VRFB Electrolyte Preparation With Reducing Agents 2013
-
Upload
cristian-fernandez -
Category
Documents
-
view
2 -
download
0
description
Transcript of VRFB Electrolyte Preparation With Reducing Agents 2013

Seediscussions,stats,andauthorprofilesforthispublicationat:http://www.researchgate.net/publication/268099758
VanadiumRedox-Flow-BatteryElectrolytePreparationwithReducingAgents
CONFERENCEPAPERinECSTRANSACTIONS·MAY2013
DOI:10.1149/05307.0093ecst
READS
15
5AUTHORS,INCLUDING:
MikeL.Perry
UnitedTechnologiesResearchCenter
32PUBLICATIONS651CITATIONS
SEEPROFILE
YingShe
UnitedTechnologiesResearchCenter
11PUBLICATIONS47CITATIONS
SEEPROFILE
Availablefrom:MikeL.Perry
Retrievedon:08October2015

Vanadium Redox-Flow-Battery Electrolyte Preparation with Reducing Agents
W. N. Li, R. Zaffou, C. Shovlin, M. Perry, and Y. She
United Technologies Research Center (UTRC), East Hartford, CT, 06108, USA
A waste-free method was developed to prepare electrolytes usingreducing agents for vanadium redox flow battery. Via thisapproach, both the electrolyte cost and waste can be reduced by33% which are favored financially and environmentally.Thermodynamic-model calculations show 100% conversion onreaction between fully-charged vanadium positive solution (VO2
+)and oxalic acid. The kinetics on the redox reaction is discussed aswell.
Introduction
To meet increasing energy demands, research effort has been devoted to thedevelopment of new and renewable energy resources. A Redox-Flow-Battery (RFB) isattractive due to its applications in large-scale energy storage [1]. RFB is a type ofrechargeable battery consisting of two tanks of soluble redox reactants, which determinethe system energy storage capacity, and the battery stack, which determines the systempower capacity. Since the Iron/Chromium (Fe/Cr) RFB was initiated by NASA in 1970s,various redox chemistries have been developed and evaluated, such aspolysulphide/bromine RFB, all-vanadium RFB, and others [2]. Among these RFBsystems, Vanadium Redox Flow Batteries (VRFB) are considered especially attractive,since the electrochemically active reactants are vanadium species in four differentoxidation states in both electrolyte solutions. As such, the undesired cross-contaminationof negative and positive electrolytes through the cell separator is eliminated, althoughreactant crossover still results in coulombic-efficiency losses.
VRFB employs the redox couples of V2+/V3+ as negative electrolyte and VO2+/VO2+
(i.e., V4+/V5+) as positive electrolyte, both dissolved in aqueous-acid solutions, which arestored in two external tanks. During charge and discharge, the two electrolyte solutionsare pumped from the storage tanks to cells in one or more cell stacks, where theconversion between chemical energy and electrical energy takes place. The reversiblepotentials of the V2+/V3+ couple and V4+/V5+ couple are -0.26 V (RHE) and 1.0 V (RHE),respectively.
The electrolyte solutions are one of the key components of a VRFB system. Therefore,research has been devoted to the development of VRFB electrolyte production, includingon a large scale [3]. However, many of the reported preparations have variousdisadvantages, which limit scalability and/or have negative cost implications. Forexample, in order to lower the electrolyte cost, the use of commodity vanadiumcompound of V2O5 as the starting vanadium material to prepare VRFB electrolytes hasattracted much research attention [4]. However, the very low solubility of V2O5 preventsthe direct production of solutions with high vanadium concentrations. Other vanadiumcompounds, such as V2O3 and VCl3, have been investigated as initial compounds.

