inside the polymer; the polystyrene itself is still stiff and hard. It has applications such as disposable cups, packing material and ice boxes.
2. Some scientists research the extraction of materials from biomass to reduce our dependence on fossil fuels
- Discuss the need for alternative sources of the compounds presently obtained from the petrochemical industry
Most synthetic polymers manufactured today are derived from fossil fuels, which are non-renewable. In order to preserve fossil fuel reserves, biopolymers derived from biomass have been suggested as a suitable alternative due to the renewability of plant material such as cellulose, or ethanol derived from fermentation.
- Explain what is meant by a condensation polymer
A condensation polymer is one formed from condensation polymerisation, in which monomers chemically combine by eliminating small molecules like water. Examples of natural condensation polymers are cellulose, starch and DNA, while synthetic condensation polymers include polyester and nylon.
- Describe the reaction involved when a condensation polymer is formed
Cellulose
Cellulose is formed from β-glucose monomers, as shown to the right.
The polymerisation of cellulose eliminates one molecule of water per pair of β-glucose monomers that have joined.
Starch
Starch is formed from α-glucose monomers, as shown to the right.
The polymerisation of starch is similar to that of cellulose, eliminating one water molecule per pair of monomers.
Down
Up
Down Down

Polyester
To produce a polyester, two monomer units must be used – a dicarboxylic acid (HOOC-R-COOH) and a dialcohol (HO-R-OH). A water molecule is eliminated and an ester link (-COO-) is formed between the two monomers.
Polyamide
Nylon is an example of a polyamide. To produce a polyamide, two monomer units must be used – a dicarboxylic acid (HOOC-R-COOH) and a diamine (H2N-R-NH2). A water molecule is eliminated and an amide link (-CONH-) is formed between the two monomers, also known as a peptide bond.
- Describe the structure of cellulose and identify it as an example of a condensation polymer found as a major component of biomass
Cellulose is a biopolymer formed by the condensation polymerisation of β-glucose monomers.
Cellulose is produced by plants for use in cell walls. It is a large component of plant matter and biomass.
- Identify that cellulose contains the basic carbon-chain structures needed to build petrochemicals and discuss its potential as a raw material
Cellulose contains the basic structure needed by the petrochemical industry – carbon chains, which give it the potential to be used as a raw material. Cellulose can be used outright to produce paper or rayon, and this is already successful. Breaking cellulose polymers into its individual monomer units, glucose, allows ethanol to be produced from biomass through fermentation, which can be used as a fuel in its own right or can be further dehydrated into ethylene, an important raw material in the petrochemical industry.
However, high temperatures and costs are needed to break cellulose into glucose, either through enzyme activity or acid hydrolysis, and it is more efficient to directly crack the fossil fuels into ethylene. Considerable energy is also needed to plant, fertilise and harvest the energy crops.
- Use available evidence to gather and present data from secondary sources and analyse progress in the recent development and use of a named biopolymer. This analysis should name the specific enzyme(s) used or organism used to synthesise the material and an evaluation of the use or potential use of the polymer produced related to its properties
BIOPOL is a trademark for a copolymer of

Polyhydroxybutyrate/Polyhydroxyvalerate (PHB/PHV). It is produced by bacteria known as Cupriavidus metallidurans (formerly known as Alcaligenes Eutrophus), which produces the biopolymer for storing carbon in an environment with surplus carbon, but limited essential nutrients such as nitrogen or phosphorus.
BIOPOL is insoluble in water, non-toxic, resistant to UV light, biocompatible and biodegradable, properties shared with other biopolymers. As a result, it is used in medicine for internal sutures which do not have to be removed and staples. It has potential uses for food trays, containers and wrapping, food utensils and nappies etc.
3. Other resources, such as ethanol, are readily available from renewable resources such as plants
- Describe the dehydration of ethanol to ethylene and identify the need for a catalyst in this process and the catalyst used
C2H5OH(g) C2H4 (g) + H2O(g)
Concentrated sulfuric acid acts as a catalyst and dehydrating agent, removing water from the ethanol molecule.
- Describe the addition of water to ethylene resulting in the production of ethanol and identify the need for a catalyst in this process and the catalyst used
C2H4 (g) + H2O(g) C2H5OH(g)
This reaction requires an acid catalyst such as dilute sulfuric acid.
- Describe and account for the many uses of ethanol as a solvent for polar and non-polar substances
Ethanol is used as an industrial solvent. It can dissolve polar and non-polar substances, leading to this use. This is due to its polar hydroxyl end (OH) which is able to form dipole-dipole, hydrogen bonds and sometimes ion-dipole with polar substances, and its non-polar ethyl end (CH3CH2) allows it to establish dispersion forces with non-polar substances. This allows it to dissolve a large variety of substances.
- Outline the use of ethanol as a fuel and explain why it can be called a renewable resource
Ethanol has the potential to be a renewable fuel since it can be fermented and produced from plants such as sugar cane. The combustion of ethanol can be used as fuel for automobiles as non-renewable fossil fuel based resources dwindle.
Concentrated H2SO4
Dilute H2SO4

