Published in Nature Materials (2016) doi:10.1038/nmat4569 ...
Vol 453 1 May 2008 doi:10.1038/nature06733 LETTERS
Transcript of Vol 453 1 May 2008 doi:10.1038/nature06733 LETTERS
LETTERS
Hydatellaceae are water lilies with gymnospermoustendenciesWilliam E. Friedman1
The flowering plant family Hydatellaceae was recently discoveredto be allied to the ancient angiosperm lineage Nymphaeales (waterlilies)1. Because of its critical phylogenetic position, members ofthe Hydatellaceae have the potential to provide insights into theorigin and early diversification of angiosperms2. Here I report thatHydatella expresses several rare embryological features that, incombination, are found only in members of the Nymphaeales.At maturity, the female gametophyte is four-celled, four-nucleateand will produce a diploid endosperm, as is characteristic ofmost early divergent angiosperm lineages3,4. As with all membersof the Nymphaeales, endosperm in Hydatella is minimallydeveloped and perisperm is the major embryo-nourishing tissuewithin the seed5,6. Remarkably, Hydatella exhibits a maternal seed-provisioning strategy that is unique among flowering plants, butcommon to all gymnosperms7: pre-fertilization allocation ofnutrients to the embryo-nourishing tissue. This exceptional caseof pre-fertilization maternal provisioning of a seed in Hydatellamay well be an apomorphic feature of Hydatellaceae alone but,given the newly discovered phylogenetic position of this family,potentially represents a plesiomorphic and transitional conditionassociated with the origin of flowering plants from gymnosper-mous ancestors.
For over a century, the flowering plant family Hydatellaceae wasthought to belong to the Poales, a highly derived monocot order thatincludes the grasses1,2. New evidence derived from DNA sequencingand phylogenetic analysis shows that this obscure group of minuteaquatic plants is closely related to water lilies (Nymphaeales)1, whichthemselves were only recently discovered to be one of the mostancient extant lineages of angiosperms8–12. As a result of these sur-prising phylogenetic insights, our understanding of the earliestphases of the radiation of angiosperms continues to be very muchin flux3,13.
Although the data are, at best, ambiguous, the original embryolo-gical studies of Hydatellaceae5,14,15 suggested that the female gameto-phyte might contain eight nuclei and seven cells, with antipodals thatdegenerate early (or are absent), and possibly two polar nuclei; as such,Hydatellaceae was thought to produce a triploid genetically biparentalendosperm. Recently, however, members of the Nymphaeales andAustrobaileyales (another ancient lineage of flowering plants) havebeen shown to produce an unusual four-celled, four-nucleate femalegametophyte (Nuphar/Schisandra-type) that lacks antipodal cells anda second polar nucleus (Fig. 1). Importantly, the target of the secondfertilization event, the central cell of the female gametophyte, is hap-loid in Nymphaeales and Austrobaileyales, and endosperm in theseancient lineages is diploid, not triploid as in most flowering plants3.Because of the recent phylogenetic insights into what constitute themost ancient angiosperm lineages1, Hydatellaceae might be predictedto share some of the rare and potentially conservative embryologicalfeatures of water lilies and other ancient lineages of flowering plants.
Working with field-collected material of Hydatella inconspicua, Iexamined female gametophyte development and ontogenetic fea-tures of the formation of embryo-nourishing reserves in the ovule/seed. The mature female gametophyte of H. inconspicua contains fouruninucleate cells: the egg cell, two synergids and a uninucleate centralcell (Fig. 2). No antipodal cells are evident, nor is a second polarnucleus present within the central cell. However, in many floweringplants with seven-celled, eight-nucleate female gametophytes(Polygonum-type), the three antipodal cells degenerate and thetwo haploid nuclei of the central cell fuse before the fertilizationprocess3. The result is a female gametophyte that is structurally indis-tinguishable, at maturity, from a ‘true’ four-celled female gameto-phyte (Nuphar/Schisandra-type) that never forms antipodal cells ora second polar nucleus. To distinguish between these two types offemale gametophyte, early developmental stages must be examined(Fig. 1).