However, their applications are restricted due to cost, low solubility and/or low stability,especially for large scale systems. Therefore, currently, a common route to make VRFBelectrolytes, especially on a lab-scale, is to dissolve vanadyl sulfate (V4+) in sulfuric-acidsolution because it readily dissovles in aqueous solutions and is an electrolyte solutionwith excellent stability. Subsequently, the vanadyl sulfate (V4+) acid solution is chargedto V2+ and V5+ in negative and positive electrolytes, respectively. However, in order toaccommodate a change of two oxidation states in the negative solution (i.e., convert V4+
to V2+), the initial volume of positive electrolyte is twice that required, and the excesspositive electrolyte (33%) must be disposed of as waste. This is obviously notenvironmentally or financially desirable.
This paper will discuss approaches to produce VRFB electrolyte solutions with highenergy densities without generating waste, which is accomplished by utilizing reducingagents at relatively benign conditions.
Experimental
1. Electrolyte preparationThis approach enables starting a VFRB with equal volumes of vanadyl sulfate (V4+) in
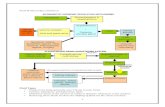
aqueous acid for the negative and positive solutions. After an initial charge of the VRFBto V3+ in the negative electrolyte and V5+ in the positive electrolyte, a reducing agent isintroduced into the positive side to reduce the produced V5+ solution back to V4+, whichprovides discharged VRFB electrolytes of V3+ on negative and V4+ in the positivesolutions, respectively. The overall process is depicted in Figure 1. The reducing agentdiscussed in this work is-oxalic acid.
2. Thermodynamic modelThe thermodynamic model is built up to evaluate the reaction conversion and
equilibrium constant for the redox process of V5+ and oxalic acid using HSCChemistry5.0 software (Outotec Research Oyj, Finland).
3. Analysis:3.1 Chemical analysisVanadium concentrations before and after the reduction are determined by titration,
which is also used to calculate the reaction conversion and monitor the kinetics.3.2 Electrochemical testElectrochemistry test was carried out on UTRC subscale cell, which are composed of
Nafion 117 membranes between identical carbon electrodes on both sides. The cell activearea was 23 cm2. The electrolyte reservoirs contain 70 mL of electrolyte on each sideunder nitrogen purge. Constant current at 87mA.cm-2 was used to charge and dischargethe cell and polarization curves were collected during the tests.

Results and Discussions
The selection of reducing agents for VRFB is dictated by several key requirementsincluding no introduction of foreign cations, mild redox reaction conditions, high reactionconversion, and high rate. To meet the requirement of no foreign cations in the system,only several reducing agents which have lower standard electrode potentials thanVO2+/VO2
+ (i.e., V4+/V5+) can be chosen for this system, such as oxalic acid, formic acid,and sulfur dioxide as shown in Equations 1-4 [5]. However, the other two requirementsfurther restrict the selection. According to the equation of Gibbs free energy and totalpotential as indicated in Equation 5, reducing agents with lower standard electrodepotentials will have a higher tendency for the redox reaction to occur spontaneously.Therefore, oxalic acid is the most favorable reducing agent in this system.
The standard electrode potentials of VO2+/VO2+ (i.e., V4+/V5+) and oxalic acid are
shown in Equations 1-2. Equation 3 indicates the chemical reaction between them, whichresults in the total potential to 1.49V (Equation 6). The Gibbs free energy of the reactionis around -144kJ at standard conditions (Equation 5) which indicates that oxalic acid cantheoretically convert V5+ solution to V4+. Equations (1), (2), and (6) can be representedby a full chemical reaction without electric charges in Equation (7). HSC Chemistry5.0thermodynamic software was used to calculate the equilibrium constant and equilibriumcompositions of the reaction presented by Equation (7). Equilibrium conversion of(VO2)2SO4 was also calculated. Figure 2 shows the equilibrium constant and theequilibrium conversion as a function of temperature. The equilibrium constant valueswere between 1*1050 and 1*1055 at temperatures between 20 °C and 100 °C, indicatingthat the reaction can be completed thermodynamically. As a result, the equilibriumconversions were 100% in the temperature range calculated. However, in practice,
Figure 1. Simple VRFB electrolyte preparation method with no waste stream.

0%
10%
20%
30%
40%
50%
60%
70%
80%
90%
100%
1.0E+50
1.0E+51
1.0E+52
1.0E+53
1.0E+54
1.0E+55
0 20 40 60 80 100 120
Equ
ilib
riu
mco
nve
rsio
n
Equ
ilib
riu
mco
nst
ant
Temperature (°C)
Equlibrium constantEquilibrium conversion
Initial amounts for equilirbiumconversion calculation:(VO2)2SO4: 1 kmoleH2SO4 : 1 kmoleH2C2O4 : 1 kmole
Pressure = 1 atm
100% conversion cannot be obtained at lower temperatures such as room temperature in ashort period of time. For instance, around 6-8 hours is necessary to reach 90-95%conversion at room temperature, compared to 90-95% in 2 hours at 60 oC. Therefore, areasonable amount of heat is preferentially provided to the reaction to trigger the light-offof the reaction. Once the reaction initiated, no additional heat was needed because thereaction was exothermic. The use of reducing agents has been discussed by others toconvert vanadium pentaoxide to V4+ (e.g., [4]). However, the reaction kinetics observedhere are significantly better than what one might expect with the two-phase reaction thanwhat was reported before [4].
VO2+ + 2H+ + e- VO2+ + H2O E0 = +1.00V [1]
1/2 CO2 + 2H+ + 2e- H2C2O4 E0 = -0.49V [2]CO2 + 2H+ + 2e- HCOOH (aq) E0= -0.199V [3]1/2 SO4
2- + 2H+ + e- 1/2 H2SO3 + 1/2 H2O E0= 0.172V [4]Go = -nEoF [5]
2VO2+ + H2C2O4 2VO2+ + 2CO2 + 2H2O E0 = +1.49V [6]
(VO2)2SO4 + H2SO4 + H2C2O4 = 2VOSO4 +2H2O + 2CO2 [7]
Also electrochemical performance of a moderate power density VFRB cell, both withand without oxalic acid, was investigated in the lab to determine if this new preparation
Figure 2. Equilibrium constant and conversion calculation on equation 7. (HSCChemistry5.0 software (Outotec Research Oyj, Finland))