- Summarise the chemistry of the fermentation process
Fermentation is a biochemical process in which glucose is turned into ethanol and carbon dioxide under the action of enzymes produced by yeast in an anaerobic environment.
Yeast is added to the starting carbohydrate, usually starch, sucrose, fructose or glucose. More complex sugars like sucrose, starch or fructose are first broken down into glucose by enzyme activity. In an anaerobic environment yeast will respire the glucose to form ethanol. The maximum concentration of ethanol is about 15% v/v before the yeast die. Distillation is then carried out to increase the ethanol concentration to 95-100%.
C6H12O6 (aq) 2C2H5OH(aq) + 2CO2 (g)
- Describe conditions under which fermentation of sugars is promoted
-Presence of alcohol-tolerant yeast- Suitable grain or fruit mashed up with water- Anaerobic environment- About 37°C for optimum enzyme activity- Suitable nutrient to aid the growth of yeast
- Define the molar heat of combustion of a compound and calculate the value for ethanol from first-hand data
The molar heat of combustion (ΔHc) of a compound is the amount of heat released per mole when it undergoes complete combustion. The accepted value for ethanol is 1367 kJ/mol.
- Assess the potential of ethanol as an alternative fuel and discuss the advantages and disadvantages of its use
Advantages of ethanol as a fuel:
- It can be produced from renewable resources- It is “greenhouse neutral” – the net amount of CO2 released into the atmosphere by the complete combustion of ethanol is zero.- Ethanol burns more cleanly than petrol, containing less carbon than octane (a major constituent of petrol) and already having an O atom in its structure, having a greater tendency to completely combust.
Disadvantages of ethanol as a fuel:
- Engines must be modified to run on fuel containing more that 20% ethanol- Large areas of land must be used to grow production crops, reducing the production of food, and leading to environmental problems such as soil erosion, salinity and deforestation.
- Although in theory, ethanol is greenhouse neutral, it does not take into account other energy inputs during its production such as energy for planting and harvesting the crops, and the energy needed for distillation.- Fermentation wastes present an environmental issue.
- Identify the IUPAC nomenclature for straight-chained alkanols from C1 to C8
The alkanols are a homologous series of compounds derived from alkane-based alcohols. The alcohol functional group is the hydroxyl –OH group.
CnH2n+1OH

4. Oxidation-reduction reactions are increasingly important as a source of energy
- Explain the displacement of metals from solution in terms of transfer of electrons
Displacement reactions are redox reactions in which one of the aqueous ions are displaced from solution, forming usually a solid or gas. Metals behave as reductants – they donate electrons to positive cations in solution. These ions are reduced and are displaced out of solution. The reductant metal is oxidised and its ions enter the solution.
- Identify the relationship between displacement of metal ions in solution by other metals to the relative activity of metals
The ability of a metal to reduce a metal ion into elemental metal can be determined by their respective reduction potentials. A more reactive metal can reduce a less reactive metal ion. If a less reactive metal is placed in a solution with a more reactive ion, there will be no reaction.
- Account for changes in the oxidation state of species in terms of their loss or gain of electrons
The oxidation state of a species is related to the extent that it is oxidised.