Critically, at the two-nucleate syncytial stage in H. inconspicua,both nuclei are located at the micropylar pole of the female gameto-phyte (Fig. 2a). This is precisely the pattern exhibited by otherfour-nucleate, four-celled gametophytes found in Nymphaealesand Austrobaileyales, and differs from the comparable ontogeneticstage of Polygonum-type (and Amborella-type13) gametophyteswhere the nuclei at the two-nucleate syncytial stage are displaced
1Department of Ecology and Evolutionary Biology, University of Colorado, Boulder, Colorado 80309, USA.
Nuphar/Schisandra-type
Polygonum-type
Figure 1 | Schematic of four-celled, four-nucleate (Nuphar/Schisandra-type) female gametophyte development in Nymphaeales andAustrobaileyales and of seven-celled, eight-nucleate (Polygonum-type)development in most other angiosperms. The micropylar pole is towardsthe top of the figure. At the two-nucleate syncytial stage (blue boxes), water-lily female gametophytes have both nuclei at the micropylar pole; inPolygonum-type female gametophytes, a nuclear migration event leads toplacement of a single nucleus at each pole. Red, synergids; yellow, egg cell;brown, antipodals. cc, central cell. pn, polar nucleus.
Vol 453 | 1 May 2008 | doi:10.1038/nature06733
94Nature Publishing Group©2008
to opposite poles of the female gametophyte (Fig. 1). Thus, Hydatellaproduces a four-celled, four-nucleate female gametophyte thatprecisely matches (developmentally and at maturity) the uniqueand potentially plesiomorphic pattern found in two of the mostancient extant angiosperm lineages (Fig. 2c)16,17. The finding that
Hydatella produces a female gametophyte identical to those foundin the water lilies is clearly concordant with both the phylogeneticplacement of Hydatellaceae as sister to the previously recognizedNymphaeales and its general position among ancient extant angio-sperm clades.
cc
pn
ecsc
a b
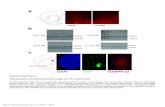
Figure 2 | Female gametophyte development in Hydatella inconspicua.a, Two-nucleate syncytial female gametophyte in H. inconspicua, with bothnuclei (red arrowheads) at micropylar pole. Grey box contains digitalsuperposition of second nucleus from adjacent histological section.
b, Mature four-celled female gametophyte in H. inconspicua. Grey boxcontains digital superposition of second synergid nucleus from adjacenthistological section. cc, central cell; ec, egg cell; pn, polar nucleus; sc, synergidcell.
Figure 3 | Pre- and post-fertilization development of perisperm inH. inconspicua. a, Longitudinal section of perisperm in pre-fertilizationovule at two-nucleate stage of female gametophyte development. b, Cross-polarization optical image demonstrating presence of starch from boxedregion in a. Starch grains, which are birefringent, appear as four whitequadrants separated by a black cross. c, Longitudinal section of perisperm inpre-fertilization ovule just before fertilization. d, Cross-polarization opticalimage demonstrating presence of large amounts of starch from boxed region
in c. Starch grains, which are birefringent, appear as four white quadrantsseparated by a black cross. e, Longitudinal section of perisperm in post-fertilization seed with three-celled embryo and minimally developedendosperm. f, Higher magnification from boxed region in e of embryosurrounded by a small endosperm tissue that lacks significant storagereserves. The perisperm below the endosperm contains large amounts ofstarch (bright circular structures). Scale bars, 10mm. em, embryo; es,endosperm; fg, female gametophyte; ps, perisperm.
NATURE | Vol 453 | 1 May 2008 LETTERS
95Nature Publishing Group©2008
Although endosperm is initiated in H. inconspicua (Fig. 3), peri-sperm, a diploid tissue derived from the maternal sporophyte, isthe major embryo-nourishing constituent within the seed5,15,18, as ischaracteristic of all members of the Nymphaeales2,19,20. The sexuallyformed endosperm ultimately occupies a very small portion of thematuring seed (Fig. 3) and does not play a significant role in thenourishment of the embryo.