process had any impact on cell performance. The baseline electrochemical performanceof the cell was done in the conventional procedure below:
1) Double volume of V4+ sulfuric acid solution was used for positive electrolyte ascompared to that of negative electrolyte.
2) The electrolytes were fully charged to obtain V2+ and V5+ on negative and positivesolutions, respectively.
3) Half volume of the positive charged V5+electrolyte was decanted as waste.4) The remaining half of positive electrolyte and the negative electrolytes are now
equal volumes, which were then used for the cell polarization measurements.Another cell experiment was accomplished by utilizing the addition of reducing agent
of oxalic acid to eliminate the waste, as follows:1) Equal volumes of V4+ solutions were employed as the initial electrolytes;2) V4+ solutions were charged until fully-charged yellow V5+ was obtained in the
positive side.3) Oxalic acid was added into the V5+ electrolyte at either 60 oC for 2 hours or at room
temperature for overnight.4) When 85-95% of conversion of V5+ to V4+ was achieved, the positive electrolyte was
poured back to the electrolyte reservoir.5) Polarization curve was performed.
During the reaction time between V5+ and oxalic acid, V2+ solution in negative sidewas stored for the subsequent use by protection of N2 blanket above solution. The colorchange of the solution can be used as an indicator to determine if the reaction iscompleted. Usually, the solution will be in dark blue color after the reaction at 60 oC for 2hours or room temperature for overnight, which indicates 90-95% V5+ was reduced to V4+.A much longer time, such as a week at room temperature, is required to obtain bright blueV4+ solution.
Figure 3 shows that the electrochemical performance of cell is not affected by theelectrolyte-production method described herein (open-black line), relative to the resultsobtained with the more conventional, waste-generating method (filled-grey line).
Figure 3. Polarization curves of VRFB using electrolytes without H2C2O4
(baseline) and with H2C2O4 addition.

Conclusions
A method to prepare waste-free VRFB electrolytes was developed via anintroduction of a reducing agent in the fully-charged positive electrolyte. Thermodynamicmodeling predicts the reaction conversion can be 100% from room temperature to 100 oC,which has been confirmed with experimental data given sufficient reaction periods. Theelectrochemical tests showed the cell performance was not affected by the oxalic acidpreparation method, which therefore offers a more benign approach for VRFBresearchers and developers to make electrolyte solutions for both lab-scale experimentand larger scale systems.

Acknowledgements
The authors would like to thank their flow-battery project colleagues at UTRC. Thework presented herein was funded, in part, by the Advanced Research Projects Agency -Energy (ARPA-E), U.S. Department of Energy (DOE) under Award Number DE-AR0000149.
The information, data, or work presented herein was funded in part by an agency ofthe United States Government. Neither the United States Government nor any agencythereof, nor any of their employees, makes any warranty, express or implied, or assumesany legal liability or responsibility for the accuracy, completeness, or usefulness of anyinformation, apparatus, product, or process disclosed, or represents that its use would notinfringe privately owned rights. Reference herein to any specific commercial product,process, or service by trade name, trademark, manufacturer, or otherwise does notnecessarily constitute or imply its endorsement, recommendation, or favoring by theUnited States Government or any agency thereof. The views and opinions of authorsexpressed herein do not necessarily state or reflect those of the United States Governmentor any agency thereof.
References1. Z. Yang, J. Zhang, M.C.W. Kintner-Meyer, X. Lu, D. Choi, J.P. Lemmon, and J. Liu,
“Electrochemical Energy Storage for Green Grid,” Chem. Reviews, 111, 3577-3613(2011).
2. A. Z. Weber, M. M. Mench, J. P. Meyers, P. N. Ross, J. T. Gostick, and Q. Liu,“Redox flow batteries: a review” J. Appl Electrochem., 41, 1137-1164 (2011).
3. G. Kear, A. A. Shah, and F. C. Walsh, “Development of the all vanadium redox flowbattery for energy storage: A review of technological, financial and policy aspects,” Int.J. Energy Res., 36, 1105–1120 (2012).
4. M. Skyllas-Kazacos and M. Kazacos. “Vanadium compound dissolution process” WO89 / 05363 (1989) (No issued patent found).
5. D. R. Lide, CRC Handbook of Chemistry and Physics, 71 Ed. P 8-16, CRC Press, Inc.(1990)