An increase in the oxidation state of a species is a loss of electrons.A decrease in the oxidation state of a species is a gain of electrons.
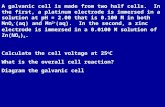
- Describe and explain galvanic cells in terms of oxidation/reduction reactions
Redox reactions can be used to generate electron flow – electricity – in a galvanic cell. A galvanic cell is a device that utilises the chemical energy released by a spontaneous redox reaction to perform electrical work.
Instead of directly placing a metal in a solution for a redox reaction to occur, in a galvanic cell the electrons are forced to travel through an external pathway rather than being transferred through direct contact.
- Outline the construction of galvanic cells and trace the direction of electron flow
A galvanic cell is constructed of two half cells – the oxidation half-cell and the reduction half-cell. Each half-cell is made up of an electrode and an electrolyte solution. The electrode used depends on whether or not the solid form of the substance is involved in the redox reaction. The two half-cells are joined by the external pathway and the salt bridge. The electrons flow from the anode in the oxidation half cell and enter the cathode in the reduction half-cell.
The salt bridge is an electrolyte that joins the two half-cells. It has two functions: to complete the circuit and to allow the migration of ions to neutralise the charges of the two half cells. As the reaction takes place, the loss of electrons in the oxidation half-cell and the gain of electrons in the reduction half-cell need to be neutralised by the migration of ions through the salt bridge. The cations migrate towards the cathode to balance the loss of the displaced cations while the anions migrate towards the anode to balance the gain in cations from the redox reaction.
- Define the terms anode, cathode, electrode and electrolyte to describe galvanic cells
An electrode is a conducting substance such as a metal or graphite that is in contact with the electrolyte in each half-cell. These conductors are connected to the external circuit.
An anode is the electrode at which oxidation occurs. It is the negative electrode and is the electrode where electrons are liberated into the external circuit.

A cathode is the electrode at which reduction occurs. It is the positive electrode and is the electrode where electrons are entering from the external circuit.
An electrolyte is a substance that conducts electricity when in solution or when molten. In a galvanic cell, the two electrolyte solutions are solutions with ions in them. The anolyte is the electrolyte in the anode compartment while the catholyte is the electrolyte present in the cathode department.
- Gather and present information on the structure and chemistry of a dry cell and evaluate it in comparison to the button cell in terms of chemistry, cost and practicality, impact on society and environmental impact
DRY CELL
The dry cell (Leclanche cell) has electrolytes in the form of a solid or paste rather than a liquid. The cell is made from a central graphite rod – the cathode – that is surrounded by manganese dioxide and powdered graphite. Around it is an aqueous ammonium chloride paste – the electrolyte – mixed with more manganese dioxide and powdered graphite. All this is surrounded by a zinc shell – the anode – which is the reductant.
Chemistry
The complete chemical equation for the reaction in the dry cell is:
Zn(s) + 2MnO2 (s) +2NH4Cl(aq) ZnCl2 (aq) + Mn2O3 (s) +2NH3 (aq) + H2O(l)
Oxidation:
Zn(s) Zn2+(aq) + 2e-
Reduction:
2MnO2 (s) +2NH4+
(aq) + 2e- Mn2O3 (s) +2NH3 (aq) + H2O(l)

The reduction involves NH4+ gaining two electrons to form H2 gas and aqueous NH3. The MnO2 then reacts with the
H2 gas to form Mn2O3 and H2O liquid. The above equation is an overall equation for these two reactions.
In the reaction, Zn is oxidised (0 to +2) and Mn is reduced (+4 to +3).
CATHODE – graphite rod surrounded by MnO2
ANODE – zinc casingELECTROLYTE – NH4Cl paste (26% w/w)VOLTAGE – 1.5 V
Cost and Practicality
The Leclanche dry cell is relatively cheap compared to other batteries, even though it is non-rechargeable. It comes in a variety of sizes to suit various needs, and is light and portable to be used in a variety of portable appliances. However, it has a shorter shelf life than some of the other batteries since NH4
+ slowly corrodes the Zn, possibly causing the cell to deteriorate and leak. This is also contributed to by the fact that the zinc casing itself is slowly thinned as it is oxidised. By the end of the 20th century, however, the storage life of zinc-carbon cells had improved 4 times of its predicted life in 1910.
Impact on Society
The dry cell had a huge impact on society – being the first commercially produced battery, it paved the way for a much more mobile society. Portable devices such as torches, radios and clocks would not be possible without dry cells. Today, dry cells are used extensively in devices which do not need high currents such as remote controls and battery powered toys due to their low cost.
Environmental Impact
Dry cells are primary cells, meaning that they are non-rechargeable and once used have to be disposed of. This means that they will end up in landfill. However, it causes minimal environmental problems despite being weakly acidic - the manganese (III) in dry cells is easily oxidised to manganese (IV) oxide which is insoluble and harmless. Ammonium salts and carbon also have no detrimental effect although the zinc may be toxic to some animals such as birds.
BUTTON CELL
There are many different button cells, including mercury (II) oxide, but one of the most commonly used ones is the silver oxide button battery.
The silver-oxide button cell is enclosed in a stainless steel case, the cathode. On the top of the button cell is zinc along with powdered zinc underneath, the