Remarkably, the embryo-nourishing tissue within the ovule/seedin Hydatella begins to acquire significant carbon resources (starch)from the maternal plant before fertilization. At the two-nucleate stageof pre-fertilization female gametophyte development, the perispermcontains reserves of starch (Fig. 3). At the four-nucleate, four-celledstage of female gametophyte development, just before fertilization,cells of the perisperm are densely packed with starch grains. Afterfertilization (Supplementary Data) the seed continues to develop andthe volume of the perisperm increases (Fig. 3). Overall seed sizeincreases from approximately 450 mm in length by 235 mm in widthat the time of fertilization to 620 mm in length by 420 mm in width atmaturity. Thus in Hydatella a significant portion of the maternalcommitment of embryo-nourishing carbon reserves to ovules/seedsoccurs before fertilization.
Angiosperms (with the exception of Hydatellaceae) differ fromextant gymnosperms (conifers, Ginkgo, cycads, Gnetales) in thatmaternal commitment of embryo-nourishing resources to seeds onlyoccurs after fertilization21,22. This ontogenetic delay in seed provi-sioning (compared with ancestral non-flowering seed plants) haslong been viewed as an evolved and adaptive mechanism to allowthe maternal plant to allocate limited resources efficiently onlyto those seeds that have been successfully fertilized. As such, post-fertilization maternal resource allocation has been hypothesized to bea key innovation associated with the origin and subsequent radiationof flowering plants22.
The exceptional case of pre-fertilization maternal provisioningof a seed in Hydatella may well be an apomorphic feature ofHydatellaceae alone. If so, Hydatellaceae stands as an essentiallyunique angiosperm clade that has reverted to allocating resourcesto an ovule/seed before the initiation of an embryo (albeit to theperisperm and not to the female gametophyte, as in gymnosperms).Because most taxa of flowering plants use a sexually formedendosperm to nourish an embryo, pre-fertilization allocation ofembryo-nourishing reserves to the seed is ontogenetically precluded.However, members of a large number of disparate clades of angio-sperms, including many basal lineages (Nymphaeaceae, Cabombaceae,Trimeniaceae, Acoraceae, Ceratophyllaceae, Saururaceae, Piperaceae,Hydnoraceae), form an embryo-nourishing perisperm23–28. None ofthese diverse perisperm-forming lineages (with the possible exceptionof Acorus) allocates significant carbon resources to the ovule/seedbefore fertilization23–29. Importantly, if pre-fertilization provisioningof embryo-nourishing reserves should prove to be an apomorphy ofHydatellaceae, it is more strong proof that the earliest phases offlowering plant evolution were marked by a tremendous diversifica-tion of reproductive features.
Alternatively, pre-fertilization maternal resource allocation toovules/seeds, and specifically to a maternally derived perisperm, inHydatella could represent a plesiomorphic and transitional con-dition associated with the origin of flowering plants. If so, the ‘under-developed’ endosperm of Hydatellaceae and Nymphaeales is notreduced, but rather represents an intermediate condition (betweengymnosperms and other angiosperms) in which the endosperm hasnot yet achieved its fully fledged role as the primary source of nutri-ents for the developing embryo. It is important to note that Amborellaand most members of the Austrobaileyales contain a well-developedendosperm20 and do not form a perisperm (Trimenia being thereported exception27). Nevertheless, the prospect that early angios-perms might have used both a perisperm and an endosperm to nour-ish the embryo within a seed (as previously hypothesized24,30), andthat the maternal plant allocated reserves to this perisperm before
fertilization, is thoroughly congruent with the data derived fromHydatella and its new-found phylogenetic position.