anode. Underneath is a moist paste of KOH which acts as the electrolyte. Finally, powdered silver (I) oxide at the bottom of the cell is reduced into solid silver.
Chemistry
The complete chemical equation for the silver-oxide button cell is:
Zn(s) + Ag2O(s) ZnO(s) + 2Ag(s)
Oxidation:
Zn(s) + 2OH-(aq) ZnO(s) + H2O(l) + 2e-
Reduction:
Ag2O(s) + H2O(l) +2e- 2Ag(s) + 2OH-(aq)
In the complete reaction, KOH(aq) also acts as a catalyst as well as the electrolyte.
In the reaction, Zn is oxidised (0 to +2) and Ag is reduced (+1to 0).
CATHODE – stainless steel case and powdered silver(I) oxideANODE – zinc top and powdered zincELECTROLYTE – KOHVOLTAGE – 1.5 to 1.6 V
Cost and Practicality
Silver oxide button cells are relatively expensive due to the cost of silver, more expensive than other button cells such as the mercury (II) oxide cell. However, very small button cells produce a steady voltage for long periods of time, making it very useful in small appliances. Unlike the dry cell, the steel case itself does not take part in the reaction, so it does not have the problem of the case thinning, extending its shelf life.
Impact on Society
The button cell allowed many miniature appliances to be powered portably. Its small size, constant voltage and long life allow it to have many applications such as in watches, calculators and hearing aids.
Environmental Impact
Silver oxide button cells have significantly less environmental impact than mercury (II) oxide cells since they do not produce the highly toxic wastes that the latter does. Like the dry cell, it is non-rechargeable meaning that one it is flat, it is disposed of. Although zinc, zinc oxide and silver oxide are all stable, insoluble and non-toxic, leakage of the highly alkaline potassium hydroxide could have many adverse effects on the environment.
5. Nuclear chemistry provides a range of materials
- Distinguish between stable and radioactive isotopes and describe the conditions under which a nucleus is unstable

Isotopes of an element have the same atomic number but a different mass number due to different numbers of neutrons. The stability of a nucleus is dependent on the ratio of neutrons to protons (n:p), and can be represented by the zone of stability.
Stable isotopes:
- Z≤20, n:p = 1- Z>20, n:p>1- 73≤Z≤83, n:p = 1.5
Unstable isotopes:- Z>83- Z≤83, n:p outside stability
zone
Too many neutrons to protons:
Beta decay occurs. A neutron decays into a proton and electron.
n→01 p1
1 + e−10 (β )
Too many protons to neutrons:
Positron decay or electron capture occurs. In positron decay, a proton is converted into a neutron and a positron (an electron with positive charge, a form of antimatter). When a positron and electron collide, the whole mass is annihilated and gamma is emitted.
p→11 n0
1 + e10 ¿
Electron capture occurs when one of the inner-orbital electrons is captured by the nucleus. It converts a proton into a neutron.
p+ e−10 →1
1 n01
Nucleus is too large:
Alpha decay occurs in nuclei that are too large, typically those with atomic number greater than 83.
e.g.
U92238 → Th90
234 + He24 (α )

Energised nucleus:
Metastable nuclei will emit gamma radiation, as will many other nuclear reactions that have excess energy.
e.g.mTc43
99 → Tc4399 +γ
- Describe how transuranic elements are produced
Transuranic elements are elements with atomic numbers greater than 92 (Uranium). Neptunium and plutonium (93 and 94) are produced in nuclear reactors, while others are produced in a particle accelerator or cyclotron to bombard the target nuclei with neutrons or nuclei of other elements.
U92238 → U92
239 → Np93239 → Pu94
239
U+ ¿92238 n0
1 → U92239 ¿
U92239 → Np93
239 + e−10
Np93239 → Pu94
239 + e−10
The latest official element, Copernicium (element 112) was produced by bombarding Pb82208 with Zn30
70 .
Pb82208 + Zn30
70 → Cn→112278 Cn112
277 + n01
- Describe how commercial radioisotopes are produced
Most commercial radioisotopes for use in industry, medicine and research are either produced in a nuclear reactor or a cyclotron, a type of particle accelerator. Nuclear reactors are sources of neutrons and are used for neutron bombardment, while cyclotrons are used to bombard a suitable targe nucleus with a small ion such as a hydrogen, helium or carbon nucleus. Linear accelerators can also be used for similar effect, but take up more space than relatively compact cyclotrons.
- Identify instruments and processes that can be used to detect radiation
Photographic film
Radioactivity darkens photographic film. The extent of darkening is proportional with the length of exposure and the intensity of the radiation. Researchers working with radiation sometimes wear a radiation badge containing photographic film to monitor their level of exposure to radiation.
Cloud chamber
A cloud chamber contains supersaturated vapours of water or alcohol. As ionising radiation passes through it, it ionises the air molecules. The supersaturates vapours condense onto these ions which creates small droplets or cloud trails which can be used to detect the radiation; alpha radiation causes thick straight trails, beta radiation forms thinner longer zigzag trails while gamma radiation forms long weak trails.
Geiger-Müller Counter

As ionising radiation passes through the window and enters the GM tube, it causes ionisation of the argon gas in the tube. A high potential difference is applied across the circuit, and the liberated electrons accelerate rapidly towards the positive electrode causing a cascade of electrons arriving at the positive electrode. This creates an electric pulse which is amplified and counted in the circuit, generating audible “clicks”.
- Identify one use of a named radioisotope in industry and in medicine

- Describe the way in which the above named industrial and medical radioisotopes are used and explain their use in terms of their properties
Sodium-24
Sodium-24 is used in detecting underground pipe leaks by adding Na-24 to the water or oil. It is produced in a nuclear reactor and is a β and γ emitter. A leak can be detected by monitoring the radioactivity of the surrounding soil; an unexpectedly high level of radiation detected indicates a possible leak. The physical properties that make it suitable for this purpose is that it is soluble in water, and it has a half-life of 15 hours so that it does not cause any serious permanent pollution to water bodies.
Na+ ¿1123 n0
1 → Na1124 ¿
Na1124 → Mg12
24 + e−10 +γ
Technetium-99m
Technetium-99m is the most widely used medical radioisotope, used for medical diagnosis. It is produced in a nuclear reactor and is a gamma emitter. The chemical property that makes Tc-99m useful is its multivalency which allows it to form many different compounds. For example, combined with a tin compound it can be injected into the bloodstream where it attaches to red blood cells. The physical property that makes it suitable is its short half-life of about 6 hours, meaning there is no permanent radioactivity after diagnosis and causes minimal damage to the patient.
Mo4298 + n0
1 → Mo4299
Mo4299 → m
Tc4399 + e−1
0
mTc43
99 → Tc4399 +γ
- Use available evidence to analyse benefits and problems associated with the use of radioactive isotopes in identified industries and medicine

Benefits
- Commercial radioisotopes have many useful applications in industry and medicine.- Allows preservation of food and sterilisation of equipment by irradiating it with γ radiation from a source such as Co-60.- Allows detection of structural faults in industry- Allows non-invasive diagnosis and radiation therapy for cancer in medicine
Problems
- Ionising radiation is harmful to organisms, causing tissue damage, disrupting cellular processes, cancer development and genetic damage.- People who are regularly exposed to radiation for extended periods of time, usually those working with radioisotopes in research, medicine and industry need to take extra cautionary measures to monitor and reduce the amount of received radiation, including radiation badges and protective clothing.