Charles Darwin was among the first to recognize the immensechasm between gymnosperms and angiosperms with respect to theirbiological characteristics (letters to Oswald Heer in 1875 and toJoseph Hooker in 1879 and 1882). Since then, inferring the vegeta-tive, floral and reproductive features that defined the first angios-perms, as well as their evolutionary (transformational) links to agymnospermous ancestor, has proved to be fraught with difficulties.Indeed, the past five years have witnessed the near-global collapse of acentury-old set of paradigms concerning the embryological featuresof the earliest angiosperms3,13,17,20. With the present finding thatmaternal plants of Hydatella provision ovules/seeds with embryo-nourishing reserves before fertilization, yet another long-standinghypothesis for a presumed angiosperm-defining biological featureappears poised to be overturned.
METHODS SUMMARY
Hydatella inconspicua was collected on 6 December 2006 at Kai Iwi Lake,
Northland, New Zealand, by P. Champion, New Zealand National Institute of
Water and Atmospheric Research.
Plants were chemically fixed in 4% glutaraldehyde, washed in phosphate
buffer and stored in water. Specimens were dehydrated through an ethanol
series, then infiltrated and embedded in glycol methacrylate (JB-4 embedding
kit, Electron Microscopy Sciences). Embedded flowers were serially sectioned
into 4-mm thick ribbons. Sectioned flowers were stained with 0.1% toluidine blue
and examined under brightfield and cross-polarization conditions. Digital
imaging was on a Zeiss Axiocam digital camera using brightfield and cross
polarization optics. Images were processed with Adobe Photoshop CS2. Image
manipulations were restricted to operations applied to the entire image, except
as noted in specific figure legends.
Received 19 November 2007; accepted 15 January 2008.Published online 19 March 2008.
1. Saarela, J. M. et al. Hydatellaceae identified as a new branch near the base of theangiosperm phylogenetic tree. Nature 446, 312–315 (2007).
2. Rudall, P. J. et al. Morphology of Hydatellaceae, an anomalous aquatic familyrecently recognized as an early-divergent angiosperm lineage. Am. J. Bot. 94,1073–1092 (2007).
3. Williams, J. H. & Friedman, W. E. Identification of diploid endosperm in an earlyangiosperm lineage. Nature 415, 522–525 (2002).
4. Tobe, H., Kimoto, Y. & Prakash, N. Development and structure of the femalegametophyte in Austrobaileya scandens (Austrobaileyaceae). J. Plant Res. 120,431–436 (2007).
5. Hamann, U. Neue Untersuchungen zur Embryologie und Systematik derCentrolepidaceae. Bot. Jahr. 96, 154–191 (1975).
6. Johri, B. M., Ambegaokar, K. B. & Srivastava, P. S. Comparative Embryology ofAngiosperms. (McGraw-Hill, New York, 2002).
7. Friedman, W. E. & Carmichael, J. S. Heterochrony and developmental innovation:evolution of female gametophyte ontogeny in Gnetum, a highly apomorphic seedplant. Evolution 52, 1016–1030 (1998).
8. Mathews, S. & Donoghue, M. J. The root of angiosperm phylogeny inferred fromduplicate phytochrome genes. Science 286, 947–950 (1999).
9. Parkinson, C. L., Adams, K. L. & Palmer, J. D. Multigene analyses identify the threeearliest lineages of extant flowering plants. Curr. Biol. 9, 1485–1488 (1999).
10. Qiu, Y.-L. et al. The earliest angiosperms: evidence from mitochondrial, plastidand nuclear genomes. Nature 402, 404–407 (1999).
11. Soltis, P. S., Soltis, D. E. & Chase, M. W. Angiosperm phylogeny inferred frommultiple genes as a research tool for comparative biology. Nature 402, 402–404(1999).
12. Graham, S. W. & Olmstead, R. G. Utility of 17 chloroplast genes for inferring thephylogeny of the basal angiosperms. Am. J. Bot. 87, 1712–1730 (2000).
13. Friedman, W. E. Embryological evidence for developmental lability during earlyangiosperm evolution. Nature 441, 337–340 (2006).
14. Hamann, U. Hydatellaceae – a new family of Monocotyledoneae. N.Z. J. Bot. 14,193–196 (1976).
15. Hamann, U. in Families and Genera of Vascular Plants vol. IV (ed. Kubitzki, K.)231–235 (Springer, Berlin, 1998).
16. Friedman, W. E., Gallup, W. N. & Williams, J. H. Female gametophytedevelopment in Kadsura: implications for Schisandraceae, Austrobaileyales, andthe early evolution of flowering plants. Int. J. Plant Sci. 164 (suppl.), S294–S305(2003).
17. Friedman, W. E., Madrid, E. N. & Williams, J. H. Origin of the fittest and survival ofthe fittest: relating female gametophyte development to endosperm genetics. Int.J. Plant Sci. 169, 79–92 (2008).
LETTERS NATURE | Vol 453 | 1 May 2008
96Nature Publishing Group©2008
18. Rudall, P. J. The nucellus and chalaza in monocotyledons: structure andsystematics. Bot. Rev. 63, 140–184 (1997).
19. Schneider, E. L. Morphological studies of the Nymphaeaceae. IX. The seed ofBarclaya longifolia Wall. Bot. Gaz. 139, 223–230 (1978).
20. Floyd, S. K. & Friedman, W. E. Developmental evolution of endosperm inbasal angiosperms: evidence from Amborella (Amborellaceae), Nuphar(Nymphaeaceae), and Illicium (Illiciaceae). Plant Syst. Evol. 228, 153–169(2001).
21. Stebbins, G. L. Flowering Plants: Evolution above the Species Level (Harvard Univ.Press, Cambridge, Massachusetts, 1974).
22. Tiffney, B. H. in Paleobotany, Paleoecology, and Evolution (ed. Niklas, K. J.) 193–230(Praeger, New York, 1981).
23. Cocucci, A. E. Estudios en el genero Prosopanche (Hydnoraceae). III Embriologia.Kurtziana 9, 19–39 (1976).
24. Rudall, P. J. & Furness, C. A. Systematics of Acorus: ovule and anther. Int. J. PlantSci. 158, 640–651 (1997).
25. Floyd, S. K. & Friedman, W. E. Evolution of endosperm developmental patternsamong basal flowering plants. Int. J. Plant Sci. 161 (suppl.), S57–S81 (2000).
26. Prakash, N. & Bak, H. K. Flower and fruit development in Piper nigrum L. cv.Kuching. Malays. J. Sci. 7, 11–19 (1982).
27. Prakash, N. in Plant Form and Function (eds Bhatia, B., Shukla, A. K. & Sharma, H. L.)207–216 (Angkor, New Delhi, 1998).
28. Johnson, D. S. On the development of Saururus cernuus L. Bull. Torrey Bot. Club 27,365–372 (1900).
29. Shamrov, I. I. in Embryology of Flowering Plants (ed. Batygina, T. B.) 169–170(Science Publishers, Enfield, New Hampshire, 2006).
30. Doyle, J. A. in Early Evolution of Flowers (eds Endress, P. K. & Friis, E. M.) 7–29(Springer, Vienna, 1994).
Supplementary Information is linked to the online version of the paper atwww.nature.com/nature.
Acknowledgements I thank: P. Champion and A. Drinnan for collecting plantmaterials; S. Holloway for histological work; S. Renner for translation of theembryological studies of U. Hamaan; and P. Diggle, L. Hufford, J. Williams andR. Robichaux for feedback on this manuscript. This work was supported by aNational Science Foundation Research Grant.
Author Information Reprints and permissions information is available atwww.nature.com/reprints. Correspondence and requests for materials should beaddressed to W.E.F. (e-mail: [email protected]).
NATURE | Vol 453 | 1 May 2008 LETTERS
97Nature Publishing Group©2008